ABSTRACT
Chronic kidney disease (CKD) in clinical is defined as a gradual loss of kidney function for more than 3 months. The pathologic course of CKD is characterized by extensive renal fibrosis; thus, preventing renal fibrosis is vital for the treatment of CKD. It has been reported that microRNA (miR)-374a-5p was under-expressed in renal venous blood samples from patients with CKD. In addition, it exhibited anti-apoptotic effects in renal tissues suggesting that miR-374a-5p may play an important role in CKD. However, it is not clear whether miR-374a-5p could be delivered to renal cells by exosomes and exerts anti-renal fibrosis effects. To mimic renal fibrosis in vitro, human renal tubular epithelial cell lines (HK-2 cells) were treated by transforming growth factor-β (TGF-β) 1. Reverse transcription-quantitative polymerase-chain reaction (RT-qPCR) or Western blot was carried out to evaluate the mechanism by which miR-374a-5p regulated the development of renal fibrosis. Next, exosomes were isolated using with ultracentrifugation method, and the relationship between miR-374a-5p and MAPK6 was evaluated using dual-Luciferase a reporter assay system. The results indicated TGF-β1 significantly down-regulated the expression of miR-374a-5p in HK-2 cells and miR-374a-5p agomir remarkably inhibited the progression of fibrosis in vitro. In addition, exosomal miR-374a-5p could be internalized by HK-2 cells and obviously enhanced the level of miR-374a-5p in HK-2 cells. Furthermore, exosomal miR-374a-5p prevented the progression of renal fibrosis in vivo by regulating MAPK6/MK5/YAP axis. In conclusion, exosomal miR-374a-5p inhibited the progression of renal fibrosis by regulating MAPK6/MK5/YAP axis.
KEYWORDS: Renal fibrosis, mesenchymal stem cells (MSC), exosomes, miR-374a-5p, MAPK6
Introduction
Chronic kidney disease (CKD) in clinical is defined as a gradual loss of kidney function for more than 3 months [1–3]. The pathologic progression of CKD is characterized by extensive renal fibrosis due to the accumulation of extracellular matrix (ECM) [4–6]. Thus, early prevention of renal fibrosis is vital for the treatment of CKD [4]. Renal fibrosis is the process of renal fibrous tissue hyperplasia due to drug poisoning, hypertension, diabetes, inflammatory stimulation, cytokines and other pathogenic factors, and finally resulting in the loss of renal function [7–9]. In addition, renal fibrosis is characterized by enhanced reflection of the kidney, unclear boundary between cortex and medulla, and even renal shrinkage [7,9,10]. Although there have been a lot of scientific studies on renal fibrosis, the specific occurrence and development mechanism of renal fibrosis remain unclear.
MicroRNAs (miRNAs) are small and non-coding RNAs [11,12]. MiRNAs play a crucial role in many diseases including cancer, liver fibrosis, renal fibrosis, etc. [13–15]. Exosomes are small vesicles mainly composed of proteins and nucleic acids with a diameter of 30–150 nm [16, 17]. As intercellular communication carriers, exosomes could mediate the transport of biological macromolecules such as nucleic acids between cells, and extensively affect the body’s pathophysiological process [18,19]. For example, bone mesenchymal stem cells-derived exosome (BMSCs-exo) could inhibit lipopolysaccharide (LPS)-induced acute uterine injury (AUI) of endothelial progenitor cells [20]. Besides, exosomal miRNA-150-5p derived from BMSCs prevents cerebral ischemia/reperfusion (I/R) injury [21]. Additionally, exosomes play an indispensable role in the pathophysiological process of renal fibrosis [16,22,23]. For instant, exosomes can transfer miR-let7c from MSCs to NRK52E cells, thereby inhibiting the process of renal fibrosis [22]. Meanwhile, exosomal miR-29 was able to alleviate renal fibrosis [23].
It has been reported that microRNA (miR)-374a-5p was under-expressed in renal venous blood samples from patients with CKD. In addition, it exhibited anti-apoptotic effects in renal tissues suggesting that miR-374a-5p may play an important role in CKD [24]. Therefore, we aimed to explore whether miR-374a-5p could be delivered to renal cells by exosomes, and exerts anti-renal fibrosis effects in the current study. The results indicated that exosomal miR-374a-5p prevented the progression of renal fibrosis by regulating MAPK6/MK5/YAP axis for the first time. Therefore, the present study might provide a new therapeutic strategy for the treatment of renal fibrosis.
