ABSTRACT
Chinese patent medicine (CPM) has been widely used in China for patients with osteoporosis (OP) but a comprehensive literature review is still important. Therefore, we performed meta-analysis using six electronic databases prior to 30 April 2021 only randomized controlled trials (RCTs) using CPM as the first-line treatment in adults with OP were included. Thirty RCTs met the inclusion criteria with a total of 2723 patients, and seven types of CPM were included. Compared with the control group, 23 studies showed significantly improved bone mineral density (BMD) (lumbar spine) (mean difference [MD] = 0.08; confidence interval [CI], 0.03 to 0.13), 15 studies showed significantly improved BMD (femoral) (MD = 0.05; 95% CI, 0.02 to 0.07), 6 studies showed significantly improved BMD (radius) (MD = 0.06; 95% CI, 0.03 to 0.09), 2 trials showed significantly improvement of BMD (ulna) (MD = 0.02; 95% CI, 0.01 to 0.03), and 4 trials showed significantly improved BMD (MD = 0.09; 95% CI, 0.09 to 0.10). The meta-analysis also showed that CPM had superior pain improvement, a higher total effectiveness rate, and a lower risk of adverse events compared with standard western treatment. The findings of this study suggest that CPM therapy may be a safe and effective alternative treatment modality for OP, it has potential benefits in relieving symptoms and improving BMD compared to western medications or placebos.
KEYWORDS: Chinese patent medicine, clinical efficacy, herbal medicine, meta-analysis, osteoporosis
Graphical Abstract
Introduction
Osteoporosis (OP) is a systemic skeletal disease characterized by decreased bone mass and microarchitectural deterioration of bone tissue, resulting in increased bone fragility and fracture risk [1]. OP imposes exorbitant financial expenditures on society, while patients suffer from serious bone fractures and physical agony [2]. According to reports, the prevalence of OP has grown, and it now affects 34.65% of adults aged over 50 years in China [3]. There are also several therapies for OP, such as bisphosphonates, which are the most often recommended drug for the illness [4]. Bisphosphonates, on the other hand, are linked to a number of possible dangers, including osteonecrosis and gastrointestinal side effects [5]. As a necessary consequence, there has been an upsurge in discovering methods to prevent and cure OP.
Traditional Chinese medicine (TCM) is heavily favored in the treatment of OP in China. According to TCM theory, OP is classified as ‘bone impediment’ or ‘bone wilting’ caused by an insufficient innate endowment and an imbalance of acquired absorption and nourishment. TCM theory asserts that an invasion of exogenous evil can induce OP, leading to a disharmony of yin-yang, qi, and blood; a deficiency of the spleen, liver, and kidney; and a loss of bone nourishment [6]. Correspondingly, the principle of Chinese medicine treatment is to tonify the kidney and strong bones. Chinese patent medicine (CPM) is composed of Chinese herbal medicines as raw materials and processed into TCM products according to the prescribed prescription and preparation process [7]. CPM includes various forms such as pills, powders, granules, and capsules [8]. Currently, there are hundreds of types of CPM used for the treatment of OP, and several recent studies have suggested that their active ingredients may exert a certain effect on bone mineral density (BMD) and overall symptoms by increasing hormone levels and regulating bone metabolism-related pathways [9,10]. Although CPM has long been regarded as a key component in China and recommended in several Chinese treatment guidelines of OP, either as a monotherapy or in combination with standard western medicine, the quality of the evidence has led to varying degrees of efficacy and safety assessments. Many new clinical studies have been published since then, but existing systematic reviews were still limited by samples, methodological quality [11,12], or specific kinds of CPM [13].
From this, it can be seen that a comprehensive review is still an important step for making recommendations in clinical practice. Thus, we systematically reviewed the a large amount of medical literature and performed a meta-analysis on randomized controlled trials (RCTs) of CPM therapy for patients with OP to understand its benefits for OP.
Methods
Protocol and registration
This study was based on the recommendations of the Cochrane handbook for systematic reviews of interventions and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [14]. This study has been registered on PROSPERO (CRD42020183795).
Search strategy
We searched the following six electronic databases to identify qualified trials published from inception to 30 April 2021: PubMed, Cochrane Library, Chinese Biomedical Databases (CBM), Chinese National Knowledge Infrastructure (CNKI), Wan Fang, and Chongqing VIP. In addition, we manually searched for publication records from the library. There were no restriction on publication language. The search strategy included the following keywords: traditional Chinese medication, traditional Chinese patent medicine, capsule, tablet, powders, pill, granules, osteoporosis, clinical trial, and randomized controlled trial.
Eligibility criteria
We only included RCTs that compared CPM with conventional western therapies and placebos for the treatment of OP and that involved interventions of CPM therapy for the duration of at least 2 weeks with more than 10 subjects in each group. The diagnostic criterion was from the OP Committee of Chinese Gerontology Society [15,16] and Chinese Medical Association [17,18]. We also accepted diagnostic criteria for primary OP in Chinese (Trial) [19]. To be eligible for this study, the experimental group had to be treated with CPM, and the control group had to only receive non-Chinese medicine interventions, such as calcium, alpha calcidol, or alendronate. There was no language restriction in document retrieval. We excluded review articles, theoretical research, case reports, animal experiments, and any control group that included traditional Chinese therapies.
Study selection
Two authors independently screened all potentially eligible studies. Titles and abstracts were first screened to exclude irrelevant citations. The full text of all potential articles was retrieved and screened according to the study qualification criteria. Disagreements were resolved by consensus or discussion with a third author.
BMD was the first outcome in this study to evaluate the clinical efficacy of CPM in the treatment of OP, including lumbar BMD, femoral BMD, ulna BMD, and radius BMD. We also used pain level and total effectiveness rate to measure the effects of CPM on clinical symptoms. Pain level was measured using the visual analogue scale (VAS), and the VAS score ranged from 0 point (no pain) to 10 points (worst possible pain), where a lower score indicates a better outcome. The total effectiveness rate [20] was used to evaluate overall pain, physical performance, and wellness. The total effectiveness rate was assessed based on the number of patients in each of the following categories: ‘Clinically cured,’ (the pain and swelling of joints had disappeared and active function had returned to normal); ‘Significant improvement,’ (the pain and swelling of joints was alleviated and active function had improved significantly); ‘Improvement,’ (the pain and swelling of joints was partially alleviated and active function had improved); and ‘Not cured,’ (the pain and swelling of joints remained unchanged and there was no improvement of active function).
Data collection and quality assessment
A pre-designed data extraction table was used to extract data from the selected studies, including publication information, gender, age, interventions, control measures, outcomes, summary of results, and adverse reactions. One author evaluated all data extraction and quality ratings for consistency and resolved discordant responses.
Statistical analysis
All analyses were conducted using RevMan V5.3 (The Nordic Cochrane Center, The Cochrane Collaboration), and study quality was assessed using the Cochrane Risk of Bias Tool. For meta-analysis of BMD and pain score, we combined studies using mean difference (MD) or standard mean difference (SMD) in the BMD score and VAS score. We calculated 95% confidence interval (CI) based on the mean change from baseline to the study endpoint, and we evaluated heterogeneity using the I2 statistic. The fixed-effect model was used if I2 < 50%, otherwise a random-effects model was applied. For a meta-analysis of the total effectiveness rate, we combined studies using risk ratio (RR) comparing CPM therapy with controls. P-value < 0.05 was considered to be statistically significant for all results.
Results
Brief introduction
A comprehensive review is still an important step in developing clinical practice recommendations. Thus, we systematically reviewed the prior medical literature and performed a meta-analysis on randomized controlled trials (RCTs) of CPM therapy for patients with OP to better understand its benefits for OP. We searched electronic databases for qualifying publications before extracting pertinent data for meta-analysis. Finally, the results showed that the CPM improves therapeutic impact while having less side effects when compared to typical western therapy.
Study selection
We screened a total of 13,110 studies from 6 databases. Following an initial review of 523 possibly relevant abstracts, we excluded 401 abstracts because they did not match the inclusion criteria. We retrieved and reviewed 122 full articles, and 92 articles were excluded due to low quality, insufficient data, no outcome of BMD, wrong intervention, or comparator measures. Finally, thirty studies [21–50] published between 2004 and 2020 were included. Only one study was published in English. Figure 1 summarizes the detailed study selection process.
