Abstract
Introduction
Despite women showing greater Alzheimer's disease (AD) prevalence, tau burden, and immune/neuroinflammatory response, whether neuroinflammation impacts cognition differently in women versus men and the biological basis of this impact remain unknown. We examined sex differences in how cerebrospinal fluid (CSF) neuroinflammation relates to cognition across the aging–mild cognitive impairment (MCI)–AD continuum and the mediating role of phosphorylated tau (p‐tau) versus other AD biomarkers.
Methods
Participants included 284 individuals from the Alzheimer's Disease Neuroimaging Initiative study. CSF neuroinflammatory markers included interleukin‐6, tumor necrosis factor α, soluble tumor necrosis factor receptor 2 (sTNFR2), and chitinase‐3‐like protein 1. AD biomarkers were CSF p‐tau181 and amyloid beta1‐42 levels and magnetic resonance imaging measures of hippocampal and white matter hyperintensity volumes.
Results
We found a sex‐by‐sTNFR2 interaction on Mini‐Mental State Examination and Clinical Dementia Rating‐Sum of Boxes. Higher levels of sTNFR2 related to poorer cognition in women only. Among biomarkers, only p‐tau181 eliminated the female‐specific relationships between neuroinflammation and cognition.
Discussion
Women may be more susceptible than men to the adverse effects of sTNFR2 on cognition with a potential etiological link with tau to these effects.
Keywords: aging, Alzheimer's disease, magnetic resonance imaging, neuroinflammation, phosphorylated tau, sex differences
1. BACKGROUND
Neuroinflammation plays a central role in Alzheimer's disease (AD) pathological processes including amyloid beta (Aß) and phosphorylated tau (p‐tau) aggregation, neurodegeneration, and also to cerebrovascular pathology, including small vessel disease. 1 While a healthy proinflammatory response is important for clearing pathogens and mitigating tissue damage, more chronic levels of neuroinflammation that occur in AD can contribute to AD pathogeneses. 2 Increased neuroinflammation occurs in the early stages of the AD trajectory. 3 There is evidence to suggest that individuals with either mild cognitive impairment (MCI) or AD dementia have higher cerebrospinal fluid (CSF) and plasma levels of certain proinflammatory proteins compared to healthy controls. 4 Specifically, CSF levels of chitinase‐3‐like protein 1 (YKL‐40), 5 tumor necrosis factor α (TNFα), 6 soluble tumor necrosis factor receptor 2 (sTNFR2), 7 and interleukin‐6 (IL‐6) 8 are increased in individuals with MCI and AD. Furthermore, elevated levels of these CSF neuroinflammatory marker levels relate to higher levels of CSF p‐tau in older adults across the AD trajectory, 5 , 9 , 10 can discriminate individuals with AD from those with other types of dementia, 11 and predict conversion from MCI to dementia. 5 , 7 Higher CSF or plasma levels of neuroinflammatory markers, such as IL‐6, 12 TNFα, 13 and YKL‐40, 5 are also associated with worse cognition in healthy older adults and predict future cognitive decline. 5 , 14
There are important sex differences in both AD 15 , 16 , 17 , 18 and in immune function. 19 , 20 , 21 Women typically have enhanced immunoreactivity compared to men. 22 Levels of plasma immune‐regulating cytokines have been reported to be higher in women than in men. 23 Relatedly, the prevalence of many autoimmune diseases is considerably higher among women, with women accounting for 80% of cases. 19 , 21 Sex differences in the neuroinflammatory response may be particularly relevant among older adults given that menopause potentiates low‐grade, chronic inflammation as evidenced by increases in levels of cytokines such as IL‐6 after menopause. 24 Women also have a higher prevalence of AD compared to men 25 and several studies suggest that women may demonstrate a more rapid cognitive decline than men after MCI diagnosis. 26 Despite these sex differences in neuroimmune response 20 , 27 and clinical trajectory, 15 , 26 , 28 it is unknown whether neuroinflammation impacts cognition differently in women versus men on the AD trajectory.
Neuroinflammation is tightly linked with multiple AD pathologic processes including Aβ and p‐tau deposition, neurodegeneration, and vascular mechanisms. 1 , 29 , 30 Thus, it is unclear whether the association between neuroinflammation and cognition is driven by a particular AD pathological process and how this mediation may differ by sex. Among biomarkers of AD pathologies, p‐tau is more strongly tied to clinical profile. 31 Given that p‐tau strongly relates to both neuroinflammation 30 and cognitive symptoms 32 in AD, 33 a mediating role of p‐tau in the relationship between neuroinflammation and cognition is plausible, although not yet tested to our knowledge. There is also growing evidence of greater p‐tau burden in women versus men in earlier disease stages, 18 , 34 , 35 , 36 which further supports the need to investigate associations among neuroinflammation, tau, and cognitive function by sex. Importantly, the relationship between p‐tau burden and cognition is sex‐specific, with evidence suggesting that women can better maintain cognitive functioning in the face of p‐tau pathology compared to men. 17
We extend previous findings of associations between neuroinflammation and cognitive outcomes in AD by examining how these associations differ by sex and whether p‐tau mediates these associations. In a secondary analysis, we examined the specificity of the mediating role of p‐tau by comparing other AD biomarkers that are also associated with neuroinflammation. To do so, we used data from the Alzheimer's Disease Neuroimaging Initiative (ADNI) to examine sex differences in the relationship between selected CSF proinflammatory markers (IL‐6, TNFα, sTNFR2, and YKL‐40) and measures of global cognitive status across AD stages and determine the mediating role of AD biomarkers in these relationships. We hypothesized that higher levels of neuroinflammatory markers would relate to poorer cognitive function, with a stronger relationship observed in women versus men, and that these relationships would be mediated more strongly by pathological tau than other AD biomarkers.
2. METHODS
2.1. Participants and data source
Baseline data were extracted from ADNI, a publicly accessible dataset available at adni.loni.usc.edu. ADNI is a longitudinal, multi‐site, cohort study that began in 2003 as a public–private partnership with the goal of integrating neuroimaging, neuropsychological, clinical, and biomarker information to identify early indicators of AD and to measure disease progression. General enrollment inclusion and exclusion criteria for ADNI are described elsewhere. 37 All participants provided informed consent. ADNI data and further information are available at adni.loni.usc.edu. This specific study was limited to participants with baseline data for (1) at least one of our examined CSF inflammatory markers, (2) neuropsychological test battery, and (3) at least one of our AD biomarkers (hippocampal volume, white matter hyperintensities [WMH], CSF‐Aβ1‐42,, or CSF p‐tau181 levels). Sample size varied by neuroinflammatory marker whereby 284 participants (40% female) were included in analyses involving the IL‐6, TNFα, and sTNFR2 markers and 141 were included in analyses involving YKL‐40 (33% female). All participants were aged 55 to 90 years and were either cognitively normal (29%), MCI (45%), or AD dementia (26%). Hippocampal volume data were missing for 57 participants and WMH data were missing for three participants; therefore, these participants were excluded from analyses examining mediating role of hippocampal volume and WMH.
