Abstract
Introduction
More women than men develop Alzheimer's disease, yet women perform better and show less decline on episodic memory measures, a contradiction that may be accounted for by modifiable risk factors for dementia.
Methods
Associations among age, sex, modifiable dementia risk factors, and cognition were measured in a cross‐sectional online sample (n = 21,840, ages 18 to 89).
Results
Across four tests of associative memory and executive functions, only a Face‐Name Association task revealed sex differences in associative memory that varied by age. Men had worse memory than women (the equivalent of performing similar to someone 4 years older) across ages. Men had larger age differences than women (ie, worse memory in older ages) among people with no to one risk factor, but not those with multiple risk factors.
Discussion
Because the relationship between dementia risk factors and age‐related memory differences varies between men and women, sex‐specific dementia prevention approaches are warranted.
1. BACKGROUND
There is a need to study sex differences in dementia risk, given findings that women are more likely than men to develop Alzheimer's disease, 1 , 2 , 3 although these differences vary across populations. 4 , 5 The higher prevalence of Alzheimer's disease in women is contradicted by findings of better memory for events (episodic memory) in women compared to men 6 , 7 and less age‐related memory decline, 6 , 7 , 8 , 9 , 10 , 11 despite greater episodic memory impairment than normal being a potential precursor to and a defining feature of Alzheimer's disease. 12 By contrast, no consistent sex differences occur in the decline of other cognitive abilities impacted by Alzheimer's disease. 13 , 14 This female memory advantage extends to mild cognitive impairment, a pre‐clinical stage of Alzheimer's disease, 15 but not to Alzheimer's disease itself. 16
The memory advantage in women suggests that sex alone does not account for the higher risk of dementia in women. Therefore, a critical question toward understanding sex‐based differences in dementia etiology is the extent to which other risk factors interact with sex to explain the difference in memory decline, and subsequently in dementia. Accumulating evidence has established that several modifiable lifestyle behaviors are associated with greater cognitive decline and the likelihood of dementia in later life, and collectively account for 40% of dementias worldwide. 17 , 18 Many of these modifiable risk factors vary by sex, leading to growing interest in how modifiable risk factors may explain sex differences in cognitive decline.
Past work shows that individual modifiable risk factors can differentially influence cognitive decline and risk of dementia in men and women. In women, hypertension 6 and low education 19 are associated with greater episodic memory decline, and hypertension increases the likelihood of dementia. 20 , 21 , 22 Diabetes is associated with greater cognitive impairment in women, 23 and smoking is associated with greater episodic memory decline in men, 6 , 24 , 25 but both factors have no sex‐specific effects on general cognitive decline or dementia likelihood. 22 , 26 Depression is associated with greater cognitive decline and dementia in women in some studies, 27 , 28 and in men in others. 6 However, newer research increasingly recommends studying combined rather than individual risk‐factor effects, based on findings that age‐related disease works in a cumulative manner, such that each factor in the aggregate may be strongly associated with dementia even when some individual factors do not have a significant effect on cognitive decline. 29 , 30
Studies of combined factors show that each additional modifiable risk factor lowers cognitive performance 29 , 30 , 31 , 32 and episodic memory, 33 regardless of the type of risk factor (a dose‐response effect). A cumulative approach also accounts for overlapping effects among individual factors. 17 , 18 Based on this evidence, in the current study, we explore the novel interaction of sex and combined modifiable risk factors on cognitive performance over the adult lifespan. Studies have confirmed effects of both sex and modifiable risk factors on cognition over the lifespan; what remains to be considered is the synergistic impact of these factors.
We examine the interactive effects using an existing web‐based data set, 34 collected using an online cognitive assessment designed for sensitivity to aging and age‐associated cognitive disorders. 35 The assessment was developed to be suitable for older adults, and was psychometrically validated in clinical 36 and non‐clinical 35 samples, showing acceptable internal consistency, test‐retest reliability, alternate version reliability, convergent validity compared to traditional neuropsychological tests of the same constructs, and diagnostic validity for mild cognitive impairment.
We use a lifespan sample, as emerging consensus recommends a life‐course perspective for modifiable risk factors, based on evidence that modifiable risk factors accumulate over life to influence likelihood of dementia later in life. 17 , 18 Similarly, the negative dose‐response association between combined modifiable risk factors and cognition increases over the adult lifespan. 32 A life‐course perspective is especially relevant to the interaction between sex and modifiable risk factors, as sex differences in risk‐factor prevalence vary with age (eg, diabetes and hypertension are more common in men in mid‐adulthood, but equal or more common in women after menopause or in older adulthood 25 , 37 , 38 ), as does the interaction between sex and individual risk factors on cognition. 3 , 7 , 25
Our aims were to (1) characterize sex differences in the number and type of modifiable risk factors at different age periods and (2) test if sex, the number of modifiable risk factors, and cross‐sectional age differences interact to influence cognitive abilities impacted in Alzheimer's disease (specifically, interference control, set shifting, working memory, and associative memory). We predicted that sex and modifiable risk factors would interact to influence cognitive performance between ages, specifically on associative memory.
