Abstract
The activity of gemifloxacin against Haemophilus influenzae and Moraxella catarrhalis was compared to those of 11 other agents. All quinolones were very active (MICs, ≤0.125 μg/ml) against 248 quinolone-susceptible H. influenzae isolates (40.7% of which were β-lactamase positive); cefixime (MICs, ≤0.125 μg/ml) and amoxicillin-clavulanate (MICs ≤4.0 μg/ml) were active, followed by cefuroxime (MICs, ≤16.0 μg/ml); azithromycin MICs were ≤4.0 μg/ml. For nine H. influenzae isolates with reduced quinolone susceptibilities, the MICs at which 50% of isolates are inhibited (MIC50s) were 0.25 μg/ml for gemifloxacin and 1.0 μg/ml for the other quinolones tested. All strains had mutations in GyrA (Ser84, Asp88); most also had mutations in ParC (Asp83, Ser84, Glu88) and ParE (Asp420, Ser458), and only one had a mutation in GyrB (Gln468). All quinolones tested were equally active (MICs, ≤0.06 μg/ml) against 50 M. catarrhalis strains; amoxicillin-clavulanate, cefixime, cefuroxime, and azithromycin were very active. Against 10 H. influenzae strains gemifloxacin, levofloxacin, sparfloxacin, and trovafloxacin at 2× the MIC and ciprofloxacin at 4× the MIC were uniformly bactericidal after 24 h, and against 9 of 10 strains grepafloxacin at 2× the MIC was bactericidal after 24 h. After 24 h bactericidal activity was seen with amoxicillin-clavulanate at 2× the MIC for all strains, cefixime at 2× the MIC for 9 of 10 strains, cefuroxime at 4× the MIC for all strains, and azithromycin at 2× the MIC for all strains. All quinolones except grepafloxacin (which was bactericidal against four of five strains) and all ß-lactams at 2× to 4× the MIC were bactericidal against five M. catarrhalis strains after 24 h; azithromycin at the MIC was bactericidal against all strains after 24 h. The postantibiotic effects (PAEs) against four quinolone-susceptible H. influenzae strains were as follows: gemifloxacin, 0.3 to 2.3 h; ciprofloxacin, 1.3 to 4.2 h; levofloxacin, 2.8 to 6.2 h; sparfloxacin, 0.6 to 3.0 h; grepafloxacin, 0 to 2.1 h; trovafloxacin, 0.8 to 2.8 h. At 10× the MIC, no quinolone PAEs were found against the strain for which quinolone MICs were increased. Azithromycin PAEs were 3.7 to 7.3 h.
Although the development of an effective vaccine against Haemophilus influenzae type b has led to the disappearance of this organism from many parts of the world, untypeable H. influenzae strains, followed by Streptococcus pneumoniae and Moraxella catarrhalis, are now considered the leading cause of acute exacerbations of chronic bronchitis and an important cause, together with S. pneumoniae and M. catarrhalis, of acute otitis media, sinusitis, and community-acquired respiratory tract infections (4, 6, 8, 18).
Current recommendations by the National Committee for Clinical Laboratory Standards (NCCLS) for the use of Haemophilus test medium (HTM) for Haemophilus susceptibility testing have been complicated by difficulty with the commercial manufacture of this medium and its short shelf-life when it is made in-house (8).
Gemifloxacin (SB 265805; LB 20304a) is a new broad-spectrum fluoronaphthyridone carboxylic acid with a novel pyrrolidone substituent. Preliminary studies have shown that this compound is very active against H. influenzae and M. catarrhalis (2, 7, 11). This study examined the activity of gemifloxacin against these species by (i) the NCCLS microdilution MIC methodology to test the activities of gemifloxacin, ciprofloxacin, levofloxacin, sparfloxacin, grepafloxacin, trovafloxacin, ampicillin, amoxicillin, amoxicillin-clavulanate, cefixime, cefuroxime, and azithromycin against 257 H. influenzae and 50 M. catarrhalis strains; (ii) testing the MICs for and the resistance mechanisms of 9 H. influenzae strains with reduced fluoroquinolone susceptibilities; (iii) testing the killing kinetics of the compounds listed above against 10 H. influenzae and 5 M. catarrhalis strains; and (iv) testing the postantibiotic effects (PAEs) of the compounds listed above against 5 H. influenzae strains.
MATERIALS AND METHODS
Bacteria and antimicrobial agents.
Strains (248 quinolone-susceptible H. influenzae strains, 9 H. influenzae strains with reduced quinolone susceptibility, and 50 M. catarrhalis strains) were isolated from clinical specimens within the past 2 years and were stored at −70°C in double-strength skim milk (Difco Laboratories, Detroit, Mich.) prior to use. H. influenzae strains with reduced fluoroquinolone susceptibilities compared to those of strains usually encountered (see Table 1) were recovered from patients in Western Europe and were obtained from David Felmingham (GR Micro, London, United Kingdom). Gemifloxacin powder for susceptibility testing was obtained from SmithKline Beecham Laboratories, Harlow, United Kingdom. The other drugs were obtained from their respective manufacturers.
TABLE 1.
