Abstract
Background:
This study was to determine the analgesic effect of ultrasound-guided erector spinae plane block (ESPB) and paravertebral block (PVB) as well as the combination of PVB and ESPB (P + E) after video-assisted thoracoscopic surgery (VATS).
Patients and Methods:
Patients were randomly assigned to receive ESPB, PVB or PVB combined with ESPB with 0.5% ropivacaine (20 ml). The primary outcomes were cumulative hydromorphone consumption and Visual Analogue Scale (VAS) scores at rest and while coughing at 0 h, 12 h, 24 h, 48 h and 72 h postoperatively. The secondary outcomes were effective PCA usage count and rescue analgesia requirement at the same time points.
Results:
The median (interquartile range) hydromorphone consumption, including converted oxycodone, was significantly different at 48 h postoperatively among the three groups (ESPB, 10.24 [9.53–11.71] mg; PVB, 9.94 [9.19–10.75] mg; P + E, 9.44 [8.96–9.97] mg; P = 0.011). Hydromorphone consumption in P + E group was lower compared with that in ESPB group at 12 h, 24 h and 48 h (P < 0.001, P = 0.004 and P = 0.003, respectively). VAS scores at rest were significantly higher for ESPB group compared to P + E group at 0 h postoperatively (P = 0.009). VAS scores while coughing were significantly higher for ESPB group compared to P + E group at 0 h and 12 h postoperatively (P = 0.015 and P < 0.001) and to the PVB group at 12 h postoperatively (P = 0.002). The effective PCA usage count in P + E group was lower than in ESPB group in 0–12 h (P < 0.001). More patients needed rescue analgesia in ESPB group compared to those in P + E group in 0–12 h, 0–24 h and 0–48 h (P = 0.022, 0.035 and 0.035, respectively).
Conclusions:
Ultrasound-guided PVB combined with ESPB provided superior analgesia to ESPB for VATS. The combination of PVB and ESPB had a similar analgesic effect compared with PVB alone.
Keywords: Erector spinae plane block, paravertebral block, post-operative pain, thoracoscopic surgery
INTRODUCTION
Video-assisted thoracoscopic surgery (VATS) is a less invasive and traumatic surgical procedure for both minor and major oncological lung surgeries, which improves post-operative respiratory function and reduces hospital length of stay. However, 25% of patients experience moderate-to-severe pain after VATS.[1] Inadequate analgesia will increase patients’ suffering and make patients difficult to cough effectively, which will delay patient recovery and prolong the hospital stays.
Many kinds of regional anaesthesia techniques including thoracic epidural analgesia (TEA) and paravertebral block (PVB) have been widely used to alleviate post-operative pain after VATS.[2,3] Erector spinae plane block (ESPB) is gaining popularity because of its simple application and safety. However, the optimal regional anaesthesia technique, which is expected to be effective, safe, simple and less invasive, remains a debate after thoracoscopic surgery. Therefore, this study investigated whether the combination of ESPB and PVB could provide adequate analgesia for VATS and reduce hydromorphone consumption compared with PVB and ESPB, respectively. The primary outcomes of this study were the hydromorphone consumption and Visual Analogue Scale (VAS) scores. The secondary outcomes were effective PCA usage count, haemodynamic changes, nerve block-related complications and post-operative adverse effects, such as pruritus, urinary retention, nausea and vomiting.
PATIENTS AND METHODS
Study design and patient randomisation
This prospective, randomised controlled study was approved by the Ethics Committee of the First Affiliated Hospital of China Medical University (2018-306-2) and was registered to Chinese Clinical Trials Registry (Registration No: ChiCTR1900023205). Written informed consents were obtained from all patients.
Patients aged 18–80 years with ASA I–II and undergoing VATS under general anaesthesia were enrolled in the study. The exclusion criteria included unwillingness to cooperate with the test, allergy to local anaesthetics, hepatic or renal insufficiency, severe heart diseases, coagulation disorders, infection of the skin at the site of needle puncture area, a history of chronic pain or chronic opioid use, pre-existing neurological deficit or psychiatric illness.
