ABSTRACT
Otitis media (OM) is a common disease of childhood and available pneumococcal conjugate vaccines (PCVs), with different compositions, could have different impact on OM reduction. This systematic literature review evaluated available data describing the efficacy, effectiveness, and impact of 10-valent pneumococcal Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) and 13-valent PCV (PCV13) on OM outcomes. Statistically significant reductions in all-cause and complicated OM, tympanostomy tube placement and OM-related hospitalizations were consistently observed after the introduction of PHiD-CV and PCV13. Impact studies with data in children <2 years of age using PCV13 report 47–51% and PHiD-CV 34–43% reduction of all-cause OM (primary care, outpatient, ambulatory, emergency department visits) compared to periods before PCV introduction. When the impact of both vaccines is assessed in comparable settings, some studies suggest PHiD-CV may offer better protection against some OM outcomes. Well-designed, head-to-head comparisons are needed to better understand the differences and guide vaccination policies.
KEYWORDS: effectiveness, impact, incidence, otitis media, pneumococcal conjugate vaccine, systematic literature review, vaccine
Plain Language Summary
What is the context?
Pneumococcal vaccines are highly effective in preventing pneumonia and meningitis in children. The two main pneumococcal vaccines are PHiD-CV (Synflorix, GSK) and PCV13 (Prevenar 13, Pfizer). Both vaccines have been shown to provide protection against otitis media despite differing in their composition.
However, it is currently unknown if both vaccines confer similar level of protection against otitis media.
What is new?
We conducted a literature review to evaluate the effects of PHiD-CV and PCV13 on otitis media.
- From 33 articles, we found that:
- ‡Both vaccines were effective in reducing doctor visits for otitis media as well as the number of severe cases and cases requiring hospitalization.
- ‡Four studies suggested a higher level of protection provided by PHiD-CV compared to PCV13, although more data is needed to confirm this finding.
What is the impact?
Available information shows that PHiD-CV and PCV13 are effective in preventing a proportion of otitis media during childhood.
Given the remaining substantial burden associated with the disease and the related significant usage of antibiotics, the development of improved vaccines with higher impact on otitis media would be welcome.
Introduction
The introduction of pneumococcal conjugate vaccines (PCVs) into national immunization programs for infants has had remarkable and sustained public health impacts. PCVs reduce the incidence of invasive pneumococcal disease (IPD) in infants and young children as well as IPD due to antibiotic-resistant strains, pneumonia and otitis media (OM), leading to reduced outpatient visits, hospitalizations, antibiotic use and deaths.1–6 By preventing nasopharyngeal colonization by Streptococcus pneumoniae, PCVs induce substantial herd protection, leading to vaccine impacts in unvaccinated age-groups including young infants and adults.7,8
For outcomes such as IPD which can be readily identified in hospital settings and culture-confirmed, the efficacy, effectiveness and wider impacts of PCVs on disease incidence have been well described.9,10 Quantitation of the impact of PCVs on OM and pneumonia has been less straightforward.6,11 Acute OM (referred to in this review as ‘OM’) is one of the most frequent illnesses of childhood. However, in practice, the diagnosis is usually made clinically, and culture confirmation is rarely undertaken. The incidence of OM and whether an etiology is sought depend strongly on the setting; being less likely in general practices (GPs), outpatients, and emergency departments (EDs) than hospitals or specialist clinics; and on local treatment practices including clinical thresholds for hospitalization, the prescription of antibiotics, myringotomy, and tympanostomy tube placement (TTP).
The first PCV (7-valent; PCV7, Prevenar/Prevnar, Pfizer Inc.) was licensed in 2000 and was replaced from 2009 by higher valency PCVs containing 10 (pneumococcal non-typeable Haemophilus influenzae [NTHi] protein D conjugate vaccine [PHiD-CV], Synflorix, GSK) or 13 (PCV13, Prevenar 13/Prevnar 13, Pfizer Inc.) capsular polysaccharide antigens. PHiD-CV contains 8 serotypes (1, 4, 5, 6B, 7F, 9V, 14, 23F) conjugated to Protein D, a recombinant non-lipidated form of a highly conserved cell-surface lipoprotein of NTHi, 1 serotype (18C) conjugated to tetanus toxoid, and 1 (19F) to diphtheria toxoid (total 16 µg polysaccharide). PCV13 has 13 serotypes (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 23F, total 30.8 µg polysaccharide) conjugated to mutant diphtheria toxoid. A second 10-valent PCV (SIIPL-PCV, Pneumosil, Serum Institute of India Private Limited) was recently pre-qualified by the World Health Organization.12 As it is too soon to evaluate its impact on pneumococcal disease, this vaccine is not discussed further in this review.
Extensive experience with PHiD-CV and PCV13 show they provide high levels of protection against IPD with no consistent evidence of a difference in their overall impact,13,14 although direct comparisons have not been possible due to inter-study heterogeneity.15
In the pre-PCV period the most frequently identified bacterial causes of OM and pneumonia were S. pneumoniae and H. influenzae.16–18 PHiD-CV is the only PCV to use Protein D as the conjugate protein. Protein D induces an immune response that could help to reduce the burden of OM caused by NTHi.19 However, most studies conducted to date were not powered to assess vaccine efficacy against OM caused by H. influenzae.19–21 On the other hand, PCV13 includes 3 additional serotypes compared to PHiD-CV. Therefore, both vaccines provide benefits as compared to PCV7.
In randomized controlled trials (RCTs) of PCV7 and PHiD-CV, vaccination prevented between 6% and 20% of all-cause or clinical OM in children <2 years of age.20–22 No RCTs to evaluate efficacy of PCV13 in preventing OM have been conducted. Despite evidence from RCTs that PCVs prevent a proportion of OM cases,20–22 quantifying and comparing the impact and effectiveness of PCVs in real-world settings is difficult due to marked heterogeneity between studies in their design, disease definitions and outcomes studied.
Because OM is such a common disease of childhood, even small differences between vaccines in OM prevention could have marked population impacts with implications for cost-effectiveness analyses and potential flow-on effects such as reduced antibiotic use. Estimation of the PHiD-CV-preventable disease incidence in Finland found that even though vaccination prevented 94% of IPD cases, this represented 75 cases per 100,000 person-years and contributed to only 0.6% of the overall vaccine effect and only 4.4% of the reduction in healthcare costs. By contrast, although vaccine efficacy was substantially less (13%) against TTP and (7%) antimicrobial prescriptions for treatment of OM, together these 2 outcomes were reduced by 12,524 per 100,000 person-years by vaccination. The 2 OM outcomes contributed to 95.7% of the overall reduction in PHiD-CV-preventable diseases and to 71.8% of the reduction in in healthcare costs.23
A systematic literature review assessed the effect of PCVs in preventing OM in children up to 12 years of age but focused exclusively on RCTs where PCV was compared to placebo or control vaccine.24 RCTs provide only one facet of the evaluation of potential vaccine impacts on infectious diseases and an assessment of the impact of PCVs in routine use on OM and related outcomes is lacking. We reviewed available data describing the efficacy, effectiveness and impact of PHiD-CV and PCV13 against OM outcomes and explored a possible comparison between both vaccines.
A summary contextualizing the relevance, the results and the impact of our study is described in the plain language summary (Figure 1).
Figure 1.
Plain language summary.
Methods
The study was conducted using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Search strategy, article selection and data extraction
A literature search was conducted for English-language publications in MEDLINE (via PubMed) and EMBASE from 01/01/2009 to 01/07/2020. The search terms are provided in Supplementary Box S1. Studies were accepted from any country. The population of interest was children aged <6 years in whom the incidence of OM is highest. Studies with data in individuals up to 21 years of age were included if they reported data specifically in age-groups less <6 years, or if the median age of the population was <6 years.
Eligibility criteria
Articles were included if they were observational studies or RCTs investigating PHiD-CV and/or PCV13 describing efficacy/effectiveness and/or impact. Vaccine efficacy refers to the percentage difference in disease in vaccinated compared to unvaccinated individuals and measures how the vaccine performs under ideal circumstances in a regimented protocol in healthy individuals; vaccine effectiveness refers to the difference in the incidence of disease in vaccinated versus unvaccinated individuals within similar populations and is a measure of real-world benefit to individuals for whom vaccination is recommended; vaccine impact refers to the fraction of disease prevented in a population. This can be assessed by comparing incidence or prevalence of disease in comparable populations in the pre- and post-vaccine eras, or by measuring population-level trends over time.
Studies were included if they provided information on OM or any related outcome or proxy, such as TTP, OM-related hospitalizations, ED or outpatient visits. Articles were excluded if they reported the burden of disease only, health-economic or cost-effectiveness estimations, modelling/simulations/extrapolation, literature reviews, or conference abstracts. References of review articles were scanned for other articles of potential relevance.
Outcome measures
The primary outcome of interest was all-cause acute OM or OM (globally referred to here as OM) episodes or outpatient visits, irrespective of causative pathogen. We considered all-cause OM as the endpoint most relevant for children, parents and physicians. Secondary outcomes included complicated and severe OM, all-cause OM-related hospitalizations, and TTP procedures.
The feasibility of comparing PHiD-CV and PCV13 based on the measured outcomes was also assessed. However, a meta-analysis could not be performed due to the very high level of heterogeneity between the studies.
Data extraction
Two reviewers independently performed title/abstract selection of the papers using Rayyan25 applying the pre-defined selection criteria. Discrepancies were discussed and if not resolved, a decision was taken by a third reviewer.
In the second phase, full length articles were assessed for eligibility by a single reviewer. A second reviewer independently examined a random sample of 10% of full-length articles. A disagreement of more than 10% between the two reviewers triggered independent full assessment of all papers by the second reviewer. Any discrepancies unable to be resolved were decided by a third reviewer.
Relevant information from each study for extraction included population characteristics, study design, study period, source of data, exposure, and estimates of vaccine efficacy/effectiveness or impact. For primary and secondary outcomes, estimates were extracted as reported (e.g., relative risks, hazard ratios and other measures with their accompanying 95% confidence intervals [CI], if available). To obtain percentage reductions in outcomes, we calculated the reduction as (1 minus the reported ratio)*100. Data extraction was carried out by a single reviewer and was checked by a second independent reviewer.
Quality assessment
The quality of each full-length study included in the data extraction was assessed using a validated risk of bias tool appropriate for the study type. The methodological risk of bias was rated by applying the ROBINS-I tool26 recommended by Cochrane Collaboration for non-randomized studies of interventions, and the ROBINS-II tool,27 a revised tool to assess risk of bias in randomized trials. Final results of the ROBINS tools led to an overall methodological rating of low, moderate, serious or critical risk of bias.
Results
We identified 33 unique articles of which 4 reported vaccine efficacy, 4 reported vaccine effectiveness, 21 reported vaccine impact, and 4 reported comparisons between PHiD-CV and PCV13 (Figure 2). Key features of each study are provided in Table 1.
Figure 2.
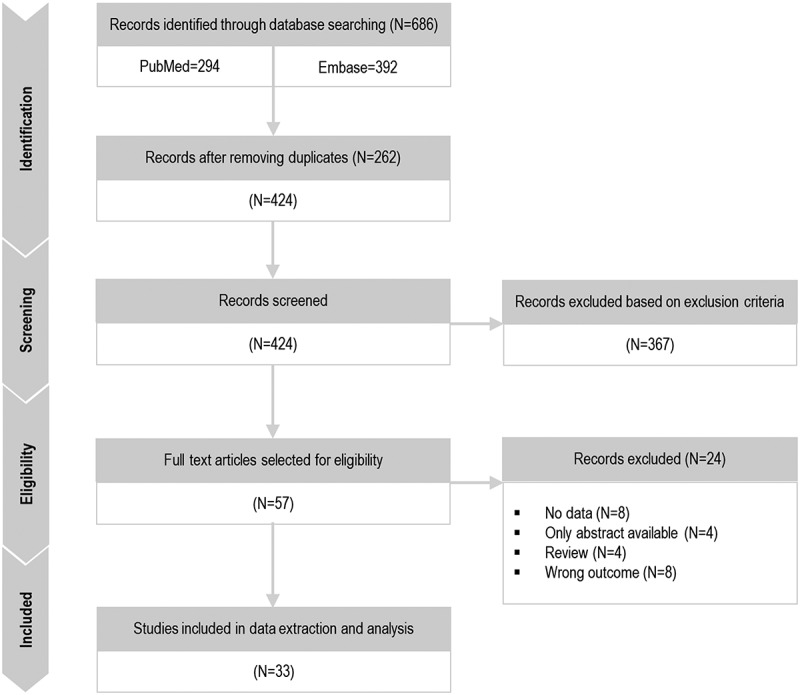
PRISMA diagram.