RESEARCH IN CONTEXT
Systematic review: Online databases were searched for articles on sex differences, (cumulative) modifiable risk factors for dementia, or their interaction, on cognition/memory or age‐related/lifespan cognitive decline. Articles were reviewed to gain insights into independent and synergistic effects of sex and modifiable risk factors on cognition and cognitive decline.
Interpretation: Our findings indicate an interaction between previously demonstrated sex differences and modifiable risk factor cumulative effects on cognition, indicating a higher vulnerability in women than men to the adverse cognitive impact of accumulating risk factors.
Future directions: The findings can be used toward sex‐related precision targeting of modifiable risk factors. This research highlights the need to integrate previously independent fields of sex differences and modifiable risk factors to understand the progression of prodromal Alzheimer's disease and promote healthy brain aging. Future work is needed on (1) objectively measured modifiable risk factors, (2) longitudinal effects of sex, modifiable risk factors, and age, and (3) mechanisms for the interaction between sex and modifiable risk factors.
2. METHODS
2.1. Participants
We used data collected from 2016 to 2019 in an existing web‐based sample. 34 Refer to Figure 1 for data exclusions and cleaning. Data were cleaned with between‐subject iterative trimming per age, sex, and cognitive task, using a recursive moving criterion for the standard deviation (SD) based on the sample size. 39 Extensive data cleaning is needed for online data, especially when not directly collected for a study, to account for low quality or inaccurate recordings. 40 The final sample was n = 21,840 (age range = 18 to 89, mean = 64 years, SD = 12; 6620 male, mean age = 64, SD = 13; 15,220 female, mean age = 65, SD = 12).
FIGURE 1.
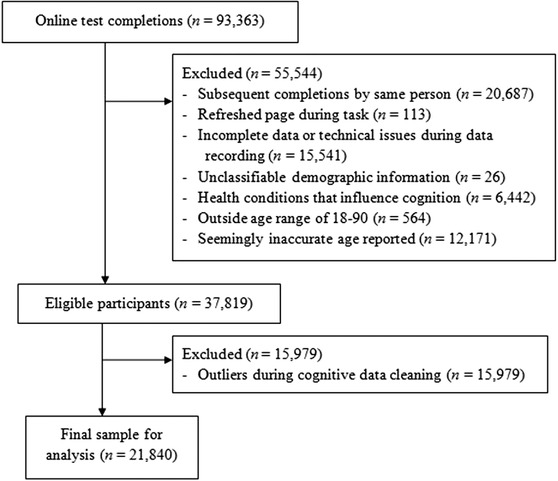
Flowchart showing exclusions and data cleaning
2.2. Online assessment
Participants completed a free web‐based assessment in their homes (Cogniciti's Brain Health Assessment, www.cogniciti.com). The assessment consists of a background questionnaire (self‐reported age, sex, level of education, and specific health conditions) and four cognitive tasks administered in the following order: (1) a Spatial Working Memory task, measured as the number of clicks to recall locations of six shape pairs over two trials; (2) a Face‐Name Association task, measured as correctly recognized face and name pairs; (3) a number‐word Stroop task of processing speed and interference control, measured as the response time when counting words for incongruent stimuli (eg, “three three”); and (4) a Letter‐Number Alternation task, an online version of the Trails B set shifting task, measured as the total completion time to click alternating numbers and letters in ascending order (details on task development and validation are provided elsewhere 35 ).
Associative recognition memory was calculated from accuracy data on the Face‐Name Association task. Process dissociation logic 41 was used to parse memory for individual items (item recognition memory) from memory for items and their associations (associative recognition memory), by comparing hits for intact face‐name pairs to recombined pairs (for details on this procedure 42 ).
2.3. Modifiable risk factors
Eight self‐reported modifiable risk factors were measured: low education, hearing loss, traumatic brain injury (TBI), alcohol or substance abuse, hypertension, smoking, diabetes, and depression. Based on previous research, 18 low education was classified as less than completion of high school, and smoking was classified as being a smoker currently or in the past 1 to 4 years.
Composite risk scores were calculated as the total number of risk factors a person reported (0 to 8) and a risk amount adjusted for variance shared among risk factors (range 0 to 1). Adjusted risk amount was calculated by multiplying the relative effect of each risk factor (the population attributable fraction for each risk factor from the life‐course model 18 ), divided by the total population attributable fraction.
2.4. Statistical analyses
Analyses were conducted with the R language and environment for statistical computing. Statistical test results were interpreted alongside effect sizes, because large sample sizes can produce low p‐values regardless of their theoretical or practical importance.