Figure 1.
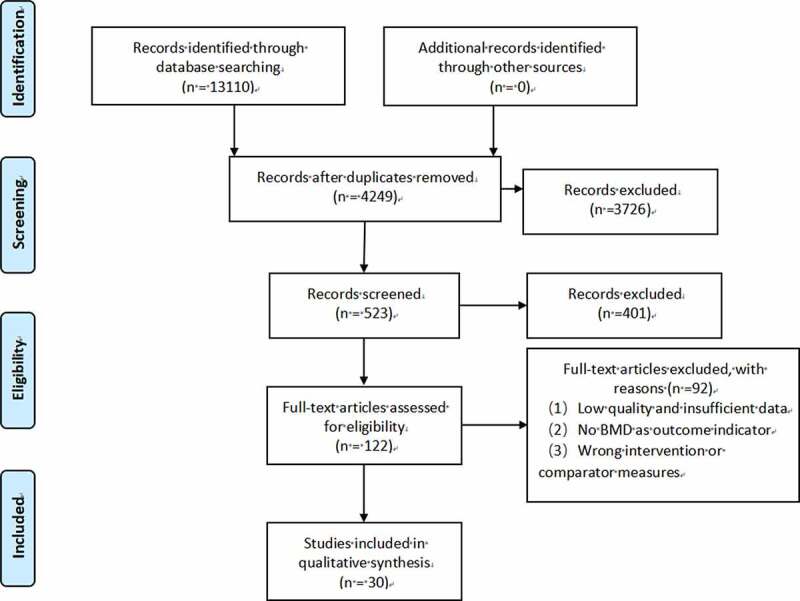
Flow diagram of literature search.
Study characteristics
The characteristics of the 30 trials are summarized in Table 1. All 30 RCTs with a total of 2723 people were carried out in China, and the total sample size of included RCTs ranged from 39 to 200 (median: 81). The participants varied in age from 47 to 75 years (median: 61.5 years), with women accounting for 32.93% to 100% (average: 74.95%) of the total. Table 2 summarizes the evidence and major impact of CPM therapies for OP.
Table 1.
Characteristics of 30 included studies of CPM for osteoporosis
| Author, (yr) | N (Female, %) | Age (yr) | CPM | Controls | Outcomes Assessments | Results (CPM vs. Control) | P value | Adverse Events (CPM vs. Control) |
|---|---|---|---|---|---|---|---|---|
| 1. Jintiange capsule | ||||||||
| Yuan, 2019 | 160 (NR) | 68 | Jintiange capsule, 3 capsules/time, 3 times/day, 12wks | Caltrate D, 600 mg, once/day, 12wks | BMD (LS) | 0.728 vs 0.535 | < 0.05 | NR |
| Zeng M, 2016 | 86 (100%) | 59 | Jintiange capsule, 2 capsules/time, 2 times/day, 24wks | Alendronate sodium, 70 mg, once /week, 24wks | BMD (radius) | 0.268 vs 0.269 | > 0.05 | NR |
| Qin, 2016 | 112 (100%) | 63 | Jintiange capsule, 3 capsules/time, 3 times/day, 12wks | Caltrate D, 1 capsule/time, once/day, 12wks | BMD Total effectiveness rate |
0.62 vs 0.51 95.6 vs 73.2 |
< 0.05 < 0.05 |
NO |
| He, 2015 | 160 (57.5%) | 65 | Jintiange capsule, 3 capsules/time, 3 times/day, 36wks | Caltrate D, 600 mg, 2 times/d, 12wks | BMD (LS) BMD (femoral) |
0.618 vs 0.5355 0.5855 vs 0.5315 |
< 0.01 < 0.01 |
8 vs 8 |
| Cai, 2015 | 64 (100%) | 61 | Jintiange capsule, 1.2 g/time, 3 times/day, 24wks | Alendronate sodium, 70 mg, once/week, 24wks | BMD (LS) BMD (femoral) |
0.9 vs 0.9 0.9 vs 0.9 |
> 0.05 > 0.05 |
0 vs 3 |
| 2. Qianggu capsule | ||||||||
| Zhao, 2004 | 69 (100%) | 56 | Qianggu capsule, 1 capsule/time, 3 times/day, 24wks | Livial, 1.25 mg, once/day, 24wks | BMD (LS) | 0.781 vs 0.826 | > 0.05 | 3 vs 8 |
| Wang, 2007 | 54 (100%) | 62 | Qianggu capsule, 1 capsule/time, 3 times/day, 24wks | α-D3 capsule, 1 capsule/time, twice/day, 24wks | BMD (LS) BMD (femoral) Total effectiveness rate |
0.797 vs 0.762 0.693 vs 0.656 92.9 vs 88.5 |
< 0.01 < 0.01 < 0.05 |
3 vs 0 |
| Ji, 2006 | 62 (70.97%) | 65 | Qianggu capsule, 1 capsule/time, 3 times/day, 12wks | Vitamin D2 calcium hydrogen phosphate tablets, 2 tablets/time, 3 times/day, 12wks | BMD (ulna) BMD (radius) Total effectiveness rate |
0.62 vs 0.565 0.625 vs 0.545 97 vs 90 |
< 0.05 < 0.05 < 0.05 |
NR |
| Gu, 2004 | 82 (32.98%) | 63 | Qianggu capsule, 1 capsule/time, 3 times/day, 12wks | Calcium gluconate, 3 tablets /time, 3 times/day, 12wks | BMD | 0.742 vs 0.684 | < 0.05 | NR |
| Wang, 2019 | 132 (100%) | 58 | Dizhong Qianggu capsule, 3 capsules/time, 3 times/day, 48wks | Alendronate sodium, 70 mg, once/week, 24wks | BMD (LS) BMD (femoral) Total effectiveness rate VAS pain |
0.836 vs 0.842 0.753 vs 0.757 84.85 vs 77.27 1.9 vs 2.3 |
< 0.05 < 0.05 < 0.05 < 0.05 |
6 vs 4 |
| Shan, 2006 | 62 (59.68%) | 61 | Qianggu capsule, 1 capsule/time, 3 times/day, 12wks | α-D3 capsule, 1 capsule/time, twice/day, 12wks | BMD (LS) BMD (femoral) Total effectiveness rate |
1.038 vs 0.936 0.7961 vs 0.7395 90.63 vs 86.67 |
> 0.05 < 0.01 > 0.05 |
2 vs 0 |
| 3. Qing’e pill, main ingredients: cortex eucommiae, fructus psoraleae, walnut, and garlic. | ||||||||
| Niu, 2012 | 39 (100%) | 47–61 | Qing’e pill, 9 g/time, 2 times/day, 8wks | Caltrate D, 600 mg, once/day, 8wks | BMD (LS/femoral) Total effectiveness rate |
−2.04 vs −2.06 (Z socre) 90 vs 89 |
< 0.01 < 0.05 |
NR |
| 4. Xianling Gubao capsule (XLGB) | ||||||||
| Jin, 2014 | 160 (59.38%) | 70 | XLGB capsule, 0.5 g, 3 capsules/time, twice/day, 6wks | Caltrate D, 600 mg, twice/day, 6wks | BMD (LS) BMD (femoral) Total effectiveness rate |
0.8 vs 0.74 0.79 vs 0.77 91.25 vs 77.5 |
< 0.05 > 0.05 < 0.05 |
NO |
| Qin, 2015 | 160 (55%) | 72 | XLGB capsule, 0.5 g, 2 capsules/time, 3times/day, 24wks | Calcium D, 1 tablet/time, twice/day, 24wks | BMD (LS) BMD (femoral) |
0.786 vs 0.742 0.673 vs 0.651 |
< 0.05 < 0.05 |
NR |
| Wu, 2013 | 84 (33.33%) | 61 | XLGB capsule, 0.5 g, 1 capsule/time, twice/day, 6wks | Calcium carbonate vitamin D3 tablet, 500 mg, twice/day, 6wks | BMD (LS) Total effectiveness rate |
0.682 vs 0.639 95.24 vs 71.43 |
< 0.05 < 0.05 |
0 vs 7 |
| Zheng, 2019 | 98 (80.61%) | 56 | XLGB capsule, 0.5 g, 3 capsules/time, twice/day, 24wks | Calcium carbonate vitamin D3 tablet, once/day, 24wks | BMD (LS) Total effectiveness rate |
0.82 vs 0.74 83.7 vs 73.8 |
< 0.05 < 0.05 |
NO |
| Le, 2020 | 80(33.75%) | XLGB capsule, 0.5 g, 3 capsules/time, twice/day, 24wks | Alendronate, 70 mg, once/7 days, 24wks | BMD (LS) BMD (femoral) VAS |