N, number of articles.
Table 1.
Key features of studies reporting impact, efficacy or effectiveness of PHiD-CV and/or PCV13
| Author, countryref | Vaccine | Schedule | Study design | Population | Sample size | Study period | Primary outcome | Outcome source | Effect |
|---|---|---|---|---|---|---|---|---|---|
| Palmu, Finland28 | PHiD-CV | <7 m: 3 + 1 or 2 + 1 7–11 m: 2 + 1 12–18 m: 2 + 0 |
RCT double-blind cluster (FinIP) |
Children <19 m who received PHiD-CV or hepatitis control vaccine | 30,527 age <7 m enrolled. 4369 TTPs reported in 3594 children |
Feb 2009 – Dec 2011 | TTP | Nationwide database, NOMESCO, reimbursement codes | Efficacy |
| Vesikari, Finland58 | PHiD-CV | <7 m: 3 + 1 or 2 + 1 7–11 m: 2 + 1 12–18 m: 2 + 0 |
RCT double-blind cluster (nested within FinIP) |
Children 6 w-18 m who received PHiD-CV or hepatitis control vaccine | 4117 vaccinated infants for OM assessment. 6161 OM episodes | Feb 2009- Dec 2011 | OM and NPC | RCT Self-reported |
Efficacy |
| Tregnaghi, Argentina, Colombia, Panama21 | PHiD-CV | 3 + 1 | RCT double-blind | Children enrolled 6–16 w who received PHiD-CV or hepatitis control vaccine | Approximately 24,000 infants enrolled. 3602 vaccinated and 3612 controls for OM assessment | Jun 2007 – Jul 2011 | OM, pneumonia, IPD | Clinical definition | Efficacy |
| Saez-Llorens, Panama20 | PHiD-CV | 3 + 1 | RCT double-blind | Children enrolled 6–16 w who received PHiD-CV or hepatitis control vaccine | 3602 vaccinated and 3612 controls for OM, 503 with MEF collected | Aug 2007–Jul 2011 | OM and NPC | Clinical definition and laboratory result | Efficacy |
| Karppinen, Finland29 | PHiD-CV | <7 m: 3 + 1 or 2 + 1 7–11 m: 2 + 1 12–18 m: 2 + 0 |
Cohort prospective | Children <19 m enrolled in FinIP vaccine trial, 2009–2010 | 424 children. 521 episodes of RTI with OM | Apr 2008–Apr 2012 | RTI with or without OM | Clinical definition | Effectiveness |
| Pichichero, US30 | PCV7 PCV13 |
3 + 1 | Cohort prospective | Healthy children ≤30 m seen as outpatients | 239 received PCV13, 348 received PCV7 (Oct 2007–Sept 2009) | Sept 2010–Sept 2013 | OM caused by PCV13-nonPCV7 serotypes | Private practice, clinical definition and laboratory result | Effectiveness |
| Ochoa-Gondar, Spain31 | PCV7 PCV13 |
Not described | Surveillance active prospective | Children ≤14 y | 78 with pneumococcal OM | 2007–2013 | Pneumococcal OM | Population-based, laboratory confirmed | Effectiveness |
| Sohn, Korea32 | PHiD-CV PCV13 |
3 + 1 | Cohort retrospective | Children born Jan 2013–Jun 2015, mean age 32.6 m ± 8.6 m |
990,224 children, 591,529 OM cases | 2013–2016 | OM, IPD, pneumonia | Nationwide database, ICD10 | Effectiveness |
| Lau, UK33 | PCV7 PCV13 |
2 + 1 | Cohort retrospective | Children <10 y | 567,275 children (240,419 OM episodes) | 2002–2012 | OM, antibiotic prescriptions |
Nationally representative database, ICD10, reimbursement codes | Impact |
| Wiese, US34 | PCV7 PCV13 |
3 + 1 | Cohort retrospective | Children <2 y | 368,063 children (618,968 OM episodes, 24,875 TTP) | 2006–2014 | OM, TTP, recurrent OM | State-wide database, ICD9, ICD10 | Impact |
| Zhou, US35 | PCV7 PCV13 |
3 + 1 | Ecological | Children <5 y | Not described | 1997–2013 | OM | Nationally representative database, ICD9-CM | Impact |
| Kawai, US36 | PCV7 PCV13 |
3 + 1 | Cohort retrospective | Children <19 y with an OPD visit for OM | 24,148 OM-related visits | 1997–2014 | OM | Nationally representative database, ICD9 | Impact |
| Fortanier, Netherlands37 | PCV7 PHiD-CV |
3 + 1 and 2 + 1 after 2013 | Cohort retrospective | Children <5 y born 2004–2015 | 18,237 children (6967 first OM episodes) | 2004–2015 | OM | Nationwide database ICPC | Impact |
| Sigurdsson, Iceland38 | PHiD-CV | 2 + 1 | Cohort retrospective | Children <3 y born 2005–2015 | 53,150 children (58,794 OM episodes) | 2005–2015 | OM | Whole population database, ICD10 | Impact |
| Sartori, Brazil39 | PHiD-CV | 3 + 1 | Cohort retrospective | Children 2–23 m | 4793 children with 6401 OM OPD visits | Aug 2008–Jul 2015 | OM | Municipal database, ICD10 | Impact |
| Rosenblut, Chile40 | PHiD-CV | 3 + 1 (2011), 2 + 1 (from 2012) | Case-control nested | Children <24 m attending ED | OM: 1907 Controls: 244,334 |
2007–2015 | OM | Hospital database, ICD10 | Impact |
| Suarez, Peru41 | PCV7 PHiD-CV |
2 + 1 | Cohort retrospective | Children <1 y | 70,670 OM-related OPD visits | 2006–2012 | OM, pneumonia | Nationwide database, ICD10 | Impact |
| Marom, Israel42 | PCV7 PCV13 |
2 + 1 | Cohort retrospective | Children <16 y (mean 1.1–3.3 y for pneumococcal infections) hospitalized with head/neck infections | 787 head/neck infections, including 529 OM | 2007–2014 | OM, head/neck infections | Hospital database, ICD9 | Impact |
| Laursen, Denmark43 | PCV7 PCV13 |
Not described | Cohort retrospective | Children <16 y with OM hospitalization or mastoiditis (median age 14–29 m) |
246 cases of complicated OM | 2001–2015 | Complicated OM | Hospital ENT clinic database | Impact |
| Tawfik, US44 | PCV7 PCV13 |
3 + 1 | Cohort retrospective | Hospitalized children mean age 4.5–7.1 y with OM/complicated OM | >7,000,000 hospitalizations annually. 3343 for OM/complicated OM in 2000, 2279 in 2012 |
2000–2012 | OM/complicated OM | Nationally representative database, ICD9 | Impact |
| Fortunato, Italy45 | PCV7 PCV13 |
2 + 1 | Case-control matched | Children <5 y hospitalized for OM | 28 pediatric wards | 2006–2012 | OM, IPD, pneumonia | Nationwide database, ICD9 | Impact |
| Leach, Australia46 | PCV7 PHiD-CV |
3 + 1 | Surveillance active prospective | Aboriginal children <6 y | 895 (451 PHID-CV vaccinated, 444 PCV7 vaccinated)** | Sep 2008–Dec 2012 | OM | Clinical definition and laboratory result | Impact |
| Sigurdsson, Iceland47 | PHiD-CV | 2 + 1 | Cohort retrospective | Children <3 y born 2005–2015 | 51,264 children (256 children OM hospitalized, 280 OM hospitalizations) | 2005–2016 | OM, IPD, pneumonia, sepsis, meningitis | Hospital database, ICD10 | Impact |
| Petousis-Harris, New Zealand48 | PCV7 PHiD-CV PCV13 |
3 + 1 | Cohort retrospective | Children born 2006–2015, <6 y | 44,545 hospitalized for OM | 2006–2015 | OM, IPD, pneumonia | Database, ICD10 | Impact |
| Ben-Shimol, Israel49 | PCV7 PCV13 | 2 + 1 | Surveillance active prospective | Children <3 y with OM resulting in MEF culture | 7705 OM episodes with MEF culture | Jul 2004–Jun 2016 | Complex OM | Hospital laboratory result | Impact |
| Ben-Shimol, Israel50 | PCV7 PCV13 |
2 + 1 | Surveillance active prospective | Children <3 y with OM resulting in MEF culture | 7475 OM episodes with MEF culture | Jul 2004–Jun 2015 | Complex OM | Hospital laboratory result | Impact |
| Ben-Shimol, Israel51 | PCV7 PCV13 |
2 + 1 | Surveillance active prospective | Children <2 y with OM resulting in MEF culture | 6122 OM episodes with MEF culture | Jul 2004–Jun 2013 | Complex OM | Hospital laboratory result | Impact |
| Palmu, Finland52 | PHiD-CV | 2 +1 | Cohort retrospective | Vaccine eligible 3–54 m old children | unknown | Jun 2010–Sept 2014 | TTP, antibiotic purchases (OM) | Nationwide database, NOMESCO, reimbursement codes | Impact |
| Eythorsson, Iceland53 | PHiD-CV | 2 + 1 | Cohort retrospective | Children <5 y born 2005–2015 | 51,247 children (14,351 underwent 20,373 procedures) | 2005–2016 | TTP | Whole population database reimbursement codes | Impact |
| Gisselsson-Solen, Sweden54 | PCV7 PHiD-CV PCV13 |
2 + 1 | Cohort retrospective | Children <4 y with OM-related diagnosis | 21 Swedish regions | 2005–2014 | OM and TTP | Whole population database, ICD10 | Comparison |
| Edmondson-Jones, Sweden55 | PCV7 PHiD-CV PCV13 |
2 + 1 | Cohort retrospective | ≤5 y living in Skåne (PCV7 then PHiD-CV) or VGR (PCV7 then PCV13) | 191,596 born in Skåne, 250,327 born in VGR; 1999–2013 | 2005–2013 | OM | Regional database, ICD10 | Comparison |
| Leach, Australia56 | PHiD-CV PCV13 |
3 + 1 | Surveillance active prospective | Aboriginal children <6 y | 651 (511 PHID-CV vaccinated, 140 PCV13 vaccinated)* | Feb 2010– Aug 2013 |
OM, NPC | Clinical definition and laboratory result | Comparison |
| Lee, Korea57 | PHiD-CV PCV13 |
3 + 1 | Cohort prospective | Healthy infants enrolled <2 m who visited 8 hospitals | 305 children, 182 received PCV13, 123 received PHiD-CV |
Nov 2014– May 2019 |
OM, NPC | Hospital clinical definition | Comparison |
ED, emergency department; ENT, ear-nose-throat; FinIP, Finnish Invasive Pneumococcal trial; ICD, International Classification of Diseases; ICD-CM, International Classification of Diseases, Clinical Modification; ICPC, International Classification of Primary Care; IPD, invasive pneumococcal disease; m, months of age; MEF, middle ear fluid; NOMESCO, Nordic Medico-Statistical Committee; NPC, nasopharyngeal carriage; OM, otitis media/acute otitis media; OPD, outpatient department; PCV7/PCV13, 7- or 13-valent pneumococcal conjugate vaccines; PHiD-CV, pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine; RCT, randomized controlled trial; RTI, respiratory tract infection; TTP, tympanostomy tube placement; VGR, Västra Götalandsregionen, w, weeks of age; y, years of age.
*Children were vaccinated with at least 2 doses of PCV13 or PHiD-CV (no more than 1 dose of the other vaccine).
**Children were vaccinated with at least 2 doses of PCV7 or PHiD-CV (no more than 1 dose of the other vaccine).
The extent of heterogeneity between studies reflects important differences between studies in terms of design, OM case ascertainment and outcomes, and populations studied. Therefore, results are presented graphically in the sections below, but each study is discussed individually to provide the underlying information necessary to interpret the results.
Vaccine efficacy
Estimates of PHiD-CV efficacy against OM outcomes come from two large phase III RCTs: one in Europe (FinIP) and one in Latina America (COMPAS).20,21,28,58 The results are presented in (Figure 3). These studies, along with efficacy studies of other PCVs including PCV7, PCV9 and PHiD-CV11 were reviewed in an updated Cochrane Review by de Sevaux et al.24 The authors concluded that PCV7 and PHiD-CV were associated with large relative risk reductions in pneumococcal OM when administered in infancy. The effect on all-cause OM was considered more uncertain because of low- to moderate-certainty evidence.
