ABSTRACT
Industrial effluents/wastewater are the main sources of hexavalent chromium (Cr (VI)) pollutants in the environment. Cr (VI) pollution has become one of the world’s most serious environmental concerns due to its long persistence in the environment and highly deadly nature in living organisms. To its widespread use in industries Cr (VI) is highly toxic and one of the most common environmental contaminants. Cr (VI) is frequently non-biodegradable in nature, which means it stays in the environment for a long time, pollutes the soil and water, and poses substantial health risks to humans and wildlife. In living things, the hexavalent form of Cr is carcinogenic, genotoxic, and mutagenic. Physico-chemical techniques currently used for Cr (VI) removal are not environmentally friendly and use a large number of chemicals. Microbes have many natural or acquired mechanisms to combat chromium toxicity, such as biosorption, reduction, subsequent efflux, or bioaccumulation. This review focuses on microbial responses to chromium toxicity and the potential for their use in environmental remediation. Moreover, the research problem and prospects for the future are discussed in order to fill these gaps and overcome the problem associated with bacterial bioremediation’s real-time applicability.
KEYWORDS: Microbial bioremediation, biosorption, environmental contaminates, health hazards, chromium toxicity
Graphical abstract
1. Introduction
Chromium (Cr) is a naturally occurring element present in volcanic dust, rocks, and soil [1]. Cr has high redox potential, and it can exist in various oxidation states ranging from (-II) to (IV). However, its most stable forms are trivalent Cr (Cr (III)) and hexavalent Cr (Cr (VI)) [2]. The physical, chemical, and toxicological properties of Cr (III) and Cr (IV) vary considerably. While Cr (III) is usually found in nature in the form of ore such as ferrochromite, Cr (VI) is mostly generated from anthropogenic activities and is highly toxic to living organisms [3]. Cr (III) exists as an insoluble hydroxide cation whereas Cr (VI) is an oxyanion species occurring in the form of divalent chromate, dichromate, or monovalent chromate depending on the solution pH [4]. Cr (VI) is the most mobile form of Cr in the aquatic environment due to its relatively higher water solubility. Chromium is a versatile element and has been used in many industrial applications since its discovery in 1797. Chromium compounds are often used in chrome plating, dye manufacturing, textile industry, aircraft industry, leather tanning, wood preservation, and mud drilling. Chromates, dichromates, chromic acid, chromic sulfate and, chromic oxides are examples of industrially relevant chromium compounds. These chromium compounds are generally produced from the mining and treatment of chromite ore. However, such mining and industrial activities generate considerable volumes of solid and liquid chromium-rich waste, as well as result in its atmospheric emissions [5]. In addition to mining and industrial activities, natural rocks such as ultramafic and mafic rocks are also a geogenic source of Cr (VI) in groundwater [4–6]. Increased concentrations of Cr (VI) have been observed in water reservoirs linked with ultramafic aquifers in California, Mexico, Brazil, Italy, Argentina, and Greece [7–10]. Moreover, Cr (VI) concentrations in igneous and meta-volcanic groundwater, as well as aquifers associated with mixed or more felsic igneous and metamorphic formations, are relatively high in North Carolina (up to 25 g/L). Environmental contamination by Cr (VI) has become a worldwide concern. In many places of the world, industries have disposed of hazardous waste in ways that benefit their bottom lines, such as illegal dumpsites, at the expense of the environment and human health. These dumpsites are the primary source of Cr pollution and long-term damage to groundwater.
The following are some of the most important sources of Cr (VI)
Dyes, paints, inks, and polymers containing chromate pigments.
Chrome plating is the process of putting chromium metal onto an item’s surface using a chromic acid solution.
Particles produced while ferrochromium ore smelting.
Welding fumes from stainless steel and nonferrous chromium alloys
Cr (VI) is classified as a group 1 carcinogen by the World Health Organization (WHO). The maximum allowed concentration of chromium in drinking water is set at 50 ug/L by the drinking water guideline. In the United States and Canada, average Cr (VI) levels in drinking water range from 0.2 to 2 µg-Cr (VI)/L [11,12]. Although the US Environmental Protection Agency (US EPA) acknowledges Cr (VI) as a harmful element. Only total chromium (Cr(T) is included in the drinking water standard, with a maximum pollutant level of 100 µg/L. To avoid the ill effects of Cr (VI) on human health, there is an urgent need to implement strict environmental restrictions to limit the amount of Cr (VI) that can be released into the environment. Several treatment procedures for Cr (VI) removal from wastewater exist, including adsorption, chemical precipitation, ion exchange, electrocoagulation, membrane separation, and electrodialysis [13]. Amongst which, chemical precipitation is the most common approach to Cr (VI) elimination. Calcium hydroxide, magnesium oxide, sodium hydroxide, and calcium magnesium carbonate are examples of some chemical precipitators used in Cr (VI) removal. All criteria or elements that affect precipitation include the type of precipitation agent, sludge volume, speed of agitation, pH, mixing duration, and complexing agents [14,15]. Advanced treatment procedures including reverse osmosis, ion exchange, membrane filtering, electrocoagulation, and electrodialysis are successful at eliminating Cr (VI), but they are costly and produce concentrated wastes that must be treated and disposed of later [16]. Bioremediation is emerging as may be an effective way technique to remove Cr (VI) from industrial effluents. Chromium bioremoval has been documented using a variety of fungal and bacterial species. Streptomyces rimosus, Actinomycetes, and Streptomyces griseus both showed promise in removing Cr (VI) from industrial effluent [17]. However, chitosan, rice husk, waste tea leaves, pomegranate husk, neem leaves, coconut shell, orange peel, watermelon rind, sawdust, and banana rachis are low-cost farm wastes with adsorption abilities to remove Cr (VI) from wastewater [18–23]. There is not a thorough investigation on the use of chromium-resistant bacteria to remediate chrome-polluted effluent. Hexavalent chromium is a well-known environmental contaminant that has the potential to cause cancer, teratogenicity, and mutation [24]. The goal of this review is to reveal the harmful effects of Cr (VI), as well as ways for remediating polluted sites by using microbes to absorb and break down Cr (VI) pollutants.
2. Hexavalent chromium (Cr (VI)) effect on human health
Heavy metal contamination has become a severe environmental hazard worldwide in recent decades [25,26]. Hexavalent chromium [Cr (VI)] is a global environmental pollutant that increases the risk for several types of cancers and is increasingly being recognized as a neurotoxicant [24]. Several kinds of plants and microbes play crucial roles in the removal of toxic metals from contaminated sites [27–32]. Cr (VI) and its metabolites, particularly chromates, take a distinct route into the human body. The main routes of Cr (VI) exposure include inhalation, ingestion, and skin contact. Depending on the duration, Cr (VI) exposure can be classified as acute (14 days), intermediate (75–364 days), and chronic (365 days) [33,34]. Cr (VI) causes toxicity in a variety of ways. It can reduce immune system activity or efficiency, compete with enzyme activity cofactor fixation sites, suppress important enzymes such as oxidative phosphorylation, and cause changes in cell architecture, notably in the lipoprotein region of the membrane. Nasal irritation and ulceration, hypersensitivity reactions and contact dermatitis, acute bronchitis and emphysema, liver and kidney disease, lung and skin cancer, internal bleeding, and DNA damage are all caused by the interaction of Cr (VI) with the DNA-polymerase enzyme [35]. Cr (VI) rapidly enters cells, but it needs to pass through several stages in the bloodstream before becoming Cr (III) in the internal organs. The Cr (VI) ion is excreted from the body, whereas the chromate ion is carried to the cell via a transport pathway that also involves the ions sulfate and phosphate. Such ions can induce oxidative stress in cells, which has been associated with a variety of chronic, cardiovascular, neurodegenerative diseases. Cr (VI) damages cells in a variety of ways, such as increased oxidative stress, the creation of DNA adducts, and chromosome breakups [36]. The World Health Organization’s International Agency for Data on Cancer (IARC) has classified Cr (VI) compounds as group one human carcinogens with several complex modes of action based on epidemiological research tying Cr (VI) to lung cancer [37,38]. Eardrum perforation, irritations, allergies, eczema, respiratory tract issues, skin irritations, ulceration, and lung cancer have all been linked to human exposure to Cr (VI) [39]. At various phases, Cr (VI) radiation can produce cytotoxic, mutagenic, and DNA mutations, as well as carcinogenic effects of Cr (VI)-containing compounds, chromosomal damage, and oxidative protein changes [40]. Nasal lining nose ulcers, irritation, anemia, and ulcers in the small intestine and stomach, and other respiratory problems like nasal blockage, coughing, wheezing, and face erythema, can all be caused by inhaling a high amount of hexavalent chromium.