Material and methods
Cell culture
HK-2 cells were provided by American Type Culture Collection (ATCC, Manassas, VA, USA). Bone marrow MSCs were provided by iCell Bioscience Inc. These cells were maintained in DMEM (Thermo Fisher Scientific, Waltham, MA, USA) with 10% fatal bovine seru (FBS), 1% penicillin and 1% streptomycin in a 5% CO2 atmosphere at 37°C. To mimic renal fibrosis in vitro, HK-2 cells were dealt with 5 ng/mL TGF-β1 for 48 h [25].
RT-qPCR
Trizol reagent (ELK Biotechnology, Wuhan, China) was carried out to measure total RNA in HK-2 cells and exosomes. EntiLink™ 1st Strand cDNA Synthesis Kit was carried out to synthesize cDNA. Then, a StepOne™ Real-Time PCR System was carried out to conduct qPCR. To analyze the expression of miR-374a-5p, the 2−ΔΔCt method was carried out [26]. Primer sequences are as Table 1. U6 or β-actin was worked as internal controls for miR-374a-5p or MAPK6, respectively.
Table 1.
Primer sequences
| Name | Primer sequences (5’-3’) | |
|---|---|---|
| miR-374a-5p | Forward | CCCGGGTTATAATACAACCTG |
| Reverse | CTCAACTGGTGTCGTGGAGTC | |
| MAPK6 | Forward | CGTCAGGAGCTTCTCAGCGT |
| Reverse | GGCTTGAAATTGGCTCATCC | |
| U6 | Forward | CTCGCTTCGGCAGCACAT |
| Reverse | AACGCTTCACGAATTTGCGT | |
| β-actin | Forward | GTCCACCGCAAATGCTTCTA |
| Reverse | TGCTGTCACCTTCACCGTTC |
Western blot assay
Radio-Immunoprecipitation Assay (RIPA) buffer (Aspen Biotechnology, Wuhan, China) was carried out to extract total proteins from cells or from tissues. (Bicinchonininc acid) BCA kit (Aspen) was carried out to quantify the concentration of proteins. Then, the samples were loaded in 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and followed by transferred to Polyvinylidene-Fluoride (PVDF) membrane. Next, the proteins were incubated with primary antibodies and then incubated with the horseradish peroxidase (HRP)-labeled secondary antibody. Finally, an efficient chemiluminescenc (ECL) kit was used to evaluate these samples [27]. In the currently study, these primary antibodies (Abcam, Cambridge, MA, USA) used were listed as follows: Collagen 1α1, Fibronectin, α-SMA, CD63, TSG101, p-MAPK6, MAPK6, p-MK5, MK5 and YAP. GAPDH was worked as internal controls [27].
Exosomes isolation
The ultracentrifugation method was carried out to extract the exosomes from MSCs. MSCs supernatant was collected and centrifuged [28,29]. Then, the exosomes were obtained.
Transmission electron microscopy (TEM) analysis
TEM was conducted to evaluate the number and morphology of collected vesicles. First, drop the exosomes sample on the carbon supporting membrane copper net for 5 min. Next, 2% phosphotungstic acid was dropped onto the carbon supported membrane copper net for 3 min. Finally, a TEM was used to observe the number and morphology of collected vesicles [29].
Nanoparticle Tracking Analysis (NTA)
NTA analysis was comducted to confirm the particle size of collected vesicles. Firstly, the exosomes sample was cleaned using deionized water. Next, ZetaView analyzer (Particle Metrix, Meerbusch, Germany) was calibrated. After that, the collected vesicles were washed with PBS buffer twice. Finally, ZetaView analyzer was carried out to evaluate the particle size of collected vesicles [30,31].
Flow cytometry assay
Annexin-V-FITC apoptosis detection kit was provided by Tianjin Sanjian Biotechnology Co., Ltd. (Tianjin, China). HK-2 cells were maintained in 6-well plates (5 × 104/mL). After that, HK-2 cells were treated by FITC‐Annexin V for 15 min. Then, cells were treated with 5 μL propidium iodide (PI) for another 15 min in the darkness. Then, the apoptosis of HK-2 was evaluated using a flow cytometry [26].
Dual-luciferase reporter assay
Either wild‐type (WT) or mutant (MT) MAPK6‐3′ Untranslated Regions (UTR) fragment was put into the pGL6-miRNA luciferase reporter vector (Beyotime, Shanghai, China). Next, MAPK6 (WT or MT) was transfected into HK-2 cells together with miR-374a-5p agomir or negative control (NC). Subsequently, dual-Luciferase a reporter assay system was carried out to confirm the relationship between miR-374a-5p and MAPK6 [32].
