Abstract
Our previous International Union of Basic and Clinical Pharmacology report on the nomenclature and classification of adenosine receptors (2011) contained a number of emerging developments with respect to this G protein-coupled receptor subfamily, including protein structure, protein oligomerization, protein diversity, and allosteric modulation by small molecules. Since then, a wealth of new data and results has been added, allowing us to explore novel concepts such as target binding kinetics and biased signaling of adenosine receptors, to examine a multitude of receptor structures and novel ligands, to gauge new pharmacology, and to evaluate clinical trials with adenosine receptor ligands. This review should therefore be considered a further update of our previous reports from 2001 and 2011.
Significance Statement
Adenosine receptors (ARs) are of continuing interest for future treatment of chronic and acute disease conditions, including inflammatory diseases, neurodegenerative afflictions, and cancer. The design of AR agonists (“biased” or not) and antagonists is largely structure based now, thanks to the tremendous progress in AR structural biology. The A2A- and A2BAR appear to modulate the immune response in tumor biology. Many clinical trials for this indication are ongoing, whereas an A2AAR antagonist (istradefylline) has been approved as an anti-Parkinson agent.
I. Introduction
A decade has passed since our last International Union of Basic and Clinical Pharmacology report on the nomenclature and classification of adenosine receptors appeared (Fredholm et al., 2011), after the first one in 2001 (Fredholm et al., 2001). The field has matured to the extent that the recommendations on the nomenclature stand firmly and require neither changes nor refinements. Substantial developments, however, took place (Fredholm et al., 2021), and these alone warrant a further update already. The adenosine A2A receptor (A2AAR) has become a test case for G protein-coupled receptor (GPCR) structure elucidation, whereas structures of the adenosine A1 receptor (A1AR) have also become available. The structures have been obtained through either X-ray crystallography or a more recent development, cryo-electron microscopy (EM). These together constitute a huge variety, most of which were determined with different antagonist ligands, a few with agonistic ligands with or without (parts of the) G protein present, and one with a partial agonist. Secondly, the increasing awareness that the study of target binding kinetics reveals more details on the interaction between ligand and receptor has had its effect on the further and more detailed kinetic characterization of adenosine receptor ligands, both agonists and antagonists. Moreover, there is ample attention again for novel ligands interacting with adenosine receptors. Some of these newer and older ligands possess a preference for biased signaling (i.e., the preferred coupling to particular signaling pathways), most notably through different G proteins or β-arrestin. Furthermore, there is an in-depth analysis of the (patho)pharmacological aspects of adenosine receptors and their ligands, both in the periphery and the central nervous system (CNS), leading to an evaluation of the receptors’ relevance in diverse disease states including COVID-19 infection and in aging. The report is concluded with a (nonexhaustive) overview of the clinical trials with adenosine receptor ligands in the last ten years. Disappointing were the outcomes for A1AR partial agonists, with lack of efficacy in heart failure noted in advanced phase 2b clinical studies. On the other hand, an A2AAR antagonist was approved in the United States as a new anti-Parkinsonian drug, and the role of adenosine receptors in immunology has led to a surge of ongoing studies in immuno-oncology, particularly with A2AAR, A2BAR, or dual A2AAR/A2BAR antagonists.
II. Receptor Ligands (for Structures, See Figs. 1, 2, 3, and 5)
Fig. 1.

Selected ligands for studying ARs.
Fig. 2.
Ligands investigated in kinetic studies.
Fig. 3.
Ligands in 3D receptor structures and ligands in biased signaling studies. (Note: In 67 and 68, the X-ray structure of “LUAA47070” was not obtained with the prodrug LUAA47070 but with the A2AAR antagonist that is released from the prodrug upon hydrolysis.)
Fig. 5.
Ligands in clinical studies.
Adenosine receptors (ARs) have become established drug targets. Adenosine (1, Fig. 1) itself is used as an injectable diagnostic for cardiac imaging to dilate the coronary arteries via A2AAR activation of patients who cannot exercise on a treadmill (Singh and McKintosh, 2020). The short half-life of under 10 seconds prevents severe side effects of concomitant A1AR activation, such as cardiac block. Moreover, adenosine is applied in supraventricular tachycardia due to its antiarrhythmic effects (Singh and McKintosh, 2020). The A2AAR-selective agonist regadenoson (2, Table 1), used for the same purpose, displays a longer half-life of 2–3 minutes and is of benefit for patients who develop bronchospasms upon treatment with adenosine (Thomas et al., 2017; Patel and Alzahrani, 2020). The natural products caffeine (3) and theophylline (4), xanthine alkaloids present in plants (e.g., Coffea arabica and Camellia sinensis), are moderately potent, nonselective AR antagonists (see Table 2 for receptor affinities) that have been used for thousands of years (Daly, 2007; Müller and Jacobson, 2011b; van Dam et al., 2020). There is epidemiologic evidence linking coffee and tea consumption with different health benefits (Grosso et al., 2016; Poole et al., 2017; van Dam et al., 2020). Caffeine, probably the most widely applied psychoactive drug in the world and broadly used for recreational purposes, is therapeutically applied as a central nervous system (CNS) stimulant, for preterm infants to support breathing function, and in combination therapeutics with analgesics to treat pain and colds (Abo-Salem et al., 2004; Lipton et al., 2017; Alhersh et al., 2020; Evans et al., 2020; van Dam et al., 2020). Several ongoing clinical trials (see also Chapter VII) are evaluating caffeine for various indications including cognition, pain, obesity, cataract prevention, and others. Theophylline, which is less brain-permeant than caffeine, is used for the treatment of asthma, but due to its narrow therapeutic window and the availability of safer and more potent alternative therapeutics, it has lost its importance and nowadays serves as a third-line treatment of add-on therapy only (Barnes, 2003; Tilley, 2011; Journey and Bentley, 2020). Both caffeine and theophylline also interact with other targets (e.g., they inhibit phosphodiesterases), but many of these effects are only observed at high, nonphysiologic concentrations. Most of the desired effects of caffeine and theophylline can in fact be explained by a blockade of ARs. It has to be noted that both xanthine derivatives are about equally potent at all four human AR subtypes, but they are inactive at rodent A3ARs (see Table 2). The A2AAR-selective antagonist istradefylline (5), a xanthine derivative that is structurally derived from caffeine, has been approved for the treatment of Parkinson’s disease (PD) in combination with levodopa, initially only in Japan (in 2013) but now also in the United States (in 2019), whereas the approval process in Europe is in progress (Takahashi et al., 2018; Chen and Cunha, 2020; Jenner et al., 2021). Due to intensive research for several decades aimed at developing AR ligands, a large number of subtype-selective agonists and antagonists has been developed (for reviews, see Müller and Jacobson, 2011a; Jacobson and Müller, 2016; Jacobson et al., 2019; Jacobson et al., 2021). The rather modest success in drug approvals despite a large number of clinical trials discouraged scientists and pharmaceutical companies. However, the recent approval of the A2AAR antagonist istradefylline in the United States and, in particular, the ‘gold rush fever’ in immuno-oncology centered around adenosine as an immunosuppressant (Boison and Yegutkin, 2019; Borah et al., 2019; Allard et al., 2020; Willingham et al., 2020; Thompson and Powell, 2021) have newly energized and fueled the field.
TABLE 1.
Affinities of selected adenosine receptor agonists
| Ki or EC50 (nM)a | |||||
|---|---|---|---|---|---|
| A1 | A2A | A2Bb | A3 | ||
| Nonselective Agonists | |||||
| 1 | Adenosinec | ca. 100 (h) 73 (r) |
310 (h) 150 (r) |
15,000 (h) 5,100 (r) |
290 (h) 6,500 (r) |
| 6 | NECA | 14 (h) 5.1 (r) 2.49 (m) |
20 (h) 9.7 (r) 43.4 (m) |
1,890 (h) 1,110 (r) 656 (m) |
25 (h) 113 (r) 13.2 (m) |
| A1AR-Selective Agonists | |||||
| 7 | CCPA | 0.83 (h) 1.3 (r) 0.269 (m) |
2270 (h) 950 (r) 988 (m) |
18,800 (h) 6,160 (r) 25.300 (m) |
38 (h) 237 (r) 15.6 (m) |
| 8 | 2′-MeCCPA | 3.3 (h) | 9,580 (h) | 37.600 (h) | 1.150 (h) |
| 9 | (S)-ENBA | n.d. (h) 0.34 (r) |
n.d. (h) 477 (r) |
n.d. | 282 (h) 915 (r) |
| A2AAR-Selective Agonists | |||||
| 10 | CGS21680 | 289 (h) 1800 (r) 961 (m) |
27 (h) 19 (r) 13.7 (m) |
>10,000 (h) >10,000 (r) >10,000 (m) |
67 (h) 584 (r) 93.0 (m) |
| 11 | UK-432,097 | n.d. | 4 | n.d. | n.d. |
| 12 | PSB-0777 | 541 (h) ≥10,000 (r) |
360 (h) 44.4 (r) |
>10,000 (h) | >10,000 (h) |
| 2 | Regadenoson | >10,000 (h) | 290 (h) | >10,000 (h) | >10,000 (h) |
| A2BAR-Selective (Partial) Agonist | |||||
| 13 | BAY 60-6583 | 387 (h) 514 (r) 351 (m) |
>10,000 (h) >10,000 (r) >10,000 (m) |
3–10 (h, EC50) 114 (h) 100 (r) 136 (m) |
223 (h) 2,750 (r) 3,920 (m) |
| A3AR-Selective Agonists | |||||
| 14 | Cl-IB-MECA (CF102, Namodenoson) |
220 (h) 280 (r) 35 (m) |
5360 (h) 470 (r) 290 (m) |
>10,000 (h) 1,210 (r) 44,300 (m) |
1.4 (h) 0.33 (r) 0.18 (m) |
| 15 | HEMADO | 330 (h) | 1200 (h) | >30,000 (h) | 1.10 (h) |
| 16 | MRS5698 | >10,000 (h) >10,000 (m) |
>10,000 (h) >10,000 (m) |
assumed to be inactive | 3.49 (h) 3.08 (m) |
h, human; Ki, inhibition constant; m, mouse; n.d., no data; r, rat.
adata (if available from Ki values from radioligand binding assays) are taken from the literature cited in the text.
bmost A2BAR data are from functional studies (cAMP accumulation).
cadenosine data are from functional studies (cAMP accumulation).
TABLE 2.
Affinities of selected, useful adenosine receptor antagonists
| Ki (nM)a | |||||
|---|---|---|---|---|---|
| A1 | A2A | A2B | A3 | ||
| Nonselective Antagonists | |||||
| 3 | Caffeine | 44,900 (h) 41,000 (r) 50,700 (m) |
23,400 (h) 43,000 (r) 11,100 (m) |
33,800 (h) 30,000 (r) 23,000 (m) |
13,300 (h) >100,000 (r) >100,000 (m) |
| 4 | Theophylline | 6,770 (h) 14,000 (r) |
6,700 (h) 22,000 (r) |
9,070 (h) 15,100 (r) 5,630 (m) |
22,300 (h) >100,000 (r) |
| A1AR-Selective Antagonists | |||||
| 17 | DPCPX (CPX) | 3.0 (h) 0.50 (r) 0.413 (m) |
129 (h) 157 (r) 263 (m) |
51 (h) 186 (r) 86.2 (m) |
243 (h) >10,000 (r) >10,000 (m) |
| 18 | PSB-36 | 0.7 (h) 0.124 (r) 1.58 (m) |
980 (h) 552 (r) 697 (m) |
187 (h) 350 (r) 704 (m) |
2,300 (h) 6,500 (r) >10,000 (m) |
| 19 | SLV320 | 1.00 (h) 2.51 (r) |
398 (h) | 3,981 (h) 501 (r) |
200 (h) |
| A2AAR-Selective Antagonists | |||||
| 5 | Istradefylline (KW6002) | 841 (h) 230 (r) 438 (m) |
12 (h) 4.46 (r) 6.83 (m) |
>10,000 (h) 5,940 (r) 3,590 (m) |
4,470 (h) >10,000 (r) >10,000 (m) |
| 20 | MSX-3 / MSX-2 (Data are for MSX-2) |
2,500 (h) 900 (r) |
5.38 (h) 8.04 (r) |
>10,000 (h) | >10,000 (h) |
| 21 | Preladenant (SCH-420814) | >1,000 (h) >1,000 (h) 462 (m) |
0.9 (h) 0.986 (r) 0.241 (m) |
>1,000 (h) >1,000 (m) >1,000 (r) |
>1,000 (h) >1,000 (m) >1,000 (r) |
| 22 | Imaradenant (AZD4635) | 160 (h) | 1.7 (h) | 64 (h) | >10,000 (h) |
| A2BAR-Selective Antagonists | |||||
| 23 | MRS1754 | 403 (h) 16.8 (r) 1.45 (m) |
503 (h) 612 (r) >10,000 (m) |
1.97 (h) 12.8 (r) 3.12 (m) |
570 (h) >1,000 (m) >1,000 (r) |
| 24 | PSB-603 | >10,000 (h) >10,000 (r) 42.4 (m) |
>10,000 (h) >10,000 (r) >10,000 (m) |
0.553 (h) 0.355 (r) 0.265 (m) |
>10,000 (h) >10,000 (r) >10,000 (m) |
| 25 | PSB-0788 | 2,240 (h) 386 (r) 118 (m) |
333 (h) 1,730 (r) 235 (m) |
0.393 (h) 2.12 (r) 1.90 (m) |
>1,000 (h) >10,000 (r) >10,000 (m) |
| 26 | PSB-1115 | >10,000 (h) 2,200 (r) 591 (m) |
3790 (h) 24,000 (r) >10,000 (m) |
53.4 (h) 3,140 (r) 1,940 (m) |
>10,000 (h) >10,000 (r) >10,000 (m) |
| 27 | GS 6201 (CVT-6883) | 1,940 (h) | 3,280 (h) | 22 (h) | 1,070 (h) |
| 28 | BAY-545 | >1,000; 1,300 (h) n.d. (r) >6,700 (m) |
>1,000; 820 (h) 750 (r) 470 (m) |
59–97 (h) 280 (r) 400 (m) |
>10,000 (h) n.d. (r) >6,700 (m) |
| 29 | ISAM-140 | >1,000 (h) | >1,000 (h) | 3.49 (h) | >1,000 (h) |
| A3AR-Selective Antagonists | |||||
| 30 | MRS1523 | >10,000 (h) 15,600 (r) >10,000 (m) |
3660 (h) 2050 (r) >10,000 (m) |
>10,000 (h) >10,000 (r) >10,000 (m) |
18.9 (h) 113 (r) 731 (m) |
| 31 | MRE3008-F20 | 1200 (h) | 141 (h) | 2100 (h) | 0.82 (h) |
| 32 | PSB-10 | 1,700 (h) 805 (r) |
2,700 (h) 6,040 (r) |
30,000 (h) | 0.441 (h) 17,000 (r) |
| 33 | VUF5574 | ≥10,000 (r) | ≥10,000 (r) | n.d. | 4.03 (h) |
| 34 | MRS7591b | >10,000 (h) 590 (m) |
>10,000 (h) n.d. |
n.d. n.d. |
10.9 (h) 17.8 (m) |
h, human; Ki, inhibition constant; m, mouse; n.d., no data; r, rat.
adata are taken from the literature cited in the text.
bpartial agonistic activity if receptor is highly expressed.
This chapter will provide guidance in selecting tool compounds for research on ARs. Rather than presenting a comprehensive collection of AR ligands for which the reader be referred to previous review articles selected (Fredholm et al., 2011; Müller and Jacobson, 2011a; Jacobson and Müller, 2016; Jacobson et al., 2021), preferably well characterized ligands will be discussed that are recommended as tool compounds. Whenever possible, not only data for human ARs but also those for rat and mouse orthologs will be listed since considerable species differences have been observed in some cases, which are most pronounced for the A3AR subtype (Alnouri et al., 2015; Du et al., 2018). For most receptor subtypes, at least two different agonists and antagonists will be included. In addition, useful physicochemical and pharmacokinetic data have been collected if available.
A. Adenosine Receptor Agonists
The physiologic agonist adenosine (1) is more potent at A1-, A2A- and A3ARs than at A2BARs in most settings (see Table 1). However, reliable radioligand binding data cannot be obtained since adenosine is present in tissues, cells, and even cell membrane preparations and is constantly produced (e.g., from released ATP by ectonucleotidases) (Zimmermann, 2021). Therefore, it usually has to be removed, which is achieved by preincubation or addition of adenosine deaminase (ADA). Thus, ADA and its reaction product inosine are typically present during incubation with radioligand and test compound. ADA itself can allosterically modulate ARs (Gracia et al., 2013). In contrast to radioligand binding data, potencies determined in functional, G protein-dependent assays such as cAMP accumulation studies depend on receptor expression levels and receptor reserve, and concentration-effect curves are shifted to the left with increased receptor expression levels (Fujioka and Omori, 2012). Therefore, EC50 values of agonists obtained in different cellular systems are not comparable. As mentioned before, adenosine has a short half-life being metabolized by ADA or adenosine kinase (AdoK) after removal by cellular uptake through nucleoside transporters, which can additionally influence results. For that reason, metabolically (more) stable adenosine analogs have been developed. Nevertheless, it becomes increasingly clear that synthetic ligands do not necessarily induce the same effects at a certain receptor as the cognate agonist (e.g., regarding the activation of intracellular signaling pathways) (see also Chapter V). Therefore, if possible, adenosine should always be included in pharmacological studies besides more stable and selective synthetic agonists. The closely related adenosine analog 5′-N-ethylcarboxamidoadenosine (NECA, 6) cannot be metabolized by ADA or AdoK. Similar to adenosine, NECA is significantly more potent at A1-, A2A-, and A3ARs than at A2BARs. There is a lack of potent, selective, and fully efficacious A2BAR agonists; NECA is still one of the more potent full agonists at the A2BAR and represents a useful tool to study A2BARs in combination with selective antagonists for the other AR subtypes (Verzijl and IJzerman, 2011; Müller et al., 2018; Franco et al., 2021b).
Potent, truly selective A1AR agonists have been developed by N6-substitution of adenosine (see Table 1 and Fig. 2). 2-chloro-N6-cyclopentyladenosine (CCPA, 7) is suitable for rat and mouse studies, where it shows >100-fold selectivity versus the other AR subtypes, whereas it is less selective in humans versus the A3AR subtype (46-fold). For studies at the human A1AR, its 2′-methyl-substituted derivative 2′-MeCCPA (8) can be used, which is more selective (>300-fold) in humans (Franchetti et al., 2009). Data at rat and mouse ARs are not available for this compound. Another potent and selective A1AR agonist is (S)-ENBA (9), possessing a bulky bicyclo[2.2.1]hept-2-yl moiety at the N6-position that confers A1AR selectivity.
A2AAR-selective agonists have been obtained by introducing large, bulky substituents into the 2-position of adenosine or NECA, in some cases in combination with an additional bulky N6-substituent. Most of the developed compounds are only moderately selective in humans versus the A1- or A3AR subtypes. CGS21680 (10) is a potent and A2AAR-selective agonist in rat and mouse but shows only moderate selectivity in humans (vs. A1- and A3ARs; see Table 1). However, in some studies on mouse brain, 10 has been observed to additionally bind to A1ARs (Lopes et al., 2004). The reason for this observation is still unclear; one explanation could be the formation of heteromeric receptor complexes showing a different pharmacology. The 2,N6-disubstituted NECA derivative 11 (UK-432,097; Table 1) is potent at the human A2AAR and was reported to also be selective. Compound 11 is a relatively large and lipophilic molecule that is less water-soluble than other adenosine derivatives and analogs. It showed a long receptor residence time of 250 minutes at 5°C (see Table 3), which probably contributed to its successful cocrystallization with the human A2AAR (Xu et al., 2011). PSB-0777 (12), bearing a phenylsulfonate group, is well soluble in water and has been useful for injection or for local application in the gut since it is not perorally absorbed due to its negative charge. It shows high selectivity in rats but not in humans and is thus useful for studies in rodents. Regadenoson (2) is only moderately potent but selective in humans and is clinically used as a diagnostic (see above). Importantly, in tissues with higher A1AR versus A2AAR density such as the brain, (moderately selective) A2AAR agonists often bind to and activate A1AR rather than A2AAR (Zhang et al., 1994; Cunha et al., 1996; Halldner et al., 2004; Pliássova et al., 2020). Thus, potent and really selective A2AAR agonists to target central A2AARs are still required.
TABLE 3.
Association and dissociation rate constants of selected AR ligands
| Compound | Target (h, human; r, rat) |
Temp (°C) | kon (M−1·min−1) | koff (min−1) | RT (min) | Kinetic KD (nM)a | Reference | |
|---|---|---|---|---|---|---|---|---|
| 7 | CCPA | hA1AR | 25 | 9.6 × 106 | 1.2 | 0.9 | 131 | (Guo et al., 2014b) |
| 6 | NECA | hA1AR | 25 | 9.0 × 105 | 0.47 | 2.1 | 522 | (Guo et al., 2014b) |
| 35 | LUF5834 | hA1AR | 25 | 2.0 × 108 | 0.92 | 1.1 | 4.6 | (Guo et al., 2014b) |
| 36 | Capadenoson | hA1AR | 25 | 2.4 × 107 | 0.036 | 28 | 1.5 | (Louvel et al., 2015) |
| 37 | LUF6976 | hA1AR | 25 | 3.9 × 108 | 0.87 | 1.1 | 2.2 | (Louvel et al., 2014) |
| 38 | LUF6941 | hA1AR | 25 | 2.6 × 106 | 0.0076 | 132 | 2.9 | (Louvel et al., 2015) |
| 39 | ABA-X-BY630 | hA1ARb | 37 | 2.6 × 107 | 2.0 | 0.5 | 77 | (May et al., 2010) |
| 17 | DPCPX | hA1AR | 25 | 1.4 × 108 | 0.21 | 4.8 | 1.5 | (Guo et al., 2013) |
| 17 | DPCPX | rA1R | 25 | 9.6 × 107 | 0.045 | 22.2 | 0.50 | (Guo et al., 2017) |
| 10 | CGS21680 | hA2AAR | 25 | 5.0 × 104 | 0.02 | 50.0 | 380 | (Guo et al., 2017) |
| 10 | CGS21680 | rA2AAR | 23 | 2.1 × 107 | 0.033 | 30.3 | 1.6 | (Guo et al., 2017) |
| 40 | LUF6057 | hA1AR | 25 | 4.8 × 108 | 3.0 | 0.3 | 6.3 | (Guo et al., 2013) |
| 6 | NECA | hA2AAR | 4 | 1.9 × 106 | 0.053 | 19 | 28 | (Guo et al., 2015) |
| 11 | UK432,097 | hA2AAR | 5 | 5.0 × 105 | 0.004 | 250 | 8.0 | (Guo et al., 2012) |
| 41 | SCH58261 | hA2AAR | 25 | 6.4 × 108 | 1.5 | 0.67 | 2.3 | (Dionisotti et al., 1997) |
| 42 | ZM241385 | hA2AAR | 4 | 1.3 × 108 | 0.014 | 71 | 0.11 | (Guo et al., 2014c) |
| 43 | LUF6632 | hA2AAR | 4 | 3.4 × 107 | 0.0031 | 323 | 0.091 | (Guo et al., 2014c) |
| 20a | MSX-2 | rA2AAR (brain striatal membranes) |
23 | 14.5 × 107 | 0.2839 | 3.52 | 1.95 | (Müller et al., 2000) |
| 6 | NECA | hA2BAR | 4 | n.d. | 2.201 | 0.45 | n.d. | (Hinz et al., 2018) |
| 23 | MRS1754 | hA2BAR | 25 | 2.2 × 107 | 0.027 | 37 | 1.2 | (Ji et al., 2001) |
| 44 | MRE2029-F20 | hA2BAR | 4 | 1.7 × 107 | 0.031 | 32 | 1.8 | (Baraldi et al., 2004) |
| 45 | OSIP339391 | hA2BAR | 22 | 9.5 × 107 | 0.039 | 26 | 0.41 | (Stewart et al., 2004) |
| 24 | PSB-603 | hA2BAR | 21 | 11.4 × 107 | 0.02279 | 44 | 0.652 | (Borrmann et al., 2009) |
| 46 | PSB-298 | hA2BAR | 25 | 3.76 × 107 | 0.9533 | 1.05 | 25 | (Bertarelli et al., 2006) |
| 47 | I-AB-MECA | hA3AR | 37 | 6.1 × 107 | 0.042 | 24 | 0.69 | (Gao et al., 2001) |
| 48 | IB-MECA | hA3AR | 10 | 3.5 × 107 | 0.011 | 95 | 0.30 | (Xia et al., 2018) |
| 14 | 2-Cl-IB-MECA | hA3AR | 10 | 2.4 × 107 | 0.0043 | 231 | 0.18 | (Xia et al., 2018) |
| 49 | MRS5127 | hA3AR | 25 | 2.4 × 108 | 0.51 | 2.0 | 2.1 | (Auchampach et al., 2010) |
| 16 | MRS5698 | hA3AR | 10 | 7.8 × 106 | 5.1 × 10−4 | 1961 | 0.068 | (Xia et al., 2018) |
| 31 | MRE3008-F20 | hA3AR | 4 | 7.6 × 107 | 0.042 | 24 | 0.55 | (Varani et al., 2000) |
| 50 | LUF7531 (cmpd 2) | hA3AR | 10 | 1.7 × 108 | 0.0036 | 315 | 0.021 | (Xia et al., 2017) |
| 51 | PSB-11 | hA3AR | 25 | 2.35 × 108 | 0.2082 | 4.80 | 0.46 | (Müller et al., 2002) |
n.d., no data.
a(kinetic) KD = koff/kon.
bwhole cells.
So far, potent and selective full agonists for the A2BAR are not available. BAY 60-6583 (13), a non-nucleoside aminopyridine derivative, behaves as a partial A2BAR agonist (Hinz et al., 2014) but was shown to act as an antagonist at other AR subtypes (Alnouri et al., 2015). In the presence of high adenosine concentrations, it can even inhibit A2BAR activation (Hinz et al., 2014; Alnouri et al., 2015). Data obtained with 13 are therefore difficult to interpret. BAY 60-6583 may induce a different A2BAR conformation than adenosine or NECA; for example, it has been shown that BAY 60-6583 does not induce calcium mobilization via A2BAR-mediated Gq protein activation in human embryonic kidney (HEK) cells with low endogenous A2BAR expression in contrast to adenosine or NECA (Hinz et al., 2014; Gao et al., 2018b). Thus, a potent, selective, efficacious, and unbiased A2BAR agonist is urgently needed. Instead of the partial agonist BAY 60-6583, the full, nonselective agonist NECA (6) may be used in the presence of antagonists for the other AR subtypes.