Hexavalent chromium exposure at work may result in the following health consequences:
If hexavalent chromium is inhaled in high quantities, it can cause irritation or injury to the nostrils, throat, and lungs (respiratory tract).
Lung cancer in workers exposed to hexavalent chromium in the air.
If hexavalent chromium comes into touch with organs in high amounts, it might cause irritation or injury.
Severe and repeated exposure to chromium and related compounds, particularly those containing hexavalent ions, can cause health issues. The toxic effects of (Cr (VI)) on human health have been provided in Figure 1 & Figure 2.
Figure 1.
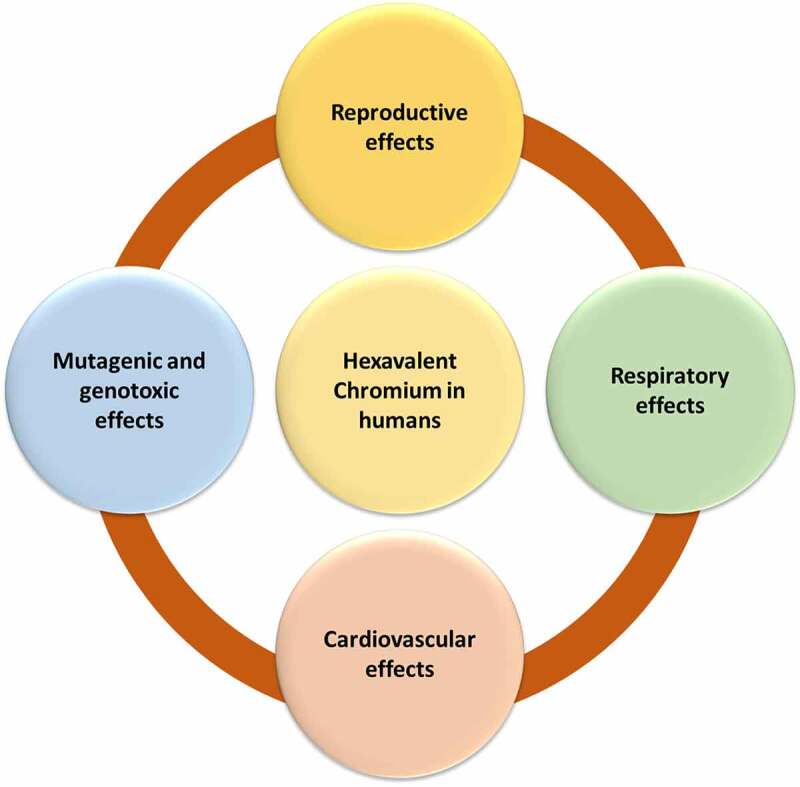
Toxicological effects of hexavalent chromium on humans.
Figure 2.
Hexavalent chromium (Cr (VI) has effects on the ecosystem and human health.
2.1. Chromium (VI) effects on macrophages
Lung shape is unaffected by chromium inhalation, but macrophages become larger, multinucleated, or vacuolated, and nodules form in intra-alveolar spaces. Higher concentrations of Cr (VI) suppress alveolar macrophage phagocytic activity and the humoral immune response, whereas lower levels of Cr(VI) stimulate alveolar macrophage phagocytic activity and the humoral immunological reaction.
2.2. Chromium (VI) effects on immune response
An early study done on human cells by Borella et al., 1983 who studied the effects of Cr (VI) and other metals on cultured human lymphocytes found that chromium induces reductions in both blastogenesis and immunoglobulin production in relation to its capability to enter the cells.
2.3. Chromium (VI) induced cell death
Chromium has been shown to be cytotoxic to cells. Vasant et al., 2001 discovered that apoptosis is the method of cell death in human lymphocytes when Cr (VI) is present.
3. Hexavalent chromium effect on plant health
Plants show signs of Cr (VI) toxicity, including delayed seed germination, damaged roots reduced root growth, reduced biomass, reduced plant height, photosynthetic impairment, membrane damage, leaf chlorosis, necrosis, low grain production, and ultimate death of the plant. The most prevalent chromium compounds in soil are HCrO4 and CrO42, which are easily absorbed by plants and contaminate soil [41]. Because of its extremely low solubility, Cr (VI) has been found to cause significant injury to living tissue [42]. Plant shoot length and biomass are affected by Cr (VI) exposure. Though low levels of Cr (3.8104 M) do not affect some crops, chromium compounds are highly poisonous to most plants and damage their growth and production [43]. According to Elahi et al., 2020 [43], Cr (VI) can be severely harmful to plants at concentrations as low as 5 mg/kg in soils and 0.5 mg/L in solution. Cr (VI) is linked to a decrease in nutrient intake and photosynthesis, which contributes to the delayed growth of plants. Various physiological, structural, and biochemical processes in plant cells are severely disturbed, resulting in the generation of reactive oxygen species. Chlorosis and plant necrosis are two symptoms of Cr poisoning [44]. Chromium affects the growth of leaves, which are part of the photosynthesis organ of plants. Increasing chromium concentration causes a considerable decrease in leaf area and biomass, as well as decreased photosynthesis and the production of chlorophyll content and necrosis in leaves. Under Cr (VI) exposure, many destructive processes take place in leaves. Chlorophyll synthesis is suppressed, the chloroplast ultrastructure is disrupted, photosynthetic electron transport is inhibited, and magnesium ions are released from the chlorophyll molecule [45]. Reduced plant development, leaf deformation, and necrosis, root tissue damage, chlorosis, decreased enzyme activity, food uptake, transport, photosynthesis, lipid peroxidation, DNA strand break, and chromosome aberration are all symptoms of Cr (VI) toxicity in plants [46–48]. As a result, chromium (VI) can interfere with photosynthesis, seed germination, and nutrient intake, as well as the overall growth and functionality of the plant. The toxic effect of Cr (VI) on plants has been provided in Figure 3.
Figure 3.
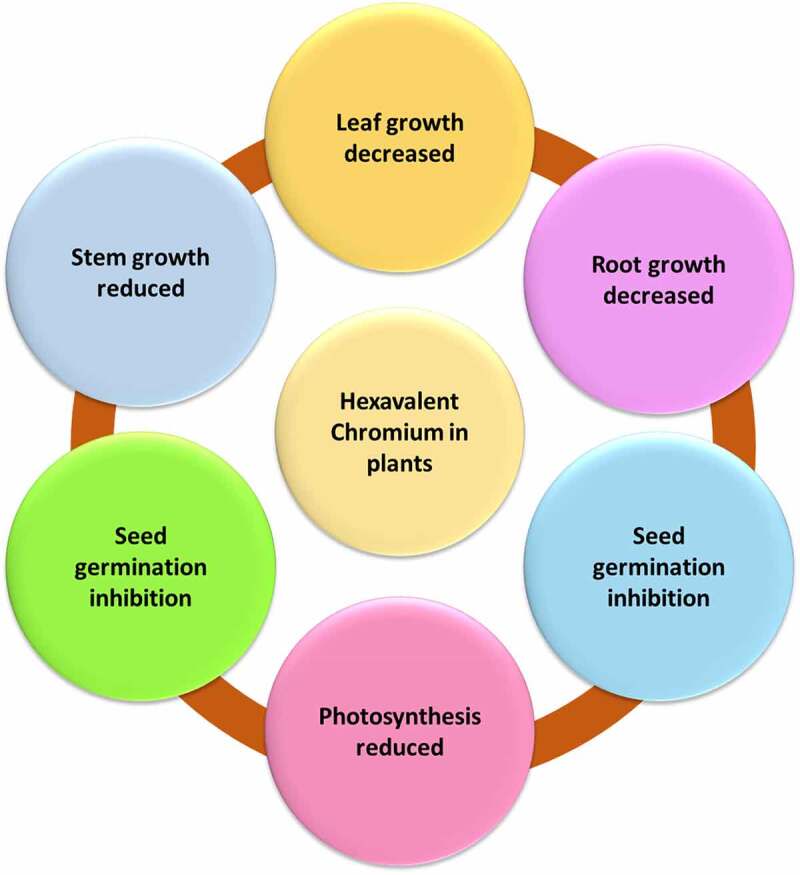
Toxicological effects of hexavalent chromium on the plant.
4. Hexavalent chromium (Cr (VI)) effect on microbiota
Chromium is the strongest metal in nature, ranking 17th in crust richness, and is primarily merged with the other elements to form trivalent and hexavalent compounds [49]. A variety of factors, including pathogens, habitat destruction, increased ultraviolet radiation, introduced non-native species, and contaminants, have all contributed to amphibian population declines. Intestinal microbial communities play a critical role in maintaining the host’s health [50]. They play a role in the regulation of numerous physiological functions [51]. Statistical analysis found that the human gut contains ~1014 bacteria, which is roughly 10 times the number of body cells [52].