0.89 vs 0.70 0.79 vs 0.62 2.01 vs 3.09 |
< 0.05 < 0.05 < 0.05 |
NR | |
| Lin, 2017 | 60 (65.00%) | 61 | XLGB capsule, 0.5 g, 3 capsules/time, twice/day, 4wks | Calcium carbonate vitamin D3 tablet, 500 mg, twice/day, 4wks | BMD (LS) Total effectiveness rate |
0.634 vs 0.867 96.67 vs 73.33 |
< 0.05 < 0.05 |
1 vs 4 |
| Xu, 2009 | 104 (100%) | 58 | XLGB capsule, 0.5 g, 1 capsule/time, 3times/day, 24wks | Alendronate, 70 mg, once/7 days, 24wks | BMD (LS) BMD (femoral) |
0.740 vs 0.740 0.440 vs 0.441 |
> 0.05 > 0.05 |
3 vs 3 |
| 5. Liuwei Dihuang pill | ||||||||
| Zhang, 2011 | 60 (75.00%) | 59 | Liuwei Dihuang pill, 8 pills/time, 3times/day, 48wks | Calcium carbonate vitamin D3 tablet, 0.6 g, once/day, 48wks | BMD (LS) BMD (femoral) |
0.771 vs 0.733 0.633 vs 0599 |
< 0.01 < 0.01 |
NO |
| Guan, 2006 | 40 (100%)) | 57 | Liuwei Dihuang pill, 8 pills/time, 3times/day, 24wks | Caltrate D, 0.6 g, once/day, 24wks | BMD (LS) BMD (femoral) BMD (radius) Total effectiveness rate |
0.661 vs 0.627 0.580 vs 0.531 0.545 vs 0.512 85 vs 60 |
< 0.05 < 0.05 < 0.05 < 0.05 |
NO |
| Wei, 2012 | 100 (100%) | 56 | Liuwei Dihuang pill, 8 pills/time, 3 times/day, 24wks | Caltrate D, 1 tablet/time, 3times/day, 24wks | BMD | 0.90 VS 0.88 | < 0.05 | 3 vs 2 |
| Ma, 2011 | 71 (100%) | 55 | Liuwei Dihuang pill, 8 pills/time, 3 times/day, 24wks | Caltrate D, 600 mg, 3times/day, 24wks | BMD (LS) | 0.94 vs 0.89 | < 0.05 | 2 vs 3 |
| Zhang, 2003 | 42 (100%) | 64 | Liuwei Dihuang pill, 8 pills/time, 3 times/day, 48wks | Calcium, 500 mg, 3times/day, 48wks | BMD (radius) BMD (ulna) |
0.045 vs 0.004 0.051 vs 0.032 |
< 0.05 < 0.05 |
NR |
| 6. Zuogui pill | ||||||||
| Li, 2018 | 200 (NR) | 65 | Zuogui pill and Yougui pill, twice/day, 24wks | Placebo, twice/day, 24wks | BMD (LS) BMD (femoral) VAS pain |
0.79 vs 0.74 0.81 vs 0.78 2.2 vs 3.4 |
< 0.05 < 0.05 < 0.05 |
3 vs 2 |
| Sun, 2002 | 90 (58%) | 65 | Jawei Zuogui pill, 3 g, 3times/day, 12wks | Calcium granule, 5 g, 3times/day, 12wks | BMD (LS) Total effectiveness rate |
0.78 vs 0.68 91.67 vs 50.00 |
< 0.05 < 0.05 |
NR |
| 7. Gusongbao capsule | ||||||||
| Zou, 2012 | 80 (61.25%) | 49–72 | Gusongbao capsule, 3 capsules, twice/day, 4wks | Calcium carbonate D1 tablets, twice/day, 4wks | BMD (LS) BMD (femoral) BMD (radius) Total effectiveness rate |
0.76 vs 0.72 0.8 vs 0.7 0.4 vs 0.33 100 vs 84 |
< 0.05 < 0.05 < 0.05 < 0.05 |
NR |
| He, 2010 | 100 (62%) | 62 | Gusongbao capsule, 2 capsules/time, 3 times/day, 24wks | Calcium D, 600 mg, once/day, 24wks | BMD (LS) BMD (femoral) BMD (radius) |
0.875 vs 0.815 0.716 vs 0.670 0.386 vs 0.341 |
< 0.05 < 0.05 < 0.05 |
NR |
| He, 2016 | 46 (54.35%) | 41 | Gusongbao capsule, 2 capsules/time, 3 times/day, 24wks | Calcium D, 2 capsules/time, 3 times/day, 24wks | BMD (LS) | 0.884 vs 0.812 | < 0.05 | NR |
| Liu, 2020 | 66(68.18%) | 41 | Gusongbao capsule, 1 g/time, 3 times/day, 24wks | Calcium Carbonate D3, 1.2 g/time, 3 times/day, 24wks | BMD | 0.876 vs 0.812 | < 0.01 | 0 vs 4 |
NR, not reported; NO, not occurred.
(1) BMD = Bone mineral density, BMD (LS) = BMD (lumbar spine).
(2) BMD (femoral): includes BMD (femoral neck), BMD (wards area), BMD (femoral great trochanter), and BMD (Totsl).
(3) VAS pain: 0–10, lower score = better outcome.
(4) Total effectiveness rate (%) was determined as the quotient of the number of cured and improved patients divided by the total number of patients.
Table 2.
Summary of evidence and effects of CPM interventions for osteoporosis
| Study Characteristic | No. of Studies |
|---|---|
| Main varieties | |
| Xianling Gubao capsule | 7 |
| Qianggu capsule | 6 |
| Jintiange capsule | 5 |
| Liuwei Dihuang pill | 5 |
| Gusongbao capsule | 4 |
| Zuogui pill | 2 |
| Qing’e pill | 1 |
| Outcomes | |
| BMD (lumbar spine) | 22 (18+,4-) |
| BMD (femoral) | 15 (12+,3-) |
| BMD (radius) | 6 (5+,1-) |
| BMD (ulna) | 2 (2+) |
| BMD | 4 (3+,1-) |
| VAS pain score | 3 (3+) |
| Total effectiveness rate | 14 (13+,1-) |
| Adverse events | 18 (10+,8-) |
+ overall beneficial effect; – no effect
The experimental groups contained 7 CPM, including Jintiange capsule (5 studies), Qianggu capsule (6 studies), Qing’e pill (1 study), Xianling Gubao capsule (7 studies), Liuwei Dihuang pill (5 studies), Zuogui pill (2 studies), and Gusongbao capsule (4 studies). The China Food and Drug Administration (CDFA) classified all medications as proprietary. An overview of CPM components utilized in OP is provided in Table 3. For 4–48 weeks, the CPM was administered orally one to three times per day. The control groups received calcium (21 studies), alpha calcidol (2 studies), alendronate (5 studies), tibolone tablet (1 study), and placebo (1 study) as therapies.
Table 3.
