Abstract
The efficacy of intravenous injection of FK463, a novel water-soluble lipopeptide, was evaluated in mouse models of disseminated candidiasis and aspergillosis and was compared with those of fluconazole (FLCZ) and amphotericin B (AMPH-B). In the candidiasis model, FK463 significantly prolonged the survival of intravenously infected mice at doses of 0.125 mg/kg of body weight or higher. In disseminated candidiasis caused by Candida species, including FLCZ-resistant Candida albicans, FK463 exhibited an efficacy 1.4 to 18 times inferior to that of AMPH-B, with 50% effective doses (ED50s) ranging from 0.21 to 1.00 mg/kg and 0.06 to 0.26 mg/kg, respectively, and was much more active than FLCZ. The protective effect of FK463 was not obviously influenced by the fungal inoculum size, the starting time of the treatment, or the immunosuppressed status of the host. The reduction in efficacy was less than that observed with FLCZ or AMPH-B. The efficacy of FK463 was also evaluated in the disseminated candidiasis target organ assay and was compared with those of FLCZ and AMPH-B. Efficacies were evaluated on the basis of a comparison between the mean log10 CFU in kidneys in the groups treated with antifungal agents and that in control group. A single dose of FK463 at 0.5 mg/kg or higher significantly reduced the viable counts in kidneys compared with the numbers of yeast cells before treatment, and its efficacy was comparable to that of AMPH-B, while FLCZ at 4 mg/kg showed only a suppressive effect on the growth of C. albicans in the kidneys. In the disseminated aspergillosis model, FK463 given at doses of 0.5 mg/kg or higher significantly prolonged the survival of mice infected intravenously with Aspergillus fumigatus conidia. The efficacy of FK463 was about 2 times inferior to that of AMPH-B, with ED50s ranging from 0.25 to 0.50 mg/kg and 0.11 to 0.29 mg/kg, respectively. These results indicate that FK463 may be a potent parenterally administered therapeutic agent for disseminated candidiasis and aspergillosis.
People who have impaired immune systems are susceptible to fungal infections which can be life-threatening. Immune deficiencies resulting from AIDS, aggressive cancer treatment, the growing use of organ transplants, and other nosocomial situations have greatly increased the incidence of serious fungal infections (2, 3, 4, 6) and have created a critical need for new, safe fungicidal agents that can be used to treat disseminated infections. Systemic mycoses are not easily diagnosed, and the patient usually has been infected for quite some time before symptoms appear. Thus, empiric therapy needs to begin immediately, but currently available treatments have problems with toxicity or resistance. Amphotericin B (AMPH-B) is the first-line therapy for systemic infections because of its broad-spectrum and fungicidal activity. However, significant side effects limit its clinical utility to controlled intravenous administration (16). Lipid AMPH-B formulations have recently attracted much attention due to significantly lower toxicity (7). The azole antifungal agents have broad spectra of activity, are orally active, and are considered less toxic than AMPH-B but are only fungistatic against most major fungal pathogens. Resistance to azole antifungals has been reported recently in several types of fungal infections worldwide (8, 15).
FK463 is a new parenterally administered antifungal drug candidate undergoing clinical development. FK463 is a semisynthetic derivative of FR901379, a water-soluble cyclic hexapeptide with a fatty acyl side chain, which is similar in structure to the echinocandin class of antifungal agents (T. Iwamoto, N. Sakamoto, M. Yamashita, M. Ezaki, S. Hashimoto, T. Furuta, M. Okuhara, and M. Kohsaka, Prog. Abstr. 33rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. 371, 1993). Modification of FR901379 improved antifungal potencies. FK463 has been shown to have potent in vitro antifungal activity against Candida and Aspergillus species (K. Maki, Y. Morishita, Y. Iguchi, E. Watabe, K. Otomo, N. Teratani, Y. Watanabe, F. Ikeda, S. Tawara, T. Goto, M. Tomishima, H. Ohki, A. Yamada, K. Kawabata, H. Takasugi, H. Tanaka, K. Sakane, F. Matsumoto, and S. Kuwahara, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F141, p. 268, 1998). FK463 is an inhibitor of synthesis of 1,3-β-d-glucan, a critical structural cell wall component in certain pathogenic fungi (5; Maki et al., 38th ICAAC). In the study described in this report, the in vivo activity of FK463 was evaluated in mouse models of disseminated candidiasis and aspergillosis.
MATERIALS AND METHODS
Compounds.