MICs for 248 H. influenzae and 50 M. catarrhalis (all quinolone susceptible) strains determined by the broth microdilution method
| Drug | MIC (μg/ml)
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ß-Lactamase-positive H. influenzae(n = 101)a
|
ß-Lactamase-negative H. influenzae(n = 147)
|
All H. influenzae strains (n = 248)
|
M. catarrhalis(n = 50)
|
|||||||||
| Range | 50% | 90% | Range | 50% | 90% | Range | 50% | 90% | Range | 50% | 90% | |
| Gemifloxacin | 0.004–0.06 | 0.008 | 0.016 | 0.004–0.03 | 0.008 | 0.016 | 0.004–0.06 | 0.008 | 0.016 | 0.008–0.03 | 0.016 | 0.016 |
| Ciprofloxacin | 0.008–0.03 | 0.016 | 0.016 | 0.008–0.12 | 0.016 | 0.016 | 0.008–0.12 | 0.016 | 0.016 | 0.016–0.06 | 0.03 | 0.03 |
| Levofloxacin | 0.016–0.03 | 0.03 | 0.03 | 0.008–0.12 | 0.03 | 0.03 | 0.008–0.12 | 0.03 | 0.03 | 0.016–0.06 | 0.06 | 0.06 |
| Sparfloxacin | 0.008–0.125 | 0.016 | 0.03 | 0.008–0.06 | 0.016 | 0.03 | 0.008–0.12 | 0.016 | 0.03 | 0.016–0.06 | 0.03 | 0.03 |
| Grepafloxacin | 0.002–0.03 | 0.008 | 0.008 | 0.002–0.03 | 0.008 | 0.008 | 0.002–0.03 | 0.008 | 0.008 | 0.004–0.016 | 0.008 | 0.016 |
| Trovafloxacin | 0.008–0.06 | 0.016 | 0.03 | 0.008–0.06 | 0.016 | 0.03 | 0.008–0.06 | 0.016 | 0.03 | 0.016–0.03 | 0.03 | 0.03 |
| Ampicillin | 1.0–>16.0 | >16.0 | >16.0 | ≤0.12–2.0 | 0.25 | 0.5 | ≤0.12–>16.0 | 0.25 | >16.0 | 0.125–16.0 | 4.0 | 8.0 |
| Amoxicillin | 2.0–>16.0 | >16.0 | >16.0 | 0.25–4.0 | 0.5 | 1.0 | 0.25–>16.0 | 0.5 | >16.0 | 0.125–16.0 | 4.0 | 8.0 |
| Amoxicillin-clavulanate | 0.25–4.0 | 1.0 | 2.0 | 0.25–4.0 | 0.5 | 1.0 | 0.25–4.0 | 0.5 | 2.0 | 0.125–0.5 | 0.125 | 0.25 |
| Cefixime | ≤0.008–0.12 | 0.03 | 0.03 | 0.016–0.12 | 0.03 | 0.03 | ≤0.008–0.12 | 0.03 | 0.03 | 0.03–0.5 | 0.25 | 0.25 |
| Cefuroxime | 0.25–8.0 | 0.5 | 2.0 | ≤0.25–16.0 | 0.5 | 2.0 | ≤0.25–16.0 | 0.5 | 2.0 | 0.25–4.0 | 2.0 | 2.0 |
| Azithromycin | 0.25–4.0 | 2.0 | 4.0 | 0.25–4.0 | 2.0 | 4.0 | 0.25–4.0 | 2.0 | 4.0 | 0.06 | 0.06 | 0.06 |
n, number of strains.
MIC determination.
ß-Lactamase production was tested by the nitrocefin disk method (Cefinase; BBL Microbiology Systems, Sparks, Md.). MICs were determined by the NCCLS microdilution method (10) with HTM in frozen 96-well trays (Medical Specialties, Inc., Cleveland, Ohio). The HTM used in the trays was freshly prepared, and its growth properties were confirmed. The quinolone-susceptible H. influenzae strains comprised 50 type b strains and 198 untypeable strains. Inocula were prepared from chocolate agar plates incubated for a full 24 h by the direct colony suspension method, as follows. In a tube of Mueller-Hinton broth (Difco), an organism suspension was made to a density of a 0.5 McFarland standard (108 CFU/ml). The latter inoculum was diluted in sterile saline such that the final organism suspensions in the trays yielded colony counts of 3 × 105 to 8 × 105 CFU/ml. Inoculum checks were performed for all strains, and testing was repeated if the inocula were not in the correct range.
Wells were inoculated by use of disposable inoculators, and the trays were incubated in ambient air at 35°C for 20 to 24 h. The lowest drug concentration at which no growth occurred was read as the MIC. Clavulanate was added to amoxicillin at a ratio of 1:2. Standard quality control strains, including H. influenzae ATCC 49766, H. influenzae ATCC 49247, Staphylococcus aureus ATCC 29213, and Escherichia coli ATCC 25922, were included with each run.
Time-kill studies.
Ten H. influenzae strains (five ß-lactamase positive strains), including one strain for which quinolone MICs were increased, and five ß-lactamase-positive M. catarrhalis strains were tested. Glass tubes containing 5 ml of HTM with doubling antibiotic concentrations were inoculated with approximately 5 × 105 of organism per ml (5 × 105 to 5 × 106 CFU/ml) and were incubated at 35°C in a shaking water bath. Antibiotic concentrations were chosen to comprise three doubling dilutions above and three dilutions below the MIC determined by the broth microdilution method. Freshly made batches of HTM were used for all tests. The dilutions required to obtain the correct inoculum (approximately 5 × 105 CFU/ml) were determined by prior viability studies with each strain (12, 13).