For the A3AR, potent and selective agonists are available. Cl-IB-MECA (14, CF102, namodenoson), a 2-chloro-N6-iodobenzyl-substituted methylcarboxamidoadenosine (MECA) derivative, is being evaluated in clinical trials for the treatment of hepatocellular carcinoma and nonalcoholic steatohepatitis (NASH). For pharmacological studies, especially in mice, the doses of 14 have to be carefully chosen in order not to activate the A1AR as well (see Table 1). HEMADO (15) is similarly potent and selective in humans. A potent and at the same time selective A3AR agonist, in human as well as in mouse, is MRS5698 (16).
B. Adenosine Receptor Antagonists
Many potent A1AR-selective antagonists have been developed based on caffeine and theophylline as lead structures, such as DPCPX (17, CPX) and PSB-36 (18) (Müller and Jacobson, 2011b). Whereas DPCPX shows only moderate selectivity in humans, PSB-36 is highly selective in all three species: human, rat, and mouse (Alnouri et al., 2015). SLV320 (19) is an A1AR antagonist with a 7-deaza-adenine core structure bearing a cyclohexyl moiety at the exocyclic amino function (Kalk et al., 2007). The compound is potent and selective in humans and displays similar potency in rat, but complete data in rat and mouse are not available.
The xanthine derivative istradefylline (5) was the first A2AAR antagonist to be approved as a drug (Shimada et al., 1992; Takahashi et al., 2018). Its potency and selectivity for the A2AAR is similar in human, rat, and mouse. Although it is highly selective versus the A2B- and A3AR subtypes, selectivity versus the A1AR is somewhat lower (50- to 70-fold) (see Table 2). Like many other A2AAR antagonists, it is moderately water-soluble. In addition, the double bond of its styryl residue can undergo light-induced E/Z-isomerization in dilute solution and is prone to light-induced dimerization in the solid state; therefore, it needs to be protected from light (Nonaka et al.,1993; Hockemeyer et al., 2004). The same is true for MSX-3 (20), a phosphate prodrug of MSX-2, which is, however, well soluble in water (Sauer et al., 2000; Faivre et al., 2018). The A2AAR selectivity of MSX-2 is higher than that of istradefylline (see Table 2). The nonxanthine A2AAR antagonist preladenant (21, SCH-420814) is one of the most potent and selective A2AAR antagonists. It has been evaluated in clinical trials for PD and was found to be well tolerated but did not show significant beneficial effects (Stocchi et al., 2017; LeWitt et al., 2020). As observed with istradefylline, the study design is most critical for these types of clinical PD studies and may have contributed to the negative outcome in the case of preladenant (Hauser et al., 2015). AZD4635 (22, imaradenant) is a potent A2AAR antagonist with moderate selectivity versus the A2B- and A3ARs subtypes. Despite its relatively low molecular weight (315.7 g/mol) the compound is not readily soluble in water.
In recent years many A2BAR-selective antagonists have been developed (Müller et al., 2018). The xanthine derivative MRS1754 (23) is a potent and selective A2BAR antagonist in humans but not in rats and mice, where it additionally blocks the A1AR (Kim et al., 2000; Alnouri et al., 2015). One of the most potent and selective A2BAR antagonists in all three species is the 8-sulfophenylxanthine derivative PSB-603 (24) (Borrmann et al., 2009; Alnouri et al., 2015). The compound is metabolically highly stable in human, rat, and mouse. Its main drawback, however, is its low water solubility. The related A2BAR antagonist PSB-0788 (25) (Borrmann et al., 2009; Alnouri et al., 2015) is better soluble, especially under weakly acidic conditions since it bears a basic nitrogen atom that can be protonated. However, it is less metabolically stable and therefore less suitable for in vivo studies. PSB-0788 is moderately selective for A2B- versus A1ARs in mouse (only about 60-fold) but highly A2BAR-selective in human and rat. PSB-1115 (26) was developed as an A2BAR antagonist with high water solubility due to its sulfonate group (Hayallah et al., 2002). Although the compound is potent and selective in human, it is not selective in rat and mouse and additionally blocks rodent A1ARs (see Table 2) (Alnouri et al., 2015). The xanthine derivative GS6201 (27, CVT6883) which shows good potency and selectivity for human A2BARs (Elzein et al., 2008), was evaluated in a phase 1 clinical trial for pulmonary diseases, but further development has not been reported (Kalla and Zablocki, 2009). The compound displayed a half-life of 4 hours and a peroral bioavailability of 35% in rat (Elzein et al., 2008); potency and selectivity in rodents have not been reported. BAY-545 (28) is a recently published A2BAR antagonist with a new scaffold identified by high-throughput screening, although its thienopyrimidinedione structure resembles the xanthine scaffold (Härter et al., 2019). The compound shows moderate affinity compared with other developed A2BAR antagonists and is more potent at human than at rat and mouse A2BARs. It is more than 10-fold selective in human but is nonselective in mouse and rat (Härter et al., 2019). Another novel scaffold, a pyrimido[1,2-a]benzimidazole, is represented by ISAM-140 (29), an A2BAR antagonist that shows high potency and selectivity in human. Unfortunately, data from other species are not available (El Maatougui et al., 2016). Subsequently, related dihydropyrimidine derivatives have been developed that are similarly potent and selective (Majellaro et al., 2021).
The A3AR typically shows large species differences for antagonists (Müller, 2003; Jacobson and Müller, 2016). Most published antagonists that are very potent at human A3ARs are inactive at the rodent (rat and mouse) orthologs. One of the best A3AR antagonists for rodent studies is MRS1523 (30). The compound is only moderately potent but very selective in human (>100-fold) and at least somewhat selective in rat (18-fold vs. A2A, >100-fold vs. the other AR subtypes) and mouse (at least 14-fold vs. the other subtypes) (van Rhee et al., 1996; Li et al., 1998; Müller and Jacobson, 2011a; Alnouri et al., 2015; Jacobson and Müller, 2016). Further potent A3AR antagonists, including MRE3008-F20 (31) (Baraldi et al., 2012; Borea et al., 2015), PSB-10 (32) (Ozola et al., 2003; Alnouri et al., 2015), and VUF5574 (33) (van Muijlwijk-Koezen et al., 2000) are highly potent and selective in human but virtually inactive at rodent A3ARs (see Table 2). As species differences are more pronounced for A3AR antagonists than for agonists, most of which are derivatives or analogs of adenosine, compounds with a truncated, furanyl, or carbocyclic moiety in place of the ribose ring of adenosine were investigated and optimized (Jeong et al., 2007; Lee et al., 2010; Nayak et al., 2014; An et al., 2020). Such adenosine analogs show reduced intrinsic activity or even block the receptors. Appropriate substitution on the adenine ring led to MRS7591 (34) showing high affinity for both human and mouse A3ARs and good selectivity in human (>1000-fold) (Tosh et al., 2020). Selectivity in mouse has only been assessed against the A1AR (33-fold). It has to be kept in mind that compound 50 behaved as a (weakly efficacious) partial agonist (Tosh et al., 2020).
C. Allosteric Modulators of Adenosine Receptors
The development of allosteric modulators for GPCRs in general is an emerging field of research (Müller et al., 2012; Gao and Jacobson, 2013; Wootten et al., 2013). Positive allosteric modulators (PAMs) increasing the potency or efficacy of agonists, and negative allosteric modulators (NAMs) acting as noncompetitive antagonists, have been reported for various AR subtypes, especially for the A1AR. The AR-PAMs that have been developed so far display only moderate potency or selectivity, and their usefulness is still unclear (Fredholm et al., 2011; Göblyös and IJzerman, 2011; Jacobson et al., 2011; Müller et al., 2012; Nguyen et al., 2016; Barresi et al., 2021). Interestingly, in a recent cryo-EM structure of the A1AR, a PAM (MIPS521) was found to be localized in an extrahelical domain (Draper-Joyce et al., 2021). MIPS521’s analgesic properties were evaluated in the same paper, reminiscent of earlier attempts to profile another PAM as a potential painkiller (Kiesman et al., 2009).
D. Inosine and Guanosine
Adenosine is metabolized to inosine by adenosine deaminases (ADA-1 and -2). Inosine has been reported by several groups to interact with ARs (e.g., with A2AAR and A3AR) but only at very high, nonphysiologic concentrations (>100 μM) (Welihinda et al., 2016). On the other hand, inosine (Lovászi et al., 2021) as well as the nucleoside guanosine (Di Liberto et al., 2016) clearly show pharmacological effects, at least some of which seemed to be exerted by interaction with GPCRs. However, it is unlikely that these effects are mediated by direct activation of ARs. As an example, the hypothermic effects of inosine disappear completely in mice lacking either all four ARs or the A3AR (Xiao et al., 2019). Alternatively, they may be due to inhibition of adenosine uptake through the equilibrative nucleoside transporter 1 (ENT1). Indirect effects are also conceivable (e.g., through allosteric modulation). Further research on inosine and guanosine as extracellular signaling molecules in their own right is warranted.
III. Receptor Binding Kinetics
It has been recognized in recent years that the study of target binding kinetics is crucial to reduce attrition rates in drug discovery (Copeland, 2016). Over the decades medicinal chemists have successfully synthesized lead compounds displaying high, often (sub)nanomolar affinity for a given target, including ARs. However, kinetic aspects of the ligand-receptor interaction have been studied in lesser detail. Although these can be very informative, the extra effort to obtain values for association (kon) and dissociation (koff) rate constants was and is substantial. This is because kinetic assays tend to be laborious although more efficient approaches (Guo et al., 2013) and methods are being developed, including scintillation proximity assays (Xia et al., 2016) and bioluminescence resonance energy transfer (BRET)-based ligand binding studies (Bouzo-Lorenzo et al., 2019; White et al., 2019). On the other hand, systematically evaluating the binding kinetics of a series of lead compounds that are otherwise chemically or biologically similar provides additional parameters for triage and advancement of molecules in the drug discovery process (Guo et al., 2016a; 2017). For instance, assessment of the lifetime of a ligand-receptor complex, coined residence time (RT = 1/koff) (Copeland et al., 2006), has been shown predictive for drug efficacy and selectivity, including on ARs (Swinney, 2006a,b; Guo et al., 2014a; Zhang, 2015; Tonge, 2018). Drugs with long target RT are likely to produce a longer duration of action by more gradually reducing the decline of target occupancy than those with short RT (Dahl and Akerud, 2013; de Witte et al., 2016). Furthermore, a direct correlation between receptor RT and functional efficacy has been observed in some cases (Sykes et al., 2009; Guo et al., 2012). A thorough review of the kinetic characteristics of AR ligands, both orthosteric ligands and allosteric modulators, has recently appeared (Guo et al., 2017); hence, we will only provide a concise summary and update here.
A. Orthosteric Ligands and Adenosine Receptor Binding Kinetics
In Table 3, kinetic data [association and dissociation rate constants, kinetic equilibrium dissociation constants (KD), and residence times] for (orthosteric) agonists and antagonists of the human adenosine receptors (hARs) are summarized. Their chemical structures, if not listed in Fig. 1, are assembled in Fig. 2. Most experiments were radioligand binding assays performed on membrane preparations, whereas lower than physiologic temperatures were employed in most cases due to practical limitations of the (radio)labeled probe used, such as a (too) fast dissociation at higher temperatures. There have been a few attempts to use surface plasmon resonance instrumentation for kinetic assays on hA2AR, but these have not become routinely available since solubilized and purified receptor material is needed (Bocquet et al., 2015; Errey et al., 2015).
It is often thought that association rate constants for bimolecular encounters readily reach high values that are diffusion-limited only (1010 ∼1011 M−1·min−1) (Smoluchowski, 1918; Alberty and Hammes, 1958). However, this is only true for reactant molecules that have isotropic reactivity, whereas the interaction between ligand and receptor, including ARs, is of a more constrained nature (e.g., due to the stereospecificity of recognition). This is obvious from Table 3, in which association rate constants vary from 5.0 × 105 (11, UK432,097) to 6.4 × 108 (41, SCH58261) M−1·min−1, an over 1000-fold difference but still far from diffusion control. The latter compound is another selective A2AAR antagonist that has been extensively characterized in rodents. Association rate constants appear correlated with the onset of clinical action, in vivo target occupancy, and target rebinding (Vauquelin, 2018). However, this has not been demonstrated for AR ligands yet.
The dissociation rate constants or, for convenience, residence times also vary significantly, up to >5000-fold. There are ligands with ultra-short residence times [e.g., only seconds for antagonists LUF6057 (40, A1AR) and SCH58261 (41, A2AAR)], whereas agonists UK432,097 (11, A2AAR), Cl-IB-MECA (14, A3AR), and MRS5698 (16, A3AR) as well as antagonists LUF6632 (43, A2AAR) and LUF7531 (50, A3AR) engage with the receptor for hours. In a recent study, Hothersall and coworkers (2017) identified UK432,097 analogs that displayed even longer target occupancy on hA2AAR. Differences in RT for a number of A2AAR antagonists have been linked to their differential modulation of the salt bridge strength between amino acids Glu169 and His264 in the egress pathway at the extracellular side of the receptor (Guo et al., 2016b; Segala et al., 2016).
B. Orthosteric Ligands Binding Covalently to Adenosine Receptors
Ligands that react covalently with ARs can be regarded as having infinite RT as long as the chemical bond between ligand and receptor “survives.” Over the decades, such ligands have been developed as probes mostly (e.g., to identify the molecular weight of AR molecules, block the physiologic function of ARs or, more recently, help in AR structure elucidation). It remains to be investigated whether such ligands might have relevant therapeutic value.
Thus, both chemoreactive and photoaffinity agonists and antagonists were synthesized in early A1AR studies and evaluated for their binding irreversibility using various assays and degrees of sophistication (Choca et al., 1985; Klotz et al., 1985; Earl et al., 1988; Patel et al., 1988; Stiles and Jacobson, 1988; Jacobson et al., 1989a; Boring et al., 1991; Scammells et al., 1994; Srinivas et al., 1996; Beauglehole et al., 2000; van Muijlwijk-Koezen et al., 2001; Jorg et al., 2016). Of these, FSCPX (52) has been most widely used, and a close derivative of it, DU172 (72) (Beauglehole et al., 2000), appeared crucial for the crystallographic structure elucidation of hA1AR (Glukhova et al., 2017) (Chapter IV). DU172, through its fluorosulfonyl moiety, forms a covalent bond with amino acid Y2717.36 at the extracellular end of the seventh transmembrane domain (TM7) of the receptor.
Likewise, similar efforts have been performed on A2AAR for agonists (Jacobson et al., 1989b; Barrington et al., 1990; Jacobson et al., 1992; Niiya et al., 1993; Luthin et al., 1995; Moss et al., 2014) and antagonists (Ji et al., 1993; Muranaka et al., 2017; Yang et al., 2017). One of the covalent antagonists, LUF7445 (53), was equipped with a click handle to act as a chemical probe (54, LUF7487) for A2AAR (Yang et al., 2018). This chemical biology approach allowed, among others, receptor visualization in hA2AAR-expressing cell membranes.
The A2BAR has not been subjected to covalent labeling yet, whereas the A3AR has been the target for such studies, sampling both irreversibly binding agonist (Ji et al., 1994) and antagonist ligands (Li et al., 1999; Baraldi et al., 2001; Yang et al., 2019).
C. Allosteric Ligands and Adenosine Receptor Binding Kinetics
Ligands binding to an allosteric site distinct from the AR orthosteric binding pocket (see also Chapter II) may influence the binding kinetics of orthosteric ligands. Indeed, on many occasions it has been shown that positive allosteric modulators (PAMs) for the A1AR retard the dissociation rate of orthosteric A1AR agonists, as summarized in a number of reviews (Göblyös and IJzerman, 2011; Kimatrai-Salvador et al., 2013; Guo et al., 2017). For instance, one of the more potent A1AR PAMs, BC-1/compound 8j (55) (Romagnoli et al., 2008), increased the residence time of CCPA up to 200-fold from 0.9 minutes (Table 2) to 172 minutes (Guo et al., 2014b). Unfortunately, the often modest, micromolar potency of PAMs and other allosteric ligands for ARs has so far precluded the assessment of the binding kinetics of these ligands per se.
D. Adenosine Receptor Target Binding Kinetics – Conclusions
Kinetic parameters are an additional factor in assessing the quality and nature of new chemical entities. Nearly all compounds in Table 3 have high affinity, but their kinetics can be very different. A striking example is the pair of LUF6976 (37, KD = 2.2 nM for A1AR, RT = 1.1 minutes) and LUF6941 (38, KD = 2.9 nM for A1AR, RT = 132 minutes), showing identical affinity but a more than 100-fold difference in residence time. Thus, many compounds are considered equivalent on the basis of affinity alone, whereas a further differentiation or even triage may be possible depending on their kinetic characteristics. For instance, A2AAR antagonists are currently in clinical trials as potential adjuvants in cancer immunotherapy (see Chapter VII) to block adenosine’s unwanted anti-inflammatory and immunosuppressive effects (Hatfield and Sitkovsky, 2016). The local adenosine concentration in the tumor may be so high that short-RT antagonists cannot productively compete, whereas a long-RT antagonist may lead to sufficient target engagement even in the presence of elevated adenosine concentrations. Likewise, A2AAR antagonists have been developed for the treatment of PD in combination with levodopa/dopaminergic agonists, although clinical success has been limited so far (Morelli et al., 2009; Hickey and Stacy, 2012). In that setting, a compound with a long receptor RT could have some advantages, as it might yield a reduction in the “wear-off” effect (e.g., of levodopa in between doses) (Hickey and Stacy, 2012). Thus, information obtained from a kinetic perspective may provide additional rationales for the design of new AR ligands. At the same time, one needs to realize that pharmacokinetic aspects are also governing in vivo effects and that an integration of aspects of target binding kinetics and of pharmacokinetics is required (Daryaee and Tonge, 2019).
IV. Receptor Structures
Over the last decade, the elucidation of receptor architecture has been one of the hallmarks in GPCR research (Venkatakrishnan et al., 2013). The A2AAR was one of the first structures solved, through X-ray crystallography (Jaakola et al., 2008), and since then many adenosine receptor structures have been reported (see Table 4 and references therein). Typical characteristics of GPCRs such as their thermolability and fragility have dictated the use of highly engineered proteins and protein constructs for structure elucidation as well as of highly sophisticated technologies (Grisshammer, 2017). At least three approaches have been used widely. First, thermostabilization of GPCRs (Magnani et al., 2016), including the A2AAR, has yielded material sufficient for crystallization by combining amino acid mutations to raise the protein melting temperature. Secondly, fusion of the A2AAR with proteins that crystallize “easily,” such as T4 lysozyme (T4L) (Jaakola et al., 2008) or apocytochrome b562RIL (bRIL) (Liu et al., 2012), has been instrumental to generate crystalline material. Thirdly, complexation of the A2AAR with antibodies raised against epitopes of the receptor provided sufficient stability to render X-ray crystallography feasible (Hino et al., 2012). In recent years, cryogenic electron microscopy (EM), particularly single-particle cryo-EM (Cheng, 2018; Ceska et al., 2019), has been employed to study membrane protein structures as well, including agonist-bound structures of the A1AR (Draper-Joyce et al., 2018; 2021) and A2AAR (Garcia-Nafria et al., 2018) in complex with G protein variants.
TABLE 4.
Reported structures of adenosine receptor subtypes
| PDB | Engineering | Ligand | Resolution (Å) | Technique | Remarks | Reference |
|---|---|---|---|---|---|---|
| A2AAR Antagonist Structures | ||||||
| 3PWH | TS | ZM241385 (42) | 3.3 | X-ray | (Dore et al., 2011) | |
| 3REY | TS | XAC (57) | 3.3 | X-ray | (Dore et al., 2011) | |
| 3RFM | TS | Caffeine (3) | 3.6 | X-ray | (Dore et al., 2011) | |
| 3UZA | TS | T4G (58) | 3.3 | X-ray | T4G: 6-(2,6-dimethylpyridin-4-yl)-5-phenyl-1,2,4-triazin-3-amine | (Congreve et al., 2012) |
| 3UZC | TS | T4E (59) | 3.3 | X-ray | T4E: 4-(3-amino-5-phenyl-1,2,4-triazin-6-yl)-2-chlorophenol | (Congreve et al., 2012) |
| 3EML | FP (T4L) | ZM241385 | 2.6 | X-ray | (Jaakola et al., 2008) | |
| 4EIY | FP (bRIL) | ZM241385 | 1.8 | X-ray | (Liu et al., 2012) | |
| 5UIG | FP (bRIL) | 8D1 (60) | 3.5 | X-ray | 8D1: 5-amino-N-[(2-methoxyphenyl)methyl]-2-(3-methylphenyl)-2H-1,2,3-triazole-4-carboximidamide | (Sun et al., 2017) |
| 5K2A | FP (bRIL) | ZM241385 | 2.5 | X-ray/SFX/XFEL, sulfur SAD phasing | SFX: serial femtosecond crystallography; XFEL: X-ray free-electron laser; SAD: single-wavelength anomalous diffraction | (Batyuk et al., 2016) |
| 5K2B | FP (bRIL) | ZM241385 | 2.5 | X-ray/SFX/XFEL, MR phasing | MR: molecular replacement | (Batyuk et al., 2016) |
| 5K2C | FP (bRIL) | ZM241385 | 1.9 | X-ray/SFX/XFEL, sulfur SAD phasing and phase extension | (Batyuk et al., 2016) | |
| 5K2D | FP (bRIL) | ZM241385 | 1.9 | X-ray/SFX/XFEL, MR phasing | (Batyuk et al., 2016) | |
| 5VRA | FP (bRIL) | ZM241385 | 2.4 | X-ray in situ | in situ: film sandwich plates at room temperature | (Broecker et al., 2018) |
| 5JTB | FP (bRIL) | ZM241385 | 2.8 | X-ray/I-SAD | I-SAD: iodide-single-wavelength anomalous diffraction | (Melnikov et al., 2017) |
| 5UVI | FP (bRIL) | ZM241385 | 3.2 | X-ray millisec | millisec: serial millisecond crystallography using synchrotron radiation | (Martin-Garcia et al., 2017) |
| 6AQF | FP (bRIL) | ZM241385 | 2.5 | X-ray | (Eddy et al., 2018b) | |
| 7RM5 | FP (bRIL) | ZM241385 | 2.8 | Microcrystal electron diffraction | (Martynowycz et al., 2021) | |
| 5NM2 | TS-FP (bRIL) | ZM241385 | 2.0 | X-ray millisec (cryo) | (Weinert et al., 2017) | |
| 5NLX | TS-FP (bRIL) | ZM241385 | 2.1 | X-ray millisec (room temp) | (Weinert et al., 2017) | |
| 5NM4 | TS-FP (bRIL) | ZM241385 | 1.7 | X-ray femtosec (room temp) | Serial femtosecond crystallography using XFEL | (Weinert et al., 2017) |
| 5MZJ | TS-FP (bRIL) | Theophylline (4) | 2.0 | X-ray | (Cheng et al., 2017) | |
| 5MZP | TS-FP (bRIL) | Caffeine (3) | 2.1 | X-ray | (Cheng et al., 2017) | |
| 5N2R | TS-FP (bRIL) | PSB-36 (18) | 2.8 | X-ray | (Cheng et al., 2017) | |
| 5IU4 | TS-FP (bRIL) | ZM241385 | 1.7 | X-ray | (Segala et al., 2016) | |
| 5IU7 | TS-FP (bRIL) | 12c (61, LUF6805) | 1.9 | X-ray | 12c: 2-(furan-2-yl)-N5-(2-(4-phenylpiperidin-1-yl)ethyl)[1,2,4]triazolo[1,5-a][1,3,5]triazine-5,7-diamine | (Segala et al., 2016) |
| 5IU8 | TS-FP (bRIL) | 12f (62, LUF6806) | 2.0 | X-ray | 12f: 2-(furan-2-yl)-N5-(2-(4-methylpiperazin-1-yl)ethyl)[1,2,4]triazolo[1,5-a][1,3,5]triazine-5,7-diamine | (Segala et al., 2016) |
| 5IUA | TS-FP (bRIL) | 12b (63, LUF6732) | 2.2 | X-ray | 12b: 2-(furan-2-yl)-N5-(3-(4-phenylpiperazin-1-yl)propyl)[1,2,4]triazolo[1,5-a][1,3,5]triazine-5,7-diamine | (Segala et al., 2016) |
| 5IUB | TS-FP (bRIL) | 12x (64, LUF6632) | 2.1 | X-ray | 12x: N5-(2-(4-(2,4-difluorophenyl)piperazin-1-yl)ethyl)-2-(furan2-yl)-[1,2,4]triazolo[1,5-a][1,3,5]triazine-5,7-diamine | (Segala et al., 2016) |
| 5OLG | TS-FP (bRIL) | ZM241385 | 1.9 | X-ray, soaking | soaking of ligand to displace theophylline in the crystals | (Rucktooa et al., 2018) |
| 5OLH | TS-FP (bRIL) | Vipadenant (65) | 2.6 | X-ray, soaking for 24 hr | (Rucktooa et al., 2018) | |
| 5OLO | TS-FP (bRIL) | Tozadenant (66) | 3.1 | X-ray, soaking for 24 hr | (Rucktooa et al., 2018) | |
| 5OLV | TS-FP (bRIL) | LUAA47070 (analog) (67/68) | 2.0 | X-ray, soaking for 24 hr | (Rucktooa et al., 2018) | |
| 5OLZ | TS-FP (bRIL) | 4e (69) | 1.9 | X-ray | 4e: 4-(3-amino-5-phenyl-1,2,4-triazin-6-yl)-2-chlorophenol | (Rucktooa et al., 2018) |
| 5OM1 | TS-FP (bRIL) | 4e | 2.1 | X-ray, soaking for 1 hr | (Rucktooa et al., 2018) | |
| 5OM4 | TS-FP (bRIL) | 4e | 2.0 | X-ray, soaking for 24 hr | (Rucktooa et al., 2018) | |
| 6LPJ/K/L | FP (bRIL) | ZM241385 | 1.8–2.0 | Serial femtosecond crystallography using XFEL | EROCOC17 + 4 as crystallization matrix | (Ihara et al., 2020) |
| 6ZDR | TS-FP (bRIL) | Chromone 4d (70) | 1.9 | X-ray | (Jespers et al., 2020) | |
| 6ZDV | TS-FP (bRIL) | Chromone 5d (71) | 2.1 | X-ray | (Jespers et al., 2020) | |
| 6GT3 | TS-FP (bRIL) | AZD4635 (22) | 2.0 | X-ray | (Borodovsky et al., 2020) | |
| 6S0Q | TS-FP (bRIL) | ZM241385 | 2.7 | Native SAD | SAD: single-wavelength anomalous diffraction | (Nass et al., 2020) |
| 3VG9 | antibody-stab | ZM241385 | 2.7 | X-ray | (Hino et al., 2012) | |
| 3VGA | antibody-stab | ZM241385 | 3.1 | X-ray | (Hino et al., 2012) | |
| A2AAR Agonist Structures | ||||||
| 2YDO | TS | Adenosine (1) | 3.0 | X-ray | (Lebon et al., 2011) | |
| 2YDV | TS | NECA (6) | 2.6 | X-ray | (Lebon et al., 2011) | |
| 4UG2 | TS | CGS21680 (10) | 2.6 | X-ray | (Lebon et al., 2015) | |
| 4UHR | TS | CGS21680 | 2.6 | X-ray | (Lebon et al., 2015) | |
| 3QAK | FP (T4L) | UK432097 (11) | 2.7 | X-ray | WT receptor | (Xu et al., 2011) |
| 5WF5 | FP (bRIL) | UK432097 | 2.6 | X-ray | D52N mutant | (White et al., 2018) |
| 5WF6 | FP (bRIL) | UK432097 | 2.9 | X-ray | S91A mutant | (White et al., 2018) |
| 5G53 | truncated and tagged WT | NECA (6) | 3.4 | X-ray | with engineered G protein (mini-Gs) | (Carpenter et al., 2016) |
| 6GDG | FP (thioredoxin) | NECA | 4.1 | cryo-EM | with engineered G protein (mini-Gs-β1γ2); also includes nanobody Nb35 | (Garcia-Nafria et al., 2018) |
| 7ARO | TS-FP (bRIL) | LUF5833 (56) | 3.1 | X-ray | LUF5833 is a partial agonist | (Amelia et al., 2021) |
| A1AR Antagonist Structures | ||||||
| 5N2S | TS | PSB-36 (18) | 3.3 | X-ray | (Cheng et al., 2017) | |
| 5UEN | FP (bRIL) | DU172 (72) | 3.2 | X-ray | (Glukhova et al., 2017) | |
| A1AR Agonist Structures | ||||||
| 6D9H | tagged WT receptor | Adenosine (1) | 3.6 | cryo-EM | with engineered Gi2 protein | (Draper-Joyce et al., 2018) |
| 7LD3/4 | tagged WT receptor | Adenosine (1) +/− MIPS521 (73) | 3.3–3.4 | cryo-EM | with engineered Gi2 protein | (Draper-Joyce et al., 2021) |
A. Resolution
The overall resolution of the AR crystal structures varies between 1.7 Å and 3.6 Å (see Table 4). A high resolution (lower Å values) provides more structural details, particularly the presence or absence of explicit water molecules. It has been shown that a minimum resolution of ∼3.0 Å is required to see any water molecules in a protein crystal structure, whereas on average one water molecule per amino acid residue can be detected at 2.0 Å (Carugo and Bordo, 1999). This means that most adenosine receptor crystal structures lack information on the role that water molecules play in ligand binding. However, >60 explicit water molecules are observed in the 1.8 Å resolution A2AAR-ZM241385 (42) complex (4EIY, Table 4), showing a wide distribution throughout the protein, including the ligand binding site, in which water molecules hydrogen-bond to both ligand and amino acid residues (Liu et al., 2012). In fact, the receptor structure is suggestive of a water-filled pore or channel. The channel has two bottlenecks around Trp2466.48 and Tyr2887.53, slightly less in size than the diameter of one water molecule. These amino acids are part of two general motifs related to GPCR activation, the “rotamer/toggle switch” and the NPxxY sequence, respectively. Interestingly, recent developments show that molecular dynamics and other calculations can make up for the absence of water molecules in a low-resolution protein structure (Matter and Gussregen, 2018). Due to its high resolution, the same 4EIY structure was the first to show the presence of an allosterically binding sodium ion, interacting in a cavity containing a strongly conserved aspartic acid (Asp522.50). As this domain is generic among most class A GPCRs, it is expected that other GPCRs bind sodium ions at this site as well (e.g., as was demonstrated for the human δ-opioid receptor) (Fenalti et al., 2014).
