Abstract
There is a vital need to understand mechanisms contributing to susceptibility to depression to improve treatments for the 11% of Americans who currently suffer from this debilitating disease. The adaptive immune system, comprising T and B cells, has emerged as a potential contributor to depression, as demonstrated in the context of lymphopenic mice. Overall, patients with depression have reduced circulating T and regulatory B cells, "immunosuppressed" T cells, and alterations in the relative abundance of T cell subtypes. T helper (Th) cells have the capacity to differentiate to various lineages depending on the cytokine environment, antigen stimulation, and costimulation. Regulatory T cells are decreased, and the Th1/Th2 ratio and the Th17 cells are increased in patients with depression. Evidence for changes in each Th lineage has been reported to some extent in patients with depression. However, the evidence is strongest for the association of depression with changes in Th17 cells. Th17 cells produce the inflammatory cytokine interleukin (IL)-17A, and the discovery of Th17 cell involvement in depression evolved from the well established link that IL-6, which is required for Th17 cell differentiation, contributes to the onset, and possibly maintenance, of depression. One intriguing action of Th17 cells is their participation in the gut-brain axis to mediate stress responses. Although the mechanisms of action of Th17 cells in depression remain unclear, neutralization of IL-17A by anti-IL-17A antibodies, blocking stress-induced production, or release of gut Th17 cells represent feasible therapeutic approaches and might provide a new avenue to improve depression symptoms.
Significance Statement
Th17 cells appear as a promising therapeutic target for depression, for which efficacious therapeutic options are limited. The use of neutralizing antibodies targeting Th17 cells has provided encouraging results in depressed patients with comorbid autoimmune diseases.
Introduction
Immunity is a critical guardian of health involving both protection against pathogens and maintenance of homeostasis. The immune response is often viewed as the cooperation between the innate immune system, which responds quickly and often unselectively to an insult, and the adaptive immune system, which takes time to be activated and requires antigen selectivity. Immune responses are often considered peripheral events in response to pathogen infections or injuries; nonetheless, they are not without effects on the central nervous system. This review will focus on the adaptive immune responses associated with depression.
Depression is a widespread and debilitating disorder. 17.3 million (7.1%) of adults and 2.3 million (9.4%) of adolescents aged 12–17 had at least one major depressive episode in the United States in 2017 (NIMH data). Women are more affected than men, and around two-thirds of patients with depression also experience severe emotional and cognitive impairments and difficulty handling daily activities such as sleeping, eating, or working. A major depressive episode is defined by the Diagnostic and Statistical Manual of Mental Disorders (DSM)-5 and is diagnosed if a person experiences a constellation of symptoms associated with depressed mood for at least 2 weeks. The etiology of depression remains mainly unknown, and the manifestation of the disease is heterogeneous. The risk factors for depression include a combination of genetic, environmental, biologic, and psychologic factors, in addition to comorbid disorders (e.g., diabetes, cardiovascular diseases, etc., and of relevance for this review autoimmune diseases). Multiple treatment options are available including medications, psychotherapy, and electroconvulsive therapies. Although efficient in around one-third of patients, antidepressant medications have the disadvantage of taking time to work (usually 2–4 weeks), and many patients develop treatment resistance (Beurel et al., 2020).
I. Dysregulation of the Adaptive Immune Response in Depression
A. Adaptive Immune System and Depression
Immune responses in patients with depression have attracted attention after findings that patients with depression have a variety of elevated inflammatory markers called cytokines, compared to healthy controls (Raison et al., 2006). Cytokines are produced by the innate immune system and participate in the quick resolution of the insult(s). In clinical trials for depression, patients have found benefit after selective anti-cytokine therapies, such as neutralization of tumor necrosis factor (TNF) or interleukin (IL)-6, although these results are not always reproduced and the sample size is small, mainly due to the expensive nature of these therapies. Yet the adaptive immune system also produces cytokines, and some promising therapies have emerged from targeting cytokines produced by the adaptive immune system (e.g., anti-IL-23 or anti-IL-17A therapies), which will be discussed in more detail later in this review.
The adaptive immune system has been well described in textbooks (Murphy et al., 2022). Briefly, it is composed of lymphocytes T and B cells and is activated when the innate immune system is overwhelmed by an infection or an insult, which is often the case of infections with pathogens. The microenvironment produced by the innate immune response to the infection or insult provides the adequate conditions to activate the adaptive immune system in an antigen specific manner. T cells are circulating in the bloodstream after they complete their development in the thymus and reach peripheral lymphoid organs through the lymphatics, where they usually encounter processed antigens by the antigen presenting cells. T cells that have not encountered antigens yet are called naïve T cells, whereas effector T cells are ready to perform their function as soon as they encounter their specific antigen. Every effector T cell can differentiate in several functional classes specialized in several different activities. Thus, cluster of differentiation (CD)8+ T cells recognize pathogens presented by major histocompatibility complex (MHC) class I molecules, which are expressed by nearly every mammalian cell type, and differentiate into cytotoxic effector T cells to kill infected cells. In contrast, CD4+ T cells recognize pathogens presented by MHC class II, in which expression is restricted to cells of the immune system and have the capacity to differentiate in a continuum of T helper (Th) lineages. B cells are in charge of the humoral immunity and when differentiated into plasma cells, produce antibodies. Antibodies often survey extracellular space to eliminate any pathogens using a variety of mechanisms (direct blockade of pathogen infectivity, induction of the complement cascade leading to phagocytosis, etc.). Altogether the adaptive immune system is critical to resolve the insult, which explains why mounting immune responses to self-antigens often lead to the development of autoimmune diseases.
Unmedicated patients with major depressive disorders often exhibit lymphopenia (reduced number of circulating lymphocytes) (Kronfol et al., 1984; Schleifer et al., 1984; Irwin et al., 1990; Herbert and Cohen, 1993; Zorrilla et al., 2001). When examining leukocyte enumeration, the data are more controversial with reports of decrease of T cells (Schleifer et al., 1984,1985), B cells (Schleifer et al., 1984; Maes et al., 1992b; Petitto et al., 1993; Ahmetspahic et al., 2018), and increase of the CD4/CD8 ratio (Darko et al., 1988; Tondo et al., 1988; Müller et al., 1989) or no change in any lymphocyte subsets (Calabrese et al., 1986; Darko et al., 1989; Kronfol and House, 1989; Schleifer et al., 1989). Other cells from the innate immune system (e.g., natural killer cells, neutrophils, and monocytes) have also been shown to be differentially present in patients with major depressive disorder (MDD) (Evans et al., 1992; Ravindran et al., 1999). Furthermore, recent studies have shown an association between low white blood cell counts and severity of depression (Beydoun et al., 2016; Köhler et al., 2017), whereas others report in larger cohorts of patients a genetic association between polygenic score and increased white blood cell counts (Shafiee et al., 2017; Sealock et al., 2021). Whether these controversial reports result from the analysis of different subpopulations of T or B cells remains to be determined. It is indeed only recently that advances in the immunophenotyping of immune cells have allowed the discovery of subsets of T or B cells associated with major depressive disorder. For example, CD24+CD38hi transitional B cells, which are associated with a regulatory phenotype, are reduced in MDD (Ahmetspahic et al., 2018). Transitional B cells originate from the bone marrow to reach the lymphoid tissues to complete maturation (Sims et al., 2005; Palanichamy et al., 2009). Similarly, memory CD4+ T cells and CD69+CD19+ activated B cells have been found reduced among peripheral blood mononuclear cells exposed to plasma of unmedicated patients with MDD, compared with exposure to plasma of healthy control subjects (Syed et al., 2018). Furthermore, telomeres of three lymphocyte subpopulations [CD8+ (T cytotoxic cells), CD20+ (B cells), and CD4+ (T helper) cells] are shorter in female patients with MDD compared with age matched female controls (Karabatsiakis et al., 2014), suggesting that these subpopulations of lymphocytes age faster in patients with depression. The different subtypes of Th cells will be discussed in greater details in the next section.
In mouse models, acute stress seems associated with increased immune responses, whereas chronic stress in contrast reduces immune responses including reduced leukocyte trafficking, neutrophil phagocytosis, and reduction of peripheral lymphocytes (Padgett and Glaser, 2003; Glaser and Kiecolt-Glaser, 2005; Fan et al., 2019). Yet, Rag1−/− or Rag2−/− mice, which are deficient in T and B cells, have been shown to exhibit attenuated depressive-like and anxiety-like behaviors (Rattazzi et al., 2013; Clark et al., 2015, 2016 Rilett et al., 2015; Fan et al., 2019). Furthermore, adoptive transfer of splenocytes/lymphocytes from chronically stressed mice protects naïve mice from exhibiting anxiety-like and depressive-like behaviors (Lewitus et al., 2009; Brachman et al., 2015). T cells are also necessary to promote the resolution of inflammation-induced depressive-like behaviors (Laumet et al., 2018). The role of B cells in depressive-like behaviors has been less studied, but some reports have shown an amelioration of depressive symptoms in patients receiving B cell neutralizing antibodies (Wittenberg et al., 2020), whereas others have reported increased incidence of depression in patients with autoimmune disorders treated with the anti-B cell activating factor (BAFF) antibodies (Belimumab) (Minnema et al., 2019), which deplete B cells, as BAFF is a B cell survival cytokine belonging to the TNF superfamily. In addition, autoantibody levels are elevated in MDD patients (Iseme et al., 2014; Postal and Appenzeller, 2015). Overall, these findings suggest that the apparent controversial effects of T and B cells in depression might in fact result from the existence of various T and B cell subtypes that might respond differentially to the environment (e.g., cytokine milieu, stress hormones, microbiome composition, etc.).
B. T Helper Cells and Depression
In response to the milieu of cytokines, the activation by an antigen, and a costimulatory signal, CD4+ T cells differentiate into a specific lineage belonging to a continuum of phenotypes. The main lineages studied in depression are: Th1, Th2, Th17, cells and regulatory T cells (Treg) (Fig. 1). Simplistically, Th1 cells require IL-12 and interferon (IFN)γ to differentiate in vitro and produce IFNγ; Th2 cells require IL-4 and produce IL-4, IL-5, and IL-13; Th17 cells require IL-6 and transforming growth factor (TGF)β and produce IL-17A; and Treg cells require TGFβ and express forkhead box P3 (Foxp3) (Zhu et al., 2010).
Fig. 1.