Figure 3.
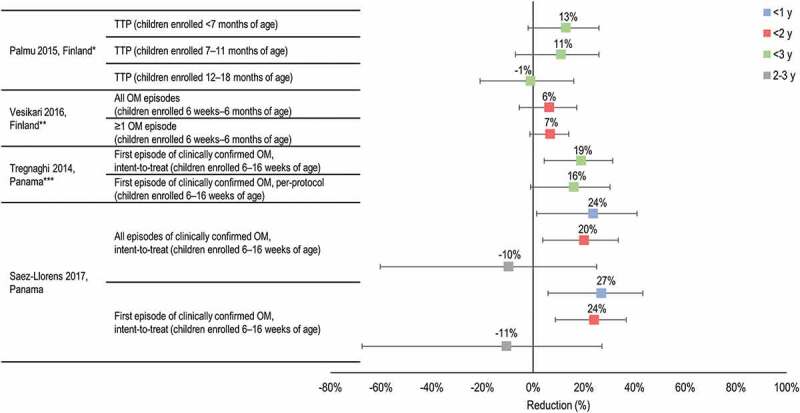
Efficacy of PHiD-CV in preventing all-cause otitis media (OM) and tympanostomy tube placement (TTP).
y, years of age. *Mean follow-up 24 months (range 14–34 months) for infants; 27 months (range 13–34 months) for catch-up. **Mean follow-up 18 months. ***Mean follow-up 28 months for the per-protocol; 31 months for the intent-to-treat. Horizontal lines show 95% confidence intervals. References: Palmu28; Vesikari58; Tregnaghi21; Saez-Llorens20.
Learnings from efficacy studies
RCTs have shown consistent trends that PHiD-CV (no efficacy data for PCV13 are available) reduced all-cause clinically diagnosed OM in infants (Figure 3), although the results were not always statistically significant and limitations of studies, such as the risk of bias and imprecise estimates of effect, reduce the certainty of the evidence.24 The vaccine efficacy estimate was 13% (95% CI −2, 26) against TTP in the FinIP trial and 6.4% (95% CI −5.5, 17.2) for the prevention of all OM episodes in a FinIP nested trial. Vaccine efficacy against the first OM episode was 16.1% (95% CI −1.1, 30.4) in the COMPAS per-protocol analysis, and was 14.8% (95% CI −1.0, 28.2; intent-to-treat analysis) for preventing all clinically confirmed OM. Available all-cause clinical OM data from COMPAS suggest that efficacy may be higher in younger children (<2 years of age) than in older age-groups.
Vaccine effectiveness
The data describing effectiveness of PHiD-CV and PCV13 come from 4 studies and the data are limited and heterogeneous. One study was conducted in clinical trial participants (FinIP trial) who received PHiD-CV using a mixed endpoint of respiratory tract infections (RTI) and OM; 1 study in the US used the endpoint of bacteriologically-confirmed serotype-specific OM based on tympanocentesis and cannot be extrapolated to real-world settings; 1 study from Spain evaluated a mixed PCV7/PCV13 endpoint for pneumococcal OM; and 1 study in Korea used a nationwide insurance database to estimate combined PHiD-CV/PCV13 effectiveness. Although 3 of these studies do not refer specifically the outcomes of interest of this review, they are included for discussion.
PHiD-CV effectiveness was estimated prospectively in a cohort of 424 children who participated in FinIP. Children were followed from birth until 2 years of age for the occurrence of RTI. Vaccine effectiveness was 23% (95% CI 0, 40) in preventing RTIs with OM.29
Pichichero et al.30 conducted a prospective observational study to evaluate the effectiveness of PCV13 in preventing OM caused by the 6 serotypes in PCV13 not included in PCV7. 239 children fully vaccinated up to 30 months of age seen as outpatients at a private pediatric practice in the US were enrolled. Episodes of OM were captured up until 30–36 months of age, and all cases of OM underwent tympanocentesis. Controls were children enrolled in an earlier similar study using PCV7. The estimated effectiveness of PCV13 in preventing OM due to the 6 additional serotypes was 86% (95% CI 61, 94).30
A population-based study in Southern Catalonia, Spain, identified 78 cases of culture-confirmed pneumococcal OM identified from spontaneous otorrhea (with or without tympanostomy tube in situ). The late-PCV7 period (January 2007 to June 2010) was compared with the early-PCV13 period (July 2010 to December 2013). No significant difference between overall incidence rates of pneumococcal OM episodes was observed between the two study periods, although the prevalence of cases caused by PCV13 serotypes showed a decreasing trend (65.5% vs 48.4%). The aggregate PCV7/PCV13 effectiveness against vaccine-type infection was 72% (95% CI −26, 94). PCV13 effectiveness against vaccine-type infection was 62% (95% CI −141, 95).31 PCV7 effectiveness could not be estimated as no OM cases due to PCV7 serotypes occurred in vaccinated children.
A retrospective nationwide database study was conducted in Korea to determine the feasibility of evaluating the effectiveness of the Korean vaccination program in which vaccination is with either PHiD-CV or PCV13. Adjusting for age, sex, comorbidity, and healthcare utilization rate, combined PHiD-CV/PCV13 vaccine effectiveness in preventing outpatient visits for OM was 19.13% (95% CI 13.42, 24.46). Vaccine effectiveness in preventing outpatient visits due to OM in a propensity score matched cohort was 33.21% (95% CI 31.01, 35.33).32
Learnings from effectiveness studies
Despite the heterogeneity of the studies, 3 of 4 studies showed statistically significant vaccine effectiveness in preventing the OM endpoint under study, confirming trends observed in efficacy studies. The fourth study by Ochoa-Gondar et al.31 did not show significant aggregate effectiveness of PCV7/PCV13 against vaccine-type infection, but the sample size was limited and the 95% CI on the effectiveness estimates were very wide, suggesting insufficient power.
Vaccine impact
The studies that assessed the impact of PHiD-CV and PCV13 on OM evaluated numerous different endpoints across a range of age-groups. ‘Like’ endpoints in age-groups of interest have been grouped in the sections below. While comparisons between PHiD-CV and PCV13 cannot always be made due to underlying study differences, some studies provide results for both vaccines that were conducted in comparable settings (see section Studies conducted in comparable settings and populations).
Clinical all-cause OM
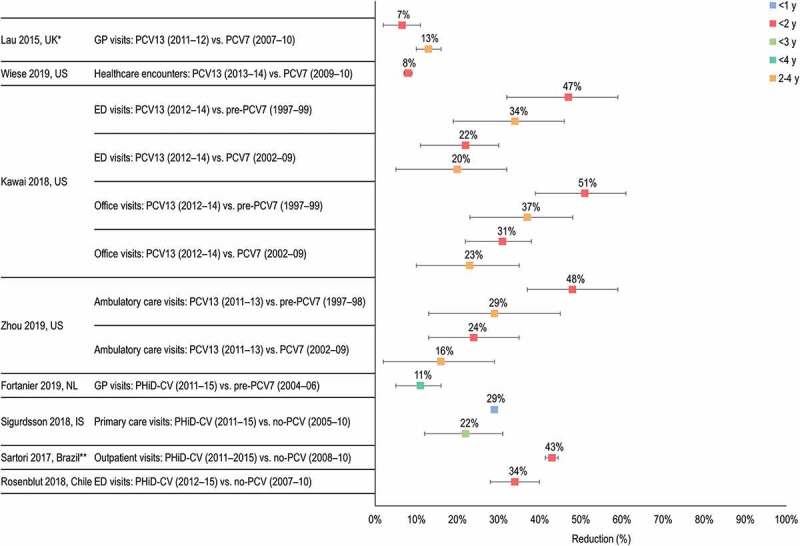
The 9 studies included in this section report on the outcome of all-cause OM diagnosed in a range of settings, including primary care/GP visits, ambulatory episodes, hospital outpatient and ED visits (Figure 4).
Figure 4.
Impact of PCV13 and PHiD-CV on clinical otitis media (OM) (all-cause OM visits).
ED, emergency department; GP, general practitioner; IS, Iceland; NL, the Netherlands; UK, United Kingdom; US, United States; y, years of age. *Monthly incidence decline of OM following transition period of 12 months after PCV13 introduction (Apr 2010–Mar 2011). **Includes children aged 2–23 months. Horizontal lines show 95% confidence intervals when available. References: Lau33; Wiese34; Kawai36; Zhou35; Fortanier37; Sigurdsson38; Sartori39; Rosenblut40.
Lau et al.33 conducted an observational cohort study using a nationally-representative primary care database (Intercontinental Medical Statistics database) in the United Kingdom (UK) to investigate trends in the incidence of GP visits for OM and associated antibiotic prescriptions in children <10 years of age. Compared to the period when PCV7 was in use (September 2007 to March 2010), the annual OM incidence in children <2 years of age reduced by 6.6% (95% CI 1.9, 11.0) after the introduction of PCV13 (April 2011 to December 2012). The annual incidence in children 2–4 years of age was also significantly reduced (13.0%, 95% CI 10.1, 15.9).33
Wiese et al.34 reported a retrospective study in the US that used claims data and ICD codes (International Classification of Diseases) to assess healthcare encounters for OM episodes in children <2 years of age before (PCV7 period, 2006 to 2010) and after PCV13 introduction (2011 to 2014). Healthcare encounters included visits to physicians, ED and hospitalizations. Compared to the PCV7 period, PCV13 was associated with an 8% decreased risk of OM (adjusted hazard ratio [HR] 0.92, 95% CI 0.91, 0.93) in children <2 years of age, and with statistically significant 12% decline in the risk of experiencing the first OM episode (adjusted HR 0.88, 95% CI 0.86, 0.89).34
Two overlapping retrospective studies from the US used the National Ambulatory Medical Care Survey and the National Hospital Ambulatory Medical Care Survey to evaluate OM-associated ambulatory visits to physicians’ offices or hospital ED and outpatient departments.35,36 Kawai et al.36 reported that office visit rates for OM decreased significantly during the PCV13 period (2012 to 2014) compared with the pre-PCV7 period (1997 to 1999), by 51% (95% CI 42, 58) in children <2 years of age and 37% (95% CI 23, 48) in children 2–4 years of age. ED visits for OM declined by 47% (95% CI 36, 55) in children <2 years of age and by 34% (95% CI 19, 46) in children 2–4 years of age. Annual visits for OM in children <5 years of age were estimated to have decreased from 14 million in 2000 to 8 million in 2014. Compared to the PCV7 period (2002 to 2009), PCV13 was associated with a 31% (95% CI 19, 41) reduction of office visits and a 22% (95% CI 7, 34) decline of ED visits for OM in children <2 years of age, respectively. In children 2–4 years of age, office visits declined by 23% (95% CI 10, 35) and ED visits by 20% (95% CI 5, 32).36 Zhou et al.35 reported that in children <5 years of age, the observed reduction in the rate of OM visits was 22% (95% CI 12, 32) for the PCV13 (2011 to 2013) vs the PCV7 period (2002 to 2009), and 41% (95% CI 30, 52) for the PCV13 vs the pre-PCV7 period (1999 to 1997). In children 2–4 years of age, the observed reduction in the rate of OM visits was 16% (95% CI 2, 29) (PCV13 vs PCV7 period) and 29% (95% CI 13, 45) (PCV13 vs pre-PCV7 period). In children <2 years of age, the observed rate reduction was 24% (95% CI 13, 35) (PCV13 vs PCV7 period) and 48% (95% CI 37, 59) (PCV13 vs pre-PCV7 period).35
Fortanier et al.37 conducted a retrospective, nationwide, observational study of GP-diagnosed OM episodes in children <4 years of age in the Netherlands. Children were assigned to the pre-PCV7 group (January 2004 to March 2006), the PCV7 group (April 2006 to February 2011) or the PHiD-CV group (March 2011 to February 2015). Children in the PHiD-CV group had a 21% lower risk of experiencing a first OM episode than those in the pre-PCV7 group (HR 0.79, 95% CI 0.70, 0.89), and an 11% lower overall risk for OM (HR 0.89, 95% CI 0.84, 0.95). PHiD-CV postponed the time to onset and reduced the risk of first OM episode compared to the PCV7 and pre-PCV7 groups (p < .001). Neither PCV7 nor PHiD-CV reduced the risk of effect of subsequent OM episodes, suggesting that the impact of PCV on overall OM is largely attributable to the prevention of the first OM episode.