B. Ligand Binding Site
The ARs’ orthosteric binding site (i.e., the binding site for endogenous agonist adenosine) accommodates a range of ligands with diverse scaffolds and different sizes (see Table 4; Fig. 4). In fact, the A2AAR is the GPCR with the most structures available in the Protein Data Bank (PDB) (Vass et al., 2018), allowing an unprecedented view on the conformational flexibility of the ligand binding site.
Fig. 4.
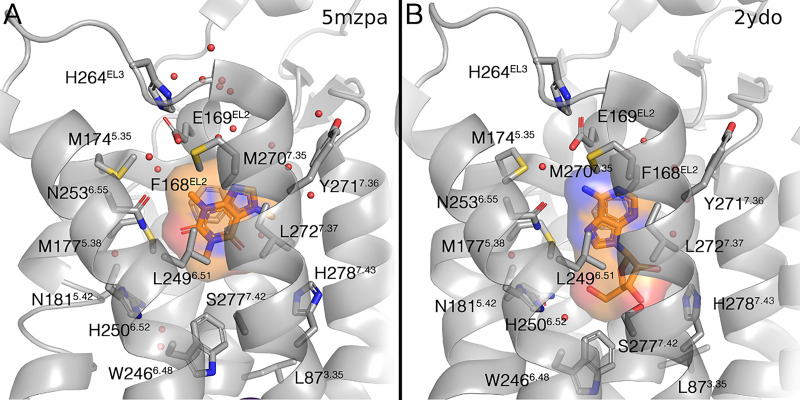
Overview of the A2AAR binding site, showing the first frame of two movies: (A) antagonist (Supplemental Video 1) and (B) agonist (Supplemental Video 2). All residues within 2Å of a given ligand in an A2AAR structure were considered as the binding pocket, and this selection was maintained in all frames. The residues are labeled according to the wild-type (WT) sequence, and modifications made to the receptor (e.g., the thermostabilizing mutant S2777.42A) are not taken into account; note that no labeling is used in the supplemental movie files. The Ballesteros-Weinstein numbering is given in superscript. Ligands are shown in orange, with a volumetric occupancy surface-colored on the atom type. Water atoms in the binding site are shown as red dots, and the sodium ion (when present) as a purple sphere. If alternate coordinates were given in the extracted PDB file, the ‘A’ coordinates were maintained, except in the case of caffeine, in which case we generated two separate frames (referred to as 5mzpa and 5mzpb) to show the two binding modes in the crystal structure. Only distinctly different binding modes of ZM241385 (as present in 4EIY and 3PWH) are included in the movie.
In total >15 different antagonists have been cocrystallized with hA2AAR so far (Table 4), compared with just one structure with ZM241385 (PDB: 3EML) in the previous update. The receptor binding site appears flexible as these antagonists take slightly different positions therein (see Supplemental Video 1). The four agonists cocrystallized with hA2AAR until now (Table 4) all have a ribose moiety, pointing deeper into the ligand binding pocket and displacing explicit water molecules present in the antagonist-occupied receptor structures (see Supplemental Video 2). The agonist-bound structures crystallized in the absence of G protein are now regarded as representing intermediate states in the process of receptor activation. The presence of an engineered G protein makes the cytoplasmic end of TM6 move away considerably from the receptor core by ∼14 Å compared with the other agonist-bound structures, with little impact on the extracellular side of the receptor and the ligand binding pocket (Carpenter et al., 2016; Garcia-Nafria et al., 2018). This is most pronounced for the NECA (6)-bound cryo-EM structure with engineered G protein and nanobody Nb35 (Garcia-Nafria et al., 2018). The thermodynamic contributions of a single, conserved water molecule bridging the 2′-hydroxyl and 3-aza groups of adenosine were analyzed, which led to the design of a modified, potent agonist containing a mimic of this water (Matricon et al., 2020). Recently, the first X-ray structure of A2AAR with a nonriboside, 3,5-dicyanopyridine partial agonist (56, LUF5833) was reported (Amelia et al., 2021).
The structure elucidation of the hA1AR is another achievement. Two crystal structures of antagonist-bound receptor are available (Cheng et al., 2017; Glukhova et al., 2017), whereas one cryo-EM structure with an agonist (adenosine, 1) bound has been reported, the latter in the presence of an engineered Gi protein (Draper-Joyce et al., 2018). The latter structure was later complemented with a positive allosteric modulator, MIPS521 (73), as well, the binding site of which appeared to be extrahelically located involving TM domains 1, 6, and 7 (Draper-Joyce et al., 2021). In the antagonist structures, it was noted that there are differences in the extracellular loop regions, particularly the second one, relative to the hA2AAR structure. The ligand binding cavity is relatively wide, again in comparison with hA2AAR. Differences in pocket shape between the two receptors may determine selectivity more than the (very similar) amino acids lining the pockets. There is a tightening of the orthosteric binding site induced by an ∼4 Å inward movement of the extracellular ends of TMs 1 and 2 in the adenosine-bound, active structure compared with the antagonist-bound, inactive hA1AR. At the intracellular surface, the engineered G protein present causes a 10.5 Å outward movement of TM6 in the hA1AR, quite comparable to the similar large shift in active hA2AAR.
All agonists and antagonists are anchored by two amino acids in particular in both receptors [i.e., Asn2536.55 (numbering as in hA2AAR) and Phe168 in EL2]. A further summary of relevant amino acids for ligand binding, also focusing on selectivity issues between ARs, has been provided recently (Jespers et al., 2018).
C. NMR Studies
Although X-ray crystallography and cryo-EM methods provide important information on AR architecture, NMR spectroscopy has the potential to reveal additional structural dynamics data. Two main approaches have been used so far: 1) indole 15N-1H chemical shifts are monitored after introducing extrinsic (15N-labeled) tryptophan residues at relevant sites, or 2) by incorporating 19F reporter tags onto cysteine residues in the protein, 19F-1H resonances are assessed. In both cases, distinct conformational A2AAR states were observed upon interaction with G protein (Prosser et al., 2017; Huang et al., 2021), cations (Ye et al., 2018), full and partial agonists (Eddy et al., 2018a; 2021; Sušac et al., 2018), and allosteric modulators/sites (Eddy et al., 2018b). The next challenge will be to begin and address other aspects of AR dynamics and functioning, such as the impact of the lipid membrane environment (Guixà-González et al., 2017).
V. Cellular Pharmacology – Biased Signaling of Adenosine Receptors
Each GPCR potentially couples to multiple G proteins, as was demonstrated for the endogenous A2AAR (Cunha et al., 1999) and A2BAR (Gao et al., 2018b), and to non-G protein dependent pathways, such as β-arrestins (Michel and Charlton, 2018; Vecchio et al., 2018). In some cases, the net effect of each of these signaling cascades induced by the same endogenously expressed AR in different cells may be opposite, such as with the A2BAR (Gao et al., 2018b). In theory, the ability of a GPCR agonist to consistently distinguish among multiple intracellular signaling pathways provides advantages when used in a therapeutic mode if the preferred pathway is associated with the beneficial action at the receptor. Such an agonist is termed biased, which implies a nonequivalence in the potency or efficacy across the signaling pathways. In principle, side effects that are associated with the nonpreferred pathways would be avoided. Signaling bias might also affect the kinetics of GPCR trafficking, as internalized receptors can also signal, or gene transcription.
Biased signaling depends on multiple active GPCR conformations, each of which would couple to its own spectrum of second messenger pathways. Thus, biased agonists, also at ARs, achieve signaling selectivity by interacting with or stabilizing a subset of the possible active receptor conformations, and this subset has characteristic pharmacology distinct from other conformations of the same receptor (Verzijl and IJzerman, 2011). Biased agonism has been reported at adenosine A1-, A2B-, and A3ARs for both nucleoside agonists and two classes of non-nucleoside AR agonists, the 3,5-dicyanopyridines and 5-cyanopyrimidines (Langemeijer et al., 2013). Tissue-dependent A2AAR signaling was observed in neurons of different brain areas through engineered optogenetic signaling (Li et al., 2015). Inhibitors of GPCR signaling could be biased as well, for example, as shown for the A1AR using a suramin derivative (Kudlacek et al., 2002). Allosteric GPCR modulators, such as A1AR enhancers (PAMs) in the 2-amino-3-benzoylthiophene family or A3AR PAMs in the imidazoquinolinamine family, can show biased effects on agonist-induced signaling (Gao et al., 2011).
A1AR: In a broad screen of AR agonists, nucleoside agonist LUF5589 (74, 2-chloro-5′-O-ethyl-N6-(3-iodobenzyl)adenosine) tended toward a signaling bias for the Gi protein-dependent pathway in comparison with the β-arrestin pathway (Langemeijer et al., 2013). Biased agonism at the A1AR was also explored by Baltos and coworkers (2016). PAM VCP520 (75) potentiated A1AR agonist-induced Ca2+ mobilization more effectively than extracellular signal-regulated kinase 1/2 activation (Valant et al., 2010). Identification of biased agonism (i.e., cardioprotective efficacy without hemodynamic side effect) associated with an A1AR PAM conjugated to an agonist, VCP746 (76), suggested that this bitopic ligand might be bridging orthosteric and allosteric sites on the receptor (Valant et al., 2014). A recent structure determined for A1AR (Glukhova et al., 2017) shows that it possesses at least one allosteric site, potentially the site that has been exploited to promote biased agonism (Valant et al., 2014).
A2AAR: The biased signaling of A2AAR is rather peculiar among ARs since it seems to be a property of the environment of the A2AAR, probably related to the numerous G protein-interacting proteins that are associated with A2AAR. In fact, at least six G protein-interacting proteins (actinin, calmodulin, NECAB2, translin-associated protein X, ARNO/cytohesin-2, and ubiquitin-specific protease-4) have been reported to interact with the long A2AAR C terminus (Keuerleber et al., 2011). The exploitation of constructs with an altered C-terminal tail revealed a biased A2AAR-mediated signaling with PSB-0777 (12) and LUF5834 (35) (Navarro et al., 2020). Also, inosine has been proposed to activate A2AAR in a biased manner in CHO-K1 cells heterologously expressing hA2AAR (Welihinda et al., 2016). Still, A2AAR agonists with biased properties have been scarcely explored, although they would be of clear interest to potentially optimize immunomodulatory functions without cytotoxic or vascular effects.
A2BAR: Nucleoside agonists distinguish among different G protein-dependent signaling pathways of the A2BAR (Gao et al., 2014). Extracellular signal-regulated kinase 1/2 activation may result from β-arrestin mobilization or from Gq- or Gs-protein coupling. In fact, entirely different signaling pathways are activated depending on whether the receptor is endogenously occurring or introduced by transfection (Gao et al., 2018b). A2BAR activation in muscle and brown fat had a beneficial effect on energy expenditure and increased muscle mass, suggesting the application of selective A2BAR agonists that principally activate cAMP for treating obesity (Gnad et al., 2020). A2BAR activation also reduces cardiac fibrosis via the PKCδ to p38-MAPK pathway and protects the ischemic heart by stabilizing HIF-1α (Campos-Martins et al., 2021). Thus, translational opportunities are conceivable if selective and biased A2BAR agonists could be developed for these signaling pathways.
A3AR: Storme and coworkers (2018), using an engineered arrestin-reporter cell line, compared the Gi-dependent and β-arrestin2-dependent signaling of 19 nucleoside agonists at the A3AR to show a tendency toward weak bias for the G protein pathway in a few analogs. Similar conclusions were reported in an earlier study comparing known A3AR agonists (Gao and Jacobson, 2008), which noted differences in the kinetics of receptor activation.
VI. Pharmacology – Novel Developments
This chapter addresses select aspects of AR pharmacology. In this update, we focus on the therapeutic targeting of ARs and elaborate on their relevance in disease states. In the previous update, dimerization/oligomerization of ARs was particularly emphasized (Fredholm et al., 2011). This potentially critical variable to selectively modulate AR activity has been detailed in a number of recent reviews (Vecchio et al., 2018; Ferré and Ciruela, 2019; Franco et al., 2021a).
A. Therapeutic Targeting of Adenosine Receptors
Adenosine receptors have been targeted in the treatment of a number of (peripheral and CNS) diseases including PD, cardiac arrhythmias, asthma, and infant apnea (Kreutzer and Bassler, 2014). Adenosine receptors are also targeted for diagnostic studies of coronary circulation in individuals unable to manage a treadmill. Over the years, targeting adenosine receptors has been tested in animal models of diabetes, inflammatory diseases, wound healing, sickle cell disease, congestive heart failure, Alzheimer’s disease, depression, and grand mal epilepsies, as well as in human trials. Other potential disease targets for agents targeted to adenosine receptors have recently been identified. Below we identify some of the most promising applications for adenosine receptor agents described over the past 10 years (Borea et al., 2018). It should be kept in mind, however, that a knowledge gap exists between advanced animal studies, which are many, and the limited number of reports on native human cells and tissues. From a translational perspective toward successful clinical studies, it seems essential to close this gap.
B. Therapeutic Targeting of Peripheral Adenosine Receptors
1. Adenosine A1 Receptors and Congestive Heart Failure
Adenosine, generated within the kidney and acting at A1AR, induces vasoconstriction of afferent arterioles reducing renal blood flow and glomerular filtration rate (GFR), further stimulating renin release. Moreover, activation of A1AR increases proximal tubular reabsorption of sodium ions (Vallon et al., 2006). In congestive heart failure A1AR activation was postulated to play a role in the reduced GFR and sodium retention that characterize congestive heart failure, and it was suggested that blockade of A1AR could alleviate the symptoms of congestive heart failure by increasing GFR and promoting sodium elimination (Vallon et al., 2008). When tested in the clinic, however, short courses of rolofylline, a selective A1AR antagonist, provided no benefit in the treatment of congestive heart failure, and a number of patients suffered seizures, a known potential adverse effect of A1AR antagonists (Massie et al., 2010). Subsequently, it was noted that A1AR stimulation could enhance cardiac myocyte function by improving mitochondrial function and the function of the Ca2+-ATPase (SERCA2a), and the use of a partial agonist could potentially avoid the cardiac dysrhythmias induced by A1AR (full) agonists (Voors et al., 2018). Unfortunately, the partial A1AR agonist neladenoson (BAY1067197) did not improve exercise tolerance (see also Chapter VII) in patients with heart failure (Shah et al., 2019).
2. Adenosine A2A Receptors and Cancer
Severe impairment of the cellular immune system was first associated with deficiency of adenosine deaminase in 1972 (Giblett et al., 1972). Whereas adenosine deaminase deficiency is toxic to T cells, in many subsequent studies the immunosuppressive effects of adenosine at concentrations that are not toxic to T cells have been further confirmed. Moreover, the A2AAR has been implicated as the mediator by which adaptive immunity is suppressed (Huang et al., 1997), the T cell subtypes affected have been identified, and the intracellular signaling mechanisms have been investigated (Cronstein and Sitkovsky, 2017). The impact of A2AAR in cancer development is best heralded by the pioneering report in which melanoma and lymphoma cell lines were completely rejected in A2AAR knockout mice (Ohta et al., 2006) through a mechanism involving the control of the antitumor effects of CD8 T cells. Although it had previously been established that high concentrations of adenosine were present in the extracellular fluid of solid tumors (Blay et al., 1997), the significance of that finding was not fully appreciated until the report by Ohta and colleagues. Moreover, a number of more recent studies suggest that A2AAR antagonists interact with anti-PD1 and anti-CTLA4 therapy to further enhance tumor immunity and promote tumor regression (Iannone et al., 2014; Beavis et al., 2015; Gessi et al., 2017). Indeed, A2AAR antagonists bolster cytokine release by CAR-T cells increasing their antitumor efficiency (Beavis et al., 2017). Currently, a number of A2AAR, A2BAR, and dual antagonists are at various stages of clinical development (see Chapter VII) (Yu et al., 2020). In addition, other therapeutic approaches targeting adenosine production from adenine nucleotides by ecto-5′nucleotidase (CD73) are making their way to the clinic as well (Congreve et al., 2018).
3. Adenosine Receptors and Autoimmune and Inflammatory Diseases
The potential anti-inflammatory effects of adenosine, acting at A2AAR, have been known since 1983 (Cronstein et al., 1983). Subsequently adenosine, acting at both A2AAR and A3AR, was shown to mediate many of the anti-inflammatory and immunosuppressive effects of low-dose methotrexate therapy, the gold standard in the therapy of rheumatoid arthritis and psoriasis (Cronstein and Sitkovsky, 2017). Administration of A2AAR agonists, although potentially useful for treatment of inflammatory diseases, would likely have too many side effects to be tolerated, mainly due to their strong hypotensive action, so other approaches have been taken. Thus, one approach has been to develop a prodrug of an A2AAR agonist that is liberated by the action of ecto-5′-nucleotidase (CD73). Such an agent was shown to suppress inflammatory arthritis in animal models (Flögel et al., 2012) and suggests a promising approach to development of new anti-inflammatory agents.
In contrast, A3AR agonists do not appear to have the same potential for systemic toxicity, as receptor expression is not as widespread as for the A2AAR. Thus, relatively selective A3AR agonists have been tested in both animal models and the clinic for their anti-inflammatory effects. Potential clinical utility with minimal toxicity has been reported for A3AR agonists in the treatment of rheumatoid arthritis, psoriasis, and liver conditions, and thus agents remain in development for the treatment of these autoimmune disorders (reviewed in Jacobson et al., 2018).
4. Adenosine Receptors and Infectious Diseases
The anti-inflammatory and immunosuppressive effects of adenosine, acting at A2AAR, have not gone unnoticed by microorganisms. Thus, adenosine has been identified as a virulence factor in Candida albicans (Smail et al., 1992; Rodrigues et al., 2016), Staphylococcus aureus, (Thammavongsa et al., 2009), and Streptococcus suis (Liu et al., 2014) that mitigates the effects of the host immune and inflammatory response on these microorganisms. Leishmania amazonensis also exploits the adenosine system to elude detection by dendritic cells, in this case through A2BAR (Figueiredo et al., 2021). To date, A2AAR or A2BAR have not been targeted as a means to enhance host responses to microorganisms for the treatment of infectious diseases for resistant organisms.
In contrast, it is increasingly clear that much of the injury associated with infections comes as a result of the active host response to the infection with tissue damage in affected tissues, much like the tissue injury triggered by inflammatory and autoimmune diseases. First postulated as a potential therapy for COVID-19 pneumonia (Abouelkhair, 2020; Falcone et al., 2020), Correale and colleagues (2020) reported on the beneficial effects of administration of aerosolized adenosine in patients with COVID-19 pneumonia. They treated 14 patients with COVID-19 interstitial pneumonitis with aerosolized adenosine and observed improved oxygenation in 13 of 14 patients (compared with 7 of 52 control patients) and improved imaging studies, although the RNA load of SARS-CoV-2 increased in 13 of 14 patients. There was one death in the adenosine-treated patients compared with 11 of 52 patients in the historic control group. Bronchospasm was observed in one of the treated patients. The authors concluded that aerosolized adenosine might be a useful adjunct to other therapies for the treatment of SARS-CoV-2 pneumonia and might be similarly effective in other types of viral pneumonia. Although it is likely that the actions of adenosine in viral pneumonitis are mediated by the actions of an adenosine receptor, it is unclear which receptor(s) that might be, although the actions of A2AAR, A2BAR, and A3AR could account for the anti-inflammatory effects observed, as noted above.
5. Adenosine A2A Receptors and Retinal Disease
The retinopathy of prematurity is the most common cause of childhood blindness. A2AAR stimulation in the retina promotes retinal vascular overgrowth, and results of recent studies indicate that A2AARs play a significant role in the development of oxygen toxicity-induced retinal angiogenesis (Taomoto et al., 2000; Liu et al., 2010; 2017). Caffeine, which is commonly used to treat apnea in neonates, was recently shown to prevent oxygen toxicity-induced retinal angiogenesis in animal models and has been suggested as a therapeutic approach to prevent retinopathy of prematurity (Zhang et al., 2017), an effect mimicked by the selective antagonism of A2AARs (Zhou et al., 2018). The antagonism of A2AARs also emerges as a novel promising strategy to dampen the local inflammatory processes involved in the degeneration of ganglion neurons in ischemic eye diseases and glaucoma that are a prevalent cause of blindness in the elderly (Liu et al., 2016; Madeira et al., 2016; Boia et al., 2017). A1AR agonists, which prevent neuronal damage from pressure and ischemia in animal models, have been tested in the treatment of glaucoma but failed in phase 3 trials to reduce intraocular pressure better than placebo (ClinicalTrials.gov Identifier: NCT02565173).
6. Adenosine Receptors and Bone
Adenosine A1-, A2A-, and A2B-ARs play a role in regulating bone biology by modulating osteoclast differentiation and bone remodeling as well as osteoblast differentiation and production of new bone (Strazzulla and Cronstein, 2016). A1AR stimulation is required for osteoclast differentiation, and A1AR knockout mice have mild osteopetrosis (Kara et al., 2010a,b). In contrast, A2AAR and A2BAR stimulation diminish osteoclast differentiation and stimulate new bone formation by osteoblasts (Mediero et al., 2012b; 2013; 2015b; Corciulo et al., 2016). More importantly, an A1AR antagonist, an A2AAR agonist, or dipyridamole, which blocks adenosine uptake via the equilibrative nucleoside transport protein ENT1 (SLC29A1) and thereby increases extracellular adenosine levels, stimulate bone regeneration in critical bone defects, whether applied topically or as a coating for 3D-printed β-tricalcium phosphate scaffolds (Mediero et al., 2015b; Ishack et al., 2017). Currently, dipyridamole-coated scaffolds are undergoing preclinical testing for restoration of bone.
Despite the remarkable success of joint replacement therapy, approximately 25% of implanted hip and knee prostheses will require revision due to erosion of the bone surrounding the prosthesis (Bozic et al., 2010). Application of A2AAR agonists markedly 1) diminishes the inflammation due to prosthesis wear particles, the most common cause of bone destruction leading to prosthetic joint replacement, and 2) by inhibiting osteoclast differentiation, diminishes wear particle-induced bone destruction in a murine model (Mediero et al., 2012a). Moreover, weekly low doses of methotrexate, a commonly used anti-inflammatory drug that inhibits inflammation by increasing local adenosine concentrations, similarly alleviates wear particle-induced bone destruction in mice (Mediero et al., 2015a) by an A2AAR-dependent mechanism.