Prevalence was measured as the percentage of each risk factor and the total risk factors per age period. For prevalence estimates, ages were grouped into young adults (ages 18 to 44, n = 1448), middle‐aged adults (ages 45 to 65, n = 8737), and older adults (ages 66 to 89, n = 11,655), based on the life‐course model for modifiable risk factors. 18 Chi‐square tests were used to test for sex differences in the frequency of each risk factor per sex and age period.
The interaction of sex and modifiable risk factors on cognition with age was measured using quadratic regression models regressing cognitive performance for each task on age (linear and polynomial terms), sex, and composite risk score (either total risk factors or adjusted risk amount) on cognitive performance. Polynomial models were used because our previous analysis of this data set indicated curvature in the relationship between age and cognition over the adult lifespan. 34 Continuous predictors (age and total risk) were mean‐centered so that coefficient estimates could be meaningfully interpreted. 19 All variables of interest were included in the final model to estimate the unique effect of each variable after accounting for any correlations among variables. To account for uneven sample sizes across ages, sex, and risk factors, regression analyses were weighted by an inverse of the sample size per age, sex, and risk amount. To ensure reliability of obtained results, risk amounts were also removed from regression analyses if there were fewer than 25 people for that amount per age decade, sex, and number of risk factors (ie, over four risk factors for all ages, over two risk factors for ages 18 to 50, and over three risk factors for ages 80 to 89; n = 94).
3. RESULTS
3.1. Prevalence of risk factors by sex
The number of risk factors increased with age. Women had fewer risk factors than men for all age periods (Figure 2). Chi‐square tests revealed significant differences and medium effect sizes 43 in the distribution of individual risk factors between sexes for young adults, χ2 (7, N = 1448) = 24.3, p = 0.001, φc = 0.33, middle aged adults, χ2 (7, N = 8737) = 136.1, p < 0.0001, φc = 0.33, and older adults, χ2 (7, N = 11,665) = 198.9, p < 0.0001, φc = 0.34 (Figure 3). Post hoc Pearson residual analyses revealed that significant differences were driven by different risk factors per age group (all p’s < 0.05). In young adults, smoking was more common in men. In middle‐aged adults, hearing loss and substance abuse were more common in men. In older adults, diabetes, hearing loss, smoking, and substance abuse were more common in men, and hypertension was more common in women. Depression was more common in women at all age periods.
FIGURE 2.
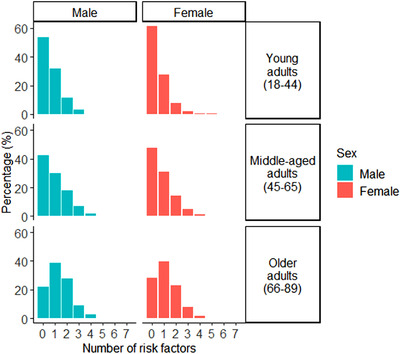
Prevalence (% frequency) of total risk factors per age period per sex
FIGURE 3.
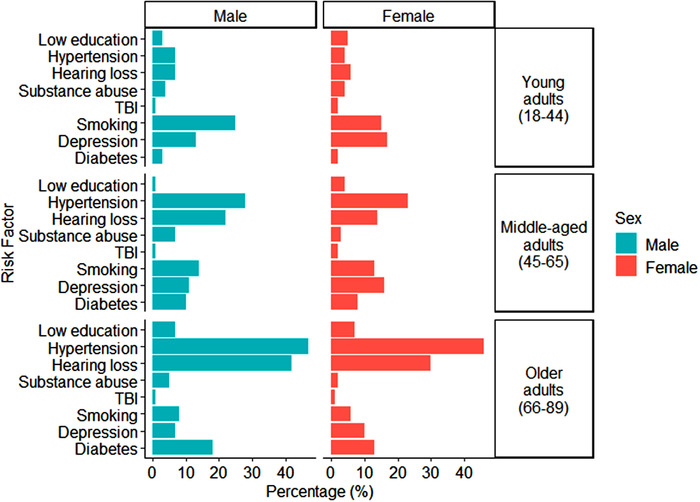
Prevalence (% frequency) of individual risk factors per age period per sex
3.2. Effect of total risk factors and sex on age‐related decline in cognition
The effects of age and risk factors were present on all cognitive tasks (Details are provided elsewhere 34 ). Likewise, sex differences in cognitive performance and age by sex differences in cognitive performance were found for all tasks, all p’s < 0.0001. However, effect sizes were minimal for all tasks except Face‐Name Association, R 2 = 0.01 (a small effect size 44 ).