To inoculate each tube of serially diluted antibiotic, 30 μl of diluted inoculum was delivered beneath the surface of the broth with a pipette. The tube was then vortexed and the contents were plated for viability counts (0 h). Only tubes containing an initial inoculum within the range of 5 × 105 to 5 × 106 CFU/ml were acceptable.
The viability counts of the antibiotic-containing suspensions were determined at 0, 3, 6, 12, and 24 h by plating 10-fold dilutions of 0.1-ml aliquots from each tube in Mueller-Hinton broth (BBL) onto chocolate agar plates. Recovery plates were incubated for up to 48 h. Colony counts were determined for plates that yielded 30 to 300 colonies (12, 13).
The lower limit of sensitivity of the colony count determinations was 300 CFU/ml. Time-kill data were analyzed by determining the number of strains which yielded changes of −1 −2, and −3 log10 CFU/ml at 0, 3, 6, 12, and 24 h compared to the counts at time zero. Antimicrobial agents were considered bactericidal if they reduced the original inoculum by ≥3 log10 CFU/ml (99.9%) at each of the time periods and bacteriostatic if they reduced the inoculum by 0 to <3 log10 CFU/ml. With the sensitivity threshold and inocula used in these studies, no problems were encountered in delineating 99.9% killing, when it was present. The problem of antibiotic carryover was addressed as described previously (12, 13). Amoxicillin and ampicillin were not tested against ß-lactamase-positive H. influenzae and M. catarrhalis strains.
Measurement of PAE.
The PAE (3) was determined by the viable plate count method with freshly made HTM. The bacterial inoculum was prepared by suspending growth from an overnight chocolate agar plate in broth. The suspension was diluted in Mueller-Hinton broth until the turbidity matched that of a no. 1 McFarland standard (approximately 5 × 108 CFU/ml) (3, 14, 15).
For PAE experiments, tubes with 5 ml of broth and the antibiotic to be tested at 10× the MIC were inoculated with 50 μl of inoculum to provide 5 × 106 CFU/ml. The tubes were then vortexed, and the contents were plated for viability count determinations. Growth controls with inoculum but no antibiotic were included with each experiment. The inoculated test tubes were then placed in a shaking water bath at 35°C for 1 h. At the end of the 1-h period, cultures were diluted 1:1,000 in prewarmed HTM broth to remove the antibiotic. An additional control culture containing bacteria and antibiotic at a concentration of 0.01× the MIC was prepared to confirm that after dilution the antibiotic is no longer bacteriostatic (3, 14, 15).
Viability counts were determined before exposure and immediately after dilution (0 h) and then every 2 h until the turbidity of the tube reached that of a no. 1 McFarland standard. Viability counts studies were performed by preparing 10-fold dilutions of 0.1-ml aliquots from each tube in Mueller-Hinton broth and plating 0.1-ml volumes onto chocolate agar plates. Recovery plates were incubated for 24 h, and colony counts were determined for plates that yielded 30 to 300 colonies (3, 14, 15).
The PAE was defined as described by Craig and Gudmundsson as T − C, where T is the time required for viability counts of an antibiotic-exposed culture to increase by 1 log10 above the counts observed immediately after dilution and C is the corresponding time for the growth control. For each experiment, viability counts, expressed as log10 CFU per milliliter, were plotted against time. Results were expressed as the means of two separate assays (3).
PCR and DNA sequencing of quinolone resistance-determining regions of parC, parE, gyrA, and gyrB.
Template DNA for PCR was prepared as follows. A colony from an overnight growth was lysed by incubation for 1 h at 37°C in lysis buffer (6 mM Tris-HCl [pH 7.4], 1 M NaCl, 10 mM EDTA [pH 8.0], 0.2% deoxycholate, 0.5% sodium lauroyl sarcosine) to which lysozyme (Sigma, St. Louis, Mo.) at 0.5 mg/ml and lysostaphin (Sigma) at 0.05 mg/ml were added fresh. DNA was isolated from the lysed cells with a Prep-A-Gene kit (Bio-Rad, Hercules, Calif.) as recommended by the manufacturer. PCR was carried out in a final volume of 100 μl containing 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, each deoxynucleoside triphosphate at a concentration of 200 μM, 5 pmol of each primer, 5 to 10 ng of DNA template, and 2.5 U of Taq DNA polymerase (Fisher Biotech). Conditions for PCR were 30 cycles of 94°C for 1 min, annealing at 53°C for 1 min, and extension at 72°C for 3 min. For parC a 370-bp region encoding residues 41 to 163 was amplified with primers HFPAR CUP (5′-TGGTTTAAAACCCGTTCA-3′; nucleotide positions 120 to 137) and HFPARCDN (5′-AGCAGGTAAATATTGTGG-3′; positions 473 to 490). For parE a 471-bp region encoding residues 335 to 491 was amplified with primers HFPAREUP (5′-GAACGCTTATCATCACGCCA-3′; positions 1003 to 1022) and HFPAREDN (5′-AGCATCCGCGAGAATACAGA-3′; positions 1454 to 1473). For gyrA a 375-bp region encoding residues 47 to 171 was amplified with primers HFGYRAUP (5′-CCGCCGCGTACTGTTCT-3′; positions 138 to 154) and HFGYRADN (5′-CCATTTGCTAAAAGTGC-3′; positions 496 to 512). For gyrB a 445-bp region encoding residues 367 to 513 was amplified with primers HFGYRBFOR (5′-GGAAAATCCTGCAGATGC-3′; positions 1095 to 1113) and HFGYRBBAC (5′-AAGCAACGTACGGATGTG-3′; positions 1522 to 1539). After amplification the PCR products were purified from excess primers and nucleotides with a QIAquick PCR purification kit (Qiagen, Valencia, Calif.) and were sequenced directly by using an Applied Biosystems model 373A DNA sequencer. The products of independent PCRs were sequenced twice in the forward and reverse directions.