2.2. Measures
2.2.1. Neuroinflammatory markers
We examined four neuroinflammatory markers—YKL‐40, IL‐6, TNFα, and sTNFR2—with proinflammatory properties that were available in CSF in ADNI and have previously shown relationships with cognition 12 , 14 , 38 and cognitive decline in individuals on the AD continuum. 39 To maintain consistency in data collection and processing across all study sites, a standardized protocol for CSF collection and assaying was used. YKL‐40 was quantified by MicroVue YKL‐40 enzyme‐linked immunosorbent assay (ELISA) assay (Quidel Corp.). 40 , 41 The lower limit of detection was 20 μg/L. 42 Commercially available multiplex immunoassays (Millipore Sigma) which were modified for CSF analyte levels were used to measure IL‐6 (pg/mL), TNFα (pg/mL), and sTNFR2 (ng/mL) in banked CSF samples from ADNI. 43 The lower limit of detection for IL‐6 was 16.25 pg/mL. The lower limit of detection for TNFα was 49.20 pg/mL. The lower limit of detection for sTNFR2 was 16.39 ng/mL.
2.2.2. Cognitive outcomes
Our cognitive outcomes were two measures sensitive to decline in this population: the Mini‐Mental State Examination (MMSE) 44 and the Clinical Dementia Rating‐Sum of Boxes (CDR‐SOB). 45 The MMSE is an assessment of global cognitive function, whereby higher scores (score range = 0–30) reflect better cognitive function. 46 The CDR‐SOB is an assessment of dementia severity whereby higher scores (score range = 0–18) reflect greater dementia severity. 47 For parsimony, we focused on standard tests of global cognitive status that are commonly used in MCI and AD diagnostic criteria.
2.2.3. AD biomarkers
Our AD biomarkers included markers of AD‐specific pathology (CSF levels of Aβ1‐42 and p‐tau181 levels), neurodegeneration (hippocampal volume), and small‐vessel vascular disease (WMH volume). CSF‐Aβ1‐42 and p‐tau181 levels were measured by the Roche Elecsys cobas e 601 fully automated immunoassay. The lower technical limit to upper technical limit for the Elecsys b‐Amyloid (1‐42) CSF immunoassay was 200 to 1700 pg/mL. The lower technical limit to upper technical limit for the Elecsys Phospho‐Tau (181P) CSF immunoassay was 8 to 120 pg/mL. Lower levels of Aβ1‐42 indicate greater amyloid plaque pathology and higher p‐tau181 levels indicate greater tau pathology. Structural magnetic resonance imaging (MRI) scans were collected on a 1.5T scanner according to a standardized protocol. 48 Hippocampal volume data were analyzed using FreeSurfer version 4.3 (https://surfer.nmr.mgh.harvard.edu) at the University of California–San Francisco 49 (http://adni.loni.ucla.edu/wp‐content/uploads/2010/12/UCSF‐FreeSurfer‐Overview‐and‐QC_‐Template_Format.pdf). WMH volumes were derived from fluid‐attenuated inversion recovery MRI images.
RESEARCH IN CONTEXT
Systematic review: Women have a higher prevalence of Alzheimer's disease (AD), greater phosphorylated tau burden in the mild cognitive impairment stage, and a steeper clinical decline versus men. Women also show a more robust immune response compared to men, and neuroinflammation is implicated in the pathogenesis of AD. Examining sex differences in the link between neuroinflammation and cognition in individuals on the AD continuum and whether tau serves as a mediator of this relationship might provide a deeper understanding of sex differences in the pathogenesis and clinical trajectory of AD.
Interpretation: Findings suggest that women are more vulnerable to the deleterious effects of neuroinflammation through a tau mechanism.
Future directions: Women on the AD continuum may benefit more than men from treatments targeting neuroinflammation. Future studies should aim to replicate female‐specific links between neuroinflammation and cognition in AD and test the potential efficacy of tailored treatments.
We adjusted hippocampal (HV/IV) and WMH (WMH/IV) volumes for intracranial volume by dividing each by intracranial volume x 103.
2.2.4. Covariates
Considered covariates included age, years of education, and apolipoprotein E (APOE) ε4 carrier status.
2.3. Statistical analyses
IL‐6, TNFα, and p‐tau181 levels were log transformed to normalize their non‐normal distributions as determined by the Shapiro‐Wilk test. Two outliers for sTNFR2 and three outliers for TNFα were identified using the explore procedure in SPSS and removed from analyses. MMSE and CDR‐SOB were cube‐root transformed to normalize their non‐normal distributions as determined by the qqPlot function in R using the “car” package. 50 Sex differences in sample characteristics were assessed using independent t‐tests for continuous variables and Chi‐square tests for categorical variables. We conducted a series of cross‐sectional analyses using linear regression to examine the relationship between individual neuroinflammatory markers and cognitive outcomes. We examined sex differences in these relationships by examining a sex by neuroinflammatory marker interaction. When the interaction term was significant, we probed the interaction by comparing effect sizes in sex‐stratified analyses. If the interaction was nonsignificant (P > .05), it was removed from the model to assess main effects of neuroinflammatory markers. All analyses adjusted for covariates of age, education, and APOE ε4 positivity. When the association between a neuroinflammatory marker and a cognitive outcome was significant, we examined CSF p‐tau181 level as a potential mediator. To do so, we used a structural equation modeling (SEM) approach to perform a mediation analysis with bootstrapping techniques using the lavaan package 51 in R. This allowed us to generate 5000 bootstrap samples to generate a 95% bias‐corrected confidence interval of the indirect effect (a × b). In our mediation analysis, the a path represented the path from neuroinflammatory marker level to CSF p‐tau181 level, and the b path represented the impact of the mediator, CSF p‐tau181 level, on cognition. The product of a × b represents the indirect effect. This approach does not assume normally distributed indirect effects, and for this and other reasons it is more powerful and more accurate in testing mediation than the commonly used Sobel test and causal steps approach. 52 Importantly, this mediational analysis cannot confirm causality because of the cross‐sectional nature of the data. 53
In secondary analyses, we examined the mediating role of other AD biomarkers (Aβ1‐42, HV/IV, and WMH/IV) to test the specificity of a potential mediating role of tau in these relationships. Analyses were performed using R version 3.5.0 (https://cran.r‐project.org/) and SPSS 26 (SPSS Inc.). Significance was defined as α = .05 (two‐sided).