Quadratic regression revealed that age, sex, total risk factors, and their interactions explained a significant amount of the variance in the associative recognition rate on the Face‐Name Association task, F(8, 21697) = 775.9, p < 0.0001, with a medium effect size, R2 = 0.22 (Table 1 and Figure 4). The complete model had main effects of age, sex, and total risk factors. Across participants, each year of age was associated with a drop in associative recognition rates (b = ‐0.9). Each additional risk factor was associated with a lowering in associative recognition rates equivalent to three and half years of aging (the main effect of total risk factors, b = ‐3.3, is over three and half times larger than the main effect of age, b = ‐0.9). Being male was also associated with lower associative recognition rates equivalent to 4 years of aging.
TABLE 1.
Regression estimates for age, sex, and total risk factors on the Face‐Name Association task
| B | SE | t | p | |
|---|---|---|---|---|
| Age | −0.88 | 0.02 | −60.0 | <0.0001*** |
| Age2 | −0.01 | 0.0005 | −25.9 | <0.0001*** |
| Total risk factors | −3.32 | 0.21 | −16.0 | <0.0001*** |
| Sex | 3.64 | 0.44 | 8.45 | <0.0001*** |
| Age × Sex | 0.09 | 0.02 | 4.49 | <0.0001*** |
| Age × Total risk factors | 0.06 | 0.01 | 4.64 | <0.0001*** |
| Sex × Total risk factors | 0.45 | 0.28 | 1.60 | 0.11 |
| Age × Sex × Total risk factors | −0.06 | 0.02 | −3.75 | 0.0002** |
*** p < 0.0001, ** p < 0.001, * p < 0.05.
FIGURE 4.
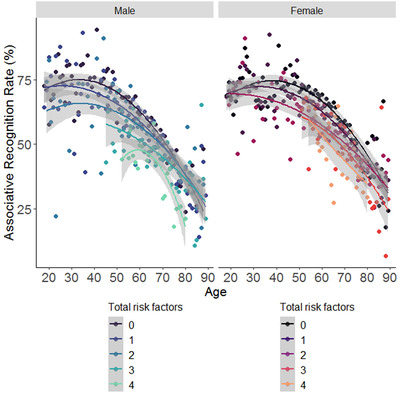
Dose‐response association of total risk factors and age per sex on a face‐name associative recognition task. Note. Each dot shows the mean performance per number of risk factors. The lines show fitted polynomial curves. The gray shading around the curves indicates a 95% confidence interval envelope. Smaller scores indicate worse performance on the Face‐Name Association task
Main effects were qualified by significant two‐way interactions of age and sex (worse performance in men as age increased), and of age and total risk factors (worse performance for each additional risk factor as age increased), although both of these were qualified by a three‐way interaction between age, sex, and total risk factors. Post hoc simple slopes analyses revealed a stronger negative effect of age on performance in men (β = ‐0.9, SE = 0.02) than in woman (β = ‐0.8, SE = 0.02), p < 0.0001, but these sex differences were moderated by the number of risk factors. Among those with no or one risk factor, men had a significantly stronger negative effect of age on performance compared to women (β = ‐0.6, SE = 0.03 for men, and β = ‐0.5, SE = 0.02 for women, p < 0.001; and β = ‐0.5, SE = 0.03 for men, and β = ‐0.9, SE = 0.03 for women, p < 0.001; respectively). By contrast, among those with two, three, or four modifiable risk factors, the negative effect of age on performance did not differ between men and women (β = ‐0.5, SE = 0.03 for men, and β = ‐0.5, SE = 0.03 for women, p > 0.05; β = ‐0.8, SE = 0.06 for men, and β = ‐1.0, SE = 0.06 for women, p > 0.05; and β = ‐1.0, SE = 0.1 for men, and β = ‐1.0, SE = 0.1 for women, p > 0.05; respectively). Thus, the cumulative effect of multiple modifiable risk factors on age‐related cognitive decline, compared to none or one, was significantly larger in women (∆β = 0.5, SE = 0.11) than men (∆β = 0.4, SE = 0.11), p < 0.0001. Similar results were found on the Face‐Name Association task for the adjusted risk amount, indicating that the relationship persists after controlling for shared variance among risk factors (Figure S1). Quadratic regression revealed that age (with linear and polynomial terms), sex, adjusted risk amount, and their interactions, explained a significant amount of the variance in the associative recognition rate on the Face‐Name Association task, F(8, 21755) = 660.6, p < 0.0001, with a medium effect size, R2 = 0.20 (Table 2).
TABLE 2.
