Abstract
This proof-of-principle study aims to find commensal oral bacteria that can produce extracellular polymeric substances (EPS), which have similar lubrication properties to saliva and could serve as saliva substitutes. Saliva and plaque samples were collected from 21 generally healthy individuals. Primary screening was done by conventional culturing and Gram-staining; all species selected for further analysis were identified by MALDI-TOF and deposited in DSMZ. Lactobacillus gasseri (DSM32453 and DSM32455), Lactobacillus rhamnosus (DSM32452), Lactobacillus paracasei (DSM32454), and Streptococcus sanguinis (DSM32456) produced 413.6, 415.7, 431.1, 426.8, and 877.6 µg/ml of EPS, respectively. At the same time calcium dissolution could not be detected for both L. gasseri strains, minimal dissolution for the other three: S. sanguinis 0.3 mm, and 3.7 mm for L. rhamnosus and L. paracasei. There were no differences found between the EPS samples and the saliva for the effect of shear rate on the viscosity and for the effect of sliding speed on lubrication properties. In conclusion, five commensal bacterial strains have been isolated, all able to produce EPS and lead to no or to low calcium dissolution. EPS produced exhibits rheological and tribological properties comparable to human saliva. A total of four out of five selected strains are probiotic and, therefore, may exhibit additional beneficial influence within the oral cavity.
Keywords: xerostomia, bacteria, extracellular polymeric substance matrix, rheology, lubrication, biotechnology
Commensal oral bacteria can produce EPS, which have similar lubrication properties to saliva and could serve as saliva substitutes.
Introduction
Saliva is a mix of fluids secreted from major and minor salivary glands and gingival crevicular region (Porcheri and Mitsiadis 2019). Saliva has numerous roles in maintaining health of the oral cavity: (1) lubrication and protection of oral mucosa from infection, (2) maintenance of tooth integrity by reducing demineralization and protection against wear, (3) buffering action and clearance, and (4) antimicrobial activity (Llena-Puy 2006, Buzalaf et al. 2012, Martins et al. 2013, Pedersen et al. 2018).
Saliva flow varies among individuals (Matsuo 2000, Chaudhari and Roper 2010). Normal stimulated salivary flow rate averages 1.5–2.0 ml/min while the unstimulated salivary flow rate is approximately 0.3–0.4 ml/min (Villa et al. 2015). Any flow rate below 0.1 ml/min and 0.7 ml/min for unstimulated and stimulated saliva, respectively, is considered as hyposalivation (Falcao et al. 2014, Velasco-Ortega et al. 2016). In hyposalivation, the lubrication of oral tissues fails, resulting in discomfort affecting quality of life (QOL) and in secondary oral health problems, such as dental erosion and infections of oral hard and soft tissues.
Xerostomia refers to a subjective feeling of oral dryness (Villa et al. 2015). While most of the patients who have this sensation suffer from hyposalivation, sometimes the mouth feels dry even with normal salivary flow (xerostomia without hyposalivation). Thus, xerostomia is an under-recognized condition, affecting approximately 20% of the adult population of industrialized countries (Furness et al. 2013, Agostini et al. 2018).
The most common cause of dry mouth are drugs in general, particularly medication with anticholinergic effect; cytotoxic effects from chemotherapy, head and neck radiotherapy, autoimmune and chronic diseases, and other conditions like nerve damage, uncontrolled diabetes, infections, and hormonal changes are more rare (Villa et al. 2015). Severity of associated symptoms vary from mild oral discomfort to remarkably compromised QOL due to difficulties in speaking, chewing and swallowing, and altered taste of food. Secondary problems include the development of oral diseases, such as caries, dental erosion, and periodontal diseases.
In general, handling of xerostomia focuses on relieving clinical symptoms and improving QOL of patients, as no curative treatment is available (Assery 2019). The available approaches include sialagogues agents promoting flow of saliva and simple procedures like sipping water or chewing gum. However, if saliva production cannot be stimulated effectively by these measures, saliva substitutes can be considered as an alternative treatment. Although they provide satisfactory short-lasting relief of discomfort, their impaired long-lasting effect results in suboptimal compliance. Therefore, a key characteristic for functional saliva substitutes is a long-term relief from xerostomia.