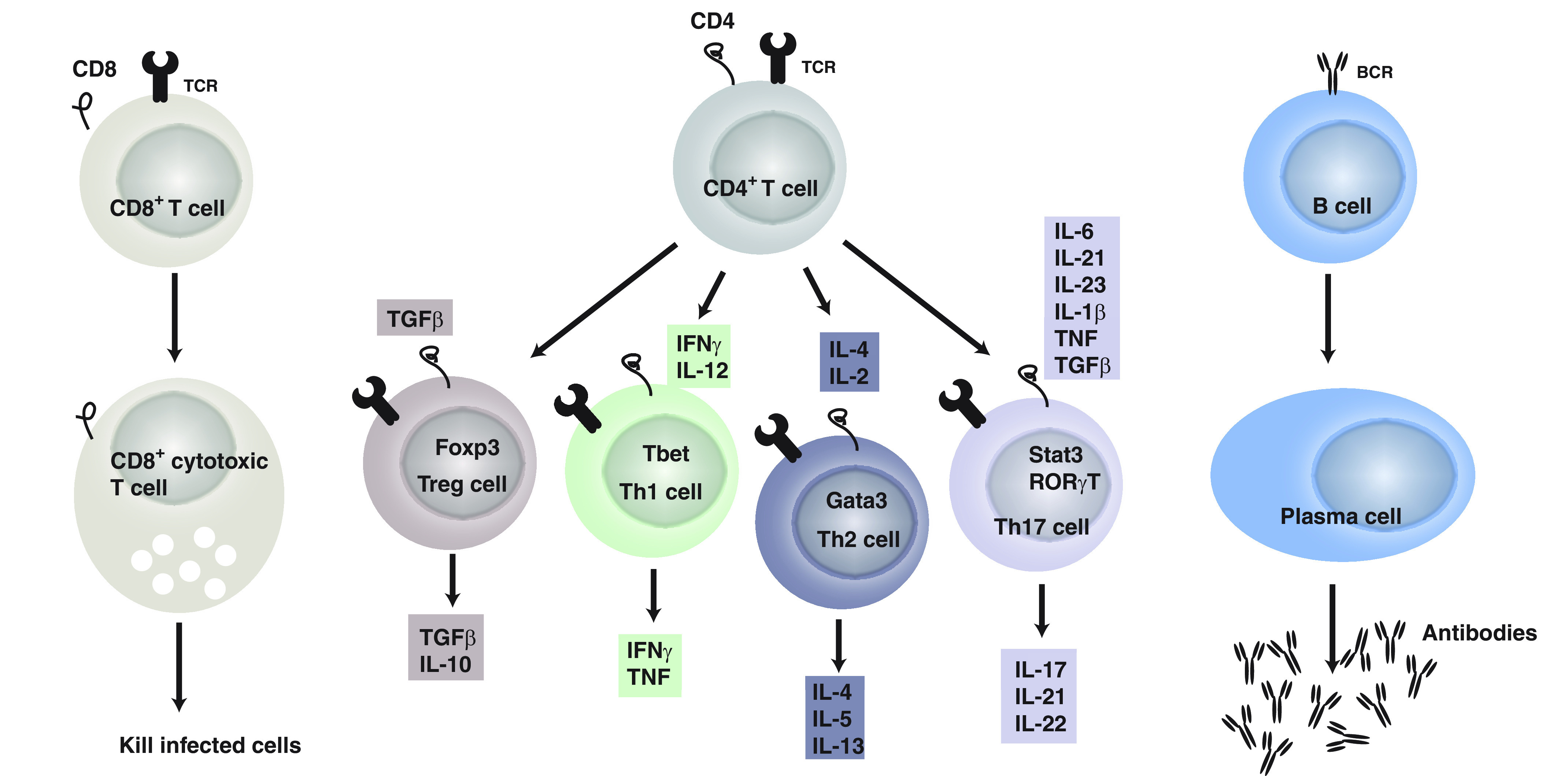
The adaptive immune system. It includes T (CD4+ and CD8+) and B cells. CD8+ T cells differentiate into cytotoxic T cells after recognizing antigen presented by MHC class I. CD4+ T cells in contrast recognize antigens presented by MHC class II on antigen-presenting cells. Once activated by their antigen and the cytokine milieu (each lineage of CD4+ T cells has a combination of cytokines required to differentiate), as well as upon receiving costimulatory signals, CD4+ T cells differentiate in the different lineages: Treg, Th1, Th2, and Th17 cells, which produce a combination of cytokines as indicated. Finally, CD4+ T cells help B cells to differentiate into plasma cells once they encounter a specific antigen and plasma cells produce antigen specific antibodies.
1. T Helper 1/T Helper 2 Cells
Although data are limited, there is growing evidence of alterations of Th cells in MDD. Some findings include evidence in patients with MDD of increased Th cells relative to T cytotoxic cells (Darko et al., 1988; Tondo et al., 1988; Müller et al., 1989), and elevated ratio of Th1/Th2 cells (Maes et al., 1992a) that is reversed by antidepressants (Kubera et al., 2001). It is generally thought that acute mild stress increases Th2 activation, whereas chronic or repeated stress leads to Th1 activation (Maes et al., 2012). Th1 cells are increased in the brains of mice subjected to learned helplessness, although their role in promoting learned helplessness is less clear (Beurel et al., 2013, 2018a). Nonetheless, Th1 cell specific transcription factor Tbet knockout mice do not exhibit depressive-like behaviors (Kim et al., 2011), suggesting a positive role of Th1 cells in promoting depressive-like behaviors. One of the possible mechanisms whereby Th1 cells promote depressive-like behaviors is through the activation of the enzyme indoleamine 2,3-dioxygenase (IDO). Indeed, IDO converts tryptophan to kynurenine, depleting the pool of tryptophan required for the synthesis of serotonin. IDO is activated in depression (Wichers and Maes, 2004) and antidepressants reduce Th1 cell level (Mohr et al., 2001). In addition, polymorphisms in the Th1 gene Tbet predict diagnosis of MDD (Wong et al., 2008). These suggest that the proinflammatory Th1 cells promote depression. In contrast, Th2 cells attenuate sepsis-associated encephalopathy-induced anxiety-like behaviors (Saito et al., 2021). Reciprocally, Th2-associated cytokine IL-4-deficient mice exhibit enhanced depressive-like behaviors (Wachholz et al., 2017). IL-4 inhibits depressive-like behaviors induced by IL-1β-mediated-astrocytic activation and IL-1β-induced alterations of serotonin and norepinephrine levels (Park et al., 2015). These suggest that Th2 cells might be anti-inflammatory and protect from depression.
2. T Helper 17 Cells
Recently, most of the studies have focused on the newly discovered Th17 cell subset of T helper cells (Harrington et al., 2005). There is accumulating evidence that Th17 cells are increased in depression and contribute to the pathology of depression. Thus, patients with MDD have elevated blood levels of Th17 cells (Chen et al., 2011; Ghosh et al., 2020), and patients at high risk of suicide exhibit the highest level of Th17 cells (Schiweck et al., 2020). Furthermore, CD4+ T cells isolated from patients with generalized anxiety disorder when activated in vitro acquire a Th17 phenotype (Vieira et al., 2010; Ferreira et al., 2011). It is important to note patients with Th17 cell-associated autoimmune diseases often exhibit comorbid MDD (Kurd et al., 2010; Patten et al., 2017; Singh et al., 2020; Sales et al., 2021). Consistent with these findings, the signature inflammatory cytokine produced by Th17 cells, IL-17A, is elevated in some patients with MDD (Chen et al., 2011; Liu et al., 2012; Kim et al., 2013a; Davami et al., 2016), but not all (Liu et al., 2012; Kim et al., 2013b), and in patients with post-traumatic stress disorder (Zhou et al., 2014). In addition, the treatment response to certain antidepressants (bupropion and escitalopram combination) is predicted by IL-17A levels (Jha et al., 2017). Depressive symptoms are reduced after anti-IL-17A treatment in 40% of patients with psoriasis experiencing MDD (Griffiths et al., 2017), whereas blocking the downstream effectors of IL-17A in patients with psoriasis by blocking its receptor using anti-IL-17RA therapy has been associated with increased psychiatric disorders and suicidality risk (Lebwohl et al., 2018).
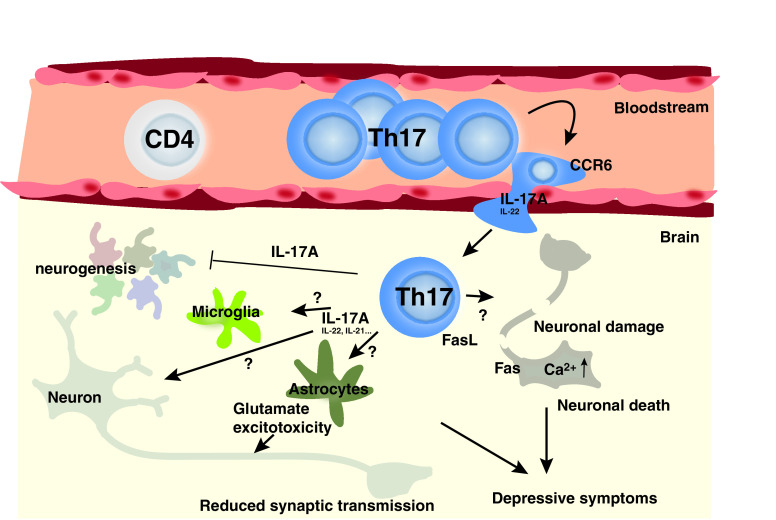
Studies in rodents provide corroborative evidence of these detrimental effects of Th17/IL-17A in depression, such as administration of IL-17A in rodents promotes depressive-like behaviors (Nadeem et al., 2017). Stress increases IL-17A levels (Lu et al., 2017; Cheng et al., 2018; Gu et al., 2018; Zhang et al., 2019). Th17 cells accumulate in the brain of learned helpless mice or mice subjected to chronic restraint stress (Beurel et al., 2013, 2018; Medina-Rodriguez et al., 2020). Th2 and Th17 responses are associated with exacerbation of fear in a severe allergen induced airway hyperreactivity mouse model (Lewkowich et al., 2020). Splenic Th17 cells are increased after stress induced by social defeat (Ambrée et al., 2019), and Th17/Treg ratio is increased in the liver and ileum of mice subjected to chronic stress (Westfall et al., 2021). The involvement of Th17 cells in promoting depressive-like behaviors has been demonstrated by using a variety of approaches: stimulation by IL-17A (Nadeem et al., 2017), neutralization of IL-17A (Beurel et al., 2013; Nadeem et al., 2017; Kim et al., 2021), pharmacological inhibition (Beurel et al., 2013), or knockout approach where the master transcription factor of Th17 cells is depleted (Beurel et al., 2013). Th17 cells increase microglial activation in the hippocampus, amygdala, and prefrontal cortex of cumulative mild prenatal stress, and anti-IL-17 treatment rescues depressive-like and anxiety-like behaviors after perinatal stress (Kim et al., 2021). Moreover, the transcription factor Retinoic acid receptors (RAR)-related orphan receptor gamma T (RORγT) is required for Th17 induced depressive-like behaviors (Beurel et al., 2013, 2018a). Little is known about the mechanisms of action of Th17 cells, as well as their mode of infiltration into the brain (Fig. 2). Nonetheless, the expression of the chemokine receptor C-C motif chemokine receptor (CCR)6 is required for Th17 cells to infiltrate the brain (Beurel et al., 2018a). Th17 cells in the brain also express the follicular marker C-X-C motif chemokine receptor 5, whose contribution to depression is less clear (Beurel et al., 2018a). The possible mechanisms of action of Th17 cells include induction of neuroinflammation by activated microglia and astrocytes and/or induction of neuronal cell death [e.g., increased IL-17A-mediated astrocytic glutamate excitotoxicity (Kostic et al., 2017)] as well as inhibition of neurogenesis by IL-17A as shown by IL-17A knockout, which increases neurogenesis (Liu et al., 2014) (Fig. 2).
Fig. 2.
Potential mechanisms whereby Th17 cells promote depressive symptoms. Upon stress, Th17 cells enter the brain. CCR6 and IL-17A seem critical for the entry of pathogenic Th17 cells into the brain after stress. Various effects of Th17 cells have been proposed in the brain. Neurons, microglia, and astrocytes express IL-17RA and are therefore regulated by the Th17 cell signature cytokine IL-17A. In astrocytes, for example, IL-17A activates glutamate excitotoxicity. IL-17A also inhibits neurogenesis. Th17 cells have also been reported to trigger neuronal cell death via direct contact leading to increased intracellular Ca2+ level or through activation of neuronal Fas by FasL produced by Th17 cells.
Th17 cells are present in healthy mice in the lamina propria of the small intestines because of the presence of the bacteria Segmented filamentous bacterium (SFB), which is required to induce the differentiation of Th17 cells (Ivanov et al., 2009). SFB level predicts sensitivity to the learned helplessness paradigm in a Th17 cell-dependent manner. Mice deficient in SFB are resistant to the learned helplessness (Medina-Rodriguez et al., 2020). Furthermore, CD4+ T cells specific to SFB are sufficient to promote depressive-like behaviors and accumulation of Th17 cells in the hippocampus of Rag2−/− mice (Medina-Rodriguez et al., 2020). This suggests that microbiome alterations might be responsible for the increased Th17 cell response in depression.
3. Treg Cells
In contrast to those inflammatory Th cell types, anti-inflammatory Treg cells are decreased in patients with MDD (Li et al., 2010; Chen et al., 2011; Grosse et al., 2016b; Jahangard and Behzad, 2020) or adolescents with high risk for mood disorders (Snijders et al., 2016), whereas other studies find increased regulatory T cells in patients with MDD (Suzuki et al., 2017; Patas et al., 2018). Treg cells are decreased after chronic unpredictable stress in rodents (Hong et al., 2013). Depletion of Treg cells is associated with higher rate of depressive-like behaviors in mice (Kim et al., 2012). Depletion of Treg cells is also associated with increased levels of Th1 (IFNγ), Th2 (IL-4), Th17 (IL-17) cytokines, and reduced serotonin level (Kim et al., 2012). In addition, Treg cells are increased after effective antidepressant treatment (Himmerich et al., 2010; Grosse et al., 2016a). Altogether, findings on T helper cells suggest that depression is associated with more proinflammatory Th cells and fewer immunosuppressing regulatory T cells.