Animal study
C57BL/6 mice weighing 18 ± 2 g were provided from Vital River (Beijing, China). This study was complies with the National Institutes of Health Guide (NIHG) for the Care and Use of laboratory animals. The mice were grouped as follows: Sham, Unilateral Uretera Obstruction (UUO), UUO + exosomes derived from MSCs (MSCs/NC-Exo) and UUO + exosomes derived from miR-374a-5p-modified MSCs (MSC/miR-374a-5p-Exo). To establish the model of UUO, mice were subjected to peritoneal injection anesthesia with pentobarbital sodium (1%). Then, mice were ligated at two points of the left ureter, and partially ligated to the ureteral-pelvic junction based on the available literature [33]. The sham group was used as a control. Twelve weeks later, mice were injected intravenously with MSCs/NC-Exo or MSC/miR-374a-5p-Exo twice weekly for 4 weeks [34]. At the end of study, mice were euthanasia via 40% volume/min CO2 and kidney tissue was collected from the mice.
Hematoxylin-eosin (HE) staining
The kidney tissue was fixed with paraformaldehyde and embedded in paraffin. Then, the tissue was dewaxed with xylene and followed by dehydrated with 70%, 80% and 90% alcohol. After that, the tissue was stained using HE. Subsequently, the tissue was dehydrated with alcohol. Finally, the staining result was observed under an optical microscope [35].
Measurement of blood urea nitrogen (BUN) and creatinine (CR)
The levels of BUN or CR were evaluated by Urea Assay Kit or Creatinine Assay kit provided by Jiancheng Bioengineering Institute respectively according to the manufacturer’s instructions [35,36].
Immunohistochemistry (IHC) staining
Mice kidney tissues were fixed with paraformaldehyde. Then, the tissues were treated with 0.01 M boiling water citric acid buffer to extract antigens. Next, the tissues were treated with fluorescent labeled primary antibody (α-SMA). Subsequently, a fluorescence microscope was used to visualize the IHC staining, according to previous literature [37].
Statistical analysis
Statistical data were analyzed by GraphPad Prism (La Jolla, CA, USA). All data were presented as mean ± standard deviation (SD). Differences in multiple groups were analyzed by one-way analysis of variance (ANOVA) and Tukey’s test [25,35].
Results
MiR-374a-5p agomir remarkably inhibits TGF-β1-induced fibrosis in vitro
In order to mimic renal fibrosis in vitro, HK-2 cells were treated with TGF-β1. As shown in (Figure 1a), the level of miR-374a-5p was significantly downregulated by TGF-β1 in HK-2 cells and miR-374a-5p agomir obviously increased the level of miR-374a-5p in HK-2 cells (Figure 1b). In addition, TGF-β1 significantly increased the levels of Collagen 1α1, Fibronectin and α-SMA in HK-2 cells; however, these phenomena were significantly reversed by miR-374a-5p agomir (Figures 1c-f). Taken together, TGF-β1 downregulated the level of miR-374a-5p in HK-2 cells and miR-374a-5p agomir remarkably inhibits the TGF-β1-induced fibrosis in vitro.
Figure 1.

MiR-374a-5p agomir remarkably inhibits TGF-β1-induced fibrosis in vitro. (a) HK-2 cells were treated with 5 ng/mL TGF-β1 for 48 h. RT-qPCR was used to evaluate the expression of miR-374a-5p in HK-2 cells. (b) HK-2 were dealed with miR-374a-5p agomir or NC using Lipofectamine® 2000 and the level of miR-374a-5p was evaluated with RT-qPCR. (C, D, E and F) Western blot were carried out to evaluate the levels of Collagen 1α1, Fibronectin and α-SMAs. **P < 0.01.
Exosomes from miR-374a-5p-modified MSC can be internalized by HK-2 cells
It has been reported that exosomes from MSCs could inhibit the progression of renal fibrosis [38,39]. In order to explore if exosomal miR-374a-5p could better prevent the renal fibrosis, MSCs were transfected with miR-374a-5p firstly. The result of RT-qPCR suggested miR-374a-5p agomir markedly upregulated the level of miR-374a-5p in MSCs (Figure 2a). Then, exosomes were collected and characterized using with TEM and NTA. The data indicated these extracellular vesicles were discoid vesicles with a phospholipid bilayer structure and are 100 to 150 nm in diameter (Figures 2b,c); meanwhile, the isolated extracellular vesicles expressed specific exosome markers CD63 and TSG101 (Figure 2d). Moreover, the level of miR-374a-5p in exosomes derived from miR-374a-5p-modified MSCs was much higher than that in MSCs/NC-Exo (Figure 2e).
Figure 2.