Thus, the aim of the study was to present a proof of principle that some bacteria can produce extracellular polymeric substances (EPS) with properties comparable to saliva. The goal was to isolate harmless oral commensal microorganisms exhibiting no or low calcium dissolution and high production of EPS with rheological and tribological properties comparable to saliva. Furthermore, EPS produced by selected bacterial strains could serve as saliva substitutes and bring relief to people suffering from xerostomia.
Materials and methods
All reagents and consumables were purchased from Sigma-Aldrich, Switzerland, unless indicated differently.
Bacterial isolates
Bacterial species were isolated from saliva and dental plaque samples collected from generally healthy individuals. All volunteers had neither oral infections nor other oral or systemic diseases. Data on saliva and plaque samples were analyzed anonymously. All leftover samples were discarded by the end of the study. Each dental plaque sample was placed in 1 ml NaCl and sonicated (Vibracell, Sonics and Materials) at 22.5 W for 2 min. A volume of 100 μl solution was plated on Columbia blood agar plates and incubated for 5 d in anaerobic conditions (10% CO2, 10% H2, and 80% N2). Colonies were investigated by Gram-staining and positive rod-shaped bacteria were sub-passaged to fresh Columbia blood agar plate and incubated for 3 d. After confirmation of purity of cultures by Gram-staining, all bacteria (including Lactobacillus spp. isolated from saliva) were identified by MALDI-TOF mass spectrometry (Biotyper, Bruker Daltonics, Germany), and stored in Microbank tubes at −70°C.
Selected strains have been deposited at German Collection of Microorganisms and Cell Cultures (DSMZ).
Saliva preparation
Saliva from generally healthy donors free from systemic or oral topical medications was collected and filtered through a 70-μm filter, sonicated for 1 min at 22.5 W, and centrifuged for 40 min at 4°C and 21 000 g. Supernatant was filtered through double filter system of 0.45 and 0.22 μm (Millex Millipore, Switzerland) and used immediately.
Pooled saliva was prepared by mixing saliva from three donors ranging in age from 32 to 58, with an average age of 47.7.
Quantification of EPS
One bead of frozen bacterial stock was inoculated in 5 ml of either MRS (Lactobacillus spp.) or LB medium (non-Lactobacillus spp.) and incubated at 37°C for 24 h in aerobic (S. mitis, S, oralis, S. sanguinis, S. mutans,andM. luteus) or anaerobic (Lactobacillus spp. andV. parvula) conditions. From each culture, 100 μl were inoculated in 5 ml of pooled saliva pool enriched with 40 g/l of glucose and incubated for 24 h in previously described conditions. OD was measured at 595 nm (Eppendorf, Switzerland) and the cultures were diluted with NaCl to OD595 0.2, corresponding to 6.0 × 107 CFU/ml. Pooled saliva with 40 g/l of glucose was diluted accordingly and served as control for each sample in order to deduct background signal. From each culture, 2 ml were transferred in 50-ml Nalgene Centrifuge Tubes and mixed with 6 ml of ice cold 99.8% ethanol, followed by 30 s of vortexing, and incubation at 4°C for 1.5 h. Samples were centrifuged for 15 min at 4°C and 21 000 g and dried out for 30 min at room temperature. A volume of 2 mL of Alcian Blue solution were added to each tube, vortexed for 10 s, and incubated for 30 min at room temperature. Tubes were centrifuged for 15 min at 4°C and 21 000 g and absorbance of supernatant was measured at 614 nm. In order to quantify the results, EPS standard curve was prepared using dilutions of 0.25% dextran solution resulting in final concentrations of 32, 64, 96, 128, 160, and 192 μg. Final amount of EPS produced by different bacterial strains was diminished by respective background signal.