RESULTS
The results of microdilution testing of quinolone-susceptible strains are presented in Table 1. All quinolones were very active (MICs, ≤0.125 μg/ml) against 248 quinolone-susceptible H. influenzae (40.9% of which were ß-lactamase positive); cefixime (MICs, ≤0.125 μg/ml) and amoxicillin-clavulanate (MICs, ≤4.0 μg/ml) were very active, followed by cefuroxime (MICs, ≤16.0 μg/ml); azithromycin MICs were all ≤4.0 μg/ml. All quinolones were equally active (MICs, ≤0.06 μg/ml) against 50 M. catarrhalis (all of which were ß-lactamase positive); amoxicillin-clavulanate, cefixime, cefuroxime, and azithromycin were also very active.
For nine H. influenzae strains with reduced fluoroquinolone susceptibilities, the MICs at which 50% of isolates are inhibited (MIC50s) were 0.25 μg/ml (highest MIC, 1.0 μg/ml) for gemifloxacin, whereas they were 1.0 μg/ml (highest MIC, 8.0 μg/ml) for the other quinolones tested (Table 2). The mechanisms of quinolone resistance in the H. influenzae strains are presented in Table 3. All nine strains had mutations at Ser84 in GyrA, with Ser84 to Leu, Phe, or Tyr being observed. Additional mutations in GyrA at Asp88 to Asn or Tyr and Ala117 to Glu were also observed in some strains. Most strains also had at least one mutation in ParC (at Asp83, Ser84, Glu88, Ser133, or Asn138) and ParE (at Gly405, Asp420, Ser458, or Ser474). Strain 4 had an in-frame insertion in parE that led to an insertion of a Ser residue between Ser458 and Thr459. Only one strain had a mutation in GyrB (at Gln468). The most resistant strain (strain 9) had double mutations in GyrA, ParC, and ParE.
TABLE 2.
Quinolone MICs for nine H. influenzae strains for which quinolone MICs were increased
| Drug | MIC (μg/ml)
|
|
|---|---|---|
| Range | 50% | |
| Gemifloxacin | 0.03–1.0 | 0.25 |
| Ciprofloxacin | 0.25–8.0 | 1.0 |
| Levofloxacin | 0.25–4.0 | 1.0 |
| Sparfloxacin | 0.25–8.0 | 1.0 |
| Grepafloxacin | 0.25–4.0 | 1.0 |
| Trovafloxacin | 0.25–8.0 | 1.0 |
TABLE 3.
Mechanisms of resistance in H. influenzae strains for which quinolone MICs were increased
| Strain | MIC (μg/ml)
|
Mutation
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gemifloxacin | Ciprofloxacin | Levofloxacin | Sparfloxacin | Grepafloxacin | Trovafloxacin | ParC | ParE | GyrA | GyrB | |
| 1 | 0.03 | 0.5 | 0.5 | 0.25 | 0.25 | 1 | S133-A, N138-S | None | S84-L | None |
| 2 | 0.125 | 0.25 | 0.25 | 0.25 | 0.5 | 0.25 | None | S458-L | S84-F | None |
| 3 | 0.125 | 1 | 1 | 0.5 | 0.5 | 1 | S84-I | None | S84-L | None |
| 4 | 0.25 | 1 | 0.5 | 0.25 | 2 | 0.5 | D83-N | Extra Sa | S84-F, D88-N | Q468-R |
| 5 | 0.25 | 1 | 1 | 1 | 1 | 1 | E88-K | G405-S | S84-Y | None |
| 6 | 0.5 | 2 | 2 | 1 | 1 | 4 | S84-R | D420-N | S84-L, A117-E | None |
| 7 | 0.5 | 2 | 2 | 1 | 1 | 4 | S84-R | D420-N | S84-L, A117-E | None |
| 8 | 0.5 | 2 | 2 | 1 | 1 | 4 | S84-R | D420-N | S84-L, A117-E | None |
| 9 | 1 | 8 | 4 | 8 | 4 | 8 | S84-R, N138-S | S458-A, S474-N | S84-F, D88-Y | None |
In-frame insertion in parE leading to insertion of serine between the serine at position 458 and the threonine at position 459.