5. Microbial remediation
Numerous microorganisms and plants have developed various strategies to counteract the toxic effects of Cr (VI) as shown in Table 1 [53–62]. Among the various methods, microbes’ enzymatic reduction of Cr (VI) into Cr (III) is the best-understood mechanism for such bioremediation [63]. For the detoxification of Cr (III) contamination and bioremediation of polluted waste, biological reduction of Cr (VI) to Cr is a potentially useful technique. Microbes that remove chromium from the environment offer a healthy and environmentally beneficial alternative to long-term manufacturing. These methods can be used to a wide range of microbial biomass, including bacteria, fungi, and algae. Biosorption of Cr (VI) has been proposed as a potential alternative to current industrial wastewater detoxification processes that use biomass (growing, resting, and dead cells), as well as biological and agricultural waste materials [64]. Chromium-resistant bacteria are responsible for or contribute to the biological reduction of Cr (VI) to less mobile Cr (III), and precipitation” of these bacteria could be a useful method for cleaning up polluted Cr (VI) areas. Through enzyme-catalyzed hazardous chemical degradation, bacteria successfully remove metal ions from the environment by using them as a source of energy and converting them to biomass [65]. Microbes in soil, underground materials, water, sludge, and residues are stimulated to break down environmentally detrimental substances to eco-friendly or acceptable levels during the microbial remediation process. The removal of chromium and other heavy metals from industrial effluents has been demonstrated using bioremediation strategies such as bioaccumulation, biotransformation, biosorption, and bioleaching [66]. Bioaccumulation is a metabolism-dependent method in which only live biomass uses cellular energy to transport hexavalent chromium (Cr (VI)) through the cell membrane. The bioaccumulation process in microorganisms is divided into the following phases. Initially, potentially hazardous heavy metal ions link themselves to the cell’s surface ligand. The metal-ligand combination that develops on the cell surface is subsequently transported inside by transporter protein. In addition, intracellularly transported complexes interact with metal-binding proteins such phytochelatins and metallothionein, causing precipitation, methylation, and other reactions. At the higher metal level, the approach only works on living cells and stops microbial cell development. Moreover, the biosorption, biotransformation, and bioaccumulation processes break down and remove harmful chromium ions from industrial effluent in an environmentally beneficial manner.
Table 1.
Different physical, chemical, and biological methods available for chromium remediation [94]
| S. No. | Biological Methods | Chemical Methods | Physical Methods |
|---|---|---|---|
| 1. | Biosorption | Hydrogen sulfide (H2S) | Adsorption |
| 2. | Bioaccumulation | Sodium metabisulfite (NaHSO3) | Membrane filtration |
| 3. | Reverse osmosis | Ferrous sulfate (FeSO4) | Extracellular precipitation |
| 4. | Bioreduction | Sodium dithionite (Na2 S2 O4) | Ion exchange |
| 5. | Electrodialysis | Calcium polysulfide (CaS5) | Biomineralization |
5.1. Biosorption of Cr (VI)
Biosorption, unlike bioaccumulation, is a metabolic rate-independent movement that can occur in both dead microbial biomass and living cells [67]. Potential hazardous ions, like Cr (VI), bind extracellularly to different functional groups of the microbial cell wall and are then eliminated by ion exchange, surface precipitation, or a complicated creation process [68]. The composition and design of bacteria’s cell walls differ. For example, bacterial cell walls are mostly peptidoglycan, fungi’s cell walls are mostly glucans, glycoproteins, chitin, melanin, and sulfonated polysaccharides, and algae’s cell walls are mostly alginate, sulfonated polysaccharides, and mannans [69]. The biosorption process’ mechanism is complicated since it is dependent on the types of biomass employed, the functional groups of the microbial cell wall, its structure, and the extracellular polymer compounds produced by bacteria [70,71]. For eliminating harmful heavy metals from polluted environments, the biosorption method is thought to have certain advantages over standard bioremediation strategies. The biosorption process has several advantages, including the presence of multifunctional groups and a homogeneous distribution of binding sites on the cell surface, biosorbent renewal, high efficiency, and the possibility of metal recovery. Because of these and other advantages, biosorption of heavy metals, notably hexavalent chromium, employing diverse microbial biomass has gotten a lot of attention. Environmental scientists, engineers, and biotechnologists have been fascinated by the ability of organisms to remove heavy metal ions induce their transformation to less dangerous forms for decades [72].
The Cr (VI) ion binds extracellularly to various functional groups of the microbial cell wall, which are eliminated via surface precipitation, ion exchange, or a similar mechanism. Microbes are organisms that have formed techniques for thousands of years to cope with environmental stress. Heavy metal-resistant defensive systems abound in microbial cells. The mechanisms involved are extracellular and intracellular sequestration, active metal ion transport, and metal ion reduction [73]. The biological process of heavy metal removal may be either biosorption or bioaccumulation, depending on the cell’s metabolism [74], the biological process of heavy metal elimination might be either biosorption or bioaccumulation. Increased membrane permeability increases intracellular uptake of heavy metals, which is a metabolism-dependent process. It can only happen in living creatures when pollutants are carried into the cell and metal ions accumulate inside the biosorbent’s cell [75]. Biosorption is a fast, autonomous, and metabolically passive mechanism that allows heavy metal ions to be selectively sequestered by dead/inactive biomaterials [76]. Heavy metals bind to cell walls extracellularly during biosorption, but once inside the cells, they bind to proteins such as metallothionein during the bioaccumulation process. A solid phase serves as the biosorbent in all of these biosorption processes. The sorbate is drawn and bonded by numerous mechanisms due to the sorbent’s increased affinity for the sorbate species [77].
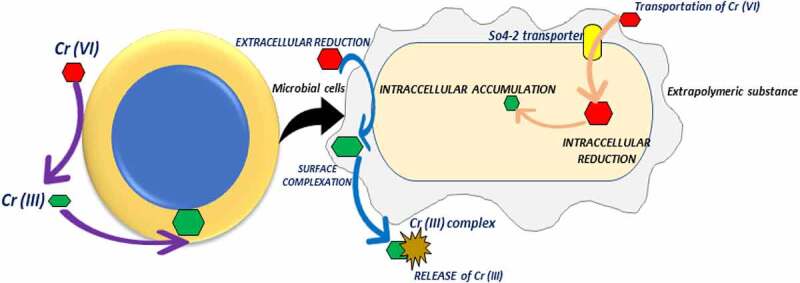
Biosorption is a physiochemical contact between metal species and biological species’ cellular components. Accumulation, adsorption, oxidation, methylation, and decrease of poisonous, Cr (III) to Cr (VI) are some of the mechanisms underpinning their resistance. Heavy metal ions can become lodged in the cellular structure of these organisms and then absorbed via binding sites. Phosphates, carboxyl, imadizole, amino, hydroxyl moieties, thioether, sulfate, phenol, amine, and sulfhydryl groups are all found in biosorbents [77]. Microorganisms interact with metal ions in a variety of ways, including cell wall-associated metals, intracellular accumulation, metal siderophore, extracellular polymeric contacts with extracellular mobilization or immobilization of metal ions, transformation, and metal volatilization. Ion exchange, complexation, adsorption, and microprecipitation are all examples of physicochemical interactions between the charged surface groups of microbes and ions in solution [78]. In the case of bioaccumulation, metal sequestration or uptake is followed by metallothionein binding, metal (Cr) localization within cell components, extracellular precipitation, metal deposition, and complexation [79]. Chromium translocation into the cell, chromium binding to the cell surface, and Cr (VI) reduction to Cr are the three phases of microbial Cr (VI) removal (III). Microbes reduce Cr (VI) on the cell surface, outside the cell, or within the cell, either directly through chromate reductase enzymes or indirectly through Cr (VI) metabolite reduction [80]. Hexavalent chromium (Cr (VI)) biosorption process by microbes has been provided in Figure 4 & Table 2 here.
Figure 4.
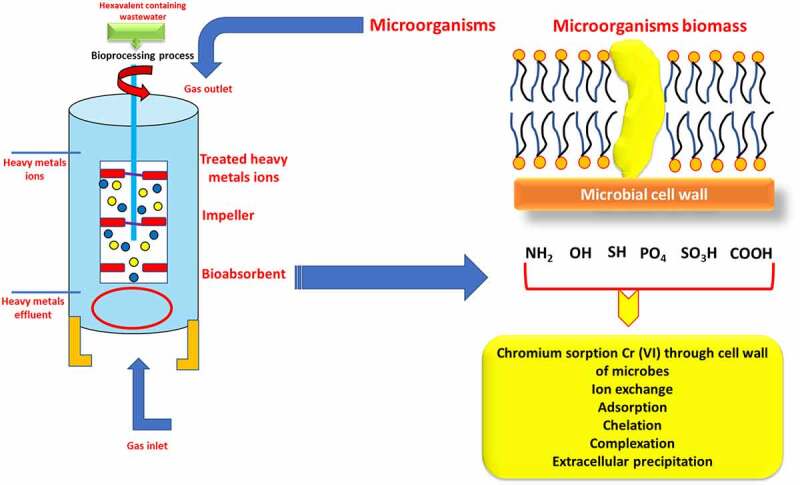
Hexavalent chromium (Cr (VI)) biosorption process by microorganisms.