Calcium phosphate dissolution
To assess dissolution of hydroxyapatite, the cultures were grown on Columbia blood agar plates at 37°C for 48 h under (an)aerobic conditions (species-specific as described above), and resulting colonies were needle inoculated on Pikovskaya's agar plates (per 1 l: 5 g Ca5(PO4)3OH, 10 g glucose, 0.5 g (NH4)2SO4, 0.2 g NaCl, 0.1 g MgSO4 × 7H2O, 0.2 g KCl, 0.5 g yeast extract, 0.002 g MnSO4× H2O, and 0.002 g FeSO4 × 7H2O) supplemented with Ca5(PO4)3OH (particle size < 1 μm) as a calcium source. Plates were incubated for 7 d at 37°C in (an)aerobic conditions before dissolution zones were measured. The average of three measures was taken as result.
Optimized bacterial media
Lactobacillus spp. were incubated in 50% LB medium supplemented with 40 g/l of glucose and 10% MRS. Streptococcus sanguinis, Streptococcus mutans,andStreptococcus mitis were incubated in 50% LB medium supplemented with 40 g/l of glucose. Veillonella parvula was incubated in 25% thioglucollate, Micrococcus luteus was incubated in 25% Brain–Heart infusion medium (both supplemented with 40 g/l of glucose).
EPS production and dialysis
In taotal, one bead of frozen bacteria stock was inoculated in 5 ml of either MRS (Lactobacillus spp.) or LB medium supplemented with 40 g/l of glucose (non-Lactobacillus spp.) and incubated at 37°C for 24 h in aerobic (S. mitis, S, oralis, S. sanguinis, S. mutans, andM. luteus) or anaerobic (Lactobacillus spp. andV. parvula) conditions. From each culture, 2 ml were further inoculated in 500 ml of optimized bacterial media. Bacterial cultures were incubated for 72 h in previously described conditions followed by two-step filtration through GF/F 1 and 0.2 μm filters. Filtrate was mixed with 1 l of ice cold 99.8% ethanol and EPS was precipitated overnight at 4°C. EPS was recovered by centrifugation at 4°C and 3900 g for 10 min. Pellets were resuspended in 10 ml of 99.8% ethanol and subjected to dialysis, as described elsewhere (Steiger et al. 2020). EPS was recovered and stored at 4°C.
Acid-based titration of EPS
EPS sample was diluted in ultrapure water (MilliQ) and mixed with KNO3 to reach a final concentration of 10 mM. Solution was placed for 10 min in vacuum to remove CO2, followed by constant injection of nitrogen preventing ambient CO2 to react at higher pH and form HCO3–. Appropriate amount of 1 M HCl was added to the solution in order to reach a pH in range of 2.3 and 2.5 followed by stepwise (20 μl) addition of 0.1 M NaOH until the pH of solution reached value of 10.5. pH of the solution was measured after each addition of sodium hydroxide. ProtoFit GUI Version 2.1 was used to estimate pK of EPS and proton binding sites density (Turner and Fein 2006).
Shear rate sweep
Rheological analyses were performed using research rheometer (DHR2, TA Instruments) fitted with 60 mm plate–plate measuring system, testing gap set to 200 μm. Solvent trap cover was employed for rheological analyses to minimize atmospheric exposure of samples at exposed edges. Following 30 s equilibration time at 37°C, samples were exposed to 30 s pre-shear at rate of 1/s, led immediately into a shear rate sweep, 1–1000/s, points spaced logarithmically, 8 points per decade of shear rate, shear applied for 30 s at each rate with viscosity calculated over final 5 s of each step. Test was performed in duplicate and results are presented as mean values. Student's t-test was performed to determine whether differences were statistically significant.
Tribological analysis
Tribology testing was performed using the same instrument fitted with custom 3 balls on plate setup with pliant lower substrate. Tribology assembly was employed that comprised a geometry of 3 glass spheres that slided against pliant lower substrate, under defined load of 1 N, onto which sample has been spread. Rotational angular velocity was ramped from 0.05 to 20 rad/s, 8 points per decade, each point maintained for 20 s with coefficient of friction averaged over final 15 s. Lower surface was made to hold 37°C throughout analysis. Test was performed in triplicate for each sample and results are presented as mean values.
Results
In order to obtain reproducible results, pooled saliva was used for EPS production throughout the study. Preliminary results revealed significant differences in bacterial growth and EPS production when using saliva from different donors. A total of 20 isolated Lactobacillus strains were preliminary screened for EPS production in pooled saliva enriched with glucose (40 g/l) and their ability to dissolve calcium from culture plates (Table 1, bacterial strains 1–20).