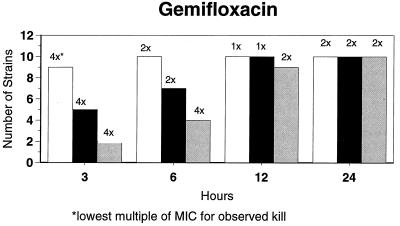
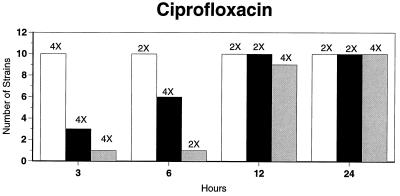
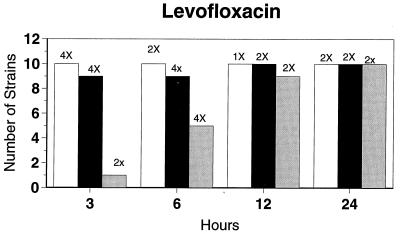
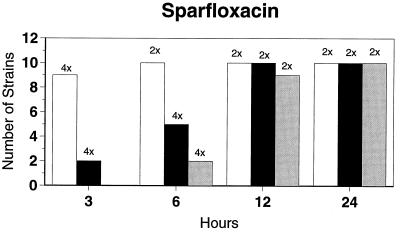
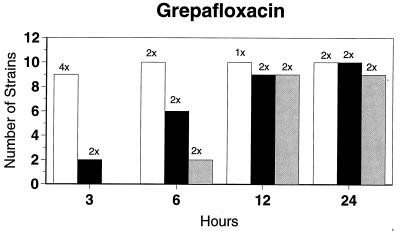
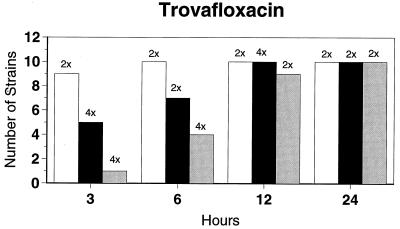
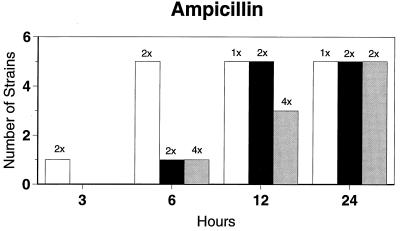
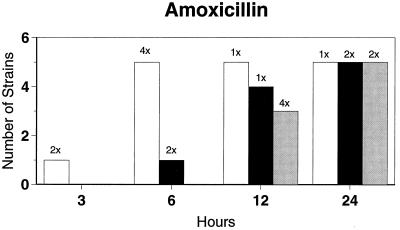
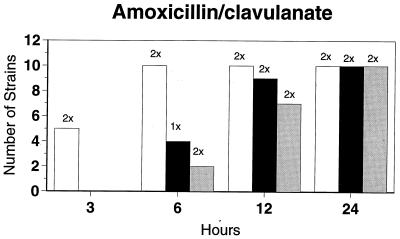
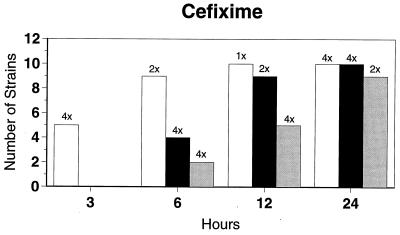
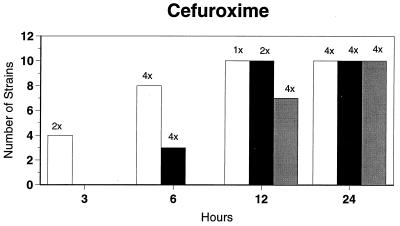
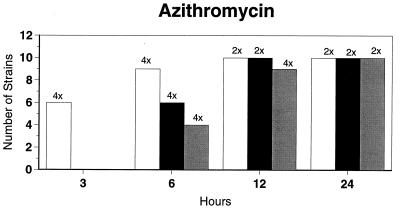
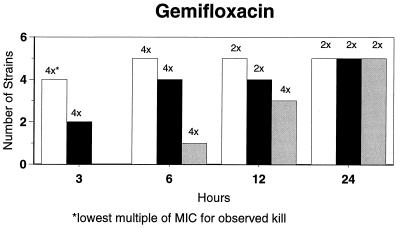
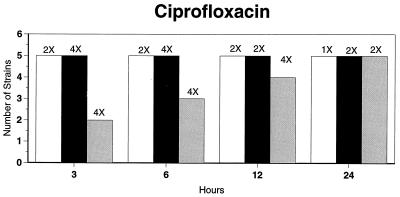
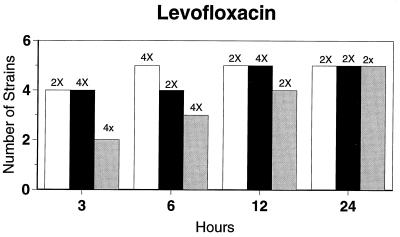
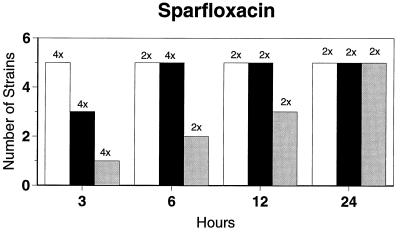
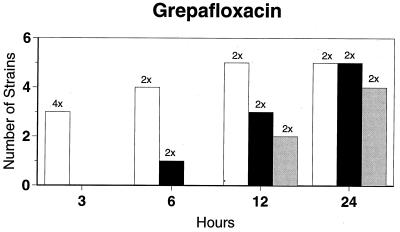
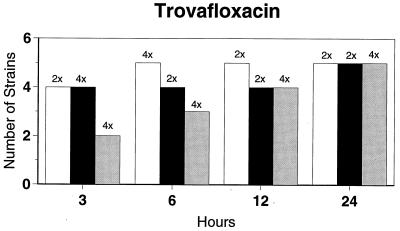
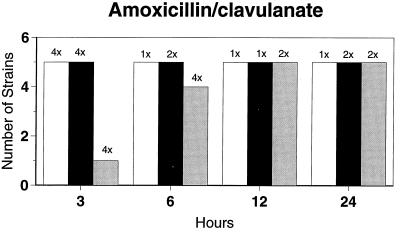
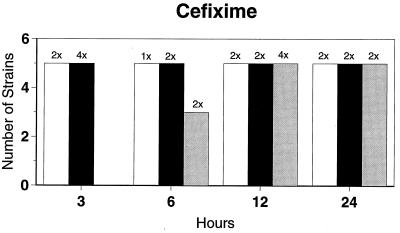
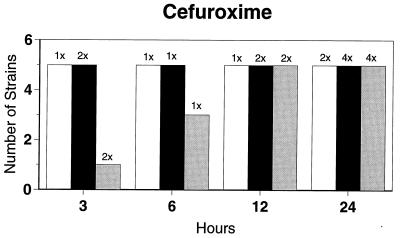
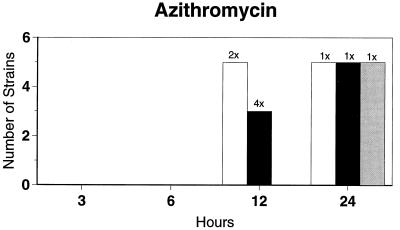
Killing kinetic results for the 10 H. influenzae strains for which killing kinetics were tested are presented in Fig. 1, and those for the 5 M. catarrhalis strains are presented in Fig. 2. Of the quinolones tested, gemifloxacin, levofloxacin, sparfloxacin, and trovafloxacin at 2× the MIC and ciprofloxacin at 4× the MIC were bactericidal against all 10 H. influenzae strains after 24 h, and grepafloxacin at 2× the MIC was bactericidal against 9 of 10 strains after 24 h. Gemifloxacin at 2× the MIC was bactericidal against 9 of 10 strains after 12 h and showed killing at earlier time periods. The killing kinetics of the other quinolones tested were similar to those of gemifloxacin except for the slightly more rapid killing of levofloxacin, especially at earlier time periods. Of the other compounds tested, bactericidal activity after 24 h was found with amoxicillin-clavulanate at 2× the MIC for all 10 strains, cefixime at 2× the MIC for 9 of 10 strains, cefuroxime at 4× the MIC for all 10 strains, and azithromycin at 2× the MIC for all strains. For the five M. catarrhalis strains, all quinolones except grepafloxacin (which was bactericidal against four of five strains) and all ß-lactams were uniformly bactericidal at 2× to 4× the MIC after 24 h, with slower but significant killing at earlier time periods; azithromycin at the MIC was bactericidal against all five strains after 24 h.
