Abstract
The efficacy of FK463, a novel water-soluble lipopeptide, was evaluated in mouse models of pulmonary aspergillosis and was compared with that of amphotericin B (AMPH-B). In the pulmonary aspergillosis models induced by intranasal inoculation, FK463 exhibited good efficacy, with 50% effective doses in the range of 0.26 to 0.51 mg/kg of body weight; these values were comparable to those of AMPH-B. In an Aspergillus target organ assay with immunosuppressed mice, under conditions of constant plasma levels of FK463, using a subcutaneously implanted osmotic pressure pump, a significant reduction in viable fungal cells was observed at plasma FK463 levels of 0.55 to 0.80 μg/ml or higher. We conclude that FK463 is highly effective in the treatment of pulmonary aspergillosis in this animal model. These results indicate that FK463 may be a potent parenterally administered antifungal agent for pulmonary aspergillosis.
Deep-seated mycoses in immunocompromised hosts are becoming an increasingly important medicinal problem (10). Candidiasis is the most common and important fungal infection in humans, and aspergillosis is the next most common (2, 8, 9). Invasive aspergillosis in immunocompromised patients is associated with significant morbidity and mortality (3, 4). In view of the well-known problems with the established agents, it is clear that newer antifungal therapies with improved efficacy and reduced toxicity are needed. FK463 is a new, parenterally administered antifungal drug candidate undergoing clinical development. This compound is a novel water-soluble lipopeptide derived by semisynthetic modification of FR901379, a naturally occurring cyclic hexapeptide with a fatty acyl side chain, similar in structure to echinocandins and pneumocandins (T. Iwamoto, N. Sakamoto, M. Yamashita, M. Ezaki, S. Hashimoto, T. Furuta, M. Okuhara, and M. Kohsaka, Prog. Abstr. 33rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. 371, 1993). FK463 has been shown to have potent in vitro antifungal activity against Candida and Aspergillus species (K. Maki, Y. Morishita, Y. Iguchi, E. Watabe, K. Otomo, N. Teratani, Y. Watanabe, F. Ikeda, S. Tawara, T. Goto, M. Tomishima, H. Ohki, A. Yamada, K. Kawabata, H. Takasugi, H. Tanaka, K. Sakane, F. Matsumoto, and S. Kuwahara, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F141, p. 268, 1998). In mouse models of disseminated candidiasis and aspergillosis, FK463 showed good efficacy (S. Matsumoto, Y. Wakai, K. Maki, E. Watabe, T. Ushitani, K. Otomo, T. Nakai, Y. Watanabe, F. Ikeda, S. Tawara, T. Goto, F. Matsumoto, and S. Kuwahara, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F142, p. 268, 1998). In the study described in this report, the activity of FK463 was evaluated in mouse models of pulmonary aspergillosis.
MATERIALS AND METHODS
Compound and animals.
FK463 was synthesized in Fujisawa Pharmaceutical Co., Ltd. Amphotericin B (AMPH-B) and fluconazole (FLCZ) were purchased from Bristol-Myers Squibb (Tokyo, Japan) and Pfizer (Tokyo, Japan), respectively. FK463 and AMPH-B were formulated in sterile saline and 5% glucose, respectively, for intravenous injection and injected as 10mL/kg. Male Slc-ICR strain mice (4 weeks old) were purchased from SLC Japan (Shizuoka, Japan).
Organisms and media.
Inocula of Aspergillus fumigatus TIMM0063, IFM40814, and IFM41209 were prepared by culturing the test organisms on potato dextrose agar. Conidia were collected in sterile saline, and the conidium concentration was standardized with a spectrophotometer at 660 nm. The viable counts of the test strains were confirmed by serially diluting the cell suspension 10-fold and plating the inoculum onto Sabouraud dextrose agar (SDA) plates.
MIC.
The MICs of FK463 and AMPH-B against A. fumigatus TIMM0063, IFM40814, and IFM41209 were determined according to guideline M-27A (Maki et al., 38th ICAAC).
Comparative efficacy studies for pulmonary aspergillosis.