7. Adenosine Receptors and Cartilage
In recent studies in both mice (Corciulo et al., 2017) and humans (St Hilaire et al., 2011), premature development of osteoarthritis has been described, and in mice, loss of A2AARs leads to spontaneous development of osteoarthritis (Corciulo et al., 2017), indicating that endogenous adenosine production acts in an autocrine fashion to maintain chondrocyte homeostasis. Moreover, treatment of rats with post-traumatic osteoarthritis with intra-articular injections of liposomal adenosine preparations prevents progression of osteoarthritis (Corciulo et al., 2017). Similarly, loss of A3ARs leads to the development of osteoarthritis in mice (Shkhyan et al., 2018), and treatment of chemically induced osteoarthritis with an A3AR agonist inhibits development of osteoarthritis (Bar-Yehuda et al., 2009). These events suggest that targeting A2ARs or A3ARs in the joint may be useful approaches to the treatment of osteoarthritis, a disabling condition affecting as many as 150 million people worldwide.
8. Adenosine Receptors and Fibrosis
Fibrosis is a common condition in a number of organs, and recent studies indicate that blockade of A2AARs can diminish excessive fibrosis in the skin, liver, and other organs in response to injury, ionizing radiation, or exposure to toxins (Shaikh and Cronstein, 2016). Indeed, in recent studies, topical application of an A2AAR antagonist prevents both scarring and radiation fibrosis in the skin (Perez-Aso et al., 2012; 2016). In some organs, A2BAR blockade can also diminish fibrosis (Shaikh and Cronstein, 2016), but recent studies suggest that in Peyronie’s disease, which involves fibrosis of the shaft of the penis, A2BAR stimulation prevents myofibroblast production of collagen (Mateus et al., 2018), suggesting that an A2BAR agonist could prevent the development of Peyronie’s disease.
9. Adenosine A2A Receptors and Sickle Cell Disease
Patients with sickle cell disease suffer from focal areas of vascular obstruction leading to localized regions of poor perfusion and resulting ischemia. In these hypoxic foci, invariant natural killer T cells can induce further tissue injury, and A2AAR stimulation inhibits invariant natural killer T cell function and tissue injury. Studies in humanized mice with sickle cell disease demonstrated that infusion of an A2AAR agonist, regadenoson, reduced the tissue injury associated with sickle cell disease (Nathan et al., 2012). Although preclinical studies showed promise in these patients, the results of a clinical trial of regadenoson infusions for sickle cell disease did not show any evidence of shortened hospital stay or reduction in respiratory symptoms or opioid use (ClinicalTrials.gov Identifier: NCT01788631).
10. Summary
ARs are expressed ubiquitously in the periphery and play a variety of roles. ARs remain targets for clinical development despite recent failures in treatment of congestive heart failure and sickle cell disease. Immunostimulatory blockade of A2AAR for the treatment of cancer shows real promise in early clinical trials, and development of other adenosine receptor targets is moving out of the laboratory into the clinic.
C. Therapeutic Targeting of Central Nervous System Adenosine Receptors
Although ARs are present throughout the human body, their density is far greater in the brain. Accordingly, manipulating ARs upon moderate intake of caffeine (Fredholm et al., 1999) mainly results in brain-associated effects, typified by increased arousal and attention with faster reaction time, decreased fatigue, more efficient working memory and memory recall, and better mood (Smith et al., 2005; McLellan et al., 2016). These effects of caffeine are mostly mediated by brain ARs, namely A1ARs and A2AARs (Fredholm et al., 2005), as heralded by the elimination of the effects of caffeine on synaptic transmission and plasticity upon blockade of A1AR and A2AAR (Lopes et al., 2019). Although also present in glia cells, A1AR and A2AAR are mostly colocated in excitatory synapses where they cooperate to encode information salience in neuronal circuits through a combined A1AR-mediated inhibition of synaptic transmission (decreasing noise) and an A2AAR-mediated facilitation of synaptic plasticity (increasing encoding) (Cunha, 2016).
1. Acute Brain Dysfunction – Ischemia and Epilepsy
Apart from their physiologic role, ARs also have an impact on brain dysfunction and damage, in accordance with the universal utilization of ATP (Rodrigues et al., 2015) and adenosine (Cunha, 2001) to signal stress or increased cellular workload in the brain. Thus, in conditions of metabolic stress such as upon ischemic stroke, both the acute A1AR activation and A2AAR blockade afford a robust neuroprotection but through different mechanisms. A1AR activation increases the hurdle for onset of brain dysfunction by hyperpolarizing neurons. In contrast, A2AAR blockade restrains neurodegeneration, probably as a result of the combined inhibition of glutamate release and decreased activation of N-methyl-D-aspartate (NMDA) receptors (Cunha, 2016), together with an attenuation of neuroinflammation (Rebola et al., 2011) and decreased neuronal apoptosis (Silva et al., 2007). A similar dual and opposite control by A1ARs and A2AARs occurs upon abnormal increased workload typified by epileptic conditions (Tescarollo et al., 2020). Acute A1AR activation attenuates the onset of seizures and, conversely, acute A1AR inhibition decreases seizures threshold, whereas A2AARs control seizure-induced neurodegeneration (Canas et al., 2018). This dual control of the onset and evolution of brain damage by A1ARs and A2AARs prompts the suggestion that a combined activation of A1ARs and blockade of A2AARs might have a superior efficacy to limit acute brain damage (Cunha, 2005). However, timing of intervention might be of key importance since A1ARs desensitize and their function decreases in the injured brain, which may result in paradoxical effects (Jacobson et al., 1996). In contrast, central A2AARs are upregulated in noxious brain condition (Cunha, 2016), justifying the interest in A2AAR antagonists to control brain damage.
2. Neurodegenerative Diseases – Parkinson’s and Motor Diseases
The particularly high density of A2AARs in the basal ganglia and their tight antagonistic interaction with dopamine D2 receptors typified by the formation of A2AAR-D2 receptor heteromers (Ferré and Ciruela, 2019) prompted targeting A2AARs to alleviate dopaminergic depletion characteristic of PD. Indeed, A2AAR antagonists dampen PD features in animal models, and the regular consumption of moderate doses of caffeine attenuates PD features in humans (Schwarzschild et al., 2006). As mentioned above, this preclinical evidence, together with the safety profile of A2AAR antagonists, supported the US Food and Drug Administration’s recent approval of istradefylline as an add-on therapy to manage PD patients (Chen and Cunha, 2020). This offers novel possibilities of carrying out phase 4 trials to directly test the role of A2AARs in the control of nonmotor PD symptoms, such as cognitive deficits and mood dysfunction. It should be mentioned that the clinical trajectory of istradefylline has been long and windy. After its introduction in Japan in 2013, market access to the United States has only recently (in 2019) been granted after an earlier rejection and is limited to treating “off” episodes with levodopa only. Other A2AAR antagonists such as preladenant have failed to obtain market authorization from the Food and Drug Administration, as clinical efficacy was not convincingly demonstrated. This might be due to our insufficient knowledge of the role that different A2AR populations have in the control of altered motor function and to lack of patient stratification in the clinical studies.
Selective A2AAR antagonists also attenuate other motor conditions, such as catalepsy and tremor (Salamone et al., 2008), akathisia (Varty et al., 2008), dystonia (Maltese et al., 2017), cocaine or MK801-induced psychomotor activity (Shen et al., 2008; Yu et al., 2008), cerebrospinal type 3 ataxia or Machado-Joseph’s disease (Gonçalves et al., 2013; Gonçalves et al., 2017), or amyotrophic lateral sclerosis (Ng et al., 2015). Their impact on Huntington’s disease is less clear and might depend on the phase of the disease (Popoli et al., 2008). This broader ability of A2AAR antagonists to control different motor disorders that might not directly result from dopaminergic depletion prompts the involvement of a control of glutamate excitotoxicity rather than only the control of dopamine D2 receptors (Schiffmann et al., 2007; Cunha, 2016).
3. Neurodegenerative Diseases – Alzheimer’s Disease and Cognitive Dysfunction
The pharmacological or the genetic blockade of A2AARs prevents memory deficits in different animal models of Alzheimer’s disease (Canas et al., 2009; Laurent et al., 2016; Viana da Silva et al., 2016). A2AAR antagonism also prevents memory dysfunction associated with other conditions, such as convulsions (Cognato et al., 2010), diabetes (Duarte et al., 2012), hypoxia (Chen et al., 2018), traumatic brain injury (Zhao et al., 2017), demyelination conditions (Akbari et al., 2018), repeated stress or depression (Batalha et al., 2013; Kaster et al., 2015; Machado et al., 2017), PD (Hu et al., 2016; Carmo et al., 2019), or cannabis exposure (Mouro et al., 2019). A2AARs are not only necessary but actually sufficient to impair memory since their increased activity driven by genetic (Temido-Ferreira et al., 2020), optogenetic (Li et al., 2015), or pharmacological strategies (Pagnussat et al., 2015) impairs memory in normal animals. This converges with several findings in humans, namely: 1) Caffeine intake prevents cognitive deterioration upon aging (Ritchie et al., 2007; Dong et al., 2020) and is inversely associated with the onset (Eskelinen et al., 2009; Sugiyama et al., 2016) or neuropathological hallmarks of dementia (Gelber et al., 2011); 2) A2AARs are upregulated in the brains of demented patients (Temido-Ferreira et al., 2020); and 3) A2AAR polymorphisms are associated with memory phenotypes (Beste et al., 2012; Horgusluoglu-Moloch et al., 2017). However, it is still unknown if A2AAR antagonists ameliorate memory deficits in dementia patients.
4. Neuropsychiatric Diseases – Major Depression and Suicide
Caffeine, A2AAR antagonists, and the genetic deletion of A2AARs selectively in forebrain neurons abrogate the onset of depressive-like symptoms and can also reverse these symptoms in mice subject to chronic unpredictable stress (Kaster et al., 2015). Accordingly, coffee intake is inversely correlated with the incidence of depression (Grosso et al., 2016; Lucas et al., 2011) and its major consequence suicide (Lucas et al., 2014), and the incidence of major depression is associated with A2AAR haplotypes (Oliveira et al., 2019). In parallel, the upregulation of A1ARs bolsters the resilience toward depressive-like behavior and, conversely, knocking out A1ARs increased depressive-like behavior and eliminated the antidepressant effects of sleep deprivation (Serchov et al., 2015). Furthermore, A1ARs in the amygdala are also involved in neuroimmune-driven depression (Fan et al., 2019).
5. Other Neuropsychiatric Diseases
The elegant work of Chen and colleagues revealed a temporal ability of A2AARs to modulate instrumental behavior, formatting the sensitivity to goal-directed valuation (Li et al., 2016; 2018). Thus, A2AAR-mediated overactivation of striatopallidal neurons disrupts the homeostatic control of goal-directed behavior, with impaired decision-making and behavioral disinhibition with loss of flexibility, which are at the core of psychiatric symptoms (Li et al., 2016; 2020; He et al., 2020). Indeed, A2AARs control addiction (Ferré, 2016; Borroto-Escuela et al., 2018) and preservative and obsessive-compulsive behaviors (Bleickardt et al., 2014; Asaoka et al., 2019) that are transversal to most neuropsychiatric diseases. Accordingly, A2AAR polymorphisms are associated with anxiety (Alsene et al., 2003; Fraporti et al., 2019), depression (Oliveira et al., 2019), phobia (Deckert et al., 1998; Hamilton et al., 2004), preservative/obsessive disorders (Freitag et al., 2010; Janik et al., 2015), or addictive profiles (Kobayashi et al., 2010). This A2AAR-mediated control of behavioral inhibition may be associated with their ability to modulate arousal (Lazarus et al., 2012) and enhanced motivation (He et al., 2020), which are founding behaviors of decision-making and cognitive performance.
6. Brain Aging
Aging is by far the major risk factor for most prevalent chronic brain diseases, namely depressive, cerebrovascular, and neurodegenerative diseases. The adenosine modulation system in the forebrain is modified upon aging with a decreased density and functional efficiency of A1ARs (Sperlagh et al., 1997; Sebastião et al., 2000; Costenla et al., 2011) and an increased density and efficiency of A2AARs (Rebola et al., 2003; Canas et al., 2009; Costenla et al., 2011). These alterations posit a contribution of the adenosine modulation system to the deterioration of brain function since hyperactivity of A2AARs is sufficient to trigger brain dysfunction (Li et al., 2015; Carvalho et al., 2019; Temido-Ferreira et al., 2020) and a hypofunction of A1ARs increases excitability (noise) of brain networks and bolsters the spreading of excitotoxicity (Tescarollo et al., 2020). Indeed, the intake of caffeinated coffee is inversely associated with memory deterioration upon aging (Hameleers et al., 2000; Ritchie et al., 2007; van Gelder et al., 2007; Arab et al, 2011; Dong et al., 2020), which was shown in animal models to be reverted by caffeine and by selective A2AAR antagonists (Prediger et al., 2005). This stresses the particular association between increased A2AAR activity with the deterioration of brain function upon aging, which might underlie the increased susceptibility for the emergence of age-associated brain diseases.
Hyperactivity of A2AARs in the aged brain is further reinforced by the parallel increase of the pathway responsible for the formation of the pool of adenosine selectively associated with the activation of A2AARs, namely ecto-5′-nucleotidase (CD73)-mediated formation of ATP-derived extracellular adenosine (Augusto et al., 2013; Carmo et al., 2019; Gonçalves et al., 2019). In fact, different studies reported a robust increase of the activity of CD73 in the aged brain (Fuchs, 1991; Cunha et al., 2001; Mackiewicz et al., 2006), as best heralded by the inverse association of CD73 activity with the probability of reaching centenarian ages (Crooke et al., 2017). In parallel, the activity of AdoK is decreased in the aged brain (Mackiewicz et al., 2006). This bolsters the availability of the extracellular adenosine (Cunha et al., 2001; Murillo-Rodriguez et al., 2004), possibly to compensate the decreased density of A1ARs. Indeed, the alteration of purinergic metabolism seems to be a prominent characteristic—a metabolic fingerprint of the aging process in different organisms (Furman et al., 2017; Gao et al., 2018a). Precocious modifications of adenosine metabolism occur in the brain of aged rodents (Ivanisevic et al., 2016) and in an animal model of accelerated aging (Sanchez-Melgar et al., 2020).
VII. Current and Recent Clinical Trials
Both AR agonists and antagonists have been in clinical trials dating back to the late 1960s for a wide range of conditions (Borah et al., 2019; Jacobson et al., 2019). Although most of these clinical trials were unsuccessful, the therapeutic focus of AR-based therapeutics has shifted since the early days, and new trials are underway for more recently identified indications (Table 5; Fig. 5). There is reason for optimism that this situation can be remedied in future clinical trials based on current pharmaceutical technology. Firstly, the availability of high-resolution experimental structures of two of the adenosine receptors allows the discovery and optimization of compounds of extremely high selectivity for each of the four receptors. Furthermore, the lack of efficacy in clinical trials often results from inadequate pharmacokinetics, which has been greatly improved in the recent generation of adenosine receptor ligands, which are also much more structurally diverse than in the past. Additionally, the expanded range of allosteric modulators of the adenosine receptors and indirect adenosine modulators (e.g., enzyme and transport inhibitors) promises to provide clinical candidate molecules that are more temporally and spatially selective than orthosteric agonists. More specifically, further research may lead to new therapeutics, including: A2A- and A2BAR antagonists for treating cancer and neurodegenerative conditions such as Parkinson’s disease and Alzheimer’s disease; A2B- and A3AR agonists for treating chronic conditions such as obesity and NASH, respectively; A1- and A3AR agonists for treating chronic pain; A3AR antagonists for treating glaucoma; and A2A- and A2BAR agonists for treating musculoskeletal conditions such as osteoporosis. These are potential therapeutic approaches that have matured scientifically in recent years.
TABLE 5.
Representative adenosine receptor modulators in clinical trials, currently and previously, according to clinicaltrials.gov
| Compound | Action | Activity | Phase, National Clinical Trial Number |
|---|---|---|---|
| Agonists | |||
| Adenosine (1) | Nonselective agonist | headache/migraine ADCY5-related dyskinesia |
-, 04577443 -, 04469283 |
| Neladenoson bialanate (77, BAY1067197) |
A1AR agonist | heart failure | 2, 02040233, PARSiFAL 2, 02992288, PANTHEON 2, 03098979, PANACHE |
| Selodenoson (78, DTI-0009, RG14202) | A1AR agonist | atrial fibrillation | 2, 00040001 |
| Trabodenoson (79, INO-8875; PJ-875) | A1AR agonist | glaucoma | 3, 02565173 |
| Regadenoson (2, CVT 3146) | A2AAR agonist | sickle cell anemia COVID-19 pulmonary hypertension glioma (disrupting BBB) |
2, 01788631 1/2, 0460609 -, 02220634 1, 03971734 |
| Tecadenoson (80, CVT-510) | A2AAR agonist | atrial fibrillation | 2, 00713401 |
| Spongosine (81, BVT.115959) | A2AAR agonist | diabetic nerve pain | 2, 00452777 |
| UK-432,097 (11) | A2AAR agonist | chronic obstructive pulmonary disease | 2, 00430300 |
| Piclodenoson (48, IB-MECA, CF-101) | A3AR agonist | rheumatoid arthritis psoriasis COVID-19 |
3, 02647762 3, 03168256 2, 04333472 |
| Namodenoson (14, Cl-IB-MECA, CF-102) |
A3AR agonist | hepatocellular carcinoma NASH (non-alcoholic steatohepatitis) |
2, 02128958 2, 02927314 |
| Antagonists | |||
| Caffeine (3) | Nonselective antagonist | hypoxic-ischemic encephalopathy ADCY5-related dyskinesia Alzheimer’s disease radiation-induced fibrosis glaucoma |
1, 03913221 -, 04469283 3, 04570085 2, 03768492 -, 03675412 |
| Theophylline (4) |
Nonselective antagonist | acute kidney injury smell in COVID-19 depression anaesthesia recovery |
3, 03897335 2, 04789499 1, 04309877 1, 04151381 |
| Rolofylline (82, KW-3902) | A1AR antagonist | congestive heart failure | 3, 00328692 (PROTECT-1) 3, 00354458 (PROTECT-2) |
| SLV320 (19) | A1AR antagonist | heart failure and renal dysfunction combined with furosemide |
2, 00744341 2, 00160134 -, 00568009a |
| PBF-680 | A1AR antagonist | asthma | 2, 03774290, ADENOASMA |
| Istradefylline (5, KW-6002) | A2AAR antagonist | Parkinson’s disease (alone) | 2, 00250393 |
| Parkinson’s disease (with l-dopa) | 3, 00955526, 6002-009 3, 01968031 |
||
| Preladenant (21, MK-3814, SCH 420814) |
A2AAR antagonist | Parkinson’s disease | 3, 01155479, PARADYSE |
| antipsychotic drug side effects | 2, 00686699, P04628 | ||
| advanced solid tumors (alone and in combination with pembrolizumab) | 1, 0309916 | ||
| BIIB014 (65) | A2AAR antagonist | Parkinson’s disease | 2, NCT00438607 |
| Tozadenant (66) | A2AAR antagonist | Parkinson’s disease | 3, NCT03051607 |
| Taminadenant (83, NIR178, PBF-509) | A2AAR antagonist | Parkinson’s disease, non-small cell lung cancer (with PDR001b) | 1, 02111330 1/2, 02403193, AdenONCO |
| various cancersc (with PDR001) | 3, 03207867 | ||
| Ciforadenant (84, CPI-444, V81444) | A2AAR antagonist | advanced cancers (in combination with CD73 blocker, CPI-0006) | 1, 03454451 |
| (in combination with PD-L1/PD-1b) | 1/2, 03337698 | ||
| Imaradenant (22, AZD4635, HTL1071) | A2AAR antagonist | cancer, alone | 1, 03980821 |
| (in combination with CD73 blocker, MEDI9447d) | 1/2, 03381274 | ||
| (in combination with anticancer drugs) | 1, 02740985 2, 04089553 |
||
| Inupadenant (85, EOS100850) | A2AAR antagonist | solid tumors | 1, 03873883 |
| Etrumadenant (86, AB928) | A2A/A2B antagonist | various cancers (with AB122b) (in combination with anticancer drugs) |
1, 03629756 1/2, 04381832 |
| PBF-1129 | A2BAR antagonist | non-small cell lung cancer | 1, 03274479 |
| PBF-677 | A3AR antagonist | glaucoma ulcerative colitis |
1, 02639975 2, 03773952, ADENOIBD |
| PBF-1650 | A3AR antagonist | psoriasis, NASH | 1, 03798236, ADENOIMMUNE |
| FM101 (87) | A3AR antag/part agonist | glaucoma NASH |
1/2, 04585100 2, 04710524 |
Note that this list is not all-inclusive (e.g., dipyridamole has been omitted). Other compounds are reviewed elsewhere (Borah et al., 2019; Jacobson et al., 2019). Structures, when disclosed, are shown in Figs. 1, 2, 3, and 5.
aterminated additional enrollment criteria made patient recruitment unfeasible.
bcheckpoint inhibitor.
ctriple negative breast cancer, pancreatic ductal adenocarcinoma, non-small cell lung cancer, renal cell cancer, urothelial cancer, head and neck cancer, diffused large B cell lymphoma, microsatellite stable colon cancer, non-Hodgkin lymphoma.
doleclumab.
A. Clinical Trials of Adenosine Receptor Agonists
AR agonists and partial agonists have been considered for pharmaceutical development in the treatment of: pain, seizures, arrhythmias, atrial fibrillation, diabetes, chronic heart failure, glaucoma (A1AR); hypertension (and diagnosis), inflammation, atrial fibrillation, ischemic conditions, sickle cell disease (A2AAR); neurodegeneration, inflammation, hepatocellular carcinoma, ischemic conditions, NASH, and chronic neuropathic pain (A3AR). There are no clinical trials of A2BAR agonists, but their use as antidiabetic agents or in cardioprotection, lung injury, diabetes, pulmonary hypertension, and other vascular conditions has been suggested (Eckle et al., 2007; Koscso et al., 2013; Merighi et al., 2015; Bessa-Gonçalves et al., 2018).
The antiarrhythmic effects of adenosine acting at the A1AR led to its approval for treating supraventricular tachycardia (Jacobson et al., 2019). More selective nucleoside-based A1AR agonists were also considered for this application, but their clinical trials failed. A partial A1AR agonist, CVT-3619, was predicted to display fewer side effects as an antiarrhythmic agent, but the clinical trial was discontinued (Jacobson et al., 2019). A clinical trial of A1AR agonist selodenoson (78, DTI-0009) for atrial fibrillation was discontinued. Non-nucleosides have also been developed as A1AR agonists. 3,5-Dicyanopyridine derivative capadenoson was in a clinical trial for heart failure, but it was later supplanted by an ongoing trial of neladenoson (77, BAY1067197), a newer, more selective prodrug derivative of the same structural class (Shah et al., 2019). However, the compound failed to meet the clinical endpoint. A phase 3 trial of A1AR agonist trabodenoson (79) also failed to demonstrate efficacy (Jacobson and Civan, 2016). A1AR agonists, including selective agonist GW493838 and intrathecally administered adenosine, and a PAM (T-62) were also studied for their application in pain, but three clinical trials failed to demonstrate efficacy (Miao et al., 2018; Jacobson et al., 2019).
The vasodilatory effects of A2AAR agonists have resulted in the approval of adenosine (1) itself and regadenoson (2, CVT-3146) for coronary stress imaging in patients not suitable for exercise-induced vasodilation. However, clinical trials for chronic obstructive pulmonary disease, asthma, and sickle cell disease, based on the anti-inflammatory effects of A2AAR agonists, were unsuccessful (Jacobson et al., 2019).
A3AR activation has been the subject of many clinical trials, and several are ongoing (Jacobson et al., 2019). Two prototypical A3AR agonists, IB-MECA (48, CF101, piclodenoson) and Cl-IB-MECA (14, CF102, namodenoson) have progressed to clinical phases 3 and 2 for autoimmune inflammatory diseases and liver diseases, respectively. A3AR agonists also have protective effects in models of chronic pain. Piclodenoson is in phase 3 trials for rheumatoid arthritis/psoriasis. Namodenoson is in phase 2 trials for hepatocellular carcinoma and nonalcoholic steatohepatitis (NASH).
B. Clinical Trials of Adenosine Receptor Antagonists
AR antagonists have been considered for pharmaceutical development in the treatment of: asthma, renal dysfunction (A1AR); neurodegeneration, cancer (A2AAR); cancer, asthma, diabetes (A2BAR); glaucoma, psoriasis, NASH, and ulcerative colitis (A3AR). There are not yet therapeutic antibodies proposed for use in blocking ARs.
Various clinical trials of A1AR antagonists for treatment of heart failure were eventually discontinued. These A1AR antagonists include rolofylline (82, KW-3902) for congestive heart failure and SLV320 (19) for renal dysfunction/heart failure.
Initially, when A2AAR antagonists were first reported in the early 1990s, the principal target was PD. Several selective antagonists were in clinical trials, some of which indicated a relatively modest effect, whereas others did not reach statistical significance. Antagonists in this group were tozadenant (66), preladenant (21), and istradefylline (5). A phase 3 trial of tozadenant was discontinued after five fatalities from agranulocytosis had occurred. The caffeine-like antagonist istradefylline was first approved in Japan for use as a cotherapy in treating PD to reduce off-time. Additional clinical evidence of a beneficial effect of istradefylline has accrued, leading to its recent approval in the United States, as mentioned before (Chen and Cunha, 2020).