FIG. 1.
Results of time-kill studies for 10 H. influenzae strains that exhibited decreases of 1 (□), 2 (■), or 3 ( ) log 10 CFU/ml compared to the numbers at time zero. *, the lowest multiple of the MIC that resulted in the observed killing for the most strains is indicated above each bar. Only five ß-lactamase-negative strains were tested with ampicillin and amoxicillin.
) log 10 CFU/ml compared to the numbers at time zero. *, the lowest multiple of the MIC that resulted in the observed killing for the most strains is indicated above each bar. Only five ß-lactamase-negative strains were tested with ampicillin and amoxicillin.
FIG. 2.
Results of time-kill studies for five M. catarrhalis strains that exhibited decreases of 1 (□), 2 (■), or 3 ( ), log10 CFU/ml compared to the numbers at time zero. *, the lowest multiple of the MIC that resulted in the observed killing for the most strains is indicated above each bar. Because all strains were ß-lactamase positive, amoxicillin and ampicillin were not tested.
), log10 CFU/ml compared to the numbers at time zero. *, the lowest multiple of the MIC that resulted in the observed killing for the most strains is indicated above each bar. Because all strains were ß-lactamase positive, amoxicillin and ampicillin were not tested.
PAEs were tested against five H. influenzae strains; four were quinolone susceptible and the quinolone MICs were increased for one strain. Three strains (including the strain for which the quinolone MICs were increased) were ß-lactamase positive. The PAEs of the antibiotics against the four quinolone-susceptible strains were as follows: gemifloxacin, 0.3 to 2.3 h; ciprofloxacin, 1.3 to 4.2 h; levofloxacin, 2.8 to 6.2; sparfloxacin, 0.6 to 3.0 h; grepafloxacin, 0 to 2.1 h; and trovafloxacin, 0.8 to 2.8 h. The PAEs of azithromycin against the five strains were 3.7 to 7.3 h. ß-Lactams had no PAEs against four strains, and PAEs of 0.2 to 1.7 h were detected against one ß-lactamase-negative strain. At 10× the MIC, no PAEs of the quinolones were found against the strain for which the quinolone MICs were increased.