Table 1.
Calcium dissolution and amount of EPS produced by tested bacterial species isolated from oral cavity.
| Number | Species | Calcium dissolution (mm) | EPS production (µg/ml) |
|---|---|---|---|
| 1 | L. paracasei | 4.7 | 427.1 |
| 2 | L. plantarum | 11 | 317.8 |
| 3 | L. rhamnosus | 3.7 | 431.1 |
| 4 | L. rhamnosus | 8.3 | 410.0 |
| 5 | L. rhamnosus | 9.3 | 413.9 |
| 6 | L. paracasei | 6.3 | 427.5 |
| 7 | L. paracasei | 8 | 412.5 |
| 8 | L. plantarum | 12.7 | 324.3 |
| 9 | L. gasseri | 0 | 413.6 |
| 10 | L. salivarius | 6.7 | 363.9 |
| 11 | L. fermentum | 7 | 436.1 |
| 12 | L. fermentum | 5 | 431.1 |
| 13 | L. gasseri | 0 | 415.7 |
| 14 | L. paracasei | 6.7 | 424.6 |
| 15 | L. paracasei | 6 | 409.3 |
| 16 | L. gasseri | 2.3 | 368.6 |
| 17 | L. gasseri | 0 | 377.5 |
| 18 | L. rhamnosus | 7 | 321.0 |
| 19 | L. paracasei | 5.3 | 401.1 |
| 20 | L. paracasei | 3.7 | 426.8 |
| 21 | S. sanguinis | 0.6 | 0.00 |
| 22 | S. sanguinis | 0.3 | 877.6 |
| 23 | S. oralis | 1.1 | 0.00 |
| 24 | S. mutans | 4.4 | 0.00 |
| 25 | M. luteus | 0 | 0.00 |
Strains selected for further studies are marked in gray.
Lactobacillus species were isolated from saliva of 18 healthy donors by conventional culturing. Streptococcus mitis, Streptococcus oralis, S. sanguinis, S. mutans, M. luteus, and V. parvula were isolated from dental plaque samples collected from 21 generally healthy individuals.
A total of four Lactobacillus spp. were selected for further study. In total, two selection criteria were applied to all the strains: (1) no or low calcium dissolution, (2) high production of EPS while cultured in saliva enriched with glucose. Selected strains included: 1x Lactobacillusrhamnosus, 2x Lactobacillus gasseri, and 1x Lactobacillus paracasei (Table 1, marked in gray). A similar study was performed for S. sanguinis, S. mutans, M. luteus, and V. parvula and revealed lack of EPS production for all strains but one of S. sanguinis (22). Additionally, S. sanguinis (22) showed relatively low calcium dissolution, and, therefore, has been also chosen for further characteristics.
EPS from each of the above-mentioned bacterial strains was isolated, dialysed, and characterized in terms of density of potential binding sites, their concentration, and dissociation constants (Table 2). Among the investigated bacteria, only V. parvula produced basic EPS with three potential binding sites. The character of EPS produced by all other bacteria was found acidic (L. rhamnosus, L. gasseri, L. paracasei, L. gasseri, S. sanguinis, S. mutans,andM. luteus) with either one (L. gasseri), two (L. rhamnosusandL. paracasei), or three (S. sanguinis, S. mutans, V. parvula,andM. luteus) potential binding sites. In optimal conditions, the amount of EPS produced by all strains ranged from 0.71 to 3.55 g of EPS in 1 l liquid culture, with lowest production for V. parvula and highest for S. mutans.
Table 2.