Currently, the most excitement surrounds the use of A2AAR antagonists as adjuvants in cancer immunotherapy. Adenosine forms an immunosuppressive “cloud” in the tumor microenvironment for both solid malignancies and hematologic cancer (Sek et al., 2018). The adenosine acts through both A2AAR and A2BAR to induce an anti-inflammatory phenotype in T cells, macrophages, and other cells; and antagonists have a beneficial effect when combined with immunotherapy (Congreve et al., 2018). Ongoing clinical trials include: NIR178 (83, PBF-509, now taminadenant) for PD, NSCLC, and various other cancers in combination with a checkpoint inhibitor; CPI-444 (84, formerly V81444, now ciforadenant) for advanced cancers in combination with a checkpoint inhibitor; phase 1/1b study of inupadenant (85, EOS100850) for solid tumors (NCT03873883) (Houthuys et al., 2018); PBF-1129 for non-small cell lung cancer (NSCLC, structure not disclosed); and mixed A2AAR/A2BAR antagonist AB928 (86, now etrumadenant) for various cancers in combination with checkpoint inhibitor AB122. A2AAR antagonist preladenant (21) has been repurposed from a failed phase 3 trial in PD to treating advanced solid tumors (also in combination with pembrolizumab) but the data did not support study endpoints. A2AAR antagonist AZD4635 (22, formerly HTL1071, now imaradenant) was developed by rational drug design based on A2AAR X-ray structures. Its envisioned application was attention deficit hyperactivity disorder (ADHD), but it is currently being applied to cancer immunotherapy (Borodovsky et al., 2020).
VIII. Concluding Remarks
The number of scientific publications with “adenosine receptor” as a topic in the Web of Science database has remained relatively stable over the years. With close to 4500 articles in each of the two decades covered by this and the previous report, one captures the field as relatively mature and significant. This number is quite comparable to other GPCRs (e.g., ∼4000 in the last decade for serotonin/5-HT and ∼6000 for dopamine), more than for histamine receptors (∼1000) but fewer than for chemokine receptors (close to 13,000 publications in the last decade). Even a relatively comprehensive report like this one can only pinpoint to some of these many references, however, with a focus on particular topics that have gained traction over the years. We invite readers to draw our attention to other focal points for future inclusion. Such focal points in this report not or hardly covered before are target binding kinetics, receptor structure, and biased signaling. It turns out that high-affinity ligands, be it agonists or antagonists, may have very divergent kinetic profiles. Some have relatively short residence times (defined as 1/koff) at one or more of the four AR subtypes, whereas others show long residence times, up to several hours. It should be mentioned that many of these studies have been performed at lower than physiologic temperature, which impedes a reliable estimation of in vivo residence times and target engagement. The ultimate in this respect is covalent binding, a feature that is now well established in clinically approved kinase inhibitors but that has not found much application yet in clinical studies of GPCRs. With only one receptor structure discussed in the previous report, remarkable progress has been made, particularly with respect to the A2AAR. By combinations of fusion proteins and thermostabilizing receptor mutations, this receptor has become one of the more easily accessed GPCRs for structure elucidation. Today it has become possible, as with cytosolic proteins, to soak/exchange receptor crystals to enable multiple crystal structures at the same time. On the other hand, the A2BAR and A3AR have not been successfully subjected to structure elucidation. Biased signaling has been a hot topic and heavily studied aspect of GPCR signaling in the last decade, but less so for ARs. Compared with the opioid receptor field, for example, most AR agonists appear to display less outspoken preferences, also with a less obvious separation between desired and side effects. Still, the potential of developing adenosine receptor agonists that are biased for particular signaling pathways associated with treatment modalities promises to alleviate the problem of side effects of adenosine agonists, as noted in multiple clinical trials. However, in most cases, the precise G protein-dependent or independent pathways involved in the salutary effects of adenosine agonists are unexplored. Recently, an A1AR agonist that was reported to selectively activate Gob versus the other five Gαi/o subtypes and without β-arrestin recruitment was discovered (Wall et al., 2020). It appears to act as a potent analgesic without sedation or cardiorespiratory depression. This can serve as a model for applying biased signaling to other adenosine receptor subtypes. Finally, clinical development takes a long time, and this is also true for AR ligands. Istradefylline was only recently allowed access to the United States (Food and Drug Administration) and is in the approval process for the European market (European Medicines Agency) for the treatment of motor effects in PD long after its introduction in Japan. It had been reviewed as an early clinical candidate in our 2001 report, whereas in the 2011 update it was again mentioned as a clinical candidate, then in large phase 3 trials. This suggests that there will be room for a fourth update a decade from now.
Acknowledgments
Figure 4 and Supplemental Videos 1 and 2 were assembled and configured by Willem Jespers (Uppsala University, Sweden/Leiden University, The Netherlands), which is gratefully acknowledged.
Abbreviations
- ADA
adenosine deaminase
- AdoK
adenosine kinase
- AR
adenosine receptor
- CCPA
2-chloro-N6-cyclopentyladenosine
- CNS
central nervous system
- EM
electron microscopy
- GFR
glomerular filtration rate
- GPCR
G protein-coupled receptor
- h
human
- KD
equilibrium dissociation constant
- MECA
methylcarboxamidoadenosine
- NASH
nonalcoholic steatohepatitis
- NECA
5′-N-ethylcarboxamidoadenosine
- PAM
positive allosteric modulator
- PD
Parkinson’s disease
- PDB
Protein Data Bank
- RT
residence time
- TM
transmembrane domain
- WT
wild-type
Authorship Contributions
Wrote or contributed to the writing of the manuscript: IJzerman, Jacobson, Müller, Cronstein, Cunha.
Footnotes
This research was supported in part by the Intramural Research Program of the National Institutes of Health [National Institute of Diabetes and Digestive and Kidney Diseases]. This work was supported by National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant ZIADK31117]; the Deutsche Forschungs Gemeinschaft [Grants FOR2372, SFB1328, and GRK1873]; La Caixa Foundation [Grant LCF/PR/HP17/52190001]; Centro 2020 [Grants CENTRO-01-0145-FEDER-000008:BrainHealth 2020 and CENTRO-01-0246-FEDER-000010]; and Fundação para a Ciência e a Tecnologia [Grant UIDB/04539/2020].
Financial disclosure: Bruce N. Cronstein is founder and Chairman of the Scientific Advisory Board of Regenosine, LLC.
 This article has supplemental material available at pharmrev.aspetjournals.org.
This article has supplemental material available at pharmrev.aspetjournals.org.
References
- Abo-Salem OM, Hayallah AM, Bilkei-Gorzo A, Filipek B, Zimmer A, Müller CE (2004) Antinociceptive effects of novel A2B adenosine receptor antagonists. J Pharmacol Exp Ther 308:358–366. [DOI] [PubMed] [Google Scholar]
- Abouelkhair MA (2020) Targeting adenosinergic pathway and adenosine A2A receptor signaling for the treatment of COVID-19: a hypothesis. Med Hypotheses 144:110012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbari A, Khalili-Fomeshi M, Ashrafpour M, Moghadamnia AA, Ghasemi-Kasman M (2018) Adenosine A2A receptor blockade attenuates spatial memory deficit and extent of demyelination areas in lyolecithin-induced demyelination model. Life Sci 205:63–72. [DOI] [PubMed] [Google Scholar]
- Alberty RA, Hammes GG (1958) Application of the theory of diffusion-controlled reactions to enzyme kinetics. J Phys Chem 62:154–159. [Google Scholar]
- Alhersh E, Abushanab D, Al-Shaibi S, Al-Badriyeh D (2020) Caffeine for the treatment of apnea in the neonatal intensive care unit: a systematic overview of meta-analyses. Paediatr Drugs 22:399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allard B, Allard D, Buisseret L, Stagg J (2020) The adenosine pathway in immuno-oncology. Nat Rev Clin Oncol 17:611–629. [DOI] [PubMed] [Google Scholar]
- Alnouri MW, Jepards S, Casari A, Schiedel AC, Hinz S, Müller CE (2015) Selectivity is species-dependent: characterization of standard agonists and antagonists at human, rat, and mouse adenosine receptors. Purinergic Signal 11:389–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsene K, Deckert J, Sand P, de Wit H (2003) Association between A2a receptor gene polymorphisms and caffeine-induced anxiety. Neuropsychopharmacology 28:1694–1702. [DOI] [PubMed] [Google Scholar]
- Amelia Tvan Veldhoven JPDFalsini MLiu RHeitman LHvan Westen GJPSegala EVerdon GCheng RKYCooke RM, et al. (2021) Crystal structure and subsequent ligand design of a nonriboside partial agonist bound to the adenosine A2A receptor. J Med Chem 64:3827–3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An SKim GKim HJAhn SKim HYKo HHyun YENguyen MJeong JLiu Z, et al. (2020) Discovery and structure-activity relationships of novel template, truncated 1′-homologated adenosine derivatives as pure dual PPARγ/δ modulators. J Med Chem 63:16012–16027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arab L, Biggs ML, O’Meara ES, Longstreth WT, Crane PK, Fitzpatrick AL (2011) Gender differences in tea, coffee, and cognitive decline in the elderly: the Cardiovascular Health Study. J Alzheimers Dis 27:553–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asaoka N, Nishitani N, Kinoshita H, Nagai Y, Hatakama H, Nagayasu K, Shirakawa H, Nakagawa T, Kaneko S (2019) An adenosine A2A receptor antagonist improves multiple symptoms of repeated quinpirole-induced psychosis. eNeuro 6:0366-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auchampach JA, Gizewski ET, Wan TC, de Castro S, Brown GG Jr, Jacobson KA (2010) Synthesis and pharmacological characterization of [(125)I]MRS5127, a high affinity, selective agonist radioligand for the A3 adenosine receptor. Biochem Pharmacol 79:967–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augusto E, Matos M, Sévigny J, El-Tayeb A, Bynoe MS, Müller CE, Cunha RA, Chen JF (2013) Ecto-5′-nucleotidase (CD73)-mediated formation of adenosine is critical for the striatal adenosine A2A receptor functions. J Neurosci 33:11390–11399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltos JA, Paoletta S, Nguyen AT, Gregory KJ, Tosh DK, Christopoulos A, Jacobson KA, May LT (2016) Structure-activity analysis of biased agonism at the human adenosine A3 receptor. Mol Pharmacol 90:12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Yehuda SRath-Wolfson LDel Valle LOchaion ACohen SPatoka RZozulya GBarer FAtar EPiña-Oviedo S, et al. (2009) Induction of an antiinflammatory effect and prevention of cartilage damage in rat knee osteoarthritis by CF101 treatment. Arthritis Rheum 60:3061–3071. [DOI] [PubMed] [Google Scholar]
- Baraldi PG, Cacciari B, Moro S, Romagnoli R, Ji Xd, Jacobson KA, Gessi S, Borea PA, Spalluto G (2001) Fluorosulfonyl- and bis-(beta-chloroethyl)amino-phenylamino functionalized pyrazolo[4,3-e]1,2,4-triazolo[1,5-c]pyrimidine derivatives: irreversible antagonists at the human A3 adenosine receptor and molecular modeling studies. J Med Chem 44:2735–2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraldi PG, Preti D, Borea PA, Varani K (2012) Medicinal chemistry of A3 adenosine receptor modulators: pharmacological activities and therapeutic implications. J Med Chem 55:5676–5703. [DOI] [PubMed] [Google Scholar]
- Baraldi PGTabrizi MAPreti DBovero AFruttarolo FRomagnoli RMoorman ARGessi SMerighi SVarani K, et al. (2004) [3H]-MRE 2029-F20, a selective antagonist radioligand for the human A2B adenosine receptors. Bioorg Med Chem Lett 14:3607–3610. [DOI] [PubMed] [Google Scholar]
- Barnes PJ (2003) Theophylline: new perspectives for an old drug. Am J Respir Crit Care Med 167:813–818. [DOI] [PubMed] [Google Scholar]
- Barresi E, Giacomelli C, Marchetti L, Baglini E, Salerno S, Greco G, Da Settimo F, Martini C, Trincavelli ML, Taliani S (2021) Novel positive allosteric modulators of A2B adenosine receptor acting as bone mineralisation promoters. J Enzyme Inhib Med Chem 36:286–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrington WW, Jacobson KA, Stiles GL (1990) Glycoprotein nature of the A2-adenosine receptor binding subunit. Mol Pharmacol 38:177–183. [PMC free article] [PubMed] [Google Scholar]
- Batalha VL, Pego JM, Fontinha BM, Costenla AR, Valadas JS, Baqi Y, Radjainia H, Müller CE, Sebastião AM, Lopes LV (2013) Adenosine A(2A) receptor blockade reverts hippocampal stress-induced deficits and restores corticosterone circadian oscillation. Mol Psychiatry 18:320–331. [DOI] [PubMed] [Google Scholar]
- Batyuk AGalli LIshchenko AHan GWGati CPopov PALee MYStauch BWhite TABarty A, et al. (2016) Native phasing of x-ray free-electron laser data for a G protein-coupled receptor. Sci Adv 2:e1600292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauglehole AR, Baker SP, Scammells PJ (2000) Fluorosulfonyl-substituted xanthines as selective irreversible antagonists for the A(1)-adenosine receptor. J Med Chem 43:4973–4980. [DOI] [PubMed] [Google Scholar]
- Beavis PAHenderson MAGiuffrida LMills JKSek KCross RSDavenport AJJohn LBMardiana SSlaney CY, et al. (2017) Targeting the adenosine 2A receptor enhances chimeric antigen receptor T cell efficacy. J Clin Invest 127:929–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beavis PA, Milenkovski N, Henderson MA, John LB, Allard B, Loi S, Kershaw MH, Stagg J, Darcy PK (2015) Adenosine receptor 2A blockade increases the efficacy of anti-PD-1 through enhanced antitumor T-cell responses. Cancer Immunol Res 3:506–517. [DOI] [PubMed] [Google Scholar]
- Bertarelli DC, Diekmann M, Hayallah AM, Rüsing D, Iqbal J, Preiss B, Verspohl EJ, Müller CE (2006) Characterization of human and rodent native and recombinant adenosine A(2B) receptors by radioligand binding studies. Purinergic Signal 2:559–71 DOI: 10.1007/s11302-006-9012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessa-Gonçalves M, Bragança B, Martins-Dias E, Correia-de-Sá P, Fontes-Sousa AP (2018) Is the adenosine A2B ‘biased’ receptor a valuable target for the treatment of pulmonary arterial hypertension? Drug Discov Today 23:1285–1292. [DOI] [PubMed] [Google Scholar]
- Beste C, Stock AK, Ness V, Epplen JT, Arning L (2012) Differential effects of ADORA2A gene variations in pre-attentive visual sensory memory subprocesses. Eur Neuropsychopharmacol 22:555–561. [DOI] [PubMed] [Google Scholar]
- Blay J, White TD, Hoskin DW (1997) The extracellular fluid of solid carcinomas contains immunosuppressive concentrations of adenosine. Cancer Res 57:2602–2605. [PubMed] [Google Scholar]
- Bleickardt CJ, Kazdoba TM, Jones NT, Hunter JC, Hodgson RA (2014) Antagonism of the adenosine A2A receptor attenuates akathisia-like behavior induced with MP-10 or aripiprazole in a novel non-human primate model. Pharmacol Biochem Behav 118:36–45. [DOI] [PubMed] [Google Scholar]
- Bocquet N, Kohler J, Hug MN, Kusznir EA, Rufer AC, Dawson RJ, Hennig M, Ruf A, Huber W, Huber S (2015) Real-time monitoring of binding events on a thermostabilized human A2A receptor embedded in a lipid bilayer by surface plasmon resonance. Biochim Biophys Acta 1848:1224–1233. [DOI] [PubMed] [Google Scholar]
- Boia RElvas FMadeira MHAires IDRodrigues-Neves ACTralhão PSzabó ECBaqi YMüller CETomé AR, et al. (2017) Treatment with A2A receptor antagonist KW6002 and caffeine intake regulate microglia reactivity and protect retina against transient ischemic damage. Cell Death Dis 8:e3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boison D, Yegutkin GG (2019) Adenosine metabolism: emerging concepts for cancer therapy. Cancer Cell 36:582–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borah P, Deka S, Mailavaram RP, Deb PK (2019) P1 receptor agonists/antagonists in clinical trials - potential drug candidates of the future. Curr Pharm Des 25:2792–2807. [DOI] [PubMed] [Google Scholar]
- Borea PA, Gessi S, Merighi S, Vincenzi F, Varani K (2018) Pharmacology of adenosine receptors: the state of the art. Physiol Rev 98:1591–1625. [DOI] [PubMed] [Google Scholar]
- Borea PA, Varani K, Vincenzi F, Baraldi PG, Tabrizi MA, Merighi S, Gessi S (2015) The A3 adenosine receptor: history and perspectives. Pharmacol Rev 67:74–102. [DOI] [PubMed] [Google Scholar]
- Boring DL, Ji XD, Zimmet J, Taylor KE, Stiles GL, Jacobson KA (1991) Trifunctional agents as a design strategy for tailoring ligand properties: irreversible inhibitors of A1 adenosine receptors. Bioconjug Chem 2:77–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borodovsky ABarbon CMWang YYe MPrickett LChandra DShaw JDeng NSachsenmeier KClarke JD, et al. (2020) Small molecule AZD4635 inhibitor of A2AR signaling rescues immune cell function including CD103+ dendritic cells enhancing anti-tumor immunity. J Immunother Cancer 8:e000417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrmann T, Hinz S, Bertarelli DC, Li W, Florin NC, Scheiff AB, Müller CE (2009) 1-alkyl-8-(piperazine-1-sulfonyl)phenylxanthines: development and characterization of adenosine A2B receptor antagonists and a new radioligand with subnanomolar affinity and subtype specificity. J Med Chem 52:3994–4006. [DOI] [PubMed] [Google Scholar]
- Borroto-Escuela DO, Wydra K, Filip M, Fuxe K (2018) A2AR-D2R Heteroreceptor complexes in cocaine reward and addiction. Trends Pharmacol Sci 39:1008–1020. [DOI] [PubMed] [Google Scholar]
- Bouzo-Lorenzo M, Stoddart LA, Xia L, IJzerman AP, Heitman LH, Briddon SJ, Hill SJ (2019) A live cell NanoBRET binding assay allows the study of ligand-binding kinetics to the adenosine A3 receptor. Purinergic Signal 15:139–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozic KJ, Kurtz SM, Lau E, Ong K, Chiu V, Vail TP, Rubash HE, Berry DJ (2010) The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res 468:45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broecker JMorizumi TOu WLKlingel VKuo AKissick DJIshchenko ALee MYXu SMakarov O, et al. (2018) High-throughput in situ X-ray screening of and data collection from protein crystals at room temperature and under cryogenic conditions. Nat Protoc 13:260–292. [DOI] [PubMed] [Google Scholar]
- Campos-Martins A, Bragança B, Correia-de-Sá P, Fontes-Sousa AP (2021) Pharmacological tuning of adenosine signal nuances underlying heart failure with preserved ejection fraction. Front Pharmacol 12:724320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canas PM, Porciúncula LO, Cunha GM, Silva CG, Machado NJ, Oliveira JM, Oliveira CR, Cunha RA (2009) Adenosine A2A receptor blockade prevents synaptotoxicity and memory dysfunction caused by beta-amyloid peptides via p38 mitogen-activated protein kinase pathway. J Neurosci 29:14741–14751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canas PM, Porciuncula LO, Simoes AP, Augusto E, Silva HB, Machado NJ, Gonçalves N, Alfaro TM, Gonçalves FQ, Araujo IM, Real JI, Coelho JE, Andrade GM, Almeida RD, Chen JF, Kofalvi A, Agostinho P, Cunha RA (2018) Neuronal adenosine a2a receptors are critical mediators of neurodegeneration triggered by convulsions. eNeuro 5:0385-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmo MGonçalves FQCanas PMOses JPFernandes FDDuarte FVPalmeira CMTomé ARAgostinho PAndrade GM, et al. (2019) Enhanced ATP release and CD73-mediated adenosine formation sustain adenosine A2A receptor over-activation in a rat model of Parkinson’s disease. Br J Pharmacol 176:3666–3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter B, Nehmé R, Warne T, Leslie AG, Tate CG (2016) Structure of the adenosine A(2A) receptor bound to an engineered G protein. Nature 536:104–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carugo O, Bordo D (1999) How many water molecules can be detected by protein crystallography? Acta Crystallogr D Biol Crystallogr 55:479–483. [DOI] [PubMed] [Google Scholar]
- Carvalho KFaivre EPietrowski MJMarques XGomez-Murcia VDeleau AHuin VHansen JNKozlov SDanis C, et al. ; NeuroCEB Brain Bank (2019) Exacerbation of C1q dysregulation, synaptic loss and memory deficits in tau pathology linked to neuronal adenosine A2A receptor. Brain 142:3636–3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceska T, Chung CW, Cooke R, Phillips C, Williams PA (2019) Cryo-EM in drug discovery. Biochem Soc Trans 47:281–293. [DOI] [PubMed] [Google Scholar]
- Chen JF, Cunha RA (2020) The belated US FDA approval of the adenosine A2A receptor antagonist istradefylline for treatment of Parkinson’s disease. Purinergic Signal 16:167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PZ, He WJ, Zhu ZR, E GJ, Xu G, Chen DW, Gao YQ (2018) Adenosine A2A receptor involves in neuroinflammation-mediated cognitive decline through activating microglia under acute hypobaric hypoxia. Behav Brain Res 347:99–107. [DOI] [PubMed] [Google Scholar]
- Cheng RKY, Segala E, Robertson N, Deflorian F, Dore AS, Errey JC, Fiez-Vandal C, Marshall FH, Cooke RM (2017) Structures of human A1 and A2A adenosine receptors with xanthines reveal determinants of selectivity. Structure 25:1275–1285.e4. [DOI] [PubMed] [Google Scholar]
- Cheng Y (2018) Single-particle cryo-EM-How did it get here and where will it go. Science 361:876–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choca JI, Kwatra MM, Hosey MM, Green RD (1985) Specific photoaffinity labelling of inhibitory adenosine receptors. Biochem Biophys Res Commun 131:115–121. [DOI] [PubMed] [Google Scholar]
- Cognato GP, Agostinho PM, Hockemeyer J, Müller CE, Souza DO, Cunha RA (2010) Caffeine and an adenosine A(2A) receptor antagonist prevent memory impairment and synaptotoxicity in adult rats triggered by a convulsive episode in early life. J Neurochem 112:453–462. [DOI] [PubMed] [Google Scholar]
- Congreve MAndrews SPDoré ASHollenstein KHurrell ELangmead CJMason JSNg IWTehan BZhukov A, et al. (2012) Discovery of 1,2,4-triazine derivatives as adenosine A(2A) antagonists using structure based drug design. J Med Chem 55:1898–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congreve M, Brown GA, Borodovsky A, Lamb ML (2018) Targeting adenosine A2A receptor antagonism for treatment of cancer. Expert Opin Drug Discov 13:997–1003. [DOI] [PubMed] [Google Scholar]
- Copeland RA (2016) The drug-target residence time model: a 10-year retrospective. Nat Rev Drug Discov 15:87–95. [DOI] [PubMed] [Google Scholar]
- Copeland RA, Pompliano DL, Meek TD (2006) Drug-target residence time and its implications for lead optimization. Nat Rev Drug Discov 5:730–739. [DOI] [PubMed] [Google Scholar]
- Corciulo C, Lendhey M, Wilder T, Schoen H, Cornelissen AS, Chang G, Kennedy OD, Cronstein BN (2017) Endogenous adenosine maintains cartilage homeostasis and exogenous adenosine inhibits osteoarthritis progression. Nat Commun 8:15019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corciulo C, Wilder T, Cronstein BN (2016) Adenosine A2B receptors play an important role in bone homeostasis. Purinergic Signal 12:537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correale PCaracciolo MBilotta FConte MCuzzola MFalcone CMangano CFalzea ACIuliano EMorabito A, et al. (2020) Therapeutic effects of adenosine in high flow 21% oxygen aereosol in patients with Covid19-pneumonia. PLoS One 15:e0239692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costenla AR, Diógenes MJ, Canas PM, Rodrigues RJ, Nogueira C, Maroco J, Agostinho PM, Ribeiro JA, Cunha RA, de Mendonça A (2011) Enhanced role of adenosine A(2A) receptors in the modulation of LTP in the rat hippocampus upon ageing. Eur J Neurosci 34:12–21. [DOI] [PubMed] [Google Scholar]
- Cronstein BN, Kramer SB, Weissmann G, Hirschhorn R (1983) Adenosine: a physiological modulator of superoxide anion generation by human neutrophils. J Exp Med 158:1160–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronstein BN, Sitkovsky M (2017) Adenosine and adenosine receptors in the pathogenesis and treatment of rheumatic diseases. Nat Rev Rheumatol 13:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooke A, Martínez-Henández J, Martínez-López J, Cruz-Jentoft A, Huete-Toral F, Pintor J (2017) Low expression of CD39 and CD73 genes in centenarians compared with octogenarians. Immun Ageing 14:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha RA (2001) Adenosine as a neuromodulator and as a homeostatic regulator in the nervous system: different roles, different sources and different receptors. Neurochem Int 38:107–125. [DOI] [PubMed] [Google Scholar]
- Cunha RA (2005) Neuroprotection by adenosine in the brain: from A(1) receptor activation to A (2A) receptor blockade. Purinergic Signal 1:111–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha RA (2016) How does adenosine control neuronal dysfunction and neurodegeneration? J Neurochem 139:1019–1055. [DOI] [PubMed] [Google Scholar]
- Cunha RA, Constantino MD, Fonseca E, Ribeiro JA (2001) Age-dependent decrease in adenosine A1 receptor binding sites in the rat brain. Effect of cis unsaturated free fatty acids. Eur J Biochem 268:2939–2947. [DOI] [PubMed] [Google Scholar]