A whole-population study evaluated the impact of PHiD-CV on primary care visits for all-cause OM in children <3 years of age in Iceland from 2005 to 2015. Birth cohorts were grouped as vaccine non-eligible (VNEC) or vaccine eligible (VEC). PHiD-CV reduced all-cause OM by 22% (95% CI 12, 31). There was a significant impact in preventing the first (16%, 95% CI 14, 18) and the second (5%, 95% CI 2, 7) OM episode, but not subsequent episodes. The proportion of children who experienced >5 OM episodes decreased significantly by 16% (95% CI 3, 27). There were significant reductions in the crude incidence rate of OM in all age brackets, including a 40% (95% CI 31, 49) reduction in children too young to receive direct vaccine protection (<4 months of age). An estimated 4187 OM episodes were prevented in the first 5 years of the vaccination program.38
A regional study conducted in Brazil measured the impact of PHiD-CV on primary care outpatient visits for all-cause OM in children 2–23 months of age. The no-PCV period was from August 2008 to July 2010 and the PHiD-CV vaccination period from August 2011 to July 2015. Primary care visits for all-cause OM decreased by 50.7% (95% CI 42.2, 59.2) in the PHiD-CV vs no-PCV periods. Correcting for a 7.7% decrease in outpatient visits for all other causes over the same period, the impact of PHiD-CV on all-cause OM was 43.0% (95% CI 41.4, 44.5).39
Rosenblut et al.40 performed a case-control study in a hospital ED in Chile. Cases were children <24 months of age with a clinical diagnosis of OM. Controls were children aged <24 months attending the ED at the same time with other diagnoses. The percentage of children with a diagnosis of clinical OM attending the ED decreased by 32% (p = .026) between the no-PCV period (2007 to 2010) and the PHiD-CV period (2012 to 2015).40 PHiD-CV exposure was associated with decreased risk of developing OM in children <24 months of age (odds ratio 0.659, 95% CI 0.60, 0.72).
A retrospective nationwide study assessed the combined effectiveness of PCV7 and PHiD-CV vaccination on OM in children <1 year of age in Peru from January 2006 to December 2012. PCV7 was introduced in Peru in 2009 and replaced by PHiD-CV in late 2011. Vaccination reduced outpatient visits due to OM in children aged <1 year by 26.2% (95% CI 16.9, 34.4).41
Learnings from impact studies of all-cause OM
In children <2 years of age, PHiD-CV and PCV13 reduced OM episodes (primary care, outpatient, ambulatory and ED visits) compared to periods before PCV introduction; PHiD-CV by 34% to 43%, and PCV13 by 47% to 51%. In the same age group, PCV13 reduced OM episodes between 7% to 31% compared to periods when PCV7 was in use. These results should be interpreted with caution. While results for PHiD-CV arise from different countries in Europe and South America, where PCV7 was mostly not in use before PHiD-CV introduction, three of 4 studies of PCV13 were conducted in the US, where the use of PCV has been longest (PCV7 introduced in 2000 and replaced by PCV13 from 2010) and implemented in an extensive 3 + 1 vaccination schedule. Both PHiD-CV and PCV13 appeared to have the greatest impact in preventing or delaying the first episode of OM.
Complex, complicated/severe, recurrent, and hospitalized OM
Estimates of PHiD-CV and PCV13 impact on complex, complicated, recurrent and hospitalized OM are shown in Figure 5.
Figure 5.
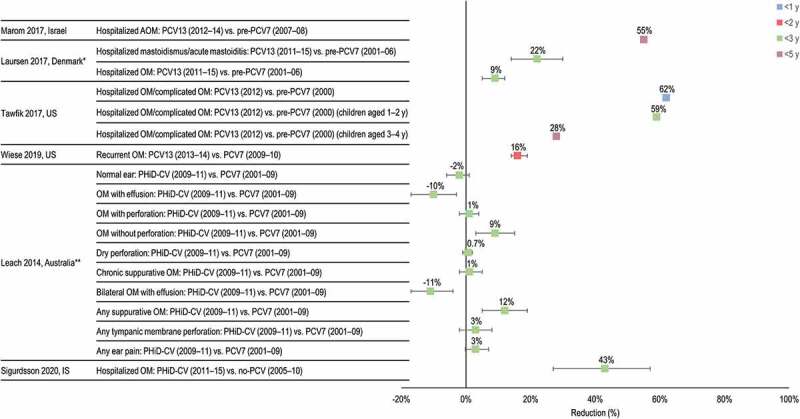
Impact of PCV13 and PHiD-CV on complex, complicated, recurrent and hospitalized otitis media (OM).
IS, Iceland; US, United States; y, years of age. *Mean age of cohorts over the study period was <3 years. **Includes children who received a primary course of at least two doses of PHiD-CV or PCV7. Horizontal lines show 95% confidence intervals when available. References: Marom42; Laursen43; Tawfik44; Wiese34; Leach46; Sigurdsson47.
A retrospective study conducted in a secondary pediatric hospital in Israel evaluated hospitalizations for head and neck infections in children <16 years of age (mean 1.1 to 3.3 years for pneumococcal infections) from 2007 to 2014. For children <5 years of age, all-cause OM hospitalizations decreased by 55% between the pre-PCV7 (2007 to 2008) and PCV13 periods (2012 to 2014).42
A retrospective cohort study in Danish children <16 years of age investigated the incidence of complicated OM, defined as OM requiring hospitalization, mastoidismus (retro-auricular redness and swelling without abscess), and acute mastoiditis, as well as the associated microbiology before (pre-PCV7 period, 2001 to 2006) and after introduction of the PCV7 (2007 to 2010) and PCV13 (2011 to 2015). The median age of children with mastoidismus or acute mastoiditis was 14 months, 15 months and 20 months in the respective periods, and the median age of children with hospitalized OM was 21, 14 and 29 months. Cases were identified from an ear-nose-and-throat (ENT) clinic at a tertiary hospital. Compared to the pre-PCV7 period, hospitalized OM decreased by 9% in the PCV13 period (IRR 0.91, 95% CI 0.88, 0.95). Compared to the pre-PCV7 period, mastoidismus or acute mastoiditis decreased by 22% in the PCV13 period (IRR 0.78, 95% CI 0.70, 0.86).43
A retrospective analysis using the Kids’ Inpatient Database in the US determined the annual prevalence of admission of US children <21 years of age hospitalized with acute suppurative OM, acute mastoiditis, suppurative labyrinthitis, and/or acute petrositis.44 The study was conducted from 2000 to 2012 and the mean age of cases was 7.1 years in 2000 and 4.5 years in 2012 (p = .14). There was a significant decline in annual prevalence of hospitalization for pediatric OM-related diagnoses between 2000 and 2012, with the most significant declines in children <2 years of age. Comparing year 2012 (PCV13 period) and year 2000 (pre-PCV7 period), there was a statistically significant decrease in admission prevalence that was most pronounced in children <1 year of age (62% decrease, p < .0001), 1–2 years of age (59% decrease, p < .0001) and 3–4 years of age (28% decrease, p = .0006).
Fortunato et al.45 conducted a matched case-control (1:3) study in 28 pediatric wards in Italy to assess the combined impact of PCV7 and PCV13 on hospitalizations for severe pneumococcal disease including suppurative and unspecified OM. Cases were children born between January 2006 and June 2012 hospitalized when aged at least 6 months for IPD or pneumococcal pneumonia. Controls were presumed healthy children matched for age, municipality and gender from the general population registry and without previous hospital admission for pneumococcal disease. Hospitalization rates in children <5 years of age for OM decreased by 39% at the national level after introduction of PCV7/PCV13 (hospitalization risk ratio 0.61, 95% CI 0.58, 0.65) and by 37% in regions that were first to introduce PCVs (hospitalization risk ratio 0.63, 95% CI 0.57, 0.70).45
The study by Wiese et al.34 described above included a secondary analysis of the impact of PCVs on recurrent OM, defined as ≥3 episodes in 6 months or ≥4 episodes in 12 months. Compared to the PCV7 period, there was a 16% decrease in the risk of recurrent OM in children <2 years of age during the PCV13 period (adjusted HR 0.84, 95% CI 0.81, 0.86).
Leach et al.46 compared OM outcomes in Australian indigenous children <36 months of age who received primary vaccination with at least 2 doses of either PCV7 or PHiD-CV. Compared to the PCV7 group, children vaccinated with PHiD-CV had a 12% reduction in any suppurative OM (OM with/without perforation and chronic suppurative OM) (p = .0004), with a 9% reduction in OM without perforation (p = .003) and an increase in OM with effusion (10%, p = .002) with an 11% increase in bilateral OM with effusion (p = .001). There was no statistically significant change in any tympanic membrane perforation or chronic suppurative OM.46 The results were similar in children who received only PCV7 or PHiD-CV. Children who received PHiD-CV had statistically significantly less any suppurative OM (16%, p < .0001), and more OM with effusion (12%, p = .001) than those vaccinated with PCV7.
A retrospective hospital-based cohort study estimated the impact of PHiD-CV on pediatric hospitalizations for IPD and RTI including OM in Iceland. Birth cohorts between 2005 and 2015 were defined as VNEC (2005 to 2010) and VEC (2011 to 2015). Follow-up continued until 3 years of age. Admission rates of OM decreased by 43% between the VNEC and VEC (HR 0.57, 95% CI 0.43, 0.73). When stratified by age < and ≥90 days, the impact was higher in children aged ≥90 days, consistent with a direct effect of vaccination (HR 0.72 [95% CI 0.33, 1.57] in children aged <90 days and 0.55 [95% CI 0.42, 0.72] for children ≥90 days).47 Despite observing a clear and statistically significant impact, with a greater impact in the VEC, a higher incidence of OM hospitalizations among the first 3 VNEC compared to all other study birth-cohorts led the authors to conclude that the observed effect may have been unrelated to vaccination. The authors also noted that the effect on OM in outpatient care has been well established in Iceland. A limitation of the study is the very high variability in OM incidence rates in the pre-vaccination years, with wide and overlapping CI, which reduces confidence in interpreting underlying trends. All observational studies are prone to bias and confounding, particularly the impact of temporal changes on disease outcomes that can occur independently of other modifiers such as vaccination. No attempts were made to use statistical methods to account for temporal trends that may have clarified the impact of vaccination in this study. Importantly, statistically significant decreases in IPD and pneumonia hospitalizations were observed, consistent with a vaccine impact.
A retrospective national cohort study used administrative databases to evaluate the impact of PCVs in children <6 years of age in New Zealand. New Zealand introduced PCV7 in June 2008, PHiD-CV in 2011, and PCV13 in 2014. OM hospitalizations declined by 25% between 2005 and 2015 in children <6 years of age (combined impact estimate). The declines were highest among Māori and Pacific children and those from socioeconomically deprived areas. OM declined by 51% in Māori children. Estimates of impact by PCV type were not made.48
Ben-Shimol et al. have conducted prospective population-based active surveillance since 2004 to assess the impact of PCV7 and PCV13 on OM.49–51 Three studies report the results from culture of middle ear fluid (MEF) obtained through tympanocentesis or spontaneous ear drum perforation over 3 sub-periods: the pre-PCV7 period from July 2004 to June 2008, the PCV7 period from July 2009 to June 2011, and the PCV13 period from July 2012, 2013 or 2014, depending on the study. These studies used specific case identification through MEF cultures and therefore, any comparison with the other studies presented in this section should be made with caution. Although these studies do not refer specifically to the outcomes of interest of this review, they are included for completeness.
Two years after the introduction of PCV13, annual all-cause OM resulting in MEF culture in children <2 years of age had reduced by 60% compared to the pre-PCV7 period (incidence rate ratio [IRR] 0.40, 95% CI 0.36, 0.45), and by 52% compared to the PCV7 period (IRR 0.48, 95% CI 0.42, 0.54).51 By 4 years after PCV13 introduction, all-cause OM resulting in MEF culture reduced by 68% compared to the pre-PCV7 period (IRR 0.32, 95% CI 0.29, 0.35) and by 62% compared to the PCV7 period (IRR 0.38, 95% CI 0.34, 0.41).50
The third study considered the source of MEF used for OM identification. There was a 69% decrease in all-cause spontaneous otorrhea in the PCV13 (July 2014 to June 2016) vs the pre-PCV7 period (IRR 0.31, 95% CI 0.24, 0.39), and a 80% decrease in the PCV13 vs PCV7 period (IRR 0.20, 95% CI 0.16, 0.25).49 All-cause tympanocentesis decreased by a similar proportion: 67% (IRR 0.33, 95% CI 0.30, 0.38) and 70% (IRR 0.30, 95% CI 0.27, 0.33), respectively.