Cyclophosphamide (Acros Organics, Springfield, N.J.) was administered intraperitoneally at 200 mg/kg of body weight 4 days before and 1 day after infection to induce a leukopenic condition in mice. The polymorphonuclear leukocyte counts were <100/μl for as long as 6 days after infection (5). On the day of infection the mice were anesthetized intravenously with sodium pentobarbital at 50 mg/kg of body weight. The inoculum, a 0.05-ml droplet, was placed into the nares of the mice. The antifungal agents (at 2.0, 1.0, 0.5, 0.25, or 0.125 mg/kg) were administered once daily for 4 days, starting at 1.5 h after infection by intravenous injection. The efficacies of antifungal agents were assessed as the 50% effective dose (ED50) calculated by probit analysis or normal probability plot based on the survival rate at 15 days after infection, and survival was analyzed by the Wilcoxon rank sum test. A P value of <0.01 was considered significant.
Determination of minimum effective concentration for reduction of A. fumigatus in pulmonary aspergillosis.
To determine the effective concentration for reduction of A. fumigatus in pulmonary aspergillosis, an Alzet osmotic pump was used to deliver the drug by continuous infusion. In brief, the Alzet miniosmotic pump, model 2001 (Palo Alto, Calif. USA) was filled with FK463 dissolved in isotonic saline according to the recommendation of the operation manual. The expected pumping rate of this model was 1 μl/h. The mass rates of FK463 infusion were 4, 2, 1, and 0.5 μg/h. Thirty ICR mice received hydrocortisone acetate (100 mg/kg, 1 day before and 1 day after infection) (Nacalai Tesque, Kyoto, Japan) subcutaneously, and polymorphonuclear leukocytes in the peripheral blood of the hydrocortisone-treated mice were dysfunctional in killing and ingesting microorganisms (6). The immunosuppressed mice were inoculated intranasally with a suspension of A. fumigatus TIMM0063 (6.0 × 104 CFU). After infection, the miniosmotic pump was implanted subcutaneously in the mouse. The lungs were aseptically removed from euthanized mice 5 days after infection and were homogenized with isotonic saline. The adequately diluted homogenates were plated onto Sabouraud agar to determine the residual CFU of viable cells in the lungs. At the same time, cardiac blood was collected and plasma was prepared for measurement of FK463. Plasma concentrations of FK463 were assayed by high-pressure liquid chromatography. The liquid chromatography utilized a TSK-GEL octyldecyl silane 80TM column (Toso, Tokyo, Japan) with a mobile phase consisting of CH3CN–0.02 M KH2PO4 in a 3:4 ratio. FK463 was detected by fluorescence (excitation wavelength, 273 nm; emission wavelength, 464 nm) (J. Azuma, I. Yamamoto, M. Ogura, T. Mukai, H. Suematsu, H. Kageyama, K. Nakahara, K. Yoshida, and T. Takaya, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F146, p. 269, 1998).
Statistics.
For analysis of quantitative culture, all values were expressed as the mean ± standard deviation of the log of each viable count. One-way layout analysis of variance and the Dunnet t test were used to compare the numbers of cells in the lungs for the treatment groups and the control group. All P values were calculated for two-tailed significance levels, and a P value of <0.05 was considered significant.
RESULTS
In vitro activities.
The MICs of FK463 and AMPH-B against A. fumigatus used in this study are presented in Table 1.
TABLE 1.
MICs of FK463 and AMPH-B against Aspergillus strains used in this study
| A. fumigatus strain | MIC (μg/ml) of:
|
|
|---|---|---|
| FK463 | AMPH-B | |
| TIMM0063 | ≤0.0039 | 1 |
| IFM40814 | 0.0078 | 0.25 |
| IFM41209 | 0.0078 | 0.5 |
Pulmonary aspergillosis.
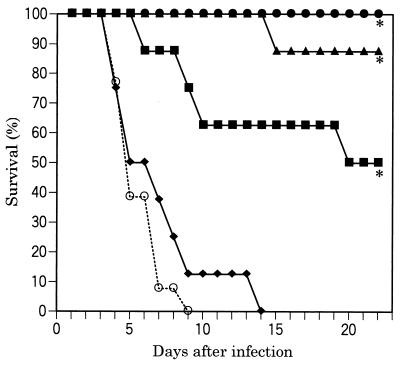
Survival curves are illustrated in Fig. 1. All untreated infected control mice died within 9 days. FK463 significantly prolonged survival at doses of 0.25 mg/kg or higher, compared with the survival of the control mice. All infected mice could survive as long as 22 days when treated with FK463 at a dose of 1.0 mg/kg.