Table 2.
Various microbes used for biosorption process of chromium (VI)
| S. No. | Microorganisms | Remediation % | References |
|---|---|---|---|
| 1. | Chelatococcus daeguensis | 94.42 (%) | [82,95] |
| 2. | Pseudomonas alcaliphila NEWG-2 | 96.60 (%) | [83,96] |
| 3. | Bacillus salmalaya | 20.35 mg/g | [84,97] |
| 4. | Bacillus sp | 75 (%) | [85,98] |
| 5. | Bacillus sp | 99 (%) | [86,99] |
| 6. | Klebsiella sp | 63.08 (%) | [87,100] |
| 7. | Sinorhizobium sp. SAR1 | 285.71 mg/g | [88,101] |
| 8. | Aspergillus sp | 92 (%) | [89,102] |
| 9. | Aspergillus terreus | 54 (%) | [90,103] |
| 10. | Pleurotus ostreatus | 100 (%) | [91,104] |
| 11. | Pseudopediastrum boryanum | 70 (%) | [91,104] |
| 12. | Scenedesmus sp | 98 (%) | [92,105] |
| 13. | Chlorella colonials | 97.8 (%) | [93,106] |
| 14. | Spirulina platensis | 45.5 mg/g | [94,107] |
| 15. | Isochrysis galbana | 335.27 mg/g | [95,108] |
| 16. | Chlamydomonas sp | 91 (%) | [36,109] |
5.2. Hexavalent chromium to tetravalent chromium reduction
On a local and big scale, textile, galvanizing, tannery, leather, metallurgical, electroplating, paint, and metal processing, and refining sectors produce dangerous metal ions in their effluents. By releasing metal ions into surrounding streams, rivers, and open pits, the above-mentioned businesses cause problems for the aquatic environment. Changes in surface and groundwater quantities are most likely to be experienced as a result of these metals’ potential impact on the environment [81]. Such hazardous elements not only endanger human health but also have an impact on other living things [82]. These source physical uneasiness and, intimidating illnesses, such as kidney damage and cancer [83]. Whereas its reduced trivalent form, (Cr3+) is less toxic, insoluble and a vital nutrient for humans. The high toxicity of Cr is stringent regulations are imposed on the release of Cr into surface water bodies to below 0.05 mg/l by the U.S. EPA and the European Union, while total Cr forms to below 2 mg/l [84]. Cr (Ⅵ) detoxification mechanism has been provided in Figure 5 & Tables 3, 4.
Figure 5.
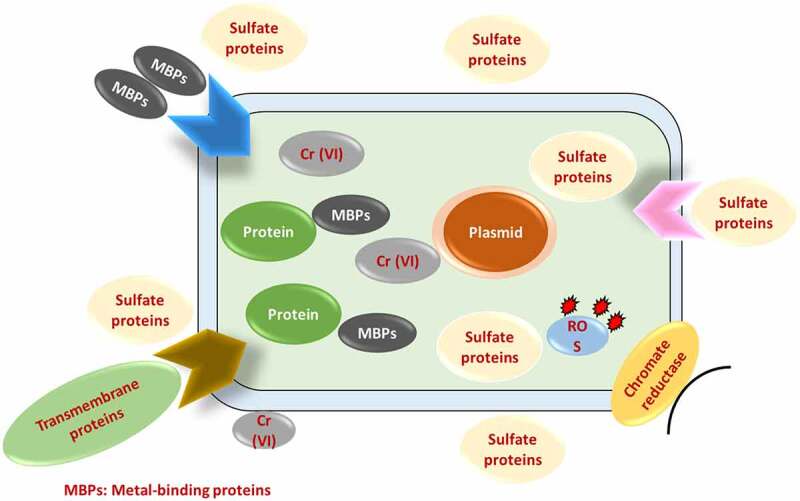
Cr(Ⅵ) detoxification mechanism.
Table 3.
Various functional groups involved in chromium (VI) binding by different microorganisms
| S. No. | Microorganisms | Functional groups | References |
|---|---|---|---|
| 1. | Bacillus marisflavi and Arthrobacter sp | -OH, -NH acetamido group, free phosphates, phosphate groups, -CN | [34,110] |
| 2. | Pseudomonas aeruginosa Rb-1 and Ochrobactrum intermedium Rb-2 | -OH, -NH, S-, -C-C- and C-Cl,- carboxylic group, | [96,111] |
| 3. | Streptomyces werraensis LD22 | O–H or N–H, C–H, C–O – | [97,112] |
| 4. | Aspergillus foetidus | C = O, C-Cl, PO4 −3 amine, N = C = S, OH, C-O | [98,113] |
| 5. | Pleurotus ostreatus | NH and COOH | [99,114] |
| 6. | Aspergillus Niger | -COOH, -OH, -NH2 | [100,115] |
| 7. | Klebsiella sp. | -NH2, O-H, -CONH-, -COOH, C = C, -CH2 | [87,100] |
| 8. | Halomonas sp. DK4 | –OH, – CH2, N-H, P–O–C, C = O | [101,116] |
| 9. | Scenedesmus sp | N-H, O-H, C-H, -COOH, C-F, C-Cl, C-Br, C-O | [31,117] |
| 10. | Chlorella miniata | O-H and N-H, C-H, -CH3, COO-, P = O, C-O – | [102,118] |
| 11. | Arthrinium malaysianum | –OH, C–O, C = O, – NO2, CxOH | [103,119] |
| 12. | Pleurotus ostreatus | NH and COOH- | [99,114] |
| 13. | Aspergillus Niger | -COOH, -OH, -NH2 | [100,115] |
Table 4.
Efficiency and mechanism of different microbes for the removal of Cr (VI)
| Microbes | Concentration (mg/L) | Carbon source | Temperature (°C) | pH | Efficiency | Mechanisms | References |
|---|---|---|---|---|---|---|---|
| Serratia sp. C8 | 20 | Glucose | 28 | 6–8 | ≈80% | Bioreduction | [120] |
| Sporosarcina saromensis M52 | 50–200 | Acetate | 35 | 7–8.5 | >90% | Bioreduction | [121] |
| Sphingopyxis macrogoltabida SUK2c | 4 | 31 | 2 | 55% | Biosorption | [122] | |
| Bacillus sp. CRB-B1 | 100 | Glucose | 37 | 7 | Completely | Bioreduction, biosorption | [123] |
| 100 | Fructose | 37 | 7 | 89.54% | Bioreduction, biosorption | [123] | |
| 100 | Sodium lactate | 37 | 7 | 4.8% | [123] | ||
| Pisolithus sp1 | 25 | Organic acid | 30 | 5–6 | 99% | Bioreduction, biosorption | [124] |
| Aspergillus sp. | 100 | Sucrose | 27 | 4 | 98.96% | Biosorption | [125] |
| Leiotrametes flavida | 1000 | 30 | 6 | 72.38% | Biosorption | [126] | |
| Bacillus cereus | 200 | Sucrose | 37 | 7.5 | Completely | Bioreduction, Biosorption | [127] |
6. Factor affecting bioremediation
The process of bacteria, fungi, and plants digesting, altering, immobilizing, and removing countless hazardous contaminants from the environment is known as biological treatment. Microbes are engaged because their enzymatic pathways operate as biocatalysts, allowing biochemical reactions to proceed more quickly and destroy the targeted contaminant. Microbes have access to a diversity of materials substances to assist them to manufacture nutrients and energy to build more cells so they act against pollution. The chemical composition and concentration of contaminants, as well as the physico-chemical features of the environment and their availability to microbes, all influence bioremediation efficacy [85]. Because bacteria and contaminants do not come into contact with each other, the rate of deterioration is influenced. Furthermore, bacteria and contaminants are not evenly distributed across the environment. Due to a variety of elements, regulating and improving bioremediation procedures is a complicated system. The availability of contaminants to the microbial community, and the presence of a bacterial community capable of decomposing the toxic pollutants.
6.1. Availability of nutrients
Nutrient supply influences the critical nutritional balance for microbial growth and reproduction, as well as the rate and effectiveness of biodegradation. By adjusting the bacterial C: N: P ratio, particularly the delivery of important nutrients like P and N, can boost degradation competence. Microorganisms require a variety of nutrients, including carbon, nitrogen, and phosphorus, to endure and remain their activity. The degree of hydrocarbon breakdown is similarly limited at low concentrations. In cold conditions, adding a suitable amount of nutrients is a good technique for enhancing microorganism metabolic activity and hence biodegradation rate. The availability of nutrients limits biodegradation in aquatic environments [86]. Such nutrients can be found in the natural world, although in little amounts [87].