- Cunha RA, Constantino MD, Ribeiro JA (1999) G protein coupling of CGS 21680 binding sites in the rat hippocampus and cortex is different from that of adenosine A1 and striatal A2A receptors. Naunyn Schmiedebergs Arch Pharmacol 359:295–302. [DOI] [PubMed] [Google Scholar]
- Cunha RA, Johansson B, Constantino MD, Sebastião AM, Fredholm BB (1996) Evidence for high-affinity binding sites for the adenosine A2A receptor agonist [3H] CGS 21680 in the rat hippocampus and cerebral cortex that are different from striatal A2A receptors. Naunyn Schmiedebergs Arch Pharmacol 353:261–271. [DOI] [PubMed] [Google Scholar]
- Dahl G, Akerud T (2013) Pharmacokinetics and the drug-target residence time concept. Drug Discov Today 18:697–707. [DOI] [PubMed] [Google Scholar]
- Daly JW (2007) Caffeine analogs: biomedical impact. Cell Mol Life Sci 64:2153–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daryaee F, Tonge PJ (2019) Pharmacokinetic-pharmacodynamic models that incorporate drug-target binding kinetics. Curr Opin Chem Biol 50:120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Witte WEA, Danhof M, van der Graaf PH, de Lange ECM (2016) In vivo target residence time and kinetic selectivity: the association rate constant as determinant. Trends Pharmacol Sci 37:831–842. [DOI] [PubMed] [Google Scholar]
- Deckert J, Nöthen MM, Franke P, Delmo C, Fritze J, Knapp M, Maier W, Beckmann H, Propping P (1998) Systematic mutation screening and association study of the A1 and A2a adenosine receptor genes in panic disorder suggest a contribution of the A2a gene to the development of disease. Mol Psychiatry 3:81–85. [DOI] [PubMed] [Google Scholar]
- Di Liberto VMudò GGarozzo RFrinchi MFernandez-Dueñas VDi Iorio PCiccarelli RCaciagli FCondorelli DFCiruela F, et al. (2016) The guanine-based purinergic system: the tale of an orphan neuromodulation. Front Pharmacol 7:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionisotti S, Ongini E, Zocchi C, Kull B, Arslan G, Fredholm BB (1997) Characterization of human A2A adenosine receptors with the antagonist radioligand [3H]-SCH 58261. Br J Pharmacol 121:353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X, Li S, Sun J, Li Y, Zhang D (2020) Association of coffee, decaffeinated coffee and caffeine intake from coffee with cognitive performance in older adults: National Health and Nutrition Examination Survey (NHANES) 2011-2014. Nutrients 12:840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dore AS, Robertson N, Errey JC, Ng I, Hollenstein K, Tehan B, Hurrell E, Bennett K, Congreve M, Magnani F, Tate CG, Weir M, Marshall FH (2011) Structure of the adenosine A(2A) receptor in complex with ZM241385 and the xanthines XAC and caffeine. Structure 19:1283–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper-Joyce CJBhola RWang JBhattarai ANguyen ATNCowie-Kent IO’Sullivan KChia LYVenugopal HValant C, et al. (2021) Positive allosteric mechanisms of adenosine A1 receptor-mediated analgesia. Nature 597:571–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper-Joyce CJKhoshouei MThal DMLiang YLNguyen ATNFurness SGBVenugopal HBaltos JAPlitzko JMDanev R, et al. (2018) Structure of the adenosine-bound human adenosine A1 receptor-Gi complex. Nature 558:559–563. [DOI] [PubMed] [Google Scholar]
- Du L, Gao ZG, Paoletta S, Wan TC, Gizewski ET, Barbour S, van Veldhoven JPD, IJzerman AP, Jacobson KA, Auchampach JA (2018) Species differences and mechanism of action of A3 adenosine receptor allosteric modulators. Purinergic Signal 14:59–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte JM, Agostinho PM, Carvalho RA, Cunha RA (2012) Caffeine consumption prevents diabetes-induced memory impairment and synaptotoxicity in the hippocampus of NONcZNO10/LTJ mice. PLoS One 7:e21899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl CQ, Patel A, Craig RH, Daluge SM, Linden J (1988) Photoaffinity labeling adenosine A1 receptors with an antagonist 125I-labeled aryl azide derivative of 8-phenylxanthine. J Med Chem 31:752–756. [DOI] [PubMed] [Google Scholar]
- Eckle TKrahn TGrenz AKöhler DMittelbronn MLedent CJacobson MAOsswald HThompson LFUnertl K, et al. (2007) Cardioprotection by ecto-5′-nucleotidase (CD73) and A2B adenosine receptors. Circulation 115:1581–1590. [DOI] [PubMed] [Google Scholar]
- Eddy MT, Gao ZG, Mannes P, Patel N, Jacobson KA, Katritch V, Stevens RC, Wüthrich K (2018a) Extrinsic tryptophans as NMR probes of allosteric coupling in membrane proteins: application to the A2A adenosine receptor. J Am Chem Soc 140:8228–8235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy MTLee MYGao ZGWhite KLDidenko THorst RAudet MStanczak PMcClary KMHan GW, et al. (2018b) Allosteric coupling of drug binding and intracellular signaling in the A2A adenosine receptor. Cell 172:68–80.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy MT, Martin BT, Wüthrich K (2021) A(2A) adenosine receptor partial agonism related to structural rearrangements in an activation microswitch. Structure 29:170–176.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Maatougui A, Azuaje J, González-Gómez M, Miguez G, Crespo A, Carbajales C, Escalante L, García-Mera X, Gutiérrez-de-Terán H, Sotelo E (2016) Discovery of potent and highly selective A2B adenosine receptor antagonist chemotypes. J Med Chem 59:1967–1983. [DOI] [PubMed] [Google Scholar]
- Elzein E, Kalla RV, Li X, Perry T, Gimbel A, Zeng D, Lustig D, Leung K, Zablocki J (2008) Discovery of a novel A2B adenosine receptor antagonist as a clinical candidate for chronic inflammatory airway diseases. J Med Chem 51:2267–2278. [DOI] [PubMed] [Google Scholar]
- Errey JC, Doré AS, Zhukov A, Marshall FH, Cooke RM (2015) Purification of stabilized GPCRs for structural and biophysical analyses. Methods Mol Biol 1335:1–15. [DOI] [PubMed] [Google Scholar]
- Eskelinen MH, Ngandu T, Tuomilehto J, Soininen H, Kivipelto M (2009) Midlife coffee and tea drinking and the risk of late-life dementia: a population-based CAIDE study. J Alzheimers Dis 16:85–91. [DOI] [PubMed] [Google Scholar]
- Evans J, Richards JR, Battisti AS (2020) Caffeine, in StatPearls, StatPearls Publishing LLC, Treasure Island, FL. [Google Scholar]
- Faivre ECoelho JEZornbach KMalik EBaqi YSchneider MCellai LCarvalho KSebda SFigeac M, et al. (2018) Beneficial effect of a selective adenosine A2A receptor antagonist in the APPswe/PS1dE9 mouse model of Alzheimer’s disease. Front Mol Neurosci 11:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcone CCaracciolo MCorreale PMacheda SVadalà EGLa Scala STescione MDanieli RFerrarelli ATarsitano MG, et al. (2020) Can adenosine fight COVID-19 acute respiratory distress syndrome? J Clin Med 9:3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan KQLi YYWang HLMao XTGuo JXWang FHuang LJLi YNMa XYGao ZJ, et al. (2019) Stress-induced metabolic disorder in peripheral CD4+ T cells leads to anxiety-like behavior. Cell 179:864–879.e19. [DOI] [PubMed] [Google Scholar]
- Fenalti G, Giguere PM, Katritch V, Huang XP, Thompson AA, Cherezov V, Roth BL, Stevens RC (2014) Molecular control of δ-opioid receptor signalling. Nature 506:191–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré S (2016) Mechanisms of the psychostimulant effects of caffeine: implications for substance use disorders. Psychopharmacology (Berl) 233:1963–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré S, Ciruela F (2019) Functional and neuroprotective role of striatal adenosine A2A receptor heterotetramers. J Caffeine Adenosine Res 9:89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo ABde Oliveira E Castro RANogueira-Paiva NCMoreira FGonçalves FQSoares RPCastro-Borges WSilva GGCunha RAGonçalves T, et al. (2021) Clustering of adenosine A2B receptors with ectonucleotidases in caveolin-rich lipid rafts underlies immunomodulation by Leishmania amazonensis. FASEB J 35:e21509. [DOI] [PubMed] [Google Scholar]
- Flögel UBurghoff Svan Lent PLTemme SGalbarz LDing ZEl-Tayeb AHuels SBönner FBorg N, et al. (2012) Selective activation of adenosine A2A receptors on immune cells by a CD73-dependent prodrug suppresses joint inflammation in experimental rheumatoid arthritis. Sci Transl Med 4:146ra108. [DOI] [PubMed] [Google Scholar]
- Franchetti PCappellacci LVita PPetrelli RLavecchia AKachler SKlotz KNMarabese ILuongo LMaione S, et al. (2009) N6-cycloalkyl- and N6-bicycloalkyl-C5‘(C2’)-modified adenosine derivatives as high-affinity and selective agonists at the human A1 adenosine receptor with antinociceptive effects in mice. J Med Chem 52:2393–2406. [DOI] [PubMed] [Google Scholar]
- Franco R, Cordomí A, Llinas Del Torrent C, Lillo A, Serrano-Marín J, Navarro G, Pardo L (2021a) Structure and function of adenosine receptor heteromers. Cell Mol Life Sci 78:3957–3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco R, Rivas-Santisteban R, Reyes-Resina I, Navarro G (2021b) The old and new visions of biased agonism through the prism of adenosine receptor signaling and receptor/receptor and receptor/protein interactions. Front Pharmacol 11:628601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraporti TT, Contini V, Tovo-Rodrigues L, Recamonde-Mendoza M, Rovaris DL, Rohde LA, Hutz MH, Salatino-Oliveira A, Genro JP (2019) Synergistic effects between ADORA2A and DRD2 genes on anxiety disorders in children with ADHD. Prog Neuropsychopharmacol Biol Psychiatry 93:214–220. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Bättig K, Holmén J, Nehlig A, Zvartau EE (1999) Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev 51:83–133. [PubMed] [Google Scholar]
- Fredholm BB, Chen JF, Cunha RA, Svenningsson P, Vaugeois JM (2005) Adenosine and brain function. Int Rev Neurobiol 63:191–270. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, IJzerman AP, Jacobson KA, Klotz KN, Linden J (2001) International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev 53:527–552. [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB, IJzerman AP, Jacobson KA, Linden J, Müller CE (2011) International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors--an update. Pharmacol Rev 63:1–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB, Frenguelli BG, Hills R, IJzerman AP, Jacobson KA, Klotz KN, Linden J, Müller CE, Schwabe U, Stiles GL (2021). Adenosine receptors in GtoPdb v.2021.2. IUPHAR/BPS Guide to Pharmacology CITE 2021 DOI: 10.2218/gtopdb/F3/2021.2. [DOI] [Google Scholar]
- Freitag CM, Agelopoulos K, Huy E, Rothermundt M, Krakowitzky P, Meyer J, Deckert J, von Gontard A, Hohoff C (2010) Adenosine A(2A) receptor gene (ADORA2A) variants may increase autistic symptoms and anxiety in autism spectrum disorder. Eur Child Adolesc Psychiatry 19:67–74. [DOI] [PubMed] [Google Scholar]
- Fuchs JL (1991) 5′-Nucleotidase activity increases in aging rat brain. Neurobiol Aging 12:523–530. [DOI] [PubMed] [Google Scholar]
- Fujioka M, Omori N (2012) Subtleties in GPCR drug discovery: a medicinal chemistry perspective. Drug Discov Today 17:1133–1138. [DOI] [PubMed] [Google Scholar]
- Furman DChang JLartigue LBolen CRHaddad FGaudilliere BGanio EAFragiadakis GKSpitzer MHDouchet I, et al. (2017) Expression of specific inflammasome gene modules stratifies older individuals into two extreme clinical and immunological states. Nat Med 23:174–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao AW, Smith RL, van Weeghel M, Kamble R, Janssens GE, Houtkooper RH (2018a) Identification of key pathways and metabolic fingerprints of longevity in C. elegans. Exp Gerontol 113:128–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao ZG, Balasubramanian R, Kiselev E, Wei Q, Jacobson KA (2014) Probing biased/partial agonism at the G protein-coupled A(2B) adenosine receptor. Biochem Pharmacol 90:297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao ZG, Inoue A, Jacobson KA (2018b) On the G protein-coupling selectivity of the native A2B adenosine receptor. Biochem Pharmacol 151:201–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao ZG, Jacobson KA (2008) Translocation of arrestin induced by human A(3) adenosine receptor ligands in an engineered cell line: comparison with G protein-dependent pathways. Pharmacol Res 57:303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao ZG, Jacobson KA (2013) Allosteric modulation and functional selectivity of G protein-coupled receptors. Drug Discov Today Technol 10:e237–e243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao ZG, Van Muijlwijk-Koezen JE, Chen A, Müller CE, IJzerman AP, Jacobson KA (2001) Allosteric modulation of A(3) adenosine receptors by a series of 3-(2-pyridinyl)isoquinoline derivatives. Mol Pharmacol 60:1057–1063. [PMC free article] [PubMed] [Google Scholar]
- Gao ZG, Verzijl D, Zweemer A, Ye K, Göblyös A, IJzerman AP, Jacobson KA (2011) Functionally biased modulation of A(3) adenosine receptor agonist efficacy and potency by imidazoquinolinamine allosteric enhancers. Biochem Pharmacol 82:658–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Nafría J, Lee Y, Bai X, Carpenter B, Tate CG (2018) Cryo-EM structure of the adenosine A2A receptor coupled to an engineered heterotrimeric G protein. eLife 7:e35946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelber RP, Petrovitch H, Masaki KH, Ross GW, White LR (2011) Coffee intake in midlife and risk of dementia and its neuropathologic correlates. J Alzheimers Dis 23:607–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessi S, Bencivenni S, Battistello E, Vincenzi F, Colotta V, Catarzi D, Varano F, Merighi S, Borea PA, Varani K (2017) Inhibition of A2A adenosine receptor signaling in cancer cells proliferation by the novel antagonist TP455. Front Pharmacol 8:888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giblett ER, Anderson JE, Cohen F, Pollara B, Meuwissen HJ (1972) Adenosine-deaminase deficiency in two patients with severely impaired cellular immunity. Lancet 2:1067–1069. [DOI] [PubMed] [Google Scholar]
- Glukhova A, Thal DM, Nguyen AT, Vecchio EA, Jörg M, Scammells PJ, May LT, Sexton PM, Christopoulos A (2017) Structure of the adenosine A1 receptor reveals the basis for subtype selectivity. Cell 168:867–877.e13. [DOI] [PubMed] [Google Scholar]
- Gnad TNavarro GLahesmaa MReverte-Salisa LCopperi FCordomi ANaumann JHochhäuser AHaufs-Brusberg SWenzel D, et al. (2020) Adenosine/A2B receptor signaling ameliorates the effects of aging and counteracts obesity. Cell Metab 32:56–70.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göblyös A, IJzerman AP (2011) Allosteric modulation of adenosine receptors. Biochim Biophys Acta 1808:1309–1318. [DOI] [PubMed] [Google Scholar]
- Gonçalves FQLopes JPSilva HBLemos CSilva ACGonçalves NTomé ARFerreira SGCanas PMRial D, et al. (2019) Synaptic and memory dysfunction in a β-amyloid model of early Alzheimer’s disease depends on increased formation of ATP-derived extracellular adenosine. Neurobiol Dis 132:104570. [DOI] [PubMed] [Google Scholar]
- Gonçalves N, Simões AT, Cunha RA, de Almeida LP (2013) Caffeine and adenosine A(2A) receptor inactivation decrease striatal neuropathology in a lentiviral-based model of Machado-Joseph disease. Ann Neurol 73:655–666. [DOI] [PubMed] [Google Scholar]
- Gonçalves N, Simões AT, Prediger RD, Hirai H, Cunha RA, Pereira de Almeida L (2017) Caffeine alleviates progressive motor deficits in a transgenic mouse model of spinocerebellar ataxia. Ann Neurol 81:407–418. [DOI] [PubMed] [Google Scholar]
- Gracia EFarré DCortés AFerrer-Costa COrozco MMallol JLluís CCanela EIMcCormick PJFranco R, et al. (2013) The catalytic site structural gate of adenosine deaminase allosterically modulates ligand binding to adenosine receptors. FASEB J 27:1048–1061. [DOI] [PubMed] [Google Scholar]
- Grisshammer R (2017) New approaches towards the understanding of integral membrane proteins: A structural perspective on G protein-coupled receptors. Protein Sci 26:1493–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosso G, Micek A, Castellano S, Pajak A, Galvano F (2016) Coffee, tea, caffeine and risk of depression: a systematic review and dose-response meta-analysis of observational studies. Mol Nutr Food Res 60:223–234. [DOI] [PubMed] [Google Scholar]
- Guixà-González RAlbasanz JLRodriguez-Espigares IPastor MSanz FMartí-Solano MManna MMartinez-Seara HHildebrand PWMartín M, et al. (2017) Membrane cholesterol access into a G-protein-coupled receptor. Nat Commun 8:14505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D, Heitman LH, IJzerman AP (2015) Importance of Drug Target Residence Time at G Protein-Coupled Receptors, Wiley-VCH Verlag GmbH & Co., Weinheim, Germany. [Google Scholar]
- Guo D, Heitman LH, IJzerman AP (2016a) The added value of assessing ligand-receptor binding kinetics in drug discovery. ACS Med Chem Lett 7:819–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D, Heitman LH, IJzerman AP (2017) Kinetic aspects of the interaction between ligand and G protein-coupled receptor: the case of the adenosine receptors. Chem Rev 117:38–66. [DOI] [PubMed] [Google Scholar]
- Guo D, Hillger JM, IJzerman AP, Heitman LH (2014a) Drug-target residence time--a case for G protein-coupled receptors. Med Res Rev 34:856–892. [DOI] [PubMed] [Google Scholar]
- Guo D, Mulder-Krieger T, IJzerman AP, Heitman LH (2012) Functional efficacy of adenosine A2A receptor agonists is positively correlated to their receptor residence time. Br J Pharmacol 166:1846–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D, Pan AC, Dror RO, Mocking T, Liu R, Heitman LH, Shaw DE, IJzerman AP (2016b) Molecular basis of ligand dissociation from the adenosine A2A receptor. Mol Pharmacol 89:485–491. [DOI] [PubMed] [Google Scholar]
- Guo D, van Dorp EJ, Mulder-Krieger T, van Veldhoven JP, Brussee J, IJzerman AP, Heitman LH (2013) Dual-point competition association assay: a fast and high-throughput kinetic screening method for assessing ligand-receptor binding kinetics. J Biomol Screen 18:309–320. [DOI] [PubMed] [Google Scholar]
- Guo D, Venhorst SN, Massink A, van Veldhoven JP, Vauquelin G, IJzerman AP, Heitman LH (2014b) Molecular mechanism of allosteric modulation at GPCRs: insight from a binding kinetics study at the human A1 adenosine receptor. Br J Pharmacol 171:5295–5312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D, Xia L, van Veldhoven JP, Hazeu M, Mocking T, Brussee J, IJzerman AP, Heitman LH (2014c) Binding kinetics of ZM241385 derivatives at the human adenosine A2A receptor. ChemMedChem 9:752–761. [DOI] [PubMed] [Google Scholar]
- Halldner L, Lopes LV, Daré E, Lindström K, Johansson B, Ledent C, Cunha RA, Fredholm BB (2004) Binding of adenosine receptor ligands to brain of adenosine receptor knock-out mice: evidence that CGS 21680 binds to A1 receptors in hippocampus. Naunyn Schmiedebergs Arch Pharmacol 370:270–278. [DOI] [PubMed] [Google Scholar]
- Hameleers PA, Van Boxtel MP, Hogervorst E, Riedel WJ, Houx PJ, Buntinx F, Jolles J (2000) Habitual caffeine consumption and its relation to memory, attention, planning capacity and psychomotor performance across multiple age groups. Hum Psychopharmacol 15:573–581. [DOI] [PubMed] [Google Scholar]
- Hamilton SP, Slager SL, De Leon AB, Heiman GA, Klein DF, Hodge SE, Weissman MM, Fyer AJ, Knowles JA (2004) Evidence for genetic linkage between a polymorphism in the adenosine 2A receptor and panic disorder. Neuropsychopharmacology 29:558–565. [DOI] [PubMed] [Google Scholar]
- Härter M, Kalthof B, Delbeck M, Lustig K, Gerisch M, Schulz S, Kast R, Meibom D, Lindner N (2019) Novel non-xanthine antagonist of the A2B adenosine receptor: from HTS hit to lead structure. Eur J Med Chem 163:763–778. [DOI] [PubMed] [Google Scholar]
- Hatfield SM, Sitkovsky M (2016) A2A adenosine receptor antagonists to weaken the hypoxia-HIF-1α driven immunosuppression and improve immunotherapies of cancer. Curr Opin Pharmacol 29:90–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser RA, Stocchi F, Rascol O, Huyck SB, Capece R, Ho TW, Sklar P, Lines C, Michelson D, Hewitt D (2015) Preladenant as an adjunctive therapy with levodopa in Parkinson disease: two randomized clinical trials and lessons learned. JAMA Neurol 72:1491–1500. [DOI] [PubMed] [Google Scholar]
- Hayallah AM, Sandoval-Ramírez J, Reith U, Schobert U, Preiss B, Schumacher B, Daly JW, Müller CE (2002) 1,8-disubstituted xanthine derivatives: synthesis of potent A2B-selective adenosine receptor antagonists. J Med Chem 45:1500–1510. [DOI] [PubMed] [Google Scholar]
- He YLi YPu ZChen MGao YChen LRuan YPan XZhou YGe Y, et al. (2020) Striatopallidal pathway distinctly modulates goal-directed valuation and acquisition of instrumental behavior via striatopallidal output projections. Cereb Cortex 30:1366–1381. [DOI] [PubMed] [Google Scholar]
- Hickey P, Stacy M (2012) Adenosine A2A antagonists in Parkinson’s disease: what’s next? Curr Neurol Neurosci Rep 12:376–385. [DOI] [PubMed] [Google Scholar]
- Hino TArakawa TIwanari HYurugi-Kobayashi TIkeda-Suno CNakada-Nakura YKusano-Arai OWeyand SShimamura TNomura N, et al. (2012) G-protein-coupled receptor inactivation by an allosteric inverse-agonist antibody. Nature 482:237–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz S, Alnouri WM, Pleiss U, Müller CE (2018) Tritium-labeled agonists as tools for studying adenosine A2B receptors. Purinergic Signal 14:223–233 DOI: 10.1007/s11302-018-9608-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz S, Lacher SK, Seibt BF, Müller CE (2014) BAY60-6583 acts as a partial agonist at adenosine A2B receptors. J Pharmacol Exp Ther 349:427–436. [DOI] [PubMed] [Google Scholar]
- Hockemeyer J, Burbiel JC, Müller CE (2004) Multigram-scale syntheses, stability, and photoreactions of A2A adenosine receptor antagonists with 8-styrylxanthine structure: potential drugs for Parkinson’s disease. J Org Chem 69:3308–3318. [DOI] [PubMed] [Google Scholar]
- Horgusluoglu-Moloch ENho KRisacher SLKim SForoud TShaw LMTrojanowski JQAisen PSPetersen RCJack CR Jr, et al. ; Alzheimer’s Disease Neuroimaging Initiative (ADNI) (2017) Targeted neurogenesis pathway-based gene analysis identifies ADORA2A associated with hippocampal volume in mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging 60:92–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hothersall JDGuo DSarda SSheppard RJChen HKeur WWaring MJIJzerman APHill SJDale IL, et al. (2017) Structure-activity relationships of the sustained effects of adenosine A2A receptor agonists driven by slow dissociation kinetics. Mol Pharmacol 91:25–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houthuys EMarillier RDeregnaucourt TBrouwer MBasilico PPirson RMarchante JPrasad SHermant ANyawouame F, et al. (2018) Abstract LB-291: EOS100850, an insurmountable and non-brain penetrant A2A receptor antagonist, inhibits adenosine-mediated T cell suppression, demonstrates anti-tumor activity and exhibits best-in class characteristics, in AACR Annual Meeting; 2018 Apr 14–18; Chicago. American Association for Cancer Research, Philadelphia. [Google Scholar]
- Hu Q, Ren X, Liu Y, Li Z, Zhang L, Chen X, He C, Chen JF (2016) Aberrant adenosine A2A receptor signaling contributes to neurodegeneration and cognitive impairments in a mouse model of synucleinopathy. Exp Neurol 283 (Pt A):213–223. [DOI] [PubMed] [Google Scholar]
- Huang S, Apasov S, Koshiba M, Sitkovsky M (1997) Role of A2a extracellular adenosine receptor-mediated signaling in adenosine-mediated inhibition of T-cell activation and expansion. Blood 90:1600–1610. [PubMed] [Google Scholar]
- Huang SK, Pandey A, Tran DP, Villanueva NL, Kitao A, Sunahara RK, Sljoka A, Prosser RS (2021) Delineating the conformational landscape of the adenosine A2A receptor during G protein coupling. Cell 184:1884–1894.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannone R, Miele L, Maiolino P, Pinto A, Morello S (2014) Adenosine limits the therapeutic effectiveness of anti-CTLA4 mAb in a mouse melanoma model. Am J Cancer Res 4:172–181. [PMC free article] [PubMed] [Google Scholar]