3. RESULTS
See Table 1 for sample characteristics. In the largest sample of 284 participants (those with IL‐6, TNFα, and sTNFR2 levels), women were significantly younger, less educated, had larger HV/IV, and had lower levels of IL‐6, TNFα, and sTNFR2 relative to men.
TABLE 1.
Sample characteristics by sex
| Women (n = 115) | Men (n = 169) | P Value | |
|---|---|---|---|
| Age (years), M (SD) | 73.8 (7.3) | 75.8 (7.2) | 0.022 |
| Education (years), M (SD) | 14.7 (2.8) | 16.2 (3.0) | <0.001 |
| White, no. (%) | 108 (93.9) | 162 (95.9) | 0.643 |
| APOE ε4 carrier, No. (%) | 57 (49.6) | 84 (49.7) | 0.999 |
| Clinical diagnosis, no. (%) | 0.354 | ||
| Cognitively normal | 35 (30.4) | 46 (27.2) | |
| MCI | 46 (40.0) | 82 (48.5) | |
| AD dementia | 34 (29.6) | 41 (24.3) | |
| IL‐6 (pg/mL), M (SD) | 1.3 (0.6) | 1.5 (0.6) | 0.008 |
| TNFα (pg/mL), M (SD) | 0.4 (0.4) | 0.6 (0.4) | 0.005 |
| sTNFR2 (ng/mL), M (SD) | 970.4 (243.3) | 1133.0 (468.9) | <0.001 |
| YKL‐40 (ng/mL), M (SD) a | 386.6 (144.4) | 420.0 (131.0) | 0.253 |
| CSF p‐tau181 (ng/l), M (SD) | 308.9 (123.3) | 294.4 (117.2) | 0.324 |
| HV/IV, M (SD) b | 4.4 (0.8) | 4.1 (0.7) | 0.004 |
| CSF‐Aβ1‐42 (ng/l), M (SD) | 953.9 (586.9) | 945.3 (546.6) | 0.896 |
| WMH/IV, M (SD) c | 5.5e‐10 (1.3e‐9) | 5.6e‐10 (1.5e‐10) | 0.917 |
| CDR‐SOB, M (SD) | 1.9 (1.9) | 1.8 (1.9) | 0.627 |
| MMSE, M (SD) | 26.6 (2.7) | 26.7 (2.6) | 0.794 |
Abbreviations: Aβ1‐42 = amyloid beta; AD, Alzheimer's disease; APOE, apolipoprotein E; CDR‐SOB, Clinical Dementia Rating–Sum of Boxes; HV/IV, hippocampal volume adjusted for intracranial volume; IL‐6, interleukin‐6; MCI, mild cognitive impairment; MMSE, Mini Mental State Examination; p‐tau181, phosphorylated tau; SD, standard deviation; sTNFR2, tumor necrosis factor receptor 2; TNFα, tumor necrosis factor α; WMH/IV, white matter hyperintensity volume adjusted for intracranial volume; YKL‐40, chitinase‐3‐like protein 1.
Note. Table displays raw means, standard deviations, and percentages. Sample characteristics by sex were assessed using independent t‐tests for continuous variables and Chi‐square tests for categorical variables.
Sample size for YKL‐40 was 141.
HV/IV was calculated by dividing total hippocampal volume by total intracranial volume x 103.
WMH/IV was calculated by dividing total hippocampal volume by total intracranial volume x 103.
3.1. Sex by inflammatory marker interactions on MMSE
Sex significantly interacted with sTNFR2 on MMSE performance (Table 2). In line with hypotheses, sex‐stratified analyses revealed that higher sTNFR2 levels related to poorer MMSE in women but not in men (Figure 1). The main effects of IL‐6, TNFα, and YKL‐40 levels on MMSE were not significant.
TABLE 2.
Results of multivariable linear regression modeling sex by neuroinflammatory marker on global cognition
| Main effects of sex and neuroinflammatory marker level | Sex x neuroinflammatory marker interaction | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcome/neuro‐inflammatory marker | Sex (male vs. female) | Neuroinflammatory marker level | |||||||
| B, β | SE | P Value | B, β | SE | P Value | B, β | SE | P Value | |
| MMSE | |||||||||
| IL‐6 | ‐0.004, ‐0.020 | 0.012 | .736 | 0.002, 0.010 | 0.020 | .861 | –0.019, –0.168 | 0.020 | .323 |
| TNFα | ‐7.304e‐4, ‐0.004 | 0.001 | .953 | –0.004, –0.116 | 0.002 | .059 | 0.056, 0.192 | 0.036 | .122 |
| sTNFR2 | ‐0.028, ‐1.241 | 0.011 | .010 | –2.196e‐04, ‐0.471 | 6.153e‐5 | .009 | 2.327e‐04, 1.406 | 8.407e‐05 | .006 |
| YKL‐40 | 0.002, 0.100 | 0.002 | .314 | –1.325e‐04, ‐0.206 | 6.084e‐05 | .032 | 1.431e‐04, 0.373 | 1.201e‐04 | .237 |
| CDR‐SOB | |||||||||
| IL‐6 | 0.006, 0.004 | 0.077 | .941 | 0.022, 0.020 | 0.061 | .723 | 0.085, 0.116 | 0.123 | .492 |
| TNFα | 0.014, 0.011 | 0.077 | .857 | 0.091, 0.047 | 0.118 | .438 | 0.183, –0.099 | 0.227 | .421 |
| sTNFR2 | 1.470, 1.097 | 0.627 | .021 | 0.001, 0.431 | 4.260e‐4 | .005 | –0.001, –1.201 | 4.993e‐4 | .018 |
| YKL‐40 | 0.001, 0.308 | 6.785e‐4 | .051 | 0.397, 0.293 | 0.375 | .292 | –0.001, –0.438 | 8.785e‐4 | .175 |
Abbreviations: CDR‐SOB, Clinical Dementia Rating–Sum of Boxes; IL‐6, interleukin‐6; MCI, mild cognitive impairment; MMSE, Mini Mental State Examination; SE, standard error; sTNFR2, tumor necrosis factor receptor 2; TNFα, tumor necrosis factor α; YKL‐40, chitinase‐3‐like protein 1.
FIGURE 1.