Regression estimates for age, sex, and adjusted risk amount on the Face‐Name Association task
| B | SE | t | p | |
|---|---|---|---|---|
| Age | −0.93 | 0.02 | −44.1 | <0.0001*** |
| Age2 | −0.01 | 0.0006 | −21.5 | <0.0001*** |
| Adjusted risk amount | −0.24 | 1.56 | −15.1 | <0.0001*** |
| Sex | 3.60 | 0.47 | 7.60 | <0.0001*** |
| Age × Sex | 0.10 | 0.02 | 4.23 | <0.0001*** |
| Age × Adjusted risk amount | 0.58 | 0.11 | 5.32 | <0.0001*** |
| Sex × Adjusted risk amount | 7.27 | 2.07 | 3.52 | 0.0004** |
| Age × Sex × Adjusted risk amount | −0.33 | 0.14 | −2.39 | 0.017* |
***p < 0.0001, **p < 0.001, *p < 0.05.
4. DISCUSSION
Using an online adult lifespan sample, we found lower associative recognition in older ages, with larger cross‐sectional age differences in men than women (the equivalent of performing similar to someone 3.5 years older), and in people with more modifiable risk factors (the equivalent of performing similar to someone 4 years older). We also found a novel small synergistic association between combined modifiable risk factors and sex. Among those with no to one risk factor, men show larger age differences in associative memory than women, but among those with multiple risk factors, both men and women show the same age differences. Thus, although women have better memory than men across all ages, and a smaller difference in associative memory in older versus younger adults than men, the female memory advantage is not found in individuals with multiple modifiable risk factors. This finding was also evident after controlling for communality among risk factors.
The elimination of the female memory advantage is particularly striking, as a larger than average episodic memory decline is a sign of Alzheimer's disease, 12 and women are more likely than men to have Alzheimer's disease. 1 , 2 , 3 The higher rates of Alzheimer's disease in women were previously contradicted by the finding of less episodic memory decline in women than men. 6 , 7 , 8 , 9 , 10 , 11 , 25 We speculate that accounting for modifiable risk factors clarifies this contradiction. There is a greater decrease in recognition rates across age from no to many risk factors in women than men. The observed sex and modifiable risk factors interaction may be at least partially explained by shifts in hormonal levels with age (such as estrogen loss in menopause) possibly interacting with modifiable risk factors. 45 , 46
The interaction of sex with age was of a smaller magnitude than the interaction of modifiable risk factors with age, which is encouraging, because modifiable risk factors are changeable by definition, whereas sex is not. People with many risk factors show the same negative association between age and associative memory regardless of sex. Thus, modifiable risk factors must be studied in men and women alike, as both sexes have an increased risk for developing Alzheimer's disease as age increases. 47
We found sex differences across age for associative recognition, but not for interference control, set shifting, or spatial working memory. Our findings align with past observations of women outperforming men and showing less age‐related decline on episodic memory, but not other cognitive abilities. 13 , 14 Further work is needed to confirm our findings using several cognitive measures per ability.
Future work could provide insights into Alzheimer's disease prevention by examining if sex also has protective effects with measured modifiable risk factors. For example, although low education and excess alcohol consumption are risk factors for dementia, 18 high education and moderate alcohol consumption are more common in older adult women who perform above normal cognitive performance for their age. 9
A limitation of the current study is the use of cross‐sectional data. Cohort effects may impact the observed effects, as age differences are also confounded by different life experiences and exposure to risk factors between participants. 48 , 49 , 50 Cross‐sectional data can also overestimate cognitive decline in women but not men when compared to longitudinal data. 50 These sex differences have been attributed to possible cohort differences in risk factors such as education. 50 Accounting for these risk factors in our study may help control for some cohort effects. Indeed, our findings extend past results by showing that sex differences in memory depend on the presence of modifiable risk factors. In addition to cohort effects, aging effects from cross‐sectional lifespan studies are not always found in longitudinal lifespan comparison studies from ages 20 to 60, but effects after age 60 are similar for both approaches. 49 Thus, our findings are more likely to generalize for individuals older than age 60.
Using a self‐administered online assessment enabled the collection of a large sample of different ages. However, the sample is limited to people who had web literacy and access to a computer and Internet connection. On the other hand, online testing is more accessible than in‐person testing, and thus may have recruited lower functioning individuals who lack the capability to visit a testing location, as well as higher functioning individuals who are too busy to visit a testing location. 48 , 49 The sample was mostly North American (37% from Canada, 20% United States of America, 2% United Kingdom, 2% Other, and 39% not reported); hence results may not generalize outside of this continent.
In addition, a representative population sample is needed to confirm the sex differences in prevalence of individual modifiable risk factors. However, our findings align with published work on depression and late‐life hypertension being more common in women, and smoking and substance abuse being more common in men. 2 , 3 , 20 , 37 The different prevalence of factors in men versus women suggests that primary prevention messages could be tailored towards sex‐specific common factors.
The prevalence of modifiable risk factors is independent of their influence on cognitive decline. Men had more risk factors than women (a large effect size), but the association among risk factors, age, and memory was larger in women (a small effect size). Similarly, past work shows a factor could occur more frequently in men but have a larger impact in women (eg, midlife hypertension21), or a factor could occur with equal frequency in men and women but have a stronger effect in women (such as apolipoprotein E [APOE] genotype, or amyloid beta burden [Aβ]47).