FK463 was synthesized by Fujisawa Pharmaceutical Co., Ltd. AMPH-B and fluconazole (FLCZ) were purchased from Bristol-Myers Squibb (Tokyo, Japan) and Pfizer (Tokyo, Japan), respectively. FK463, AMPH-B, and FLCZ were formulated in sterile saline or 5% glucose for intravenous injection and injected as 10 ml/kg of body weight.
Animals.
Male Slc-ICR mice (age, 4 weeks) were purchased from SLC Japan (Shizuoka, Japan). Eight mice were allocated to each dosage level.
Organisms and media.
The strains of Candida species were cultured on Sabouraud dextrose agar (SDA). Each test strain was suspended in sterile saline, and the inoculum was standardized by spectrophotometer. The inocula of Aspergillus fumigatus strains were prepared by culturing the test organisms on potato dextrose agar. Conidia were collected in sterile saline, and the conidium concentration was standardized by spectrophotometer. The viable counts of test strains were confirmed by serially diluting the cell suspension 10-fold and plating the inoculum onto SDA plates.
MICs.
The MICs of FK463, FLCZ, and AMPH-B were determined according to guideline M27-A of the National Committee for Clinical Laboratory Standards (NCCLS) (Maki et al., 38th ICAAC).
Systemic mouse infection.
Disseminated candidiasis and aspergillosis were induced in ICR mice by the intravenous inoculation of 0.2 ml of a cell suspension of each test strain via their lateral tail veins. Cyclophosphamide (Acros Organics, Springfield, N.J.) was administered intraperitoneally at 200 mg/kg 4 days before infection for A. fumigatus TIMM0063; 4 days before and 1 day after infection for the Candida albicans strains, C. tropicalis, C. krusei, C. parapsilosis, and A. fumigatus IFM41209; and 4 days before and 1 day and 6 days after infection for C. glabrata.
For the experiment on the influence of immunosuppression on efficacy against disseminated candidiasis, mice received either cyclophosphamide at 200 mg/kg administered intraperitoneally 4 days before and 1 day after infection or hydrocortisone (Nacalai Tesque, Kyoto, Japan) at 100 mg/kg administered subcutaneously 1 day before and 3 h, 1 day, and 2 days after infection to induce a continuously immunosuppressed condition in the host. The immunosuppressed and normal mice were challenged intravenously with a saline suspension of C. albicans FP633, containing 1.9 × 106 CFU for normal mice, 3.1 × 104 CFU for cyclophosphamide-treated mice, and 1.3 × 105 CFU for hydrocortisone-treated mice.
Treatment regimens.
The antifungal agents were administered once daily or twice daily for 4 days, starting at 1 h after infection by intravenous injection. The regimens used for the influence of the starting time of treatment on efficacy against disseminated candidiasis were as follows: (i) once daily for 4 days starting at 1 h after infection and (ii) once daily for 3 days starting at 24 h after infection. The efficacies of antifungal agents were assessed as the 50% effective doses (ED50), calculated by probit analysis or normal probability plot based on the survival rate at 15 days after infection.
Target organ assays.
Disseminated candidiasis was induced by the intravenous inoculation of a 0.2-ml saline suspension containing 1.0 × 105 CFU of C. albicans FP633 into the lateral tail veins of mice. Cyclophosphamide was administered intraperitoneally at 200 mg/kg 4 days before infection, and the antifungal agents were administered intravenously 1 h after infection. The target organ assay for C. albicans monitors the number of CFU in the kidneys at 1 day after infection. Kidneys from sacrificed mice were removed and placed in glass vials containing 10 ml of sterile saline. The kidneys were homogenized and were serially diluted in saline, and aliquots were plated onto SDA plates. The plates were incubated at 37°C for 2 days.
Statistics.
Survival was compared by the Wilcoxon rank sum test. One-way layout analysis of variance and the Dunnet t test were used to compare numbers of cells in the kidney for the treatment groups and the control group.
RESULTS
Efficacy in the C. albicans and A. fumigatus disseminated infection survival models.