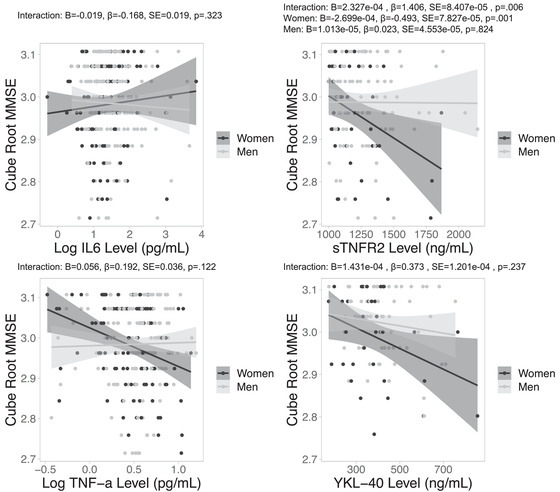
Sex‐specific relationships between neuroinflammatory marker levels and MMSE. Note. Negative associations between CSF neuroinflammatory marker level sTNFR2, but not IL‐6, TNFα, or YKL‐40, and MMSE were significant in women, but not in men. CSF, cerebrospinal fluid; IL‐6, interlukein‐6; MMSE, Mini Mental State Examination; sTNFR2, tumor necrosis factor receptor 2; TNFα, tumor necrosis factor α; YKL‐40, chitinase‐3‐like protein 1
3.2. Sex by inflammatory marker interactions on CDR‐SOB
There were significant sex by YKL‐40 and sTNFR2 interactions on CDR‐SOB. Sex‐stratified analyses revealed that higher YKL‐40 and sTNFR2 levels related to higher CDR‐SOB in women but not men (Figure 2). The main effects of IL‐6, TNFα, and YKL‐40 levels on CDR‐SOB were not significant in the total sample.
FIGURE 2.
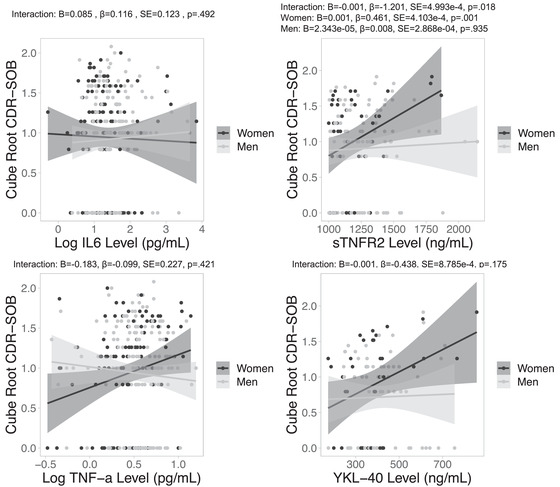
Sex‐specific relationships between neuroinflammatory marker levels and CDR‐SOB. Note. Positive associations between CSF neuroinflammatory marker levels sTNFR2, but not IL‐6, TNFα, or YKL‐40 and CDR‐SOB were significant in women, but not in men. CDR‐SOB, Clinical Dementia Rating–Sum of Boxes; CSF, cerebrospinal fluid; IL‐6, interlukein‐6; sTNFR2, tumor necrosis factor receptor 2; TNFα, tumor necrosis factor α; YKL‐40, chitinase‐3‐like protein 1
3.3. Mediating role of AD biomarkers in the neuroinflammation and cognition relationships
We examined the mediating role of CSF p‐tau181 in the significant, female‐specific relationships between neuroinflammatory markers and cognition. In an SEM model examining the mediating role of CSF p‐tau181 in the relationship between sTNFR2 level and MMSE among women, we found a significant indirect effect of sTNFR2 through CSF p‐tau181 on MMSE, and a nonsignificant effect of sTNFR2 on MMSE taking into account CSF p‐tau181, suggesting a full mediating effect of tau in the female‐specific relationships between sTFNR2 level and MMSE (see Figure 3).
FIGURE 3.
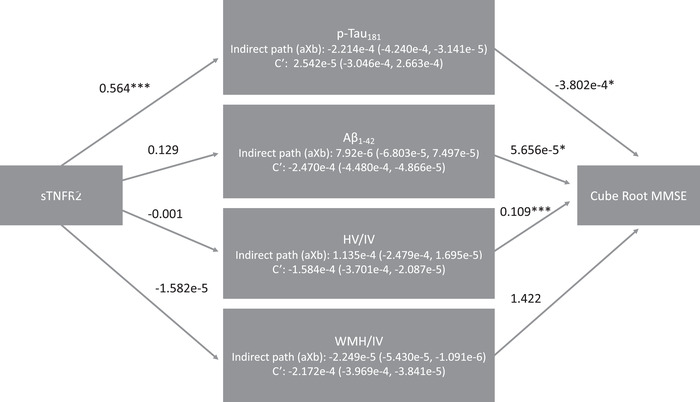
Mediators of the sTNFR2 level link with MMSE among women. Note. Mediation of relationship between sTNRF2 level and cubed root MMSE score through p‐tau. Unstandardized coefficients are reported for each path. Path c′ is the effect of sTNRF2 level and cubed root MMSE score while accounting for the mediator. A significant aXb estimate suggests mediation, denoted by 0 falling outside the CI. The c’ path accounts for each hypothesized mediator. * denotes significance P < .05. ** denotes significance P < .01. *** denotes significance P < .001. CI, confidence interval; CSF, cerebrospinal fluid; IL‐6, interlukein‐6; MMSE, Mini Mental State Examination; p‐tau, phosphorylated tau; sTNFR2, tumor necrosis factor receptor 2; TNFα, tumor necrosis factor α; YKL‐40, chitinase‐3‐like protein 1
In an SEM model examining the mediating role of role of CSF p‐tau181 in the relationship between sTNFR2 level and CDR‐SOB among women, we found a significant indirect effect of sTNFR2 through CSF p‐tau181 on CDR‐SOB, and a nonsignificant effect of sTNFR2 on MMSE taking into account CSF p‐tau181, suggesting a full mediating effect of tau in the female‐specific relationships between sTFNR2 level and CDR‐SOB (see Figure 4).
FIGURE 4.