6.2. Environmental factors
All through the procedure, the metabolic capabilities of the microorganisms and the physico-chemical features of the targeted pollutants determine probable interactions. The ambient variables at the interaction location, on the other hand, impact the actual success of the interaction between the two. Temperature, site features, solubility in water, redox potential, nutrients, pH, moisture, soil structure, and oxygen content, as well as physico-chemical bioavailability of contaminants and all influence microorganism development and activities. The parameters listed above determine the kinetics of deterioration [88,89]. Bioremediation can occur in a wide pH range; however, in most aquatic and terrestrial systems, a pH of 6.5 to 8.5 is generally ideal for microbial degradation. Pollutant metabolism is influenced by the types and amounts of soluble materials present, as well as the pH of terrestrial and aquatic ecosystems [90].
Temperature is the most essential physical factor in determining microbe survival and hydrocarbon content [91]. The sub-zero temperature of the water in this region causes transport channels within microbial cells to shut down or even freeze, rendering them metabolically dormant, rendering most oleophilic bacteria metabolically inert [92,93]. The metabolic cycle of biological enzymes involved in the degradation process has an optimal temperature and will not be the same at all temperatures. Furthermore, the degradation of a certain substance necessitates a specific temperature. Temperature influences microbial physiological features; hence it can speed up or slow down the bioremediation process. The rate of microbial activity increases as the temperature rises, peaking at the optimal temperature. It began to diminish abruptly when the temperature increased or decreased, eventually coming to a halt after reaching a set degree. The pH of a chemical, which refers to its acidity, basicity, and alkalinity, has an impact on microbial metabolic activity and the rate at which it is eliminated. The potential for microbial growth in the soil can be determined by measuring pH. Increase or decrease pH values resulted in poor results; metabolic processes are extremely sensitive to even minor pH variations. Because hazardous characteristics of some contaminants are present in high concentrations, toxic effects on microorganisms might occur, slowing clean-up. The degree and processes of toxicity differ depending on the toxicants, their concentrations, and the microbes exposed. Targeted life types are poisonous to some organic and inorganic chemicals [88].
7. Future prospects
The challenging problem of removing Cr (VI) pollutants from the environment has resulted in the development of numerous bioremediation strategies, particularly competent reduction techniques by microbes. Microbial degradation is a very profitable and appealing technology for cleaning, controlling, and restoring contaminated habitats via bacterial metabolism. Through autogenous enzymes or externally added reducing substances, microorganisms provide electrons to reduce Cr (VI). The rate at which undesired waste chemicals degrade is determined by competition with biological agents, insufficient food supply, unpleasant external abiotic conditions (aeration, moisture, pH, temperature), and limited pollutant bioavailability. Because of these characteristics, biodegradation under natural conditions is less successful, resulting in less favorable effects. Because bioremediation is only successful when the environment allows for microbial growth development. Bioremediation has been employed in a variety of locations around the world with variable degrees of effectiveness. In most instances, the benefits outweigh the disadvantages, as seen by the expanding number of sites that use this technology and its growing popularity over time. Usually, many species from various areas are researched and determined to be effective regulatory processes. Previous reports on bioremediation advanced technologies focused primarily on water bodies; however, arable land is currently suffering from severe heavy metal pollution, so future microbial remediation technology should also target the soil and environment. In response to the research deficiencies proposed, the following recommendations are made:
This is difficult to achieve the goal of governance using purely cultured microbes due to the complexity of the actual environmental conditions, particularly the soil. Through the synergy of microbes, the use of mixed cultures of microorganisms can improve the ability to adapt to the environment and treatment effectiveness.
Because polluted sites contain more than one type of heavy metals, this is appropriate to screen microbes for their potential to reduce or bind numerous toxic metals.
Bioremediation has a lower performance than physical and chemical materials, and it also takes a long time to remove heavy metals. Future studies could concentrate on the combination of microbes in the formation of a consortium to improve process efficiency.
8. Conclusions
The bioremediation and biosorption of chromium (VI) by microorganisms are discussed in this paper, as well as the parameters that metal accumulation mechanisms impact on metal’s elimination. The microbial remedy of Cr (VI) is among the most efficient and protracted strategies for decreasing excess Cr (VI) levels in the ecosystem. To survive in such a hazardous environment, these microorganisms have evolved amazing systems to maintain equilibrium and resistance to toxic metals. The biosorption technique is a microbe technology for eliminating chromium from the aquatic environment, it is safe and cost-effective, and has a great deal of potential in terms of future applications. The process of biosorption requires transport across the precipitation, complexation, cell membrane, ion exchange, and physical adsorption. The pH, contact time, temperature, biomass, and metal concentration parameters can all affect the biosorption ability of the biosorbent. Because industrial wastewaters, unlike laboratory solutions, hazardous heavy metals, simultaneous removal of multiple coexisting contaminants may be difficult. Conclusion of this review more research on this topic is required to fully exploit the benefits of microbial biotechnology in the ecosystem.
Acknowledgements
This research is supported by the National Research Foundation, Prime Minister’s Office, Singapore under its Campus for Research Excellence and Technological Enterprise (CREATE) program.
Funding Statement
This work was supported by the National Research Foundation, Prime Minister’s Office, Singapore under its Campus for Research Excellence and Technological Enterprise (CREATE) programme. [NA].
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- [1].Sharma A, Kapoor D, Wang J, et al. Chromium bioaccumulation and its impacts on plants: an overview. Plants. 2020a;9(1):100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Jiang B, Liu Y, Zheng J, et al. Synergetic transformations of multiple pollutants driven by Cr (VI)–sulfite reactions. Environ Sci Technol. 2015;49(20):12363–12371. [DOI] [PubMed] [Google Scholar]
- [3].Liang J, Huang X, Yan J, et al. A review of the formation of Cr(VI) via Cr(III) oxidation in soils and groundwater. SciTotal Environ. 2021;774:145762. [Google Scholar]
- [4].Kazakis N, Kougias I, Patsialis T.. Assessment of flood hazard areas at a regional scale using an index-based approach and analytical hierarchy process: application in Rhodope–Evros region, Greece. SciTotal Environ. 2015;538:555–563. [DOI] [PubMed] [Google Scholar]
- [5].Oze C, Bird DK, Fendorf S. Genesis of hexavalent chromium from natural sources in soil and groundwater. Proc Nat Acad Sci. 2007;104(16):6544–6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Vengosh A, Jackson RB, Warner N, et al. A critical review of the risks to water resources from unconventional shale gas development and hydraulic fracturing in the United States. Environ Sci Technol. 2014;48(15):8334–8348. [DOI] [PubMed] [Google Scholar]
- [7].Ball JW, Izbicki JA. Occurrence of hexavalent chromium in ground water in the western Mojave Desert, California. Appl Geochem. 2004;19(7):1123–1135. [Google Scholar]