Patients were allocated randomly to ESPB group, PVB group or PVB combined with ESPB group (P + E group) according to computer-generated random number table. All patients and an investigator who was responsible for follow-up were blinded to the randomisation groups. The investigator familiarised the patients how to use the patient-controlled analgesia (PCA) device during pre-operative visits.
Ultrasound-guided nerve block
Sufentanil 5 μg was injected as premedication before nerve block. The skin over the needle insertion site was anaesthetised with 2 ml of 1% lidocaine. For ESPB group, a 21G needle (Pajunk, Germany) was inserted into the interfacial plane between the erector spinae muscle and T5 transverse process in the lateral decubitus position with the diseased side up. For PVB group, the needle was inserted into the paravertebral space at T5–T6 level. The correct position was confirmed by injecting 0.5–1 ml of saline and visualising the saline spread in the interfacial plane or displacement of pleura. Then, 20 ml of 0.5% ropivacaine was injected for each group. For P + E group, the needle was inserted into the paravertebral space. After confirming the correct position by injecting 0.5–1 ml of saline and visualising displacement of pleura, 10 ml of 0.5% ropivacaine was injected. Then, the needle was pulled back to the interfacial plane between the erector spinae muscle and T5 transverse process and 10 ml of 0.5% ropivacaine was injected.
General anaesthesia
On arrival at the operation room, standard monitors were applied and radial artery was catheterised before anaesthesia induction. Then, the nerve block was performed. General anaesthesia was induced with propofol 2.0 mg·kg−1, sufentanil 0.4 μg·kg−1 and cis-atracurium 0.2 mg·kg−1. A double-lumen tube was intubated and the position was determined by fibre-optic bronchoscopy. Patients were mechanically ventilated using constant-flow volume-controlled ventilation. The tidal volume was 6–8 ml·kg−1, and the ventilatory frequency was adjusted to maintaining an end-tidal carbon dioxide tension of 35–45 mmHg and airway pressure below 30 cm H2O. Anaesthesia was maintained with inhaled sevoflurane 1.5% in 50% oxygen and intravenous infusion of propofol at 6 mg·kg−1·h−1. At the end of the surgery, tropisetron 5 mg was given intravenously to prevent post-operative nausea and vomiting. Hypotension (mean arterial pressure <80% of baseline) during the surgery was treated with phenylephrine 0.05–0.1 mg once intravenously. Bradycardia (heart rate [HR]: <50 bpm) was treated with atropine 0.3 mg. Patients were extubated at the end of surgery. When the Ramsay sedation score was 2–4, the patients were transferred to post-anaesthesia care unit (PACU) for a 1-h observation period.
Post-operative analgesia and rescue analgesia
The pain levels at rest and while coughing using VAS (0 cm = no pain and 10 cm = worst pain imaginable) were evaluated on arrival at PACU. If VAS score was >3 at rest, oxycodone 3 mg was administered intravenously as rescue analgesia. The PCA device (0.1 mg·ml−1 hydromorphone) was applied, which was set to deliver 2 ml·h−1 background rate, 6 ml bolus doses and 10 min-lockout interval. If VAS score was >3 at rest at the ward, flurbiprofen 50 mg was given intravenously as rescue analgesia.
Outcome measurements
The primary outcomes were cumulative hydromorphone consumption (rescue oxycodone was converted to intravenous hydromorphone equivalents for final analysis) at 12 h, 24 h and 48 h postoperatively and VAS scores at rest and while coughing at 0 h, 12 h, 24 h, 48 h and 72 h postoperatively. The secondary outcomes included rescue analgesic requirement, effective PCA usage count at the same time points, vasoactive drug consumption as well as post-operative adverse effects, such as pruritus, urinary retention, nausea and vomiting. Blood pressure and HRs at five time points (enter the operation room, before operation, 5 min after operation, before extubating and arriving at PACU) were also recorded.