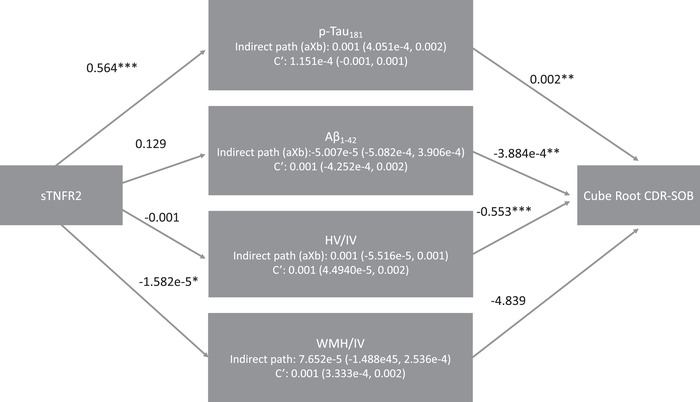
Mediators of the sTNFR2 level link with CDR‐SOB among women. Note. Mediation of relationship between sTNRF2 level and cubed root CDR‐SOB score through p‐tau. Unstandardized coefficients are reported for each path. Path c′ is the effect of sTNRF2 level and cubed root CDR‐SOB score while accounting for the mediator. A significant aXb estimate suggests mediation, denoted by 0 falling outside the CI. The c’ path accounts for each hypothesized mediator. * denotes significance P < .05. ** denotes significance P < .01. *** denotes significance P < .001. CI, confidence interval; CDR‐SOB, Clinical Dementia Rating–Sum of Boxes; CSF, cerebrospinal fluid; IL‐6, interlukein‐6; p‐tau, phosphorylated tau; sTNFR2, tumor necrosis factor receptor 2; TNFα, tumor necrosis factor α; YKL‐40, chitinase‐3‐like protein 1
Taken together, results show a significant direct effect of sTNFR2 on both cognitive measures absent CSF p‐tau181, with a significant indirect effect of sTNFR2 on both cognitive measures through CSF p‐tau181, and a nonsignificant effect of sTNFR2 on both cognitive measures taking into account CSF p‐tau181. Per conventions established by Baron and Kenny, 54 results of these two mediation analyses indicate a complete mediation of the female‐specific relationships between sTFNR2 level and cognition by p‐tau181.
In secondary analyses, we examined the mediating role of other AD biomarkers in the significant, female‐specific relationships between neuroinflammatory markers and cognition. Across all models, the indirect effects of sTNFR2 through HV/IV, WMH/IV, or CSF‐Aβ1‐42 on either MMSE and CDR‐SOB were not significant, suggesting an absence of a mediating role of these AD biomarkers (HV/IV, WMH/IV, or CSF‐Aβ1‐42).
4. DISCUSSION
We investigated sex differences in the relationship between levels of CSF neuroinflammatory markers and cognition, and the mediating role of CSF p‐tau181 versus other AD biomarkers. In partial support of our hypotheses that neuroinflammatory levels would inversely relate to cognition more strongly in women versus men; we found that these relationships were actually female‐specific. Specifically, we found that higher sTNFR2 related to worse MMSE performance and worse CDR‐SOB performance in women but not men.
As hypothesized, the indirect relationship of sTNFR2 levels to cognition through the mediator of p‐tau181 in women was significant, suggesting that p‐tau serves as a critical etiological link between neuroinflammation and cognition in women on the AD spectrum. The potential mediating role of p‐tau stands to reason given that p‐tau has demonstrated strong associations with both neuroinflammation 30 and cognitive performance. 31 , 32 The relationship between neuroinflammation and tau pathology appears to be bidirectional 1 , 30 , 55 whereby proinflammatory proteins, such as IL‐6, YKL‐40, and TNFα, enhance tau aggregation and neuronal loss, and, in turn, tau aggregation stimulates microglia activation and release of proinflammatory cytokines. 30 Our findings indicate the importance of future investigations into whether interventions aimed at reducing neuroinflammation may have more beneficial effects on tau burden and, in turn, cognition in women.
As a secondary analysis, we examined the potential mediating role of other AD biomarkers to determine the specificity of tau as a possible mechanism of the relationship between neuroinflammation and cognition in women. Aβ1‐42 did not mediate the relationship between sTFNR2 and cognition, consistent with another study documenting an absence of relationship between Aβ1‐42 and neuroinflammation among older adults with MCI. 56 Similarly, neither HV/IV nor WMH/IV mediated the link between sTFNR2 and cognition.
All relationships between sTNFR2 levels and cognition were female‐specific and were attenuated by p‐tau181. TNFR2 is a transmembrane TNFα receptor expressed in immune cells and activated brain endothelial cells that is cleaved to sTNFR2 under inflammatory conditions. 57 , 58 Circulating sTNFR2 is upregulated in biological fluids of inflammatory diseases, such as rheumatoid arthritis, colitis, and diabetic kidney disease and can be used as a biomarker for inflammation, because it correlates with increased levels of TNFα and disease activity. 59 , 60 , 61 Overall, our findings indicate that sTNFR2 may play a larger role in cognitive dysfunction in women versus men on the AD trajectory and may potentially play a contributing role to the more aggressive clinical trajectory of AD in women. 15 , 26 , 28
sTNFR2 levels only related to cognition in women. The reason for the specificity of these relationships to women remains to be determined. Given evidence that women on the AD continuum show greater tau burden than men 18 as well as recent evidence of sex differences in gene expression of proteins related to tau phosphorylation, 62 the reciprocal interactions of tau and neuroinflammation and their adverse cognitive effects may be more prominent in women. One contributing mechanism could be related to sex hormones, particularly testosterone. Our prior work in ADNI showed that lower levels of circulating testosterone relate to higher CSF p‐tau level in both men and women 16 and to poorer cognitive function specifically among female APOE ε4 allele carriers. 63 Given that testosterone's actions are largely anti‐inflammatory, 64 these results suggest that the lower testosterone levels in women on average may be a contributing factor to the sex differences in neuroinflammation, tau, and cognitive links. In the current study, the sample size did not afford the statistical power to test three‐way neuroinflammatory marker by sex by APOE ε4 status interactions or to further stratify by APOE ε4 status. Future studies with larger sample sizes should consider sex by APOE ε4 interactions when examining associations among neuroinflammatory markers, p‐tau, and cognition.