Characteristics of EPS binding sites for selected bacterial strains.
| Amount of produced EPS (g/l) | ||||||
|---|---|---|---|---|---|---|
| Species | Binding sites | pKa | Concentration of binding sites (mol/kg) | Character | In saliva | In optimized medium |
| L. rhamnosus (DSM 32452) | 2 | 1.63 | 0.209 | Acidic | 0.43 | 0.93 |
| 6.69 | 0.007 | |||||
| L. gasseri (DSM 32453) | 1 | 1.6 | 0.135 | Acidic | 0.41 | 1.01 |
| L. gasseri (DSM 32455) | 1 | 5.79 | 0.132 | Acidic | 0.42 | 0.89 |
| L. paracasei (DSM 32454) | 2 | 1.66 | 0.219 | Acidic | 0.43 | 0.89 |
| 5.71 | 0.007 | |||||
| S. sanguinis (DSM 32456) | 3 | 1.6 | 0.126 | Acidic | 0.88 | 2.07 |
| 5.7 | 0.013 | |||||
| 9.66 | 0.012 | |||||
In order to investigate the rheological and tribological properties of the above-mentioned five EPS samples, set of tests comparing their properties with two saliva samples (stimulated and unstimulated saliva) were performed. To this end, two tests were employed: shear rate sweeps (Fig. 1 and Table 3) and tribological analysis (Fig. 2).
Figure 1.
Effect of shear rate on the viscosity of EPS and saliva samples. Each sample has been tested in duplicate and results are presented as mean values.
Table 3.
Viscosity of EPS and saliva samples at two defined shear rates: 100/s corresponding to slow movements in oral cavity and 1000/s mimic movements during swallowing.
| Viscosity (mPa.s) | P-value | |||
|---|---|---|---|---|
| Sample | At 100/s | At 1000/s | Vs. stimulated saliva | Vs. unstimulated saliva |
| Stimulated saliva | 0.947 | 0.718 | - | .93 |
| Unstimulated saliva | 1.04 | 0.697 | .93 | - |
| S. sanguinis EPS (DSM 32456) | 0.887 | 0.709 | .86 | .93 |
| L. gasseri EPS (DSM 32453) | 0.862 | 0.638 | .79 | .75 |
| L. gasseri EPS (DSM 32455) | 0.954 | 0.678 | .63 | .62 |
| L. paracasei EPS (DSM 32454) | 1.02 | 0.663 | .89 | .83 |
| L. rhamnosus EPS (DSM 32452) | 1.11 | 0.683 | .97 | .92 |
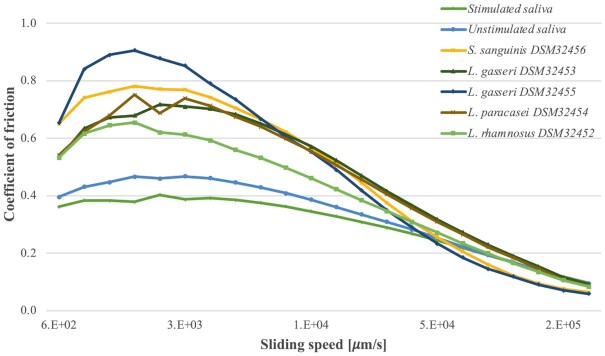
Figure 2.
Effect of sliding speed on the lubrication properties (coefficient of friction) of EPS and saliva samples. Each sample has been tested in triplicate and results are presented as mean values.
The shear rate sweeps involved applying a range of shear rates to the samples and measuring the viscosity. Higher shear rates are thought to be more relevant to swallowing processes and lower shear rates to slower movements. The results (Fig. 1) revealed that, when exposed to high shear rates, all five EPS samples tested have similar viscosity values in comparison to saliva. At low shear rates, however, there were some measurable differences in value and behavior (Table 3). However, the differences are not statistically significant (P<> 0.05i >). There was also no statistically significant difference between stimulated and unstimulated saliva (P<> 0.05i >).
Tribological test analyzed the lubricating properties of the samples by applying a layer of the test sample to a substrate and forcing an upper geometry to slide against it at a number of speeds while under a defined load. High sliding speeds are thought to be more relevant to in-mouth conditions during the swallowing process. Tribological analysis results (Fig. 2) indicated that at low sliding speeds the human saliva samples provide a greater degree of lubrication than all EPS samples. However, at high sliding speeds, corresponding to conditions during the swallowing process, it looks exactly opposite—saliva samples show lower lubrication than five EPS samples. Nevertheless, all observed differences were not statistically significant.
Discussion
The aim of the present work was to: (1) isolate and identify oral commensal bacterial strains, that produce high amounts of EPS, (2) select strains exhibiting no or low calcium dissolution, and (3) characterize the rheological and tribological properties of the isolated EPS in comparison to saliva.