Sample size
According to our preliminary study data, the 48 h mean hydromorphone consumptions in group ESPB, PVB and P + E were 10.73 mg, 10.36 mg and 9.48 mg. Twenty patients per group were required to achieve a significance level of 0.05 with a power of 80%. The statistical test used for sample size calculation was one-way analysis of variance (ANOVA) power analysis. The power calculation was completed using PASS (NCSS LLC, Kaysville, Utah).
Statistical analysis
Statistical analysis was performed using IBM SPSS version 25 (IBM Corp., Armonk, NY, USA). Continuous variables were presented as mean ± standard deviation or median (interquartile range: [IQR]) and categorical variables were presented as number (percentage). Kolmogorov–Smirnov tests were used to evaluate data distribution. Demographic and perioperative data were analysed using one-way ANOVA and Chi-square test. Hydromorphone consumption, VAS, haemodynamic parameters and effective PCA usage count were analysed using Kruskal–Wallis test among the groups. Mann–Whitney U-test was used in pairwise comparison and P < 0.0167 (Bonferroni correction) was considered significant. The frequency of rescue analgesia and the incidence of post-operative adverse effects were compared with Chi-square test or Fisher's exact test. P < 0.05 was considered significant.
RESULTS
Patient characteristics
A total of 62 patients were recruited in the final analysis [Figure 1]. The demographical data of the three groups were similar [Table 1].
Figure 1.
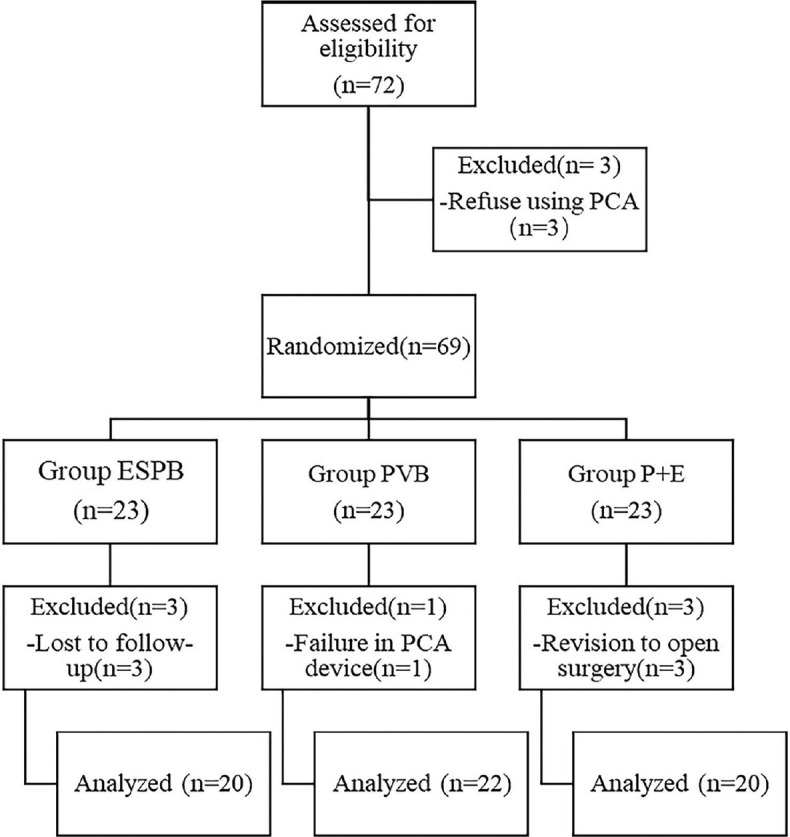
Flow chart of the study
Table 1.