Our study has limitations. The data available for our variables of interest is cross‐sectional only, limiting our ability to test causality and observe how these relationships unfold over time. However, our cross‐sectional findings are novel in that they are the first of their kind to examine sex differences in how neuroinflammatory markers relate to cognitive outcomes and potential underlying mechanisms. We are in the process of expanding these analyses to a longitudinal design and hope that these findings will spur additional longitudinal studies that will more definitively test these relationships (e.g., changes in p‐tau aggregation with changes in CSF levels of sTNFR2). Other limitations include generalizability of the findings, given that ADNI sample is predominantly White and well‐educated. Future studies should aim to replicate these findings in a more diverse sample and examine the intersection between sex and race in the relationship among neuroinflammation, tau, and cognition in AD. We did not correct for multiple comparisons given that we had a priori hypotheses about the direction of the relationships, and our results aligned with these hypotheses. However, we note that the sex by sTNFR2 interaction on MMSE survives a Bonferroni correction adjusting for eight comparisons (four neuroinflammatory markers by two outcomes: α = .05/8 = .0063). CSF levels of IL‐6 and TNFα are low in AD, and were close to the limit of quantification by the multiplex assays used to measure them. Further studies using more sensitive assays and a larger panel of neuroinflammatory markers may provide clearer insight about neuroinflammatory mediators. Our sample also comprised individuals across the disease trajectory; therefore, the possibility that this group includes some individuals with atypical clinical presentations, such as logopenic primary progressive aphasia, cannot be ruled out, although these cases are typically selected out in ADNI. Last, we limited our investigation to proinflammatory markers that were available in CSF in ADNI and have previously shown relationships with cognition 12 , 14 , 38 and cognitive decline in individuals on the AD continuum. 39 We hope that these findings can motivate more extensive analyses of sex differences in the effects of neuroinflammation in AD using more neuroinflammatory markers. Our study also has a number of strengths. Because of our focus on cognition, we used CSF‐derived neuroinflammatory markers, as they may more accurately reflect neuroinflammation in the brain than peripheral levels. 65 Further, data from this study were from a large sample with comprehensive neurocognitive and AD biomarker data that allowed us to compare the mediating role of different pathological processes in the link between neuroinflammation and cognition.
In summary, our results suggest that women may be more vulnerable than men to the effects of neuroinflammation, specifically sTNFR2, on cognition and these effects seem to be driven by p‐tau. The novelty of our study is a major strength in that we are the first to show sex differences in how sTNFR2 relates to cognition in individuals on the AD continuum and identify the AD pathology driving these relationships in women. Our findings highlight the need to examine sex‐specific effects of interventions targeting specific inflammatory mechanisms, on pathological tau and, in turn, cognitive function, with the hypothesis that these interventions may be more beneficial in women. The identification of sex differences in AD‐related mechanisms such as with the current findings will lead to important enhancements in understanding of AD in general and the development of precision medicine targets that can be optimized for both women and men.
AUTHOR CONTRIBUTIONS
Erin E. Sundermann, Sarah J. Banks, Matthew S. Panizzon: study concept. Erin E. Sundermann, Sarah J. Banks, Matthew S. Panizzon, Rachel A. Bernier, Murray J. Andrews, Emily G. Jacobs, Douglas R. Galasko, Alyx L. Shepherd: study design. Erin E. Sundermann, Sarah J. Banks, Murray J. Andrews: data acquisition. Erin E. Sundermann, Murray J. Andrews, Rachel A. Bernier: statistical analysis. Erin E. Sundermann, Rachel A. Bernier, Sarah J. Banks, Emily G. Jacobs, Katerina Akassoglou: data interpretation. Rachel A. Bernier, Erin E. Sundermann: initial manuscript preparation. All authors provided a critical review of manuscript and contributed to the final manuscript.
CONFLICTS OF INTEREST
All authors report no disclosures.
ROLE OF THE FUNDER/SPONSOR
The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
ADDITIONAL INFORMATION
Data used in this study were obtained from the Alzheimer's Disease Neuroimaging Initiative (ADNI) database (adni.loni.ucla.edu). Investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or preparation of this manuscript. A complete listing of ADNI investigators can be found at http://adni.loni.usc.edu/wp‐content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf.
Supporting information
Supporting information
ACKNOWLEDGMENTS
This study was supported by the California Department of Public Health (19‐10613 & 19‐10612) as well as NIH R01 (AG05064). Dr. Bernier's effort was supported by the Alzheimer's Association (AARG‐20‐644036). Dr. Akassoglou is supported by grants from the Simon Family Trust, the Dagmar Dolby Family Fund, Edward and Pearl Fein, NIA RF1 AG064926, and NIH/NINDS R35 NS097976. Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (NIH grant U01 AG024904). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering and for‐profit and non‐profit partners. The for‐profit and non‐profit organizations that help to fund ADNI include the Alzheimer's Association and Alzheimer's Drug Discovery Foundation, with participation from the U.S. Food and Drug Administration and from the following: Abbott; Alzheimer's Association; Alzheimer's Drug Discovery Foundation; Amorfix Life Sciences Ltd.; AstraZeneca; Bayer HealthCare; BioClinica, Inc.; Biogen Idec Inc.; Bristol‐Myers Squibb Company; Eisai Inc.; Elan Pharmaceuticals Inc.; Eli Lilly and Company; F. Hoffmann‐La Roche Ltd and its affiliated company Genentech, Inc.; GE Healthcare; Innogenetics, N.V.; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research and Development, LLC; Johnson and Johnson Pharmaceutical Research and Development LLC; Medpace, Inc.; Merck and Co., Inc.; Meso Scale Diagnostics, LLC; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Servier; Synarc Inc.; and Takeda Pharmaceutical Company.