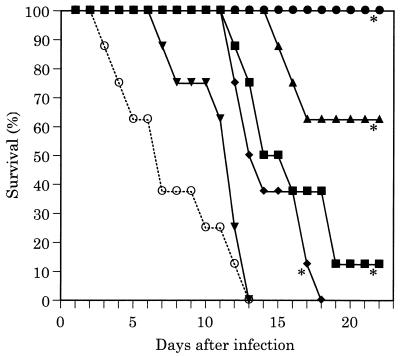
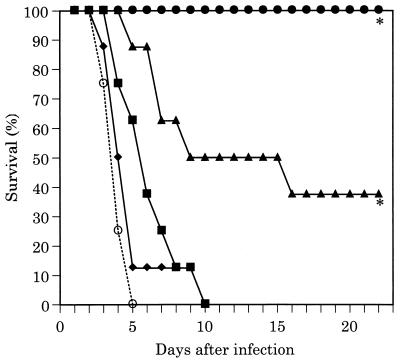
Survival curves in the C. albicans disseminated infection model are illustrated in Fig. 1. All untreated infected control mice died within 13 days. FK463 significantly prolonged the survival of infected mice at doses of 0.125 mg/kg or higher. All of the infected mice could survive as long as 22 days when treated with FK463 at 1.0 mg/kg. Survival curves in the A. fumigatus disseminated infection model are illustrated in Fig. 2. All untreated infected control mice died within 5 days. FK463 levels at or greater than 0.5 mg/kg significantly prolonged the survival of infected mice. All of the infected mice treated with 1.0 mg/kg of FK463 survived at day 22 after challenge.
FIG. 1.
Efficacy of FK463 in disseminated C. albicans 16010 infection (intravenous challenge with 4.0 × 105 CFU/mouse) in ICR mice (cyclophosphamide was administered intraperitoneally at 200 mg/kg 4 days before and 1 day after infection). FK463 was administered intravenously once daily for 4 days, starting at 1 h after infection. Symbols: ●, 1.0 mg/kg; ▴, 0.5 mg/kg; ■, 0.25 mg/kg; ⧫, 0.125 mg/kg; ▾, 0.0625 mg/kg; ○, sham treatment; ∗, significantly different from the control (P < 0.01 by the Wilcoxon rank sum test).
FIG. 2.
Efficacy of FK463 in disseminated A. fumigatus IFM41209 infection (intravenous challenge with 3.4 × 104 CFU/mouse) in ICR mice (cyclophosphamide was administered intraperitoneally at 200 mg/kg 4 days before and 1 day after infection). FK463 was administered intravenously once daily for 4 days, starting at 1 h after infection. Symbols: ●, 1.0 mg/kg; ▴, 0.5 mg/kg; ■, 0.25 mg/kg; ⧫, 0.125 mg/kg; ○, sham treatment; ∗, significantly different from the control (P < 0.01 by the Wilcoxon rank sum test).
Comparative efficacy studies in disseminated candidiasis and aspergillosis.
The efficacy of FK463 against disseminated fungal infection was compared with those of AMPH-B and FLCZ. The MICs of FK463, FLCZ, and AMPH-B against the test organisms used in these studies are presented in Table 1. Table 2 shows the ED50 calculated on the basis of the survival rate at 15 days after infection. The ED50 of FK463 against disseminated infections with C. albicans, C. glabrata, C. tropicalis, and C. krusei ranged from 0.14 to 0.77 mg/kg. Although the efficacies of FK463 were 1.4 to 3.1 times inferior to those of AMPH-B (0.09 to 0.26 mg/kg), they were 9.6 to >77 times superior to those of FLCZ. The ED50 of FK463 against disseminated C. parapsilosis infection was 1.0 mg/kg, which was 11 times superior to that of FLCZ (10.9 mg/kg) and 18 times inferior to that of AMPH-B (0.06 mg/kg). The ED50 of FK463 and AMPH-B given twice daily (BID) against C. albicans FP633 were comparable to once daily treatment (UID), but for FLCZ, twice daily treatment was slightly superior to once daily treatment (Table 2).
TABLE 1.
MICs of FK463, FLCZ, and AMPH-B against Candida and Aspergillus strains used in this study
| Organism | MIC (μg/ml) of:
|
||
|---|---|---|---|
| FK463 | FLCZ | AMPH-B | |
| C. albicans FP633 | 0.0156 | 0.5 | 0.5 |
| C. albicans 16010 | 0.0078 | 0.5 | 0.5 |
| C. albicans FP1839a | 0.0156 | 32 | 0.25 |
| C. glabrata 13002 | 0.0156 | 4 | 0.5 |
| C. tropicalis 16009 | 0.0156 | 0.25 | 0.0625 |
| C. krusei FP1866 | 0.125 | 32 | 0.5 |
| C. parapsilosis FP1946 | 0.5 | 1 | 0.0313 |
| A. fumigatus TIMM0063 | ≤0.0039 | >64 | 1 |
| A. fumigatus IFM41209 | 0.0078 | >64 | 0.5 |
FLCZ-resistant C. albicans.