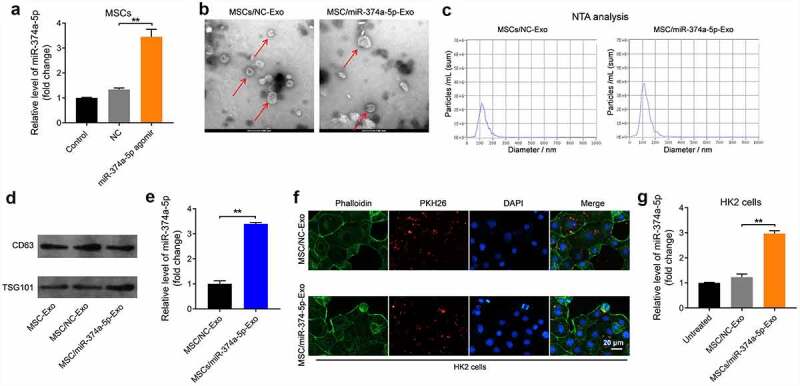
Exosomes from miR-374a-5p-modified MSC can be internalized by HK-2 cells. (a) MSCs were transfected with miR-374a-5p agomir NC or miR-374a-5p agomir using Lipofectamine® 2000 and RT-qPCR was conducted to evaluate the level of miR-374a-5p in MSCs. (b, c) TEM and NTA analysis were used to characterize the morphology and particle size of vesicles. (d) Western blot was used to evaluate the levels of CD63 and TSG101. (e) The level of miR-374a-5p in MSC/miR-374a-5p-Exo or MSCs/NC-Exo was evaluated by RT-qPCR. (f) HK-2 cells were incubated with PKH26-labeled MSC/miR-374a-5p-Exo or PKH26-labeled MSCs/NC-Exo with for 24 h. Then, a fluorescence microscope was conducted to observe PKH26 staining. Green color: HK-2 cells, Red color: exosome, blue color: nucleus. (g) HK-2 cells were treated with MSC/miR-374a-5p-Exo or MSCs/NC-Exo and the level of miR-374a-5p in HK-2 cells was evaluated by RT-qPCR. **P < 0.01.
Next, to investigate whether these exosomes could be internalized by HK-2 cells, a cell membrane staining dye PKH26 was used. The data of staining indicated that both MSC/miR-374a-5p-Exo and MSCs/NC-Exo could be absorbed by HK-2 cells (figure 2f). After absorbing, MSC/miR-374a-5p-Exo obviously increased the expression of miR-374a-5p in HK-2 cells (Figure 2g). All in all, MSC/miR-374a-5p-Exo could be internalized by HK-2 cells and obviously upregulated the level of miR-374a-5p in cells.
MSC/miR-374a-5p-Exo markedly inhibits the progression of fibrosis in vitro
With the aim of investigating the effect of MSC/miR-374a-5p-Exo on the apoptosis of HK-2 cells, flow cytometry was carried out. As indicated in (Figure 3a), TGF-β1 clearly induced the apoptosis of HK-2 cells, and this phenomenon was reversed by MSC/miR-374a-5p-Exo. Meanwhile, TGF-β1 upregulated the levels of Collagen 1α1, Fibronectin and α-SMA in HK-2 cells, while these upregulations were partly reversed by MSC/miR-374a-5p-Exo (Figures 3b-e).
Figure 3.

MSC/miR-374a-5p-Exo markedly inhibits the progression of fibrosis in vitro. (a) Flow cytometry was carried out to measure the apoptosis. (b, c, d and e) Western blot assay were carried out to evaluate the levels of Collagen 1α1, Fibronectin and α-SMA in HK-2 cells. (Supplementary Figure 1A) Flow cytometry was carried out to measure the apoptosis. (Supplementary Figure 1B) Western blot assay were carried out to evaluate the levels of Collagen 1α1 and Fibronectin in HK-2 cells. **P < 0.01.
In order to verify the effect of miR-374a-5p on the progression of fibrosis, miR-374a-5p inhibitor was used. As suggested in supplementary Figure 1A, the effect of MSC/miR-374a-5p-Exo on the apoptosis of HK-2 cells was abolished by miR-374a-5p inhibitor. Expectantly, the effect of MSC/miR-374a-5p-Exo on the expressions of Collagen 1α1 and Fibronectin in HK-2 cells was abolished when miR-374a-5p was eliminated (Supplementary Figure 1B). All these results suggested that MSC/miR-374a-5p-Exo was able to inhibit TGF-β1-induced apoptosis of HK-2 cells and prevent the progression of fibrosis in vitro.
MiR-374a-5p regulates MAPK6/MK5/YAP axis
To study the mechanism by which MSC/miR-374a-5p-Exo mediated the development of renal fibrosis, miRDB (http://www.mirdb.org/cgi-bin/search.cgi) and TargetScan (http://www.targetscan.org/vert_72/) online databases were used. These two databases commonly predicted that MAPK6 was the downstream targets of miR-374a-5p (Figure 4a); in addition, it has been reported that MAPK6 have a close relationship with kidney-related diseases [40,41]. Thus, we focused on investigating the relationship between miR-374a-5p and MAPK6 in the current study.