II. Possible Origins of These Immune Dysregulations in Depression
Although it remains to be determined whether all the dysregulations of the adaptive immune system observed in patients with depression occur in the same individual at the same time, it is possible to envision that factors specific to depression might affect the adaptive immune response. Thus, for example, it is possible that the plasticity of Th cells is responsible for some of the differences observed between the different Th subtypes in depression. Late stage Th17 cells can indeed differentiate into Th1 cells (Lee et al., 2009) or regulatory T cells (Gagliani et al., 2015), for example. It is also possible that previous treatments with antidepressants affect the proportion of T helper cells. In addition, Th cells are differently affected by stress, including the type of stress (acute versus chronic), the stress hormones, as well as indirect effects of stress such as alterations of the microbiota, which is known to control immunity. Thus, acute stress promotes leukocyte mobilization to the blood (Dhabhar et al., 2012) and infiltration into the site of inflammation (Viswanathan and Dhabhar, 2005) and mostly activates proliferative responses of immune cells (Rinner et al., 1992). In addition, increased activity of natural killer and CD8+T cells is found in humans subjected to acute stress. Chronic stress in contrast is immunosuppressant in animals and humans (Kiecolt-Glaser et al., 1996; Dhabhar, 2000). Some of the pathways contributing to these different actions of stress are described below (Fig. 3).
Fig. 3.
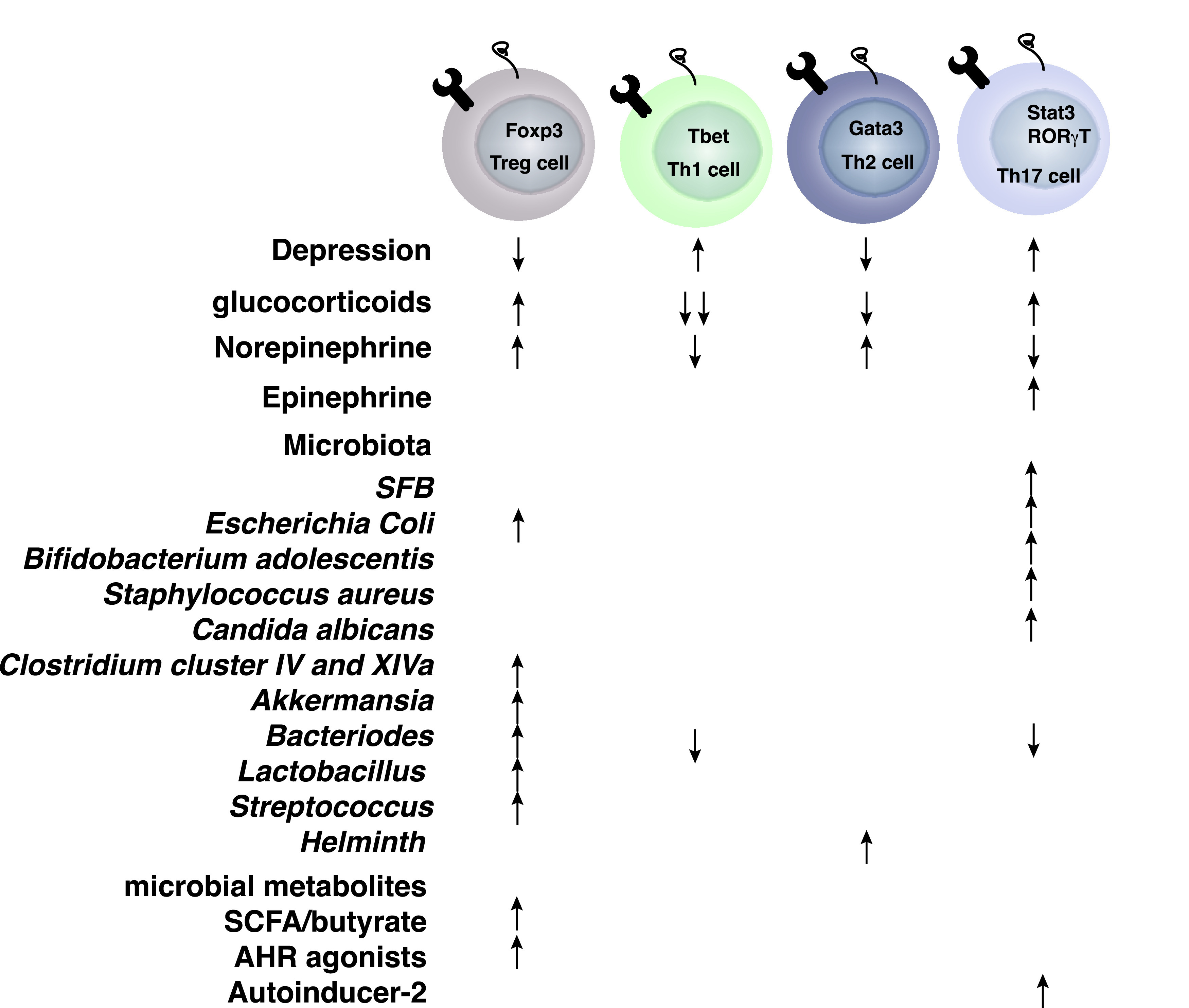
Effects of glucocorticoids, catecholamines, and microbiota on T helper cells.
A. Glucocorticoids
Glucocorticoids are small lipid hormones that are produced by the adrenal glands and are immunosuppressant in periphery and, therefore, have been used as anti-inflammatory and immunosuppressive therapies. Stress and circadian alterations change the level of glucocorticoids to regulate metabolism, neuronal and cardiovascular functions, as well as immune activity. Exogenous glucocorticoid administration such as corticosteroid treatment in mice provides immunosuppression in part by inhibiting the secretion of T cell growth factors; blocking the gene expression of a wide variety of cytokines including IL-1, IL-2, IL-3, IL-4, IL-5, IL-6, IL-8, IL-10, IL-13, granulocyte-macrophage colony-stimulating factor (GM-CSF), TNF, and IFNγ; and shaping the cytokine milieu required for T cell differentiation (for review Taves and Ashwell, 2021). Endogenous glucocorticoids, however, can promote or suppress immunity. Glucocorticoids have been implicated in the selection of the appropriate T cell receptor repertoire in the thymus, suppressing Th1 cells (Franchimont et al., 2000) while moderately inhibiting Th2 cells and favoring Th17 cells (Schewitz-Bowers et al., 2015), regulating T cell trafficking, and promoting T cell memory (Taves and Ashwell, 2021). Glucocorticoid receptors are expressed on all T cells, but different T cells have different sensitivity to glucocorticoids. The difference is mainly attributed to the glucocorticoid-induced loss of expression of B-cell lymphoma 2 (Bcl-2), and greater expression of Bim in Th1 cells, whereas Bcl-2 is moderately affected in Th2 cells and increased in Th17 cells (Banuelos et al., 2017). Furthermore, Treg cell differentiation is also promoted by glucocorticoids due to the upregulation of TGFβ receptors and Foxp3 (Karagiannidis et al., 2004), and the promotion of regulatory T cells by glucocorticoids might be one of the major mechanisms mediating immunosuppression (Fig. 4). These wide effects of glucocorticoids on immunity might explain the enrichment in certain T cell subtypes during depression, e.g., Th17 cells are the most resistant cells to glucocorticoid-induced apoptosis and are found elevated in depression. Depression is associated with increased level of cortisol/corticosterone in humans and animal models (Pariante, 2009). It is important to note, that although of potential benefit to patients with MDD because glucocorticoids reduce inflammation and inflammation is elevated in patients with MDD, glucocorticoid treatment only provides minimal improvement to patients with MDD with short-term treatments (DeBattista et al., 2000). Furthermore, patients with MDD exhibit a dysregulated response to glucocorticoids (Carvalho et al., 2015), which complicates the interpretation of the role of glucocorticoids in T cell-mediated behavioral alterations. Nonetheless, several studies have pointed toward a blunted leukocyte responsiveness to glucocorticoids in patients with MDD (Bauer et al., 2002, 2003; Raison and Miller, 2003; Carvalho et al., 2014; Grosse et al., 2015; Pariante and Miller, 2001), whereas another study has not (Hasselmann et al., 2018). Similarly, T and B cell responses are modulated by exposure to stress and glucocorticoids in animal models (Roque et al., 2011; Clark et al., 2019), suggesting a possible mechanism whereby lymphocytes become immunosuppressed in depression.
Fig. 4.
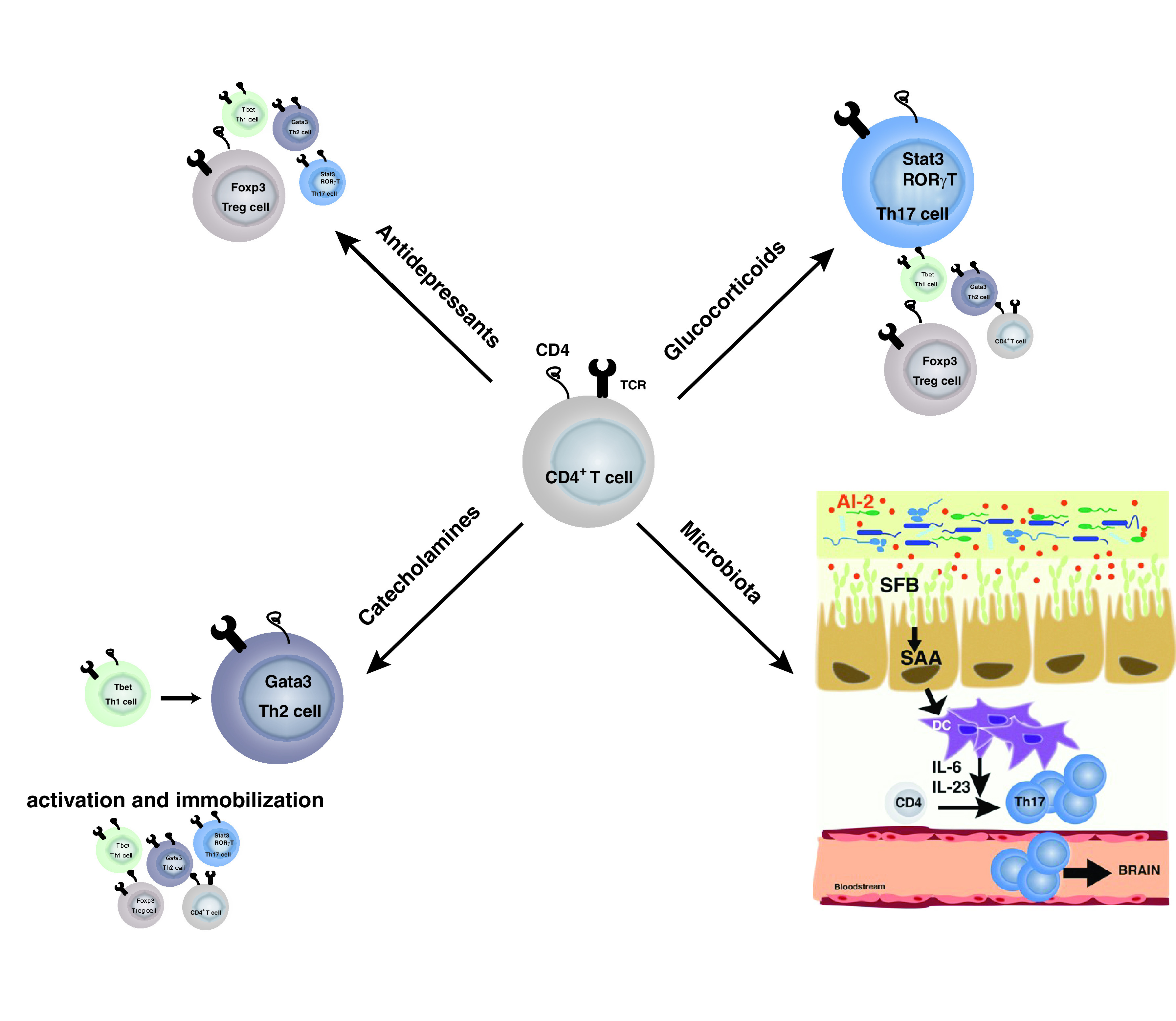
Overall selection of T cells associated with depression. Glucocorticoid treatment and microbiota alterations in depression have been associated with a shift toward Th17 cells. Catecholamines seem to favor Th2 cells. However, catecholamines promote T helper cell activation, whereas they block leukocyte migration. Antidepressant treatments in contrast seem to reduce T helper cells in a nonspecific manner and favor regulatory T cells.