- Ihara KHato MNakane TYamashita KKimura-Someya THosaka TIshizuka-Katsura YTanaka RTanaka TSugahara M, et al. (2020) Isoprenoid-chained lipid EROCOC17 + 4: a new matrix for membrane protein crystallization and a crystal delivery medium in serial femtosecond crystallography. Sci Rep 10:19305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishack S, Mediero A, Wilder T, Ricci JL, Cronstein BN (2017) Bone regeneration in critical bone defects using three-dimensionally printed β-tricalcium phosphate/hydroxyapatite scaffolds is enhanced by coating scaffolds with either dipyridamole or BMP-2. J Biomed Mater Res B Appl Biomater 105:366–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanisevic JStauch KLPetrascheck MBenton HPEpstein AAFang MGorantla STran MHoang LKurczy ME, et al. (2016) Metabolic drift in the aging brain. Aging (Albany NY) 8:1000–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaakola VP, Griffith MT, Hanson MA, Cherezov V, Chien EY, Lane JR, IJzerman AP, Stevens RC (2008) The 2.6 angstrom crystal structure of a human A2A adenosine receptor bound to an antagonist. Science 322:1211–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, Barone S, Kammula U, Stiles GL (1989a) Electrophilic derivatives of purines as irreversible inhibitors of A1 adenosine receptors. J Med Chem 32:1043–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, Civan MM (2016) Ocular purine receptors as drug targets in the eye. J Ocul Pharmacol Ther 32:534–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, Gao ZG, Göblyös A, IJzerman AP (2011) Allosteric modulation of purine and pyrimidine receptors. Adv Pharmacol 61:187–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, IJzerman AP, Müller CE (2021) Medicinal chemistry of P2 and adenosine receptors: common scaffolds adapted for multiple targets. Biochem Pharmacol 187:114311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KAMerighi SVarani KBorea PABaraldi SAghazadeh Tabrizi MRomagnoli RBaraldi PGCiancetta ATosh DK, et al. (2018) A3 adenosine receptors as modulators of inflammation: from medicinal chemistry to therapy. Med Res Rev 38:1031–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, Müller CE (2016) Medicinal chemistry of adenosine, P2Y and P2X receptors. Neuropharmacology 104:31–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, Pannell LK, Ji XD, Jarvis MF, Williams M, Hutchison AJ, Barrington WW, Stiles GL (1989b) Agonist derived molecular probes for A2 adenosine receptors. J Mol Recognit 2:170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, Stiles GL, Ji XD (1992) Chemical modification and irreversible inhibition of striatal A2a adenosine receptors. Mol Pharmacol 42:123–133. [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, Tosh DK, Jain S, Gao ZG (2019) Historical and current adenosine receptor agonists in preclinical and clinical development. Front Cell Neurosci 13:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, von Lubitz DK, Daly JW, Fredholm BB (1996) Adenosine receptor ligands: differences with acute versus chronic treatment. Trends Pharmacol Sci 17:108–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janik P, Berdyński M, Safranow K, Żekanowski C (2015) Association of ADORA1 rs2228079 and ADORA2A rs5751876 polymorphisms with Gilles de la Tourette Syndrome in the Polish population. PLoS One 10:e0136754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenner P, Mori A, Aradi SD, Hauser RA (2021) Istradefylline - a first generation adenosine A2A antagonist for the treatment of Parkinson's disease. Expert Rev Neurother 21:317–333. [DOI] [PubMed] [Google Scholar]
- Jeong LSChoe SAGunaga PKim HOLee HWLee SKTosh DKPatel APalaniappan KKGao ZG, et al. (2007) Discovery of a new nucleoside template for human A3 adenosine receptor ligands: D-4′-thioadenosine derivatives without 4′-hydroxymethyl group as highly potent and selective antagonists. J Med Chem 50:3159–3162. [DOI] [PubMed] [Google Scholar]
- Jespers W, Schiedel AC, Heitman LH, Cooke RM, Kleene L, van Westen GJP, Gloriam DE, Müller CE, Sotelo E, Gutiérrez-de-Terán H (2018) Structural mapping of adenosine receptor mutations: ligand binding and signaling mechanisms. Trends Pharmacol Sci 39:75–89. [DOI] [PubMed] [Google Scholar]
- Jespers WVerdon GAzuaje JMajellaro MKeränen HGarcía-Mera XCongreve MDeflorian Fde Graaf CZhukov A, et al. (2020) X-ray crystallography and free energy calculations reveal the binding mechanism of A2A adenosine receptor antagonists. Angew Chem Int Ed Engl 59:16536–16543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji X, Kim YC, Ahern DG, Linden J, Jacobson KA (2001) [3H]MRS 1754, a selective antagonist radioligand for A(2B) adenosine receptors. Biochem Pharmacol 61:657–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji XD, Gallo-Rodriguez C, Jacobson KA (1993) 8-(3-Isothiocyanatostyryl)caffeine is a selective, irreversible inhibitor of striatal A(2)-adenosine receptors. Drug Dev Res 29:292–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji XD, Gallo-Rodriguez C, Jacobson KA (1994) A selective agonist affinity label for A3 adenosine receptors. Biochem Biophys Res Commun 203:570–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jörg M, Glukhova A, Abdul-Ridha A, Vecchio EA, Nguyen AT, Sexton PM, White PJ, May LT, Christopoulos A, Scammells PJ (2016) Novel irreversible agonists acting at the A1 adenosine receptor. J Med Chem 59:11182–11194. [DOI] [PubMed] [Google Scholar]
- Journey JD, Bentley TP(2020) Theophylline Toxicity, in StatPearls, StatPearls Publishing LLC, Treasure Island, FL. [PubMed] [Google Scholar]
- Kalk P, Eggert B, Relle K, Godes M, Heiden S, Sharkovska Y, Fischer Y, Ziegler D, Bielenberg GW, Hocher B (2007) The adenosine A1 receptor antagonist SLV320 reduces myocardial fibrosis in rats with 5/6 nephrectomy without affecting blood pressure. Br J Pharmacol 151:1025–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalla RV, Zablocki J (2009) Progress in the discovery of selective, high affinity A(2B) adenosine receptor antagonists as clinical candidates. Purinergic Signal 5:21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kara FM, Chitu V, Sloane J, Axelrod M, Fredholm BB, Stanley ER, Cronstein BN (2010a) Adenosine A1 receptors (A1Rs) play a critical role in osteoclast formation and function. FASEB J 24:2325–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kara FM, Doty SB, Boskey A, Goldring S, Zaidi M, Fredholm BB, Cronstein BN (2010b) Adenosine A(1) receptors regulate bone resorption in mice: adenosine A(1) receptor blockade or deletion increases bone density and prevents ovariectomy-induced bone loss in adenosine A(1) receptor-knockout mice. Arthritis Rheum 62:534–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaster MPMachado NJSilva HBNunes AArdais APSantana MBaqi YMüller CERodrigues ALPorciúncula LO, et al. (2015) Caffeine acts through neuronal adenosine A2A receptors to prevent mood and memory dysfunction triggered by chronic stress. Proc Natl Acad Sci USA 112:7833–7838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keuerleber S, Gsandtner I, Freissmuth M (2011) From cradle to twilight: the carboxyl terminus directs the fate of the A(2A)-adenosine receptor. Biochim Biophys Acta 1808:1350–1357. [DOI] [PubMed] [Google Scholar]
- Kiesman WF, Elzein E, Zablocki J (2009) A1 adenosine receptor antagonists, agonists, and allosteric enhancers. Handb Exp Pharmacol 193:25–58. [DOI] [PubMed] [Google Scholar]
- Kim YC, Ji X, Melman N, Linden J, Jacobson KA (2000) Anilide derivatives of an 8-phenylxanthine carboxylic congener are highly potent and selective antagonists at human A(2B) adenosine receptors. J Med Chem 43:1165–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimatrai-Salvador M, Baraldi PG, Romagnoli R (2013) Allosteric modulation of A1-adenosine receptor: a review. Drug Discov Today Technol 10:e285–e296. [DOI] [PubMed] [Google Scholar]
- Klotz KN, Cristalli G, Grifantini M, Vittori S, Lohse MJ (1985) Photoaffinity labeling of A1-adenosine receptors. J Biol Chem 260:14659–14664. [PubMed] [Google Scholar]
- Kobayashi HUjike HIwata NInada TYamada MSekine YUchimura NIyo MOzaki NItokawa M, et al. (2010) The adenosine A2A receptor is associated with methamphetamine dependence/psychosis in the Japanese population. Behav Brain Funct 6:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koscsó B, Trepakov A, Csóka B, Németh ZH, Pacher P, Eltzschig HK, Haskó G (2013) Stimulation of A2B adenosine receptors protects against trauma-hemorrhagic shock-induced lung injury. Purinergic Signal 9:427–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzer K, Bassler D (2014) Caffeine for apnea of prematurity: a neonatal success story. Neonatology 105:332–336. [DOI] [PubMed] [Google Scholar]
- Kudlacek O, Waldhoer M, Kassack MU, Nickel P, Salmi JI, Freissmuth M, Nanoff C (2002) Biased inhibition by a suramin analogue of A1-adenosine receptor/G protein coupling in fused receptor/G protein tandems: the A1-adenosine receptor is predominantly coupled to Goalpha in human brain. Naunyn Schmiedebergs Arch Pharmacol 365:8–16. [DOI] [PubMed] [Google Scholar]
- Langemeijer EV, Verzijl D, Dekker SJ, IJzerman AP (2013) Functional selectivity of adenosine A1 receptor ligands? Purinergic Signal 9:91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent CBurnouf SFerry BBatalha VLCoelho JEBaqi YMalik EMarciniak EParrot SVan der Jeugd A, et al. (2016) A2A adenosine receptor deletion is protective in a mouse model of Tauopathy. Mol Psychiatry 21:149. [DOI] [PubMed] [Google Scholar]
- Lazarus M, Huang ZL, Lu J, Urade Y, Chen JF (2012) How do the basal ganglia regulate sleep-wake behavior? Trends Neurosci 35:723–732. [DOI] [PubMed] [Google Scholar]
- Lebon G, Edwards PC, Leslie AG, Tate CG (2015) Molecular determinants of CGS21680 binding to the human adenosine A2A receptor. Mol Pharmacol 87:907–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebon G, Warne T, Edwards PC, Bennett K, Langmead CJ, Leslie AG, Tate CG (2011) Agonist-bound adenosine A2A receptor structures reveal common features of GPCR activation. Nature 474:521–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HW, Kim HO, Choi WJ, Choi S, Lee JH, Park SG, Yoo L, Jacobson KA, Jeong LS (2010) Design, synthesis, and binding of homologated truncated 4′-thioadenosine derivatives at the human A3 adenosine receptors. Bioorg Med Chem 18:7015–7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeWitt PA, Aradi SD, Hauser RA, Rascol O (2020) The challenge of developing adenosine A2A antagonists for Parkinson disease: istradefylline, preladenant, and tozadenant. Parkinsonism Relat Disord 80 (Suppl 1):S54–S63. [DOI] [PubMed] [Google Scholar]
- Li AH, Chang L, Ji Xd, Melman N, Jacobson KA (1999) Functionalized congeners of 1,4-dihydropyridines as antagonist molecular probes for A3 adenosine receptors. Bioconjug Chem 10:667–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li AH, Moro S, Melman N, Ji XD, Jacobson KA (1998) Structure-activity relationships and molecular modeling of 3, 5-diacyl-2,4-dialkylpyridine derivatives as selective A3 adenosine receptor antagonists. J Med Chem 41:3186–3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li PRial DCanas PMYoo JHLi WZhou XWang Yvan Westen GJPayen MPAugusto E, et al. (2015) Optogenetic activation of intracellular adenosine A2A receptor signaling in the hippocampus is sufficient to trigger CREB phosphorylation and impair memory. Mol Psychiatry 20:1339–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YHe YChen MPu ZChen LLi PLi BLi HHuang ZLLi Z, et al. (2016) Optogenetic activation of adenosine A2A receptor signaling in the dorsomedial striatopallidal neurons suppresses goal-directed behavior. Neuropsychopharmacology 41:1003–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YPan XHe YRuan YHuang LZhou YHou ZHe CWang ZZhang X, et al. (2018) Pharmacological blockade of adenosine A2A but not A1 receptors enhances goal-directed valuation in satiety-based instrumental behavior. Front Pharmacol 9:393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YRuan YHe YCai QPan XZhang YLiu CPu ZYang JChen M, et al. (2020) Striatopallidal adenosine A2A receptors in the nucleus accumbens confer motivational control of goal-directed behavior. Neuropharmacology 168:108010. [DOI] [PubMed] [Google Scholar]
- Lipton RB, Diener HC, Robbins MS, Garas SY, Patel K (2017) Caffeine in the management of patients with headache. J Headache Pain 18:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Pian Y, Li X, Liu R, Xie W, Zhang C, Zheng Y, Jiang Y, Yuan Y (2014) Streptococcus suis adenosine synthase functions as an effector in evasion of PMN-mediated innate immunit. J Infect Dis 210:35–45. [DOI] [PubMed] [Google Scholar]
- Liu WChun EThompson AAChubukov PXu FKatritch VHan GWRoth CBHeitman LHIJzerman AP, et al. (2012) Structural basis for allosteric regulation of GPCRs by sodium ions. Science 337:232–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Huang P, Wang J, Yang Z, Huang S, Luo X, Qi J, Shen X, Zhong Y (2016) The effect of A2A receptor antagonist on microglial activation in experimental glaucoma. Invest Ophthalmol Vis Sci 57:776–786. [DOI] [PubMed] [Google Scholar]
- Liu XL, Zhou R, Pan QQ, Jia XL, Gao WN, Wu J, Lin J, Chen JF (2010) Genetic inactivation of the adenosine A2A receptor attenuates pathologic but not developmental angiogenesis in the mouse retina. Invest Ophthalmol Vis Sci 51:6625–6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZYan SWang JXu YWang YZhang SXu XYang QZeng XZhou Y, et al. (2017) Endothelial adenosine A2a receptor-mediated glycolysis is essential for pathological retinal angiogenesis. Nat Commun 8:584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes JP, Pliássova A, Cunha RA (2019) The physiological effects of caffeine on synaptic transmission and plasticity in the mouse hippocampus selectively depend on adenosine A1 and A2A receptors. Biochem Pharmacol 166:313–321. [DOI] [PubMed] [Google Scholar]
- Lopes LV, Halldner L, Rebola N, Johansson B, Ledent C, Chen JF, Fredholm BB, Cunha RA (2004) Binding of the prototypical adenosine A(2A) receptor agonist CGS 21680 to the cerebral cortex of adenosine A(1) and A(2A) receptor knockout mice. Br J Pharmacol 141:1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louvel JGuo DAgliardi MMocking TAKars RPham TPXia Lde Vries HBrussee JHeitman LH, et al. (2014) Agonists for the adenosine A1 receptor with tunable residence time. A Case for nonribose 4-amino-6-aryl-5-cyano-2-thiopyrimidines. J Med Chem 57:3213–3222. [DOI] [PubMed] [Google Scholar]
- Louvel J, Guo D, Soethoudt M, Mocking TA, Lenselink EB, Mulder-Krieger T, Heitman LH, IJzerman AP (2015) Structure-kinetics relationships of Capadenoson derivatives as adenosine A1 receptor agonists. Eur J Med Chem 101:681–691. [DOI] [PubMed] [Google Scholar]
- Lovászi M, Németh ZH, Gause WC, Beesley J, Pacher P, Haskó G (2021) Inosine monophosphate and inosine differentially regulate endotoxemia and bacterial sepsis. FASEB J 35:e21935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas M, Mirzaei F, Pan A, Okereke OI, Willett WC, O’Reilly ÉJ, Koenen K, Ascherio A (2011) Coffee, caffeine, and risk of depression among women. Arch Intern Med 171:1571–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas M, O’Reilly EJ, Pan A, Mirzaei F, Willett WC, Okereke OI, Ascherio A (2014) Coffee, caffeine, and risk of completed suicide: results from three prospective cohorts of American adults. World J Biol Psychiatry 15:377–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthin DR, Lee KS, Okonkwo D, Zhang P, Linden J (1995) Photoaffinity labeling with 2(-)[2-(4-azido-3(-)[125I]- iodophenyl)ethylamino]adenosine and autoradiography with 2(-)[2-(4-amino-3(-)[125I]iodophenyl)ethylamino]adenosine of A2a adenosine receptors in rat brain. J Neurochem 65:2072–2079. [DOI] [PubMed] [Google Scholar]
- Machado NJSimões APSilva HBArdais APKaster MPGarção PRodrigues DIPochmann DSantos AIAraújo IM, et al. (2017) Caffeine reverts memory but not mood impairment in a depression-prone mouse strain with up-regulated adenosine A2A receptor in hippocampal glutamate synapses. Mol Neurobiol 54:1552–1563. [DOI] [PubMed] [Google Scholar]
- Mackiewicz M, Nikonova EV, Zimmermann JE, Romer MA, Cater J, Galante RJ, Pack AI (2006) Age-related changes in adenosine metabolic enzymes in sleep/wake regulatory areas of the brain. Neurobiol Aging 27:351–360. [DOI] [PubMed] [Google Scholar]
- Madeira MH, Boia R, Elvas F, Martins T, Cunha RA, Ambrósio AF, Santiago AR (2016) Selective A2A receptor antagonist prevents microglia-mediated neuroinflammation and protects retinal ganglion cells from high intraocular pressure-induced transient ischemic injury. Transl Res 169:112–128. [DOI] [PubMed] [Google Scholar]
- Magnani F, Serrano-Vega MJ, Shibata Y, Abdul-Hussein S, Lebon G, Miller-Gallacher J, Singhal A, Strege A, Thomas JA, Tate CG (2016) A mutagenesis and screening strategy to generate optimally thermostabilized membrane proteins for structural studies. Nat Protoc 11:1554–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majellaro MJespers WCrespo ANúñez MJNovio SAzuaje JPrieto-Díaz RGioé CAlispahic BBrea J, et al. (2021) 3,4-Dihydropyrimidin-2(1H)-ones as antagonists of the human A2B adenosine receptor: optimization, structure-activity relationship studies, and enantiospecific recognition. J Med Chem 64:458–480. [DOI] [PubMed] [Google Scholar]
- Maltese MMartella GImbriani PSchuermans JBillion KSciamanna GFarook FPonterio GTassone ASantoro M, et al. (2017) Abnormal striatal plasticity in a DYT11/SGCE myoclonus dystonia mouse model is reversed by adenosine A2A receptor inhibition. Neurobiol Dis 108:128–139. [DOI] [PubMed] [Google Scholar]
- Martin-Garcia JMConrad CENelson GStander NZatsepin NAZook JZhu LGeiger JChun EKissick D, et al. (2017) Serial millisecond crystallography of membrane and soluble protein microcrystals using synchrotron radiation. IUCrJ 4:439–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martynowycz MW, Shiriaeva A, Ge X, Hattne J, Nannenga BL, Cherezov V, Gonen T (2021) MicroED structure of the human adenosine receptor determined from a single nanocrystal in LCP. Proc Natl Acad of Sci U S A 118:e2106041118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massie BMO’Connor CMMetra MPonikowski PTeerlink JRCotter GWeatherley BDCleland JGGivertz MMVoors A, et al. ; PROTECT Investigators and Committees (2010) Rolofylline, an adenosine A1-receptor antagonist, in acute heart failure. N Engl J Med 363:1419–1428. [DOI] [PubMed] [Google Scholar]
- Mateus M, Ilg MM, Stebbeds WJ, Christopher N, Muneer A, Ralph DJ, Cellek S (2018) Understanding the role of adenosine receptors in the myofibroblast transformation in Peyronie’s disease. J Sex Med 15:947–957. [DOI] [PubMed] [Google Scholar]
- Matricon P, Suresh RR, Gao ZG, Panel N, Jacobson KA, Carlsson J (2020) Ligand design by targeting a binding site water. Chem Sci (Camb) 12:960–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matter H, Güssregen S (2018) Characterizing hydration sites in protein-ligand complexes towards the design of novel ligands. Bioorg Med Chem Lett 28:2343–2352. [DOI] [PubMed] [Google Scholar]
- May LT, Self TJ, Briddon SJ, Hill SJ (2010) The effect of allosteric modulators on the kinetics of agonist-G protein-coupled receptor interactions in single living cells. Mol Pharmacol 78:511–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan TM, Caldwell JA, Lieberman HR (2016) A review of caffeine’s effects on cognitive, physical and occupational performance. Neurosci Biobehav Rev 71:294–312. [DOI] [PubMed] [Google Scholar]
- Mediero A, Frenkel SR, Wilder T, He W, Mazumder A, Cronstein BN (2012a) Adenosine A2A receptor activation prevents wear particle-induced osteolysis. Sci Transl Med 4:135ra65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mediero A, Kara FM, Wilder T, Cronstein BN (2012b) Adenosine A(2A) receptor ligation inhibits osteoclast formation. Am J Pathol 180:775–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mediero A, Perez-Aso M, Cronstein BN (2013) Activation of adenosine A(2A) receptor reduces osteoclast formation via PKA- and ERK1/2-mediated suppression of NFκB nuclear translocation. Br J Pharmacol 169:1372–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mediero A, Perez-Aso M, Wilder T, Cronstein BN (2015a) Brief report: Methotrexate prevents wear particle-induced inflammatory osteolysis via activation of the adenosine A2A receptor. Arthritis Rheumatol 67:849–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mediero A, Wilder T, Perez-Aso M, Cronstein BN (2015b) Direct or indirect stimulation of adenosine A2A receptors enhances bone regeneration as well as bone morphogenetic protein-2. FASEB J 29:1577–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnikov IPolovinkin VKovalev KGushchin IShevtsov MShevchenko VMishin AAlekseev ARodriguez-Valera FBorshchevskiy V, et al. (2017) Fast iodide-SAD phasing for high-throughput membrane protein structure determination. Sci Adv 3:e1602952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merighi S, Borea PA, Gessi S (2015) Adenosine receptors and diabetes: focus on the A(2B) adenosine receptor subtype. Pharmacol Res 99:229–236. [DOI] [PubMed] [Google Scholar]
- Miao Y, Bhattarai A, Nguyen ATN, Christopoulos A, May LT (2018) Structural basis for binding of allosteric drug leads in the adenosine A1 receptor. Sci Rep 8:16836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel MC, Charlton SJ (2018) Biased agonism in drug discovery-is it too soon to choose a path? Mol Pharmacol 93:259–265. [DOI] [PubMed] [Google Scholar]
- Morelli M, Carta AR, Jenner P (2009) Adenosine A2A receptors and Parkinson’s disease. Handb Exp Pharmacol 193:589–615. [DOI] [PubMed] [Google Scholar]
- Moss SM, Jayasekara PS, Paoletta S, Gao ZG, Jacobson KA (2014) Structure-based design of reactive nucleosides for site-specific modification of the A2A adenosine receptor. ACS Med Chem Lett 5:1043–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouro FM, Köfalvi A, André LA, Baqi Y, Müller CE, Ribeiro JA, Sebastião AM (2019) Memory deficits induced by chronic cannabinoid exposure are prevented by adenosine A2AR receptor antagonism. Neuropharmacology 155:10–21. [DOI] [PubMed] [Google Scholar]
- Müller CE (2003) Medicinal chemistry of adenosine A3 receptor ligands. Curr Top Med Chem 3:445–462. [DOI] [PubMed] [Google Scholar]
- Müller CE, Baqi Y, Hinz S, Namasivayam V (2018) Medicinal chemistry of A2B adenosine receptors, in The Adenosine Receptors (Borea PA, Varani K, Gessi S, Merighi S, Vincenzi F, eds) in The Receptors, vol 34, pp 137–168, Springer International Publishing, Cham, Switzerland. [Google Scholar]
- Müller CE, Diekmann M, Thorand M, Ozola V (2002) [(3)H]8-Ethyl-4-methyl-2-phenyl-(8R)-4,5,7,8-tetrahydro-1H-imidazo[2,1-i]-purin-5-one ([(3)H]PSB-11), a novel high-affinity antagonist radioligand for human A(3) adenosine receptors. Bioorg Med Chem Lett 12:501–503 DOI: 10.1016/s0960-894x(01)00785-5. [DOI] [PubMed] [Google Scholar]
- Müller CE, Jacobson KA (2011a) Recent developments in adenosine receptor ligands and their potential as novel drugs. Biochim Biophys Acta 1808:1290–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller CE, Jacobson KA (2011b) Xanthines as adenosine receptor antagonists. Handb Exp Pharmacol 200:151–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller CE, Maurinsh J, Sauer R (2000) Binding of [3H]MSX-2(3-(3-hydroxypropyl)-7-methyl-8-(m-methoxystyryl)-1-propargylxanthine) to rat striatal membranes—a new, selective antagonist radioligand for A(2A) adenosine receptors. Eur J Pharm Sci 10:259–65 DOI: 10.1016/s0928-0987(00)00064-6. [DOI] [PubMed] [Google Scholar]
- Müller CE, Schiedel AC, Baqi Y (2012) Allosteric modulators of rhodopsin-like G protein-coupled receptors: opportunities in drug development. Pharmacol Ther 135:292–315. [DOI] [PubMed] [Google Scholar]
- Muranaka H, Momose T, Handa C, Ozawa T (2017) Photoaffinity labeling of the human A2A adenosine receptor and cross-link position analysis by mass spectrometry. ACS Med Chem Lett 8:660–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murillo-Rodriguez E, Blanco-Centurion C, Gerashchenko D, Salin-Pascual RJ, Shiromani PJ (2004) The diurnal rhythm of adenosine levels in the basal forebrain of young and old rats. Neuroscience 123:361–370. [DOI] [PubMed] [Google Scholar]
- Nass KCheng RVera LMozzanica ARedford SOzerov DBasu SJames DKnopp GCirelli C, et al. (2020) Advances in long-wavelength native phasing at X-ray free-electron lasers. IUCrJ 7:965–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan DG, Field J, Lin G, Neuberg D, Majerus E, Onyekwere O, Keefer J, Okam M, Ross A, Linden J (2012) Sickle cell disease (SCD), iNKT cells, and regadenoson infusion. Trans Am Clin Climatol Assoc 123:312–317, discussion 317–318. [PMC free article] [PubMed] [Google Scholar]
- Navarro G, Gonzalez A, Campanacci S, Rivas-Santisteban R, Reyes-Resina I, Casajuana-Martin N, Cordomí A, Pardo L, Franco R (2020) Experimental and computational analysis of biased agonism on full-length and a C-terminally truncated adenosine A2A receptor. Comput Struct Biotechnol J 18:2723–2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak AChandra GHwang IKim KHou XKim HOSahu PKRoy KKYoo JLee Y, et al. (2014) Synthesis and anti-renal fibrosis activity of conformationally locked truncated 2-hexynyl-N(6)-substituted-(N)-methanocarba-nucleosides as A3 adenosine receptor antagonists and partial agonists. J Med Chem 57:1344–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng SK, Higashimori H, Tolman M, Yang Y (2015) Suppression of adenosine 2a receptor (A2aR)-mediated adenosine signaling improves disease phenotypes in a mouse model of amyotrophic lateral sclerosis. Exp Neurol 267:115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen ATVecchio EAThomas TNguyen TDAurelio LScammells PJWhite PJSexton PMGregory KJMay LT, et al. (2016) Role of the second extracellular loop of the adenosine A1 receptor on allosteric modulator binding, signaling, and cooperativity. Mol Pharmacol 90:715–725. [DOI] [PubMed] [Google Scholar]
- Niiya K, Jacobson KA, Silvia SK, Olsson RA (1993) Covalent binding of a selective agonist irreversibly activates guinea pig coronary artery A2 adenosine receptors. Naunyn Schmiedebergs Arch Pharmacol 347:521–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka Y, Shimada J, Nonaka H, Koike N, Aoki N, Kobayashi H, Kase H, Yamaguchi K, Suzuki F (1993) Photoisomerization of a potent and selective adenosine A2 antagonist, (E)-1,3-Dipropyl-8-(3,4-dimethoxystyryl)-7-methylxanthine. J Med Chem 36:3731–3733. [DOI] [PubMed] [Google Scholar]