Learnings from impact studies of severe OM
The majority of studies evaluating the spectrum of severe OM were conducted in countries using PCV13. All studies showed statistically significant impacts of vaccination on severe OM: either complex, complicated, recurrent or hospitalized, as defined in the individual papers. Individual studies that evaluated recurrent or suppurative OM suggested that PHiD-CV and PCV13 can prevent 16% of recurrent OM,34 and 12% of suppurative OM.46 Hospital admission rates for OM decreased consistently after the introduction of PCVs, and were reduced from 9% to 59% in children younger than 3 years of age, compared to the period before PCVs were in use. Most studies did not untangle the relative impacts of PHiD-CV or PCV13 vs PCV7 but confirmed the impact of the higher valency PCVs on OM.
Several of the studies examined specific populations that might not be transferable more generally. Ben-Shimol et al., for example, used specific case identification through MEF cultures. Therefore, these studies do not reflect the overall impact of vaccination on complex OM.49–51 The study by Laursen et al.43 was limited to a single tertiary hospital-based ENT clinic in Denmark and may not be representative of disease patterns across the country.43 PHiD-CV was studied in Australian indigenous children in whom chronic suppurative OM is widespread.46 Nevertheless, patterns of reduced severity of OM were consistent across all studies, suggesting that PHiD-CV and PCV13 have consistent positive impacts on the occurrence of the most severe cases of OM.
Tympanostomy tube placement procedures
The study by Wiese et al.34 already reported above found that compared to the PCV7 period, PCV13 was associated with a 24% decreased risk of TTP among children <2 years of age (adjusted HR 0.76, 95% CI 0.72, 0.80).
A nationwide database study in Finland evaluated the impact of PHiD-CV on TTP after introduction of PHiD-CV into the national immunization schedule in September 2010. The relative and absolute reduction in the national vaccination program (NVP)-eligible target cohort was compared with a season and age-matched (3–54 months) cohort before NVP introduction. Rates of TTP decreased from 5.41 to 4.56 per 100 person-years in the pre to post-NVP periods. The relative rate reduction was 14.8% (95% CI 13.1, 16.5) and the absolute rate reduction was 0.86 TTP per 100 person-years.52
A study in Iceland estimated the impact of PHiD-CV on TTP in children <5 years of age. Children born 2005 to 2010 were included in the VNEC and from 2011 to 2015 in the VEC cohort. The crude incidence rate of TTP was significantly higher in the VEC group compared to the VNEC group (10.6 vs 8.7 per 100 person-years). The vaccine impact on TTP was estimated at −6% (95% CI −16, 2.7). The VEC and VNEC differed, with milder disease and fewer children who had an OM diagnoses and who had received antimicrobials in the VEC. Therefore TTP appeared to increase in Iceland during the study period.53
Learnings from studies of TTP
Two of three studies that evaluated vaccine impact on TTP observed statistically significant reductions in the incidence of TTP of 14.8% (PHiD-CV) and 24% (PCV13). One study unexpectedly noted an increase in TTP after PHiD-CV introduction,53 despite previous evidence of positive impacts of vaccination on rates of all-cause OM, associated antimicrobial prescriptions and OM with treatment failure.59,60 Iceland has a high rate of TTP compared to other countries.53 Limitations of the study (potential unmeasured confounding, exclusion of specialist visits, absence of clinical data or the indication for TTP) mean that reasons for the increase over time remain speculative.53
Studies conducted in comparable settings and populations
We identified 4 studies designed to compare PHiD-CV with PCV13 or used PHiD-CV and PCV13 in comparable settings.54–57
From 2010, Swedish regions were free to use either PHiD-CV or PCV13 allowing comparisons of regions using the different vaccines. One study investigated the incidence of OM-related diagnoses (OM, TTP, myringotomy, acute mastoiditis and mastoidectomy) in children <4 years of age in the pre-PCV7 period from 2007 to 2008, and the higher valency PCV period from 2013 to 2014. Compared to the pre-PCV7 period, the national incidence of OM decreased in the higher valency PCV period by 39% in outpatients (rate ratio [RR] 0.61, 95% CI 0.60, 0.61) and 42% in inpatients (RR 0.58, 95% CI 0.54, 0.64). Inpatient myringotomies decreased by 15% (RR 0.85, 95% CI 0.75, 0.96) and TTP by 18% (RR 0.82, 95% CI 0.80, 0.85). The decline in outpatient OM and TTP was more pronounced in areas that used PHiD-CV (Figure 6). However, geographical differences were large also before vaccine introduction and factors such as more hospital beds per capita in regions using PHiD-CV and a higher number of pediatricians and otorhinolaryngologists in regions using PCV13 may have influenced the results in these areas.54
Figure 6.
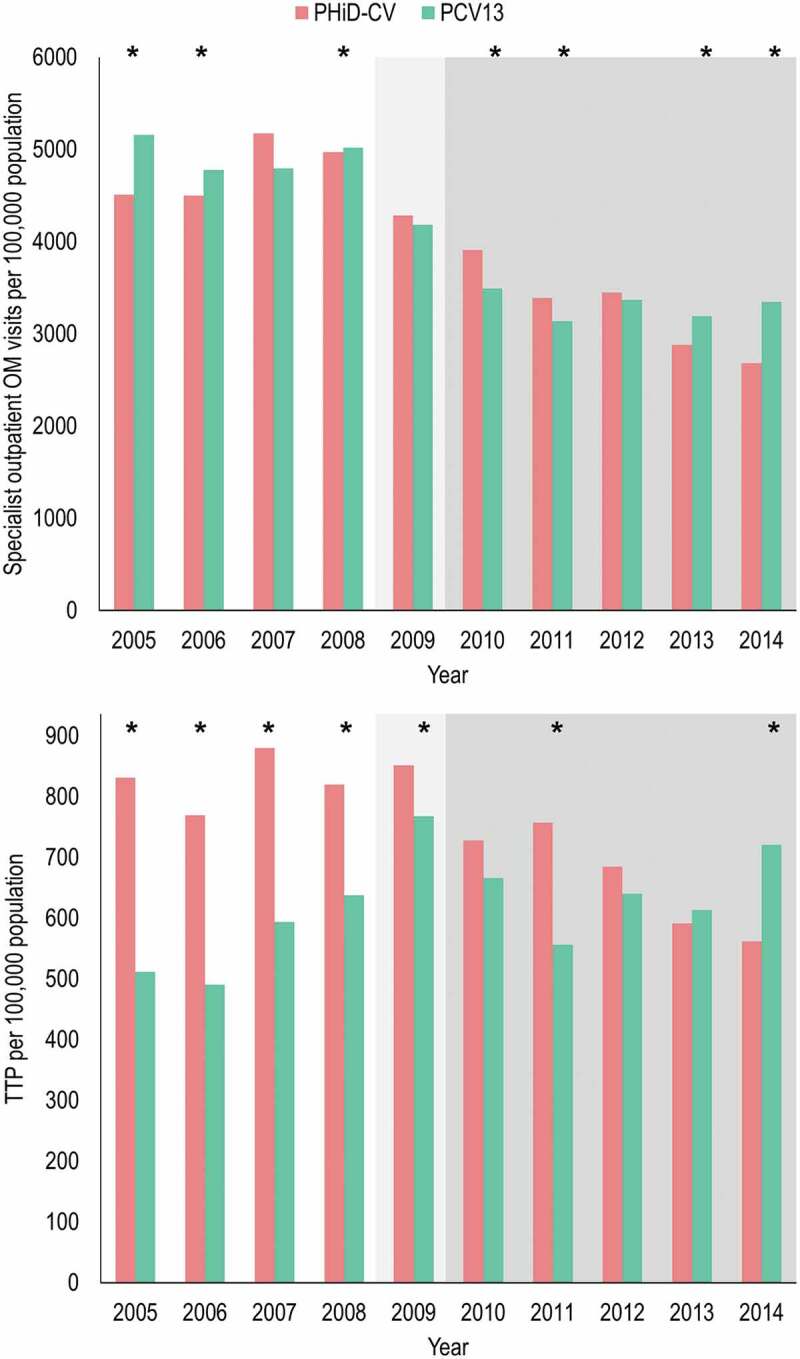
Differences in the incidences of specialist outpatients visits for otitis media (OM) and in tympanostomy tube placement (TTP) in children <4 years of age in Swedish regions using either PHiD-CV or PCV13. Data from Gisselsson-Solen et al.54
White = pre-PCV7 period (note that 3/21 regions commenced use of PCV7 during 2008), light gray = PCV7 period, darker gray = PHiD-CV or PCV13 periods. *Statistically significant difference between regions using PHiD-CV or PCV13 p < .05. Note that data was extracted from the original graph of the cited reference.
The impact of PCVs on OM was assessed in a retrospective cohort study in Sweden in children ≤5 years of age living in Skåne (PCV7 then PHiD-CV) or Västra Götalandsregionen (PCV7 then PCV13) between 2005 and 2013. The study used regional primary care databases that were linked with the National Patient Registry. Compared to the pre-PCV7 period, the incidence of OM decreased in children ≤2 years of age by 42% in Skåne post PHiD-CV introduction, and by 25% in Västra Götalandsregionen post PCV13 introduction. Of note, baseline OM incidence and duration of PCV7 use differed between regions which could confound interpretation of these findings. Using an adjusted age-period-cohort Poisson model, only the PHiD-CV cohort was associated with a statistically significant reduction in OM incidence in children aged 5 years or younger compared to the pre-PCV7 and PCV7 cohorts.55
Leach et al.56 performed a cross-sectional surveillance study of 25 remote indigenous communities in Australia. PHiD-CV was introduced in October 2009 and PCV13 in October 2011. The study evaluated indigenous children <36 months of age between February 2010 and August 2013 who had received at least 2 priming doses of PHiD-CV or PCV13. Transition from PHiD-CV to PCV13 did not show substantial further improvement against middle ear diseases. There was no significant difference between the PHiD-CV and PCV13 group in the prevalence of suppurative OM, tympanic membrane perforation, or OM with effusion (Figure 7). Analysis of ear discharges from children with a tympanic membrane perforation or chronic suppurative OM showed higher rates of NTHi in PCV13-vaccinees vs. PHiD-CV (64% vs 36%, p = .05), higher rates of S. pneumoniae (43% vs 17%, p = .03) and higher rates of S. pneumoniae and NTHi co-infection (43% vs 12%, p = .006), but lower rates of S. aureus infection (7% vs 40%, p = .02).56
Figure 7.
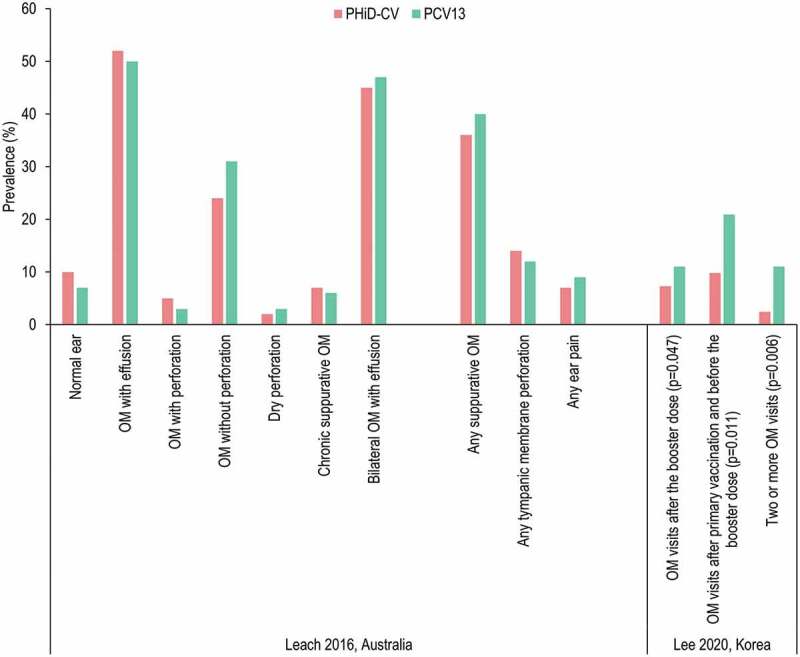
Comparison of otitis media (OM) outcomes in children who received PHiD-CV or PCV13.
Leach et al.56 Australian indigenous children aged <36 months who received at least 2 primary doses of PHiD-CV or PCV13. No comparisons between PHiD-CV and PCV13 were statistically significant (p ≥ .07 for all). Lee et al.57 Korean children aged <2 years. All comparisons were statistically significant (p < .05).