Overview of ingredients of CPM for osteoporosis
| Main varieties | Drug composition (Chinese pinyin/Latin name) | Approval number of SFDA | Prescription functions (TCM patterns) |
|---|---|---|---|
| Jintiange capsule | Artificial tiger bone meal. | Z20030080 | Strengthen the bones |
| Qianggu capsule | The total flavonoids of Rhizoma Drynariae (Gusuibu, Davallia mariesii Moore ex Bak.). | Z20030007 | Replenish the kidney, strengthen the bones, and relieve pain |
| Qing’e pill | Cortex Eucommia (Duzhong, Eucommia ulmoides Oliv.), Fructus Psoraleae (Buguzhi, Psoralea corylifolia Linn.), Walnut Kernel (Hetaoren, Juglans regia.), Allium Garlic (Dasuan, Allium sativum L.). | Z32020099 | Tonify kidney, strengthen the bones |
| Xianling Gubao capsule | Herba epimedii (Yinyanghuo, Epimedium brevicornu Maxim.), Radix Dipsaci (Xuduan, Dipsacus asper Wall.ex Henry), Fructus Psoraleae (Buguzhi, Psoralea corylifolia Linn.), Radix Rehmanniae (Dihuang, Rehmannia glutinosa Libosch.), Radix Salviae miltiorrhizae (Danshen, Salvia miltiorrhiza Bge.), Rhizoma Anemarrhena (Zhimu, Anemarrhena asphodeloides Bge.). | Z20025337 | Tonify the liver and kidney, promote blood circulation, remove blood stasis, and strengthen the bones |
| Liuwei Dihuang pill | Rehmannia glutinosa (Dihuang, Rehmannia glutinosa (Gaetn.) Libosch. ex Fisch. et Mey.), Fructus corni (Shanzhuyu, Cornus officinalis Sieb. et Zucc.), Rhizoma dioscoreae (Shanyao, Dioscorea oppositifolia L.), Cortex moutan (Danpi, Paeonia suffruticosa Andr.), Tuckahoe (Fuling, Poria cocos(Schw.) Wolf), Rhizoma alismatis (Zexie, Alisma orientalis (Sam.) Juzep.) | Z19993068 | Nourish both yin and kidney |
| Zuogui pill | Rehmannia glutinosa (Dihuang, Rehmannia glutinosa (Gaetn.) Libosch. ex Fisch. et Mey.), Semen cuscutae (Tusizi, Cuscuta chinensis Lam.), Twotooth achyranthes root (Niuxi, Radix Achyranthis Bidentatae), Tortoise-plastron glue (Guibanjiao, Colla Carapacis et Plastri Tes), Deerhorn Glue (Lujiaojiao, Colla Cervi Cornus), Rhizoma dioscoreae (Shanyao, Dioscorea oppositifolia L.), Fructus corni (Shanzhuyu, Cornus officinalis Sieb. et Zucc.), Wolfberry fruit (Gouqi, Fructus Lycii). | Z41020696 Z11020735 |
Nourish both yin and kidney |
| Gusongbao capsule | Herba epimedii (Yinyanghuo, Epimedium brevicornu Maxim.), Red Peony Root (Chishao, Radix Paeoniae Rubra), Rhizoma Sparganii (Sanleng, Sparganium stoloniferum Buch.-Ham), Curcuma zedoaria (Ezhu, Rhizoma Curcumate zedoariae), Dried rehmannia root (Shengdihuang, Rehmannia glutinosa Libosch.), Rhizoma Anemarrhenae (Zhimu, Anemarrhena asphodeloides Bunge), Radix Dipsaci (Xuduan, Dipsacus asper Wall.ex Henry), Szechuan Lovage Rhizome (Chuanxiong, Ligusticum chuanxiong Hort.), Oyster Shell (Muli, Concha Ostreae). | Z20030084 | Tonify the kidney, promote the blood circulation, strengthen the bones and gluten |
Quality assessment
The quality (risk of bias) assessment of trials was performed using a modified version of The Cochrane Collaboration’s tool [51]. Figure 2 and Figure 3 depict the risk of bias distribution and research quality within this evidence base. Overall, the trials’ bias quality was modest. In 11 studies (36.67%), randomization was satisfactory, but in 19 trials (63.33%), it was questionable. Although one research reported satisfactory allocation concealment, the remaining 29 trials (96.67%) were ambiguous. Blinding of participants and personnel happened in 1 trial (3.33%), but was unclear in 2 trials (6.67%) and high risk in 27 trials (90%). Blinding of the outcome happened in 1 trial (3.33%) but was unclear in the other 29 (96.67%). All studies reported the similarity of study groups at the baseline (100%). There was no study that mentioned selective reporting.
Figure 2.
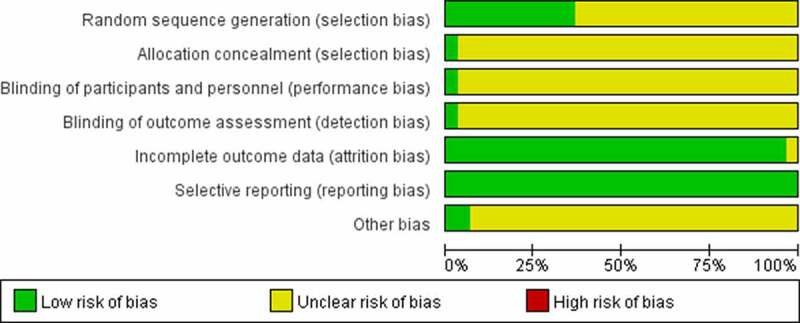
Risk of bias distribution graph.
Figure 3.
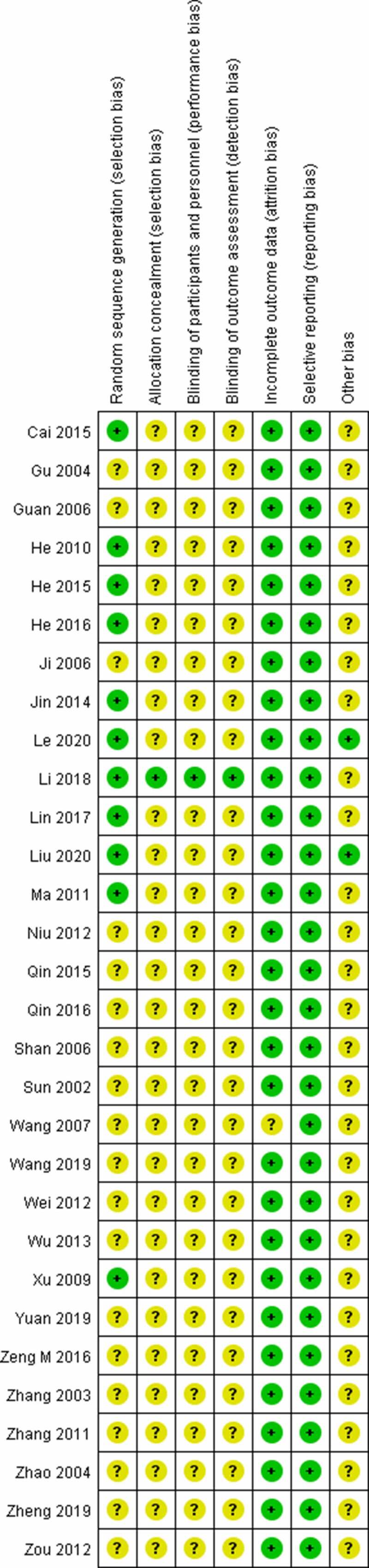
Risk of bias summary.
Meta-analysis
We used the BMD to assess the quantitative treatment effects in the 30 eligible RCTs.
Twenty-three trials used BMD (lumbar spine) (Figure 4), 15 trials used BMD (femoral) (FIGURE 5), 6 trials used BMD (radius) (FIGURE 6), 2 trials used BMD (ulna) (Figure 7), and 4 trials used BMD (Figure 8). At the same time, 5 trials used the VAS pain score (Figure 9) to measure the pain levels, and 14 trials assessed overall pain, physical performance, and wellness using the total effectiveness rate (Figure 10).
Figure 4.
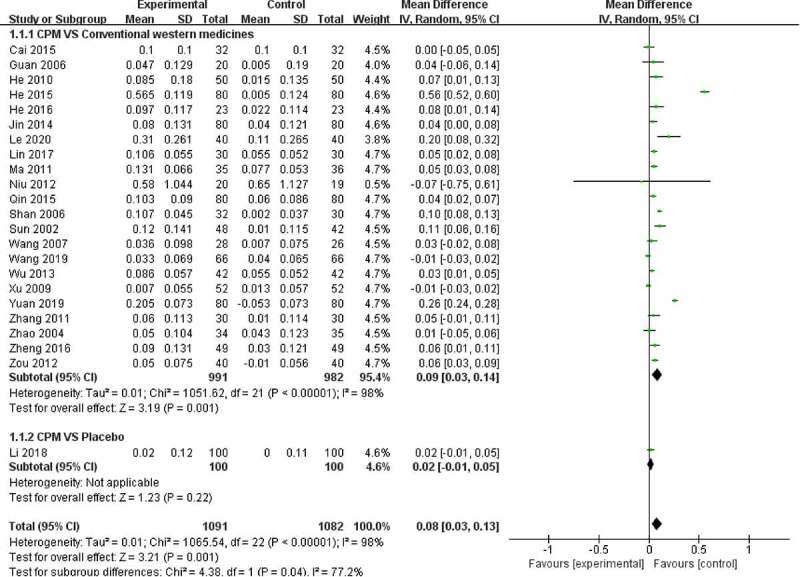
Effect of CPM therapy on BMD (lumbar spine).
