ABSTRACT
Enterotoxigenic Escherichia coli (ETEC) is a major cause of diarrhea in children and travelers in developing countries. ETEC is characterized by the ability to produce major virulence factors including colonization factors (CFs) and enterotoxins, that bind to specific receptors on epithelial cells and induce diarrhea. The gut microbiota is a stable and sophisticated ecosystem that performs a range of beneficial functions for the host, including protection against pathogen colonization. Understanding the pathogenic mechanisms of ETEC and the interaction between the gut microbiota and ETEC represents not only a research need but also an opportunity and challenge to develop precautions for ETEC infection. Herein, this review focuses on recent discoveries about ETEC etiology, pathogenesis and clinical manifestation, and discusses the colonization resistances mediated by gut microbiota, as well as preventative strategies against ETEC with an aim to provide novel insights that can reduce the adverse effect on human health.
KEYWORDS: Enterotoxigenic Escherichia coli, gut microbiota, pathogenesis, enterotoxin, colonization resistance
Introduction
Enterotoxigenic Escherichia coli (ETEC) is the major enteric pathogen that account for the tens of millions of diarrheal disease each year.1 Children under 5 years are susceptible to ETEC, particularly in endemic areas, which was responsible for an estimated 100 million diarrhea episodes and 60,000 deaths in 2015.1,2 ETEC is also the key etiology for traveler’s diarrhea that affects travelers visiting low-income regions of the world, and approximately one-third of all traveler’s diarrhea patients seeking medical care were diagnosed with gastrointestinal disturbance.3 ETEC infection is caused by ingestion of contaminated food and water in developing countries, where lack the infrastructure to supply clean drinking water and disposal of excrement. Previous study has shown that ETEC can survive in feces for more than half a year, and generally occur in water in the form of biofilms which provides a greater potential to survive.4 (Figure 1) In low-income regions, infrastructure and sanitation associated to people’s health are difficult to dramatically improve in a short period of time, the risk of diarrhea caused by ETEC is hard to be effectively controlled.
Figure 1.
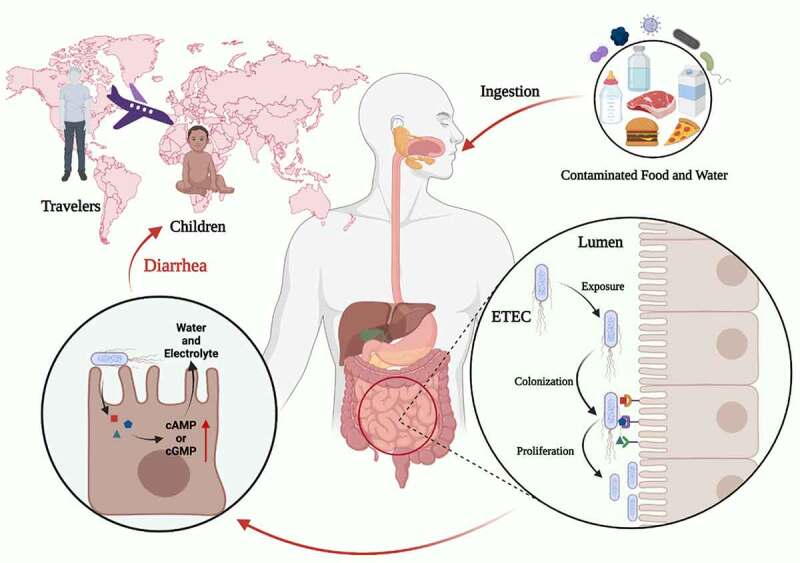
Characteristics of the ETEC infection. ETEC is the major enteric pathogen that account for the diarrhea that occurs in travelers and children in developing countries. ETEC infection is caused by ingestion of contaminated food and water, ETEC through the gastrointestinal tract, and eventually colonization in the small intestine. When ETEC is exposed in the small intestine, it colonizes intestinal epithelial cells via CFs, and ETEC proliferates on the intestinal epithelial after colonization. ETEC produces and delivers heat-labile (LT) and/or heat-stable (ST) enterotoxins to exert toxic effect. Image created with BioRender.
ETEC is characterized by the ability to produce heat-labile (LT) and/or heat-stable (ST) enterotoxins.5 LT is a high-molecular-weight (84 kDa) enterotoxin with an active alpha subunit surrounded by five identical binding B subunits, ST is a low-molecular-weight peptide consisting of 18 to 19 amino acid residues.6 The colonization of ETEC to the surface of the intestinal epithelium is a critical step to exert its toxicity. Apart from the two types of enterotoxins, the colonization factors (CFs) are also major virulence of ETEC.6 Once ETEC has colonized in the small intestine epithelia through CFs, effective enterotoxins delivery activity commences, which is responsible for the secretion of water and electrolytes in the intestinal lumen.7
In the cases of ETEC infection, the clinical manifestations are characterized by acute watery diarrhea leading to rapid dehydration and prostration within a few hours, which is similar to the clinical manifestations of cholera.8 ETEC infection is followed by a variety of symptoms, including vomiting, stomach cramps, headache, and, in rare cases, a slight fever.9 Some existing findings suggested that ETEC infection may be associated with some sequelae, such as raising the risk of childhood stunting due to immunological deficiencies and malnutrition, increasing the likelihood of contracting other infectious illnesses, and even influencing cognitive development.5,10,11 Furthermore, there is a link between traveler’s diarrhea and post-infectious irritable bowel syndrome.3 So far, antibiotics and oral rehydration are the most popular treatments, while antibiotics create a large number of resistant strains and eliminate beneficial bacteria in the gut, making it imperative to find alternative treatments.
ETEC has adapted to its environment through a variety of ways, including a cluster of varied strains that express a variety of CFs to cling to the intestinal epithelium and secrete a variety of enterotoxins. To avoid ETEC infection, it is necessary to thoroughly understand the pathogenic mechanism of ETEC infection and to identify certain targets for developing novel precautions. Although the gut microbiota of mammals performs a variety of beneficial functions for the host, the connection between the gut microbiota and ETEC infection is not well known. This paper reviews the characteristics of ETEC infection in terms of the pathogenic mechanism of major virulence factors, from toxin secretion to eventual diarrhea, as well as the process through which gut microbiota resist ETEC colonization. It also summarizes the available preventive strategies for ETEC infection as reported in recent studies. Our objective is to raise awareness about ETEC infection and to give a more comprehensive view of ETEC pathogenesis that will lead to new strategy for preventing ETEC infection.
Pathogenesis of virulence factors
Pathogens must adapt to the hostile environment of the gastrointestinal system in order to get nutrients and express virulence factors. In a nutshell, the classical paradigm for ETEC pathogenesis requires two processes to initiate an infection (Figure 1). The first process is ETEC colonizes on the small intestinal epithelium by adhesins, which is necessary for ETEC to release enterotoxin. Secondly, various toxins mainly contain LT and ST enterotoxin, as the essential weapon, released into host epithelia. It is well accepted that both LT and ST produce host diarrhea by increasing cyclic nucleotide synthesis, resulting in electrolyte and water loss (Figure 1).
Colonization factors
Attachment of ETEC to the small intestinal epithelium is a complex process that is dependent on a variety of CFs located on the bacterial surface. The majority of CFs are fimbria or fimbria-related extracellular filamentous protein polymers known as pili or fimbriae, and the morphology of CFs is classified as fimbrial, fibrillar, helical and afimbrial.12 Over 25 distinct types of CFs have been found and described to far (Table 1).48 Although ETEC strains can express one or more CFs that have been discovered, around 20–40% of ETEC isolates from clinical patients still have no detectable CFs. This could be due to the true absence of CFs, currently unknown CFs, a dearth of specialized techniques for detecting unknown CFs, and loss of CF properties on subculture of strains.6,49,50 With the in-depth development of research, more and more unknown CFs will be investigated clearly. The naming of CFs in the past was complicated and inconsistent. To simplify and standardize the nomenclature of CFs, ETEC CFs are designated by the abbreviation “CS” (Coli Surface antigen), followed by an Arabic numeral. Most of CFs are encoded on intracellular plasmids of ETEC.51 The colonization phase is an important node in the process of potentially intervening ETEC infection, and therapies aiming at preventing ETEC colonization have attracted the interest of a significant number of researchers.
Table 1.
Assembly form and morphology of identified colonization factors
| Colonization factor | Assembly class a | Morphology | MW (kDa) | ETEC strain | Accession number | References |
|---|---|---|---|---|---|---|
| CFA/I | CU | Fimbrial | 15.0 | H10407 | M55661 | 13,14 |
| CS1 | CU | Fimbrial | 16.5 | JEF100 | CR942285 | 15,16 |
| CS2 | CU | Fimbrial | 15.3 | C91f | Z47800 | 17 |
| CS3 | CU | Fribrillae | 15.1 | PB176 | X16944 | 18,19 |
| CS4 | CU | Fimbrial | 17.0 | WS2560B | AY281092 | 20,21 |
| CS5 | CU | Helical | 21.0 | O115:H40 | AJ224079 | 22–24 |
| CS6 | CU | Nonfimbrial | 14.5/16.0 | E8775 | U04846 | 25 |
| CS7 | CU | Helical | 21.5 | E29101A | AY009095 | 26,27 |
| CS8 | Type-IV | Fimbrial | 18.0 | 260–1 | AB049751 | 28 |
| CS10 | U | Nonfimbrial | 16.0 | None | None | 29,30 |
| CS11 | U | Fibrillae | None | None | None | 31 |
| CS12 | CU | Fimbrial | 19.0 | 350C1 | AY009096 | 32 |
| CS13 | CU | Fibrillae | 27.0 | ESEI_597 | OU964063 | 33 |
| CS14 | CU | Fimbrial | 15.5/17.0 | WS3294A | AY283611 | 21,29 |
| CS15 | CU | Nonfimbrial | 16.3 | None | None | 34 |
| CS17 | CU | Fimbrial | 17.5 | WS6788A | AY515609 | 35 |
| CS18 | CU | Fimbrial | 25.0 | ARG-2 | AF335469 | 36,37 |
| CS19 | CU | Fimbrial | 16.0 | WS0115A | AY288101 | 38 |
| CS20 | CU | Fimbrial | 20.8 | H721A | AF438155 | 39,40 |
| CS21 | Type-IV | Fimbrial | 22.0 | E9034A | EF595770 | 41,42 |
| CS22 | CU | Fibrillae | 15.7 | ARG-3 | AF145205 | 43 |
| CS23 | CU | Nonfimbrial | None | 1766a | JQ434477 | 44 |
| CS26 | CU | Fimbrial | None | MH2416 | HQ203050 | 45,46 |
| CS30 | CU | Fimbrial | None | E873 | LT174529 | 47 |
| PCFO71 | CU | Fimbrial | None | WS2173A | AY513487 | 21 |
aCU, chaperone-usher; U, unknown.
The common classification methods for CFs are morphology, antigenic type, and mode of assembly.52 ETEC CFs are classified into two types based on the process of CF assembly: chaperone-usher (CU) pili and Type IV pili. The CU pathway assembles pili was found in a wide range of Gram-negative bacteria, and the majority of ETEC pili are assembled in this manner.53 Two proteins are required for the CU pathway to function properly: one is a periplasmic chaperone protein that promotes pilins folding, inhibits their polymerization in the periplasm, and direct them to the usher; the other is an outer membrane protein named “usher” that convenes and coordinates chaperone-subunit complexes forming into a pilus.54 Type IV pili (T4P) contribute to a variety of biological processes, including adhesion between host and bacteria, twitching motility, biofilm formation, phage, DNA uptake, and signal transduction.55 Two CFs of ETEC, CS8 (CFA/III) and CS21 (longus), belong to T4P assembled by pilin subunits.12 T4P systems are similar to type II secretion systems, which translocate the pilin subunit from the periplasm of Gram-negative bacteria into the extracellular environment to form the pilus filament.53
Numerous adhesins were identified on the tip of CFs that detect carbohydrate receptors, leading in optimal colonization of the target region. Typically, the receptors for CFs are tested in vitro by combing CFs with glycoproteins or glycosphingolipids isolated from intestinal cells. CFs are considered to be species-specific, which explains why ETEC from animals does not induce human infection. Although CS30 can bind to human and porcine intestinal cells via a binding sulfatide, a putative glycosphingolipid receptor, this does not suggest that ETEC isolates carrying CS30 are capable of infecting both people and pigs.56 The Global Enteric Multicenter Study (GEMS) has shown that CFA/I and CS1-CS6 were the most common colonization factors, which was examined among ETEC isolates from children under 5 years with moderate-to-severe diarrhea in developing country.57 As for traveler’s diarrhea, the previous study detected ETEC isolates causing traveler’s diarrhea in Spanish travelers abroad and showed that the most common CFs were CS21 (58%), CS6 (27%), and CS3 (23%), and EAST1 (65%) and EatA (48%) were the most common nonclassical virulence factors.58
Heat-labile enterotoxin
Structures and main features of LT
LT is a high-molecular-weight enterotoxin encoded by the eltAB operon on virulence plasmid, and it is structurally and functionally identical to cholera toxin. LT is a heterohexameric molecule composed of a single A subunit that serves as catalytic component and a pentamer B subunit that is responsible for binding to the glycoconjugates on epithelia (Figure 2a).59 The A subunit has two domains linked by a disulfide bond (Figure 3): A1 is the active enzymatic activity of LTA and has a stimulatory function on G protein, whereas A2 is regarded as a bridge between the A1 domain and the B subunit, which could anchor the A1 domain into B the subunit.60 Additionally, the A2 domain possesses a cell-penetrating function, transporting protein through the membrane to the intracellular regardless of cell types.61
Figure 2.