FIG. 1.
Efficacy of FK463 in a pulmonary A. fumigatus IFM40814 infection (intranasal challenge with 7.0 × 105 CFU/mouse) in ICR mice (cyclophosphamide was administered intraperitoneally at 200 mg/kg 4 days before and 1 day after infection). FK463 was administered intravenously once daily for 4 days starting at 1.5 h after infection. Symbols: ●, 1.0 mg/kg; ▴, 0.5 mg/kg; ■, 0.25 mg/kg; ⧫, 0.125 mg/kg; ○, sham treatment; ∗, significantly different from the control (P < 0.01 by the Wilcoxon rank sum test).
The ED50s of FK463 and AMPH-B agents against A. fumigatus strains causing pulmonary infections are shown in Table 2. FK463 exhibited good efficacy against pulmonary aspergillosis, with ED50s in the range of 0.26 to 0.51 mg/kg; this was comparable to the efficacy of AMPH-B (ED50, 0.26 to 0.46 mg/kg).
TABLE 2.
Efficacy of FK463 in mouse models of pulmonary fungal infection
| A. fumigatus strain | Inoculum (105 CFU) | ED50 (mg/kg/day)a of:
|
|
|---|---|---|---|
| FK463 | AMPH-B | ||
| TIMM0063 | 8.8 | 0.35 (0.24–0.51) | 0.36 (0.24–0.52) |
| IFM40814 | 7.0 | 0.26 (0.18–0.36) | 0.26 (0.18–0.36) |
| IFM41209 | 3.5 | 0.51 (0.34–0.75) | 0.46 (0.32–0.67) |
Values in parentheses are 95% confidence intervals.
Minimum effective concentration.
Table 3 shows the viable counts of fungal cells in lungs and the concentration of FK463 in plasma on day 5 after infection. A significant reduction in viable fungal cells compared with the numbers of viable cells in the control group was observed at plasma FK463 levels of 0.55 μg/ml or higher. In addition, the total FK463 concentrations in plasma from mice in which pumps containing 4 mg of FK463/ml were implanted were 2.24 and 1.55 μg/ml at 1 day and 5 days after the treatment, respectively (data not shown). Since the total concentration at 1 day after treatment was 1.4 times higher than that after 5 days, the minimum effective concentration in plasma for murine pulmonary aspergillosis was estimated to be 0.55 to 0.80 μg/ml.
TABLE 3.
Efficacy of continuous FK463 infusion in mouse models of pulmonary aspergillosis
| Concn of FK463 (mg/ml) injected into pump | Plasma FK463 concna (μg/ml) | Viable count (log CFU/lung)b |
|---|---|---|
| Control | 3.99 (0.39) | |
| 0.5 | 0.23 | 3.71 (0.48) |
| 1 | 0.55 | 2.82 (0.48)* |
| 2 | 0.91 | 2.14 (0.79)** |
| 4 | 1.55 | 1.71 (0.64)** |
The lower limit of quantification of each compound was 0.05 μg/ml.
Numbers in parentheses represent standard deviations. ∗, P < 0.05 (comparison with control); ∗∗, P < 0.01 (comparison with control).
DISCUSSION
FK463 appears to possess a good profile as a new antifungal agent, showing potent activity against clinically important fungal pathogens, good water solubility, favorable pharmacokinetic properties, and relatively low toxicity (Azuma et al., 38th ICAAC; Maki et al., 38th ICAAC; Matsumoto et al., 38th ICAAC; S. Suzuki, M. Terakawa, F. Yokobayashi, F. Fujiwara, and T. Hata, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F144, p. 269, 1998). In recent years, a new generation of echinocandins has been developed. LY303366 and MK-0991 are the lead compounds in this class and are currently in clinical trials (1, 7). In vivo studies have demonstrated that both of these compounds are effective against animal models of pulmonary aspergillosis (7; E. Bernard, T. Ishimaru, and D. Armstrong, Prog. Abstr. 36th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F39, p. 106, 1996). In this study, the efficacy of FK463 in a multiple-dosage regimen in mouse models of pulmonary fungal infection was compared with that of AMPH-B. FK463 also exhibited good efficacy against pulmonary aspergillosis, with ED50s in the range of 0.26 to 0.51 mg/kg; this was comparable to that of AMPH-B. Since FK463 did not show complete inhibition of the growth of A. fumigatus in the broth dilution assay and appears to be fungistatic, this good in vivo activity against aspergillosis appears to correlate with its suppressive effect on hyphal elongation observed at low concentrations in vitro (Maki et al., 38th ICAAC).