Some commensal bacteria, especially these qualified as probiotic, are being considered as a tool to modulate oral microbiota and to reduce the prevalence of oral diseases. Accumulating evidence on oral probiotics has shown promising results in reducing halitosis, preventing periodontal disease or candidiasis (Anusha et al. 2015, Penala et al. 2016). Lactobacilli and bifidobacteria are typical probiotics that show beneficial influence on general condition of the oral cavity. By means of the action of their metabolites and cellular components, including EPS, they are able to enhance oral immunity, modulate oral microbiota, and improve oral health (Lin et al. 2021). Therefore, our focus was to isolate either Lactobacillus or Bifidobacterium strains. Based on colony appearance on blood agar plates indicating significant production of EPS, we have selected and identified 20 lactobacilli. Additionally, five non-Lactobacillus strains exhibiting high potential for EPS production were also included in the study.
In nature, many EPS types are highly hygroscopic and can provide protection against dehydration of microbes in biofilms by keeping moisture in the surrounding of living cells (Costa et al. 2018, Seviour et al. 2019). Bound water can reach up to 98% of EPS total mass (Pattem et al. 2021). In a clinical setting, oral biofilms experience dynamic cycles of de- and re-hydration due to fluid consumption (Signoretto et al. 2010) and saliva stimulation (Thurnheer and Belibasakis 2018). Therefore, it has been assumed that EPS produced by probiotics in the oral cavity may absorb water from drinks, foods, and air, and retain water resulting in moistening, lubrication, and protection of the oral tissues against dehydration. That was why the focus was on those bacterial strains, which produce a significant amount of EPS.
Care should be taken as EPS has always been considered to have a high affinity to calcium and to act as a calcium chelator resulting in demineralization of oral hard tissues, such as tooth enamel and dentin (Jin et al. 2004, Bradshaw and Lynch 2013, Kulshrestha et al. 2016). Cariogenic bacteria live in biofilm and attack dental enamel by converting sugar and starch into acids that dissolve out calcium from the enamel (Stoodley et al. 2008, Jaffar et al. 2016, Deng et al. 2019, Steiger et al. 2020). The dissolution of calcium increases the concentration of calcium locally, creating an environment, i.e. hostile to bacterial life (Astasov-Frauenhoffer et al. 2017). Even though, in general EPS is considered as a potential co-factor in the pathogenesis of caries and dental erosion, this disadvantageous property can be overcome by choosing strains that do not lead to calcium dissolution.
In order to consider EPS-producing bacteria as a possible future treatment for dry mouth, the potential problem of demineralization of tooth surface had to be precluded. Therefore, in our screening, we have focused on strains with no or low calcium dissolution. As a result, five commensal bacteria (four probiotic Lactobacillus strains and one non-Lactobacillus strain—S. sanguinis) have been chosen, all with the capability of producing significant amounts of EPS and at the same time with either no or very low dissolution of calcium.
All the purified EPS showed a very low denisty of acidic proton binding sites (from 0.126 to 0.219 mol/kg) when compared to the EPS purified from cariogenic strains where values can go up to 1.5 mol/kg (Astasov-Frauenhoffer et al. 2017). The other weakly acidic sites do not represent a significant fraction. Therefore, there is also much smaller chance for calcium binding. For some purified EPS, additional ITC measurment was performed. The calcium binding affinity determined ranged between 1.0 × 104 (for L. rhamnosus) and 3.2 × 103 (for L. paracasei). Again, these values are much lower than those observed for cariogenic pathogens. Overall this supports the hypothesis that EPS isolated here will not further enhance caries formation and confirms that such EPS have different properties than the ones from cariogenic microbes.
Isolated and purified EPS were tested in terms of their rheological and tribological similarities to generic saliva. All tested EPS and saliva samples showed similar responses to applied shear rate—there was no difference neither at higher shear rates that are thought to be more relevant to swallowing processes nor lower shear rates, which are related to slower movements. The tribological analysis revealed some differences between EPS and saliva samples: the stimulated and unstimulated saliva produced lower coefficient of friction than any of the EPS samples at low sliding speeds, which corresponds to the resting conditions in the mouth. Whereas, at high sliding speeds that are thought to be more relevant to in-mouth conditions during the swallowing process, the results were reversed, and the stimulated and unstimulated saliva were amongst the highest friction coefficients measured. However, as none of the differences were found statistically significant, it can be concluded that all of the tested bacterial EPS samples here demonstrated high similarity in lubrication properties to tested saliva.