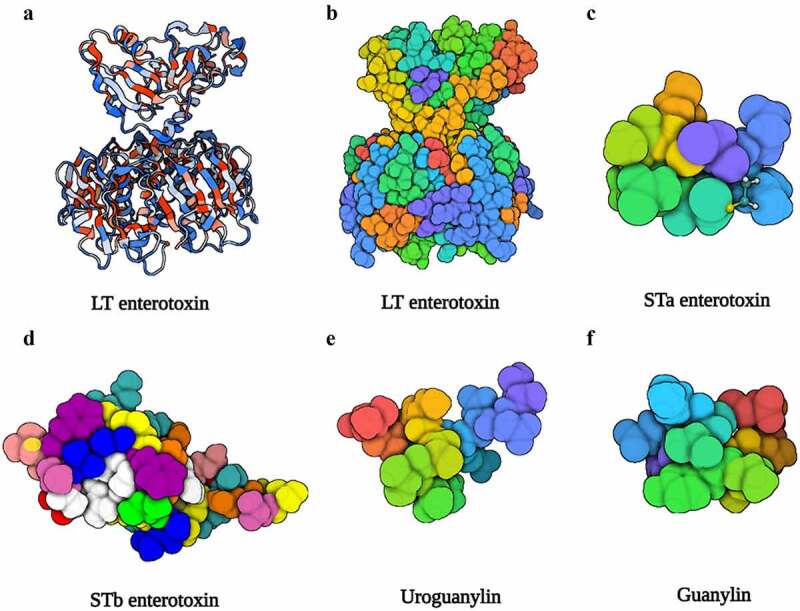
The structure of LT, STa, STb, uroguanylin, and guanylin. (a), (b) Three-dimensional structure of the LT (PDB accession no. 1LTB). (c) Three-dimensional structure of the STa (PDB accession no. 1ETN). (d) Three-dimensional structure of the STb (PDB accession no. 1EHS). (e) Three-dimensional structure of the uroguanylin (PDB accession no. 1UYA). (f) Three-dimensional structure of the guanylin (PDB accession no. 1GNA). Image created with BioRender software.
Figure 3.
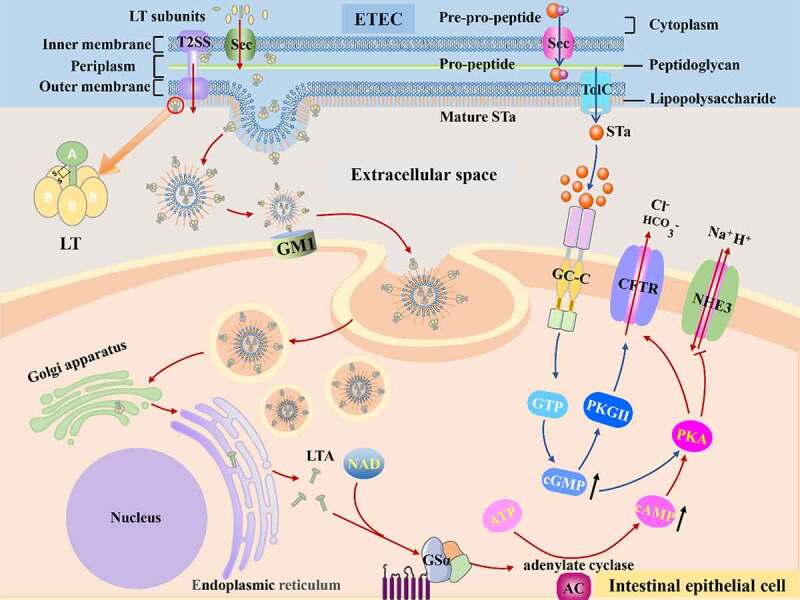
The mechanisms of disease caused by ETEC. Once ETEC established in the small intestinal epithelia via CFs, subsequent efficient enterotoxins delivery activity begins. The ST and LT of ETEC activate adenylyl and guanylate cyclase lead to high level of cAMP and cGMP, which stimulates water and electrolytes secretion in the intestinal lumen.
According to its antigenic capability and accompanying genetic sequence, LT is categorized into two primary categories (Table 2),66 including type I LT isolated from human (LT-Ih) and porcine (LT-Ip), and type II LT (LT-IIa, b, c) predominantly isolated from animals.67 Surprisingly, researchers utilized toxicity of LT-II to treat triple negative breast cancer (TNBC) cells, discovering that LT-IIc can cause selective cytotoxicity in TNBC cells but not in non-tumorigenic breast epithelial cells through modulating autophagy.68 This finding demonstrates that LT has distinct binding characteristics (Table 2). GM1 ganglioside is the primary receptor of LT-I, and LT-I can bind less stably to GD1b, GM2 and asialo-GM1. The three subtypes of LT-II have separate preferent receptors (Table 2), and LT-II B subunit could bind to toll-like receptor.62,63,67 Several residues of LTB directly impact the binding affinity and specificity of LT.69 Except for glycolipids on host cells, LT possesses the affinity with lipopolysaccharide (LPS), the main component of cell walls of Gram-negative bacteria.70
Table 2.
Characteristic of LT produced by ETEC
| LTA (amino acid) |
LTB (amino acid) |
Encoding Gene | Receptors | Host | References | |
|---|---|---|---|---|---|---|
| LT-Ih | 240 | 103 | eltAB | GM1, GM2, GD1b, LPS, asialo-GM1 | Humans | 62,63 |
| LT-Ip | 240 | 103 | eltAB | GM1, GM2, GD1b, LPS | Pigs | 62,63 |
| LT-IIa | 241 | 100 | eltAB | GM1, GM2, GM3, GD1a, GD1b, GD2, GT1b | Buffalo | 62,64 |
| LT-IIb | 243 | 99 | eltAB | GM2, GM3, GD1a, GD1α, GM1b, GT1b | Unknown | 62,64 |
| LT-IIc | 241 | 98 | eltAB | GM1, GM2, GM3, GD1a, GD1α, GQ1b | Claves | 60,65 |
Molecular mechanism of LT
Before LT cause toxicity in the host, it must be released from ETEC; protein secretion is required for the procaryotic organism to transfer toxin from the cytoplasm to the extracellular or host cell cytoplasm, which is not a simple task due to the presence of two phospholipid membranes.71 ETEC transfer enterotoxin via a Sec-dependent protein secretion system (Figure 3). Above all, enterotoxins rely on Sec secretion systems to be transported across the inner membrane into periplasm, and then enterotoxins are incised and folded into AB5 structure by identifying the N terminal Sec-type cleavable signal sequence, the folded enterotoxin can be transported from periplasm to extracellular milieu in virtue of GspD of T2SS, a large double-β-barrel pore on the outer membrane.72,73 T2SS possesses features that span inner and outer membranes and export folded proteins.74 Following export, LT bind to lipopolysaccharide (LPS) on the surface of ETEC through the pentamer B subunit.75
ETEC has been shown to produce natural Outer membrane vesicles (OMVs) that contain LT and bind to their external surface, and the construction materials of OMVs are similar to outer membrane components.76 After OMVs budded, LT located on the external surface of vesicles binds to monosialoganglioside GM1 on the host cells via LTB, forming a tether between host and vesicle.77 Thus, LT is primarily delivered by OMVs secretion, and this delivery pathway facilitates LT interaction with host cells. There are several factors that influence LT delivery. EatA, a highly immunogenic secreted serine protease, could degrade adhesin EtpA and then accelerate the delivery of LT.78 Another study proved that EatA facilitates the entry of toxins to their receptors by degrading major mucin, which restricts bacteria contact to host cells to a certain extent.79 Additionally, previous study demonstrated that highly conserved metalloprotease YghJ, a secreted ETEC antigen, shown the potential to accelerate the efficient delivery of LT by degrading the major mucins in the small intestine.80
Subsequently, vesicles were internalized depending on lipid rafts.81 Once within the cells, these vesicles transferred LT to the endoplasmic reticulum and cytoplasm. Then, A1 subunit was hydrolyzed by protease and released from A2 subunit. Consequently, A subunit with ADP ribosylating transferase activity catalyzes stimulates G protein α subunit (GSα), activating adenylate cyclase (AC) and leading to the increase of cyclic adenosine monophosphate (cAMP).82 Following, cAMP-dependent protein kinase A (PKA) is activated, leading the cystic fibrosis transmembrane conductance regulator (CFTR) being opened. As a result, electrolytes and water are secreted into the intestinal lumen.
Other new functions of LT
Additional possible roles for LT are being revealed as study progresses. LT is not only a toxin but also plays a role in facilitating bacterial attachment and intestinal colonization, delivering foreign molecules into cells, and upregulating vaccine antigenicity as an adjuvant.83 Additionally, LT was found to have a function in reducing intestinal epithelial viability and inducing intestinal epithelial apoptosis in a time-dependent and dose-dependent manner.84 A recent study explored the effect of AB5 toxins including LT on intestinal bacteria and discovered that LTB inhibited the growth of intestinal bacteria capable of mimicking GM1 gangliosides, despite these bacteria may affect human health.85 Additionally, research has revealed that LT can inhibit intestinal absorption of vitamin C,86 making intestine more vulnerable to be infected.
Heat-stable enterotoxin
Structures and main features of ST
ST-ETEC is the primary cause of infantile diarrhea in underdeveloped nations.87 ST is small, non-immunogenic peptide that is opposite to the LT, and classified into two categories based on its structure and function, known as STa and STb (Table 3). STa contains six cysteine residues to form three disulfide bonds and shares a highly sequence and structural similarity with guanylin and uroguanylin, both of them contained two disulfide bonds (Figure 2). STa contains two subtypes consisting of STaH (19aa) which is only isolated from human ETEC strains and STaP (18aa) widely found in porcine, bovine, as well as humans.88 STb consists of 48 amino acid peptides and it presents in cattle but not in humans.90
Table 3.
Characteristic of ST produced by ETEC
Molecular mechanism of STa
The synthesis of STa experiences a complex deformation process that begins with a 72 amino acids pre-pro-peptide precursor generated in the cytoplasm and progresses to a pro-peptide consisting of a signal peptide, a pro sequence, and a carboxy-terminal region, and eventually to a mature peptide (Figure 3).92 As a leader peptide, The 19 amino acids signal peptide is proteolytically cleaved first during the transfer process across the inner membrane into periplasm via Sec general export pathway.93 Subsequently, the pro sequence is cleaved in the periplasm.93 While there is considerable disagreement about this process, one study pointed out that the pro-peptide form can penetrate the outer membrane,94 and current research may suggest that some pro-peptide processing occurs outside of bacteria.95 Before connecting to their receptors, they must be folded into specific forms analogous to guanylin and uroguanylin on host cells. The specific shapes need two key elements, one is toxin’s structure including cysteine residues, and the other is disulfide oxidoreductase DsbA that catalyzes cysteine residues to form disulfide bonds.96 Additionally, the folding procedure also occurs in the periplasm.96 After that, mature STa were secreted. STa secretion requires the efflux protein TolC on the outer membrane, according to the previous research.95 In addition, EtpA not only accelerated LT delivery, but also essential for effective delivery of ST.95
STa performs function through the guanylate cyclase C (GC-C) signal transduction pathway. GC-C receptor is a transmembrane protein, and contains an extracellular domain, a transmembrane region, a kinase homology domain, a hinge region, and a catalytic domain.97 STa was identified and coupled to its extracellular ligand binding domain by GC-C receptors expressed on the brush border membrane of small intestine epithelia. This binding activates the intracellular catalytic portion of the GC-C receptors that converts GTP to cyclic GMP (cGMP), resulting in dramatically rise of cGMP.92 Accumulation of cGMP mediates diarrhea in two ways. One is opening the CFTR channel by directly activating protein kinase G II (PKGII), directly activating PKA, or indirectly activating PKA by inhibiting phosphodiesterase 3 inhibitor, resulting in a large amount of chloride and bicarbonate release into intestinal lumen.98–100 The other is to block the brush border Na/H exchanger NHE3 indirectly, reducing sodium reabsorption, and it has been discovered that the intracellular signaling mechanisms of NHE3 inhibition differ between cell types.101,102 Finally, salts ions and water accumulated in the intestinal lumen, leading to ultimate diarrhea. Nevertheless, recent research aiming at another angle, intercellular second messenger signaling, pointed that cGMP outside cell may play a role during infection as ST-induced cGMP were principally exported into basolateral part rather than staying within the cells in human jejunal organoid monolayers, which may complicate the mechanism of ETEC.103
According to a recent study, the pathogenesis of ST may be associated with the mucosal metal condition as ST can bind to zinc and iron, and the metal-binding ST weakens greatly to induce cGMP, which may beneficial for host detoxification or good for ETEC to reduce luminal metal concentrates.104 In an in vitro investigation that cultured human jejunum cells under mechanical forces to create a condition close to the real intestine environment, researchers discovered that flow application increased apical and basolateral cGMP secretion but did not alter intracellular cGMP content.105 Because GC-C was a tumor suppressor, some researchers used ST-expressing ETEC to fight tumors, and the results revealed that it reduced the incidence of colorectal cancer by recovering repressed GC-C signal during carcinogenesis.106 Furthermore, as GC-C activation is a crucial role in the onset of watery diarrhea, GC-C inhibitors that decrease STa-induced cGMP accumulation may be employed in the development ST-elicit diarrhea therapies.107
Non-canonical virulence factors
Tia and TibA
Tia and TibA, two outer membrane proteins, appear to be involved in the initial infection mediated by CFs.12 Tia is a 25 kD outer membrane protein encoded on a large pathogenicity Island with a lower GC content in ETEC strain H10407.108 Elsinghorst et al. discovered that while ETEC strain H10407 could invade cultured small intestine epithelial cells, it is unable to replicate within the cell.109 Subsequent research demonstrated that antisera recognizing Tia blocked invasion by E. coli expressing Tia,110 indicating that Tia had invasive property in addition to its adhesion function. However, existing researchers have not identified invasion as a characteristic of ETEC, this phenomenon of cell invasion observed in vitro requires further investigation.