Bernier RA, Banks SJ, Panizzon MS, et al., The neuroinflammatory marker sTNFR2 relates to worse cognition and tau in women across the Alzheimer's disease spectrum. Alzheimer's Dement. 2022;14:e12284. 10.1002/dad2.12284
REFERENCES
- 1. Laurent C, Buée L, Blum D. Tau and neuroinflammation: what impact for Alzheimer's disease and tauopathies?. Biomed J. 2018;41(1):21‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lyman M, Lloyd DG, Ji X, Vizcaychipi MP, Ma D. Neuroinflammation: the role and consequences. Neurosci Res. 2014;79:1‐12. [DOI] [PubMed] [Google Scholar]
- 3. Bradburn S, Murgatroyd C, Ray N. Neuroinflammation in mild cognitive impairment and Alzheimer's disease: a meta‐analysis. Ageing Res Rev. 2019;50:1‐8. [DOI] [PubMed] [Google Scholar]
- 4. Rota E, Bellone G, Rocca P, Bergamasco B, Emanuelli G, Ferrero P. Increased intrathecal TGF‐beta1, but not IL‐12, IFN‐gamma and IL‐10 levels in Alzheimer's disease patients. Neurol Sci Off J Ital Neurol Soc Ital Soc Clin Neurophysiol. 2006;27(1):33‐39. [DOI] [PubMed] [Google Scholar]
- 5. Janelidze S, Mattsson N, Stomrud E, et al. CSF biomarkers of neuroinflammation and cerebrovascular dysfunction in early Alzheimer's disease. Neurology. 2018;91(9):e867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jia J, Meng R, Sun Y, Sun W, Ji X, Jia L. Cerebrospinal fluid tau, Aβ1‐42 and inflammatory cytokines in patients with Alzheimer's disease and vascular dementia. Neurosci Lett. 2005;383(1‐2):12‐16. [DOI] [PubMed] [Google Scholar]
- 7. Jiang H, Hampel H, Prvulovic D, et al. Elevated CSF levels of TACE activity and soluble TNF receptors in subjects with mild cognitive impairment and patients with Alzheimer's disease. Mol Neurodegener. 2011;6(1):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Martínez M, Fernández‐Vivancos E, Frank A, De la Fuente M, Hernanz A. Increased cerebrospinal fluid Fas (Apo‐1) levels in Alzheimer's disease: relationship with IL‐6 concentrations. Brain Res. 2000;869(1‐2):216‐219. [DOI] [PubMed] [Google Scholar]
- 9. Bettcher BM, Johnson SC, Fitch R, et al. CSF and plasma levels of inflammation differentially relate to CNS markers of Alzheimer's disease pathology and neuronal damage. J Alzheimers Dis JAD. 2018;62(1):385‐397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhao A, Li Y, Deng Y. TNF receptors are associated with tau pathology and conversion to Alzheimer's dementia in subjects with mild cognitive impairment. Neurosci Lett. 2020;738:135392. [DOI] [PubMed] [Google Scholar]
- 11. Wennström M, Surova Y, Hall S, et al. The inflammatory marker YKL‐40 is elevated in cerebrospinal fluid from patients with Alzheimer's but not Parkinson's disease or dementia with lewy bodies. PloS One. 2015;10(8):e0135458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yaffe K, Lindquist K, Penninx BW, et al. Inflammatory markers and cognition in well‐functioning African‐American and white elders. Neurology. 2003;61(1):76‐80. [DOI] [PubMed] [Google Scholar]
- 13. Lindbergh CA, Casaletto KB, Staffaroni AM, et al. Systemic tumor necrosis factor‐alpha trajectories relate to brain health in typically aging older adults. J Gerontol Ser A. 2020;75(8):1558‐1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de Pablo‐Bernal RS, Cañizares J, Rosado I, et al. Monocyte phenotype and polyfunctionality are associated with elevated soluble inflammatory markers, cytomegalovirus infection, and functional and cognitive decline in elderly adults. J Gerontol Ser Biomed Sci Med Sci. 2016;71(5):610‐618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sundermann EE, Biegon A, Rubin LH, Lipton RB, Landau S, Maki PM. Does the female advantage in verbal memory contribute to underestimating Alzheimer's disease pathology in women versus men?. J Alzheimers Dis. 2017;56(3):947‐957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sundermann E, Panizzon MS, Chen X, Andrews M, Galasko D, Banks SJ, Sex differences in Alzheimer's‐related Tau biomarkers and a mediating effect of testosterone. Published online 2020. [DOI] [PMC free article] [PubMed]
- 17. Digma LA, Madsen JR, Rissman RA, Jacobs DM, Brewer JB, Banks SJ. Women can bear a bigger burden: ante‐and post‐mortem evidence for reserve in the face of tau. Brain Commun. 2020;2(1):fcaa025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Buckley RF, Mormino EC, Rabin JS, et al. Sex differences in the association of global amyloid and regional tau deposition measured by positron emission tomography in clinically normal older adults. JAMA Neurol. 2019;76(5):542‐551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kivity S, Ehrenfeld M. Can we explain the higher prevalence of autoimmune disease in women?. Expert Rev Clin Immunol. 2010;6(5):691‐694. [DOI] [PubMed] [Google Scholar]
- 20. Bekhbat M, Neigh GN. Sex differences in the neuro‐immune consequences of stress: focus on depression and anxiety. Brain Behav Immun. 2018;67:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gleicher N, Barad DH. Gender as risk factor for autoimmune diseases. J Autoimmun. 2007;28(1):1‐6. [DOI] [PubMed] [Google Scholar]
- 22. Cannon JG, Pierre BAS. Gender differences in host defense mechanisms. J Psychiatr Res. 1997;31(1):99‐113. [DOI] [PubMed] [Google Scholar]
- 23. Araneo BA, Dowell T, Diegel M, Daynes RA, Dihydrotestosterone exerts a depressive influence on the production of interleukin‐4 (IL‐4), IL‐5, and gamma‐interferon, but not IL‐2 by activated murine T cells. Published online 1991. [PubMed]
- 24. Jilka RL, Hangoc G, Girasole G, et al. Increased osteoclast development after estrogen loss: mediation by interleukin‐6. Science. 1992;257(5066):88‐91. [DOI] [PubMed] [Google Scholar]
- 25. Alzheimer's Association . 2017 Alzheimer's disease facts and figures. Alzheimers Dement. 2017;13(4):325‐373. [Google Scholar]
- 26. Lin KA, Choudhury KR, Rathakrishnan BG, et al. Marked gender differences in progression of mild cognitive impairment over 8 years. Alzheimers Dement Transl Res Clin Interv. 2015;1(2):103‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Osborne BF, Turano A, Schwarz JM. Sex differences in the neuroimmune system. Curr Opin Behav Sci. 2018;23:118‐123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Caldwell JZ, Berg J‐L, Cummings JL, Banks SJ. Alzheimer's disease neuroimaging initiative. Moderating effects of sex on the impact of diagnosis and amyloid positivity on verbal memory and hippocampal volume. Alzheimers Res Ther. 2017;9(1):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fu Y, Yan Y. Emerging role of immunity in cerebral small vessel disease. Front Immunol. 2018;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vogels T, Murgoci A‐N, Hromádka T. Intersection of pathological tau and microglia at the synapse. Acta Neuropathol Commun. 2019;7(1):109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Aschenbrenner AJ, Gordon BA, Benzinger TLS, Morris JC, Hassenstab JJ. Influence of tau PET, amyloid PET, and hippocampal volume on cognition in Alzheimer's disease. Neurology. 2018;91(9):e859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Giannakopoulos P, Herrmann F, Bussière T, et al. Tangle and neuron numbers, but not amyloid load, predict cognitive status in Alzheimer's disease. Neurology. 2003;60(9):1495‐1500. [DOI] [PubMed] [Google Scholar]
- 33. Ossenkoppele R, Schonhaut DR, Schöll M, et al. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer's disease. Brain. 2016;139(5):1551‐1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hohman TJ, Dumitrescu L, Barnes LL, et al. Sex‐specific association of apolipoprotein E with cerebrospinal fluid levels of tau. JAMA Neurol. 2018;75(8):989‐998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Altmann A, Tian L, Henderson V, Greicius M, Investigators ASDNI. Sex Modif APOE‐Relat Risk Dev Alzheimer Dis Ann Neurol. 2014;75:563‐573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Oveisgharan S, Arvanitakis Z, Yu L, Farfel J, Schneider JA, Bennett DA. Sex differences in Alzheimer's disease and common neuropathologies of aging. Acta Neuropathol (Berl). 2018;136(6):887‐900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Petersen RC, Aisen P, Beckett LA, et al. Alzheimer's disease neuroimaging initiative (ADNI): clinical characterization. Neurology. 2010;74(3):201‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Windham BG, Simpson BN, Lirette S, et al. Associations of inflammation to cognitive function in African Americans and European Americans. J Am Geriatr Soc. 2014;62(12):2303‐2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Holmes C, Cunningham C, Zotova E, et al. Systemic inflammation and disease progression in Alzheimer's disease. Neurology. 2009;73(10):768‐774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Craig‐Schapiro R, Perrin RJ, Roe CM, et al. YKL‐40: a novel prognostic fluid biomarker for preclinical Alzheimer's disease. Biol Psychiatry. 2010;68(10):903‐912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang H, Ginghina C, Shi M, Aro P, Zhang J, Human PS129 Luminex Assay in Cerebrospinal Fluid. Published online 2016.