In conclusion, five commensal bacterial strains have been isolated, all able to produce a significant amount of EPS and with no or low ability to dissolve calcium. EPS produced by identified bacteria exhibit rheological and tribological properties comparable to human saliva. A total of four out of five selected commensal strains are probiotic and, therefore, may exhibit additional beneficial influence on the condition of the oral cavity. Furthermore, isolated bacteria might bring relief to people suffering from xerostomia by production of EPS, which can substitute saliva. However, further toxicological tests of EPS, long-term behavioral investigation of bacteria (interaction with oral microbiome) and clinical trials are required.
ACKNOWLEDGEMENTS
The authors acknowledge support from the Centre for Industrial Rheology in the Long Barn, Great Britain.
Contributor Information
Piotr Kardas, Department Research, University Center for Dental Medicine Basel UZB, University of Basel, Mattenstrasse 40, 4058 Basel, Switzerland.
Monika Astasov-Frauenhoffer, Department Research, University Center for Dental Medicine Basel UZB, University of Basel, Mattenstrasse 40, 4058 Basel, Switzerland.
Olivier Braissant, Center of Biomechanics and Biocalorimetry, Department of Biomedical Engineering (DBE), University of Basel, Gewerbestrasse 14, 4123 Allschwil, Switzerland.
Michael M Bornstein, Department Research, University Center for Dental Medicine Basel UZB, University of Basel, Mattenstrasse 40, 4058 Basel, Switzerland; Department of Oral Health and Medicine, University Center for Dental Medicine Basel UZB, University of Basel, Mattenstrasse 40, 4058 Basel, Switzerland.
Tuomas Waltimo, Department of Oral Health and Medicine, University Center for Dental Medicine Basel UZB, University of Basel, Mattenstrasse 40, 4058 Basel, Switzerland; Center of Salivary Diagnostics and Hyposalivation, University Center for Dental Medicine Basel UZB, University of Basel, Basel, Mattenstrasse 40, 4058, Switzerland.
Funding
No external funding was received for this study.
Conflicts of interest statement
None declared
References
- Agostini BA, Cericato GO, Silveira ERD. et al. How common is dry mouth? Systematic review and meta-regression analysis of prevalence estimates. Braz Dent J. 2018;29:606–18. [DOI] [PubMed] [Google Scholar]
- Anusha RL, Umar D, Basheer B. et al. The magic of magic bugs in oral cavity: probiotics. J Adv Pharm Technol Res. 2015;6:43–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assery MKA. Efficacy of artificial salivary substitutes in treatment of xerostomia: a systematic review. J Pharm Bioallied Sci. 2019;11:S1–S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astasov-Frauenhoffer M, Varenganayil MM, Decho AW. et al. Exopolysaccharides regulate calcium flow in cariogenic biofilms. PLoS ONE. 2017;12:e0186256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw DJ, Lynch RJ. Diet and the microbial aetiology of dental caries: new paradigms. Int Dent J. 2013;63:64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzalaf MA, Hannas AR, Kato MT. Saliva and dental erosion. J Appl Oral Sci. 2012;20:493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhari N, Roper SD. The cell biology of taste. J Cell Biol. 2010;190:285–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa OYA, Raaijmakers JM, Kuramae EE. Microbial extracellular polymeric substances: ecological function and impact on soil aggregation. Front Microbiol. 2018;9:1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng L, Li W, He Y. et al. Cross-kingdom interaction of Candida albicans and Actinomyces viscosus elevated cariogenic virulence. Arch Oral Biol. 2019;100:106–12. [DOI] [PubMed] [Google Scholar]