- [8].Manning P, de Vries FT, Tallowin JRB, et al. Simple measures of climate, soil properties and plant traits predict national-scale grassland soil carbon stocks. J Appl Ecol. 2015;52(5):1188–1196. [Google Scholar]
- [9].Bourotte C, Bertolo R, Almodovar M, et al. Natural occurrence of hexavalent chromium in a sedimentary aquifer in Urânia, State of São Paulo, Brazil. Anais da Academia Brasileira de Ciências. 2009;81(2):227–242. [Google Scholar]
- [10].Tziritis E, Kelepertzis E, Korres G, et al. Hexavalent chromium contamination in groundwaters of Thiva basin, central Greece. Bull Environ Contam Toxicol. 2012;89(5):1073–1077. [DOI] [PubMed] [Google Scholar]
- [11].US EPA (US Environmental Protection Agency) . Data summary of the third unregulated contaminant monitoring rule (UCMR 3). EPA 815-S-17-001. Washington: USEPA; 2017. [Google Scholar]
- [12].Moffat I, Martinova N, Seidel C, et al. Hexavalent chromium in drinking water. J Am water works assoc. 2018;110(5):E22–E35. [Google Scholar]
- [13].Fathima NN, Aravindhan R, Rao JR, et al. Solid waste removes toxic liquid waste: adsorption of chromium (VI) by iron complexed protein waste. Environ Sci Technol. 2005;39(8):2804–2810. [DOI] [PubMed] [Google Scholar]
- [14].Abdulla HM, Kamal EM, and Mohamed AH, et al., 2010. Chromium removal from tannery wastewater using chemical and biological techniques aiming at zero discharge of pollution. In Proceeding of Fifth Scientific Environmental Conference. Zagazig University, Egypt. (pp. 171–183). [Google Scholar]
- [15].Kocaoba S, Akcin G. Removal and recovery of chromium and chromium speciation with MINTEQA2. Talanta. 2002;57(1):23–30. [DOI] [PubMed] [Google Scholar]
- [16].Komori K, Rivas A, Toda K, et al. A method for removal of toxic chromium using dialysis-sac cultures of a chromate-reducing strain of Enterobacter cloacae. Appl Microbiol Biotechnol. 1990;33(1):117–119. [DOI] [PubMed] [Google Scholar]
- [17].Poopal AC, Laxman RS. Studies on biological reduction of chromate by Streptomyces griseus. J Hazard Mater. 2009;169(1–3):539–545. [DOI] [PubMed] [Google Scholar]
- [18].Yasmeen S, Kabiraz MK, Saha B, et al. Chromium (VI) ions removal from tannery effluent using chitosan-microcrystalline cellulose composite as adsorbent. Int Res J Pure Appl Chem. 2016;1–14. DOI: 10.9734/IRJPAC/2016/23315 [DOI] [Google Scholar]
- [19].El Nemr A. Pomegranate husk as an adsorbent in the removal of toxic chromium from wastewater. Chem Ecol. 2007;23(5):409–425. [Google Scholar]
- [20].Amir A, Abd Rahim RNR, Abdul-Talib S. Removal of chromium hexavalent using agriculture waste. Int J Environ Sci Dev. 2017;8(4):260. [Google Scholar]
- [21].Stoller M, Sacco O, Vilardi G, et al., 2017. Chromium recovery by membranes for process reuse in the tannery industry. In 15th international conference on environmental science and technology, Rhodes. [Google Scholar]
- [22].Payel S, Sarker M, and Hashem MA, 2018, February. Banana rachis charcoal to remove chromium from tannery wastewater. In 4th International Conference on Civil and Environmental Engineering for Sustainability (IConCEES 2017)4–5 December 2017, Langkawi, Malaysia (pp. 9–11). [Google Scholar]
- [23].Prado A, Moura A, Andrade R, et al. Application of Brazilian sawdust samples for chromium removal from tannery wastewater. J Therm Anal Calorim. 2010;99(2):681–687. [Google Scholar]
- [24].Wise JP Jr, Young JL, Cai J, et al. Current understanding of hexavalent chromium [Cr(VI)] neurotoxicity and new perspectives. Environ Int. 2022;158:106877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sharma P, Purchase D, Chandra R. Residual pollutants in treated pulp paper mill wastewater and their phytotoxicity and cytotoxicity in Allium cepa. Environ Geochem Health. 2021a;43(5):2143–2164. [DOI] [PubMed] [Google Scholar]
- [26].Sharma P, and Singh SP. Pollutants characterization and toxicity assessment of pulp and paper industry sludge for safe environmental disposal. In Ming H. Wong: Emerging Treatment Technologies for Waste Management. Singapore: Springer; 2021a. p. 207–223. [Google Scholar]
- [27].Sharma P, Tripathi S, Chandra R. Phytoremediation potential of heavy metal accumulator plants for waste management in the pulp and paper industry. Heliyon. 2020b;6(7):e04559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sharma P, Tripathi S, Chandra R. Highly efficient phytoremediation potential of metal and metalloids from the pulp paper industry waste employing Eclipta alba (L) and Alternanthera philoxeroide (L): biosorption and pollution reduction. Bioresour Technol. 2021b;319:124147. [DOI] [PubMed] [Google Scholar]
- [29].Sharma P, Tripathi S, Chaturvedi P, et al. Newly isolated Bacillus sp. PS-6 assisted phytoremediation of heavy metals using Phragmites communis: potential application in wastewater treatment. Bioresour Technol. 2021c;320:124353. [DOI] [PubMed] [Google Scholar]
- [30].Sharma P, Tripathi S, Chandra R. Metagenomic analysis for profiling of microbial communities and tolerance in metal-polluted pulp and paper industry wastewater. Bioresour Technol. 2021d;324:124681. [DOI] [PubMed] [Google Scholar]
- [31].Sharma P, Pandey AK, Udayan A, et al. Role of microbial community and metal-binding proteins in phytoremediation of heavy metals from industrial wastewater. Bioresour Technol. 2021e;326:124750. [DOI] [PubMed] [Google Scholar]
- [32].Sharma P. Efficiency of bacteria and bacterial assisted phytoremediation of heavy metals: an update. Bioresour Technol. 2021a;328:124835. [DOI] [PubMed] [Google Scholar]
- [33].Yang W, Song W, Li J, et al. Bioleaching of heavy metals from wastewater sludge with the aim of land application. Chemosphere. 2020;249:126134. [DOI] [PubMed] [Google Scholar]
- [34].Shekhawat K, Chatterjee S, Joshi B. Chromium toxicity and its health hazards. Int J Adv Res. 2015;3(7):167–172. [Google Scholar]
- [35].Vendruscolo F, da Rocha Ferreira GL, Antoniosi Filho NR. Biosorption of hexavalent chromium by microorganisms. Int Biodeterior Biodegrad. 2017;119:87–95. [Google Scholar]
- [36].DesMarias TL, Costa M. Mechanisms of chromium-induced toxicity. Curr Opin Toxicol. 2019;14:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Junaid M, Hashmi MZ, Malik RN, et al. Toxicity and oxidative stress induced by chromium in workers exposed from different occupational settings around the globe: a review. Environ Sci Pollut Res. 2016;23(20):20151–20167. [DOI] [PubMed] [Google Scholar]
- [38].Seidler A, Jähnichen S, Hegewald J, et al. Systematic review and quantification of respiratory cancer risk for occupational exposure to hexavalent chromium. Int Arch Occup Environ Health. 2013;86(8):943–955. [DOI] [PubMed] [Google Scholar]
- [39].Focardi S, Pepi M, Focardi SE. Microbial reduction of hexavalent chromium as a mechanism of detoxification and possible bioremediation applications. Biodegrad Life Sci. 2013;321–347. [Google Scholar]
- [40].Al Osman M, Yang F, Massey IY. Exposure routes and health effects of heavy metals on children. Biometals. 2019;32(4):563–573. [DOI] [PubMed] [Google Scholar]
- [41].Elahi A, Arooj I, Bukhari DA, et al. Successive use of microorganisms to remove chromium from wastewater. Appl Microbiol Biotechnol. 2020;104(9):3729–3743. [DOI] [PubMed] [Google Scholar]
- [42].Cervantes C, Campos-García J, Devars S, et al. Interactions of chromium with microorganisms and plants. FEMS Microbiol Rev. 2001;25(3):335–347. [DOI] [PubMed] [Google Scholar]
- [43].Shanker AK, Cervantes C, Lozatavera H, et al. Chromium toxicity in plants. Environ Int. 2005;31(5):739–753. [DOI] [PubMed] [Google Scholar]
- [44].Jobby R, Jha P, Yadav AK, et al. Biosorption and biotransformation of hexavalent chromium [Cr(VI)]: a comprehensive review. Chemosphere. 2018;207:255–266. [DOI] [PubMed] [Google Scholar]
- [45].Sharma P, Tripathi S, Vadakedath N, et al. In-situ toxicity assessment of pulp and paper industry wastewater on Trigonella foenum-graecum L: potential source of cytotoxicity and chromosomal damage. Environ Technol Innovation. 2021d;21:101251. [Google Scholar]
- [46].Stambulska UY, Bayliak MM, Lushchak VI. Chromium(VI) toxicity in legume plants: modulation effects of rhizobial symbiosis. Biomed Res Int. 2018;2018:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Ina S. Chromium, an essential nutrient and pollutant: a review. Afr J Pure Appl Chem. 2013;7(9):310–317. [Google Scholar]