A limitation was that modifiable risk factors were self‐reported, although the obtained prevalence estimates align with previously published work of low risk factor prevalence in healthy adults. 31 The sample had few risk factors, so it is notable that these findings are demonstrated even in a relatively healthy sample. The current correlational findings do not allow us to determine the direction of the effect between modifiable risk factors and cognition. Although a review of past longitudinal work has confirmed causal effects between combined risk factors and cognitive impacts, 29 similar work would be needed to draw causal conclusions regarding the present findings. Longitudinal studies also have limitations, such as attrition and practice effects; thus a combination of both approaches is ideal.
Modifiable risk factors in the current study were selected using an evidence‐based model of dementia risk factors. 18 Several other risk factors have been linked to dementia, such as diet and sleep disturbances, but these were not included in the model as they lack strong or consistent evidence. 18 The life‐course model also includes other contributing factors such as physical and social isolation, but data on these factors were not acquired in the current investigation.
Sex is a key variable in explaining the heterogeneity of Alzheimer`s disease and developing a precision medicine approach. 37 Because sex is not modifiable, considering how it interacts with modifiable risk factors will help target early prevention and treatment of Alzheimer's disease. Our findings align with existing recommendations to keep risk factors to a minimum, and avoid accumulation of new risk factors. 29 , 30 The observed novel synergistic association of sex differences and modifiable risk factors on age‐related decline in associative memory underscores the preventative importance of modifying lifestyle behaviors linked to dementia in both women and men.
DECLARATIONS OF INTEREST
None for all authors.
Supporting information
Supporting Information
ACKNOWLEDGMENT
This work was supported by a research grant from the Natural Sciences and Engineering Council (NSERC) of Canada (RGPIN‐2017‐06057) to NA; and an Alzheimer Society of Canada PDF(20–16) to AL.
LaPlume AA, McKetton L, Anderson ND, Troyer AK. Sex differences and modifiable dementia risk factors synergistically influence memory over the adult lifespan. Alzheimer's Dement. 2022;14:e12301. 10.1002/dad2.12301
REFERENCES
- 1. Alzheimer's Association . 2020 Alzheimer's disease facts and figures. Alzheimers Dement. 2020; 16(3): 391‐460. [Google Scholar]
- 2. Andrew MK, Tierney MC. The puzzle of sex, gender and Alzheimer's disease: why are women more often affected than men? Women's Health. 2018; 14:1745506518817995. 10.1177/1745506518817995 [DOI] [Google Scholar]
- 3. Podcasy JL, Epperson CN. Considering sex and gender in Alzheimer disease and other dementias. Dialogues Clin Neurosci. 2016; 18(4): 437‐446. 10.31887/DCNS.2016.18.4/cepperson [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fiest KM, Roberts JI, Maxwell CJ, et al. The prevalence and incidence of dementia due to Alzheimer's disease: a systematic review and meta‐analysis. Can J Neurol Sci. 2016; 43(S1): S51‐S82. [DOI] [PubMed] [Google Scholar]
- 5. Perera G, Pedersen L, Ansel D, et al. Dementia prevalence and incidence in a federation of European Electronic Health Record databases: the European Medical Informatics Framework resource. Alzheimers Dement. 2018; 14(2): 130‐139. 10.1016/j.jalz.2017.06.2270 [DOI] [PubMed] [Google Scholar]
- 6. Anstey KJ, Peters R, Mortby ME, et al. Association of sex differences in dementia risk factors with sex differences in memory decline in a population‐based cohort spanning 20‐76 years. Sci Rep. 2021; 11(1): 1‐10. 10.1038/s41598-021-86397-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bloomberg M, Dugravot A, Dumurgier J, et al. Sex differences and the role of education in cognitive ageing: analysis of two UK‐based prospective cohort studies. Lancet Public Health. 2021; 6(2): e106‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. MacAulay RK, Halpin A, Cohen AS, et al. Predictors of heterogeneity in cognitive function: APOE‐e4, sex, education, depression, and vascular risk. Arch Clin Neuropsychol. 2020. ; 35(6): 660‐670. 10.1093/arclin/acaa014 [DOI] [PubMed] [Google Scholar]
- 9. Maccora J, Peters R, Anstey KJ. Gender differences in superior‐memory SuperAgers and associated factors in an Australian cohort. J Appl Gerontol. 2021; 40(4): 433‐442. 10.1177/0733464820902943 [DOI] [PubMed] [Google Scholar]