To estimate the minimum effective plasma FK463 concentrations in murine pulmonary aspergillosis, various concentrations of FK463 were infused continuously via a subcutaneously implanted pump containing the compound into immunosuppressed mice infected with A. fumigatus. The minimum effective plasma FK463 concentration in pulmonary aspergillosis was estimated to be 0.55 to 0.80 μg/ml by a viable-cell reduction assay in the target organs. The clinical dosage regimen of FK463 should be designed to maintain plasma levels or trough concentration above this minimum effective concentration.
In conclusion, the present data indicate that the new lipopeptide FK463 is efficacious in the treatment of pulmonary aspergillosis, and further studies to evaluate the compound should be considered.
ACKNOWLEDGMENT
We are grateful to David Barrett, Medicinal Chemistry Research Laboratories, for kind help and advice.
REFERENCES
- 1.Abruzzo G K, Flattery A M, Gill C J, Kong L, Smith J G, Pikounis V B, Balkovec J M, Bouffard A F, Dropinski J F, Rosen H, Kropp H, Bartizal K. Evaluation of the echinocandin antifungal MK-0991 (L-743,872): efficacies in mouse models of disseminated aspergillosis, candidiasis, and cryptococcosis. Antimicrob Agents Chemother. 1997;41:2333–2338. doi: 10.1128/aac.41.11.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beck-Sague C M, Jarvis W R the National Nosocomial Infections Surveillance System. Secular trends in the epidemiology of nosocomial fungal infections in the United States, 1980–1990. J Infect Dis. 1993;167:1247–1251. doi: 10.1093/infdis/167.5.1247. [DOI] [PubMed] [Google Scholar]
- 3.DeGregorio M W, Lee W M F, Linker C A, Jacobs R A, Ries C A. Fungal infections in patients with acute leukemia. Am J Med. 1982;73:543–548. doi: 10.1016/0002-9343(82)90334-5. [DOI] [PubMed] [Google Scholar]
- 4.Denning D W, Stevens D A. Antifungal and surgical treatment of invasive aspergillosis: review of 2,121 published cases. Rev Infect Dis. 1990;12:1147–1201. doi: 10.1093/clinids/12.6.1147. [DOI] [PubMed] [Google Scholar]
- 5.Denning D W, Hall L, Jackson M, Hollis S. Efficacy of D0870 compared with those of itraconazole and amphotericin B in two murine models of invasive aspergillosis. Antimicrob Agents Chemother. 1995;39:1809–1814. doi: 10.1128/aac.39.8.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graybill J R, Bocanegra R, Najvar L K, Loebenberg D, Luther M F. Granulocyte colony-stimulating factor and azole antifungal therapy in murine aspergillosis: role of immune suppression. Antimicrob Agents Chemother. 1998;42:2467–2473. doi: 10.1128/aac.42.10.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petraitis V, Petraitiene R, Groll A H, Bell A, Callender D P, Sein T, Schufele R L, McMillian C L, Bacher J, Walsh T J. Antifungal efficacy, safety, and single-dose pharmacokinetics of LY303366, a novel echinocandin B, in experimental pulmonary aspergillosis in persistently neutropenic rabbits. Antimicrob Agents Chemother. 1998;42:2898–2905. doi: 10.1128/aac.42.11.2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rogers A L, Kennedy M J. Opportunistic hyaline hyphomycetes. In: Balows A, Hausler W J Jr, Herrmann K L, Isenberg H D, Shadomy H J, editors. Manual of clinical microbiology. 5th ed. Washington, D.C.: American Society for Microbiology; 1991. pp. 659–673. [Google Scholar]
- 9.Warren N G, Shadomy H J. Yeasts of medical importance. In: Balows A, Hausler W J Jr, Herrmann K L, Isenberg H D, Shadomy H J, editors. Manual of clinical microbiology. 5th ed. Washington, D.C.: American Society for Microbiology; 1991. pp. 617–629. [Google Scholar]
- 10.Young L S. Aspergillus infection in the neutropenic host. Hosp Pract. 1989;30(May):37–43. [PubMed] [Google Scholar]