Figure 4.

MiR-374a-5p regulates MAPK6/MK5/YAP axis. (a) MiRDB and TargetScan databases were used to predict the downstream targets of miR-374a-5p. (b) The relationship between miR-374a-5p and MAPK6 was explored by dual-luciferase reporter assay. (c) RT-qPCR was used to evaluate the level of MAPK6p in HK-2 cells. (d, e, f and g) Western blot assay were carried out to measure the expressions of p-MAPK6, MAPK6, p-MK5, MK5 and YAP in HK-2 cells. **P < 0.01.
In addition, miR-374a-5p agomir notably downregulated the luciferase activity of cell harboring WT of MAPK6; however, it had no effect on the luciferase activity of cell transfected with mutant of MAPK6 (Figure 4b). Consistently, miR-374a-5p agomir markedly inhibited the gene expression of MAPK6 in HK-2 cells (Figure 4c). Moreover, TGF-β1 obviously increased the levels of p-MAPK6, p-MK5 and YAP in HK-2 cells, and these increases were all reversed by MSC/miR-374a-5p-Exo (Figures 4d-g). Taken together, miR-374a-5p regulate MAPK6/MK5/YAP axis by directly binding with mRNA of MAPK6 in HK-2 cells.
MSC/miR-374a-5p-Exo inhibits the progression of renal fibrosis in vivo
Next, to further investigate the effect of MSC/miR-374a-5p-Exo on the process of renal fibrosis in vivo, UUO mouse model was established. As indicated in Figure 5a, the levels of BUN and CR were notably increased in UUO mice, while MSC/miR-374a-5p-Exo treatment brought them back to the normal situation (Figure 5a). In addition, the areas of inflammatory infiltration and fibrosis were upregulated in the UUO group, whereas, MSC/miR-374a-5p-Exo alleviated these lesion (Figures 5b,c). Consistently, the level of α-SMA was upregulated in UUO mouse renal tissue, while this upregulation was visibly reversed by MSC/miR-374a-5p-Exo (Figure 5d). Moreover, MSC/miR-374a-5p-Exo significantly promoted the expression of miR-374a-5p in UUO mouse renal tissue (Figure 5e). To sum up, MSC/miR-374a-5p-Exo significantly prevented the development of renal fibrosis.
Figure 5.
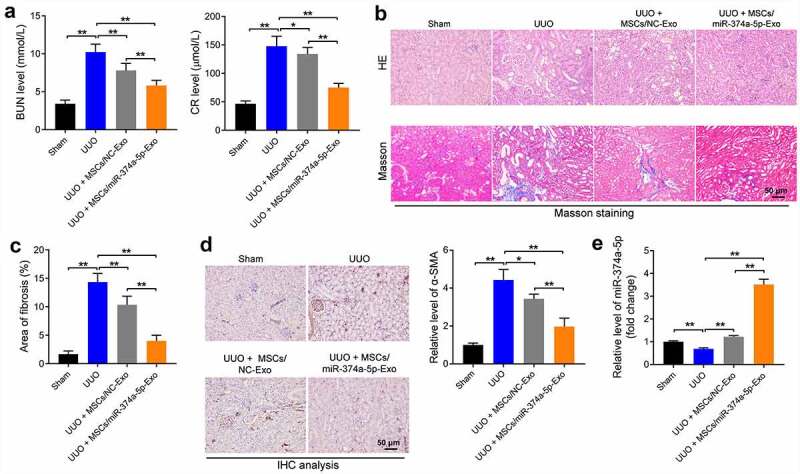
MSC/miR-374a-5p-Exo prevents the progression of renal fibrosis. (a) The level of BUN or CR was evaluated by urea assay kit or creatinine assay kit. (b, c) The tissue structure, cell morphology, inflammatory infiltration and fibrosis of kidney tissue were observed by HE and Masson staining. (d) The level of α-SMA in UUO mouse renal tissue was evaluated by IHC staining. (e) RT-qPCR was conducted to evaluate the expression of miR-374a-5p in mouse renal tissue. **P < 0.01.
MSC/miR-374a-5p-Exo blocks the progression of renal fibrosis by regulating MAPK6/MK5/YAP axis in vivo
Finally, we further explored the mechanism by which MSC/miR-374a-5p-Exo regulated the development of renal fibrosis in vivo. As revealed in (Figures 6a-d), the expressions of p-MAPK6, p-MK5 and YAP were remarkably upregulated in UUO group, whereas these upregulations were notably reversed by MSC/miR-374a-5p-Exo treatment. All in all, MSC/miR-374a-5p-Exo could inhibit the progression of renal fibrosis in vivo by regulating MAPK6/MK5/YAP axis.