Figure 5.
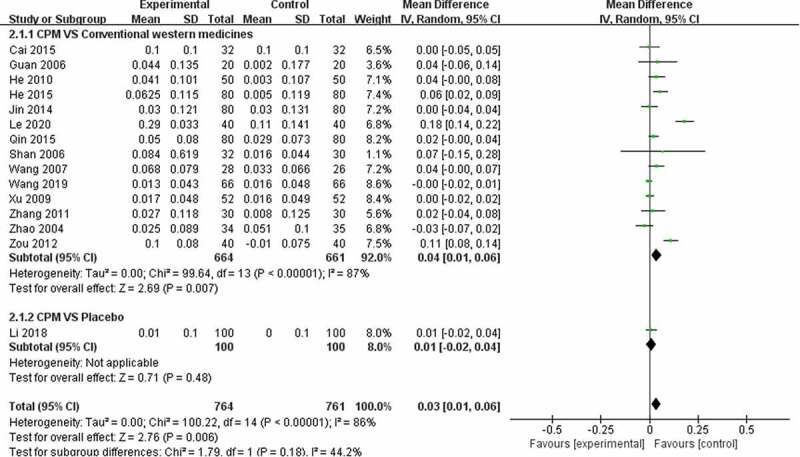
Effect of CPM therapy on BMD (femoral).
Figure 6.
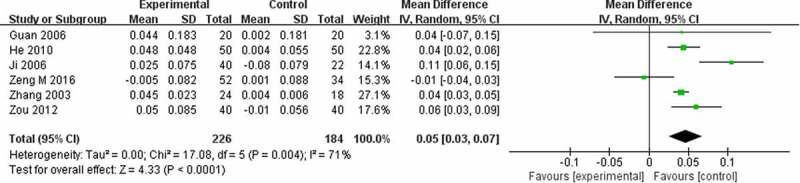
Effect of CPM therapy on BMD (radius).
Figure 7.
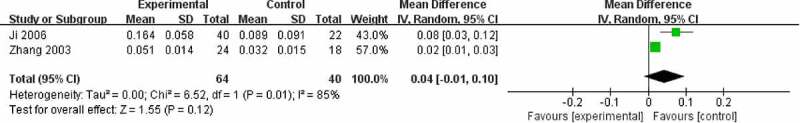
Effect of CPM therapy on BMD (ulna).
Figure 8.
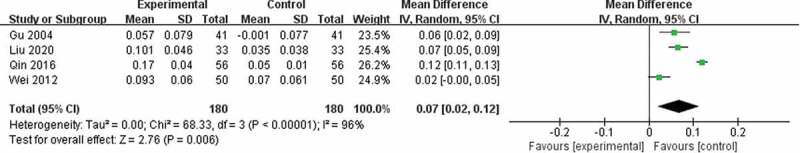
Effect of CPM therapy on BMD.
Figure 9.
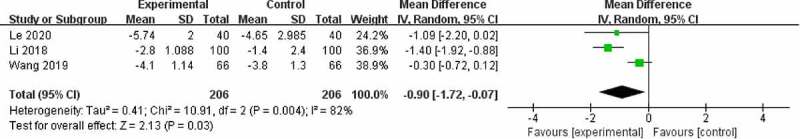
Effect of CPM therapy on VAS pain score.
Figure 10.
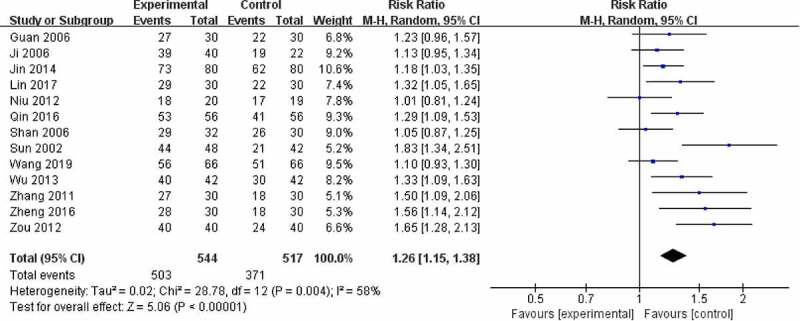
Effect of CPM therapy on total effectiveness rate.
BMD (lumbar spine)
Twenty-three trials involving 2173 patients were used to perform a meta-analysis of clinical efficiency using BMD (lumbar spine). The heterogeneity (I2) score of BMD (lumbar spine) was high. The results of the random-effects meta-analysis indicated that patients in the CPM groups had significantly higher BMD (lumbar spine) than those in the control groups of calcium, alpha calcidol, and alendronate (MD = 0.08; 95% CI, 0.03–0.13) after 4 to 48 weeks of treatment. Further subgroup analysis exploring the improvement of different controls on BMD (lumbar spine) showed that CPM therapy had a better effect compared with conventional western medicines (MD = 0.09; 95% CI, 0.03–0.14), and there was no difference between CPM and placebo control groups (MD = 0.02; 95% CI, −0.01–0.05) after 24 to 48 weeks of treatment (Figure 4).
BMD (femoral)
Fifteen trials involving 1525 patients were used to perform a meta-analysis of clinical efficiency using BMD (femoral). The results of the random-effects meta-analysis indicated that patients in the CPM groups had significantly higher BMD (femoral) than those in the control groups of calcium, alpha calcidol, and alendronate (MD = 0.03; 95% CI, 0.01–0.06) after 4 to 36 weeks of treatment. The heterogeneity (I2) score of BMD (femoral) was 86%. Further subgroup analysis exploring the improvement of different controls on BMD (femoral) showed that CPM therapy has a better effect compared with conventional western medicines (MD = 0.04; 95% CI, 0.01–0.06), and there was no difference between CPM group and placebo control groups (MD = 0.01; 95% CI, −0.02–0.04) after 12 to 48 weeks of treatment (Figure 5).
BMD (radius)
Six trials evaluated clinical efficiency using BMD (radius), involving 410 patients and 4 CPMs. The results of the random-effects meta-analysis indicated that the BMD (radius) elevation in the CPM group was much more significant than the group taking conventional western medicines (MD = 0.05; 95% CI, 0.03–0.07) after 4 to 48 weeks of treatment. (Figure 6).
BMD (ulna)
Two trials additionally evaluated clinical efficiency using BMD (ulna), involving 104 patients and 2 CPMs. The results of the random-effects meta-analysis indicated that there was no difference between the CPM group and calcium (MD = 0.04; 95% CI, −0.01–0.10) after 12 to 48 weeks of treatment, what suggested the improvement effect on BMD was very weak (Figure 7).
BMD
Four trials involving 360 patients and were used to perform a meta-analysis of clinical efficiency by BMD of unspecified site. With a very high heterogeneity (I2) score, the results of the random-effects meta-analysis indicated that patients in the CPM groups had significantly higher BMD than conventional western medicines (SMD = 1.67; 95% CI, 0.26–3.08) after 12 to 24 weeks of treatment (Figure 8).
VAS pain score
To investigate the improvement effect of CPM on pain in patients, we extracted VAS scores from three trials including 412 patients. The results of the random-effects meta-analysis indicated that patients in the CPM groups had significantly lower pain scores than the groups taking conventional western medicines (MD = −0.90; 95% CI, −1.72 – −0.07) after 24 to 48 weeks treatment, which means CPM does relieve pain in OP patients. (Figure 9).
Total effectiveness rate
Thirteen trials involving 1061 patients assessed the overall response of CPM in patients using the total effectiveness rate compared to conventional western medicine. The overall clinical effectiveness rate in the CPM groups was 92.46% (risk ratio [RR] = 1.26; 95% CI, 1.15 to 1.38), with a high degree of heterogeneity (I2 = 58%). Our meta-analysis outcome showed that CPM therapy of 4 to 48 weeks could improve clinical symptoms including overall pain, physical performance, and wellness for patients with OP (Figure 10). Funnel plot suggests that there might be publication bias (Figure 11).