B. Catecholamines
The catecholamines include neurotransmitters such as epinephrine, norepinephrine, and dopamine and act through the autonomic nervous system. Traditionally, the sympathetic nervous system is activated after stress to increase blood epinephrine and norepinephrine levels. Immune cells express both α-adrenergic and β-adrenergic receptors, and their level of expression depends on the state of the cells, their maturation, and activation (Elenkov et al., 2000; Scanzano and Cosentino, 2015). Consistent with this, norepinephrine promotes a shift from Th1 cells to Th2 cells (Wahle et al., 2006; Hou et al., 2013), whereas the overall role of catecholamines on Th17 cells is less clear. Epinephrine promotes Th17 cells via secretion of cytokines by the dendritic cells (Kim and Jones, 2010; Manni et al., 2011), whereas norepinephrine inhibits Th17 cells directly through β2-adrenergic receptors (Liu et al., 2018). Furthermore, effector T cells are activated, whereas naive splenic T cells are suppressed by increased levels of catecholamines (Case and Zimmerman, 2015). These suggest that catecholamines affect activation of T cells. Nevertheless, the duration and concentration of exposure to the catecholamines, the interaction with other factors induced during stress (glucocorticoids, etc.), and the role of adrenergic receptors in the various T cell subtypes need to be also taken into consideration. Maybe the most striking effect of catecholamines on T cells is their effects on migration (Fig. 4). Indeed, T cell migration is temporarily paralyzed in response to stress-induced sympathetic nervous system activation (Devi et al., 2021), which might explain the effect of acute stress on leukocyte migration. Inflammation has been shown to reduce dopamine synthesis and availability (Felger and Treadway, 2017), suggesting that in depression, where inflammation is increased and dopamine level reduced, T cell migration might be enhanced. Other nervous systems also affect T cells, including the parasympathetic nervous system with the release of acetylcholine and the sensory nervous system with the release of neuropeptides and glutamate, which are also known to modulate T cell responses. Of interest for this review, skin sensory neuron activation induces IL-17 response (Cohen et al., 2019).
These suggest that T cells are the point of convergence of many pathways associated with the stress response.
C. Microbiome
Host microbiota interactions influence the development of the adaptive immune system. These interactions are required for homeostasis. Yet, changes in the environment or genetic defects lead to disruption of these interactions and promote diseases. Depression has been associated with changes in the relative abundance of Firmicutes, Actinobacteria, and Bacteroidetes compared to healthy individuals [(Zheng et al., 2016); for review, see (Cheung et al., 2019)], but the pathways and underlying mechanisms used by the microbiota to promote diseases are just emerging.
The adaptive immune system is in close contact with the microbiota, and mutual regulatory mechanisms are in place. Thus, to protect the gut mucosal epithelial layer from microbes, B cells secrete microbial antigen specific IgA into the gut lumen. The production of IgA is critical for shaping the microbial communities and enhancing regulatory T cell expansion (Kawamoto et al., 2014). Th17 cells, which are only present in the small intestinal lamina propria of healthy mice, also participate in the maintenance of the epithelial barrier by producing low amounts of IL-17A (Cypowyj et al., 2012). Th17 cells are absent in germ-free mice, showing a major impact of the microbiota on Th17 cells (Amatya et al., 2017) and suggesting that Th17 cells might be one of the major Th subtypes dependent on the regulation by the microbiota. SFB is one of the first discovered inducers of Th17 cells (Ivanov et al., 2009). Other bacteria [e.g., Escherichia coli (Viladomiu et al., 2017), Bifidobacterium adolescentis (Tan et al., 2016), Staphylococcus aureus (Zielinski et al., 2012), and Candida albicans (Hernández-Santos and Gaffen, 2012)] also increase Th17 cells. However, it is important to note that these different microbes induce different flavors of Th17 cells (Tan et al., 2016). This suggests that other signals are required besides the microbial antigen to promote the differentiation of Th17 cells (Yang et al., 2014). Thus, for example, transdifferentiation of Th17 cells into regulatory T cells is partially dependent on the aryl hydrocarbon receptor (Ahr), whose agonists are produced by the microbiota to promote anti-inflammatory responses comprising of tryptophan metabolites (Hirota et al., 2011; Wlodarska et al., 2017). The abundance of aryl hydrocarbon receptor agonists in the microbiota is often diet dependent. Although Th17 cells might mediate certain actions of the microbiome in depression, the role of the microbiota-induced Th17 cells in depression remains largely unclear. The limited data show Th17 cells mediate SFB-dependent depressive-like behaviors. Stress increases SFB, which promotes the production of the quorum sensing molecule autoinducer-2, which in turn enhances the production of serum amyloid A (SAA)1/2 that is required for Th17 cell differentiation and promotion of depressive-like behavior (Medina-Rodriguez et al., 2020) (Fig. 4).
In contrast, spore forming Clostridium clusters IV and XIVa, Escherichia, Akkermansia, Bacteroides, Lactobacillus, and Streptococcus are potent inducers of regulatory T cells (Atarashi et al., 2013; Ahern et al., 2014; Geva-Zatorsky et al., 2017). Microbial metabolites (e.g., short chain fatty acids, especially butyrate) also promote regulatory T cells (Smith et al., 2013). Butyrate is mainly produced by Clostridiales species such as Ruminococcaceae and Lachnospiraceae. Polysaccharide A produced by Bacteroides species promotes Treg anti-inflammatory effects while inhibiting Th1 and Th17 cell differentiation (Round and Mazmanian, 2010; Round et al., 2011). The overall effect of the microbiota on the balance between Th17 and Treg cells depends on the composition of the microbiota. Thus, by promoting Th17/Treg imbalance microbiota metabolites promote depressive-like behaviors (Westfall et al., 2021). Although Th17 cells and Treg cells seem to be the main Th subtypes modulated by the microbiota, other Th cells have been associated with changes of the microbiota, such as helminth infection, which promotes a Th2 response (Reynolds et al., 2015).
Although the understanding of the effects of the microbiota on T cell plasticity remains to be fully understood, it is conceivable that small changes in the microbiota can have dramatic consequences on the adaptive immune cells that in turn impact mood. Although stress is known to affect microbiota composition, other factors affect the microbiota composition such as diet, disease, environment, etc. and will need to be considered. Recent evidence has pointed toward a dysregulated gut-brain axis including a choroid plexus vascular barrier contributing to the mental symptoms associated with inflammatory bowel disease (Carloni et al., 2021).
Overall, various stress signals converge on the adaptive immune cells, and it remains to be determined which signal(s) can be targeted to improve depression.
III. Implications for Patients with Depression
A. Increased Infections and Comorbidities
“We tend to get sick after stressful events” (Elstad and Vabø, 2008), prompts the question of whether the dysregulated adaptive immune response is responsible for higher sensitivity of patients with depression to infections, since the adaptive immune system is decisive in eradicating infections. A past history of depression is in fact associated with increased risks of infections (Troidle et al., 2003; Adams et al., 2008; Irwin et al., 2011; Seminog and Goldacre, 2013; Andersson et al., 2016). The effectiveness of the immune activity in depression is reduced and has been associated with increased mortality (Wulsin et al., 1999). Similarly, there is an increased risk of developing autoimmune diseases (multiple sclerosis, psoriasis, inflammatory bowel disease, rheumatoid arthritis, and systemic lupus erythematosus) in patients with depression (Kurina et al., 2001; Dickens et al., 2002; Andersson et al., 2015; Euesden et al., 2017; Patten et al., 2017). The autoreactive T cells, especially Th1 and Th17 cells, have been thought to be the culprit, and this is consistent with anti-IL-17A treatment ameliorating depression symptoms in patients with psoriasis (Griffiths et al., 2017). Reciprocally, antidepressant treatments (e.g., Sertraline, psychotherapy, and cognitive behavioral therapy) are associated with Th1-dependent improvement of multiple sclerosis (Mohr et al., 2001). Ketamine reduces the differentiation and proliferation of Th17 cells in a mouse model of experimental autoimmune encephalitis (Lee et al., 2017). Furthermore, there are reports of depression affecting the recovery from surgery (Cheng et al., 2020), and B regulatory cells are reduced in patients with hip fracture who develop depression compared with non-depressed patients with hip fracture or healthy individuals (Duggal et al., 2016). These suggest that the alterations of the immune system, especially of the adaptive immune system in depression are associated with negative outcomes in the long-term.
B. How Does the Adaptive Immune System Promote Depression?
Reciprocally, it also raises the question on how the adaptive immune alteration promotes depression. The adaptive immune system produces cytokines that can impede neuronal circuitries involved in mood regulation. Thus, for example IL-17A, by activating IL-17RA and IL-17RC receptors, leads to the production of cytokines and chemokines, matrix metalloproteinases, and anti-microbial peptides via the activation of TNF receptor associated factor (Traf)6, Traf4, nuclear factor kappa light chain enhancer of activated B cells (NF-κB), CCAAT-enhancer-binding proteins (C-EBP)β, C-EBPδ, and mitogen-activated protein kinase (MAPK) pathways [(for review (Milovanovic et al., 2020)]. Cytokines, especially IL-6, are sufficient to promote depressive-like behaviors. In addition, IL-17RA receptors are expressed on a variety of neuron subtypes, astrocytes, microglia, and endothelial cells in the brain (Das Sarma et al., 2009), broadening even more the effects of IL-17A (Fig. 2). However, it remains to be determined whether these cell specific pathways are all contributing to depression. Astrocytes mediate Th17 cell effects in a mouse model of multiple sclerosis (Kang et al., 2010). Th17 cells disrupt the blood brain barrier by acting on endothelial cells (Kebir et al., 2007), whereas they promote the production of cytokines by microglia (Murphy et al., 2010). Th17 cells promote neuronal damage (Siffrin et al., 2010) (Fig. 2). Although most of these findings were associated with autoimmune diseases for which the number of Th17 cells is higher than in depression, recent studies in autism suggest a Th17 cell-dependent neuronal damage in psychiatric diseases even when the number of Th17 cells is lower. Thus, IL-17A produced by Th17 cells induces abnormal cortical phenotype in a mouse model of maternal immune activation-inducing autistic traits (Choi et al., 2016; Reed et al., 2020), and the abnormal cortical phenotype is microbiota dependent (Kim et al., 2017). Similarly, IL-17A produced by meningeal γδT cells has been shown recently to promote anxiety-like behaviors by activating IL-17RA receptors on cortical glutamatergic neurons (Alves de Lima et al., 2020). Furthermore, Th17 cells induced by high salt diet promote neurovascular dysfunction in an IL-17A-dependent reduction of nitric oxide production by cerebral endothelial cells (Faraco et al., 2018). These data suggest that the action of Th17 cells might be specific depending on the disease and the brain region they infiltrate. Although similar mechanisms of action of Th17 cells in depression are conceivable, the specific mechanisms of action of Th17 cells in depression remain to be determined.
C. Potential Pharmacological Outcomes
Lastly, antidepressant drugs affect directly T cell proliferation and activity. Thus, fluoxetine reduces the proliferation of T cells (Fazzino et al., 2009; Ahern, 2011), leading to a decrease in number of blood T cells (Rosso et al., 2016). Fluoxetine (Himmerich et al., 2010; Grosse et al., 2016a) and ketamine (Hou et al., 2016) increase anti-inflammatory regulatory T cells. Ketamine also decreases Th2 cell differentiation (Gao et al., 2011), inhibits leukocyte adhesion and migration (Larsen et al., 1998; Kawasaki et al., 2001), and induces T cell apoptosis (Braun et al., 2010). Therefore, detangling the contribution of T cells in depression might be compromised by the use of an antidepressant treatment, and further studies will be required to understand the role of the adaptive immune cell response in medication-free depression.
Maybe one of the most promising approaches is the use of cytokine neutralizing antibodies targeting Th17 cells in depression. Indeed, few anti-IL-17A antibodies (Secukinumab, Ixekizumab) and anti-IL-17RA (Brodalumab) are available for psoriasis and have been tested on depressive symptoms associated with autoimmune diseases. Ixekizumab reduces by 40% depressive symptoms in patients with psoriasis (Griffiths et al., 2017). Brodalumab used in psoriasis, in contrast, promotes risk of suicide and of developing psychiatric illnesses, leading the Food and Drug Administration (FDA) to issue a warning (Lebwohl et al., 2018). Furthermore, anti-IL-12/IL-23 (Ustekinumab) treatment significantly improves depressive symptoms compared with placebo in patients with psoriasis (Wittenberg et al., 2020). Using monoclonal antibodies to deplete B cells, such as anti-BAFF (anti-BLγS, Belimumab) or anti-CD20 (Ofatumumab), to treat systemic lupus erythematosus and rheumatoid arthritis, respectively, have been shown to slightly improve depressive symptoms (Wittenberg et al., 2020). However, other reports have found, in contrast, an increase in risk to develop depression and suicidal ideation in patients treated with Belimumab or Ofatumumab (Minnema et al., 2019). These suggest that monoclonal neutralizing antibodies are potential therapies to alleviate depressive symptoms. However, a better understanding of the contribution of the adaptive immune system in depression is a prerequisite to design the most efficacious drug.