- [48].Guo S, Xiao C, Zhou N, et al. Speciation, toxicity, microbial remediation and phytoremediation of soil chromium contamination. Environ Chem Lett. 2021;19(2):1413–1431. [Google Scholar]
- [49].Van Hoeck V, Sonawane M, Sanchez ALG, et al. Chromium propionate improves performance and carcass traits in broilers. Anim Nutr. 2020;6(4):480–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Tran NT, Zhang J, Xiong F, et al. Altered gut microbiota associated with intestinal disease in grass carp (Ctenopharyngodon idellus). World J Microbiol Biotechnol. 2018;34(6):1–9. [DOI] [PubMed] [Google Scholar]
- [51].Jin Y, Wu S, Zeng Z, et al. Effects of environmental pollutants on gut microbiota. Environ Pollut. 2017;222:1–9. [DOI] [PubMed] [Google Scholar]
- [52].Li A, Liu B, and He Y, et al. Integrated bacterial and fungal diversity analysis reveals the gut microbial alterations in diarrheic giraffes. Front Microbiol. 2021;2241 12 1–15 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Rahman Z, Thomas L. Chemical-assisted microbially mediated chromium (Cr) (VI) reduction under the influence of various electron donors, redox mediators, and other additives: an outlook on enhanced Cr(VI) removal. Front Microbiol. 2021;11:3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Sharma P, Rath SK. Potential applications of fungi in the remediation of toxic effluents from pulp and paper industries. In: Fungi bio-prospects in sustainable agriculture, environment and nano-technology. Academic Press; 2021. p. 193–211. [Google Scholar]
- [55].Sharma P, Kumar S, Pandey A. Bioremediated techniques for remediation of metal pollutants using metagenomics approaches: a review. J Environ Chem Eng. 2021e;9(4):105684. [Google Scholar]
- [56].Sharma P, Pandey AK, Kim S-H, et al. Critical review on microbial community during in-situ bioremediation of heavy metals from industrial wastewater. Environ Technol Innovation. 2021f;24:101826. [Google Scholar]
- [57].Sharma P, Tripathi S, Purchase D, et al. Integrating phytoremediation into treatment of pulp and paper industry wastewater: field observations of native plants for the detoxification of metals and their potential as part of a multidisciplinary strategy. J Environ Chem Eng. 2021;9(4):105547. [Google Scholar]
- [58].Sharma P, Ngo HH, Khanal S, et al. Efficiency of transporter genes and proteins in hyperaccumulator plants for metals tolerance in wastewater treatment: sustainable technique for metal detoxification. Environ Technol Innovation. 2021g;23:101725. [Google Scholar]
- [59].Tripathi S, Sharma P, Purchase D, et al. Biodegradation of organo-metallic pollutants in distillery wastewater employing a bioaugmentation process. Environ Technol Innovation. 2021a;23:101774. [Google Scholar]
- [60].Tripathi S, Sharma P, Chandra R. Degradation of organometallic pollutants of distillery wastewater by autochthonous bacterial community in biostimulation and bioaugmentation process. Bioresour Technol. 2021b;338:125518. [DOI] [PubMed] [Google Scholar]
- [61].Sharma P, Kumar S. Bioremediation of heavy metals from industrial effluents by endophytes and their metabolic activity: recent advances. Bioresour Technol. 2021;339:125589. [DOI] [PubMed] [Google Scholar]
- [62].Sharma P. Role and significance of biofilm-forming microbes in phytoremediation—A review. Environ Technol Innovation. 2021b. 25 ;102182. [Google Scholar]
- [63].Singh S, Kang SH, Mulchandani A, et al. Bioremediation: environmental clean-up through pathway engineering. Curr Opin Biotechnol. 2008;19(5):437–444. [DOI] [PubMed] [Google Scholar]
- [64].Bolisetty S, Peydayesh M, Mezzenga R. Sustainable technologies for water purification from heavy metals: review and analysis. Chem Soc Rev. 2019;48(2):463–487. [DOI] [PubMed] [Google Scholar]
- [65].GracePavithra K, Jaikumar V, Kumar PS, et al. A review on cleaner strategies for chromium industrial wastewater: present research and future perspective. J Clean Prod. 2019;228:580–593. [Google Scholar]
- [66].Fernández PM, Viñarta SC, Bernal AR, et al. Bioremediation strategies for chromium removal: current research, scale-up approach and future perspectives. Chemosphere. 2018;208:139–148. [DOI] [PubMed] [Google Scholar]
- [67].Dhir B. Potential of biological materials for removing heavy metals from wastewater. Environ Sci Pollut Res. 2014;21(3):1614–1627. [DOI] [PubMed] [Google Scholar]
- [68].Rezaei H. Biosorption of chromium by using Spirulina sp. Arabian J Chem. 2016;9(6):846–853. [Google Scholar]
- [69].Garcia-Rubio R, de Oliveira HC, Rivera J, et al. The fungal cell wall: candida, cryptococcus, and aspergillus species. Front Microbiol. 2020;10:2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Chojnacka K. Biosorption and bioaccumulation – the prospects for practical applications. Environ Int. 2010;36(3):299–307. [DOI] [PubMed] [Google Scholar]
- [71].Farooq U, Kozinski JA, Khan MA, et al. Biosorption of heavy metal ions using wheat based biosorbents – a review of the recent literature. Bioresour Technol. 2010;101(14):5043–5053. [DOI] [PubMed] [Google Scholar]
- [72].Mustapha MU, Halimoon N. Microorganisms and biosorption of heavy metals in the environment: a review paper. Journal of Microbial & Biochemical Technology. 2015;7(5):253–256. [Google Scholar]
- [73].Igiri BE, Okoduwa SIR, Idoko GO, et al. Toxicity and bioremediation of heavy metals contaminated ecosystem from tannery wastewater: a review. J Toxicol. 2018;2018:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Hansda A, Kumar V. A comparative review towards potential of microbial cells for heavy metal removal with emphasis on biosorption and bioaccumulation. World J Microbiol Biotechnol. 2016;32(10):1–14. [DOI] [PubMed] [Google Scholar]
- [75].Velásquez L, Dussan J. Biosorption and bioaccumulation of heavy metals on dead and living biomass of Bacillus sphaericus. J Hazard Mater. 2009;167(1–3):713–716. [DOI] [PubMed] [Google Scholar]
- [76].Zabochnicka-Świątek M, and Krzywonos M. Potentials of biosorption and bioaccumulation processes for heavy metal removal. Polish J Environ Stud. 2014;23(2 551–561). [Google Scholar]
- [77].Garg SK, Tripathi M, Srinath T. Strategies for chromium bioremediation of tannery effluent. Rev Environ Contam Toxicol. 2012;217:75–140. [DOI] [PubMed] [Google Scholar]
- [78].Malaviya P, Singh A. Bioremediation of chromium solutions and chromium containing wastewaters. Crit Rev Microbiol. 2016;42(4):607–633. [DOI] [PubMed] [Google Scholar]
- [79].Bhattacharya A, Gupta A, Kaur A, et al. Alleviation of hexavalent chromium by using microorganisms: insight into the strategies and complications. Water Sci Technol. 2019;79(3):411–424. [DOI] [PubMed] [Google Scholar]
- [80].Joutey NT, Sayel H, Bahafid W, et al. Mechanisms of hexavalent chromium resistance and removal by microorganisms. Rev Environ Contam Toxicol. 2015;233:45–69. [DOI] [PubMed] [Google Scholar]
- [81].Javed A. Pollution level analysis in tannery effluents collected from three different cities of Punjab. J Ind Pollut Control Board. 2004. 9 3 418–421 . [Google Scholar]
- [82].Devi BD, Thatheyus AJ, Ramya D. Bioremoval of hexavalent chromium, using Pseudomonas fluorescens. J Microbiol Biotechnol Res. 2012;2(5):727–735. [Google Scholar]
- [83].Abhipsa S, Chandraraj K. Enzymatic reduction of hexavalent chromium in bacteria. ENVIS Newslett. 2009;7:2–5. [Google Scholar]
- [84].Ganguli A, Tripathi AK. Survival and chromate reducing ability of Pseudomonas aeruginosa in industrial effluents. Lett Appl Microbiol. 1999;28(1):76–80. [DOI] [PubMed] [Google Scholar]
- [85].El Fantroussi S, Agathos SN. Is bioaugmentation a feasible strategy for pollutant removal and site remediation? Curr Opin Microbiol. 2005;8(3):268–275. [DOI] [PubMed] [Google Scholar]
- [86].Thavasi R, Sharma S, and Jayalakshmi S. Evaluation of screening methods for the isolation of biosurfactant producing marine bacteria. J Pet Environ Biotechnol S. 2011;1(2 1–7). [Google Scholar]
- [87].Asira EE. Factors that determine bioremediation of organic compounds in the soil. Acad J Interdiscip Stud. 2013;2(13):125. [Google Scholar]
- [88].Naik MG, Duraphe MD. Review paper on-Parameters affecting bioremediation. Int J Life Sci Pharma Res. 2012;2(3):L77–L80. [Google Scholar]