Figure 6.

MSC/miR-374a-5p-Exo blocks the renal fibrosis in vivo by regulating MAPK6/MK5/YAP axis. (a, b, c and d) The levels of p-MAPK6, MAPK6, p-MK5, MK5 and YAP in mouse renal tissue were evaluated by Western blot. **P < 0.01.
Discussion
It has been reported that miR-374a-5p was under-expressed in renal venous blood samples from the patients with CKD [24]. We found that the expression of miR-374a-5p was downregulated by TGF-β1 in HK-2 cells, illustrating that miR-374a-5p may be crucial in kidney-related diseases.
So far, there’s a lot of research that demonstrates that exosome may serve as a carrier for the treatment of many diseases [23,42]. For example, exosome-mediated delivery of miR-125a-5p derived from BMSCs might serve as a new therapeutic strategy for the treatment of osteoarthritis (OA) [29]. In addition, exosomes are vital in kidney pathophysiology by facilitating cell-to-cell transport of miRNAs [43]. Meanwhile, exosomes from miR-let7c-modified MSCs relieves the progression of renal fibrosis [22]. In the present study, we found exosomes was able to transfer miR-374a-5p from MSCs to HK-2 cells and exert anti-fibrosis effects for the first time, indicating that exosomal miR-374a-5p was a hopeful therapeutic method for renal fibrosis.
It has been reported that MAPK6 play a vital role during the pathologic of kidney-related diseases [40]. For instant, high expression of MAPK6 in diabetic nephropathy could induce podocyte injury [40]. In this study, we found that TGF-β1 obviously promoted the level of p-MAPK6 in HK-2 cells. As we know, MK5 could be activated by MAPK6, and YAP could be activated by MK5 [44]. For example, Nawaito et al. showed that MK5 was a downstream protein of MAPK6 [44] and Seo indicated that YAP was a downstream protein of MK5 [45]. Current data had concluded that MSC/miR-374a-5p-Exo markedly inhibited TGF-β1-induced apoptosis of HK-2 cells and prevented the progression of renal fibrosis in vivo by regulating MAPK6/MK5/YAP axis, suggesting that MAPK6/MK5/YAP was vital in the pathogenesis of renal fibrosis.
Frankly speaking, there are some limitations needed to be improved in the coming study. For example, we only confirmed the relationship between miR-374a-5p and MAPK6 in the pathological process of renal fibrosis. Indeed, other potential targets such as WNT5A, BMP2 and WNT3 were found in the database, which were not verified yet. Moreover, the current studies have found that MAPK6/MK5/YAP axis was involved in the process of renal fibrosis inhibited by miR-374a-5p modified exosomes, and it is worth exploring whether other axes are also involved.
Conclusion
In conclusion, exosomes from miR-374a-5p-modified MSCs prevented the progression of renal fibrosis by regulating MAPK6/MK5/YAP axis. Thus, our study might provide a new therapeutic way for renal fibrosis.
Supplementary Material
Funding Statement
This work was supported by The Construction of Key Projects by Zhejiang Provincial Ministry (WKJ-ZJ-2017), The Zhejiang Province Chinese Medicine Modernization Program (2020ZX001), The Key Project of Scientific Research Foundation of Chinese Medicine (2022ZZ002) and Clinical and Experimental Research of YSHS Granule.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Availability of data and materials
The datasets used and/or analyzed are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This study was approved by the Zhejiang Provincial People’s Hospital and Affiliated People’s Hospital, Hangzhou Medical College, which complies with the National Institutes of Health Guide for the Care and Use of laboratory animals.