Some probiotics regimens have also been shown to ameliorate depressive symptoms (Yong et al., 2020). Of relevance for this review, oleic acid, one of the major components of olive oil, provides antidepressant effects (Lin et al., 2012; Kiecolt-Glaser et al., 2013) and reduces Th17 cell accumulation into the brain (Medina-Rodriguez et al., 2020). This suggests that targeting Th17 cells in periphery might be sufficient to induce antidepressant actions. Other Th17 cell-targeting compounds such as the RAR-related orphan receptor gamma T inhibitor SR1001 block Th17 cell-induced depressive-like behaviors (Beurel et al., 2013). Although all the characteristics of Th17 cells associated with depression remain to be determined, it is conceivable that other features of pathogenic Th17 cells could be targeted in depression to block Th17 cell entry to brain, for example (e.g., blockade of CCR6). Indeed, blocking all Th17 cells might have a negative impact on intestinal mucosal health.
Although limited, the arsenal of drugs to target Th17 cells in depression is expanding.
IV. Conclusion
Altogether, there is substantial evidence for a contribution of the adaptive immune system to depression. On one hand, there is a reduction of the lymphopoiesis in depression due to elevated level of norepinephrine and glucocorticoids (Wohleb et al., 2016). This is accompanied with a switch toward inflammatory T helper cells at the expense of the anti-inflammatory Treg cells, which might be exacerbated by gut microbial changes, which favor proinflammatory Th17 cells in depression. Th17 cells appear to be central in promoting depressive symptoms, and although the mechanisms of action of Th17 cells in depression remain to be fully elucidated, neutralizing the signature cytokine of Th17 cells, IL-17A, provides unexpected antidepressant effects.
Abbreviations
- BAFF
B cell activating factor
- CCR6
chemokine receptor C-C motif chemokine receptor
- CD
cluster of differentiation
- IDO
indoleamine 2,3-dioxygenase
- IFN
interferon
- IL
interleukin
- MDD
major depressive disorder
- MHC
major histocompatibility complex
- SFB
segmented filamentous bacterium
- TGF
transforming growth factor
- Th
T helper
- TNF
tumor necrosis factor
- Treg
regulatory T cells
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Beurel, Medina-Rodriguez, Jope.
Footnotes
The work was supported by National Institutes of Health National Institute of Mental Health [Grant R01-MH104656] and [Grant R01-MH110415] (to E.B.) and by a Merit Award from the Veterans Administration [BX003678] (to R.S.J.).
No author has an actual or perceived conflict of interest with the contents of this article.
References
- Adams TB, Wharton CM, Quilter L, Hirsch T (2008) The association between mental health and acute infectious illness among a national sample of 18- to 24-year-old college students. J Am Coll Health 56:657–663. [DOI] [PubMed] [Google Scholar]
- Ahern GP (2011) 5-HT and the immune system. Curr Opin Pharmacol 11:29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahern PP, Faith JJ, Gordon JI (2014) Mining the human gut microbiota for effector strains that shape the immune system. Immunity 40:815–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmetspahic DSchwarte KAmbrée OBürger CFalcone VSeiler KKooybaran MRGrosse LRoos FScheffer J, et al. (2018) Altered B cell homeostasis in patients with major depressive disorder and normalization of CD5 surface expression on regulatory B cells in treatment responders. J Neuroimmune Pharmacol 13:90–99. [DOI] [PubMed] [Google Scholar]
- Alves de Lima KRustenhoven JDa Mesquita SWall MSalvador AFSmirnov IMartelossi Cebinelli GMamuladze TBaker WPapadopoulos Z, et al. (2020) Meningeal γδ T cells regulate anxiety-like behavior via IL-17a signaling in neurons. Nat Immunol 21:1421–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amatya N, Garg AV, Gaffen SL (2017) IL-17 signaling: the yin and the yang. Trends Immunol 38:310–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrée O, Ruland C, Zwanzger P, Klotz L, Baune BT, Arolt V, Scheu S, Alferink J (2019) Social defeat modulates T helper cell percentages in stress susceptible and resilient mice. Int J Mol Sci 20:3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson NW, Goodwin RD, Okkels N, Gustafsson LN, Taha F, Cole SW, Munk-Jørgensen P (2016) Depression and the risk of severe infections: prospective analyses on a nationwide representative sample. Int J Epidemiol 45:131–139. [DOI] [PubMed] [Google Scholar]
- Andersson NW, Gustafsson LN, Okkels N, Taha F, Cole SW, Munk-Jørgensen P, Goodwin RD (2015) Depression and the risk of autoimmune disease: a nationally representative, prospective longitudinal study. Psychol Med 45:3559–3569. [DOI] [PubMed] [Google Scholar]
- Atarashi KTanoue TOshima KSuda WNagano YNishikawa HFukuda SSaito TNarushima SHase K, et al. (2013) Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 500:232–236. [DOI] [PubMed] [Google Scholar]
- Banuelos J, Cao Y, Shin SC, Lu NZ (2017) Immunopathology alters Th17 cell glucocorticoid sensitivity. Allergy 72:331–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer ME, Papadopoulos A, Poon L, Perks P, Lightman SL, Checkley S, Shanks N (2002) Dexamethasone-induced effects on lymphocyte distribution and expression of adhesion molecules in treatment-resistant depression. Psychiatry Res 113:1–15. [DOI] [PubMed] [Google Scholar]
- Bauer ME, Papadopoulos A, Poon L, Perks P, Lightman SL, Checkley S, Shanks N (2003) Altered glucocorticoid immunoregulation in treatment resistant depression. Psychoneuroendocrinology 28:49–65. [DOI] [PubMed] [Google Scholar]
- Beurel E, Harrington LE, Jope RS (2013) Inflammatory T helper 17 cells promote depression-like behavior in mice. Biol Psychiatry 73:622–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurel E, Lowell JA, Jope RS (2018) Distinct characteristics of hippocampal pathogenic TH17 cells in a mouse model of depression. Brain Behav Immun 73:180–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurel E, Toups M, Nemeroff CB (2020) The bidirectional relationship of depression and inflammation: double trouble. Neuron 107:234–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beydoun MA, Beydoun HA, Dore GA, Canas JA, Fanelli-Kuczmarski MT, Evans MK, Zonderman AB (2016) White blood cell inflammatory markers are associated with depressive symptoms in a longitudinal study of urban adults. Transl Psychiatry 6:e895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brachman RA, Lehmann ML, Maric D, Herkenham M (2015) Lymphocytes from chronically stressed mice confer antidepressant-like effects to naive mice. J Neurosci 35:1530–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun S, Gaza N, Werdehausen R, Hermanns H, Bauer I, Durieux ME, Hollmann MW, Stevens MF (2010) Ketamine induces apoptosis via the mitochondrial pathway in human lymphocytes and neuronal cells. Br J Anaesth 105:347–354. [DOI] [PubMed] [Google Scholar]
- Calabrese JR, Skwerer RG, Barna B, Gulledge AD, Valenzuela R, Butkus A, Subichin S, Krupp NE (1986) Depression, immunocompetence, and prostaglandins of the E series. Psychiatry Res 17:41–47. [DOI] [PubMed] [Google Scholar]
- Carloni SBertocchi AMancinelli SBellini MErreni MBorreca ABraga DGiugliano SMozzarelli AMManganaro D, et al. (2021) Identification of a choroid plexus vascular barrier closing during intestinal inflammation. Science 374:439–448. [DOI] [PubMed] [Google Scholar]
- Carvalho LA, Bergink V, Sumaski L, Wijkhuijs J, Hoogendijk WJ, Birkenhager TK, Drexhage HA (2014) Inflammatory activation is associated with a reduced glucocorticoid receptor alpha/beta expression ratio in monocytes of inpatients with melancholic major depressive disorder. Transl Psychiatry 4:e344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho LA, Urbanova L, Hamer M, Hackett RA, Lazzarino AI, Steptoe A (2015) Blunted glucocorticoid and mineralocorticoid sensitivity to stress in people with diabetes. Psychoneuroendocrinology 51:209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case AJ, Zimmerman MC (2015) Redox-regulated suppression of splenic T-lymphocyte activation in a model of sympathoexcitation. Hypertension 65:916–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Jiang T, Chen P, Ouyang J, Xu G, Zeng Z, Sun Y (2011) Emerging tendency towards autoimmune process in major depressive patients: a novel insight from Th17 cells. Psychiatry Res 188:224–230. [DOI] [PubMed] [Google Scholar]
- Cheng AL, Schwabe M, Doering MM, Colditz GA, Prather H (2020) The effect of psychological impairment on outcomes in patients with prearthritic hip disorders: a systematic review and meta-analysis. Am J Sports Med 48:2563–2571. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Desse S, Martinez A, Worthen RJ, Jope RS, Beurel E (2018) TNFα disrupts blood brain barrier integrity to maintain prolonged depressive-like behavior in mice. Brain Behav Immun 69:556–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung SG, Goldenthal AR, Uhlemann AC, Mann JJ, Miller JM, Sublette ME (2019) Systematic review of gut microbiota and major depression. Front Psychiatry 10:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HS, Im S, Park JW, Suh HJ (2016) Protective effect of deer bone oil on cartilage destruction in rats with monosodium iodoacetate (MIA)-induced osteoarthritis. Biol Pharm Bull 39:2042–2051. [DOI] [PubMed] [Google Scholar]
- Clark SM, Michael KC, Klaus J, Mert A, Romano-Verthelyi A, Sand J, Tonelli LH (2015) Dissociation between sickness behavior and emotionality during lipopolysaccharide challenge in lymphocyte deficient Rag2(-/-) mice. Behav Brain Res 278:74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark SM, Song C, Li X, Keegan AD, Tonelli LH (2019) CD8+ T cells promote cytokine responses to stress. Cytokine 113:256–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark SM, Soroka JA, Song C, Li X, Tonelli LH (2016) CD4(+) T cells confer anxiolytic and antidepressant-like effects, but enhance fear memory processes in Rag2(-/-) mice. Stress 19:303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JAEdwards TNLiu AWHirai TJones MRWu JLi YZhang SHo JDavis BM, et al. (2019) Cutaneous TRPV1+ neurons trigger protective innate type 17 anticipatory immunity. Cell 178:919–932.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypowyj S, Picard C, Maródi L, Casanova JL, Puel A (2012) Immunity to infection in IL-17-deficient mice and humans. Eur J Immunol 42:2246–2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darko DF, Gillin JC, Risch SC, Bulloch K, Golshan S, Tasevska Z, Hamburger RN (1989) Mitogen-stimulated lymphocyte proliferation and pituitary hormones in major depression. Biol Psychiatry 26:145–155. [DOI] [PubMed] [Google Scholar]
- Darko DF, Lucas AH, Gillin JC, Risch SC, Golshan S, Hamburger RN, Silverman MB, Janowsky DS (1988) Cellular immunity and the hypothalamic-pituitary axis in major affective disorder: a preliminary study. Psychiatry Res 25:1–9. [DOI] [PubMed] [Google Scholar]
- Das Sarma J, Ciric B, Marek R, Sadhukhan S, Caruso ML, Shafagh J, Fitzgerald DC, Shindler KS, Rostami A (2009) Functional interleukin-17 receptor A is expressed in central nervous system glia and upregulated in experimental autoimmune encephalomyelitis. J Neuroinflammation 6:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davami MH, Baharlou R, Ahmadi Vasmehjani A, Ghanizadeh A, Keshtkar M, Dezhkam I, Atashzar MR (2016) Elevated IL-17 and TGF-β serum levels: a positive correlation between T-helper 17 cell-related pro-inflammatory responses with major depressive disorder. Basic Clin Neurosci 7:137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBattista C, Posener JA, Kalehzan BM, Schatzberg AF (2000) Acute antidepressant effects of intravenous hydrocortisone and CRH in depressed patients: a double-blind, placebo-controlled study. Am J Psychiatry 157:1334–1337. [DOI] [PubMed] [Google Scholar]