DISCUSSION
Previous studies have shown that gemifloxacin is 32- to 64-fold more active than ciprofloxacin, ofloxacin, sparfloxacin, and trovafloxacin against S. pneumoniae, methicillin-susceptible and -resistant S. aureus, and methicillin-resistant Staphylococcus epidermidis. Gemifloxacin was also highly active against most members of the family Enterobacteriaceae, with gemifloxacin having activity more potent than those of sparfloxacin and ofloxacin and activity comparable to that of ciprofloxacin. Gemifloxacin was the most active agent against gram-positive species resistant to other quinolones and glycopeptides. Gemifloxacin has variable activity against anaerobes (2, 7, 11).
In our study, all quinolones were very active against quinolone-susceptible strains of H. influenzae and M. catarrhalis. However, only gemifloxacin had MICs of ≤1.0 μg/ml for the rare strains of H. influenzae for which quinolone MICs were increased. Previous studies (1, 5, 16) have shown that the primary target of quinolones in H. influenzae is GyrA; low-level resistance is associated with a mutation in GyrA (Ser84 or Asp88), and high-level resistance is associated with an additional mutation in ParC (Asp83, Ser84, or Glu88). Sequencing results from our study were in agreement with those reports, as all nine strains had at least one mutation in GyrA and the most resistant strains (ciprofloxacin MICs, ≥1.0 μg/ml) had an additional mutation in ParC. In addition, mutations were found in GyrA (Ala117) and ParC (Ser133, Asn138), which have not been previously reported. This is also the first study of which we are aware that examined mutations in GyrB and ParE in H. influenzae: most strains had mutations in ParE, but only one strain had a mutation in GyrB. Of particular interest was insertion of a serine between the serine at position 458 and the threonine at position 459 of ParE in one strain. It therefore appears that ParE may be more important than GyrB in quinolone resistance in H. influenzae.
All quinolone and nonquinolone compounds tested had good killing activities relative to their MICs for the H. influenzae and M. catarrhalis strains tested, with azithromycin having the slowest killing kinetics. At an MIC of 0.5 μg/ml, gemifloxacin also showed good killing of the one H. influenzae strain for which quinolone MICs were increased. A slightly improved killing activity of levofloxacin at earlier time periods compared to those of the other quinolones tested and a uniform bactericidal activity of azithromycin at the MIC or 2× the MIC against H. influenzae after 24 h have been described (12, 17).
All quinolones had PAEs against all quinolone-susceptible strains tested, and azithromycin had PAEs against all strains. A lack of significant PAEs of ß-lactams against gram-negative rods has been described before (3). The PAEs of the quinolones and azithromycin against H. influenzae detected in the present study are similar to those previously reported for other organisms (9, 14, 15).
Although strains of H. influenzae with decreased fluoroquinolone susceptibilities are extremely rare at present, isolated reports of such strains have appeared in the literature (1, 16). For an isolate from one patient (16), an increase in quinolone MICs occurred while the patient was receiving ofloxacin therapy. If the broad-spectrum quinolones are overused, and particularly if their use is extended to the pediatric population, the likelihood of an increase in the numbers of quinolone-resistant H. influenzae isolates (and perhaps M. catarrhalis isolates) is great.
The results of this study indicate that gemifloxacin has good activity (comparable to those of other quinolones) against quinolone-susceptible H. influenzae and M. catarrhalis strains according to MIC determinations and time-kill studies and also that gemifloxacin had PAEs (albeit slightly shorter than those of the other quinolones tested) against quinolone-susceptible H. influenzae strains. Because of the wide spectrum of activity of gemifloxacin against other respiratory pathogens, such as pneumococci (including quinolone-resistant strains), Legionella, mycoplasmas, and chlamydia, this compound represents an attractive addition to other quinolone and nonquinolone agents for empiric treatment of community-acquired respiratory tract infections, particularly community-acquired pneumonia. Clinical studies will be necessary to validate this hypothesis.
ACKNOWLEDGMENTS
This study was supported by a grant from SmithKline Beecham Laboratories, Collegeville, Md.
We thank D. Felmingham (London, United Kingdom) for provision of H. influenzae strains for which quinolone MICs were increased.