Lee et al.57 conducted a longitudinal study of nasopharyngeal carriage and OM in healthy Korean children visiting one of 8 hospitals for routine checkup or vaccination. Families were offered PHiD-CV or PCV13 and were allowed to choose between them. Parents were encouraged during 4 follow-up visits and by text messages to bring their child to hospital if they developed symptoms of OM. A total of 305 subjects were enrolled of whom 182 received PCV13 and 123 received PHiD-CV. The occurrence and recurrence of OM was consistently statistically significantly lower in the PHiD-CV group than the PCV13 group after primary and booster vaccination (Figure 7), possibly related to differences in nasopharyngeal carriage of S. pneumoniae and NTHi after vaccination, which were also statistically significantly lower in the PHiD-CV group.57 Analysis indicates that the study is at high risk of bias due to missing data (Figure S1), and the results should be considered cautiously.
Learnings from comparative studies
Only 4 studies allow some degree of direct comparison between PCV13 and PHiD-CV.54–57 PHiD-CV and PCV13 appeared to have comparable impacts in preventing OM. However, despite the limitations of the studies, all 4 show some outcomes where the protection afforded by PHiD-CV appeared to be higher than PCV13. The consistency of the trend suggests that a difference between the vaccines may exist. Any such difference can be hypothesized to be due to an impact on NTHi since it is unlikely that PHiD-CV is superior to PCV13 in terms of pneumococcal outcomes. Nevertheless, all four studies are subject to substantial limitations in design and the findings should be considered with caution. Additional data are necessary to better characterize and understand any potential differences.
Study quality assessment
None of the studies was judged to have an extremely serious and significant risk of bias (Supplementary Figure S1 and Supplementary text). All impact studies were however judged to have the possibility of unmeasured confounding which might have significant impact on the results. The results with regard to temporal trends in assessed impact studies must be interpreted with caution.
Limitations of the data
The studies examined here encompass a range of designs including RCTs, and numerous types of observational studies such as case-control and cohort designs conducted in single-centers or through national databases. We were unable to exclude the potential for bias in data selection/presentation due to heterogeneity of the outcomes and age groups investigated in the different studies.
Although RCTs provide robust estimates of vaccine efficacy, they do so under artificial conditions that can influence diagnosis, treatment and the behavior of patients in their propensity to seek care, and the behavior of physicians in their propensity to treat (perform TTP or prescribe antibiotics). Observational studies on the other hand, are valuable in confirming the results of efficacy studies under real-world conditions, although a major limitation is the inability to directly attribute the observed reductions to PCV introduction. Before–after study designs can be prone to many sources of bias, most commonly fluctuations in factors coinciding with the introduction of PCV that affect the incidence of OM, such as changes in health-seeking behavior, changes in diagnostic or administrative practices (e.g. changes in coding practices) and changes to access to care (number of hospitals, clinics specialists and hospital beds) or treatment guidelines, for example guidelines that evolve in response to local antibiotic resistance trends. Observational studies may also be limited in how they are able to control for confounding, such the presence of risk factors that predispose children to OM such as socioeconomic index, breastfeeding, influenza vaccination, exposure to cigarette smoke, and daycare attendance.61 Case-control and cohort studies are also at risk of under-estimating vaccine efficacy due to herd protection among unvaccinated persons.
OM is usually a clinical diagnosis made by primary care providers without microbiological confirmation, and accurate diagnosis of OM and complete capture of all episodes in clinical practice remains challenging. Healthcare and claims databases provide access to potentially large cohorts and may allow a degree of adjustment for potential confounding but may be subject to coding errors due to changes in practices over time, they lack patient-level validation, and preferences for some diagnostic codes over others can occur to support clinical decision-making or reimbursement.
Of the four comparative studies, information about OM etiology was only reported for one study,56 and the impact on carriage was only reported by one study.57 Although both were suggestive of a higher impact of PHiD-CV on NTHi OM/carriage, respectively, these studies were not designed to assess the impact of PHiD-CV on OM caused by NTHi.
Discussion
In the studies reviewed here, a positive impact of PHiD-CV and PCV13 vaccination in reducing OM-related outcomes in children was consistently observed. RCTs of high-valency PCVs have to date only been conducted with PHiD-CV. Two large phase III efficacy studies and associated sub-studies showed consistent evidence that PHiD-CV reduced all-cause clinically diagnosed OM and TTP procedures in children. The nested-trial within the FinIP study did not demonstrate statistically significant efficacy in preventing parent-reported physician-diagnosed OM in 4117 infants.58 However, statistical significance was reached in the larger COMPAS study of 7000 infants,20,21 with efficacy that was similar to the estimates from FinIP and earlier trials of PCV7 and a prototype 11-valent PCV.22,24,62,63 Both studies have limitations that potentially reduce the certainty of these observations.58 Nevertheless the studies point to higher efficacy in younger than in older children, and higher efficacy in preventing the first rather than subsequent OM episodes, suggesting the PCVs may exert their greatest effect by preventing or delaying the first OM episode during childhood.
Only 4 studies assessed vaccine effectiveness of PCV13 or PHiD-CV against a diverse set of endpoints that precludes any comparison of their results. Two studies that enrolled several hundred participants evaluated specific endpoints of RTI with OM and bacteriologically-confirmed OM due to the 6 additional serotypes in PCV13 compared with PCV7,29,30 and a third study evaluated the effectiveness against pneumococcal OM31 The fourth study used the Korean National Health Insurance System database and included in excess of 990,000 children. This study used propensity score matching to account for potential confounding baseline factors such as age, sex, co-morbidity, and healthcare utilization. The combined PHiD-CV/PCV13 vaccine effectiveness in preventing outpatient visits for OM in the propensity score matched cohort was 33.21% (95% CI 31.01, 35.33).32
There were 21 studies that have evaluated the impact of PCV13 and/or PHiD-CV on OM-related outcomes: all-cause or severe/complicated OM and TTP, diagnosed in a range of settings, including primary care/GP visits, ambulatory episodes, specialist clinics, hospital outpatient and ED visits, and hospitalizations. Twenty studies showed consistent statistically significant impacts of PCV13 and/or PHiD-CV in reducing the burden of disease due to OM in children. One study paradoxically observed an increase in TTP after PHiD-CV introduction, which contradicted previous findings of vaccine impact against all-cause OM, associated antimicrobial prescriptions and OM with treatment failure.53,59,60 In children aged <2 years, impact studies reported reductions of all-cause OM (primary care, outpatient, ambulatory, emergency department visits) between 47–51% for PCV13 and 34–43% for PHiD-CV compared to periods before PCV introduction. Although showing a consistently positive benefit, these studies were not conducted in comparable settings and the results cannot be directly compared.
Only 4 studies allow some degree of direct comparison between PCV13 and PHiD-CV.54–57 These studies suggest PHiD-CV may offer better protection against some OM outcomes than PCV13 but more data are needed to confirm potential differences as these studies are subject to substantial limitations in design and data.
PCVs have had a vast impact on human diseases caused by S. pneumoniae. Their major impact has been in the prevention of morbidity and mortality due to severe diseases such as IPD and meningitis. Impacts are lower for diseases of the respiratory tract, a feature also common to other conjugate vaccines such as H. influenzae type b conjugate vaccines.64 Nevertheless, even modest vaccine impacts on OM, one of the most common diseases of childhood and an important driver of antibiotic consumption, can have vast effects on healthcare utilization, antibiotic resistance, and associated healthcare costs. Monasta et al.65 estimated that in 2005 there were about 709 million cases of OM worldwide, with 51% in children under 5 years of age. Even when applying the moderate vaccine efficacy reported in the COMPAS study of 14.8% against all episodes of clinical OM,20 53.5 million cases of OM could be prevented annually by PCVs currently licensed to protect children against OM.
Considering the entirety of available data, it is reasonable to conclude that PHiD-CV and PCV13 show consistent, albeit variable, impacts in reducing all-cause OM, complicated and severe OM, OM-related hospitalizations, and TTP procedures. Nevertheless, the burden and costs associated with OM remain substantial and there is a space for improved vaccines capable of delivering improved efficacy in the prevention of middle ear diseases. Options could include higher-valency vaccines; although these are complex to manufacture66 and limited by the total dose of polysaccharide and carrier protein able to be administered and potential immunological interferences, and by eventual serotype replacement which has been demonstrated for all PCVs to date.67 Alternatively, new vaccines containing novel adjuvants or pneumococcal surface proteins could extend coverage to be serotype independent, and improve mucosal immune responses.68 Ideally, an OM vaccine would provide extended protection against other common pathogens implicated in OM, such as NTHi and Moraxella catarrhalis.
One of the key differentiating features between PCV13 and PHiD-CV, aside from 3 fewer serotypes in PHiD-CV, is the presence of the Protein D conjugate in PHiD-CV. Protein D was included to reduce the potential for bystander interference and carrier-induced epitopic suppression observed with CRM-conjugate vaccines,69 and the hypothesis that immunity induced to the carrier protein could itself convey protection; in this case against NTHi, one of the most common causes of OM. The results of studies that have assessed the impact of PHiD-CV on NTHi outcomes have been equivocal. An initial efficacy study of a prototype 11-valent Protein D conjugate PCV showed statistically significant efficacy in preventing NTHi OM, which was not later confirmed in the COMPAS study, although the study was not powered for this endpoint.20,21,62 The comparative study by Leach et al.56 noted higher rates of NTHi in ear discharges of PCV13-vaccinees than PHiD-CV-vaccinees (64% vs 36%), and the authors of the comparative Korean study put forward that statistically significantly lower rates of OM in PHiD-CV than PCV13 recipients could be linked to statistically significantly lower NTHi carriage after PHiD-CV vaccination.57 A review of the data supporting Protein D effects concluded that the available evidence suggests that Protein D may decrease NTHi OM, but that more evidence was needed.19 PCV13 contains 3 additional serotypes compared to PHiD-CV (serotypes 3, 6A and 19A) which suggests that protection against these serotypes might be expected to be enhanced compared to PHiD-CV. Thus, PCV13 vaccine effectiveness in preventing OM may be higher in settings of low NTHi prevalence but where these serotypes cause an important disease burden. The choice of either vaccine needs to take into consideration the local epidemiology of IPD and OM, and weigh up the potential advantages of one over the other.
At present health authorities choosing between PCVs have very little comparative data on which to base their decisions. Efficacy, effectiveness, and impact studies of single vaccines are important but do not allow direct comparison of vaccines due to very different design, endpoint and environment. As new vaccines come to market, well-designed, head-to-head comparisons between different vaccines targeting similar diseases will be needed to guide public policy. This is particularly the case for conditions such as OM where no serological correlate of protection exists, and where study results clearly differ according to case ascertainment and definitions. OM is a key outcome in development of new PCVs by Pfizer (20-valent) and Merck (15-valent); however, while they cover more serotypes than the current available PCVs, they do not integrate components against NTHi and M. catarrhalis, the other important pathogens causative of OM.
The next 5 years will likely see the development of new generation pneumococcal vaccine options with advanced capacity to prevent pneumococcal OM and other respiratory infections. Prevalent thinking that including more pneumococcal serotypes in conjugate vaccines may improve protection against OM may not be accurate, as impacts on carriage of OM-causing pneumococcal serotypes, cross-protection, replacement patterns, or additional pathogens (e.g., NTHi) may be equally relevant. Furthermore, considering innovative concepts that could improve effectiveness against OM, such as novel adjuvants, specific proteins, or alternative administration routes, may prove more effective in broadening efficacy against OM. While PHiD-CV and PCV13 differ in serotype composition, the evidence analyzed suggest both vaccines provide some protection against OM. Nevertheless, in studies where direct comparison of the 2 vaccines may be possible, and within the recognized limitations of these studies, there is a consistent trend suggesting that protection may be somewhat higher with PHiD-CV. Contribution from other factors than serotype composition (e.g., conjugated proteins and conjugation methods, cross-protection, serotype replacement, etc.) play an important role to vaccines’ efficacy, effectiveness and impact against all-cause OM.
Supplementary Material
Acknowledgments
The authors would like to thank Jozica Skufca (P95) and Margarita Riera (P95) in the form of literature search, data extraction, and analyses. The authors would like to thank Modis for editorial assistance and manuscript coordination, on behalf of GSK: Writing support was provided by Joanne Wolter; editorial support and publication management was provided by Stéphanie Deroo.
Funding Statement
GlaxoSmithKline Biologicals SA funded all costs associated with the development and the publishing of the present manuscript. P95 received consultancy fees from GSK for the performed source reports.