- 10. McCarrey AC, An Y, Kitner‐Triolo MH, Ferrucci L, Resnick SM. Sex differences in cognitive trajectories in clinically normal older adults. Psychol Aging. 2016;31(2): 166‐175. 10.1037/pag0000070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Olaya B, Bobak M, Haro JM, Demakakos P. Trajectories of verbal episodic memory in middle‐aged and older adults: evidence from the English Longitudinal Study of Ageing. J Am Geriatr Soc. 2017; 65(6): 1274‐1281. 10.1111/jgs.14789 [DOI] [PubMed] [Google Scholar]
- 12. Gallagher M, Koh MT. Episodic memory on the path to Alzheimer's disease. Curr Opin Neurobiol. 2011; 21(6): 929‐934. 10.1016/j.conb.2011.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ferreira L, Ferreira Santos‐Galduróz R, Ferri CP, Fernandes Galduroz JC. Rate of cognitive decline in relation to sex after 60 years‐of‐age: a systematic review. Geriatr Gerontol Int. 2014; 14(1): 23‐31. 10.1111/ggi.12093 [DOI] [PubMed] [Google Scholar]
- 14. Li R, Singh M. Sex differences in cognitive impairment and Alzheimer's disease. Front Neuroendocrinol. 2014; 35(3): 385‐403. 10.1016/j.yfrne.2014.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sundermann EE, Biegon A, Rubin LH, Lipton RB, Landau S, Maki PM. Does the female advantage in verbal memory contribute to underestimating Alzheimer's disease pathology in women versus men? J Alzheimer's Dis. 2017;56(3):947‐957. 10.3233/JAD-160716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tensil M, Hessler JB, Gutsmiedl M, Riedl L, Grimmer T, Diehl‐Schmid J. Sex differences in neuropsychological test performance in Alzheimer's disease and the influence of the ApoE genotype. Alzheimer Dis Assoc Disord. 2018; 32(2):145‐149. 10.1097/WAD.0000000000000229 [DOI] [PubMed] [Google Scholar]
- 17. Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017; 390(10113): 2673‐2734. 10.1016/S0140-6736(17)31363-6 [DOI] [PubMed] [Google Scholar]
- 18. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020; 396(10248): 413‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Reifegerste J, Veríssimo J, Rugg MD, et al. Early‐life education may help bolster declarative memory in old age, especially for women. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2021; 28(2): 218‐252. 10.1080/13825585.2020.1736497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Blanken AE, Nation DA. Does gender influence the relationship between high blood pressure and dementia? Highlighting areas for further investigation. J Alzheimer's Dis. 2020; 78(1): 23‐48. 10.3233/JAD-200245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gilsanz P, Mayeda ER, Glymour MM, et al. Female sex, early‐onset hypertension, and risk of dementia. Neurology. 2017; 89(18): 1886‐1893. 10.1212/WNL.0000000000004602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gong J, Harris K, Peters SA, Woodward M. Sex differences in the association between major cardiovascular risk factors in midlife and dementia: a cohort study using data from the UK Biobank. BMC Med. 2021; 19(1): 1‐11. 10.1186/s12916-021-01980- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chireh B, D'Arcy C. A comparison of the prevalence of and modifiable risk factors for cognitive impairment among community‐dwelling Canadian seniors over two decades, 1991‐2009. PLoS One. 2020; 15(12): e0242911. 10.1371/journal.pone.0242911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lipnicki DM, Sachdev PS, Crawford J, et al. Risk factors for late‐life cognitive decline and variation with age and sex in the Sydney Memory and Ageing Study. PLoS One. 2013; 8(6): e65841. 10.1371/journal.pone.0065841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van Zutphen EM, Rijnhart JJ, Rhebergen D, et al. Do cardiovascular risk factors and cardiovascular disease explain sex differences in cognitive functioning in old age? J Alzheimer's Dis. 2021; 80(4): 1643‐1655. 10.3233/JAD-201173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Arntzen KA, Schirmer H, Wilsgaard T, Mathiesen EB. Impact of cardiovascular risk factors on cognitive function: the Tromsø study. Eur J Neurol. 2011;18(5):737‐43. 10.1111/j.1468-1331.2010.03263.x [DOI] [PubMed] [Google Scholar]
- 27. Gong J, Harris K, Peters SA, Woodward M. Sex differences in the association between major cardiovascular risk factors in midlife and dementia: a cohort study using data from the UK Biobank. BMC Med. 2021; 19(1): 1‐11. 10.1186/s12916-021-01980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim S, Kim MJ, Kim S, et al. Gender differences in risk factors for transition from mild cognitive impairment to Alzheimer's disease: a CREDOS study. Compr Psychiatry. 2015; 62: 114‐122. 10.1016/j.comppsych.2015.07.002 [DOI] [PubMed] [Google Scholar]