- Devi SAlexandre YOLoi JKGillis RGhazanfari NCreed SJHolz LEShackleford DMackay LKHeath WR, et al. (2021) Adrenergic regulation of the vasculature impairs leukocyte interstitial migration and suppresses immune responses. Immunity 54:1219–1230.e7. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS (2000) Acute stress enhances while chronic stress suppresses skin immunity. The role of stress hormones and leukocyte trafficking. Ann N Y Acad Sci 917:876–893. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, Malarkey WB, Neri E, McEwen BS (2012) Stress-induced redistribution of immune cells--from barracks to boulevards to battlefields: a tale of three hormones--Curt Richter Award winner. Psychoneuroendocrinology 37:1345–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickens C, McGowan L, Clark-Carter D, Creed F (2002) Depression in rheumatoid arthritis: a systematic review of the literature with meta-analysis. Psychosom Med 64:52–60. [DOI] [PubMed] [Google Scholar]
- Duggal NA, Upton J, Phillips AC, Lord JM (2016) Development of depressive symptoms post hip fracture is associated with altered immunosuppressive phenotype in regulatory T and B lymphocytes. Biogerontology 17:229–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES (2000) The sympathetic nerve--an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev 52:595–638. [PubMed] [Google Scholar]
- Elstad JI, Vabø M (2008) Job stress, sickness absence and sickness presenteeism in Nordic elderly care. Scand J Public Health 36:467–474. [DOI] [PubMed] [Google Scholar]
- Euesden J, Danese A, Lewis CM, Maughan B (2017) A bidirectional relationship between depression and the autoimmune disorders - new perspectives from the National Child Development Study. PLoS One 12:e0173015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DL, Folds JD, Petitto JM, Golden RN, Pedersen CA, Corrigan M, Gilmore JH, Silva SG, Quade D, Ozer H (1992) Circulating natural killer cell phenotypes in men and women with major depression. Relation to cytotoxic activity and severity of depression. Arch Gen Psychiatry 49:388–395. [DOI] [PubMed] [Google Scholar]
- Fan KQLi YYWang HLMao XTGuo JXWang FHuang LJLi YNMa XYGao ZJ, et al. (2019) Stress-induced metabolic disorder in peripheral CD4+ T cells leads to anxiety-like behavior. Cell 179:864–879.e19. [DOI] [PubMed] [Google Scholar]
- Faraco GBrea DGarcia-Bonilla LWang GRacchumi GChang HBuendia ISantisteban MMSegarra SGKoizumi K, et al. (2018) Dietary salt promotes neurovascular and cognitive dysfunction through a gut-initiated TH17 response. Nat Neurosci 21:240–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazzino F, Urbina M, Cedeño N, Lima L (2009) Fluoxetine treatment to rats modifies serotonin transporter and cAMP in lymphocytes, CD4+ and CD8+ subpopulations and interleukins 2 and 4. Int Immunopharmacol 9:463–467. [DOI] [PubMed] [Google Scholar]
- Felger JC, Treadway MT (2017) Inflammation effects on motivation and motor activity: role of dopamine. Neuropsychopharmacology 42:216–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira TB, Kasahara TM, Barros PO, Vieira MM, Bittencourt VC, Hygino J, Andrade RM, Linhares UC, Andrade AF, Bento CA (2011) Dopamine up-regulates Th17 phenotype from individuals with generalized anxiety disorder. J Neuroimmunol 238:58–66. [DOI] [PubMed] [Google Scholar]
- Franchimont D, Galon J, Gadina M, Visconti R, Zhou Y, Aringer M, Frucht DM, Chrousos GP, O’Shea JJ (2000) Inhibition of Th1 immune response by glucocorticoids: dexamethasone selectively inhibits IL-12-induced Stat4 phosphorylation in T lymphocytes. J Immunol 164:1768–1774. [DOI] [PubMed] [Google Scholar]
- Gagliani NAmezcua Vesely MCIseppon ABrockmann LXu HPalm NWde Zoete MRLicona-Limón PPaiva RSChing T, et al. (2015) Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature 523:221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M, Jin W, Qian Y, Ji L, Feng G, Sun J (2011) Effect of N-methyl-D-aspartate receptor antagonist on T helper cell differentiation induced by phorbol-myristate-acetate and ionomycin. Cytokine 56:458–465. [DOI] [PubMed] [Google Scholar]
- Geva-Zatorsky NSefik EKua LPasman LTan TGOrtiz-Lopez AYanortsang TBYang LJupp RMathis D, et al. (2017) Mining the human gut microbiota for immunomodulatory organisms. Cell 168:928–943.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh R, Kumar PK, Mitra P, Purohit P, Nebhinani N, Sharma P (2020) Circulating T helper 17 and IFN-γ positive Th17 cells in major depressive disorder. Behav Brain Res 394:112811. [DOI] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK (2005) Stress-induced immune dysfunction: implications for health. Nat Rev Immunol 5:243–251. [DOI] [PubMed] [Google Scholar]
- Griffiths CEM, Fava M, Miller AH, Russell J, Ball SG, Xu W, Acharya N, Rapaport MH (2017) Impact of ixekizumab treatment on depressive symptoms and systemic inflammation in patients with moderate-to-severe psoriasis: an integrated analysis of three phase 3 clinical studies. Psychother Psychosom 86:260–267. [DOI] [PubMed] [Google Scholar]
- Grosse L, Carvalho LA, Birkenhager TK, Hoogendijk WJ, Kushner SA, Drexhage HA, Bergink V (2016a) Circulating cytotoxic T cells and natural killer cells as potential predictors for antidepressant response in melancholic depression. Restoration of T regulatory cell populations after antidepressant therapy. Psychopharmacology (Berl) 233:1679–1688. [DOI] [PubMed] [Google Scholar]
- Grosse L, Carvalho LA, Wijkhuijs AJ, Bellingrath S, Ruland T, Ambrée O, Alferink J, Ehring T, Drexhage HA, Arolt V (2015) Clinical characteristics of inflammation-associated depression: monocyte gene expression is age-related in major depressive disorder. Brain Behav Immun 44:48–56. [DOI] [PubMed] [Google Scholar]
- Grosse L, Hoogenboezem T, Ambrée O, Bellingrath S, Jörgens S, de Wit HJ, Wijkhuijs AM, Arolt V, Drexhage HA (2016b) Deficiencies of the T and natural killer cell system in major depressive disorder: T regulatory cell defects are associated with inflammatory monocyte activation. Brain Behav Immun 54:38–44. [DOI] [PubMed] [Google Scholar]
- Gu M, Li Y, Tang H, Zhang C, Li W, Zhang Y, Li Y, Zhao Y, Song C (2018) Endogenous omega (n)-3 fatty acids in fat-1 mice attenuated depression-like behavior, imbalance between microglial M1 and M2 phenotypes, and dysfunction of neurotrophins induced by lipopolysaccharide administration. Nutrients 10:1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT (2005) Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol 6:1123–1132. [DOI] [PubMed] [Google Scholar]
- Hasselmann HGamradt STaenzer ANowacki JZain RPatas KRamien CPaul FWingenfeld KPiber D, et al. (2018) Pro-inflammatory monocyte phenotype and cell-specific steroid signaling alterations in unmedicated patients with major depressive disorder. Front Immunol 9:2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert TB, Cohen S (1993) Depression and immunity: a meta-analytic review. Psychol Bull 113:472–486. [DOI] [PubMed] [Google Scholar]
- Hernández-Santos N, Gaffen SL (2012) Th17 cells in immunity to Candida albicans. Cell Host Microbe 11:425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himmerich H, Milenović S, Fulda S, Plümäkers B, Sheldrick AJ, Michel TM, Kircher T, Rink L (2010) Regulatory T cells increased while IL-1β decreased during antidepressant therapy. J Psychiatr Res 44:1052–1057. [DOI] [PubMed] [Google Scholar]
- Hirota KDuarte JHVeldhoen MHornsby ELi YCua DJAhlfors HWilhelm CTolaini MMenzel U, et al. (2011) Fate mapping of IL-17-producing T cells in inflammatory responses. Nat Immunol 12:255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong M, Zheng J, Ding ZY, Chen JH, Yu L, Niu Y, Hua YQ, Wang LL (2013) Imbalance between Th17 and Treg cells may play an important role in the development of chronic unpredictable mild stress-induced depression in mice. Neuroimmunomodulation 20:39–50. [DOI] [PubMed] [Google Scholar]
- Hou M, Zhou NB, Li H, Wang BS, Wang XQ, Wang XW, Wang KG, Xue FS (2016) Morphine and ketamine inhibit immune function of gastric cancer patients by increasing percentage of CD4(+)CD25(+)Foxp3(+) regulatory T cells in vitro. J Surg Res 203:306–312. [DOI] [PubMed] [Google Scholar]
- Hou N, Zhang X, Zhao L, Zhao X, Li Z, Song T, Huang C (2013) A novel chronic stress-induced shift in the Th1 to Th2 response promotes colon cancer growth. Biochem Biophys Res Commun 439:471–476. [DOI] [PubMed] [Google Scholar]
- Irwin M, Patterson T, Smith TL, Caldwell C, Brown SA, Gillin JC, Grant I (1990) Reduction of immune function in life stress and depression. Biol Psychiatry 27:22–30. [DOI] [PubMed] [Google Scholar]
- Irwin MRLevin MJCarrillo COlmstead RLucko ALang NCaulfield MJWeinberg AChan ISClair J, et al. (2011) Major depressive disorder and immunity to varicella-zoster virus in the elderly. Brain Behav Immun 25:759–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iseme RA, McEvoy M, Kelly B, Agnew L, Attia J, Walker FR (2014) Autoantibodies and depression: evidence for a causal link? Neurosci Biobehav Rev 40:62–79. [DOI] [PubMed] [Google Scholar]
- Ivanov IIAtarashi KManel NBrodie ELShima TKaraoz UWei DGoldfarb KCSantee CALynch SV, et al. (2009) Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139:485–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahangard L, Behzad M (2020) Diminished functional properties of T regulatory cells in major depressive disorder: the influence of selective serotonin reuptake inhibitor. J Neuroimmunol 344:577250. [DOI] [PubMed] [Google Scholar]
- Jha MK, Minhajuddin A, Gadad BS, Greer TL, Mayes TL, Trivedi MH (2017) Interleukin 17 selectively predicts better outcomes with bupropion-SSRI combination: novel T cell biomarker for antidepressant medication selection. Brain Behav Immun 66:103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang ZAltuntas CZGulen MFLiu CGiltiay NQin HLiu LQian WRansohoff RMBergmann C, et al. (2010) Astrocyte-restricted ablation of interleukin-17-induced Act1-mediated signaling ameliorates autoimmune encephalomyelitis. Immunity 32:414–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karabatsiakis A, Kolassa IT, Kolassa S, Rudolph KL, Dietrich DE (2014) Telomere shortening in leukocyte subpopulations in depression. BMC Psychiatry 14:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagiannidis CAkdis MHolopainen PWoolley NJHense GRückert BMantel PYMenz GAkdis CABlaser K, et al. (2004) Glucocorticoids upregulate FOXP3 expression and regulatory T cells in asthma. J Allergy Clin Immunol 114:1425–1433. [DOI] [PubMed] [Google Scholar]
- Kawamoto SMaruya MKato LMSuda WAtarashi KDoi YTsutsui YQin HHonda KOkada T, et al. (2014) Foxp3(+) T cells regulate immunoglobulin a selection and facilitate diversification of bacterial species responsible for immune homeostasis. Immunity 41:152–165. [DOI] [PubMed] [Google Scholar]
- Kawasaki C, Kawasaki T, Ogata M, Nandate K, Shigematsu A (2001) Ketamine isomers suppress superantigen-induced proinflammatory cytokine production in human whole blood. Can J Anaesth 48:819–823. [DOI] [PubMed] [Google Scholar]
- Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, Giuliani F, Arbour N, Becher B, Prat A (2007) Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med 13:1173–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Epel ES, Belury MA, Andridge R, Lin J, Glaser R, Malarkey WB, Hwang BS, Blackburn E (2013) Omega-3 fatty acids, oxidative stress, and leukocyte telomere length: a randomized controlled trial. Brain Behav Immun 28:16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Glaser R, Gravenstein S, Malarkey WB, Sheridan J (1996) Chronic stress alters the immune response to influenza virus vaccine in older adults. Proc Natl Acad Sci USA 93:3043–3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BJ, Jones HP (2010) Epinephrine-primed murine bone marrow-derived dendritic cells facilitate production of IL-17A and IL-4 but not IFN-γ by CD4+ T cells. Brain Behav Immun 24:1126–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Suh YH, Chang KA (2021) Interleukin-17 induced by cumulative mild stress promoted depression-like behaviors in young adult mice. Mol Brain 14:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JW, Kim YK, Hwang JA, Yoon HK, Ko YH, Han C, Lee HJ, Ham BJ, Lee HS (2013a) Plasma levels of IL-23 and IL-17 before and after antidepressant treatment in patients with major depressive disorder. Psychiatry Investig 10:294–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim OY, Hong BS, Park KS, Yoon YJ, Choi SJ, Lee WH, Roh TY, Lötvall J, Kim YK, Gho YS (2013b) Immunization with Escherichia coli outer membrane vesicles protects bacteria-induced lethality via Th1 and Th17 cell responses. J Immunol 190:4092–4102. [DOI] [PubMed] [Google Scholar]