REFERENCES
- 1.Bootsma H J, Troelstra A, van Veen-Rutgers A, Mooi F R, de Neeling A J, Overbeek B P. Isolation and characterization of a ciprofloxacin-resistant isolate of Haemophilus influenzae from The Netherlands. J Antimicrob Chemother. 1997;39:292–293. doi: 10.1093/jac/39.2.292. [DOI] [PubMed] [Google Scholar]
- 2.Cormican M G, Jones R N. Antimicrobial activity and spectrum of LB 20304, a novel fluoronaphthyridone. Antimicrob Agents Chemother. 1997;41:204–211. doi: 10.1128/aac.41.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Craig W A, Gudmundsson S. Postantibiotic effect. In: Lorian V, editor. Antibiotics in laboratory medicine. Baltimore, Md: The Williams & Wilkins Co.; 1996. pp. 296–329. [Google Scholar]
- 4.Fang G D, Fine M, Orloff J, Arisumi D, Yu V L, Kapoor W, Grayston J T, Wang S P, Kohler R, Muder R R, Yee Y C, Rihs J D, Vickers R M. New and emerging etiologies for community-acquired pneumonia with implications for therapy. A prospective multicenter study of 359 cases. Medicine (Baltimore) 1990;69:307–316. doi: 10.1097/00005792-199009000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Georgiou M, Munoz R, Roman F, Canton R, Gomez-Lus R, Campos J, De la Campa A G. Ciprofloxacin-resistant Haemophilus influenzae strains possess mutations in analagous positions of gyrA and parC. Antimicrob Agents Chemother. 1996;40:1741–1744. doi: 10.1128/aac.40.7.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoberman A, Paradise J L, Block S, Burch D J, Jacobs M R, Balanescu M I. Efficacy of amoxicillin/clavulanate for acute otitis media: relation to Streptococcus pneumoniae. Pediatr Infect Dis. 1996;10:955–962. doi: 10.1097/00006454-199610000-00034. [DOI] [PubMed] [Google Scholar]
- 7.Hohl A F, Frei R, Pünter V, von Graevenitz A, Knapp C, Washington J, Johnson D, Jones R N. International multicenter investigation of LB20304, a new fluoronaphthyridone. Clin Microbiol Infect. 1998;4:280–284. doi: 10.1111/j.1469-0691.1998.tb00057.x. [DOI] [PubMed] [Google Scholar]
- 8.Jacobs M R, Bajaksouzian S, Zilles A, Lin G, Pankuch G A, Appelbaum P C. Susceptibilities of Streptococcus pneumoniae and Haemophilus influenzae to 10 oral antimicrobial agents based on pharmacodynamic parameters: 1997 U.S. surveillance study. Antimicrob Agents Chemother. 1999;43:1901–1908. doi: 10.1128/aac.43.8.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Licata L, Smith C E, Goldschmidt R M, Barrett J F, Frosco M. Comparison of the postantibiotic and postantibiotic sub-MIC effects of levofloxacin and ciprofloxacin on Staphylococcus aureus and Streptococcus pneumoniae. Antimicrob Agents Chemother. 1997;41:950–955. doi: 10.1128/aac.41.5.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. 3rd ed. 1997. Approved standard. NCCLS publication no. M7-A4. National Committee for Clinical Laboratory Standards, Wayne, Pa. [Google Scholar]
- 11.Oh J-I, Paek K-S, Ahn M-J, Kim M-Y, Hong C Y, Kim I-C, Kwak J-H. In vitro and in vivo evaluations of LB20304, a new fluoronaphthyridone. Antimicrob Agents Chemother. 1996;40:1564–1568. doi: 10.1128/aac.40.6.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pankuch G A, Hoellman D B, Lin G, Bajaksouzian S, Jacobs M R, Appelbaum P C. Activity of HMR 3647 compared to those of five agents against Haemophilus influenzae and Moraxella catarrhalis by MIC determination and time-kill assay. Antimicrob Agents Chemother. 1998;42:3032–3034. doi: 10.1128/aac.42.11.3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pankuch G A, Jacobs M R, Appelbaum P C. Study of comparative antipneumococcal activities of penicillin G, RP 59500, erythromycin, sparfloxacin, ciprofloxacin and vancomycin by using time-kill methodology. Antimicrob Agents Chemother. 1994;38:2065–2072. doi: 10.1128/aac.38.9.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spangler S K, Lin G, Jacobs M R, Appelbaum P C. Postantibiotic effect of sanfetrinem compared with those of six other agents against 12 penicillin-susceptible and -resistant pneumococci. Antimicrob Agents Chemother. 1997;41:2173–2176. doi: 10.1128/aac.41.10.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spangler S K, Lin G, Jacobs M R, Appelbaum P C. Postantibiotic effect and postantibiotic sub-MIC effect of levofloxacin compared to those of ofloxacin, ciprofloxacin, erythromycin, azithromycin and clarithromycin against 20 pneumococci. Antimicrob Agents Chemother. 1998;42:1253–1255. doi: 10.1128/aac.42.5.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vila J, Ruiz J, Sanchez F, Navarro F, Mirelis B, Jimenez de Anta M, Prats G. Increase in quinolone resistance in a Haemophilus influenzae strain from a patient with recurrent respiratory infections treated with ofloxacin. Antimicrob Agents Chemother. 1999;43:161–162. doi: 10.1128/aac.43.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Visalli M A, Jacobs M R, Appelbaum P C. MIC and time-kill study of activities of DU-6859a, ciprofloxacin, levofloxacin, sparfloxacin, cefotaxime, imipenem, and vancomycin against nine penicillin-susceptible and -resistant pneumococci. Antimicrob Agents Chemother. 1996;40:362–366. doi: 10.1128/aac.40.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeckel M L, Jacobson K D, Guerra F J, Therasse D G, Farlow D. Loracarbef ( LY163892) versus amoxicillin/clavulanate in the treatment of acute bacterial exacerbations of chronic bronchitis. Clin Ther. 1992;14:214–229. [PubMed] [Google Scholar]