TABLE 2.
Efficacy of FK463 in mouse models of disseminated candidiasis and aspergillosis
| Organism | Inoculum (CFU) | Treatment regimen | ED50 (mg/kg/day) ofa:
|
||
|---|---|---|---|---|---|
| FK463 | FLCZ | AMPH-B | |||
| C. albicans FP633 | 1.0 × 104 | UIDb | 0.14 (0.10–0.19) | 2.15 (1.32–3.23) | 0.08 (0.05–0.10) |
| BIDc | 0.19 (0.14–0.26) | 1.82 (1.26–2.50) | 0.11 (0.04–0.16) | ||
| C. albicans 16010 | 4.0 × 105 | UID | 0.21 (0.14–0.31) | 4.51 (3.04–8.14) | 0.12 (0.08–0.18) |
| C. albicans FP1839d | 4.6 × 104 | UID | 0.26 (NE) | >20.0 (NE) | 0.18 (NE) |
| C. glabrata 13002 | 3.5 × 107 | UID | 0.30 (0.22–0.42) | 6.27 (4.08–10.1) | 0.11 (0.08–0.16) |
| C. tropicalis 16009 | 1.6 × 104 | UID | 0.28 (0.20–0.39) | 3.71 (2.47–6.06) | 0.09 (0.06–0.12) |
| C. krusei FP1866 | 6.0 × 107 | UID | 0.77 (0.55–1.08) | 9.52 (6.12–17.4) | 0.26 (0.12–0.42) |
| C. parapsilosis FP1946 | 8.6 × 107 | UID | 1.00 (0.70–1.43) | 10.9 (7.62–17.5) | 0.06 (0.04–0.08) |
| A. fumigatus TIMM0063 | 2.1 × 105 | UID | 0.25 (0.18–0.36) | >20.0 (NE) | 0.11 (0.07–0.16) |
| A. fumigatus IFM41209 | 3.4 × 104 | UID | 0.50 (NE) | >20.0 (NE) | 0.29 (NE) |
Values in parentheses are 95% confidence intervals. NE, confidence interval could not be estimated.
Once daily treatment for 4 days, starting at 1 h after infection.
Twice daily treatment for 4 days, starting at 1 h after infection.
FLCZ resistant.
FK463 showed good activity against disseminated A. fumigatus infection, with ED50 in the range of 0.25 to 0.50 mg/kg. The efficacies of FK463 were 1.7 to 2.3 times inferior to those of AMPH-B (0.11 to 0.29 mg/kg) and >80 times superior to those of FLCZ.
Efficacy in immunosuppressed mouse models of disseminated candidiasis.
Table 3 shows the ED50 of FK463 and other antifungal agents against disseminated C. albicans infection models using normal mice and mice in a state of continuous immunosuppression induced by cyclophosphamide (200 mg/kg; intraperitoneal administration) or hydrocortisone (100 mg/kg; subcutaneous administration). Although the efficacies of FK463 were reduced to 1/2.2 to 1/3.5 in continuously immunosuppressed mice compared to normal mice, FK463 showed good activity in both types of immunosuppressed mice, with ED50 of 0.28 to 0.45 mg/kg. The reduction in the efficacy of FK463 was less than that observed for FLCZ (efficacy reduced to 1/2.6 to 1/9.9) and AMPH-B (efficacy reduced to 1/3.2 to 1/5.6).
TABLE 3.
Influence of immunosuppression and starting time of treatment on efficacy of FK463 in mouse models of disseminated candidiasis
| Host condition | Starting time of treatment after infection (h) | Inoculum (CFU) | ED50 (mg/kg/day)a of:
|
||
|---|---|---|---|---|---|
| FK463 | FLCZ | AMPH-B | |||
| Normal (untreated) | 1b | 1.9 × 106 | 0.13 (0.09–0.18) | 1.5 (1.10–2.15) | 0.04 (0.03–0.06) |
| Hydrocortisone treated | 1b | 1.3 × 105 | 0.45 (0.32–0.64) | 14.9 (9.91–38.3) | 0.23 (0.16–0.32) |
| Cyclophosphamide treated | 1b | 3.1 × 104 | 0.28 (NE) | ≥4.0d (NE) | 0.13 (NE) |
| 1b | 1.2 × 104 | 0.25 (NE) | 2.0 (1.40–2.85) | 0.07 (0.05–0.10) | |
| 24c | 1.2 × 104 | 0.32 (0.24–0.45) | 6.2 (4.49–8.43) | 0.21 (0.15–0.29) | |
Values in parentheses are 95% confidence intervals. NE, confidence interval could not be estimated.