The tib locus consists of four genes, which were tibDBCA.111 tibA encodes a glycosylated outer membrane protein of 104 kD, which is a member of the autotransporter family.112 tibC encodes a 45 kD heptosyltransferase that glycosylates the TibA precursor by the addition of residues.111,113 However, the role of tibB and tibC is uncertain, although it has been suggested that they participate in gene regulation. The glycosylated form of TibA facilitates the binding of ETEC to the particular receptor on intestinal epithelial cells.112 Additionally, TibA promotes bacterial aggregation and biofilm formation, which is independent of TibA glycosylation.114
EtpA
EtpA, a key secreted adhesin, can serve as a bridge between ETEC and the epithelial surface by attaching to the tips of ETEC flagella and interacting with N-acetylgalactosamine (GalNAc) containing glycans expressed on the intestinal mucosa.115 EtpA could interact with flagellin which is required for H10407 to adhere optimally in vitro and promotes ETEC colonization in a murine model.116 Interestingly, LT can induce the synthesis of mucin MUC2 to intensify the attachment mediated by EtpA.115 EtpA is a blood group A-specific lectin/hemagglutinin that interacted with blood group A-related glycans expressed on the surface of epithelia, facilitating bacterial adhesion and effective delivery of ETEC enterotoxins, which explains why severe diarrhea caused by ETEC was more prevalent in blood group A individuals.117
Other non-canonical virulence factors
Previously published research established that the majority of ETEC, even those with unknown CFs, expressed Type I pili that were highly conserved and coordinated with CFs to enhance adhesion.118 Another highly conserved adhesin on the chromosome, EaeH, attaches to the surface of intestinal epithelial cells and assists in ETEC colonization, and the eaeH gene expression increases significantly when the host comes into contact with the pathogen.119 The eatA gene encodes EatA, a member of the serine protease autotransporter of Enterobacteriaceae family, which possessed highly immunogenic.120 Recent data suggest that EatA may facilitate ETEC access to intestinal epithelial cells by degrading MUC2.79 Apart from the pili related to CFs, LT harbors non-pili adhesins that are straightforward outer membrane adhesins.
Colonization resistance for ETEC mediated by gut microbiota
Although watery diarrhea is the most prominent clinical manifestation of ETEC infection, not all individuals challenged with ETEC suffer diarrhea symptoms, even though ETEC can be found in the feces and intestinal contents of asymptomatic individuals.121 In a previous study, investigators assessed changes in gut microbiota during volunteers challenged with ETEC H10407, and identified some biomarkers based on microbial sequencing data that could predict whether an individual will develop diarrhea following ETEC infection with reasonable accuracy.122 According to the researchers, these microbial taxa may help prevent ETEC colonization in the gut. However, these findings are hypotheses rather than conclusions, as too many complicating factors were neglected during the investigation. It is worth recognizing that this discovery inspires a new perspective that gut microbiota may interfere with ETEC infection. The gut microbiota could affect the colonization of ETEC in the small intestine, streptomycin pre-treatment prior to ETEC H10407 inoculation to eradicate normal resident bacterial flora in the intestinal tract, which is required for construction of a mouse model of human ETEC infection.123 In addition, the environmental factors of the intestine could also influence the ETEC virulence gene expression, recent work examining the transcriptome of stool samples from volunteers challenged with ETEC H10407, this study demonstrated that ETEC virulence gene expression is likely repressed in the low-oxygen lumen and identified the corresponding transcriptional regulator fumarate and nitrate reduction (FNR) regulator.124
The gut microbiota is a dynamic and diverse ecosystem composed of trillions of microorganisms that performs a variety of activities, including metabolic regulation, nutritional digestion, immune response regulation, and protection against enteric bacteria.125–127 The gut microbiota possessed the ability to inhibit enteric pathogen colonization and expansion, a property referred to as colonization resistance.128 Existing research is elucidating how the composition of the gut microbiota can offer resistance to enteric pathogens with the development of next-generation sequencing and metabolomics.129 In comparison to other prevalent enteric pathogen, such as Clostridium difficile, Salmonella enterica serovar Typhimurium, and Enterohemorrhagic Escherichia coli, there are a limited studies on colonization resistance of gut microbiota against ETEC. Colonization resistance against ETEC is achieved by the use of numerous mechanisms that remain poorly understood, however there is evidence that both direct pathogen inhibition and indirect pathogen inhibition via host systems may be involved (Figure 4).130
Figure 4.
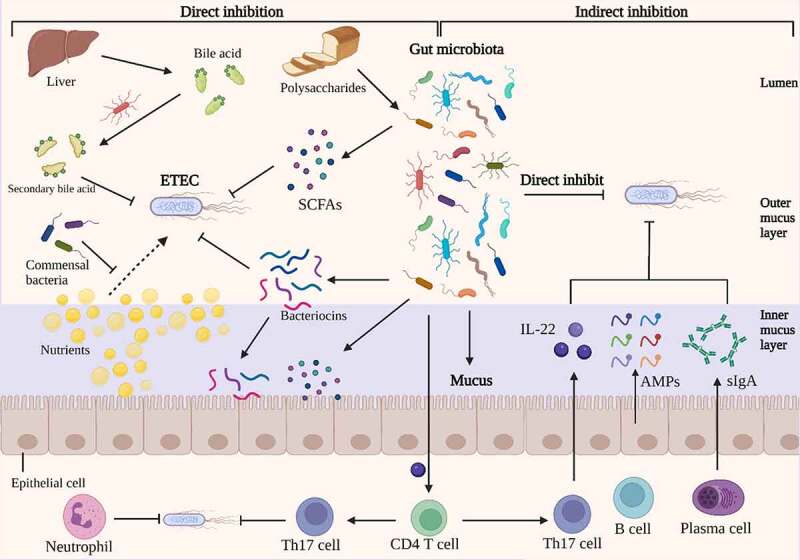
Direct and indirect inhibition mediated by gut microbiota against ETEC infections. On the left, an illustration depicts the direct inhibition against ETEC mediated by gut bacteria. Gut bacteria directly impede ETEC colonization and proliferation. Certain antibacterial compounds, such as bacteriocins, SCFAs, and secondary bile acid, generated by the gut microbiota have been shown to directly inhibit ETEC. Additionally, gut microbiota can compete with ETEC for nutrients, which could limit the growth of ETEC. On the right, indirect methods of competition between gut microbiota and ETEC are depicted. The antimicrobial molecules produced by gut microbiota, such as SCFAs and bacteriocins, which could release into inner mucus layer and stimulate the barrier function. The commensal microbiota induces the differentiation of CD4 T cells into Th17 cells, which contribute to colonization resistance against ETEC by the release of cytokines such as IL-22. Under the stimulation of gut microbiota, intestinal epithelia secrete inflammatory factors, AMPs and sIgA into the mucus, which inhibits the colonization of ETEC. Image created with BioRender.
3.1. Direct inhibition
Numerous bioactive small molecules produced by the gut microbiota can impede the growth of enteric pathogens, including short-chain fatty acids (SCFAs), secondary bile acids, and Bacteriocins.131 SCFAs are saturated fatty acids with a chain length ranging from one to six carbon atoms and are the main metabolites produced by bacterial fermentation of non-digestible polysaccharides in the gastrointestinal tract.132 Acetate (C2), propionate (C3) and butyrate (C4) are the most abundant SCFAs in the human body.133 The concentration of SCFAs is inversely related to gut pH, which can be altered to prevent the growth of pathogenic E. coli.134,135 Acetate is the most abundant SCFAs in the intestinal lumen, which has a role in inhibiting the expansion of pathogenic E. coli by depleting intracellular methionine pools.136,137 A previous study demonstrated that addition of SCFAs into the medium could reduce the production of LT from ETEC, which may be due to the disturbance of the biosynthesis of LT.138 Additionally, SCFAs are a primary fuel for the colonic epithelium, and directly affect the health of intestinal epithelia by enhancing intestinal barrier function.139 Bile acids are synthesized in the liver and secreted into small intestine to aid in digestion of dietary lipids. In the intestine tract, bile acids are modified by the gut microbiota into secondary bile acids with antimicrobial property.140 And bile acids inhibited the binding of ETEC heat-labile enterotoxins to GM1 receptor, mitigating the toxicity effect of LT.141
Bacteriocins are bacterially produced peptides that are active against other bacteria and against which the producer has a specific immunity mechanism.142,143 Probiotics could produce bacteriocins to facilitate its probiotic function in a number of ways.142 For example, bacteriocins may function as antimicrobial peptides, directly eradicating pathogens;144 they may act as colonizing peptides, helping the colonization of a probiotic in the intestine trat;145 or they may serve as signaling peptides, signaling other bacteria or the immune system of the host.146 Mircocins produced by Gram-negative bacteria belong to the large class of bacteriocins.147 Probiotic Escherichia coli Nissle 1917 could utilize microcins to limit the expansion and colonization of pathogenic E. coli in infected mice.148
3.2. Indirect inhibition
Indirectly, gut microbiota also inhibit pathogen colonization infection by increasing host defense mechanisms, such as promoting mucosal barrier function and enhancing innate immune response.127 The gut microbiota has the ability to stimulate the epithelium to create antibacterial compounds and mucus, which serves as the first line of defense against pathogen colonization.149 The notion that gut bacteria increase epithelial barrier function is mostly supported by indirect evidence. For example, germ-free mice had decreased antimicrobial peptide synthesis and higher intestinal mucus penetrability in the small intestine.150,151 Along with physically preventing pathogens from reaching the epithelial surface, the mucus layer protects the epithelium barrier by storing antimicrobial agents such as antimicrobial peptides and secretory IgA generated by intestinal epithelial cells.152 Additionally, by improving the function of the epithelial barrier via metabolites produced by the gut microbiota, such as SCFAs, the epithelium is able to block the translocation of enterotoxins produced by pathogenic E. coli.153
Not only does the gut microbiota improve mucosal barrier function, but it also boosts host immunity to protect against enteric infection, modifying the host’s vulnerability to diarrheal pathogens. The commensal microbiota stimulates CD4 T cell development into Th17 cells, which may contribute to colonization resistance against enteric pathogen via cytokine production such as IL-22.154 The gut microbiota is densely harbored by trillions of bacteria belonging to several hundreds of different species, which making it difficult to investigate the specific function of probiotic in the colonization resistance. The current approach to this challenge is to separate probiotics that play a critical role in diarrhea resistance from feces and chyme and to research their unique mechanisms of inhibition of ETEC infection using in vitro epithelial cell co-culture and in vivo animal model. In a sterile pig model, Lactobacillus plantarum can protect against ETEC infection by decreasing IL-1α and IL-8 expression and increasing IL-10 expression in the small intestine.155
Strategies to prevent ETEC infection
4.1. Vaccines
Considering protective immunity against ETEC develops after natural and experimental infection, indicating that vaccine-induced ETEC immunity should be feasible. Vaccination is now recognized as the most effective method of preventing ETEC infection, given public hygiene concerns in developing nations cannot be resolved quickly.156 Following vaccination, individuals should be protected from at least common strains.156 Interdicting ETEC adhesion to the intestinal surface and neutralizing the toxin are the primary goals of vaccine development, and the vaccine for ETEC is often administered orally. Nevertheless, highly variable of CFs and lack of suitable animal model to evaluate vaccine efficacy make it so complex to design a vaccine that there is no licensed vaccine up to now.157,158 Sufficient coverage of CFs remains a significant issue in producing an effective vaccination, and a suitable technique that targets those prevalent adhesins is necessary. According to a previous study, approximately 66% of pediatric moderate-to-severe diarrhea cases caused by ETEC expressing only ST or LT could be prevented in developing countries if effective ETEC vaccine candidates based on major CFs such as CFA/I and CS1-CS6 are developed, and the rate would increase to 77% if CS14 is added to CF-based ETEC vaccine candidates.57 Data from a systematic review of ETEC epidemiology also demonstrated that CFA/I-expressing strains were common in all regions (17%), and the results were obtained by analyzing the17205 ETEC isolates abstracted from 136 studies.49 In recent years, multi-epitope fusion antigen technology has been employed to develop multivalent vaccinations, which may aid in the development of vaccines against common CFs.159
Current research on vaccine target ST is primarily focused on modifying it to eliminate its high toxicity, identifying a protein carrier couple to ST to increase its immunogenicity, and minimizing potential immunological cross-reactivity.160 Due to the high immunogenicity of LT as a vaccine antigen, LT is frequently employed as an effective adjuvant or carrier protein for multivalent vaccine development.161 Along with multivalent vaccine, another major area of vaccine research is the quest for conserved antigens. A recent study found that EtpA and EatA are high conserved virulence molecules, as they both present in about half of 1159 globally ETEC isolates and do not exhibit obvious regional distribution differences.162 ETVAX is the most advanced ETEC vaccine candidate at the moment,163 since it consists of four inactivated recombinant E. coli strains hyper-expressing CFA/I, CS3, CS5, and CS6 adhesins, along with a recombinant subunit protein LCTBA.164 LCTBA is a hybrid B subunit of LT and cholera toxin, previous study demonstrated that cholera toxin B subunit was the immunogen in cholera vaccine which induced cross-protective immunity against LT-producing ETEC,165 due to homology between LT B subunit and cholera toxin B subunit.166 ETVAX was shown safe and immunogenic in adults from Swedish and ETEC endemic regions.167,168 Firdausi Qadri et al. reported that ETVA induced a broad protective response in Bangladesh children older than 12 months.164 Additionally, researchers found that ETVAX-induced antibodies to cross-react to CS1, CS14, CS17, and CS7 adhesins, which may result in expanded ETEC strain coverage of ETVAX vaccine.169 According to the ETVAX clinic experiment, targeting common antigens to the greatest extent possible may be feasible.164 Although ETVAX has demonstrated a favorable preventive impact in clinical trials, the true preventive benefit may be compromised if it does not also develop protective immunity against ETEC strains that produce ST. Data from recent studies suggest that ETEC producing ST is easier to cause moderate-to-severe diarrhea in young children compared with the ETEC producing LT.87,170 In addition, previous study reported that over two-thirds of ETEC strains isolated from patients express ST alone or together with LT.171 Therefore, a broadly protective vaccine should carry multiple the most common CFAs to induce anti-adhesin immunity and toxoid antigens to induce antitoxin immunity against LT and ST.156,172 Above all, the process has been well explored for a long period of time, and researchers have been developing vaccines based on the major virulence.