Disclosure statement
P Izurieta, M Scherbakov, J Nieto Guevara, V Vetter, and L Soumahoro are employees of the GSK group of companies and declare financial (including shares) and non-financial relationships and activities.
Authors’ contribution
P Izurieta and M Scherbakov contributed to the conceptualization, the methodology and the formal analysis. L Soumahoro contributed to the conceptualization, the methodology and the data curation. All authors contributed to the data interpretation and review and editing of the manuscript.
Trademark statement
Synflorix is a trademark owned by or licensed to the GSK group of companies. Prevenar/Prevnar and Prevenar 13/Prevnar 13 are trademarks of Pfizer Inc. Pneumosil is a trademark of Serum Institute of India Private Limited.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2021.2013693.
References
- 1.Tin Tin Htar M, van Den Biggelaar AHJ, Sings H, Ferreira G, Moffatt M, Hall-Murray C, Verstraeten T, Gessner BD, Schmitt H-J, Jodar L, et al. The impact of routine childhood immunization with higher-valent pneumococcal conjugate vaccines on antimicrobial-resistant pneumococcal diseases and carriage: a systematic literature review. Expert Rev Vaccines. 2019;18(10):1069–18. doi: 10.1080/14760584.2019.1676155. [DOI] [PubMed] [Google Scholar]
- 2.Wiese AD, Griffin MR, Grijalva CG.. Impact of pneumococcal conjugate vaccines on hospitalizations for pneumonia in the United States. Expert Rev Vaccines. 2019;18:327–41. doi: 10.1080/14760584.2019.1582337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen R, Cohen JF, Chalumeau M, Levy C. Impact of pneumococcal conjugate vaccines for children in high- and non-high-income countries. Expert Rev Vaccines. 2017;16:625–40. doi: 10.1080/14760584.2017.1320221. [DOI] [PubMed] [Google Scholar]
- 4.Alicino C, Paganino C, Orsi A, Astengo M, Trucchi C, Icardi G, Ansaldi F. The impact of 10-valent and 13-valent pneumococcal conjugate vaccines on hospitalization for pneumonia in children: a systematic review and meta-analysis. Vaccine. 2017;35:5776–85. doi: 10.1016/j.vaccine.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Buckley BS, Henschke N, Bergman H, Skidmore B, Klemm EJ, Villanueva G, Garritty C, Paul M. Impact of vaccination on antibiotic usage: a systematic review and meta-analysis. Clin Microbiol Infect. 2019;25:1213–25. doi: 10.1016/j.cmi.2019.06.030. [DOI] [PubMed] [Google Scholar]
- 6.de Oliveira LH, Shioda K, Valenzuela MT, Janusz CB, Rearte A, Sbarra AN, Warren JL, Toscano CM, Weinberger DM. Declines in pneumonia mortality following the introduction of pneumococcal conjugate vaccines in Latin American and Caribbean countries. Clin Infect Dis. 2021;73(2):306–13. doi: 10.1093/cid/ciaa614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsaban G, Ben-Shimol S. Indirect (herd) protection, following pneumococcal conjugated vaccines introduction: a systematic review of the literature. Vaccine. 2017;35:2882–91. doi: 10.1016/j.vaccine.2017.04.032. [DOI] [PubMed] [Google Scholar]
- 8.Loo JD, Conklin L, Fleming-Dutra KE, Knoll MD, Park DE, Kirk J, Goldblatt D, O’Brien KL, Whitney CG. Systematic review of the indirect effect of pneumococcal conjugate vaccine dosing schedules on pneumococcal disease and colonization. Pediatr Infect Dis J. 2014;33(Suppl 2):S161–71. doi: 10.1097/INF.0000000000000084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berman-Rosa M, O’Donnell S, Barker M, Quach C. Efficacy and effectiveness of the PCV-10 and PCV-13 vaccines against invasive pneumococcal disease. Pediatrics. 2020;145:e20190377. doi: 10.1542/peds.2019-0377. [DOI] [PubMed] [Google Scholar]
- 10.Izurieta P, Bahety P, Adegbola R, Clarke C, Hoet B. Public health impact of pneumococcal conjugate vaccine infant immunization programs: assessment of invasive pneumococcal disease burden and serotype distribution. Expert Rev Vaccines. 2018;17:479–93. doi: 10.1080/14760584.2018.1413354. [DOI] [PubMed] [Google Scholar]
- 11.Vojtek I, Nordgren M, Hoet B. Impact of pneumococcal conjugate vaccines on otitis media: a review of measurement and interpretation challenges. Int J Pediatr Otorhinolaryngol. 2017;100:174–82. doi: 10.1016/j.ijporl.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 12.Newhouse, L. PATH new pneumococcal vaccine from Serum Institute of India achieves WHO prequalification. 2019. Dec 19 [accessed 2020 Aug 21]. https://www.path.org/media-center/new-pneumococcal-vaccine-serum-institute-india-achieves-who-prequalification/
- 13.de Oliveira LH, Camacho LA, Coutinho ESF, Martinez-Silveira MS, Carvalho AF, Ruiz-Matus C, Toscano CM. Impact and effectiveness of 10 and 13-valent pneumococcal conjugate vaccines on hospitalization and mortality in children aged less than 5 years in Latin American countries: a systematic review. PLoS One. 2016;11(12):e0166736. doi: 10.1371/journal.pone.0166736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen O, Knoll M, O’Brien K, Ramakrishnan M, Constenla D, Privor-Dumm L, Buss-Younkin J, Farrar J, Pilishvili T, Whitney C. Pneumococcal conjugate vaccine (PCV) product assessment 2017 April. [accessed 2020 Jul 10]. https://www.jhsph.edu/ivac/wp-content/uploads/2018/05/pcv-product-assessment-april-25-2017.pdf.
- 15.McGirr A, Iqbal SM, Izurieta P, Talarico C, Luijken J, Redig J, Newson RS. A systematic literature review and network meta-analysis feasibility study to assess the comparative efficacy and comparative effectiveness of pneumococcal conjugate vaccines. Hum Vaccin Immunother. 2019;15(11):2713–24. doi: 10.1080/21645515.2019.1612667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, Lee E, Mulholland K, Levine OS, Cherian T. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374(9693):893–902. doi: 10.1016/S0140-6736(09)61204-6. [DOI] [PubMed] [Google Scholar]
- 17.Fortanier AC, Venekamp RP, Boonacker CW, Hak E, Schilder AG, Sanders EA, Damoiseaux RA. Pneumococcal conjugate vaccines for preventing acute otitis media in children. Cochrane Database Syst Rev. 2019;5:Cd001480. doi: 10.1002/14651858.CD001480.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leibovitz E, Jacobs MR, Dagan R. Haemophilus influenzae: a significant pathogen in acute otitis media. Pediatr Infect Dis J. 2004;23:1142–52. [PubMed] [Google Scholar]
- 19.Clarke C, Bakaletz LO, Ruiz-Guinazu J, Borys D, Mrkvan T. Impact of protein D-containing pneumococcal conjugate vaccines on non-typeable Haemophilus influenzae acute otitis media and carriage. Expert Rev Vaccines. 2017;16:1–14. doi: 10.1080/14760584.2017.1333905. [DOI] [PubMed] [Google Scholar]
- 20.Saez-Llorens X, Rowley S, Wong D, Rodriguez M, Calvo A, Troitino M, Salas A, Vega V, Castrejón MM, Lommel P. Efficacy of 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine against acute otitis media and nasopharyngeal carriage in Panamanian children – a randomized controlled trial. Hum Vaccin Immunother. 2017;13:1–16. doi: 10.1080/21645515.2017.1287640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tregnaghi MW, Saez-Llorens X, Lopez P, Abate H, Smith E, Posleman A, Calvo A, Wong D, Cortes-Barbosa C, Ceballos A, et al. Efficacy of pneumococcal nontypable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) in young Latin American children: a double-blind randomized controlled trial. PLoS Med. 2014;11(6):e1001657. doi: 10.1371/journal.pmed.1001657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eskola J, Kilpi T, Palmu A, Jokinen J, Haapakoski J, Herva E, Herva E, Takala A, Käyhty H, Karma P, et al. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N Engl J Med. 2001;344:403–09. doi: 10.1056/NEJM200102083440602. [DOI] [PubMed] [Google Scholar]
- 23.Palmu AA, Jokinen J, Nieminen H, Rinta-Kokko H, Ruokokoski E, Puumalainen T, Moreira M, Schuerman L, Borys D, Kilpi TM, et al. Vaccine-preventable disease incidence of pneumococcal conjugate vaccine in the Finnish invasive pneumococcal disease vaccine trial. Vaccine. 2018;36(14):1816–22. doi: 10.1016/j.vaccine.2018.02.088. [DOI] [PubMed] [Google Scholar]
- 24.de Sevaux JL, Venekamp RP, Lutje V, Hak E, Schilder AG, Sanders EA, Damoiseaux RA. Pneumococcal conjugate vaccines for preventing acute otitis media in children. Cochrane Database Syst Rev. 2020;11:CD001480. doi: 10.1002/14651858.CD001480.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sterne JAC, Hernán MA, McAleenan A, Reeves BC, Higgins JPT. Chapter 25: assessing risk of bias in a non-randomized study. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane handbook for systematic reviews of interventions version 6.0 (updated July 2019). Cochrane; 2019. https://methods.cochrane.org/methods-cochrane/robins-i-tool. [Google Scholar]
- 27.Higgins JPT, Savović J, Page MJ, Elbers RG, Sterne JAC. Chapter 8: assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane handbook for systematic reviews of interventions version 6.0 (updated July 2019). Cochrane; 2019. https://methods.cochrane.org/risk-bias-2. [Google Scholar]
- 28.Palmu AA, Jokinen J, Nieminen H, Rinta-Kokko H, Ruokokoski E, Puumalainen T, Traskine M, Moreira M, Borys D, Schuerman L. Effectiveness of the ten-valent pneumococcal conjugate vaccine against tympanostomy tube placements in a cluster-randomized trial. Pediatr Infect Dis J. 2015;34(11):1230–35. doi: 10.1097/inf.0000000000000857. [DOI] [PubMed] [Google Scholar]
- 29.Karppinen S, Toivonen L, Schuez-Havupalo L, Teros-Jaakkola T, Waris M, Auranen K, Palmu AA, Peltola V. Effectiveness of the ten-valent pneumococcal Haemophilus influenzae protein D conjugate vaccine (PHiD-CV10) against all respiratory tract infections in children under two years of age. Vaccine. 2019;37:2935–41. doi: 10.1016/j.vaccine.2019.04.026. [DOI] [PubMed] [Google Scholar]
- 30.Pichichero M, Kaur R, Scott DA, Gruber WC, Trammel J, Almudevar A, Center KJ. Effectiveness of 13-valent pneumococcal conjugate vaccination for protection against acute otitis media caused by Streptococcus pneumoniae in healthy young children: a prospective observational study. Lancet Child Adolesc Health. 2018;2(8):561–68. doi: 10.1016/s2352-4642(18)30168-8. [DOI] [PubMed] [Google Scholar]
- 31.Ochoa-Gondar O, Figuerola-Massana E, Vila-Corcoles A, Aguirre CA, de Diego C, Satue E, Gomez F, Raga X. Epidemiology of Streptococcus pneumoniae causing acute otitis media among children in Southern Catalonia throughout 2007-2013: incidence, serotype distribution and vaccine’s effectiveness. Int J Pediatr Otorhinolaryngol. 2015;79:2104–08. doi: 10.1016/j.ijporl.2015.09.022. [DOI] [PubMed] [Google Scholar]
- 32.Sohn S, Hong K, Chun BC. Evaluation of the effectiveness of pneumococcal conjugate vaccine for children in Korea with high vaccine coverage using a propensity score matched national population cohort. Int J Infect Dis. 2020;93:146–50. doi: 10.1016/j.ijid.2020.01.034. [DOI] [PubMed] [Google Scholar]
- 33.Lau WC, Murray M, El-Turki A, Saxena S, Ladhani S, Long P, Sharland M, Wong ICK, Hsia Y. Impact of pneumococcal conjugate vaccines on childhood otitis media in the United Kingdom. Vaccine. 2015;33(39):5072–79. doi: 10.1016/j.vaccine.2015.08.022. [DOI] [PubMed] [Google Scholar]