Figure 11.
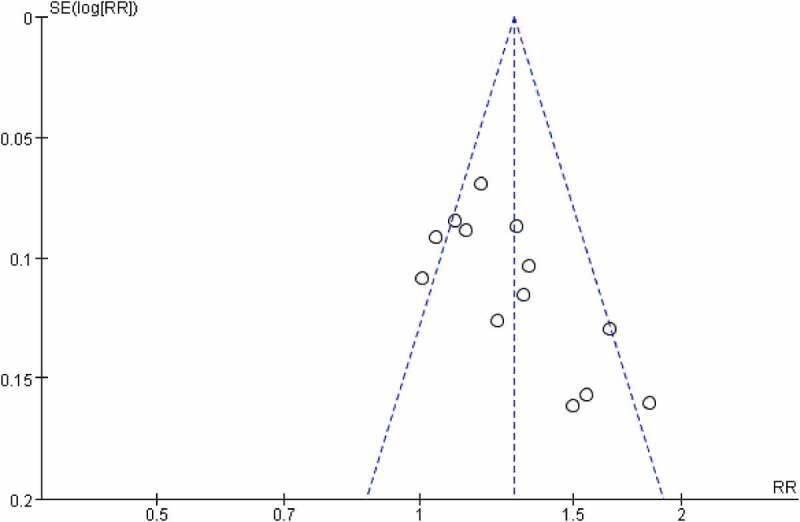
Funnel plots for publication bias.
Adverse events
Eighteen trials provided information on adverse events, while 12 trials did not. Of the 18 trials, 10 reported that 34 patients had adverse events in the CPM group and 48 patients had adverse events in the control group, and 7 trials reported that no adverse events occurred. The reported minor adverse events included dry mouth, constipation, abdominal distension, diarrhea, gastrointestinal discomfort, rash, nausea, vomiting, and muscle soreness. No serious adverse events occurred in the CPM group, but Zhao et al. stated that three patients experienced vaginal bleeding in the control group [23]. The incidence of adverse events in the CPM group was less than that in the control group, and the adverse events disappeared after stopping medication.
Discussion
The results of our meta-analysis indicate that CPM therapy is more effective than general oral medicine or placebos in relieving symptoms and improving the BMD of OP and does not pose significant safety risks. Overall, CPM therapy appears to be safe and effective for people who suffer from OP.
The functional imbalance of osteoblasts and osteoclasts can directly lead to bone loss. In women, postmenopausal decline in estrogen levels is critical to the pathogenesis of OP, in addition to calcium and vitamin D deficiencies can also accelerate this process [52,53]. The management of OP focuses on two tasks: prevention and treatment – both of which Chinese medicine can play a role. On the one hand, the specific components contained in Chinese herb medicine play a key role in bone metabolism. On the other hand, on the basis of Chinese Medicine theory, the required Chinese herb medicines mainly tonify kidney (Shen) and spleen (Pi), strengthen bones, improve cell metabolism, and invigorate Qi and blood. The Guidelines for the Diagnosis and Management of Primary Osteoporosis (2017) in China recognize the total flavonoids of Drynariae, icariin, and artificial tiger bone meal as the ingredients of Chinese medicine with anti-osteoporosis pharmacological effects. The Guidelines also list the Qing’e pill, Liuwei Dihuang pill, Zuogui pill, and Yougui pill as recommended drugs54.
A growing body of evidence is beginning to shed light on the potential biological mechanisms through which CPM therapy works in OP. Various clinical trials and animal studies of different kinds of CPM have demonstrated that kidney-tonifying Chinese herbal medicine can prevent and treat bone loss by increasing bone density, promote bone resorption decreased the level of urine Ca/Cr [55]. The primary ingredient in the Jintiange capsule, artificial tiger bone meal, contains a variety of trace elements and amino acids essential for bone production. The total flavonoids of Rhizoma Drynariae in the Qianggu capsule, and the icariin, fructus psoraleae, radix dipsaci, and rehmannia glutinosa in other CPMs all directly boost blood calcium levels and stimulate bone cells [56,57]. According to the findings of this study, CPMs can dramatically improve BMD and are more effective than alendronate, calcium, and vitamin D. In addition, the radix salviae miltiorrhizae in Xianling Gubao capsule contains tanshinone, as does the twotooth achyranthes root, which is the principal element in the Zuogui Pill and contains complete achyranthes saponins. Both of these lessen the VAS score by acting anti-inflammatory, analgesic, and blood flow improvers.
Furthermore, numerous studies indicate that CPM may have the anti-osteoporosis benefits in OP patients via a variety of targets and pathways. According to certain research, the Zuogui pill can prevent OP by rectifying the imbalance of bone formation and bone resorption through different targets and pathways, including Wnt1, LRP-5, Wnt β-catenin, and TGF-β-Smad signal [58,59,]. The network pharmacology analysis approach demonstrated that Xianling Gubao had a therapeutic impact by the regulation of osteoclastic differentiation modulation, inhibition of inflammatory responses, and involvement hub genes (AKT1, MAPK1, MAPK8, TP53, and STAT3) [60]. Some studies of the Liuwei Dihuang pill investigations hypothesized that some genes may play critical roles in OP therapeutic processes, including ATF2, FBXW7, RDX, NCOA3, TCF4, DUSP6, PELI2, and STX7 [61,62,]. It may also have effects through the up-regulation of cardiotrophin-like cytokine factor 1 (CLCF1) gene expression and activating the Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling pathway [63]. In addition, a previous study that focused on holistic quality control in a specific kind of CPM provided valuable information for guaranteeing the safety, effectiveness, and controllability of CPM therapy [64]. Overall, further research is warranted to explore the underlying biological mechanisms of CPM therapy for OP.
The most serious risk about using Chinese medicine is its toxicity and adverse effects. Several studies have found that the adverse responses experienced by OP patients receiving CPM are quite mild and can be alleviated by stopping CPM or using symptomatic therapy. As a result, whether compared to oral calcium, vitamin D, or Alendronate, CPMs have no extra adverse effects and pose minimal harm to patients. This might be an advantage of CPM.
These findings are consistent with a number of recent reviews of CPM therapy. Jing Sun et al., for example, revealed that CPM was a favorable choice for treating patients with OP in terms of increasing BMD, decreasing pain, and lowering adverse events in 31 trials utilizing Jintiange capsules alone and in conjunction with other medications[65]. Another review of 10 trials by Xu Wei et al. suggested that Qianggu capsules were associated with the improvement of BMD for primary OP[66]. In addition, 3 reviews have shown that Xianling Gubao capsules are effective in improving BMD and serum calcium levels, increasing the clinical effectiveness rate, and reducing pain [67–69]. Indeed, current research on CPM therapy and its capacity to enhance BMD, reduce pain, and raise clinical effectiveness rates in OP supports our findings.
Our study has limitations, despite its merits. For starters, several of the included RCTs have a significant risk of bias. There has only been one double-blinding and placebo-controlled trial recorded. Second, treatment durations vary amongst studies, spanning from 1 to 12 months; consequently, lengthier and more consistent follow-ups will be warranted in future research. Third, there are many kinds of TCMP utilized in clinical practice to treat OP, but only 11 types were included in this study. We also observed high heterogeneity due to diverse kinds, formulations, and control groups. Lastly, despite CPM’s statistically significant effects on BMD and symptom improvement in OP patients, the clinically essential advantages of CPM therapy remain to be determined. Thus, the potential benefits of CPM for OP need to be further evaluated through high-quality clinical trials with more rigorous methodologies.
Conclusions
The results of this meta-analysis indicate that CPM therapy may be a valuable treatment regimen for OP by improving BMD and symptoms while reducing the risk of adverse events, but it is a pity that the quality of trials included is moderate. Due to this deficiency, more rigorously designed and well-controlled RCTs are warranted to support the clinical application of CPM therapy for OP patients. Future clinical research should focus on their potential to reduce these patients’ risks of serious adverse events.
Acknowledgements
This study was supported by the National Key Research and Development Program of China (2018YFC1704703), China Association of Traditional Chinese Medicine (SATCM-2015-BZ402), and Beijing Municipal Commission of Science and Technology (Z151100003815028).