- 42. Østergaard C, Johansen JS, Benfield T, Price PA, Lundgren JD. YKL‐40 is elevated in cerebrospinal fluid from patients with purulent meningitis. Clin Vaccine Immunol. 2002;9(3):598‐604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hu WT, Howell JC, CSF inflammatory proteins methods–Hu Lab. Published online 2011.
- 44. Teri L, McCurry SM, Edland SD, Kukull WA, Larson EB. Cognitive decline in Alzheimer's disease: a longitudinal investigation of risk factors for accelerated decline. J Gerontol Ser A. 1995;50A(1):M49. [DOI] [PubMed] [Google Scholar]
- 45. Banks SJ, Qiu Y, Fan CC, et al. Enriching the design of Alzheimer's disease clinical trials: application of the polygenic hazard score and composite outcome measures. Alzheimers Dement Transl Res Clin Interv. 2020;6(1):e12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Folstein MF, Folstein SE, McHugh PR. Mini‐mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189‐198. [DOI] [PubMed] [Google Scholar]
- 47. Morris JC. The clinical dementia rating (cdr): current version and. Young. 1991;41:1588‐1592. [DOI] [PubMed] [Google Scholar]
- 48. Jack CR, Bernstein MA, Fox NC, et al. The Alzheimer's disease neuroimaging initiative (ADNI): mRI methods. J Magn Reson Imaging JMRI. 2008;27(4):685‐691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hsu Y‐Y, Schuff N, Du A‐T, et al. Comparison of automated and manual MRI volumetry of hippocampus in normal aging and dementia. J Magn Reson Imaging JMRI. 2002;16(3):305‐310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fox J, Weisberg S. An {R} companion to applied regression. Thurd. Sage; 2019. https://socialsciences.mcmaster.ca/jfox/Books/Companion/. [Google Scholar]
- 51. Rosseel Y. {lavaan}: an {R} package for structural equation modeling. J Statis Softwar. 2012;48(2):1‐36. [Google Scholar]
- 52. Zhao X, Chen Q. Reconsidering Baron and Kenny: myths and truths about mediation analysis. J Consum Res. 2010;37(2):197‐206. [Google Scholar]
- 53. Chmura Kraemer H, Kiernan M, Essex M, Kupfer DJ. How and why criteria defining moderators and mediators differ between the Baron & Kenny and MacArthur approaches. Health Psychol. 2008;27(2S):S101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Baron RM, Kenny DA. The moderator–mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173. [DOI] [PubMed] [Google Scholar]
- 55. Eikelenboom P, Van Exel E, Hoozemans JJ, Veerhuis R, Rozemuller AJ, Van Gool WA. Neuroinflammation–an early event in both the history and pathogenesis of Alzheimer's disease. Neurodegener Dis. 2010;7(1‐3):38‐41. [DOI] [PubMed] [Google Scholar]
- 56. Clark AL, Weigand AJ, Thomas KR, et al. Elevated inflammatory markers and arterial stiffening exacerbate tau but not amyloid pathology in older adults with mild cognitive impairment. J Alzheimers Dis. 2021;Preprint:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Akassoglou K, Douni E, Bauer J, Lassmann H, Kollias G, Probert L. Exclusive tumor necrosis factor (TNF) signaling by the p75TNF receptor triggers inflammatory ischemia in the CNS of transgenic mice. Proc Natl Acad Sci U S A. 2003;100(2):709‐714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Faustman D, Davis M. TNF receptor 2 pathway: drug target for autoimmune diseases. Nat Rev Drug Discov. 2010;9(6):482‐493. [DOI] [PubMed] [Google Scholar]
- 59. Cope AP, Aderka D, Doherty M, et al. Increased levels of soluble tumor necrosis factor receptors in the sera and synovial fluid of patients with rheumatic diseases. Arthritis Rheum. 1992;35(10):1160‐1169. [DOI] [PubMed] [Google Scholar]
- 60. Mizoguchi E, Subramaniam R, Okada T, Mizoguchi A. A review of selected IBD biomarkers: from animal models to bedside. Diagn Basel Switz. 2021;11(2):207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Murakoshi M, Gohda T, Suzuki Y. Circulating tumor necrosis factor receptors: a potential biomarker for the progression of diabetic kidney disease. Int J Mol Sci. 2020;21(6):E1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cáceres A, González JR. Female‐specific risk of Alzheimer's disease is associated with tau phosphorylation processes: a transcriptome‐wide interaction analysis. Neurobiol Aging. 2020;96:104‐108. [DOI] [PubMed] [Google Scholar]
- 63. Sundermann EE, Panizzon MS, Bernier RA, Galasko DR, Andrews M, Banks SJ. Elucidating the relationship between testosterone and cognitive function: moderating roles of APOE4 and sex.
- 64. Uchoa MF, Moser VA, Pike CJ. Interactions between inflammation, sex steroids, and Alzheimer's disease risk factors. Front Neuroendocrinol. 2016;43:60‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Aluise CD, Sowell RA, Butterfield DA. Peptides and proteins in plasma and cerebrospinal fluid as biomarkers for the prediction, diagnosis, and monitoring of therapeutic efficacy of Alzheimer's disease. Biochim Biophys Acta BBA‐Mol Basis Dis. 2008;1782(10):549‐558. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information