Once daily for 4 days, starting at 1 h after infection.
Once daily for 3 days, starting at 24 h after infection.
Fifty-percent survival was observed at 4.0 mg/kg of FLCZ.
Influence of starting time of treatment on efficacy in mouse models of disseminated candidiasis.
To investigate the influence of the starting time of treatment on the efficacy of FK463 and other antifungal agents against C. albicans disseminated infection, compounds were administered intravenously starting at 1 or 24 h after infection (Table 3). The protective effect of FK463 was reduced to 1/1.3 with the delay of the starting of treatment, while the reduction of efficacy was less than that observed for FLCZ (reduced to 1/3.1) or AMPH-B (reduced to 1/3.1).
Target organ assays.
FK463 and other antifungal agents were tested for their activities in reducing the numbers of recoverable yeast cells from the kidneys of mice challenged intravenously with C. albicans FP633. The antifungal agents were administered once, at 1 h postinfection. Table 4 shows the viable counts of yeast cells in kidneys at 1 day after infection. FK463 significantly reduced the counts of yeast cells recovered from the kidneys when it was administered intravenously at doses of 0.5 mg/kg or higher, and its efficacy was comparable to that of AMPH-B. FLCZ at a dose of 4 mg/kg did not reduce the counts in the kidneys compared with the numbers of yeast cells before treatment.
TABLE 4.
Effect of single administration of FK463 on fungal titers in the kidneys of mice in the disseminated C. albicans infection model
| Compound | Dose (mg/kg) | Viable count (log CFU/kidneys)a |
|---|---|---|
| Control | ||
| 0 h | 3.35 ± 0.10 | |
| 24 h | 4.93 ± 0.09 | |
| FK463 | 1 | 1.73 ± 0.17* |
| 0.5 | 2.62 ± 0.34* | |
| 0.25 | 4.20 ± 0.12 | |
| AMPH-B | 1 | 2.35 ± 0.16* |
| 0.5 | 2.98 ± 0.20* | |
| 0.25 | 3.50 ± 0.26 | |
| FLCZ | 4 | 3.86 ± 0.13 |
Mean log10 CFU ± standard deviation per paired kidneys at 24 h after infection. *, significantly different from the control at 0 h (P < 0.01) by one-way layout analysis of variance and the Dunnet t test.
DISCUSSION
FK463 appears to possess a good profile as a new antifungal agent, displaying potent activity against clinically important fungal pathogens, good water solubility, favorable pharmacokinetic properties, and low toxicity (J. Azuma, I. Yamamoto, M. Ogura, T. Mukai, H. Suematsu, H. Kageyama, H. Nakahara, K. Yoshida, and T. Takaya, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F146, p. 269, 1998; Maki et al., 38th ICAAC; S. Matsumoto, Y. Wakai, K. Maki, E. Watabe, T. Ushitani, K. Otomo, T. Nakai, Y. Watanabe, F. Ikeda, S. Tawara, T. Goto, F. Matsumoto, and S. Kuwahara, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F142, p. 268, 1998; S. Suzuki, M. Terakawa, F. Yokobayashi, F. Fujiwara, and T. Hata, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F144, p. 269, 1998; Y. Wakai, S. Matsumoto, K. Maki, E. Watabe, K. Otomo, T. Nakai, K. Hatano, Y. Watanabe, F. Ikeda, S. Tawara, T. Goto, F. Matsumoto, and S. Kuwahara, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F143, p. 268, 1998). In this study, the efficacy of intravenous injection of FK463 and other antifungal agents was investigated in mouse models of disseminated Candida and Aspergillus infection, since these compounds are administered by intravenous infusion in clinical settings.