Funding Statement
This work was supported by the National Key R&D Program of China [2018YFC1704703], and High-level Talent Scientific Research Startup Project of Beijing University of Chinese Medicine [2021-XJ-KYQD-001].
Statement of Ethics
Ethics were approved by the Institutional Review Board of the Third Affiliated Hospital of Beijing University of Chinese Medicine (No. BZYSY-2019KYKTPJ-22) and the Institutional Review Board of Wangjing Hospital, China Academy of Chinese Medical Sciences (No. WJEC-YJS-2020-025-P002).
Abbreviations
- TCM
traditional Chinese medicine
- CPM
Chinese patent medicine
- OP
osteoporosis
- RCTs
randomized controlled trials
- BMD
bone mineral density
- MD
mean difference
- CI
confidence interval
- RR
risk ratio
- VAS
visual analogue scale
- CDFA
China Food and Drug Administration.
Disclosure statement
The authors do not have any competing interests.
Author contributions
Conception and design of study: Weiheng Chen, Rongtian Wang.
Acquisition of data: Kaiqiang Tang, Ruizheng Zhu.
Analysis and interpretation of data: Yan Zhao and Wei Zhao.
Drafting the manuscript: Jigao Sun, Yan Zhao, Yan Jia.
Revising the manuscript: Weiheng Chen, Na Lin, Yanqiong Zhang, Yan Jia
All authors read and approved the final manuscript.
References
- [1].Kanis JA, Melton LJ, Christiansen C, et al. The diagnosis of osteoporosis. J Bone Miner Res. 1994;9:1137–1141. [DOI] [PubMed] [Google Scholar]
- [2].Hopkins RB, Burke N, and Von Keyserlingk C, et al. The current economic burden of illness of osteoporosis in Canada. Osteoporos Int. 2016;27:3023–3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chen P, Li Z, Hu Y.. Prevalence of osteoporosis in China: a meta-analysis and systematic review. BMC Public Health. 2016;16:1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ensrud KE, Crandall CJ. Osteoporosis. Ann Intern Med. 2017;167:ITC17–ITC32. [DOI] [PubMed] [Google Scholar]
- [5].Ensrud KE, Crandall CJ. Bisphosphonates for postmenopausal osteoporosis. JAMA. 2019;322:2017–2018. [DOI] [PubMed] [Google Scholar]
- [6].Ge JR, Wang HM, Zheng HX, et al. Traditional Chinese Medicine Expert Consensus on the prevention and treatment of primary osteoporosis (2020). Chin J Osteoporosis. 2020;26. 1717–1725. [Google Scholar]
- [7].Sun S, Mo X, Li Y, et al. Effectiveness comparisons of Chinese patent medicine on treating premature ejaculation: a systematic review and meta-analysis. Medicine (Baltimore). 2019;98:e17729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Liu Y, Li Z, Shen D, et al. Adjuvant treatment of coronary heart disease angina pectoris with Chinese patent medicine: a prospective clinical cohort study. Medicine (Baltimore). 2019;98:e16884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wang SJ, Yue W, Rahman K, et al. Mechanism of treatment of kidney deficiency and osteoporosis is similar by traditional Chinese medicine. Curr Pharm Des. 2016;22(3):312–320. [DOI] [PubMed] [Google Scholar]
- [10].Lin J, Zhu J, Wang Y, et al. Chinese single herbs and active ingredients for postmenopausal osteoporosis: from preclinical evidence to action mechanism. Biosci Trends. 2017;11(5):496–506. [DOI] [PubMed] [Google Scholar]
- [11].Zhan KJ, An YJ, Mou X. Efficacy of kidney-tonifying Chinese patent medicine on bone turnover makers in postmenopausal osteoporosis: a meta-analysis. Chin J Osteoporosis. 2019;25:202–211. [Google Scholar]
- [12].Zhang BS, Dong KF. A meta-analysis of efficacy of two kinds of the Bushen medicine on BMD and VAS in osteoporosis patients. Clin J Chin Med. 2020;12:139–143. [Google Scholar]
- [13].Jiang YQ, Yu GS, Tong PJ. A meta-analysis of Zuogui pills in the treatment of osteoporosis. J New Chin Med. 2019;51:52–56. [Google Scholar]
- [14].Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Osteoporosis Committee of Chinese Gerontology Society . Recommended diagnostic criteria for osteoporosis in China (second draft). Chin J Osteoporos. 2000;1–3. [Google Scholar]
- [16].Osteoporosis Committee of Chinese Gerontology Society . Expert consensus on diagnostic criteria for osteoporosis in Chinese (third draft). Chin J Osteoporos. 2014;20:1007–1010. [Google Scholar]
- [17].Chinese Medical Association . Clinical practice guidelines·Osteoporosis and bone mineral salt diseases. Beijing: People’s Medical Publishing House; 2006. p. 1–8. [Google Scholar]
- [18].The Osteoporosis and Bone Mineral Salt Disease Branch of Chinese Medical Association . Guidelines for diagnosis and treatment in primary osteoporosis. Chin J Osteoporosis Bone Mineral Res. 2011;4:2–17. [Google Scholar]
- [19].Liu ZH. Diagnostic criteria for primary osteoporosis in China (trial). Chin J Osteoporos. 1999: 4–6. [Google Scholar]
- [20].Zheng XY. Guiding principle of clinical research on new drugs of traditional Chinese medicine. Beijing China; 2002. p. 349–353. [Google Scholar]
- [21].Liu YB. Observation on effect of gusongbao capsule in treating osteoporotic spinal compression fracture. Chin J Clin Rational Drug Use. 2020;13:77–78. [Google Scholar]
- [22].Le SH. Effect of xianling gubao capsule on postoperative recovery of senile patients with osteoporosis complicated with fracture undergoing surgical treatment. Chin J Clin Rational Drug Use. 2020;13:96–97. [Google Scholar]
- [23].Yuan YF, Huang XS, Yang YR, et al. Study on effect of Jintiange capsule on lower limb functionin patients with primary type □ osteoporosis. Chin J Tradition Med Traumatol Orthoped. 2019;27. 24–27+31. [Google Scholar]
- [24].Zeng M, Lu P, Yang B, et al. The efficacy of Jintiange capsule on the treatment of postmenopausal osteoporosis. Chin J Osteoporosis. 2016;22. 480–482. [Google Scholar]
- [25].Qin Y, Qiu B, Zhu SG, et al. Clinical observation of Jintiange capsule on treating postmenopausal osteoporosis. J Qiannan Med Coll Nationalities. 2016;29. 19–23. [Google Scholar]
- [26].He BY, Teng T, Liu BG, et al. The clinical efficacy of Jintiange capsule on the treatment of primary osteoporosis. Chin J Osteoporosis. 2015;21. 168–174. [Google Scholar]
- [27].Cai D, Lin ZX, Huang ZH, et al. Clinical effect observation of Alendronate combined with Jintiange capsule in the treatment of postmenopausal osteoporosis. China Pharm. 2015;18:1352–1354. [Google Scholar]
- [28].Zhao G, Xu ZL, Shao QX, et al. Confarison of livial and kidney-invigorating traditional Chinese medicine in prevention and treatment of postmenopausal osteoporosis. Chin J Osteoporosis. 2004;03. 87–89. [Google Scholar]
- [29].Wang J, Zhang WK, Wang ZH. Qianggu capsule was used to treat 28 cases of postmenopausal osteoporosis. Herald Med. 2007;11:1325–1327. [Google Scholar]
- [30].Ji XR. Clinical observation of Qianggu Capsule in the treatment of senile osteoporosis. Shanxi Medical J. 2006;04:341–342. [Google Scholar]
- [31].Gu M, Guo JN. The Rhizoma Drynariae therapy for primary osteoporosis. Chin J Rehabilitation. 2004;05:297. [Google Scholar]
- [32].Wang Y, Liu J, Hou YX, et al. Study on the safety and efficacy of Dizhongqianggu capsule in the treatment of osteoporosis. Chin J Osteoporosis. 2019;25. 1454–1457. [Google Scholar]
- [33].Shan S, Zhou G. Clinical effect of Qianggu Capsule on primary osteoporosis. Northwest Pharmaceutical J. 2006;04:177–178. [Google Scholar]