4.2. Specific antibody
Extensive study has been conducted on the usage of antibody that can prevent diarrhea caused by comparable toxin antigens. Evidence demonstrated that volunteers challenged with the ETEC strain B7A, 50% protection against moderate and severe diarrhea was seen in individuals who received bovine serum immunoglobulins targeting to the whole ETEC strain BA7.173 In a nonhuman primate trial, oral administration of secretory IgA against colonization factor CFA/I significantly decreased the risk of diarrhea caused by ETEC H10407.174 Hyperimmune bovine colostrum (HBC) is produced by repeated immunization of pregnant cows, which is high in specific antibodies and immunomodulatory components, has an effective prophylactic function against gastrointestinal illnesses.175 In addition, the use of HBC rich in microbe-specific IgG for the prevention and treatment of gastrointestinal infections is a safe precaution, which is unlike antibiotics, they do not disturb the gut microbiota.176 An early clinical study conducted by Tacket et al. showed that daily consumption of an ETEC hyperimmune bovine milk concentrate shortly after each meal protected volunteers from an experimental oral challenge with 1.2 × 109 CFU of ETEC H10407.177 In a subsequent study by Otto and colleagues, an ETEC HBC delivered prior to each meal reduced the incidence of diarrhea in volunteers orally challenged with ETEC H10407.178 In a controlled human infection model (CHIM) study, volunteers received oral bovine colostrum IgG antibodies against CS17 significantly prevent diarrhea caused by ETEC strain LSN03-016011/A expressing CS17.179 Maternal natural IgG antibodies evoked by the maternal microbiota can protect new-born mice lacking the ability to make IgG against ETEC infection, regardless of whether the antibodies are transmitted through the placenta or breast milk.180 In view of these considerations, oral delivery of antibody may enhance passive immunization and provide a novel immunoprophylaxis technique that is effective against ETEC. At the same time, the future challenges of specific antibody including stability, inexpensive cost, and availability need to be taken into consideration.181
4.3. Antimicrobial molecule
Antimicrobial peptides (AMPs), which are synthesized by diverse organisms or synthetically, are used to fight bacterial infection due to their broad-spectrum antimicrobial activity.182,183 Saliva antimicrobial peptide histatin-5 was shown to inhibit ETEC adhesion and colonization, demonstrating that saliva components combat pathogens introduced through the mouth, which is a component of the innate immune system.184 In an ETEC challenged mouse model, the Lasso peptide Microcin J25 improved epithelial barrier function by increasing tight junctions expression in the small intestine, and alleviated gut inflammatory responses.185 Application of AMPs has the potential to be a beneficial and protective method for ETEC infection. Furthermore, AMPs benefited the intestinal barrier function, inflammatory response, and gut microbiota when ETEC was challenge.186,187
Additionally, natural products exhibited a variety of beneficial functions, including antibacterial, anti-inflammatory, and antioxidant capabilities. Dietary macleaya cordata plays a preventative role with respect to ETEC infection by alleviating ETEC-induced oxidative stress and enhancing immunological function.188 Icariin and its phosphorylated derivatives can help reduce inflammation and oxidative stress produced by ETEC K88 by inhibiting the production of the p38 MAPK.189 Certain polyphenol extracts have been identified as potential candidates for preventative method because to their ability to block the conjunction of LT and its receptor.190
Conclusion
The available research demonstrates the mechanism of major virulence factors of ETEC. With the deepening of researches in this filed, more and more novel virulence factors and antigens have been discovered. The identification of specific virulence factors involved in the pathogenic process of ETEC and specific antigens on the surface of ETEC will allow the development of novel vaccine. Given the critical role of the gut microbiota in protection against ETEC, targeting the microbiota to develop preventive strategy has attracted the interest of a large number of researchers. Clearly, considerable work remains to be done not only to gain a greater knowledge of the interaction between the gut microbiota and ETEC, but also to determine the most effective strategy to utilize this relationship to prevent or cure ETEC both inside and outside the intestine.
Funding Statement
This study was supported by the National Natural Science Foundation of China (31930106, 31829004, and 31722054), the National Ten-thousand Talents Program of China (23070201), the Henan Province Public Benefit Research Foundation (201300111200-05), the 2115 Talent Development Program of China Agricultural University (1041-00109019), the Developmental Fund by Henan Wofengde Biological Technology Co., Ltd. (201905410411435), and the 111 Project (B16044).
Disclosure Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Author Contributions
The review was mainly conceived and designed by XM. Literatures were collected and analyzed by YZ and YZ. The manuscript was mainly written by YZ and YZ, and edited by PT and XM. XM resourced the project. All the authors contributed to, read and approved the final manuscript.
Abbreviations
ETEC, Enterotoxigenic Escherichia coli; LT, heat-labile enterotoxin; ST, heat-stable enterotoxin; CFs, colonization factors; CS, Coli Surface antigen; CU, Cheperone-usher; T4P, Type IV pili; GalNAc, N-acetylgalactosamine; LTA, heat-labile enterotoxin A subunit; LTB, heat-labile enterotoxin B subunit; LT-Ih, type I LT isolated from human; LT-Ip, type I LT isolated from porcine; LT-IIa, b, c, type II heat-labile enterotoxin a, b,c; TNBC, triple negative breast cancer; LPS, lipopolysaccharide; OMVs, outer membrane vesicles; GSα, G protein α subunit; AC, adenylate cyclase; cAMP, cyclic adenosine monophosphate; PKA, protein kinase A; CFTR, cystic fibrosis transmembrane conductance regulator; STaH, type a ST isolated from human; STaP, type a ST isolated from porcine; GC-C, guanylate cyclase C; cAMP, cyclic adenosine monophosphate; cGMP, cyclic guanosine monophosphate; PKG, protein kinase G II; FNR, fumarate and nitrate reduction regulator; HBC, hyperimmune bovine colostrum; Amps, antimicrobial peptides; FNR, fumarate and nitrate reduction.
References
- 1.Anderson JD, Bagamian KH, Muhib F, Amaya MP, Laytner LA, Wierzba T, Rheingans R.. Burden of enterotoxigenic Escherichia coli and shigella non-fatal diarrhoeal infections in 79 low-income and lower middle-income countries: a modelling analysis. Lancet Glob Health. 2019;7(3):e321–22. doi: 10.1016/s2214-109x(18)30483-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagamian KH, Anderson JD, Muhib F, Cumming O, Laytner LA, Wierzba TF, Rheingans R. Heterogeneity in enterotoxigenic Escherichia coli and shigella infections in children under 5 years of age from 11 African countries: a subnational approach quantifying risk, mortality, morbidity, and stunting. Lancet Glob Health. 2020;8(1):e101–e12. doi: 10.1016/s2214-109x(19)30456-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steffen R, Hill DR, DuPont HL. Traveler’s diarrhea: a clinical review. JAMA. 2015;313(1):71–80. doi: 10.1001/jama.2014.17006. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed D, Islam MS, Begum YA, Janzon A, Qadri F, Sjoling A. Presence of enterotoxigenic Escherichia coli in biofilms formed in water containers in poor households coincides with epidemic seasons in Dhaka. J Appl Microbiol. 2013;114(4):1223–1229. doi: 10.1111/jam.12109. [DOI] [PubMed] [Google Scholar]
- 5.Qadri F, Saha A, Ahmed T, Al Tarique A, Begum YA, Svennerholm AM. Disease burden due to enterotoxigenic Escherichia coli in the first 2 years of life in an urban community in bangladesh. Infect Immun. 2007;75(8):3961–3968. doi: 10.1128/IAI.00459-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qadri F, Svennerholm AM, Faruque AS, Sack RB. Enterotoxigenic Escherichia col in developing countries: epidemiology, microbiology, clinical features, treatment, and prevention. Clin Microbiol Rev. 2005;18(3):465–483. doi: 10.1128/CMR.18.3.465-483.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fleckenstein JM, Hardwidge PR, Munson GP, Rasko DA, Sommerfelt H, Steinsland H. Molecular mechanisms of enterotoxigenic Escherichia coli infection. Microbes Infect. 2010;12(2):89–98. doi: 10.1016/j.micinf.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finkelstein RA, Vasil ML, Jones JR, Anderson RA, Barnard Tjjo CM. Clinical cholera caused by enterotoxigenic Escherichia coli. J Clin Microbiol. 1976;3(3):382–384. doi: 10.1128/jcm.3.3.382-384.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Youmans BP, Ajami NJ, Jiang Z-D, Campbell F, Wadsworth WD, Petrosino JF, DuPont HL, Highlander SK. Characterization of the human gut microbiome during travelers’ diarrhea. Gut Microbes. 2015;6(2):110–119. doi: 10.1080/19490976.2015.1019693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Troeger C, Colombara DV, Rao PC, Khalil IA, Brown A, Brewer TG, Guerrant RL, Houpt ER, Kotloff KL, Misra K, et al. Global disability-adjusted life-year estimates of long-term health burden and undernutrition attributable to diarrhoeal diseases in children younger than 5 years. Lancet Glob Health. 2018;6:e255–e69. doi: 10.1016/s2214-109x(18)30045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Platts-Mills JA, Taniuchi M, Uddin MJ, Sobuz SU, Mahfuz M, Gaffar SA, Mondal D, Hossain MI, Islam MM, Ahmed AS; JTAjocn . Association between enteropathogens and malnutrition in children aged 6–23 mo in Bangladesh: a case-control study. Am J Clin Nutr. 2017;105(5):1132–1138. doi: 10.3945/ajcn.116.138800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Madhavan TP, Sakellaris H. Colonization factors of enterotoxigenic Escherichia coli. Adv Appl Microbiol. 2015;90:155–197. doi: 10.1016/bs.aambs.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Karjalainen TK, Evans D, So M, Lee C-H. Molecular cloning and nucleotide sequence of the colonization factor antigen I gene of Escherichia coli. Infect Immun. 1989;57(4):1126–1130. doi: 10.1128/iai.57.4.1126-1130.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans DG, Evans JDJ, Clegg S, Pauley JA. Purification and characterization of the CFA/I antigen of enterotoxigenic Escherichia coli. Infect Immun. 1979;25(2):738–748. doi: 10.1128/iai.25.2.738-748.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Froehlich BJ, Karakashian A, Meisen LR, Wakefield JC, Scott JR. CooC and CooD are required for assembly of CS1 pili. Mol Microbiol. 1994;12(3):387–401. doi: 10.1111/j.1365-2958.1994.tb01028.x. [DOI] [PubMed] [Google Scholar]
- 16.Perez-Casal J, Swartley JS, Scott JR. Gene encoding the major subunit of CS1 pili of human enterotoxigenic Escherichia coli. Infect Immun. 1990;58(11):3594–3600. doi: 10.1128/iai.58.11.3594-3600.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Froehlich BJ, Karakashian A, Sakellaris H, Scott JR. Genes for CS2 pili of enterotoxigenic Escherichia coli and their interchangeability with those for CS1 pili. Infect Immun. 1995;63(12):4849–4856. doi: 10.1128/iai.63.12.4849-4856.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jalajakumari M, Thomas C, Halter R, Manning P. Genes for biosynthesis and assembly of CS3 pili of CFA/II enterotoxigenic Escherichia coli: novel regulation of pilus production by bypassing an amber codon. Mol Microbiol. 1989;3(12):1685–1695. doi: 10.1111/j.1365-2958.1989.tb00154.x. [DOI] [PubMed] [Google Scholar]