The efficacy of FK463 in a multiple-dosage regimen against disseminated fungal infection was compared with those of AMPH-B and FLCZ. The efficacy of FK463 against disseminated infections with Candida species, except C. parapsilosis, was 1.4 to 3.1 times inferior to that of AMPH-B, and it was obviously superior to that of FLCZ. Although the efficacy of FK463 against disseminated C. parapsilosis infection was 11 times superior to that of FLCZ, it was 18 times inferior to that of AMPH-B. These results were correlated with the MICs of FK463 against C. parapsilosis, which were higher than those against C. albicans (Maki et al., 38th ICAAC).
The half-life (t1/2) and area under the concentration-time curve (AUC) for FK463 at 1 mg/kg in mice were 5.8 h and 48 μg · h/ml (Suzuki et al., 38th ICAAC), respectively, and the levels of FK463 in the plasma of mice were higher than those of FLCZ and AMPH-B (10, 11). These data indicated that the ED50 of FK463 against disseminated candidiasis in mice, except for C. parapsilosis infection, were not related to the in vitro MICs in comparison to those of AMPH-B. These results suggest that high protein binding of FK463 (Suzuki et al., 38th ICAAC) may reduce the in vivo efficacy. Once daily treatment with FLCZ in disseminated candidiasis displayed a relative lack of efficacy in comparison to twice daily treatment, and the best regimen for mice is twice daily treatment, due to rapid excretion. However, our data indicated that, due to the excellent fungicidal activities of FK463, both once daily treatment and twice daily treatment with FK463 were obviously superior to comparable treatment with FLCZ.
The protective effect of FK463 against disseminated C. albicans infection was not obviously influenced by the starting time of the treatment and the immunosuppressed status of the host. The reduction in efficacy was less than that observed with FLCZ or AMPH-B. Furthermore, the ability of a single dose of FK463 to sterilize the kidneys of mice was superior to that of FLCZ and comparable to that of AMPH-B. The fungicidal activity of FK463 against C. albicans appears to contribute to this favorable in vivo activity (Maki et al., 38th ICAAC).
LY303366 and MK0991 have been reported to inhibit the hyphal elongation of A. fumigatus and have profound morphological effects, although they do not give MICs for Aspergillus species in a classic broth dilution assay (9, 12, 14, 18). These compounds have been shown to be effective in experimental disseminated and pulmonary aspergillosis models in rodents (1, 13, 17). FK463 did not completely inhibit the growth of A. fumigatus in the broth microdilution assay, but it showed a suppressive effect on hyphal growth and a morphological effect (Maki et al., 38th ICAAC), similar to pneumocandins. The efficacy of FK463 against disseminated aspergillosis was in excellent correlation with its partial inhibitory effect on growth at low concentrations, although it was slightly inferior to that of AMPH-B.
In conclusion, the present data suggest that FK463 is efficacious in the treatment of disseminated candidiasis and aspergillosis, and further studies to evaluate the compound should be considered.
ACKNOWLEDGMENT
We are grateful to David Barrett, Medicinal Chemistry Research Laboratories, for kind help and advice.
REFERENCES
- 1.Abruzzo G K, Flattery A M, Gill C J, Kong L, Smith J G, Pikounis V B, Balkovec J M, Bouffard A F, Dropinski J F, Rosen H, Kropp H, Bartizal K. Evaluation of the echinocandin antifungal MK-0991 (L-743,872): efficacies in mouse models of disseminated aspergillosis, candidiasis, and cryptococcosis. Antimicrob Agents Chemother. 1997;41:2333–2338. doi: 10.1128/aac.41.11.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anaisse E, Bodey G P, Kantarjian H, Ro J, Vartivarian S E, Hopfer R, Hoy J, Polston K. New spectrum of fungal infections in patients with cancer. Rev Infect Dis. 1989;11:369–378. doi: 10.1093/clinids/11.3.369. [DOI] [PubMed] [Google Scholar]
- 3.Anaisse E, Bodey G P, Rinaldi M G. Emerging fungal pathogens. Eur J Clin Microbiol Infect Dis. 1989;8:323–330. doi: 10.1007/BF01963467. [DOI] [PubMed] [Google Scholar]