Demographical data
| ESPB (n=20) | PVB (n=22) | P+E (n=20) | P | |
|---|---|---|---|---|
| Age (year) | 57.25±11.25 | 58.63±6.04 | 58.35±11.60 | 0.880 |
| Weight (kg) | 65.45±11.02 | 64.32±10.01 | 64.3±7.57 | 0.880 |
| Height (m) | 1.67±0.08 | 1.64±0.09 | 1.65±0.06 | 0.499 |
| BMI (kg/m2) | 23.4±2.46 | 23.79±2.78 | 1.65±0.63 | 0.953 |
| ASA (I/II) | 12/8 | 14/8 | 13/7 | 0.945 |
| Duration of surgery (min) | 109.6±36.16 | 105.31±39.74 | 106.15±49.12 | 0.863 |
| Sex (M/F) | 14/6 | 8/14 | 9/11 | 0.081 |
Data are presented as mean (SD) or n. BMI: Body mass index, F: Female, M: Male, ASA: American Society of Anaesthesiologists, SD: Standard deviation
Post-operative Visual Analogue Scale
As shown in Figures 2 and 3, there was no statistically significant difference for VAS at rest or while coughing between P + E and PVB group at any time point. There were statistically significant differences between P + E and ESPB group at 0 h at rest (P = 0.009) and at 0 h and 12 h while coughing (P = 0.015 and P < 0.001, respectively). A statistically significant difference for VAS while coughing was found between PVB and ESPB group at 12 h (P = 0.002).
Figure 2.
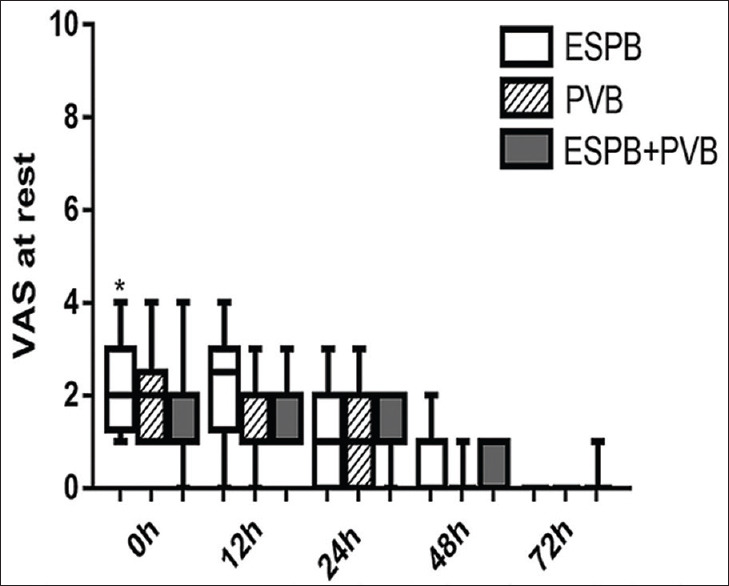
Visual Analogue Scale at rest. Data are expressed as median (horizontal bar), interquartile range (box) and the maximum and minimum values (whiskers). *P < 0.0167 when erector spinae plane block compared with P + E at 0 h (P = 0.009)
Figure 3.
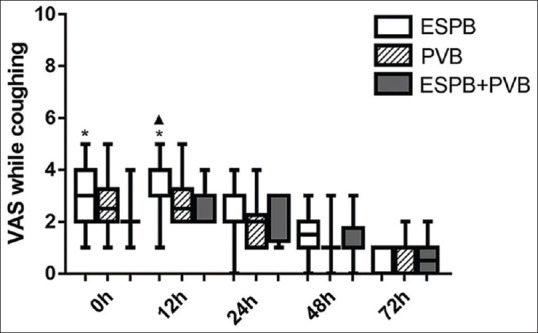
Visual Analogue Scale while coughing. Data are expressed as median (horizontal bar), interquartile range (box) and the maximum and minimum values (whiskers). *P < 0.0167 when erector spinae plane block compared with P + E at 0 h and 12 h (P = 0.015, P < 0.001). ▴P < 0.0167 when erector spinae plane block compared with paravertebral block at 12 h (P = 0.002)
Post-operative analgesia
The median [IQR] hydromorphone consumption, including converted oxycodone, was significantly different at 48 h postoperatively among the three groups (ESPB, 10.24 [9.53–11.71] mg; PVB, 9.94 [9.19–10.75] mg and P + E, 9.44 [8.96–9.97] mg; P = 0.011). There was a statistically significant difference for hydromorphone consumption between ESPB and P + E group (P = 0.003). Moreover, hydromorphone consumption in P + E group was lower compared with that in ESPB group at 12 h, 24 h and 48 h. No statistically significant difference in hydromorphone consumption was found between ESPB and PVB group or between PVB and P + E group at all time points [Table 2].