- Kim SKim HYim YSHa SAtarashi KTan TGLongman RSHonda KLittman DRChoi GB, et al. (2017) Maternal gut bacteria promote neurodevelopmental abnormalities in mouse offspring. Nature 549:528–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Lee H, Joung HY, Lee G, Lee HJ, Shin MK, Kim SH, Shim I, Bae H (2011) T-bet deficient mice exhibit resistance to stress-induced development of depression-like behaviors. J Neuroimmunol 240-241:45–51. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Lee H, Lee G, Oh SJ, Shin MK, Shim I, Bae H (2012) CD4+CD25+ regulatory T cell depletion modulates anxiety and depression-like behaviors in mice. PLoS One 7:e42054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler OSylvia LGBowden CLCalabrese JRThase MShelton RCMcInnis MTohen MKocsis JHKetter TA, et al. (2017) White blood cell count correlates with mood symptom severity and specific mood symptoms in bipolar disorder. Aust N Z J Psychiatry 51:355–365. [DOI] [PubMed] [Google Scholar]
- Kostic M, Zivkovic N, Cvetanovic A, Stojanovic I, Colic M (2017) IL-17 signalling in astrocytes promotes glutamate excitotoxicity: indications for the link between inflammatory and neurodegenerative events in multiple sclerosis. Mult Scler Relat Disord 11:12–17. [DOI] [PubMed] [Google Scholar]
- Kronfol Z, House JD (1989) Lymphocyte mitogenesis, immunoglobulin and complement levels in depressed patients and normal controls. Acta Psychiatr Scand 80:142–147. [DOI] [PubMed] [Google Scholar]
- Kronfol Z, Turner R, Nasrallah H, Winokur G (1984) Leukocyte regulation in depression and schizophrenia. Psychiatry Res 13:13–18. [DOI] [PubMed] [Google Scholar]
- Kubera M, Lin AH, Kenis G, Bosmans E, van Bockstaele D, Maes M (2001) Anti-inflammatory effects of antidepressants through suppression of the interferon-gamma/interleukin-10 production ratio. J Clin Psychopharmacol 21:199–206. [DOI] [PubMed] [Google Scholar]
- Kurd SK, Troxel AB, Crits-Christoph P, Gelfand JM (2010) The risk of depression, anxiety, and suicidality in patients with psoriasis: a population-based cohort study. Arch Dermatol 146:891–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurina LM, Goldacre MJ, Yeates D, Gill LE (2001) Depression and anxiety in people with inflammatory bowel disease. J Epidemiol Community Health 55:716–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen B, Hoff G, Wilhelm W, Buchinger H, Wanner GA, Bauer M (1998) Effect of intravenous anesthetics on spontaneous and endotoxin-stimulated cytokine response in cultured human whole blood. Anesthesiology 89:1218–1227. [DOI] [PubMed] [Google Scholar]
- Laumet G, Edralin JD, Chiang AC, Dantzer R, Heijnen CJ, Kavelaars A (2018) Resolution of inflammation-induced depression requires T lymphocytes and endogenous brain interleukin-10 signaling. Neuropsychopharmacology 43:2597–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebwohl MGPapp KAMarangell LBKoo JBlauvelt AGooderham MWu JJRastogi SHarris SPillai R, et al. (2018) Psychiatric adverse events during treatment with brodalumab: analysis of psoriasis clinical trials. J Am Acad Dermatol 78:81–89.e5. [DOI] [PubMed] [Google Scholar]
- Lee JE, Lee JM, Park YJ, Kim BS, Jeon YT, Chung Y (2017) Inhibition of autoimmune Th17 cell responses by pain killer ketamine. Oncotarget 8:89475–89485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, Weaver CT (2009) Late developmental plasticity in the T helper 17 lineage. Immunity 30:92–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewitus GM, Wilf-Yarkoni A, Ziv Y, Shabat-Simon M, Gersner R, Zangen A, Schwartz M (2009) Vaccination as a novel approach for treating depressive behavior. Biol Psychiatry 65:283–288. [DOI] [PubMed] [Google Scholar]
- Lewkowich I, Ahlbrand R, Johnson E, McAlees J, Nawreen N, Raman R, Lingel I, Hargis J, Hoover C, Sah R (2020) Modulation of fear behavior and neuroimmune alterations in house dust mite exposed A/J mice, a model of severe asthma. Brain Behav Immun 88:688–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Xiao B, Qiu W, Yang L, Hu B, Tian X, Yang H (2010) Altered expression of CD4(+)CD25(+) regulatory T cells and its 5-HT(1a) receptor in patients with major depression disorder. J Affect Disord 124:68–75. [DOI] [PubMed] [Google Scholar]
- Lin PY, Mischoulon D, Freeman MP, Matsuoka Y, Hibbeln J, Belmaker RH, Su KP (2012) Are omega-3 fatty acids antidepressants or just mood-improving agents? The effect depends upon diagnosis, supplement preparation, and severity of depression. Mol Psychiatry 17:1161–1163, author reply 1163–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Xin W, He P, Turner D, Yin J, Gan Y, Shi FD, Wu J (2014) Interleukin-17 inhibits adult hippocampal neurogenesis. Sci Rep 4:7554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Ho RC, Mak A (2012) The role of interleukin (IL)-17 in anxiety and depression of patients with rheumatoid arthritis. Int J Rheum Dis 15:183–187. [DOI] [PubMed] [Google Scholar]
- Liu Y, Rui XX, Shi H, Qiu YH, Peng YP (2018) Norepinephrine inhibits Th17 cells via β2-adrenergic receptor (β2-AR) signaling in a mouse model of rheumatoid arthritis. Med Sci Monit 24:1196–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Ho CS, Liu X, Chua AN, Wang W, McIntyre RS, Ho RC (2017) Chronic administration of fluoxetine and pro-inflammatory cytokine change in a rat model of depression. PLoS One 12:e0186700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M, Mihaylova I, Kubera M, Ringel K (2012) Activation of cell-mediated immunity in depression: association with inflammation, melancholia, clinical staging and the fatigue and somatic symptom cluster of depression. Prog Neuropsychopharmacol Biol Psychiatry 36:169–175. [DOI] [PubMed] [Google Scholar]
- Maes M, Stevens W, DeClerck L, Bridts C, Peeters D, Schotte C, Cosyns P (1992a) Immune disorders in depression: higher T helper/T suppressor-cytotoxic cell ratio. Acta Psychiatr Scand 86:423–431. [DOI] [PubMed] [Google Scholar]
- Maes M, Stevens WJ, DeClerck LS, Bridts CH, Peeters D, Schotte C, Cosyns P (1992b) A significantly increased number and percentage of B cells in depressed subjects: results of flow cytometric measurements. J Affect Disord 24:127–134. [DOI] [PubMed] [Google Scholar]
- Manni M, Granstein RD, Maestroni G (2011) β2-Adrenergic agonists bias TLR-2 and NOD2 activated dendritic cells towards inducing an IL-17 immune response. Cytokine 55:380–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina-Rodriguez EMMadorma DO’Connor GMason BLHan DDeo SKOppenheimer MNemeroff CBTrivedi MHDaunert S, et al. (2020) Identification of a signaling mechanism by which the microbiome regulates Th17 cell-mediated depressive-like behaviors in mice. Am J Psychiatry 177:974–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milovanovic J, Arsenijevic A, Stojanovic B, Kanjevac T, Arsenijevic D, Radosavljevic G, Milovanovic M, Arsenijevic N (2020) Interleukin-17 in chronic inflammatory neurological diseases. Front Immunol 11:947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnema LA, Giezen TJ, Souverein PC, Egberts TCG, Leufkens HGM, Gardarsdottir H (2019) Exploring the association between monoclonal antibodies and depression and suicidal ideation and behavior: a vigibase study. Drug Saf 42:887–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr DC, Goodkin DE, Islar J, Hauser SL, Genain CP (2001) Treatment of depression is associated with suppression of nonspecific and antigen-specific T(H)1 responses in multiple sclerosis. Arch Neurol 58:1081–1086. [DOI] [PubMed] [Google Scholar]
- Müller S, Chang HC, Köhler H (1989) Perturbation of the idiotypic network. I. Induction with multiple alloantigen stimulation. Cell Immunol 119:353–372. [DOI] [PubMed] [Google Scholar]
- Murphy AC, Lalor SJ, Lynch MA, Mills KH (2010) Infiltration of Th1 and Th17 cells and activation of microglia in the CNS during the course of experimental autoimmune encephalomyelitis. Brain Behav Immun 24:641–651. [DOI] [PubMed] [Google Scholar]
- Murphy KM, Weaver CT, Berg LJ (2022) Janeway's Immunobiology, 10th ed, W.W. Norton & Company, New York. [Google Scholar]
- Nadeem A, Ahmad SF, Al-Harbi NO, Fardan AS, El-Sherbeeny AM, Ibrahim KE, Attia SM (2017) IL-17A causes depression-like symptoms via NFκB and p38MAPK signaling pathways in mice: implications for psoriasis associated depression. Cytokine 97:14–24. [DOI] [PubMed] [Google Scholar]
- Padgett DA, Glaser R (2003) How stress influences the immune response. Trends Immunol 24:444–448. [DOI] [PubMed] [Google Scholar]
- Palanichamy A, Barnard J, Zheng B, Owen T, Quach T, Wei C, Looney RJ, Sanz I, Anolik JH (2009) Novel human transitional B cell populations revealed by B cell depletion therapy. J Immunol 182:5982–5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariante CM (2009) Risk factors for development of depression and psychosis. Glucocorticoid receptors and pituitary implications for treatment with antidepressant and glucocorticoids. Ann N Y Acad Sci 1179:144–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariante CM, Miller AH (2001) Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biol Psychiatry 49:391–404. [DOI] [PubMed] [Google Scholar]
- Park HJ, Shim HS, An K, Starkweather A, Kim KS, Shim I (2015) IL-4 inhibits IL-1β-induced depressive-like behavior and central neurotransmitter alterations. Mediators Inflamm 2015:941413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patas KWilling ADemiralay CEngler JBLupu ARamien CSchäfer TGach CStumm LChan K, et al. (2018) T cell phenotype and t cell receptor repertoire in patients with major depressive disorder. Front Immunol 9:291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten SB, Marrie RA, Carta MG (2017) Depression in multiple sclerosis. Int Rev Psychiatry 29:463–472. [DOI] [PubMed] [Google Scholar]
- Petitto JM, Folds JD, Evans DL (1993) Abnormal diurnal variation of B lymphocyte circulation patterns in major depression. Biol Psychiatry 34:268–270. [DOI] [PubMed] [Google Scholar]
- Postal M, Appenzeller S (2015) The importance of cytokines and autoantibodies in depression. Autoimmun Rev 14:30–35. [DOI] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH (2006) Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol 27:24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Miller AH (2003) When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry 160:1554–1565. [DOI] [PubMed] [Google Scholar]
- Rattazzi L, Piras G, Ono M, Deacon R, Pariante CM, D’Acquisto F (2013) CD4+ but not CD8+ T cells revert the impaired emotional behavior of immunocompromised RAG-1-deficient mice. Transl Psychiatry 3:e280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindran AV, Griffiths J, Merali Z, Anisman H (1999) Circulating lymphocyte subsets in obsessive compulsive disorder, major depression and normal controls. J Affect Disord 52:1–10. [DOI] [PubMed] [Google Scholar]
- Reed MDYim YSWimmer RDKim HRyu CWelch GMAndina MKing HOWaisman AHalassa MM, et al. (2020) IL-17a promotes sociability in mouse models of neurodevelopmental disorders. Nature 577:249–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds LA, Finlay BB, Maizels RM (2015) Cohabitation in the intestine: interactions among helminth parasites, bacterial microbiota, and host immunity. J Immunol 195:4059–4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilett KC, Friedel M, Ellegood J, MacKenzie RN, Lerch JP, Foster JA (2015) Loss of T cells influences sex differences in behavior and brain structure. Brain Behav Immun 46:249–260. [DOI] [PubMed] [Google Scholar]