Supplementary material
Supplemental data for this article can be accessed here
References
- [1].Webster AC, Nagler EV, Morton RL, et al. Chronic kidney disease. Vol. 389. (London England): Lancet; 2017. p. 1238–1252. [DOI] [PubMed] [Google Scholar]
- [2].Glassock RJ, Warnock DG, Delanaye P.. The global burden of chronic kidney disease: estimates, variability and pitfalls. Nat Rev Nephrol. 2017;13(2):104–114. [DOI] [PubMed] [Google Scholar]
- [3].Hercz D, Jiang SH, Webster AC. Interventions for itch in people with advanced chronic kidney disease. Cochrane Database Syst Rev. 2020;12:Cd011393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kuppe C, Ibrahim MM, Kranz J, et al. Decoding myofibroblast origins in human kidney fibrosis. Nature. 2021;589(7841):281–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sun YB, Qu X, Caruana G, et al. The origin of renal fibroblasts/myofibroblasts and the signals that trigger fibrosis. Differentiation. 2016;92(3):102–107. [DOI] [PubMed] [Google Scholar]
- [6].Nogueira A, Pires MJ, Oliveira PA. pathophysiological mechanisms of renal fibrosis: a review of animal models and therapeutic strategies. In Vivo (Athens, Greece). 2017;31(1):1–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chen PS, Li YP, Ni HF. Morphology and evaluation of renal fibrosis. Adv Exp Med Biol. 2019;1165:17–36. [DOI] [PubMed] [Google Scholar]
- [8].Zhang QF. Ulinastatin inhibits renal tubular epithelial apoptosis and interstitial fibrosis in rats with unilateral ureteral obstruction. Mol Med Rep. 2017;16(6):8916–8922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lan JZ, Pan Q, Tao Q, et al. TMEM45A is involved in renal fibrosis in rats by regulating Jagged1/Notch pathway. Front Biosci. 2020;25(4):593–605. (Landmark edition). [DOI] [PubMed] [Google Scholar]
- [10].Barnett LMA, Cummings BS. Nephrotoxicity and renal pathophysiology: a contemporary perspective. Toxicol Sci. 2018;164(2):379–390. [DOI] [PubMed] [Google Scholar]
- [11].Tafrihi M, Hasheminasab E. MiRNAs: biology, biogenesis, their web-based tools, and databases. MicroRNA (Shariqah, United Arab Emirates). 2019;8(1):4–27. [DOI] [PubMed] [Google Scholar]
- [12].Saliminejad K, Khorram Khorshid HR, Soleymani Fard S, et al. An overview of microRNAs: biology, functions, therapeutics, and analysis methods. J Cell Physiol. 2019;234(5):5451–5465. [DOI] [PubMed] [Google Scholar]
- [13].Fan Y, Chen H, Huang Z, et al. Emerging role of miRNAs in renal fibrosis. RNA Biol. 2020;17(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Loboda A, Sobczak M, Jozkowicz A, et al. TGF-β1/Smads and miR-21 in Renal fibrosis and Inflammation. Mediators Inflamm. 2016;2016:8319283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lv W, Fan F, Wang Y, et al. Therapeutic potential of microRNAs for the treatment of renal fibrosis and CKD. Physiol Genomics. 2018;50(1):20–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zhang W, Zhou X, Zhang H, et al. Extracellular vesicles in diagnosis and therapy of kidney diseases. Am J Physiol Renal Physiol. 2016;311(5):F844–f51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zhang H, Wang L, Li C, et al. Exosome-induced regulation in Inflammatory bowel disease. Front Immunol. 2019;10:1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Simons M, Raposo G. Exosomes–vesicular carriers for intercellular communication. Curr Opin Cell Biol. 2009;21(4):575–581. [DOI] [PubMed] [Google Scholar]
- [19].Barile L, Vassalli G. Exosomes: therapy delivery tools and biomarkers of diseases. Pharmacol Ther. 2017;174:63–78. [DOI] [PubMed] [Google Scholar]
- [20].Liu Y, Zhang S, Xue Z, et al. Bone mesenchymal stem cells-derived miR-223-3p-containing exosomes ameliorate lipopolysaccharide-induced acute uterine injury via interacting with endothelial progenitor cells. Bioengineered. 2021;12(2):10654–10665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yang S, Li X, Bi T. Exosomal microRNA-150-5p from bone marrow mesenchymal stromal cells mitigates cerebral ischemia/reperfusion injury via targeting toll-like receptor 5. Bioengineered. 2021. DOI: 10.1080/21655979.2021.2012402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wang B, Yao K, Huuskes BM, et al. Mesenchymal stem cells deliver exogenous MicroRNA-let7c via exosomes to attenuate renal fibrosis. Mol Ther. 2016;24(7):1290–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wang H, Wang B, Zhang A, et al. Exosome-mediated miR-29 transfer reduces muscle atrophy and kidney fibrosis in mice. Mol Ther. 2019;27(3):571–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lee MS, Lee FY, Chen YL, et al. Investigated the safety of intra-renal arterial transfusion of autologous CD34+ cells and time courses of creatinine levels, endothelial dysfunction biomarkers and micro-RNAs in chronic kidney disease patients-phase I clinical trial. Oncotarget. 2017;8(11):17750–17762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Feng T, Li W, Li T, et al. Circular RNA_0037128 aggravates high glucose-induced damage in HK-2 cells via regulation of microRNA-497-5p/nuclear factor of activated T cells 5 axis. Bioengineered. 