Table 2.
Hydromorphone consumption
| ESPB (n=20) | PVB (n=22) | P + E (n=20) | Pairwise comparisons (P) | |||
|---|---|---|---|---|---|---|
|
| ||||||
| ESPB vs PVB | ESPB vs P + E | PVB vs P + E | ||||
| 12 h | 3.14 (2.64-3.36) | 2.85 (2.69-3.31) | 2.53 (2.43-2.69) | 0.127 | <0.001* | 0.021 |
| 24 h | 5.86 (5.13-7.02) | 5.56 (4.65-6) | 5 (4.69-5.79) | 0.031 | 0.004* | 0.495 |
| 48 h | 10.24 (9.53-11.71) | 9.94 (9.19-10.75) | 9.44 (8.96-9.97) | 0.267 | 0.003* | 0.07 |
Data are presented as median (IQR). All groups were compared using Kruskal-Wallis test. Pairwise comparisons were analysed using Mann-Whitney U-test and. *P<0.0167 (Bonferroni correction)
As shown in Table 3, the effective PCA usage count in 0–12 h was significantly different among the three groups (P = 0.001). The PCA usage count was lower in P + E group compared to ESPB group in 0–12 h (P < 0.001). There was no statistically significant difference between ESPB and PVB group (P = 0.08) or between PVB and P + E group in 0–12 h (P = 0.034) and there was no statistically significant difference in 12–24 h (P = 0.542) and 24–48 h (P = 0.435) among the three groups.
Table 3.
Effective patient-controlled intravenous analgesia usage count
| ESPB (n=20) | PVB (n=22) | P+E (n=20) | Pairwise comparisons (P) | |||
|---|---|---|---|---|---|---|
|
| ||||||
| ESPB vs PVB | ESPB vs P + E | PVB vs P + E | ||||
| 0-12 h | 2 (1-2) | 1 (1-2) | 1 (0-1) | 0.080 | <0.001* | 0.034 |
| 12-24 h | 1 (0-1.75) | 0 (0-1) | 1 (0-1) | 0.285 | 0.718 | 0.478 |
| 24-48 h | 0 (0-0.75) | 0 (0-0.25) | 0 (0-0) | 0.865 | 0.429 | 0.275 |
Data are presented as median (IQR). *P<0.0167 when ESPB compared with P + E. IQR: Interquartile range, ESPB: Erector spinae plane block
The frequency of rescue analgesia requirement is shown in Table 4. More patients in ESPB group needed rescue analgesia compared to those in P + E group in 0–12 h (P = 0.022), 0–24 h (P = 0.035) and 0–48 h (P = 0.035). There was no statistically significant difference between ESPB and PVB group or between PVB and P + E group in 0–12 h, 0–24 h and 0–48 h.
Table 4.