- [89].Adams GO, Fufeyin PT, Okoro SE, et al. Bioremediation, Biostimulation and Bioaugmention: a Review. Int J Environ Bioremed Biodegrad. 2020;3(1):28–39. [Google Scholar]
- [90].Cases I, and Lorenzo VD. Genetically modified organisms for the environment: stories of success and failure and what we have learned from them International Microbiology . 2005. 8(3) 213 . [PubMed] [Google Scholar]
- [91].Das N, Chandran P. Microbial degradation of petroleum hydrocarbon contaminants: an overview. Biotechnol Res Int. 2011;2011:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Macaulay BM. Understanding the behaviour of oil-degrading micro-organisms to enhance the microbial remediation of spilled petroleum. Appl Ecol Environ Res. 2015;13(1):247–262. [Google Scholar]
- [93].Yang S-Z, Jin J, Wei Z, et al. Bioremediation of oil spills in cold environments: a review. Pedosphere. 2009;19(3):371–381. [Google Scholar]
- [94].Singh A. ”Hexavalent Chromium: toxic and genotoxic effects and its bioremediation strategies”. Biomed J Sci Tech Res. 2021;35(3):27637–27643. [Google Scholar]
- [95].Li H, Huang S, Zhang Y. Cr(VI) removal from aqueous solution by thermophilic denitrifying bacterium Chelatococcus daeguensis TAD1 in the presence of single and multiple heavy metals. J Microbiol. 2016;54(9):602–610. [DOI] [PubMed] [Google Scholar]
- [96].El-Naggar NE-A, El-Khateeb AY, Ghoniem AA, et al. Innovative low-cost biosorption process of Cr6+ by Pseudomonas alcaliphila NEWG-2. Sci Rep. 2020;10(1):1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Dadrasnia A, Chuan Wei KS, Shahsavari N, et al. Biosorption potential of bacillus salmalaya strain 139SI for removal of Cr(VI) from aqueous solution. Int J Environ Res Public Health. 2015;12(12):15321–15338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Upadhyay N, Vishwakarma K, Singh J, et al. Tolerance and Reduction of Chromium(VI) by Bacillus sp. MNU16 Isolated from Contaminated Coal Mining Soil. Front Plant Sci. 2017;8:778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Pun R, Raut P, Pant BR. Removal of chromium (VI) from leachate using bacterial biomass. Sci World. 2013;11(11):63–65. [Google Scholar]
- [100].Hossan S, Hossain S, Islam MR, et al. Bioremediation of hexavalent chromium by chromium resistant bacteria reduces phytotoxicity. Int J Environ Res Public Health. 2020;17(17):6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Jobby R, Jha P, Gupta A, et al. Biotransformation of chromium by root nodule bacteria Sinorhizobium sp. SAR1. PloS one. 2019;14(7):e0219387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Congeevaram S, Dhanarani S, Park J, et al. Biosorption of chromium and nickel by heavy metal resistant fungal and bacterial isolates. J Hazard Mater. 2007;146(1–2):270–277. [DOI] [PubMed] [Google Scholar]
- [103].Kumaran MB, Prasathkumar M, Kumar DM, et al. Utilization of Aspergillus terreus for the biosorption of hexavalent chromium ions. Asian J Biol Sci. 2013;6(7):312–321. [Google Scholar]
- [104].Da Rocha Ferreira GL, Vendruscolo F, Antoniosi Filho NR. Biosorption of hexavalent chromium by Pleurotus ostreatus. Heliyon. 2019;5(3):e01450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Ballén-Segura M, Hernández Rodríguez L, Parra Ospina D, et al. Using Scenedesmus sp. for the phycoremediation of tannery wastewater. Tecciencia. 2016;11(21):69–75. [Google Scholar]
- [106].Jaafari J, Yaghmaeian K. Optimization of heavy metal biosorption onto freshwater algae (Chlorella coloniales) using response surface methodology (RSM). Chemosphere. 2019;217:447–455. [DOI] [PubMed] [Google Scholar]
- [107].Nithya K, Sathish A, Pradeep K, et al. Algal biomass waste residues of Spirulina platensis for chromium adsorption and modeling studies. J Environ Chem Eng. 2019;7(5):103273. [Google Scholar]
- [108].Kadimpati KK, Mondithoka KP, Bheemaraju S, et al. Entrapment of marine microalga, Isochrysis galbana, for biosorption of Cr (III) from aqueous solution: isotherms and spectroscopic characterization. Appl Water Sci. 2013;3(1):85–92. [Google Scholar]
- [109].Ayele A, Godeto YG. Bioremediation of chromium by microorganisms and its mechanisms related to functional groups. J Chem. 2021;2021:1–21. [Google Scholar]
- [110].Mishra S, Bharagava RN. Toxic and genotoxic effects of hexavalent chromium in environment and its bioremediation strategies. J Environ Sci Health, Part C. 2016;34(1):1–32. [DOI] [PubMed] [Google Scholar]
- [111].Batool R, Yrjälä K, Hasnain S. Impact of environmental stress on biochemical parameters of bacteria reducing chromium. Braz J Microbiol. 2014;45:573–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Latha S, Vinothini G, Dhanasekaran D. Chromium [Cr (VI)] biosorption property of the newly isolated actinobacterial probiont Streptomyces werraensis LD22. 3 Biotech. 2015;5(4):423–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Ahluwalia SS, Goyal D. Removal of Cr (VI) from aqueous solution by fungal biomass. Eng Life Sci. 2010;10(5):480–485. [Google Scholar]
- [114].Arbanah M, Miradatul NMR, Halim KKH. Utilization of Pleurotus ostreatus in the removal of Cr (VI) from chemical laboratory waste. Int Refreed J Eng Sci. 2013;2(4):29–39. [Google Scholar]
- [115].Chhikara S, Hooda A, Rana L, et al. Chromium (VI) biosorption by immobilized Aspergillus Niger in continuous flow system with special reference to FTIR analysis. J Environ Biol. 2010;31(5):561–566. [PubMed] [Google Scholar]
- [116].Kalola V, Desai C. Biosorption of Cr (VI) by Halomonas sp. DK4, a halotolerant bacterium isolated from chrome electroplating sludge. Environ Sci Pollut Res. 2020;27(22):27330–27344. [DOI] [PubMed] [Google Scholar]
- [117].Pradhan D, Sukla LB, Sawyer M, et al. Recent bioreduction of hexavalent chromium in wastewater treatment: a review. J Ind Eng Chem. 2017;55:1–20. [Google Scholar]
- [118].Han X, Wong YS, Wong MH, et al. Feasibility of using microalgal biomass cultured in domestic wastewater for the removal of chromium pollutants. Water Environ Res. 2008;80(7):647–653. [PubMed] [Google Scholar]
- [119].Majumder R, Sheikh L, Naskar A, et al. Depletion of Cr (VI) from aqueous solution by heat dried biomass of a newly isolated fungus Arthrinium malaysianum: a mechanistic approach. Sci Rep. 2017;7(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].González PS, Ambrosio LF, Paisio CE, et al. Chromium (VI) remediation by a native strain: effect of environmental conditions and removal mechanisms involved. Environ Sci Pollut Res. 2014;21(23):13551–13559. [DOI] [PubMed] [Google Scholar]
- [121].Ran ZHAO, Bi WANG, Cai QT, et al. Bioremediation of hexavalent chromium pollution by Sporosarcina saromensis M52 isolated from offshore sediments in Xiamen, China. Biomedical and Environmental Sciences. 2016;29(2):127–136. [DOI] [PubMed] [Google Scholar]
- [122].Prabhakaran DC, Bolanos-Benitez V, Sivry Y, et al. Mechanistic studies on the bioremediation of Cr (VI) using Sphingopyxis macrogoltabida SUK2c, a Cr (VI) tolerant bacterial isolate. Biochem Eng J. 2019;150:107292. [Google Scholar]
- [123].Tan H, Wang C, Zeng G, et al. Bioreduction and biosorption of Cr (VI) by a novel Bacillus sp. CRB-B1 strain. J Hazard Mater. 2020;386:121628. [DOI] [PubMed] [Google Scholar]
- [124].Shi L, Xue J, Liu B, et al. Hydrogen ions and organic acids secreted by ectomycorrhizal fungi, Pisolithus sp1, are involved in the efficient removal of hexavalent chromium from waste water. Ecotoxicol Environ Saf. 2018;161:430–436. [DOI] [PubMed] [Google Scholar]
- [125].Chakraborty V, Sengupta S, Chaudhuri P, et al. Assessment on removal efficiency of chromium by the isolated manglicolous fungi from Indian Sundarban mangrove forest: removal and optimization using response surface methodology. Environ Technol Innovation. 2018;10:335–344. [Google Scholar]
- [126].Antony GS, Manna A, Baskaran S, et al. Non-enzymatic reduction of Cr (VI) and it’s effective biosorption using heat-inactivated biomass: a fermentation waste material. J Hazard Mater. 2020;392:122257. [DOI] [PubMed] [Google Scholar]
- [127].Banerjee S, Misra A, Chaudhury S, et al. A Bacillus strain TCL isolated from Jharia coalmine with remarkable stress responses, chromium reduction capability and bioremediation potential. J Hazard Mater. 2019;367:215–223. [DOI] [PubMed] [Google Scholar]