- Falcao DP, Leal SC, Vieira CN. et al. Sialometry of upper labial minor glands: a clinical approach by the use of weighing method Schirmer's test strips paper. Sci World J. 2014;2014:268634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness S, Bryan G, McMillan R. et al. Interventions for the management of dry mouth: non-pharmacological interventions. Cochrane Database Syst Rev. 2013;9:CD009603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffar N, Miyazaki T, Maeda T. Biofilm formation of periodontal pathogens on hydroxyapatite surfaces: implications for periodontium damage. J Biomedi Mater Res Part A. 2016;104:2873–80. [DOI] [PubMed] [Google Scholar]
- Jin Y, Samaranayake LP, Samaranayake Y. et al. Biofilm formation of Candida albicans is variably affected by saliva and dietary sugars. Arch Oral Biol. 2004;49:789–98. [DOI] [PubMed] [Google Scholar]
- Kulshrestha S, Khan S, Hasan S. et al. Calcium fluoride nanoparticles induced suppression of Streptococcus mutans biofilm: an in vitro and in vivo approach. Appl Microbiol Biotechnol. 2016;100:1901–14. [DOI] [PubMed] [Google Scholar]
- Lin CW, Chen YT, Ho HH. et al. Lozenges with probiotic strains enhance oral immune response and health. Oral Dis. 2021. Online ahead of print. DOI: 10.1111/odi.13854. [DOI] [PubMed] [Google Scholar]
- Llena-Puy C. The role of saliva in maintaining oral health and as an aid to diagnosis. Med Oral Patol Oral Cir Bucal. 2006;11:E449–55. [PubMed] [Google Scholar]
- Martins C, Castro GF, Siqueira MF. et al. Effect of dialyzed saliva on human enamel demineralization. Caries Res. 2013;47:56–62. [DOI] [PubMed] [Google Scholar]
- Matsuo R. Role of saliva in the maintenance of taste sensitivity. Crit Rev Oral Biol Med. 2000;11:216–29. [DOI] [PubMed] [Google Scholar]
- Pattem J, Davrandi M, Aguayo S. et al. Dependency of hydration and growth conditions on the mechanical properties of oral biofilms. Sci Rep. 2021;11:16234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen A, Sorensen CE, Proctor GB. et al. Salivary functions in mastication, taste and textural perception, swallowing and initial digestion. Oral Dis. 2018;24:1399–416. [DOI] [PubMed] [Google Scholar]
- Penala S, Kalakonda B, Pathakota KR. et al. Efficacy of local use of probiotics as an adjunct to scaling and root planing in chronic periodontitis and halitosis: a randomized controlled trial. J Res Pharm Pract. 2016;5:86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcheri C, Mitsiadis TA. Physiology, pathology and regeneration of salivary glands. Cells. 2019;8:976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seviour T, Derlon N, Dueholm MS. et al. Extracellular polymeric substances of biofilms: suffering from an identity crisis. Water Res. 2019;151:1–7. [DOI] [PubMed] [Google Scholar]
- Signoretto C, Bianchi F, Burlacchini G. et al. Drinking habits are associated with changes in the dental plaque microbial community. J Clin Microbiol. 2010;48:347–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiger EL, Muelli JR, Braissant O. et al. Effect of divalent ions on cariogenic biofilm formation. BMC Microbiol. 2020;20:287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley P, Wefel J, Gieseke A. et al. Biofilm plaque and hydrodynamic effects on mass transfer, fluoride delivery and caries. J Am Dent Assoc. 2008;139:1182–90. [DOI] [PubMed] [Google Scholar]
- Thurnheer T, Belibasakis GN. Effect of sodium fluoride on oral biofilm microbiota and enamel demineralization. Arch Oral Biol. 2018;89:77–83. [DOI] [PubMed] [Google Scholar]
- Turner BF, Fein JB. Protofit: a program for determining surface protonation constants from titration data. Comput Geosci. 2006;32:1344–56. [Google Scholar]
- Velasco-Ortega E, Delgado-Ruiz RA, Lopez-Lopez J. Dentistry and diabetes: the influence of diabetes in oral diseases and dental treatments. J Diabetes Res. 2016;2016:6073190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa A, Connell CL, Abati S. Diagnosis and management of xerostomia and hyposalivation. Ther Clin Risk Manag. 2015;11:45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]