Rescue analgesia at ward
| ESPB (n=20) | PVB (n=22) | P+E (n=20) | P | |
|---|---|---|---|---|
| 0-12h | 7 (35%) | 3 (13.6%) | 1 (5%) | 0.022* |
| 0-24h | 8 (40%) | 3 (13.6%) | 2 (10%) | 0.035* |
| 0-48h | 8 (40%) | 3 (13.6%) | 2 (10%) | 0.035* |
Values are presented as number (%). *P<0.05 when ESPB compared with P + E. ESPB: Erector spinae plane block
Extreme pain caused by drainage tube stimulation when patients moved was found in 8 patients in ESPB group, 6 in PVB group and 7 in P + E group postoperatively. There was no statistically significant difference among the three groups.
Haemodynamic changes
There were no statistically significant differences in the mean SBP, DPB and intraoperative HRs among the three groups at each time point. There were no significant differences in vasoactive drug consumption (Data were not shown).
Adverse effects
A total of 13 patients experienced nausea, two patients in ESPB group, six patients in PVB group and five patients in P + E group (P = 0.337). There were no statistically significant differences in vomiting in three groups (P = 0.638). No patients developed pneumothorax, pruritus and urinary retention.
DISCUSSION
This randomised, double-blinded study demonstrated PVB combined with ESPB provided superior analgesia to ESPB in patients undergoing VATS. The combination of ESPB and PVB provided a promising alternative to PVB in perioperative pain management in VATS. We chose to combine ESPB with PVB for four reasons. First, using ESPB alone might cause insufficient analgesia. A cadaveric study about ESPB reported that there was no spread of dye anteriorly to the paravertebral space after ESPB.[4] Another study reported that there was no discernable cutaneous sensory block on pinprick testing after ESPB.[5] In our study, the VAS scores while coughing were higher in ESPB (VAS ≥4 at 0 h and 12 h), which also indicated ESPB failed to provide sufficient analgesia. Second, although PVB had comparable analgesic efficacy with TEA,[6,7] single-injection PVB had a failure rate of 13% and it produced an unpredictable block.[8,9] The combination could provide a supplement in case of the insufficient analgesia. Third, PVB might cause haemodynamic fluctuation. According to the report, the incidence of hypotension was 4%.[10] The combination could reduce the dose of local anaesthetic in PVB, which might reduce the incidence of hypotension. Furthermore, the combination did not make the block more complicated or traumatic. In the sagittal section of the ultrasound, both erector spinae plane and paravertebral space could be obtained, which means ESPB and PVB could be performed with only one puncture.
Optimal volume and concentration of local anaesthetics for nerve blocks remain to be a problem. According to the report, bilateral injection of 0.375% ropivacaine 20 ml or 25 ml per side[11] and unilateral injection of 0.5% ropivacaine 20 ml[12] or 0.5% bupivacaine 20 ml[13] or 0.375% bupivacaine 20 ml[14] were safe and effective in ESPB. PVB could block five segments with a single injection volume of 10 ml local anaesthetic.[15] 0.2%–0.5% bupivacaine 10–20 ml or 0.375%–0.5% ropivacaine 10–20 ml was safe and effective for analgesia.[16] Hence, we chose 0.5% ropivacaine 20 ml as the total amount in all three groups and the ropivacaine was divided equally into two parts in P + E group.
Our present study indicated that patients in ESPB group had more hydromorphone consumption than P + E group. However, in clinical practice, the difference in hydromorphone consumption seemed relatively small. Four reasons may account for the results. First, it was probably the less trauma in VATS which led to less post-operative pain. More invasive surgery such as thoracotomy might reveal a statistically significant difference in hydromorphone consumption for different nerve blocks. Second, the PCA devices in this study were set the same background infusion which might have already satisfied some patients’ analgesic needs. Third, in China, patients thought pain after the surgery was inevitable and many of them were unaware of the importance of effective pain management.[17] Hence, patients were used to enduring the pain and seldom pressed the PCA button. Furthermore, opioid side effects such as nausea and vomiting caused by hydromorphone occurred to some patients. Hence, they were unwilling to press the PCA button to use more hydromorphone.