- 19.Levine MM, Ristaino P, Marley G, Smyth C, Knutton S, Boedeker E, Black R, Young C, Clements ML, Cheney C. Coli surface antigens 1 and 3 of colonization factor antigen II-positive enterotoxigenic Escherichia coli: morphology, purification, and immune responses in humans. Infect Immun. 1984;44(2):409–420. doi: 10.1128/iai.44.2.409-420.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolf MK, Andrews G, Tall B, McConnell M, Levine M, Boedeker E. Characterization of CS4 and CS6 antigenic components of PCF8775, a putative colonization factor complex from enterotoxigenic Escherichia coli E8775. Infect Immun. 1989;57(1):164–173. doi: 10.1128/iai.57.1.164-173.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anantha RP, McVeigh AL, Lee LH, Agnew MK, Cassels FJ, Scott DA, Whittam TS, Savarino SJ. Evolutionary and functional relationships of colonization factor antigen I and other class 5 adhesive fimbriae of enterotoxigenic Escherichia coli. Infect Immun. 2004;72(12):7190–7201. doi: 10.1128/IAI.72.12.7190-7201.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manning PA, Higgins GD, Lumb R, Lanser JA. Colonization factor antigens and a new fimbrial type, CFA/V, on O115: H40 and H⁻ strains of enterotoxigenic Escherichia coli in Central Australia. J Infect Dis. 1987;156(5):841–844. doi: 10.1093/infdis/156.5.841. [DOI] [PubMed] [Google Scholar]
- 23.Clark CA, Heuzenroeder MW, Manning PA. Colonization factor antigen CFA/IV (PCF8775) of human enterotoxigenic Escherichia coli: nucleotide sequence of the CS5 determinant. Infect Immun. 1992;60(3):1254–1257. doi: 10.1128/iai.60.3.1254-1257.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duthy TG, Staendner LH, Manning PA, Heuzenroeder MW. CS5 pilus biosynthesis genes from enterotoxigenic Escherichia coli O115:H40. J Bacteriol. 1999;181(18):5847–5851. doi: 10.1128/JB.181.18.5847-5851.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolf MK, De Haan LA, Cassels FJ, Willshaw GA, Warren R, Boedeker EC, Gaastra W. The CS6 colonization factor of human enterotoxigenic Escherichia coli contains two heterologous major subunits. FEMS Microbiol Lett. 1997;148(1):35–42. doi: 10.1111/j.1574-6968.1997.tb10263.x. [DOI] [PubMed] [Google Scholar]
- 26.Hibberd ML, Mcconnell MM, Field AM, Rowe B. The fimbriae of human enterotoxigenic Escherichia coli strain 334 are related to CS5 fimbriae. J Gen Microbiol. 1990;136(12):2449–2456. doi: 10.1099/00221287-136-12-2449. [DOI] [PubMed] [Google Scholar]
- 27.Hibberd ML, McConnell MM, Willshaw GA, Smith HR, Rowe B. Positive regulation of colonization factor antigen I (CFA/I) production by enterotoxigenic Escherichia coli producing the colonization factors CS5 CS6, CS7, CS17, PCFO9, PCFO159: H4 and PCFO166. J Gen Microbiol. 1991;137(8):1963–1970. doi: 10.1099/00221287-137-8-1963. [DOI] [PubMed] [Google Scholar]
- 28.Taniguchi T, Akeda Y, Haba A, Yasuda Y, Yamamoto K, Honda T, Tochikubo K. Gene cluster for assembly of pilus colonization factor antigen III of enterotoxigenic Escherichia coli. Infect Immun. 2001;69(9):5864–5873. doi: 10.1128/IAI.69.9.5864-5873.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Darfeuille-Michaud A, Forestier C, Joly B, Cluzel R. Identification of a nonfimbrial adhesive factor of an enterotoxigenic Escherichia coli strain. Infect Immun. 1986;52(2):468–475. doi: 10.1128/iai.52.2.468-475.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Forestier C, Welinder KG, Darfeuille-Michaud A, Klemm P. Afimbrial adhesin from Escherichia coli strain 2230: purification, characterization and partial covalent structure. FEMS Microbiol Lett. 1987;40(1):47–50. doi: 10.1111/j.1574-6968.1987.tb01980.x. [DOI] [Google Scholar]
- 31.Knutton S, Lloyd DR, AS M. Identification of a new fimbrial structure in enterotoxigenic Escherichia coli (ETEC) serotype O148: H28 which adheres to human intestinal mucosa: a potentially new human ETEC colonization factor. Infect Immun. 1987;55(1):86–92. doi: 10.1128/iai.55.1.86-92.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tacket C, Maneval D, Levine M. Purification, morphology, and genetics of a new fimbrial putative colonization factor of enterotoxigenic Escherichia coli O159: H4. Infect Immun. 1987;55(5):1063–1069. doi: 10.1128/iai.55.5.1063-1069.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heuzenroeder MW, Elliot TR, Thomas CJ, Halter R, Manning PA. A new fimbrial type (PCFO9) on enterotoxigenic Escherichia coli 09: h− LT+ isolated from a case of infant diarrhea in central Australia. FEMS Microbiol Lett. 1990;66:55–60. doi: 10.1016/0378-1097(90)90258-r. [DOI] [PubMed] [Google Scholar]
- 34.Aubel D, Darfeuille-Michaud A, Joly B. New adhesive factor (antigen 8786) on a human enterotoxigenic Escherichia coli O117: H4 strain isolated in Africa. Infect Immun. 1991;59(4):1290–1299. doi: 10.1128/iai.59.4.1290-1299.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McConnell MM, Hibberd M, Field AM, Chart H, Rowe B. Characterization of a new putative colonization factor (CS17) from a human enterotoxigenic Escherichia coli of serotype 0114: 821 which produces only heat-labile enterotoxin. J Infect Dis. 1990;161(2):343–347. doi: 10.1093/infdis/161.2.343. [DOI] [PubMed] [Google Scholar]
- 36.Viboud GI, Binsztein N, Svennerholm A. A new fimbrial putative colonization factor, PCFO20, in human enterotoxigenic Escherichia coli. Infect Immun. 1993;61(12):5190–5197. doi: 10.1128/iai.61.12.5190-5197.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Viboud GI, Jonson G, Dean-Nystrom E, Svennerholm A-M. The structural gene encoding human Enterotoxigenic Escherichia coli PCFO20 is homologous to that for porcine 987P. Infect Immun. 1996;64(4):1233–1239. doi: 10.1128/iai.64.4.1233-1239.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grewal H, Valvatne H, Bhan MK, van Dijk L, Gaastra W, Sommerfelt H. A new putative fimbrial colonization factor, CS19, of human Enterotoxigenic Escherichia coli. Infect Immun. 1997;65(2):507–513. doi: 10.1128/iai.65.2.507-513.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Valvatne H, Sommerfelt H, Gaastra W, Bhan MK, Grewal H. Identification and characterization of CS20, a new putative colonization factor of Enterotoxigenic Escherichia coli. Infect Immun. 1996;64(7):2635–2642. doi: 10.1128/iai.64.7.2635-2642.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Valvatne H, Steinsland H, Grewal HM, Mølbak K, Vuust J, Sommerfelt H. Identification and molecular characterization of the gene encoding coli surface antigen 20 of Enterotoxigenic Escherichia coli. FEMS Microbiol Lett. 2004;239(1):131–138. doi: 10.1016/j.femsle.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 41.Girón JA, Levine MM, Kaper JB. Longus: a long pilus ultrastructure produced by human Enterotoxigenic Escherichia coli. Mol Microbiol. 1994;12(1):71–82. doi: 10.1111/j.1365-2958.1994.tb00996.x. [DOI] [PubMed] [Google Scholar]
- 42.Gomez-Duarte OG, Chattopadhyay S, Weissman SJ, Giron JA, Kaper JB, Sokurenko EV. Genetic diversity of the gene cluster encoding longus, a type IV pilus of enterotoxigenic Escherichia coli. J Bacteriol. 2007;189(24):9145–9149. doi: 10.1128/JB.00722-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pichel M, Binsztein N, Viboud G, O’Brien AD. CS22, a novel human Enterotoxigenic Escherichia coli adhesin, is related to CS15. Infect Immun. 2000;68(6):3280–3285. doi: 10.1128/IAI.68.6.3280-3285.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Del Canto F, Botkin DJ, Valenzuela P, Popov V, Ruiz-Perez F, Nataro JP, Levine MM, Stine OC, Pop M, Torres AG. Identification of coli surface antigen 23, a novel adhesin of Enterotoxigenic Escherichia coli. Infect Immun. 2012;80(8):2791–2801. doi: 10.1128/IAI.00263-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cádiz L, Torres A, Valdés R, Vera G, Gutiérrez D, Levine MM, Montero DA, O’Ryan M, Rasko DA, Stine OC. Coli surface antigen 26 acts as an adherence determinant of Enterotoxigenic Escherichia coli and is cross-recognized by anti-CS20 antibodies. Front Microbiol. 2018;9:2463. doi: 10.3389/fmicb.2018.02463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nada RA, Shaheen HI, Khalil SB, Mansour A, El-Sayed N, Touni I, Weiner M, Armstrong AW, Klena JD. Discovery and phylogenetic analysis of novel members of class b Enterotoxigenic Escherichia coli adhesive fimbriae. J Clin Microbiol. 2011;49(4):1403–1410. doi: 10.1128/JCM.02006-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.von Mentzer A, Tobias J, Wiklund G, Nordqvist S, Aslett M, Dougan G, Å S, Svennerholm A-M. Identification and characterization of the novel colonization factor CS30 based on whole genome sequencing in Enterotoxigenic Escherichia coli (ETEC). Sci Rep. 2017;7(1):1–11. doi: 10.1038/s41598-017-12743-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bourgeois AL, Wierzba TF, Walker RIJV. Status of vaccine research and development for Enterotoxigenic Escherichia coli. Vaccine. 2016;34(26):2880–2886. doi: 10.1016/j.vaccine.2016.02.076. [DOI] [PubMed] [Google Scholar]
- 49.Isidean S, Riddle M, Savarino S, Porter C. A systematic review of ETEC epidemiology focusing on colonization factor and toxin expression. Vaccine. 2011;29(37):6167–6178. doi: 10.1016/j.vaccine.2011.06.084. [DOI] [PubMed] [Google Scholar]
- 50.Gaastra W, Svennerholm A-M, Svennerholm A-M. Colonization factors of human Enterotoxigenic Escherichia coli (ETEC). Trends Microbiol. 1996;4(11):444–452. doi: 10.1016/0966-842x(96)10068-8. [DOI] [PubMed] [Google Scholar]
- 51.Croxen MA, Law RJ, Scholz R, Keeney KM, Wlodarska M, Finlay BB. Recent advances in understanding enteric pathogenic Escherichia coli. Clin Microbiol Rev. 2013;26(4):822–880. doi: 10.1128/CMR.00022-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paranchych W, Frost LS. The physiology and biochemistry of pili. Adv Microb Physiol. 1988;29:53–114. doi: 10.1016/s0065-2911(08)60346-x. [DOI] [PubMed] [Google Scholar]
- 53.Hospenthal MK, Costa TRD, Waksman G. A comprehensive guide to pilus biogenesis in gram-negative bacteria. Nat Rev Microbiol. 2017;15(6):365–379. doi: 10.1038/nrmicro.2017.40. [DOI] [PubMed] [Google Scholar]
- 54.Waksman G, Hultgren SJ. Structural biology of the chaperone-usher pathway of pilus biogenesis. Nat Rev Microbiol. 2009;7(11):765–774. doi: 10.1038/nrmicro2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Craig L, Pique ME, Tainer JA. Type IV pilus structure and bacterial pathogenicity. Nat Rev Microbiol. 2004;2(5):363–378. doi: 10.1038/nrmicro885. [DOI] [PubMed] [Google Scholar]
- 56.Von Mentzer A, Zalem D, Chrienova Z, Teneberg S. Colonization factor CS30 from Enterotoxigenic Escherichia coli binds to sulfatide in human and porcine small intestine. Virulence. 2020;11(1):381–390. doi: 10.1080/21505594.2020.1749497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vidal RM, Muhsen K, Tennant SM, Svennerholm A-M, Sow SO, Sur D, Zaidi AK, Faruque AS, Saha D, Adegbola R; JPntd . Colonization factors among enterotoxigenic Escherichia coli isolates from children with moderate-to-severe diarrhea and from matched controls in the Global Enteric Multicenter Study (GEMS). PLoS Negl Trop Dis. 2019;13(1):e0007037. doi: 10.1371/journal.pntd.0007037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rivera FP, Medina AM, Aldasoro E, Sangil A, Gascon J, Ochoa TJ, Vila J, Ruiz J. Genotypic characterization of enterotoxigenic Escherichia coli strains causing traveler’s diarrhea. J Clin Microbiol. 2013;51(2):633–635. doi: 10.1128/JCM.02572-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beddoe T, Paton AW, Le Nours J, Rossjohn J, Paton JC; JTibs . Structure, biological functions and applications of the AB5 toxins. Trends Biochem Sci. 2010;35(7):411–418. doi: 10.1016/j.tibs.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sixma TK, Pronk SE, Kalk KH, Wartna ES, van Zanten BA, Witholt B, Hoi WG. Crystal structure of a cholera toxin-related heat-labile enterotoxin from E. coli. Nature. 1991;351(6325):371–377. doi: 10.1038/351371a0. [DOI] [PubMed] [Google Scholar]
- 61.Liu D, Guo H, Zheng W, Zhang N, Wang T, Wang P, Ma X. Discovery of the cell-penetrating function of A 2 domain derived from LTA subunit of Escherichia coli heat-labile enterotoxin. Appl Microbiol Biotechnol. 2016;100(11):5079–5088. doi: 10.1007/s00253-016-7423-x. [DOI] [PubMed] [Google Scholar]