- 4.Banerjee S N, Emori T G, Culver D H, Gaynes R P, Jarvis W R, Horan T, Edwards J R, Tolson J, Henderson T, Martone W J the National Nosocomial Infections Surveillance System. Secular trends in nosocomial primary bloodstream infections in the United States. Am J Med. 1991;91(Suppl. 3B):86S–89S. doi: 10.1016/0002-9343(91)90349-3. [DOI] [PubMed] [Google Scholar]
- 5.Bartnicki-Garcia S. Cell wall chemistry, morphogenesis, and taxonomy of fungi. Annu Rev Microbiol. 1968;22:87–108. doi: 10.1146/annurev.mi.22.100168.000511. [DOI] [PubMed] [Google Scholar]
- 6.Beck-Sague C M, Jarvis W R the National Nosocomial Infections Surveillance System. Secular trends in the epidemiology of nosocomial fungal infections in the United States, 1980–1990. J Infect Dis. 1993;167:1247–1251. doi: 10.1093/infdis/167.5.1247. [DOI] [PubMed] [Google Scholar]
- 7.Hiemenz J W, Walsh T J. Lipid formulations of amphotericin B: recent progress and future directions. Clin Infect Dis. 1996;22(Suppl. 2):S133–S144. doi: 10.1093/clinids/22.supplement_2.s133. [DOI] [PubMed] [Google Scholar]
- 8.Hitchcock C A, Pye G W, Troke D F, Johnson E M, Warnock D W. Fluconazole resistance in Candida glabrata. Antimicrob Agents Chemother. 1993;37:1962–1965. doi: 10.1128/aac.37.9.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ingroff A E. Comparison of the in vitro activities of the new triazole SCH56592 and the echinocandin MK-0991 (L-743,872) and LY303366 against opportunistic filamentous and dimorphic fungi and yeasts. J Clin Microbiol. 1998;36:2950–2956. doi: 10.1128/jcm.36.10.2950-2956.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim H, Loebenberg D, Marco A, Symchowicz S, Lin C. Comparative pharmacokinetics of Sch 28191 and amphotericin B in mice, rats, dogs, and cynomolgus monkeys. Antimicrob Agents Chemother. 1984;26:446–449. doi: 10.1128/aac.26.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Louie A, Drusano G L, Banerjee P, Liu Q, Liu W, Kaw P, Shayegani M, Taber H, Miller M H. Pharmacodynamics of fluconazole in a murine model of systemic candidiasis. Antimicrob Agents Chemother. 1998;42:1105–1109. doi: 10.1128/aac.42.5.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oakley K L, Moore C B, Denning D W. In vitro activity of the echinocandin antifungal agent LY303,366 in comparison with itraconazole and amphotericin B against Aspergillus spp. Antimicrob Agents Chemother. 1998;42:2726–2730. doi: 10.1128/aac.42.10.2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petraitis V, Petraitiene R, Groll A H, Bell A, Callender D P, Sein T, Schufele R L, McMillian C L, Bacher J, Walsh T J. Antifungal efficacy, safety, and single-dose pharmacokinetics of LY303366, a novel echinocandin B, in experimental pulmonary aspergillosis in persistently neutropenic rabbits. Antimicrob Agents Chemother. 1998;42:2898–2905. doi: 10.1128/aac.42.11.2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poeta M D, Schell W A, Perfect J R. In vitro antifungal activity of pneumocandin L-743,872 against a variety of clinically important molds. Antimicrob Agents Chemother. 1997;41:1835–1836. doi: 10.1128/aac.41.8.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruhnke M, Eigler A, Tennagen I, Geiseler B, Engelmann E, Trautmann M. Emergence of fluconazole-resistant strains of Candida albicans in patients with recurrent oropharyngeal candidosis and human immunodeficiency virus infection. J Clin Microbiol. 1994;32:2092–2098. doi: 10.1128/jcm.32.9.2092-2098.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomas A H. Suggested mechanisms for the antimycotic activity of the polyene antibiotics and the N-substituted imidazoles. J Antimicrob Chemother. 1986;17:269–279. doi: 10.1093/jac/17.3.269. [DOI] [PubMed] [Google Scholar]
- 17.Verweiji P E, Oakley K L, Morrissey J, Morrissey G, Denning D W. Efficacy of LY303366 against amphotericin B-susceptible and -resistant Aspergillus fumigatus in a murine model of invasive aspergillosis. Antimicrob Agents Chemother. 1998;42:873–878. doi: 10.1128/aac.42.4.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhanel G G, Karlowsky J A, Harding G A J, Balko T V, Zelenitsky S A, Friesen M, Kobani A, Turik M, Hoban D J. In vitro activity of a new semisynthetic echinocandin, LY-303366, against systemic isolates of Candida species, Cryptococcus neoformans, Blastomyces dermatidis, and Aspergillus species. Antimicrob Agents Chemother. 1997;41:863–865. doi: 10.1128/aac.41.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]