- 29. Peters R, Booth A, Rockwood K, Peters J, D'Este C, Anstey KJ. Combining modifiable risk factors and risk of dementia: a systematic review and meta‐analysis. BMJ Open. 2019; 9(1): e022846. 10.1136/bmjopen-2018-022846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Song X, Mitnitski A, Rockwood K. Age‐related deficit accumulation and the risk of late‐life dementia. Alz Res Therapy. 2014; 6: 54. 10.1186/s13195-014-0054-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Adams ML, Grandpre J. Dose‐response gradients between a composite measure of six risk factors and cognitive decline and cardiovascular disease. Prev Med. 2016; 91: 329‐34. 10.1016/j.ypmed.2016.09.004 [DOI] [PubMed] [Google Scholar]
- 32. LaPlume AA, McKetton L, Levine B, Anderson ND, Troyer AKD. The adverse effect of modifiable dementia risk factors on cognition amplify across the adult lifespan. In review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Olaya B, Moneta MV, Bobak M, Haro JM, Demakakos P. Cardiovascular risk factors and memory decline in middle‐aged and older adults: the English Longitudinal Study of Ageing. BMC Geriatr. 2019; 19(1): 337. 10.1186/s12877-019-1350-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. LaPlume AA, Anderson ND, McKetton L, Levine B, Troyer AK. When I'm 64: age‐related variability in over 40,000 online cognitive test takers. J Gerontol B Psychol Sci Soc Sci. gbab143. 2021. 10.1093/geronb/gbab143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Troyer AK, Rowe G, Murphy KJ, Levine B, Leach L, Hasher L. Development and evaluation of a self‐administered on‐line test of memory and attention for middle‐aged and older adults. Front Aging Neurosci. 2014; 6: 335. 10.3389/fnagi.2014.00335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Paterson TS, Sivajohan B, Gardner S, et al. Accuracy of a self‐administered online cognitive assessment in detecting amnestic mild cognitive impairment. J Gerontol B Psychol Sci Soc Sci. gbab097. Advance online publication. 10.1093/geronb/gbab097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ferretti MT, Iulita MF, Cavedo E, et al. Sex differences in Alzheimer disease—the gateway to precision medicine. Nat Rev Neurol. 2018; 14(8): 457‐469. 10.1038/s41582-018-0032-9 [DOI] [PubMed] [Google Scholar]
- 38. Kim MY, Kim K, Hong CH, Lee SY, Jung YS. Sex differences in cardiovascular risk factors for dementia. Biomol Ther. 2018; 26(6): 521‐532 10.4062/biomolther.2018.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Grange JA. trimr: an implementation of common response time trimming methods. R package version 1.0.1. 2015. https://CRAN.R‐project.org/package=trimr
- 40. Buchanan EM, Scofield JE. Methods to detect low quality data and its implication for psychological research. Behav Res Methods. 2018; 50(6): 2586‐2596. 10.3758/s13428-018-1035-6 [DOI] [PubMed] [Google Scholar]
- 41. Jacoby LL. A process dissociation framework: separating automatic from intentional uses of memory. J Mem Lang. 1991; 30(5): 513‐541. [Google Scholar]
- 42. Troyer AK, Murphy KJ, Anderson ND, et al. Associative recognition in mild cognitive impairment: relationship to hippocampal volume and apolipoprotein E. Neuropsychologia. 2012. ; 50(14): 3721‐3728. 10.1016/j.neuropsychologia.2012.10.018 [DOI] [PubMed] [Google Scholar]
- 43. Cohen J. Statistical Power Analysis for the Behavioral Sciences. Lawrence Erlbaum; 1977. [Google Scholar]
- 44. Gravetter FJ, Wallnau LB, Wardsworth T. Essentials of Statistics for the Behavioral Sciences (8th US Edition). Wadsworth CENGAGE Learning. 2009. [Google Scholar]
- 45. Georgakis MK, Kalogirou EI, Diamantaras AA, et al. Age at menopause and duration of reproductive period in association with dementia and cognitive function: a systematic review and meta‐analysis. Psychoneuroendocrinology. 2016; 73: 224‐243. 10.1016/j.psyneuen.2016.08.003 [DOI] [PubMed] [Google Scholar]
- 46. Gurvich C, Hoy K, Thomas N, Kulkarni J. Sex differences and the influence of sex hormones on cognition through adulthood and the aging process. Brain Sci. 2018; 8(9): 163 10.3390/brainsci8090163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mielke MM. Sex and gender differences in Alzheimer's disease dementia. Psychiatr Times. 2018; 35(11): 14‐17. [PMC free article] [PubMed] [Google Scholar]
- 48. Harada CN, Love MC, Triebel KL. Normal cognitive aging. Clin Geriatr. 2013;29(4):737‐752. 10.1016/j.cger.2013.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hedden T, Gabrieli JD. Insights into the ageing mind: a view from cognitive neuroscience. Nat Rev Neurosci. 2004; 5(2):87‐96. 10.1038/nrn1323 [DOI] [PubMed] [Google Scholar]
- 50. Singh‐Manoux A, Kivimaki M, Glymour MM, et al. Timing of onset of cognitive decline: results from Whitehall II prospective cohort study. BMJ. 2012;344. 10.1136/bmj.d7622 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