- [34].Niu Y. The therapeutic effect of Qing’e Wan therapy in postmenopausal women with osteoporosis. Shenzhen J Integr Traditional Chin West Med. 2012;22:101–103. [Google Scholar]
- [35].Jin JF, and Zhang JW. Clinical efficacy and safety evaluation of Xianlinggubao capsule treatment of osteoporosis pain. Chin Arch Tradition Chin Med. 2014;32:3050–3052. [Google Scholar]
- [36].Qin Y, Qiu B, Zhu SG. Analysis of the efficacy of Xianlinggubao capsule on the treatment of osteoporosis and its influences in the markers of bone metabolism and bone turnover. Chin J Osteoporos. 2015;21:1056–1060+1064. [Google Scholar]
- [37].Wu YJ. Clinical effect observation of Xianlinggubao in the treatment of osteoporosis. J Clin Exp Medi. 2013;12:1494–1495. [Google Scholar]
- [38].Zheng HQ. Clinical observation of xianlinggubao in the treatment of primary osteoporosis. J China Prescription Drug. 2016;14:83–84. [Google Scholar]
- [39].Lin H, Yang CS. Efficacy of Xianlinggubao in the treatment of primary osteoporosis. Shenzhen J Integr Traditional Chin West Med. 2017;27:54–56. [Google Scholar]
- [40].Xu M, Liu BX, Huang CJ, et al. Clinical observation of xianlinggubao plus alendronate on postmenopausal osteoporosis. J Liaoning Univ Traditional Chin Med. 2009;11:94–95. [Google Scholar]
- [41].Zhang HB, Li CY. Effect of Liuwei Dihuang Pill on bone mineral density of the primary osteoporosis (kidney yin deficiency). Chin J Tradition Med Traumatol Orthoped. 2011;19:18–20. [Google Scholar]
- [42].Guan J, Tang JF, Guo GR. Intervention of Liuwei Dihuang Pill on bone density in patients with postmenopausal osteoporosis. Chin J Inf Traditional Chin Med. 2006;12:70–71. [Google Scholar]
- [43].Wei LJ. Liuwei Dihuang Pill Combined with Caltrate D in treatment of postmenopausal osteoporosis comparative observation. J Pract Tradition Chin Internal Med. 2012;26:54–55. [Google Scholar]
- [44].Ma C. Clinical analysis of Liuwei Dihuang Pill combined with calcium in the treatment of postmenopausal osteoporosis. China Med Pharm. 2011;1:74–75. [Google Scholar]
- [45].Zhang J, Yin SL, Yin HQ, et al. Efficacy observation of Liuwei Dihuang Pill in treating postmenopausal osteoporosis. J Clin Inter Med. 2003;10. 558. [Google Scholar]
- [46].Li WX, Zhang KQ, Liu Z, et al. Effect of Zuogui pill and Yougui pill on osteoporosis: a randomized controlled trial. J Tradit Chin Med. 2018;38:33–42. [PubMed] [Google Scholar]
- [47].Sun X. Clinical observation of Jiawei Zuogui Pills in treating osteoporosis low back pain. Chin J Tradition Med Traumatol Orthoped. 2002;05:36–38. [Google Scholar]
- [48].Zou WN, Tian WJ. Clinical observation of Gusongbao capsule in treating osteoporosis. Clin J Med Officers. 2012;40:384. [Google Scholar]
- [49].He X, Shang P, Jiang YF, et al. Clinic effect of Gusongbao capsule in the treatment of osteoporotic vertebral compression fracture. J Liaoning Univ Traditional Chin Med. 2010;12:145–147. [Google Scholar]
- [50].He MR, Yuan CY, Li J. Clinical analysis of 46 cases of osteoporotic spine compression fractures treated with Gusongbao capsule. Nei Mongol J Traditional Chin Med. 2016;35:45. [Google Scholar]
- [51].Higgins JPT, Altman DG, Sterne JAC. Chapter 8: assessing risk of bias in included studies. Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions version 5.1.0. The Cochrane Collaboration; 2011. Available from www.cochrane-handbook.org [Google Scholar]
- [52].Noh J-Y, Yang Y, Jung H. Molecular mechanisms and emerging therapeutics for osteoporosis. Int J Mol Sci. 2020;21(20):7623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Raisz LG. Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Invest. 2005;115(12):3318–3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Xia WB, Zhang ZL, Lin H, et al. Guidelines for the diagnosis and management of primary osteoporosis (2017). Chin J Osteoporos. 2019;03. 281–309. [Google Scholar]
- [55].Li E, Kong DJ, Yang XH, et al. Effects of kidney-tonifying Chinese medicinal herbs on prevention of rat osteoporosis. Chin J Osteoporos. 2002;02. 73–77. [Google Scholar]
- [56].Zhao YR, Wei X, Jiang JJ, et al. Systemic review of Jintiange capsule in treatment of postmenopausal osteoporosis. Chin J Chin Mater Med. 2019;44:186–192. [DOI] [PubMed] [Google Scholar]
- [57].Wang QY, Zhang YL, Xie YM. Meta-analysis of total flavonoids from Rhizoma Drynariae (Gusuibu) in treatment of postmenopausal osteoporosis. Chin J Osteoporos. 2019;25:465–471. [Google Scholar]
- [58].Yin H, Wang S, Zhang Y, et al. Zuogui Pill improves the dexamethasone-induced osteoporosis progression in zebrafish larvae. Biomed Pharmacother. 2018;97:995–999. [DOI] [PubMed] [Google Scholar]
- [59].Liu MJ, Li Y, Pan JH, et al. Effects of zuogui pill (see text) on Wnt singal transduction in rats with glucocorticoid-induced osteoporosis. J Tradit Chin Med. 2011;31:98–102. [DOI] [PubMed] [Google Scholar]
- [60].Zhu N, Hou J. Exploring the mechanism of action Xianlingubao prescription in the treatment of osteoporosis by network pharmacology. Comput Biol Chem. 2020;85:107240. [DOI] [PubMed] [Google Scholar]
- [61].Xu F, Gao F. Liuwei Dihuang pill cures postmenopausal osteoporosis with kidney-Yin deficiency: potential therapeutic targets identified based on gene expression profiling. Medicine (Baltimore). 2018;97:e11659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Gong R, Ren S, Chen M, et al. Bioinformatics analysis reveals the altered gene expression of patients with postmenopausal osteoporosis using liuweidihuang pills treatment. Biomed Res Int. 2019;2019:1907906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Ge JR, Xie LH, Chen J, et al. Liuwei Dihuang pill treats postmenopausal osteoporosis with Shen (Kidney) Yin deficiency via janus kinase/signal transducer and activator of transcription signal pathway by up-regulating cardiotrophin-like cytokine factor 1 expression. Chin J Integr Med. 2018;24(6):415–422. [DOI] [PubMed] [Google Scholar]
- [64].Yao ZH, Qin ZF, Cheng H, et al. Simultaneous quantification of multiple representative components in the Xianlinggubao capsule by ultra-performance liquid chromatography coupled with quadrupole time-of-flight tandem mass spectrometry. Molecules. 2017;22:927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Sun J, Yang XG, and Hu YC.. Efficacy of Jintiange capsule in the treatment of osteoporosis: a network meta-analysis. Orthop Surg, 2019;11(2):176–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Wei X, Xu A, Shen H, and Xie Y. (2017). Qianggu capsule for the treatment of primary osteoporosis: evidence from a Chinese patent medicine. BMC Complement Altern Med. 2017;17(1):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Zeng XJ, Chen JN, and Jiang HQ, et al. Bayesian network meta-analysis of therapeutic efficacy of Xianlinggubao capsule combined with routine therapy for postmenopausal osteoporosis. China Pharm. 2019;30(18):2556–2562. [Google Scholar]
- [68].An YF, Zhang YL, Xie YM, et al. The effectiveness of Xianlinggubao capsule in the treatment of postmenopausal osteoporosis: a systematic review and meta-analysis. Chin J Osteoporos. 2019;25. 47–61. [Google Scholar]
- [69].Zhao YJ, HU J, Chen J, et al. A systematic evaluation on Xianlinggubao capsule for postmenopausal osteoporosis. Tianjin J Traditional Chin Med. 2010;27:279–282. [Google Scholar]