- 62.Fukuta S, Magnani JL, Twiddy EM, Holmes RK, Ginsburg V. Comparison of the carbohydrate-binding specificities of cholera toxin and Escherichia coli heat-labile enterotoxins LTh-I, LT-IIa, and LT-IIb. Infect Immun. 1988;56(7):1748–1753. doi: 10.1128/iai.56.7.1748-1753.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ma Y. Recent advances in nontoxic Escherichia coli heat-labile toxin and its derivative adjuvants. Expert Rev Vaccines. 2016;15(11):1361–1371. doi: 10.1080/14760584.2016.1182868. [DOI] [PubMed] [Google Scholar]
- 64.Jobling MG, Frisan T. The chromosomal nature of LT-II enterotoxins solved: a lambdoid prophage encodes both LT-II and one of two novel pertussis-toxin-like toxin family members in type II Enterotoxigenic Escherichia coli. Pathog Dis. 2016;74(3):ftw001. doi: 10.1093/femspd/ftw001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Norton EB, Lawson LB, Mahdi Z, Freytag LC, Clements JD, Payne SM. The A subunit of Escherichia coli heat-labile enterotoxin functions as a mucosal adjuvant and promotes IgG2a, IgA, and Th17 responses to vaccine antigens. Infect Immun. 2012;80(7):2426–2435. doi: 10.1128/IAI.00181-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mirhoseini A, Amani J, Nazarian S. Review on pathogenicity mechanism of Enterotoxigenic Escherichia coli and vaccines against it. Microb Pathog. 2018;117:162–169. doi: 10.1016/j.micpath.2018.02.032. [DOI] [PubMed] [Google Scholar]
- 67.Nawar HF, King-Lyons ND, Hu JC, Pasek RC, Connell TD. LT-IIc, a new member of the type II heat-labile enterotoxin family encoded by an Escherichia coli sst. Infect Immun. 2010;78(11):4705–4713. doi: 10.1128/IAI.00730-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Masso-Welch P, Girald Berlingeri S, King-Lyons ND, Mandell L, Hu J, Greene CJ, Federowicz M, Cao P, Connell TD, Heakal Y. LT-IIc, A bacterial type II heat-labile enterotoxin, induces specific lethality in triple negative breast cancer cells by modulation of autophagy and induction of apoptosis and necroptosis. Int J Mol Sci. 2018;20. doi: 10.3390/ijms20010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Heggelund JE, Heim JB, Bajc G, Hodnik V, Anderluh G, Krengel U. Specificity of Escherichia coli heat-labile enterotoxin investigated by single-site mutagenesis and crystallography. Int J Mol Sci. 2019;20. doi: 10.3390/ijms20030703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Horstman AL, Kuehn MJ. Bacterial surface association of heat-labile enterotoxin through lipopolysaccharide after secretion via the general secretory pathway. J Biol Chem. 2002;277(36):32538–32545. doi: 10.1074/jbc.M203740200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Green ER, Mecsas J, Kudva IT. Bacterial secretion systems: an overview. Microbiol Spectr. 2016;4(1). doi: 10.1128/microbiolspec.VMBF-0012-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yan Z, Yin M, Xu D, Zhu Y, Li X. Structural insights into the secretin translocation channel in the type II secretion system. Nat Struct Mol Biol. 2017;24(2):177–183. doi: 10.1038/nsmb.3350. [DOI] [PubMed] [Google Scholar]
- 73.Tauschek M, Gorrell RJ, Strugnell RA, Robins-Browne RM. Identification of a protein secretory pathway for the secretion of heat-labile enterotoxin by an enterotoxigenic strain of Escherichia coli. Proc Natl Acad Sci U S A. 2002;99:7066–7071. doi: 10.1073/pnas.092152899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Korotkov KV, Sandkvist M, Hol WG. The type II secretion system: biogenesis, molecular architecture and mechanism. Nat Rev Microbiol. 2012;10(5):336–351. doi: 10.1038/nrmicro2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mudrak B, Rodriguez DL, Kuehn MJ. Residues of heat-labile enterotoxin involved in bacterial cell surface binding. J Bacteriol. 2009;191(9):2917–2925. doi: 10.1128/JB.01622-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Horstman AL, Kuehn MJ. Enterotoxigenic Escherichia coli secretes active heat-labile enterotoxin via outer membrane vesicles. J Biol Chem. 2000;275(17):12489–12496. doi: 10.1074/jbc.275.17.12489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ellis TN, Kuehn MJ. Virulence and immunomodulatory roles of bacterial outer membrane vesicles. Microbiol Mol Biol Rev. 2010;74(1):81–94. doi: 10.1128/MMBR.00031-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Roy K, Kansal R, Bartels SR, Hamilton DJ, Shaaban S, Fleckenstein JM. Adhesin degradation accelerates delivery of heat-labile toxin by enterotoxigenic Escherichia coli. J Biol Chem. 2011;286(34):29771–29779. doi: 10.1074/jbc.M111.251546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kumar P, Luo Q, Vickers TJ, Sheikh A, Lewis WG, Fleckenstein JM, Payne SM. EatA, an immunogenic protective antigen of enterotoxigenic Escherichia coli, degrades intestinal mucin. Infect Immun. 2014;82(2):500–508. doi: 10.1128/IAI.01078-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Luo Q, Kumar P, Vickers TJ, Sheikh A, Lewis WG, Rasko DA, Sistrunk J, Fleckenstein JM, Payne SM. Enterotoxigenic Escherichia coli secretes a highly conserved mucin-degrading metalloprotease to effectively engage intestinal epithelial cells. Infect Immun. 2014;82(2):509–521. doi: 10.1128/IAI.01106-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kesty NC, Mason KM, Reedy M, Miller SE, Kuehn MJ. Enterotoxigenic Escherichia coli vesicles target toxin delivery into mammalian cells. EMBO J. 2004;23(23):4538–4549. doi: 10.1038/sj.emboj.7600471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Croxen MA, Finlay BB. Molecular mechanisms of Escherichia coli pathogenicity. Nat Rev Microbiol. 2010;8(1):26–38. doi: 10.1038/nrmicro2265. [DOI] [PubMed] [Google Scholar]
- 83.Duan Q, Xia P, Nandre R, Zhang W, Zhu G. Review of newly identified functions associated with the heat-labile toxin of Enterotoxigenic Escherichia coli. Front Cell Infect Microbiol. 2019;9:292. doi: 10.3389/fcimb.2019.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lu X, Li C, Li C, Li P, Fu E, Xie Y, Jin F. Heat-labile enterotoxin-induced PERK-CHOP pathway activation causes intestinal epithelial cell apoptosis. Front Cell Infect Microbiol. 2017;7:244. doi: 10.3389/fcimb.2017.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Patry RT, Stahl M, Perez-Munoz ME, Nothaft H, Wenzel CQ, Sacher JC, Coros C, Walter J, Vallance BA, Szymanski CM. Bacterial AB 5 toxins inhibit the growth of gut bacteria by targeting ganglioside-like glycoconjugates. Nat Commun. 2019;10(1):1–13. doi: 10.1038/s41467-019-09362-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Subramenium GA, Sabui S, Marchant JS, Said HM, Subramanian VS. Enterotoxigenic Escherichia coli heat labile enterotoxin inhibits intestinal ascorbic acid uptake via a cAMP-dependent NF-κB-mediated pathway. Am J Physiol Gastrointest Liver Physiol. 2019;316(1):G55–G63. doi: 10.1152/ajpgi.00259.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013;382(9888):209–222. doi: 10.1016/s0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 88.Joffre E, von Mentzer A, Svennerholm AM, Sjoling A. Identification of new heat-stable (STa) enterotoxin allele variants produced by human Enterotoxigenic Escherichia coli (ETEC). Int J Med Microbiol. 2016;306(7):586–594. doi: 10.1016/j.ijmm.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 89.Basu N, Arshad N, Ssjm V; biochemistry c . Receptor guanylyl cyclase C (GC-C): regulation and signal transduction. Mol Cell Biochem. 2010;334(1–2):67–80. doi: 10.1007/s11010-009-0324-x. [DOI] [PubMed] [Google Scholar]
- 90.Weiglmeier PR, Rosch P, Berkner H. Cure and curse: e. coli heat-stable enterotoxin and its receptor guanylyl cyclase C. Toxins (Basel). 2010;2(9):2213–2229. doi: 10.3390/toxins2092213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Foreman DT, Martinez Y, Coombs G, Torres A, Kupersztoch YM. ToIC and DsbA are needed for the secretion of STB, a heat-stable enterotoxin of Escherichia coli. Mol Microbiol. 1995;18(2):237–245. doi: 10.1111/j.1365-2958.1995.mmi_18020237.x. [DOI] [PubMed] [Google Scholar]
- 92.Wang H, Zhong Z, Luo Y, Cox E, Devriendt B. Heat-Stable enterotoxins of Enterotoxigenic Escherichia coli and their impact on host immunity. Toxins (Basel). 2019;11. doi: 10.3390/toxins11010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yamanaka H, Nomura T, Fujii Y, Okamoto K. Extracellular secretion of Escherichia coli heat-stable enterotoxin I across the outer membrane. J Bacteriol. 1997;179(11):3383–3390. doi: 10.1128/jb.179.11.3383-3390.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yang Y, Gao Z, Guzmán‐Verduzco LM, Tachias K, Kupersztoch YM. Secretion of the STA3 heat-stable Enterotoxin of Escherichia coli: extracellular delivery of Pro-STA is accomplished by either Pro or STA. Mol Microbiol. 1992;6(23):3521–3529. doi: 10.1111/j.1365-2958.1992.tb01787.x. [DOI] [PubMed] [Google Scholar]
- 95.Zhu Y, Luo Q, Davis SM, Westra C, Vickers TJ, Fleckenstein JM. Molecular determinants of Enterotoxigenic Escherichia coli heat-stable toxin secretion and delivery. Infect Immun. 2018;86(11):e00526–18. doi: 10.1128/IAI.00526-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yamanaka H, Kameyama M, Baba T, Fujii Y, Okamoto K. Maturation pathway of Escherichia coli heat-stable enterotoxin I: requirement of DsbA for disulfide bond formation. J Bacteriol. 1994;176(10):2906–2913. doi: 10.1128/jb.176.10.2906-2913.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vaandrager ABJM. biochemistry c . Structure and function of the heat-stable enterotoxin receptor/guanylyl cyclase C. Mol Cell Biochem. 2002;230(1/2):73–83. doi: 10.1023/A:1014231722696. [DOI] [PubMed] [Google Scholar]
- 98.Vaandrager AB, Bot A, DeJonge HRJG. Guanosine 3’, 5’-cyclic monophosphate-dependent protein kinase II mediates heat-stable enterotoxin-provoked chloride secretion in rat intestine. Gastronterology. 1997;112(2):437–443. doi: 10.1053/gast.1997.v112.pm9024297. [DOI] [PubMed] [Google Scholar]
- 99.Forte L, Thorne P, Eber S, Krause W, Freeman R, Francis S, Corbin J. Stimulation of intestinal Cl-transport by heat-stable enterotoxin: activation of cAMP-dependent protein kinase by cGMP. Am J Physiol. 1992;263(3):C607–C15. doi: 10.1152/ajpcell.1992.263.3.C607. [DOI] [PubMed] [Google Scholar]
- 100.Vaandrager AB, Bot AG, Ruth P, Pfeifer A, Hofmann F, De Jonge HRJG. Differential role of cyclic GMP–dependent protein kinase II in ion transport in murine small intestine and colon. Gastronterology. 2000;118(1):108–114. doi: 10.1016/s0016-5085(00)70419-7. [DOI] [PubMed] [Google Scholar]
- 101.Bagorda A, Guerra L, Di Sole F, Hemle-Kolb C, Cardone RA, Fanelli T, Reshkin SJ, Gisler SM, Murer H, Casavola V. Reciprocal protein kinase A regulatory interactions between cystic fibrosis transmembrane conductance regulator and Na+/H+ exchanger isoform 3 in a renal polarized epithelial cell model. J Biol Chem. 2002;277(24):21480–21488. doi: 10.1074/jbc.M112245200. [DOI] [PubMed] [Google Scholar]
- 102.Chen T, Lin R, Avula L, Sarker R, Yang J, Cha B, Tse CM, McNamara G, Seidler U, Waldman S, et al. NHERF3 is necessary for Escherichia coli heat-stable enterotoxin-induced inhibition of NHE3: differences in signaling in mouse small intestine and Caco-2 cells. Am J Physiol Cell Physiol. 2019;317(4):C737–C48. doi: 10.1152/ajpcell.00351.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Foulke-Abel J, Yu H, Sunuwar L, Lin R, Fleckenstein JM, Kaper JB, Donowitz M. Phosphodiesterase 5 (PDE5) restricts intracellular cGMP accumulation during Enterotoxigenic Escherichia coli infection. Gut Microbes. 2020;12(1):1752125. doi: 10.1080/19490976.2020.1752125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kiefer MC, Motyka NI, Clements JD, Bitoun JP. Enterotoxigenic Escherichia coli heat-stable toxin increases the rate of zinc release from metallothionein and is a zinc- and iron-binding peptide. mSphere. 2020;5(2):e00146–20. doi: 10.1128/mSphere.00146-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sunuwar L, Yin J, Kasendra M, Karalis K, Kaper J, Fleckenstein J, Donowitz M. Mechanical stimuli affect Escherichia coli heat-stable enterotoxin-cyclic GMP signaling in a human enteroid intestine-chip model. Infect Immun. 2020;88(3):e00866–19. doi: 10.1128/IAI.00866-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li P, Lin JE, Snook AE, Waldman SA. ST-producing E. coli oppose carcinogen-induced colorectal tumorigenesis in mice. Toxins (Basel). 2017;9(9):279. doi: 10.3390/toxins9090279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bijvelds MJ, Loos M, Bronsveld I, Hellemans A, Bongartz JP, Ver Donck L, Cox E, de Jonge HR, Schuurkes JA, De Maeyer JH. Inhibition of heat-stable toxin-induced intestinal salt and water secretion by a novel class of guanylyl cyclase C inhibitors. J Infect Dis. 2015;212(11):1806–1815. doi: 10.1093/infdis/jiv300. [DOI] [PubMed] [Google Scholar]