No serious complications such as pneumothorax were found in the three groups. In a retrospective study,[18] the incidence of adverse effects in 1427 PVB was 0.7%. These complications were hypotension, bradycardia and toxicity of local anaesthetics. Puncture of the pleura, which was mostly concerned by many clinicians, was not found. This kind of complication was not found in our study as well. This might be attributed to the better ultrasound equipment which allowed the anaesthesiologist to complete the block under visual conditions and reduce the possibility of puncturing the pleura. ESPB was also a safe technique. A systematic review analysed 125 articles about ESPB and made a conclusion that ESPB had very few complications,[19] which was consistent with our study. Patients in all three groups had side effects of nausea and vomiting, but there was no statistically significant difference among the three groups. This might be due to the opioid-sparing effect of three nerve blocks. We also found sudden transient pain caused by stimulation of the chest drainage tube occurred to some patients when they moved (such as sitting up from bed or getting out of bed). The chest drainage tube stimulated the intercostal nerve, pleura and diaphragm, which caused severe pain. In our study, ESPB, PVB or the combination of both techniques failed to produce effective analgesia for drainage tube stimulation. Frequent drainage tube stimulation might be caused by the placement of the drainage tube. The surgeon could adjust the position of the drainage tube to reduce the occurrence of pain induced by the drainage tube.
There were some limitations in our study. Not to affect the turnover of the operation room, we did not have enough time to measure the block plane. The relationship between P + E and PVB was not clear in this study and a bigger sample size might be needed for further study. The differences among the three groups in haemodynamic changes were not found in our study. This could be related to the small doses of local anaesthetics. We only recorded five time points of haemodynamic changes in our study. More observed time might be needed for further study. The relationship between volume and concentration of local anaesthetic with analgesic effect of P + E needed to be further studied.
CONCLUSIONS
This study demonstrated that the ultrasound-guided PVB combined with ESPB provided superior analgesia to ESPB for VATS. The combination of ESPB and PVB might be an effective alternative to PVB in alleviating post-operative pain in VATS.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Umari M, Carpanese V, Moro V, Baldo G, Addesa S, Lena E, et al. Postoperative analgesia after pulmonary resection with a focus on video-assisted thoracoscopic surgery. Eur J Cardiothorac Surg. 2018:53932–8. [Google Scholar]
- 2.Steinthorsdottir KJ, Wildgaard L, Hansen HJ, Petersen RH, Wildgaard K. Regional analgesia for video-assisted thoracic surgery: A systematic review. Eur J Cardiothorac Surg. 2014;45:959–66. doi: 10.1093/ejcts/ezt525. [DOI] [PubMed] [Google Scholar]
- 3.Ciftci B, Ekinci M, Celik EC, Tukac IC, Bayrak Y, Atalay YO. Efficacy of an ultrasound-guided erector spinae plane block for postoperative analgesia management after video-assisted thoracic surgery: A prospective randomized study. J Cardiothorac Vasc Anesth. 2020;34:444–9. doi: 10.1053/j.jvca.2019.04.026. [DOI] [PubMed] [Google Scholar]
- 4.Ivanusic J, Konishi Y, Barrington MJ. A cadaveric study investigating the mechanism of action of erector spinae blockade. Reg Anesth Pain Med. 2018;43:567–71. doi: 10.1097/AAP.0000000000000789. [DOI] [PubMed] [Google Scholar]