- Ohta AGorelik EPrasad SJRonchese FLukashev DWong MKHuang XCaldwell SLiu KSmith P, et al. (2006) A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci USA 103:13132–13137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira S, Ardais AP, Bastos CR, Gazal M, Jansen K, de Mattos Souza L, da Silva RA, Kaster MP, Lara DR, Ghisleni G (2019) Impact of genetic variations in ADORA2A gene on depression and symptoms: a cross-sectional population-based study. Purinergic Signal 15:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozola V, Thorand M, Diekmann M, Qurishi R, Schumacher B, Jacobson KA, Müller CE (2003) 2-Phenylimidazo[2,1-i]purin-5-ones: structure-activity relationships and characterization of potent and selective inverse agonists at human A3 adenosine receptors. Bioorg Med Chem 11:347–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagnussat N, Almeida AS, Marques DM, Nunes F, Chenet GC, Botton PH, Mioranzza S, Loss CM, Cunha RA, Porciúncula LO (2015) Adenosine A(2A) receptors are necessary and sufficient to trigger memory impairment in adult mice. Br J Pharmacol 172:3831–3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A, Craig RH, Daluge SM, Linden J (1988) 125I-BW-A844U, an antagonist radioligand with high affinity and selectivity for adenosine A1 receptors, and 125I-azido-BW-A844U, a photoaffinity label. Mol Pharmacol 33:585–591. [PubMed] [Google Scholar]
- Patel JJ, Alzahrani T (2020) Myocardial perfusion scan, in StatPearls, StatPearls Publishing LLC, Treasure Island, FL. [PubMed] [Google Scholar]
- Perez-Aso M, Chiriboga L, Cronstein BN (2012) Pharmacological blockade of adenosine A2A receptors diminishes scarring. FASEB J 26:4254–4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Aso M, Mediero A, Low YC, Levine J, Cronstein BN (2016) Adenosine A2A receptor plays an important role in radiation-induced dermal injury. FASEB J 30:457–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pliássova A, Henriques M, Silva H, Agostinho P, Cunha R, Ferreira S (2020) Control of NMDA receptor-mediated currents by adenosine A1 and A2A receptors within the basolateral amygdala. J Caffeine Adenosine Res 10:61–70. [Google Scholar]
- Poole R, Kennedy OJ, Roderick P, Fallowfield JA, Hayes PC, Parkes J (2017) Coffee consumption and health: umbrella review of meta-analyses of multiple health outcomes. BMJ 359:j5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popoli P, Blum D, Domenici MR, Burnouf S, Chern Y (2008) A critical evaluation of adenosine A2A receptors as potentially “druggable” targets in Huntington’s disease. Curr Pharm Des 14:1500–1511. [DOI] [PubMed] [Google Scholar]
- Prediger RD, Fernandes D, Takahashi RN (2005) Blockade of adenosine A2A receptors reverses short-term social memory impairments in spontaneously hypertensive rats. Behav Brain Res 159:197–205. [DOI] [PubMed] [Google Scholar]
- Prosser RS, Ye L, Pandey A, Orazietti A (2017) Activation processes in ligand-activated G protein-coupled receptors: a case study of the adenosine A2A receptor. BioEssays 39:1700072. [DOI] [PubMed] [Google Scholar]
- Rebola N, Coelho JE, Costenla AR, Lopes LV, Parada A, Oliveira CR, Soares-da-Silva P, de Mendonça A, Cunha RA (2003) Decrease of adenosine A1 receptor density and of adenosine neuromodulation in the hippocampus of kindled rats. Eur J Neurosci 18:820–828. [DOI] [PubMed] [Google Scholar]
- Rebola N, Simões AP, Canas PM, Tomé AR, Andrade GM, Barry CE, Agostinho PM, Lynch MA, Cunha RA (2011) Adenosine A2A receptors control neuroinflammation and consequent hippocampal neuronal dysfunction. J Neurochem 117:100–111. [DOI] [PubMed] [Google Scholar]
- Ritchie K, Carrière I, de Mendonca A, Portet F, Dartigues JF, Rouaud O, Barberger-Gateau P, Ancelin ML (2007) The neuroprotective effects of caffeine: a prospective population study (the Three City Study). Neurology 69:536–545. [DOI] [PubMed] [Google Scholar]
- Rodrigues L, Miranda IM, Andrade GM, Mota M, Cortes L, Rodrigues AG, Cunha RA, Gonçalves T (2016) Blunted dynamics of adenosine A2A receptors is associated with increased susceptibility to Candida albicans infection in the elderly. Oncotarget 7:62862–62872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues RJ, Tomé AR, Cunha RA (2015) ATP as a multi-target danger signal in the brain. Front Neurosci 9:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnoli RBaraldi PGCarrion MDCara CLCruz-Lopez OIaconinoto MAPreti DShryock JCMoorman ARVincenzi F, et al. (2008) Synthesis and biological evaluation of 2-amino-3-(4-chlorobenzoyl)-4-[N-(substituted) piperazin-1-yl]thiophenes as potent allosteric enhancers of the A1 adenosine receptor. J Med Chem 51:5875–5879. [DOI] [PubMed] [Google Scholar]
- Rucktooa P, Cheng RKY, Segala E, Geng T, Errey JC, Brown GA, Cooke RM, Marshall FH, Doré AS (2018) Towards high throughput GPCR crystallography: in meso soaking of adenosine A2A receptor crystals. Sci Rep 8:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JDBetz AJIshiwari KFelsted JMadson LMirante BClark KFont LKorbey SSager TN, et al. (2008) Tremorolytic effects of adenosine A2A antagonists: implications for parkinsonism. Front Biosci 13:3594–3605. [DOI] [PubMed] [Google Scholar]
- Sánchez-Melgar A, Albasanz JL, Pallàs M, Martín M (2020) Adenosine metabolism in the cerebral cortex from several mice models during aging. Int J Mol Sci 21:7300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer R, Maurinsh J, Reith U, Fülle F, Klotz KN, Müller CE (2000) Water-soluble phosphate prodrugs of 1-propargyl-8-styrylxanthine derivatives, A(2A)-selective adenosine receptor antagonists. J Med Chem 43:440–448. [DOI] [PubMed] [Google Scholar]
- Scammells PJ, Baker SP, Belardinelli L, Olsson RA (1994) Substituted 1,3-dipropylxanthines as irreversible antagonists of A1 adenosine receptors. J Med Chem 37:2704–2712. [DOI] [PubMed] [Google Scholar]
- Schiffmann SN, Fisone G, Moresco R, Cunha RA, Ferré S (2007) Adenosine A2A receptors and basal ganglia physiology. Prog Neurobiol 83:277–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzschild MA, Agnati L, Fuxe K, Chen JF, Morelli M (2006) Targeting adenosine A2A receptors in Parkinson’s disease. Trends Neurosci 29:647–654. [DOI] [PubMed] [Google Scholar]
- Sebastião AM, Cunha RA, de Mendonça A, Ribeiro JA (2000) Modification of adenosine modulation of synaptic transmission in the hippocampus of aged rats. Br J Pharmacol 131:1629–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segala EGuo DCheng RKBortolato ADeflorian FDoré ASErrey JCHeitman LHIJzerman APMarshall FH, et al. (2016) Controlling the dissociation of ligands from the adenosine A2A receptor through modulation of salt bridge strength. J Med Chem 59:6470–6479. [DOI] [PubMed] [Google Scholar]
- Sek K, Mølck C, Stewart GD, Kats L, Darcy PK, Beavis PA (2018) Targeting adenosine receptor signaling in cancer immunotherapy. Int J Mol Sci 19:3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serchov TClement HWSchwarz MKIasevoli FTosh DKIdzko MJacobson KAde Bartolomeis ANormann CBiber K, et al. (2015) Increased signaling via adenosine A1 receptors, sleep deprivation, imipramine, and ketamine inhibit depressive-like behavior via induction of Homer1a. Neuron 87:549–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah SJ, Voors AA, McMurray JJV, Kitzman DW, Viethen T, Bomfim Wirtz A, Huang E, Pap AF, Solomon SD (2019) Effect of neladenoson bialanate on exercise capacity among patients with heart failure with preserved ejection fraction: a randomized clinical trial. JAMA 321:2101–2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh G, Cronstein B (2016) Signaling pathways involving adenosine A2A and A2B receptors in wound healing and fibrosis. Purinergic Signal 12:191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HYCoelho JEOhtsuka NCanas PMDay YJHuang QYRebola NYu LBoison DCunha RA, et al. (2008) A critical role of the adenosine A2A receptor in extrastriatal neurons in modulating psychomotor activity as revealed by opposite phenotypes of striatum and forebrain A2A receptor knock-outs. J Neurosci 28:2970–2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada J, Suzuki F, Nonaka H, Ishii A, Ichikawa S (1992) (E)-1,3-dialkyl-7-methyl-8-(3,4,5-trimethoxystyryl)xanthines: potent and selective adenosine A2 antagonists. J Med Chem 35:2342–2345. [DOI] [PubMed] [Google Scholar]
- Shkhyan RLee SGullo FLi LPeleli MCarlstrom MChagin ASBanks NWLimfat SLiu NQ, et al. (2018) Genetic ablation of adenosine receptor A3 results in articular cartilage degeneration. J Mol Med (Berl) 96:1049–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva CG, Porciúncula LO, Canas PM, Oliveira CR, Cunha RA (2007) Blockade of adenosine A(2A) receptors prevents staurosporine-induced apoptosis of rat hippocampal neurons. Neurobiol Dis 27:182–189. [DOI] [PubMed] [Google Scholar]
- Singh S, McKintosh R (2020) Adenosine, in StatPearls, StatPearls Publishing LLC, Treasure Island, FL. [PubMed] [Google Scholar]
- Copyright © 2020, StatPearls Publishing LLC., Treasure Island (FL). [Google Scholar]
- Smail EH, Cronstein BN, Meshulam T, Esposito AL, Ruggeri RW, Diamond RD (1992) In vitro, Candida albicans releases the immune modulator adenosine and a second, high-molecular weight agent that blocks neutrophil killing. J Immunol 148:3588–3595. [PubMed] [Google Scholar]
- Smith A, Sutherland D, Christopher G (2005) Effects of repeated doses of caffeine on mood and performance of alert and fatigued volunteers. J Psychopharmacol 19:620–626. [DOI] [PubMed] [Google Scholar]
- Smoluchowski MV (1918) Versuch einer mathematischen Theorie der Koagulationskinetik kolloider Lösungen. Z Phys Chem 92:129–168. [Google Scholar]
- Sperlágh B, Zsilla G, Baranyi M, Kékes-Szabó A, Vizi ES (1997) Age-dependent changes of presynaptic neuromodulation via A1-adenosine receptors in rat hippocampal slices. Int J Dev Neurosci 15:739–747. [DOI] [PubMed] [Google Scholar]
- Srinivas M, Shryock JC, Scammells PJ, Ruble J, Baker SP, Belardinelli L (1996) A novel irreversible antagonist of the A1-adenosine receptor. Mol Pharmacol 50:196–205. [PubMed] [Google Scholar]
- St Hilaire CZiegler SGMarkello TCBrusco AGroden CGill FCarlson-Donohoe HLederman RJChen MYYang D, et al. (2011) NT5E mutations and arterial calcifications. N Engl J Med 364:432–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart M, Steinig AG, Ma C, Song JP, McKibben B, Castelhano AL, MacLennan SJ (2004) [3H]OSIP339391, a selective, novel, and high affinity antagonist radioligand for adenosine A2B receptors. Biochem Pharmacol 68:305–312. [DOI] [PubMed] [Google Scholar]
- Stiles GL, Jacobson KA (1988) High affinity acylating antagonists for the A1 adenosine receptor: identification of binding subunit. Mol Pharmacol 34:724–728. [PMC free article] [PubMed] [Google Scholar]
- Stocchi FRascol OHauser RAHuyck STzontcheva ACapece RHo TWSklar PLines CMichelson D, et al. ; Preladenant Early Parkinson Disease Study Group (2017) Randomized trial of preladenant, given as monotherapy, in patients with early Parkinson disease. Neurology 88:2198–2206. [DOI] [PubMed] [Google Scholar]
- Storme J, Tosh DK, Gao ZG, Jacobson KA, Stove CP (2018) Probing structure-activity relationship in β-arrestin2 recruitment of diversely substituted adenosine derivatives. Biochem Pharmacol 158:103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strazzulla LC, Cronstein BN (2016) Regulation of bone and cartilage by adenosine signaling. Purinergic Signal 12:583–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama K, Tomata Y, Kaiho Y, Honkura K, Sugawara Y, Tsuji I (2016) Association between coffee consumption and incident risk of disabling dementia in elderly Japanese: the Ohsaki cohort 2006 study. J Alzheimers Dis 50:491–500. [DOI] [PubMed] [Google Scholar]
- Sun B, Bachhawat P, Chu ML, Wood M, Ceska T, Sands ZA, Mercier J, Lebon F, Kobilka TS, Kobilka BK (2017) Crystal structure of the adenosine A2A receptor bound to an antagonist reveals a potential allosteric pocket. Proc Natl Acad Sci USA 114:2066–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sušac L, Eddy MT, Didenko T, Stevens RC, Wüthrich K (2018) A2A adenosine receptor functional states characterized by 19F-NMR. Proc Natl Acad Sci USA 115:12733–12738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinney DC (2006a) Biochemical mechanisms of new molecular entities (NMEs) approved by United States FDA during 2001-2004: mechanisms leading to optimal efficacy and safety. Curr Top Med Chem 6:461–478. [DOI] [PubMed] [Google Scholar]
- Swinney DC (2006b) Can binding kinetics translate to a clinically differentiated drug? From theory to practice. Lett Drug Des Discov 3:569–574. [Google Scholar]
- Sykes DA, Dowling MR, Charlton SJ (2009) Exploring the mechanism of agonist efficacy: a relationship between efficacy and agonist dissociation rate at the muscarinic M3 receptor. Mol Pharmacol 76:543–551. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Fujita M, Asai N, Saki M, Mori A (2018) Safety and effectiveness of istradefylline in patients with Parkinson’s disease: interim analysis of a post-marketing surveillance study in Japan. Expert Opin Pharmacother 19:1635–1642. [DOI] [PubMed] [Google Scholar]
- Taomoto M, McLeod DS, Merges C, Lutty GA (2000) Localization of adenosine A2a receptor in retinal development and oxygen-induced retinopathy. Invest Ophthalmol Vis Sci 41:230–243. [PubMed] [Google Scholar]
- Temido-Ferreira MFerreira DGBatalha VLMarques-Morgado ICoelho JEPereira PGomes RPinto ACarvalho SCanas PM, et al. (2020) Age-related shift in LTD is dependent on neuronal adenosine A2A receptors interplay with mGluR5 and NMDA receptors. Mol Psychiatry 25:1876–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tescarollo FC, Rombo DM, DeLiberto LK, Fedele DE, Alharfoush E, Tomé AR, Cunha RA, Sebastião AM, Boison D (2020) Role of adenosine in epilepsy and seizures. J Caffeine Adenosine Res 10:45–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thammavongsa V, Kern JW, Missiakas DM, Schneewind O (2009) Staphylococcus aureus synthesizes adenosine to escape host immune responses. J Exp Med 206:2417–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas GS, Cullom SJ, Kitt TM, Feaheny KM, Ananthasubramaniam K, Gropler RJ, Jain D, Thompson RC (2017) The EXERRT trial: “EXErcise to Regadenoson in Recovery Trial”: a phase 3b, open-label, parallel group, randomized, multicenter study to assess regadenoson administration following an inadequate exercise stress test as compared to regadenoson without exercise for myocardial perfusion imaging using a SPECT protocol. J Nucl Cardiol 24:788–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson EA, Powell JD (2021) Inhibition of the adenosine pathway to potentiate cancer immunotherapy: potential for combinatorial approaches. Annu Rev Med 72:331–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilley SL (2011) Methylxanthines in asthma. Handb Exp Pharmacol 200:439–456. [DOI] [PubMed] [Google Scholar]
- Tonge PJ (2018) Drug-target kinetics in drug discovery. ACS Chem Neurosci 9:29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosh DKSalmaso VRao HBitant AFisher CLLieberman DIVorbrüggen HReitman MLGavrilova OGao ZG, et al. (2020) Truncated (N)-methanocarba nucleosides as partial agonists at mouse and human A3 adenosine receptors: affinity enhancement by N6-(2-phenylethyl) substitution. J Med Chem 63:4334–4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valant C, Aurelio L, Urmaliya VB, White P, Scammells PJ, Sexton PM, Christopoulos A (2010) Delineating the mode of action of adenosine A1 receptor allosteric modulators. Mol Pharmacol 78:444–455. [DOI] [PubMed] [Google Scholar]
- Valant C, May LT, Aurelio L, Chuo CH, White PJ, Baltos JA, Sexton PM, Scammells PJ, Christopoulos A (2014) Separation of on-target efficacy from adverse effects through rational design of a bitopic adenosine receptor agonist. Proc Natl Acad Sci USA 111:4614–4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallon V, Miracle C, Thomson S (2008) Adenosine and kidney function: potential implications in patients with heart failure. Eur J Heart Fail 10:176–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallon V, Mühlbauer B, Osswald H (2006) Adenosine and kidney function. Physiol Rev 86:901–940. [DOI] [PubMed] [Google Scholar]
- van Dam RM, Hu FB, Willett WC (2020) Coffee, caffeine, and health. N Engl J Med 383:369–378. [DOI] [PubMed] [Google Scholar]
- van Gelder BM, Buijsse B, Tijhuis M, Kalmijn S, Giampaoli S, Nissinen A, Kromhout D (2007) Coffee consumption is inversely associated with cognitive decline in elderly European men: the FINE Study. Eur J Clin Nutr 61:226–232. [DOI] [PubMed] [Google Scholar]
- van Muijlwijk-Koezen JE, Timmerman H, van der Goot H, Menge WM, Frijtag Von Drabbe Künzel J, de Groote M, IJzerman AP (2000) Isoquinoline and quinazoline urea analogues as antagonists for the human adenosine A(3) receptor. J Med Chem 43:2227–2238. [DOI] [PubMed] [Google Scholar]
- van Muijlwijk-Koezen JE, Timmerman H, van der Sluis RP, van de Stolpe AC, Menge WM, Beukers MW, van der Graaf PH, de Groote M, IJzerman AP (2001) Synthesis and use of FSCPX, an irreversible adenosine A1 antagonist, as a ‘receptor knock-down’ tool. Bioorg Med Chem Lett 11:815–818. [DOI] [PubMed] [Google Scholar]
- van Rhee AM, Jiang JL, Melman N, Olah ME, Stiles GL, Jacobson KA (1996) Interaction of 1,4-dihydropyridine and pyridine derivatives with adenosine receptors: selectivity for A3 receptors. J Med Chem 39:2980–2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varani K, Merighi S, Gessi S, Klotz KN, Leung E, Baraldi PG, Cacciari B, Romagnoli R, Spalluto G, Borea PA (2000) [(3)H]MRE 3008F20: a novel antagonist radioligand for the pharmacological and biochemical characterization of human A(3) adenosine receptors. Mol Pharmacol 57:968–975. [PubMed] [Google Scholar]
- Varty GB, Hodgson RA, Pond AJ, Grzelak ME, Parker EM, Hunter JC (2008) The effects of adenosine A2A receptor antagonists on haloperidol-induced movement disorders in primates. Psychopharmacology (Berl) 200:393–401. [DOI] [PubMed] [Google Scholar]
- Vass M, Kooistra AJ, Yang D, Stevens RC, Wang MW, de Graaf C (2018) Chemical diversity in the G protein-coupled receptor superfamily. Trends Pharmacol Sci 39:494–512. [DOI] [PubMed] [Google Scholar]
- Vauquelin G (2018) Link between a high k on for drug binding and a fast clinical action: to be or not to be? MedChemComm 9:1426–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecchio EA, Baltos JA, Nguyen ATN, Christopoulos A, White PJ, May LT (2018) New paradigms in adenosine receptor pharmacology: allostery, oligomerization and biased agonism. Br J Pharmacol 175:4036–4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatakrishnan AJ, Deupi X, Lebon G, Tate CG, Schertler GF, Babu MM (2013) Molecular signatures of G-protein-coupled receptors. Nature 494:185–194. [DOI] [PubMed] [Google Scholar]
- Verzijl D, IJzerman AP (2011) Functional selectivity of adenosine receptor ligands. Purinergic Signal 7:171–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viana da Silva SHaberl MGZhang PBethge PLemos CGonçalves NGorlewicz AMalezieux MGonçalves FQGrosjean N, et al. (2016) Early synaptic deficits in the APP/PS1 mouse model of Alzheimer’s disease involve neuronal adenosine A2A receptors. Nat Commun 7:11915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voors AAShah SJBax JJButler JGheorghiade MHernandez AFKitzman DWMcMurray JJVWirtz ABLanius V, et al. (2018) Rationale and design of the phase 2b clinical trials to study the effects of the partial adenosine A1-receptor agonist neladenoson bialanate in patients with chronic heart failure with reduced (PANTHEON) and preserved (PANACHE) ejection fraction. Eur J Heart Fail 20:1601–1610. [DOI] [PubMed] [Google Scholar]
- Wall MJHill EHuckstepp RBarkan KDeganutti GLeuenberger MPreti BWinfield IWei HImlach W, et al. (2020) A biased adenosine A1R agonist elicits analgesia without cardiorespiratory depression. bioRxiv:2020.2004.2004.023945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinert TOlieric NCheng RBrünle SJames DOzerov DGashi DVera LMarsh MJaeger K, et al. (2017) Serial millisecond crystallography for routine room-temperature structure determination at synchrotrons. Nat Commun 8:542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welihinda AA, Kaur M, Greene K, Zhai Y, Amento EP (2016) The adenosine metabolite inosine is a functional agonist of the adenosine A2A receptor with a unique signaling bias. Cell Signal 28:552–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White CW, Johnstone EKM, See HB, Pfleger KDG (2019) NanoBRET ligand binding at a GPCR under endogenous promotion facilitated by CRISPR/Cas9 genome editing. Cell Signal 54:27–34. [DOI] [PubMed] [Google Scholar]
- White KL, Eddy MT, Gao ZG, Han GW, Lian T, Deary A, Patel N, Jacobson KA, Katritch V, Stevens RC (2018) Structural connection between activation microswitch and allosteric sodium site in GPCR signaling. Structure 26:259–269.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham SB, Hotson AN, Miller RA (2020) Targeting the A2AR in cancer; early lessons from the clinic. Curr Opin Pharmacol 53:126–133. [DOI] [PubMed] [Google Scholar]
- Wootten D, Christopoulos A, Sexton PM (2013) Emerging paradigms in GPCR allostery: implications for drug discovery. Nat Rev Drug Discov 12:630–644. [DOI] [PubMed] [Google Scholar]
- Xia L, Burger WAC, van Veldhoven JPD, Kuiper BJ, van Duijl TT, Lenselink EB, Paasman E, Heitman LH, IJzerman AP (2017) Structure-affinity relationships and structure-kinetics relationships of pyrido[2,1-f]purine-2,4-dione derivatives as human adenosine A3 receptor antagonists. J Med Chem 60:7555–7568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia L, de Vries H, IJzerman AP, Heitman LH (2016) Scintillation proximity assay (SPA) as a new approach to determine a ligand’s kinetic profile. A case in point for the adenosine A1 receptor. Purinergic Signal 12:115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia L, Kyrizaki A, Tosh DK, van Duijl TT, Roorda JC, Jacobson KA, IJzerman AP, Heitman LH (2018) A binding kinetics study of human adenosine A3 receptor agonists. Biochem Pharmacol 153:248–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Liu N, Jacobson KA, Gavrilova O, Reitman ML (2019) Physiology and effects of nucleosides in mice lacking all four adenosine receptors. PLoS Biol 17:e3000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Wu H, Katritch V, Han GW, Jacobson KA, Gao ZG, Cherezov V, Stevens RC (2011) Structure of an agonist-bound human A2A adenosine receptor. Science 332:322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Dong G, Michiels TJM, Lenselink EB, Heitman L, Louvel J, IJzerman AP (2017) A covalent antagonist for the human adenosine A2A receptor. Purinergic Signal 13:191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Michiels TJM, de Jong C, Soethoudt M, Dekker N, Gordon E, van der Stelt M, Heitman LH, van der Es D, IJzerman AP (2018) An affinity-based probe for the human adenosine A2A receptor. J Med Chem 61:7892–7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, van Veldhoven JPD, Offringa J, Kuiper BJ, Lenselink EB, Heitman LH, van der Es D, IJzerman AP (2019) Development of covalent ligands for G protein-coupled receptors: a case for the human adenosine A3 receptor. J Med Chem 62:3539–3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye LNeale CSljoka ALyda BPichugin DTsuchimura NLarda STPomès RGarcía AEErnst OP, et al. (2018) Mechanistic insights into allosteric regulation of the A2A adenosine G protein-coupled receptor by physiological cations. Nat Commun 9:1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F, Zhu C, Xie Q, Wang Y (2020) Adenosine A2A receptor antagonists for cancer immunotherapy. J Med Chem 63:12196–12212. [DOI] [PubMed] [Google Scholar]
- Yu LShen HYCoelho JEAraújo IMHuang QYDay YJRebola NCanas PMRapp EKFerrara J, et al. (2008) Adenosine A2A receptor antagonists exert motor and neuroprotective effects by distinct cellular mechanisms. Ann Neurol 63:338–346. [DOI] [PubMed] [Google Scholar]
- Zhang G, Franklin PH, Murray TF (1994) Activation of adenosine A1 receptors underlies anticonvulsant effect of CGS21680. Eur J Pharmacol 255:239–243. [DOI] [PubMed] [Google Scholar]
- Zhang R (2015) Pharmacodynamics: which trails are your drugs taking? Nat Chem Biol 11:382–383. [DOI] [PubMed] [Google Scholar]
- Zhang SZhou RLi BLi HWang YGu XTang LWang CZhong DGe Y, et al. (2017) Caffeine preferentially protects against oxygen-induced retinopathy. FASEB J 31:3334–3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao ZAZhao YNing YLYang NPeng YLi PChen XYLiu DWang HChen X, et al. (2017) Adenosine A2A receptor inactivation alleviates early-onset cognitive dysfunction after traumatic brain injury involving an inhibition of tau hyperphosphorylation. Transl Psychiatry 7:e1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, Zhang S, Gu X, Ge Y, Zhong D, Zhou Y, Tang L, Liu XL, Chen JF (2018) Adenosine A2A receptor antagonists act at the hyperoxic phase to confer protection against retinopathy. Mol Med 24:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann H (2021) Ectonucleoside triphosphate diphosphohydrolases and ecto-5′-nucleotidase in purinergic signaling: how the field developed and where we are now. Purinergic Signal 17:117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]