- 108.Fleckenstein JM, Lindler LE, Elsinghorst EA, Dale JB. Identification of a gene within a pathogenicity Island of Enterotoxigenic Escherichia coli H10407 required for maximal secretion of the heat-labile enterotoxin. Infect Immun. 2000;68(5):2766–2774. doi: 10.1128/IAI.68.5.2766-2774.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Elsinghorst EA, Kopecko D. Molecular cloning of epithelial cell invasion determinants from Enterotoxigenic Escherichia coli. Infect Immun. 1992;60(6):2409–2417. doi: 10.1128/iai.60.6.2409-2417.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mammarappallil JG, Elsinghorst EA, O’Brien AD. Epithelial cell adherence mediated by the Enterotoxigenic Escherichia coli Tia protein. Infect Immun. 2000;68(12):6595–6601. doi: 10.1128/IAI.68.12.6595-6601.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lindenthal C, Elsinghorst EA, Orndorff PE. Identification of a glycoprotein produced by enterotoxigenic Escherichia coli. Infect Immun. 1999;67(8):4084–4091. doi: 10.1128/IAI.67.8.4084-4091.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lindenthal C, Elsinghorst EA. Enterotoxigenic Escherichia coli TibA glycoprotein adheres to human intestine epithelial cells. Infect Immun. 2001;69(1):52–57. doi: 10.1128/IAI.69.1.52-57.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Moormann C, Benz I, Schmidt MA. Functional substitution of the TibC protein of enterotoxigenic Escherichia coli strains for the autotransporter adhesin heptosyltransferase of the AIDA system. Infect Immun. 2002;70(5):2264–2270. doi: 10.1128/IAI.70.5.2264-2270.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sherlock O, Vejborg RM, Klemm P. The TibA adhesin/invasin from enterotoxigenic Escherichia coli is self recognizing and induces bacterial aggregation and biofilm formation. Infect Immun. 2005;73(4):1954–1963. doi: 10.1128/IAI.73.4.1954-1963.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kumar P, Kuhlmann FM, Bhullar K, Yang H, Vallance BA, Xia L, Luo Q, Fleckenstein JM. Dynamic interactions of a conserved Enterotoxigenic Escherichia coli adhesin with intestinal mucins govern epithelium engagement and toxin delivery. Infect Immun. 2016;84(12):3608–3617. doi: 10.1128/IAI.00692-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Roy K, Hamilton D, Allen KP, Randolph MP, Fleckenstein JM. The EtpA exoprotein of Enterotoxigenic Escherichia coli promotes intestinal colonization and is a protective antigen in an experimental model of murine infection. Infect Immun. 2008;76:2106–2112. doi: 10.1128/IAI.01304-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kumar P, Kuhlmann FM, Chakraborty S, Bourgeois AL, Foulke-Abel J, Tumala B, Vickers TJ, Sack DA, DeNearing B, Harro CD, et al. Enterotoxigenic Escherichia coli-blood group A interactions intensify diarrheal severity. J Clin Invest. 2018;128(8):3298–3311. doi: 10.1172/JCI97659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sheikh A, Rashu R, Begum YA, Kuhlman FM, Ciorba MA, Hultgren SJ, Qadri F, Fleckenstein JM, Yang R. Highly conserved type 1 pili promote enterotoxigenic E. coli pathogen-host interactions. PLoS Negl Trop Dis. 2017;11(5):e0005586. doi: 10.1371/journal.pntd.0005586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sheikh A, Luo Q, Roy K, Shabaan S, Kumar P, Qadri F, Fleckenstein JM, Payne SM. Contribution of the highly conserved EaeH surface protein to Enterotoxigenic Escherichia coli pathogenesis. Infect Immun. 2014;82(9):3657–3666. doi: 10.1128/IAI.01890-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Patel SK, Dotson J, Allen KP, Fleckenstein JM. Identification and molecular characterization of eatA, an autotransporter protein of enterotoxigenic Escherichia coli. Infect Immun. 2004;72(3):1786–1794. doi: 10.1128/IAI.72.3.1786-1794.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bin P, Tang Z, Liu S, Chen S, Xia Y, Liu J, Wu H, Zhu G. Intestinal microbiota mediates enterotoxigenic Escherichia coli-induced diarrhea in piglets. BMC Vet Res. 2018;14(1):1–13. doi: 10.1186/s12917-018-1704-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pop M, Paulson JN, Chakraborty S, Astrovskaya I, Lindsay BR, Li S, Bravo HC, Harro C, Parkhill J, Walker AW. Individual-specific changes in the human gut microbiota after challenge with Enterotoxigenic Escherichia coli and subsequent ciprofloxacin treatment. BMC Genomics. 2016;17(1):1–11. doi: 10.1186/s12864-016-2777-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Allen KP, Randolph MM, Fleckenstein JM. Importance of heat-labile enterotoxin in colonization of the adult mouse small intestine by human enterotoxigenic Escherichia coli strains. Infect Immun. 2006;74(2):869–875. doi: 10.1128/IAI.74.2.869-875.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Crofts AA, Giovanetti SM, Rubin EJ, Poly FM, Gutierrez RL, Talaat KR, Porter CK, Riddle MS, DeNearing B, Brubaker J, et al. Enterotoxigenic E. coli virulence gene regulation in human infections. Proc Natl Acad Sci U S A. 2018;115(38):E8968–E76. doi: 10.1073/pnas.1808982115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wu Y, Ma N, Song P, He T, Levesque C, Bai Y, Zhang A and Ma X. Grape Seed Proanthocyanidin Affects Lipid Metabolism via Changing Gut Microflora and Enhancing Propionate Production in Weaned Pigs. The Journal of Nutrition. 2019;149(9):1523–1532. 10.1093/jn/nxz102. [DOI] [PubMed] [Google Scholar]
- 126.Sommer F, Bäckhed F. The gut microbiota—masters of host development and physiology. Nat Rev Microbiol. 2013;11(4):227–238. doi: 10.1038/nrmicro2974. [DOI] [PubMed] [Google Scholar]
- 127.Kamada N, Seo S-U, Chen GY, Núñez G. Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol. 2013;13(5):321–335. doi: 10.1038/nri3430. [DOI] [PubMed] [Google Scholar]
- 128.Lawley TD, Walker AW. Intestinal colonization resistance. Immunology. 2013;138(1):1–11. doi: 10.1111/j.1365-2567.2012.03616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bäumler AJ, Sperandio V. Interactions between the microbiota and pathogenic bacteria in the gut. Nature. 2016;535(7610):85–93. doi: 10.1038/nature18849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.McKenney PT, Pamer EG. From hype to hope: the gut microbiota in enteric infectious disease. Cell. 2015;163(6):1326–1332. doi: 10.1016/j.cell.2015.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Milshteyn A, Schneider JS, Brady SF. Mining the metabiome: identifying novel natural products from microbial communities. Chem Biol. 2014;21:1211–1223. doi: 10.1016/j.chembiol.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Dalile B, Van Oudenhove L, Vervliet B, Verbeke K. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat Rev Gastroenterol Hepatol. 2019;16(8):461–478. doi: 10.1038/s41575-019-0157-3. [DOI] [PubMed] [Google Scholar]
- 133.Macfarlane S, Macfarlane GT. Regulation of short-chain fatty acid production. Proc Nutr Soc. 2003;62(1):67–72. doi: 10.1079/PNS2002207. [DOI] [PubMed] [Google Scholar]
- 134.Cummings J, Pomare E, Branch W, Naylor C, MacFarlane G. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28(10):1221–1227. doi: 10.1136/gut.28.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shin R, Suzuki M, Morishita Y. Influence of intestinal anaerobes and organic acids on the growth of Enterohaemorrhagic Escherichia coli O157: H7. J Med Microbiol. 2002;51(3):201–206. doi: 10.1099/0022-1317-51-3-201. [DOI] [PubMed] [Google Scholar]
- 136.Roe AJ, O’Byrne C, McLaggan D, Booth IR. Inhibition of Escherichia coli growth by acetic acid: a problem with methionine biosynthesis and homocysteine toxicity. Microbiology. 2002;148(7):2215–2222. doi: 10.1099/00221287-148-7-2215. [DOI] [PubMed] [Google Scholar]
- 137.Han K, Hong J, Lim HC. Relieving effects of glycine and methionine from acetic acid inhibition in Escherichia coli fermentation. Biotechnol Bioeng. 1993;41(3):316–324. doi: 10.1002/bit.260410305. [DOI] [PubMed] [Google Scholar]
- 138.Takashi K, Fujita I, Kobari K. Effects of short chain fatty acids on the production of heat-labile enterotoxin from enterotoxigenic Escherichia coli. Jpn J Pharmacol. 1989;50(4):495–498. doi: 10.1254/jjp.50.495. [DOI] [PubMed] [Google Scholar]
- 139.Tan J, McKenzie C, Potamitis M, Thorburn AN, Mackay CR, Macia L. The role of short-chain fatty acids in health and disease. Adv Immunol. 2014;121:91–119. doi: 10.1016/B978-0-12-800100-4.00003-9. [DOI] [PubMed] [Google Scholar]
- 140.Devlin AS, Fischbach MA. A biosynthetic pathway for a prominent class of microbiota-derived bile acids. Nat Chem Biol. 2015;11(9):685–690. doi: 10.1038/nchembio.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chatterjee A, Chowdhury R. Bile and unsaturated fatty acids inhibit the binding of cholera toxin and Escherichia coli heat-labile enterotoxin to GM1 receptor. Antimicrob Agents Chemother. 2008;52(1):220–224. doi: 10.1128/AAC.01009-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dobson A, Cotter PD, Ross RP, Hill C. Bacteriocin production: a probiotic trait? Appl Environ Microbiol. 2012;78(1):1–6. doi: 10.1128/AEM.05576-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cotter PD, Hill C, Ross RP. Bacteriocins: developing innate immunity for food. Nat Rev Microbiol. 2005;3(10):777–788. doi: 10.1038/nrmicro1273. [DOI] [PubMed] [Google Scholar]
- 144.Soltani S, Hammami R, Cotter PD, Rebuffat S, Said LB, Gaudreau H, Bédard F, Biron E, Drider D, Fliss I. Bacteriocins as a new generation of antimicrobials: toxicity aspects and regulations. FEMS Microbiol Rev. 2021;45(1):fuaa039. doi: 10.1093/femsre/fuaa039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gillor O, Giladi I, Riley MA. Persistence of colicinogenic Escherichia coli in the mouse gastrointestinal tract. BMC Microbiol. 2009;9(1):1–7. doi: 10.1186/1471-2180-9-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kleerebezem M, Quadri LE, Kuipers OP, De Vos WM. Quorum sensing by peptide pheromones and two‐component signal‐transduction systems in gram‐positive bacteria. Mol Microbiol. 1997;24(5):895–904. doi: 10.1046/j.1365-2958.1997.4251782.x. [DOI] [PubMed] [Google Scholar]
- 147.Duquesne S, Destoumieux-Garzón D, Peduzzi J, Rebuffat S. Microcins, gene-encoded antibacterial peptides from enterobacteria. Nat Prod Rep. 2007;24(4):708–734. doi: 10.1039/b516237h. [DOI] [PubMed] [Google Scholar]
- 148.Sassone-Corsi M, Nuccio S-P, Liu H, Hernandez D, Vu CT, Takahashi AA, Edwards RA, Raffatellu M. Microcins mediate competition among Enterobacteriaceae in the inflamed gut. Nature. 2016;540(7632):280–283. doi: 10.1038/nature20557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pelaseyed T, Bergström JH, Gustafsson JK, Ermund A, Birchenough GM, Schütte A, van der Post S, Svensson F, Rodríguez‐Piñeiro AM, Nyström EE. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol Rev. 2014;260(1):8–20. doi: 10.1111/imr.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Johansson ME, Jakobsson HE, Holmén-Larsson J, Schütte A, Ermund A, Rodríguez-Piñeiro AM, Arike L, Wising C, Svensson F, Bäckhed F. Normalization of host intestinal mucus layers requires long-term microbial colonization. Cell Host Microbe. 2015;18(5):582–592. doi: 10.1016/j.chom.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Vaishnava S, Behrendt CL, Ismail AS, Eckmann L, Hooper LV. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc Natl Acad Sci U S A. 2008;105(52):20858–20863. doi: 10.1073/pnas.0808723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.McGuckin MA, Lindén SK, Sutton P, Florin TH. Mucin dynamics and enteric pathogens. Nat Rev Microbiol. 2011;9(4):265–278. doi: 10.1038/nrmicro2538. [DOI] [PubMed] [Google Scholar]