- 5.Forero M, Adhikary SD, Lopez H, Tsui C, Chin KJ. The erector spinae plane block: A novel analgesic technique in thoracic neuropathic pain. Reg Anesth Pain Med. 2016;41:621–7. doi: 10.1097/AAP.0000000000000451. [DOI] [PubMed] [Google Scholar]
- 6.Yeung JH, Gates S, Naidu BV, Wilson MJ, Gao Smith F. Paravertebral block versus thoracic epidural for patients undergoing thoracotomy. Cochrane Database Syst Rev. 2016;2:CD009121. doi: 10.1002/14651858.CD009121.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Komatsu T, Kino A, Inoue M, Sowa T, Takahashi K, Fujinaga T. Paravertebral block for video-assisted thoracoscopic surgery: Analgesic effectiveness and role in fast-track surgery. Int J Surg. 2014;12:936–9. doi: 10.1016/j.ijsu.2014.07.272. [DOI] [PubMed] [Google Scholar]
- 8.Thavaneswaran P, Rudkin GE, Cooter RD, Moyes DG, Perera CL, Maddern GJ. Brief reports: Paravertebral block for anesthesia: A systematic review. Anesth Analg. 2010;110:1740–4. doi: 10.1213/ANE.0b013e3181da82c8. [DOI] [PubMed] [Google Scholar]
- 9.Cheema S, Richardson J, McGurgan P. Factors affecting the spread of bupivacaine in the adult thoracic paravertebral space. Anaesthesia. 2003;58:684–7. doi: 10.1046/j.1365-2044.2003.03189_1.x. [DOI] [PubMed] [Google Scholar]
- 10.Naja Z, Lönnqvist PA. Somatic paravertebral nerve blockade.Incidence of failed block and complications. Anaesthesia. 2001;56:1184–8. doi: 10.1046/j.1365-2044.2001.02084-2.x. [DOI] [PubMed] [Google Scholar]
- 11.Melvin JP, Schrot RJ, Chu GM, Chin KJ. Low thoracic erector spinae plane block for perioperative analgesia in lumbosacral spine surgery: A case series. Can J Anaesth. 2018;65:1057–65. doi: 10.1007/s12630-018-1145-8. [DOI] [PubMed] [Google Scholar]
- 12.Forero M, Rajarathinam M, Adhikary S, Chin KJ. Continuous erector spinae plane block for rescue analgesia in thoracotomy after epidural failure: A case report. A A Case Rep. 2017;8:254–6. doi: 10.1213/XAA.0000000000000478. [DOI] [PubMed] [Google Scholar]
- 13.Luis-Navarro JC, Seda-Guzmán M, Luis-Moreno C, Chin KJ. Erector spinae plane block in abdominal surgery: Case series. Indian J Anaesth. 2018;62:549–54. doi: 10.4103/ija.IJA_57_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ueshima H, Inagaki M, Toyone T, Otake H. Efficacy of the erector spinae plane block for lumbar spinal surgery: A retrospective study. Asian Spine J. 2019;13:254–7. doi: 10.31616/asj.2018.0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saito T, Den S, Cheema SP, Tanuma K, Carney E, Carlsson C, et al. A single-injection, multi-segmental paravertebral block-extension of somatosensory and sympathetic block in volunteers. Acta Anaesthesiol Scand. 2001;45:30–3. doi: 10.1034/j.1399-6576.2001.450105.x. [DOI] [PubMed] [Google Scholar]
- 16.D’Ercole F, Arora H, Kumar PA. Paravertebral block for thoracic surgery. J Cardiothorac Vasc Anesth. 2018;32:915–27. doi: 10.1053/j.jvca.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Wang H, Li S, Liang N, Liu W, Liu H, Liu H. Postoperative pain experiences in Chinese adult patients after thoracotomy and video-assisted thoracic surgery. J Clin Nurs. 2017;26:2744–54. doi: 10.1111/jocn.13789. [DOI] [PubMed] [Google Scholar]
- 18.Pace MM, Sharma B, Anderson-Dam J, Fleischmann K, Warren L, Stefanovich P. Ultrasound-guided thoracic paravertebral blockade: A retrospective study of the incidence of complications. Anesth Analg. 2016;122:1186–91. doi: 10.1213/ANE.0000000000001117. [DOI] [PubMed] [Google Scholar]
- 19.Kot P, Rodriguez P, Granell M, Cano B, Rovira L, Morales J, et al. The erector spinae plane block: A narrative review. Korean J Anesthesiol. 2019;72:209–20. doi: 10.4097/kja.d.19.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]