- 34.Wiese AD, Huang X, Yu C, Mitchel EF, Kyaw MH, Griffin MR, Grijalva CG. Changes in otitis media episodes and pressure equalization tube insertions among young children following introduction of the 13-valent pneumococcal conjugate vaccine: a birth-cohort based study. Clin Infect Dis. 2019;69:2162–69. doi: 10.1093/cid/ciz142. [DOI] [PubMed] [Google Scholar]
- 35.Zhou X, de Luise C, Gaffney M, Burt CW, Scott DA, Gatto N, Center KJ. National impact of 13-valent pneumococcal conjugate vaccine on ambulatory care visits for otitis media in children under 5 years in the United States. Int J Pediatr Otorhinolaryngol. 2019;119:96–102. doi: 10.1016/j.ijporl.2019.01.023. [DOI] [PubMed] [Google Scholar]
- 36.Kawai K, Adil EA, Barrett D, Manganella J, Kenna MA. Ambulatory visits for otitis media before and after the introduction of pneumococcal conjugate vaccination. J Pediatr. 2018;201:122–127.e1. doi: 10.1016/j.jpeds.2018.05.047. [DOI] [PubMed] [Google Scholar]
- 37.Fortanier AC, Venekamp RP, Hoes AW, Schilder AGM. Does pneumococcal conjugate vaccination affect onset and risk of first acute otitis media and recurrences? A primary care-based cohort study. Vaccine. 2019;37:1528–32. doi: 10.1016/j.vaccine.2019.01.064. [DOI] [PubMed] [Google Scholar]
- 38.Sigurdsson S, Eythorsson E, Hrafnkelsson B, Erlendsdottir H, Kristinsson KG, Haraldsson A. Reduction in all-cause acute otitis media in children <3 years of age in primary care following vaccination with 10-valent pneumococcal haemophilus influenzae protein-D conjugate vaccine: a whole-population study. Clin Infect Dis. 2018;67:1213–19. doi: 10.1093/cid/ciy233. [DOI] [PubMed] [Google Scholar]
- 39.Sartori AL, Minamisava R, Bierrenbach AL, Toscano CM, Afonso ET, Morais-Neto OL, Antunes JLF, Cristo EB, Andrade AL. Reduction in all-cause otitis media-related outpatient visits in children after PCV10 introduction in Brazil. PLoS One. 2017;12(6):e0179222. doi: 10.1371/journal.pone.0179222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosenblut A, Rosenblut M, Garcia K, Maul X, Santolaya ME. Frequency of acute otitis media in children under 24 months of age before and after the introduction of the 10-valent pneumococcal conjugate vaccine into the national immunization program in Chile. Pediatr Infect Dis J. 2018;37:132–34. doi: 10.1097/inf.0000000000001722. [DOI] [PubMed] [Google Scholar]
- 41.Suarez V, Michel F, Toscano CM, Bierrenbach AL, Gonzales M, Alencar AP, Ruiz Matus C, Andrus JK, de Oliveira LH. Impact of pneumococcal conjugate vaccine in children morbidity and mortality in Peru: time series analyses. Vaccine. 2016;34:4738–43. doi: 10.1016/j.vaccine.2016.07.027. [DOI] [PubMed] [Google Scholar]
- 42.Marom T, Bookstein Peretz S, Schwartz O, Goldfarb A, Oron Y, Tamir SO. Impact of pneumococcal conjugate vaccines on selected head and neck infections in hospitalized Israeli children. Pediatr Infect Dis J. 2017;36:314–18. doi: 10.1097/inf.0000000000001425. [DOI] [PubMed] [Google Scholar]
- 43.Laursen BB, Danstrup CS, Hoffmann S, Norskov-Lauritsen N, Christensen ALB, Ovesen T. The effect of pneumococcal conjugate vaccines on incidence and microbiology associated with complicated acute otitis media. Int J Pediatr Otorhinolaryngol. 2017;101:249–53. doi: 10.1016/j.ijporl.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 44.Tawfik KO, Ishman SL, Altaye M, Meinzen-Derr J, Choo DI. Pediatric acute otitis media in the era of pneumococcal vaccination. Otolaryngol Head Neck Surg. 2017;156:938–45. doi: 10.1177/0194599817699599. [DOI] [PubMed] [Google Scholar]
- 45.Fortunato F, Martinelli D, Cappelli MG, Cozza V, Prato R. Impact of pneumococcal conjugate universal routine vaccination on pneumococcal disease in Italian children. J Immunol Res. 2015;2015:206757. doi: 10.1155/2015/206757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leach AJ, Wigger C, Andrews R, Chatfield M, Smith-Vaughan H, Morris PS. Otitis media in children vaccinated during consecutive 7-valent or 10-valent pneumococcal conjugate vaccination schedules. BMC Pediatr. 2014;14:200. doi: 10.1186/1471-2431-14-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sigurdsson S, Eythorsson E, Erlendsdottir H, Hrafnkelsson B, Kristinsson KG, Haraldsson A. Impact of the 10-valent pneumococcal conjugate vaccine on hospital admissions in children under three years of age in Iceland. Vaccine. 2020;38:2707–14. doi: 10.1016/j.vaccine.2020.01.094. [DOI] [PubMed] [Google Scholar]
- 48.Petousis-Harris H, Howe AS, Paynter J, Turner N, Griffin J. Pneumococcal conjugate vaccines turning the tide on inequity: a retrospective cohort study of New Zealand children born 2006-2015. Clin Infect Dis. 2019;68:818–26. doi: 10.1093/cid/ciy570. [DOI] [PubMed] [Google Scholar]
- 49.Ben-Shimol S, Givon-Lavi N, Leibovitz E, Greenberg D, Dagan R. Studying PCV impact on clinical presentation of otitis media helps to understand its pathogenesis. Vaccine. 2019;37:1–6. doi: 10.1016/j.vaccine.2018.11.054. [DOI] [PubMed] [Google Scholar]
- 50.Ben-Shimol S, Givon-Lavi N, Leibovitz E, Raiz S, Greenberg D, Dagan R. Impact of widespread introduction of pneumococcal conjugate vaccines on pneumococcal and nonpneumococcal otitis media. Clin Infect Dis. 2016;63:611–18. doi: 10.1093/cid/ciw347. [DOI] [PubMed] [Google Scholar]
- 51.Ben-Shimol S, Givon-Lavi N, Leibovitz E, Raiz S, Greenberg D, Dagan R. Near-elimination of otitis media caused by 13-valent pneumococcal conjugate vaccine (PCV) serotypes in southern Israel shortly after sequential introduction of 7-valent/13-valent PCV. Clin Infect Dis. 2014;59:1724–32. doi: 10.1093/cid/ciu683. [DOI] [PubMed] [Google Scholar]
- 52.Palmu AA, Rinta-Kokko H, Nohynek H, Nuorti JP, Jokinen J. Impact of national ten-valent pneumococcal conjugate vaccine program on reducing antimicrobial use and tympanostomy tube placements in Finland. Pediatr Infect Dis J. 2018;37:97–102. doi: 10.1097/inf.0000000000001810. [DOI] [PubMed] [Google Scholar]
- 53.Eythorsson E, Sigurdsson S, Erlendsdottir H, Hrafnkelsson B, Kristinsson KG, Haraldsson A. Increase in tympanostomy tube placements despite pneumococcal vaccination, a population-based study. Acta Paediatr. 2019;108:1527–34. doi: 10.1111/apa.14724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gisselsson-Solen M. Trends in otitis media incidence after conjugate pneumococcal vaccination: a national observational study. Pediatr Infect Dis J. 2017;36:1027–31. doi: 10.1097/inf.0000000000001654. [DOI] [PubMed] [Google Scholar]
- 55.Edmondson-Jones M, Dibbern T, Hultberg M, Anell B, Medin E, Feng Y, Talarico C. The effect of pneumococcal conjugate vaccines on otitis media from 2005 to 2013 in children aged ≤5 years: a retrospective cohort study in two Swedish regions. Hum Vaccin Immunother. 2021;17:517–26. doi: 10.1080/21645515.2020.1775455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leach AJ, Wigger C, Beissbarth J, Woltring D, Andrews R, Chatfield MD, Smith-Vaughan H, Morris PS. General health, otitis media, nasopharyngeal carriage and middle ear microbiology in northern territory aboriginal children vaccinated during consecutive periods of 10-valent or 13-valent pneumococcal conjugate vaccines. Int J Pediatr Otorhinolaryngol. 2016;86:224–32. doi: 10.1016/j.ijporl.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 57.Lee J, Kim KH, Jo DS, Ma SH, Kim JH, Kim CS, Kim HM, Kang JH. A longitudinal hospital-based epidemiology study to assess acute otitis media incidence and nasopharyngeal carriage in Korean children up to 24 months. Hum Vaccin Immunother. 2020;16:3090–97. doi: 10.1080/21645515.2020.1748978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vesikari T, Forsten A, Seppa I, Kaijalainen T, Puumalainen T, Soininen A, Traskine M, Lommel P, Schoonbroodt S, Hezareh M, et al. Effectiveness of the 10-valent pneumococcal nontypeable Haemophilus influenzae protein D–conjugated vaccine (PHiD-CV) against carriage and acute otitis media – a double-blind randomized clinical trial in Finland. J Pediatr Infect Dis Soc. 2016;5:237–48. doi: 10.1093/jpids/piw010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eythorsson E, Sigurdsson S, Hrafnkelsson B, Erlendsdottir H, Haraldsson A, Kristinsson KG. Impact of the 10-valent pneumococcal conjugate vaccine on antimicrobial prescriptions in young children: a whole population study. BMC Infect Dis. 2018;18:505. doi: 10.1186/s12879-018-3416-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eythorsson E, Hrafnkelsson B, Erlendsdottir H, Gudmundsson SA, Kristinsson KG, Haraldsson A. Decreased acute otitis media with treatment failure after introduction of the ten-valent pneumococcal Haemophilus influenzae Protein D conjugate vaccine. Pediatr Infect Dis J. 2018;37:361–66. doi: 10.1097/inf.0000000000001870. [DOI] [PubMed] [Google Scholar]
- 61.Kong K, Coates HL. Natural history, definitions, risk factors and burden of otitis media. Med J Aust. 2009;191:S39–43. doi: 10.5694/j.1326-5377.2009.tb02925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Prymula R, Peeters P, Chrobok V, Kriz P, Novakova E, Kaliskova E, Kohl I, Lommel P, Poolman J, Prieels JP, Schuerman L. Pneumococcal capsular polysaccharides conjugated to protein D for prevention of acute otitis media caused by both Streptococcus pneumoniae and non-typable Haemophilus influenzae: a randomised double-blind efficacy study. Lancet. 2006;367:740–48. doi: 10.1016/S0140-6736(06)68304-9. [DOI] [PubMed] [Google Scholar]
- 63.Fletcher MA, Fritzell B. Pneumococcal conjugate vaccines and otitis media: an appraisal of the clinical trials. Int J Otolaryngol. 2012;2012:312935. doi: 10.1155/2012/312935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Griffiths UK, Clark A, Gessner B, Miners A, Sanderson C, Sedyaningsih ER, Mulholland KE. Dose-specific efficacy of Haemophilus influenzae type b conjugate vaccines: a systematic review and meta-analysis of controlled clinical trials. Epidemiol Infect. 2012;140:1343–55. doi: 10.1017/S0950268812000957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Monasta L, Ronfani L, Marchetti F, Montico M, Vecchi Brumatti L, Bavcar A, Grasso D, Barbiero C, Tamburlini G. Burden of disease caused by otitis media: systematic review and global estimates. PLoS One. 2012;7:e36226. doi: 10.1371/journal.pone.0036226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Alderson MR. Status of research and development of pediatric vaccines for Streptococcus pneumoniae. Vaccine. 2016;34:2959–61. doi: 10.1016/j.vaccine.2016.03.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Balsells E, Guillot L, Nair H, Kyaw MH. Serotype distribution of Streptococcus pneumoniae causing invasive disease in children in the post-PCV era: a systematic review and meta-analysis. PLoS One. 2017;12:e0177113. doi: 10.1371/journal.pone.0177113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lagousi T, Basdeki P, Routsias J, Spoulou V. Novel protein-based pneumococcal vaccines: assessing the use of distinct protein fragments instead of full-length proteins as vaccine antigens. Vaccines (Basel). 2019;7:9. doi: 10.3390/vaccines7010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Borrow R, Dagan R, Zepp F, Hallander H, Poolman J. Glycoconjugate vaccines and immune interactions, and implications for vaccination schedules. Expert Rev Vaccines. 2011;10:1621–31. doi: 10.1586/erv.11.142. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