- 153.Fukuda S, Toh H, Hase K, Oshima K, Nakanishi Y, Yoshimura K, Tobe T, Clarke JM, Topping DL, Suzuki T. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011;469(7331):543–547. doi: 10.1038/nature09646. [DOI] [PubMed] [Google Scholar]
- 154.Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139(3):485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chytilová M, Mudroňová D, Nemcová R, Gancarčíková S, Buleca V, Koščová J, Tkáčiková Ľ. Anti-inflammatory and immunoregulatory effects of flax-seed oil and lactobacillus plantarum–Biocenol™ LP96 in gnotobiotic pigs challenged with Enterotoxigenic Escherichia coli. Res Vet Sci. 2013;95(1):103–109. doi: 10.1016/j.rvsc.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 156.Seo H, Zhang W. Development of effective vaccines for Enterotoxigenic Escherichia coli. Lancet Infect Dis. 2020;20(2):150–152. doi: 10.1016/S1473-3099(19)30631-0. [DOI] [PubMed] [Google Scholar]
- 157.Riddle M, Chen W, Kirkwood C, MacLennan C. Update on vaccines for enteric pathogens. Clin Microbiol Infect. 2018. 1016/j.cmi.2018.06.023;24(10):1039–1045. doi: 10.1016/j.cmi.2018.06.023. [DOI] [PubMed] [Google Scholar]
- 158.Seo H, Duan Q, Zhang W. Vaccines against gastroenteritis, current progress and challenges. Gut Microbes. 2020;11(6):1486–1517. doi: 10.1080/19490976.2020.1770666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Nandre RM, Ruan X, Duan Q, Sack DA, Zhang W. Antibodies derived from an Enterotoxigenic Escherichia coli (ETEC) adhesin tip MEFA (multiepitope fusion antigen) against adherence of nine ETEC adhesins: CFA/I, CS1, CS2, CS3, CS4, CS5, CS6, CS21 and EtpA. Vaccine. 2016;34(31):3620–3625. doi: 10.1016/j.vaccine.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zegeye ED, Govasli ML, Sommerfelt H, Puntervoll P. Development of an Enterotoxigenic Escherichia coli vaccine based on the heat-stable toxin. Hum Vaccin Immunother. 2019;15(6):1379–1388. doi: 10.1080/21645515.2018.1496768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Huang J, Duan Q, Zhang W, Dudley EG. Significance of Enterotoxigenic Escherichia coli (ETEC) heat-labile toxin (LT) enzymatic subunit epitopes in LT enterotoxicity and immunogenicity. Appl Environ Microbiol. 2018;84(15):e00849–18. doi: 10.1128/AEM.00849-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kuhlmann FM, Martin J, Hazen TH, Vickers TJ, Pashos M, Okhuysen PC, Gomez-Duarte OG, Cebelinski E, Boxrud D, Del Canto F, et al. Conservation and global distribution of non-canonical antigens in enterotoxigenic Escherichia coli. PLoS Negl Trop Dis. 2019;13(11):e0007825. doi: 10.1371/journal.pntd.0007825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Khalil I, Walker R, Porter CK, Muhib F, Chilengi R, Cravioto A, Guerrant R, Svennerholm A-M, Qadri F, Baqar S. Enterotoxigenic Escherichia coli (ETEC) vaccines: priority activities to enable product development, licensure, and global access. Vaccine. 2021;39(31):4266–4277. doi: 10.1016/j.vaccine.2021.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Qadri F, Akhtar M, Bhuiyan TR, Chowdhury MI, Ahmed T, Rafique TA, Khan A, Rahman SIA, Khanam F, Lundgren A, et al. Safety and immunogenicity of the oral, inactivated, Enterotoxigenic Escherichia coli vaccine ETVAX in Bangladeshi children and infants: a double-blind, randomised, placebo-controlled phase 1/2 trial. Lancet Infect Dis. 2020;20(2):208–219. doi: 10.1016/s1473-3099(19)30571-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Jertborn M, Åhrén C, Holmgren J, Svennerholm A-M. Safety and immunogenicity of an oral inactivated Enterotoxigenic Escherichia coli vaccine. Vaccine. 1998;16(2–3):255–260. doi: 10.1016/s0264-410x(97)00169-2. [DOI] [PubMed] [Google Scholar]
- 166.Lebens M, Shahabi V, Bäckström M, Houze T, Lindblad N, Holmgren J. Synthesis of hybrid molecules between heat-labile enterotoxin and cholera toxin B subunits: potential for use in a broad-spectrum vaccine. Infect Immun. 1996;64(6):2144–2150. doi: 10.1128/iai.64.6.2144-2150.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lundgren A, Bourgeois L, Carlin N, Clements J, Gustafsson B, Hartford M, Holmgren J, Petzold M, Walker R, Svennerholm A-M. Safety and immunogenicity of an improved oral inactivated multivalent Enterotoxigenic Escherichia coli (ETEC) vaccine administered alone and together with dmLT adjuvant in a double-blind, randomized, placebo-controlled Phase I study. Vaccine. 2014;32(52):7077–7084. doi: 10.1016/j.vaccine.2014.10.069. [DOI] [PubMed] [Google Scholar]
- 168.Akhtar M, Chowdhury MI, Bhuiyan TR, Kaim J, Ahmed T, Rafique TA, Khan A, Rahman SI, Khanam F, Begum YA. Evaluation of the safety and immunogenicity of the oral inactivated multivalent Enterotoxigenic Escherichia coli vaccine ETVAX in Bangladeshi adults in a double-blind, randomized, placebo-controlled phase I trial using electrochemiluminescence and ELISA assays for immunogenicity analyses. Vaccine. 2019;37(37):5645–5656. doi: 10.1016/j.vaccine.2018.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Leach S, Lundgren A, Carlin N, Löfstrand M, Svennerholm A-M. Cross-reactivity and avidity of antibody responses induced in humans by the oral inactivated multivalent Enterotoxigenic Escherichia coli (ETEC) vaccine ETVAX. Vaccine. 2017;35(32):3966–3973. doi: 10.1016/j.vaccine.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 170.Kotloff KL, Platts-Mills JA, Nasrin D, Roose A, Blackwelder WC, Levine MM. Global burden of diarrheal diseases among children in developing countries: incidence, etiology, and insights from new molecular diagnostic techniques. Vaccine. 2017;35(49):6783–6789. doi: 10.1016/j.vaccine.2017.07.036. [DOI] [PubMed] [Google Scholar]
- 171.Wolf MK. Occurrence, distribution, and associations of O and H serogroups, colonization factor antigens, and toxins of Enterotoxigenic Escherichia coli. Clin Microbiol Rev. 1997;10(4):569–584. doi: 10.1128/CMR.10.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zhang W, Sack DA. Progress and hurdles in the development of vaccines against Enterotoxigenic Escherichia coli in humans. Expert Rev Vaccines. 2012;11(6):677–694. doi: 10.1586/erv.12.37. [DOI] [PubMed] [Google Scholar]
- 173.Talaat KR, Porter CK, Bourgeois AL, Lee TK, Duplessis CA, Maciel M Jr., Gutierrez RL, DeNearing B, Adjoodani B, Adkinson R, et al. Oral delivery of hyperimmune bovine serum antibodies against CS6-expressing enterotoxigenic Escherichia coli as a prophylactic against diarrhea. Gut Microbes. 2020;12(1):1732852. doi: 10.1080/19490976.2020.1732852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Stoppato M, Gaspar C, Regeimbal J, Nunez RG, Giuntini S, Schiller ZA, Gawron MA, Pondish JR, Martin JC 3rd, Schneider MI, et al. Oral administration of an anti-CfaE secretory IgA antibody protects against Enterotoxigenic Escherichia coli diarrheal disease in a nonhuman primate model. Vaccine. 2020;38(10):2333–2339. doi: 10.1016/j.vaccine.2020.01.064. [DOI] [PubMed] [Google Scholar]
- 175.Rathe M, Muller K, Sangild PT, Husby S. Clinical applications of bovine colostrum therapy: a systematic review. Nutr Rev. 2014;72(4):237–254. doi: 10.1111/nure.12089. [DOI] [PubMed] [Google Scholar]
- 176.Sears KT, Tennant SM, Reymann MK, Simon R, Konstantopoulos N, Blackwelder WC, Barry EM, Pasetti MF. Bioactive immune components of anti-diarrheagenic Enterotoxigenic Escherichia coli hyperimmune bovine colostrum products. Clin Vaccine Immunol. 2017;24(8):e00186–16. doi: 10.1128/CVI.00186-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Tacket CO, Losonsky G, Link H, Hoang Y, Guesry P, Hilpert H, Levine MM. Protection by milk immunoglobulin concentrate against oral challenge with Enterotoxigenic Escherichia coli. N Engl J Med. 1988;318(19):1240–1243. doi: 10.1056/NEJM198805123181904. [DOI] [PubMed] [Google Scholar]
- 178.Otto W, Najnigier B, Stelmasiak T, Robins-Browne RM. Randomized control trials using a tablet formulation of hyperimmune bovine colostrum to prevent diarrhea caused by Enterotoxigenic Escherichia coli in volunteers. Scand J Gastroenterol. 2011;46(7–8):862–868. doi: 10.3109/00365521.2011.574726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Savarino SJ, McKenzie R, Tribble DR, Porter CK, O’Dowd A, Sincock SA, Poole ST, DeNearing B, Woods CM, Kim H, et al. hyperimmune bovine colostral anti-CS17 antibodies protect against Enterotoxigenic Escherichia coli diarrhea in a randomized, doubled-blind, placebo-controlled human infection model. J Infect Dis. 2019;220(3):505–513. doi: 10.1093/infdis/jiz135. [DOI] [PubMed] [Google Scholar]
- 180.Zheng W, Zhao W, Wu M, Song X, Caro F, Sun X, Gazzaniga F, Stefanetti G, Oh S, Mekalanos JJ, et al. Microbiota-targeted maternal antibodies protect neonates from enteric infection. Nature. 2020;577(7791):543–548. doi: 10.1038/s41586-019-1898-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hu Y, Kumru OS, Xiong J, Antunez LR, Hickey J, Wang Y, Cavacini L, Klempner M, Joshi SB, Volkin DB. Preformulation characterization and stability assessments of secretory IgA monoclonal antibodies as potential candidates for passive immunization by oral administration. J Pharm Sci. 2020;109(1):407–421. doi: 10.1016/j.xphs.2019.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Tan P, Tang Q, Xu S, Zhang Y, Fu H and Ma X. Designing Self‐Assembling Chimeric Peptide Nanoparticles with High Stability for Combating Piglet Bacterial Infections. Advanced Science. 2022:2105955. 10.1002/advs.202105955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Tan P, Fu H, Ma X. Design, optimization, and nanotechnology of antimicrobial peptides: from exploration to applications. Nano Today. 2021;39:101229. doi: 10.1016/j.nantod.2021.101229. [DOI] [Google Scholar]
- 184.Brown JW, Badahdah A, Iticovici M, Vickers TJ, Alvarado DM, Helmerhorst EJ, Oppenheim FG, Mills JC, Ciorba MA, Fleckenstein JM, et al. A role for salivary peptides in the innate defense against Enterotoxigenic Escherichia coli. J Infect Dis. 2018;217(9):1435–1441. doi: 10.1093/infdis/jiy032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ding X, Yu H, Qiao S. Lasso peptide microcin J25 effectively enhances gut barrier function and modulates inflammatory response in an enterotoxigenic Escherichia coli-challenged mouse model. Int J Mol Sci. 2020;21(18):6500. doi: 10.3390/ijms21186500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Yu H, Ding X, Shang L, Zeng X, Liu H, Li N, Huang S, Wang Y, Wang G, Cai S, et al. Protective ability of biogenic antimicrobial peptide microcin J25 against Enterotoxigenic Escherichia Coli-induced intestinal epithelial dysfunction and inflammatory responses IPEC-J2 cells. Front Cell Infect Microbiol. 2018;8:242. doi: 10.3389/fcimb.2018.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Huang G, Li X, Lu D, Liu S, Suo X, Li Q, Li N. Lysozyme improves gut performance and protects against Enterotoxigenic Escherichia coli infection in neonatal piglets. Vet Res. 2018;49(1):20. doi: 10.1186/s13567-018-0511-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Guan G, Ding S, Yin Y, Duraipandiyan V, Al-Dhabi NA, Liu G. Macleaya cordata extract alleviated oxidative stress and altered innate immune response in mice challenged with Enterotoxigenic Escherichia coli. Sci China Life Sci. 2019;62(8):1019–1027. doi: 10.1007/s11427-018-9494-6. [DOI] [PubMed] [Google Scholar]
- 189.Xiong W, Huang J, Li X, Zhang Z, Jin M, Wang J, Xu Y, Wang Z. Icariin and its phosphorylated derivatives alleviate intestinal epithelial barrier disruption caused by Enterotoxigenic Escherichia coli through modulate p38 MAPK in vivo and in vitro. FASEB J. 2020;34(1):1783–1801. doi: 10.1096/fj.201902265R. [DOI] [PubMed] [Google Scholar]
- 190.Verhelst R, Schroyen M, Buys N, Niewold TAE. coli heat labile toxin (LT) inactivation by specific polyphenols is aggregation dependent. Vet Microbiol. 2013;163(3–4):319–324. doi: 10.1016/j.vetmic.2012.12.039. [DOI] [PubMed] [Google Scholar]