- Rinner I, Schauenstein K, Mangge H, Porta S, Kvetnansky R (1992) Opposite effects of mild and severe stress on in vitro activation of rat peripheral blood lymphocytes. Brain Behav Immun 6:130–140. [DOI] [PubMed] [Google Scholar]
- Roque S, Oliveira TG, Nobrega C, Barreira-Silva P, Nunes-Alves C, Sousa N, Palha JA, Correia-Neves M (2011) Interplay between depressive-like behavior and the immune system in an animal model of prenatal dexamethasone administration. Front Behav Neurosci 5:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso DA, Amaral D, Latella A, Chantada G, Braier JL (2016) Reduced doses of cladribine and cytarabine regimen was effective and well tolerated in patients with refractory-risk multisystem Langerhans cell histiocytosis. Br J Haematol 172:287–290. [DOI] [PubMed] [Google Scholar]
- Round JL, Lee SM, Li J, Tran G, Jabri B, Chatila TA, Mazmanian SK (2011) The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science 332:974–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Round JL, Mazmanian SK (2010) Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci USA 107:12204–12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M, Fujinami Y, Ono Y, Ohyama S, Fujioka K, Yamashita K, Inoue S, Kotani J (2021) Infiltrated regulatory T cells and Th2 cells in the brain contribute to attenuation of sepsis-associated encephalopathy and alleviation of mental impairments in mice with polymicrobial sepsis. Brain Behav Immun 92:25–38. [DOI] [PubMed] [Google Scholar]
- Sales MCKasahara TMSacramento PMRossi ADCafasso MOSDOyamada HAAHygino JAlvim FAndrade RMCristina Vasconcelos C, et al. (2021) Selective serotonin reuptake inhibitor attenuates the hyperresponsiveness of TLR2+ and TLR4+ Th17/Tc17-like cells in multiple sclerosis patients with major depression. Immunology 162:290–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanzano A, Cosentino M (2015) Adrenergic regulation of innate immunity: a review. Front Pharmacol 6:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schewitz-Bowers LPLait PJCopland DAChen PWu WDhanda ADVistica BPWilliams ELLiu BJawad S, et al. (2015) Glucocorticoid-resistant Th17 cells are selectively attenuated by cyclosporine A. Proc Natl Acad Sci USA 112:4080–4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiweck C, Valles-Colomer M, Arolt V, Müller N, Raes J, Wijkhuijs A, Claes S, Drexhage H, Vrieze E (2020) Depression and suicidality: a link to premature T helper cell aging and increased Th17 cells. Brain Behav Immun 87:603–609. [DOI] [PubMed] [Google Scholar]
- Schleifer SJ, Keller SE, Bond RN, Cohen J, Stein M (1989) Major depressive disorder and immunity. Role of age, sex, severity, and hospitalization. Arch Gen Psychiatry 46:81–87. [DOI] [PubMed] [Google Scholar]
- Schleifer SJ, Keller SE, Meyerson AT, Raskin MJ, Davis KL, Stein M (1984) Lymphocyte function in major depressive disorder. Arch Gen Psychiatry 41:484–486. [DOI] [PubMed] [Google Scholar]
- Schleifer SJ, Keller SE, Siris SG, Davis KL, Stein M (1985) Depression and immunity. Lymphocyte function in ambulatory depressed patients, hospitalized schizophrenic patients, and patients hospitalized for herniorrhaphy. Arch Gen Psychiatry 42:129–133. [DOI] [PubMed] [Google Scholar]
- Sealock JMLee YHMoscati AVenkatesh SVoloudakis GStraub PSingh KFeng YAGe TRoussos P, et al. (2021) Use of the PsycheMERGE network to investigate the association between depression polygenic scores and white blood cell count. JAMA Psychiatry 78:1365–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminog OO, Goldacre MJ (2013) Risk of pneumonia and pneumococcal disease in people hospitalized with diabetes mellitus: English record-linkage studies. Diabet Med 30:1412–1419. [DOI] [PubMed] [Google Scholar]
- Shafiee MTayefi MHassanian SMGhaneifar ZParizadeh MRAvan ARahmani FKhorasanchi ZAzarpajouh MRSafarian H, et al. (2017) Depression and anxiety symptoms are associated with white blood cell count and red cell distribution width: a sex-stratified analysis in a population-based study. Psychoneuroendocrinology 84:101–108. [DOI] [PubMed] [Google Scholar]
- Siffrin VRadbruch HGlumm RNiesner RPaterka MHerz JLeuenberger TLehmann SMLuenstedt SRinnenthal JL, et al. (2010) In vivo imaging of partially reversible th17 cell-induced neuronal dysfunction in the course of encephalomyelitis. Immunity 33:424–436. [DOI] [PubMed] [Google Scholar]
- Sims GP, Ettinger R, Shirota Y, Yarboro CH, Illei GG, Lipsky PE (2005) Identification and characterization of circulating human transitional B cells. Blood 105:4390–4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M, Singh V, Schurman JV, Colombo JM, Friesen CA (2020) The relationship between mucosal inflammatory cells, specific symptoms, and psychological functioning in youth with irritable bowel syndrome. Sci Rep 10:11988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, Glickman JN, Garrett WS (2013) The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341:569–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijders G, Schiweck C, Mesman E, Grosse L, De Wit H, Nolen WA, Drexhage HA, Hillegers MHJ (2016) A dynamic course of T cell defects in individuals at risk for mood disorders. Brain Behav Immun 58:11–17. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Savitz J, Kent Teague T, Gandhapudi SK, Tan C, Misaki M, McKinney BA, Irwin MR, Drevets WC, Bodurka J (2017) Altered populations of natural killer cells, cytotoxic T lymphocytes, and regulatory T cells in major depressive disorder: association with sleep disturbance. Brain Behav Immun 66:193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed SABeurel ELoewenstein DALowell JACraighead WEDunlop BWMayberg HSDhabhar FDietrich WDKeane RW, et al. (2018) Defective inflammatory pathways in never-treated depressed patients are associated with poor treatment response. Neuron 99:914–924.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan TGSefik EGeva-Zatorsky NKua LNaskar DTeng FPasman LOrtiz-Lopez AJupp RWu HJ, et al. (2016) Identifying species of symbiont bacteria from the human gut that, alone, can induce intestinal Th17 cells in mice. Proc Natl Acad Sci USA 113:E8141–E8150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taves MD, Ashwell JD (2021) Glucocorticoids in T cell development, differentiation and function. Nat Rev Immunol 21:233–243. [DOI] [PubMed] [Google Scholar]
- Tondo L, Burrai C, Scamonatti L, Weissenburger J, Rush J (1988) Comparison between clinician-rated and self-reported depressive symptoms in Italian psychiatric patients. Neuropsychobiology 19:1–5. [DOI] [PubMed] [Google Scholar]
- Troidle L, Watnick S, Wuerth DB, Gorban-Brennan N, Kliger AS, Finkelstein FO (2003) Depression and its association with peritonitis in long-term peritoneal dialysis patients. Am J Kidney Dis 42:350–354. [DOI] [PubMed] [Google Scholar]
- Vieira MMFerreira TBPacheco PABarros POAlmeida CRAraújo-Lima CFSilva-Filho RGHygino JAndrade RMLinhares UC, et al. (2010) Enhanced Th17 phenotype in individuals with generalized anxiety disorder. J Neuroimmunol 229:212–218. [DOI] [PubMed] [Google Scholar]
- Viladomiu M, Bassaganya-Riera J, Tubau-Juni N, Kronsteiner B, Leber A, Philipson CW, Zoccoli-Rodriguez V, Hontecillas R (2017) Cooperation of gastric mononuclear phagocytes with helicobacter pylori during colonization. J Immunol 198:3195–3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan K, Dhabhar FS (2005) Stress-induced enhancement of leukocyte trafficking into sites of surgery or immune activation. Proc Natl Acad Sci USA 102:5808–5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachholz S, Knorr A, Mengert L, Plümper J, Sommer R, Juckel G, Friebe A (2017) Interleukin-4 is a participant in the regulation of depressive-like behavior. Behav Brain Res 326:165–172. [DOI] [PubMed] [Google Scholar]
- Wahle M, Hanefeld G, Brunn S, Straub RH, Wagner U, Krause A, Häntzschel H, Baerwald CG (2006) Failure of catecholamines to shift T-cell cytokine responses toward a Th2 profile in patients with rheumatoid arthritis. Arthritis Res Ther 8:R138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westfall S, Caracci F, Zhao D, Wu QL, Frolinger T, Simon J, Pasinetti GM (2021) Microbiota metabolites modulate the T helper 17 to regulatory T cell (Th17/Treg) imbalance promoting resilience to stress-induced anxiety- and depressive-like behaviors. Brain Behav Immun 91:350–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichers MC, Maes M (2004) The role of indoleamine 2,3-dioxygenase (IDO) in the pathophysiology of interferon-alpha-induced depression. J Psychiatry Neurosci 29:11–17. [PMC free article] [PubMed] [Google Scholar]
- Wittenberg GMStylianou AZhang YSun YGupta AJagannatha PSWang DHsu BCurran MEKhan S, et al. ; MRC ImmunoPsychiatry Consortium (2020) Effects of immunomodulatory drugs on depressive symptoms: a mega-analysis of randomized, placebo-controlled clinical trials in inflammatory disorders. Mol Psychiatry 25:1275–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wlodarska MLuo CKolde Rd’Hennezel EAnnand JWHeim CEKrastel PSchmitt EKOmar ASCreasey EA, et al. (2017) Indoleacrylic acid produced by commensal peptostreptococcus species suppresses inflammation. Cell Host Microbe 22:25–37.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Franklin T, Iwata M, Duman RS (2016) Integrating neuroimmune systems in the neurobiology of depression. Nat Rev Neurosci 17:497–511. [DOI] [PubMed] [Google Scholar]
- Wong ML, Dong C, Maestre-Mesa J, Licinio J (2008) Polymorphisms in inflammation-related genes are associated with susceptibility to major depression and antidepressant response. Mol Psychiatry 13:800–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulsin LR, Vaillant GE, Wells VE (1999) A systematic review of the mortality of depression. Psychosom Med 61:6–17. [DOI] [PubMed] [Google Scholar]
- Yang YTorchinsky MBGobert MXiong HXu MLinehan JLAlonzo FNg CChen ALin X, et al. (2014) Focused specificity of intestinal TH17 cells towards commensal bacterial antigens. Nature 510:152–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong SJ, Tong T, Chew J, Lim WL (2020) Antidepressive mechanisms of probiotics and their therapeutic potential. Front Neurosci 13:1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Zhang YP, Li YY, Liu BP, Wang HY, Li KW, Zhao S, Song C (2019) Minocycline ameliorates depressive behaviors and neuro-immune dysfunction induced by chronic unpredictable mild stress in the rat. Behav Brain Res 356:348–357. [DOI] [PubMed] [Google Scholar]
- Zheng PZeng BZhou CLiu MFang ZXu XZeng LChen JFan SDu X, et al. (2016) Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol Psychiatry 21:786–796. [DOI] [PubMed] [Google Scholar]
- Zhou J, Nagarkatti P, Zhong Y, Ginsberg JP, Singh NP, Zhang J, Nagarkatti M (2014) Dysregulation in microRNA expression is associated with alterations in immune functions in combat veterans with post-traumatic stress disorder. PLoS One 9:e94075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Yamane H, Paul WE (2010) Differentiation of effector CD4 T cell populations (*). Annu Rev Immunol 28:445–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski CE, Mele F, Aschenbrenner D, Jarrossay D, Ronchi F, Gattorno M, Monticelli S, Lanzavecchia A, Sallusto F (2012) Pathogen-induced human TH17 cells produce IFN-γ or IL-10 and are regulated by IL-1β. Nature 484:514–518. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Luborsky L, McKay JR, Rosenthal R, Houldin A, Tax A, McCorkle R, Seligman DA, Schmidt K (2001) The relationship of depression and stressors to immunological assays: a meta-analytic review. Brain Behav Immun 15:199–226. [DOI] [PubMed] [Google Scholar]