2021;12(2):10959–10970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Chen H, Jin G. Downregulation of Salusin-β protects renal tubular epithelial cells against high glucose-induced inflammation, oxidative stress, apoptosis and lipid accumulation via suppressing miR-155-5p. Bioengineered. 2021;12(1):6155–6165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Pei L, Lv X, Jia G, et al. Silencing circular RNA circ_0054537 and upregulating microRNA-640 suppress malignant progression of renal cell carcinoma via regulating neuronal pentraxin-2 (NPTX2). Bioengineered. 2021;12(1):8279–8295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ji Y, Ji J, Yin H, et al. Exosomes derived from microRNA-129-5p-modified tumor cells selectively enhanced suppressive effect in malignant behaviors of homologous colon cancer cells. Bioengineered. 2021;12(2):12148–12156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Xia Q, Wang Q, Lin F, et al. miR-125a-5p-abundant exosomes derived from mesenchymal stem cells suppress chondrocyte degeneration via targeting E2F2 in traumatic osteoarthritis. Bioengineered. 2021;12(2):11225–11238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Dong H, Wang M, and Li Q. Exosomal miR-4488 and miR-1273g-5p inhibit the epithelial-mesenchymal transition of transforming growth factor β2-mediated retinal pigment epithelial cells by targeting ATP-binding cassette A4. Bioeng. 2021;12(2):9693–9706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Shen C, Tao C, and Zhang A, et al. Exosomal microRNA-93-3p secreted by bone marrow mesenchymal stem cells downregulates apoptotic peptidase activating factor 1 to promote wound healing. Bioeng. 2021; 13(1):27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wang B, Wu W, Xu K, et al. MicroRNA-223-3p is involved in fracture healing by regulating fibroblast growth factor receptor 2. Bioeng. 2021;12(2):12040–12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Patel S. Polycystic ovary syndrome (PCOS), an inflammatory, systemic, lifestyle endocrinopathy. J Steroid Biochem Mol Biol. 2018;182:27–36. [DOI] [PubMed] [Google Scholar]
- [34].Lou G, Song X, Yang F, et al. Exosomes derived from miR-122-modified adipose tissue-derived MSCs increase chemosensitivity of hepatocellular carcinoma. J Hematol Oncol. 2015;8(1):122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Shang J, Sun S, Zhang L, et al. miR-211 alleviates ischaemia/reperfusion-induced kidney injury by targeting TGFβR2/TGF-β/SMAD3 pathway. Bioeng. 2020;11(1):547–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Meng X, Huang W, Mo W, et al. ADAMTS-13-regulated nuclear factor E2-related factor 2 signaling inhibits ferroptosis to ameliorate cisplatin-induced acute kidney injuy. Bioeng. 2021;12(2):11610–11621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Yu T, Zhao C, Hou S, et al. Exosomes secreted from miRNA-29b-modified mesenchymal stem cells repaired spinal cord injury in rats. Braz J Med Biol Res = Rev Bras Pesqui Med Biol. 2019;52(12):e8735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Chen L, Wang Y, Li S, et al. Exosomes derived from GDNF-modified human adipose mesenchymal stem cells ameliorate peritubular capillary loss in tubulointerstitial fibrosis by activating the SIRT1/eNOS signaling pathway. Theranostics. 2020;10(20):9425–9442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Yang Y, Wang J, and Zhang Y, et al. Exosomes derived from mesenchymal stem cells ameliorate renal fibrosis via delivery of miR-186-5p. Hum Cell. 2022;35(1):83–97. [DOI] [PubMed] [Google Scholar]
- [40].Yao T, Zha D, Hu C, et al. Circ_0000285 promotes podocyte injury through sponging miR-654-3p and activating MAPK6 in diabetic nephropathy. Gene. 2020;747:144661. [DOI] [PubMed] [Google Scholar]
- [41].Srivastav RK, Schwede S, Klaus M, et al. Monitoring protein–protein interactions in mammalian cells by trans-SUMOylation. Biochem J. 2011;438(3):495–503. [DOI] [PubMed] [Google Scholar]
- [42].Lv LL, Feng Y, Wu M, et al. Exosomal miRNA-19b-3p of tubular epithelial cells promotes M1 macrophage activation in kidney injury. Cell Death Differ. 2020;27(1):210–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Chen BY, Sung CW, Chen C, et al. Advances in exosomes technology. Clin Chim Acta. 2019;493:14–19. [DOI] [PubMed] [Google Scholar]
- [44].Nawaito SA, Sahadevan P, Clavet-Lanthier M, et al. MK5 haplodeficiency decreases collagen deposition and scar size during post-myocardial infarction wound repair. Am J Physiol Heart Circ Physiol. 2019;316(6):H1281–h96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Seo J, Kim MH, Hong H, et al. MK5 regulates YAP stability and is a molecular target in YAP-Driven cancers. Cancer Res. 2019;79(24):6139–6152. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed are available from the corresponding author on reasonable request.


