Abstract
A disintegrin and metalloprotease 12 (ADAM12), an essential transmembrane protein with metalloprotease, cell binding and intracellular signal-regulating capabilities, has been reported to play a crucial role in various types of cancers. However, the biological function of ADAM12 in gastric cancer (GC) remains unclear. Bioinformatic and experimental analyses were used to determine the expression level and prognostic value of ADAM12 in GC. The level of DNA methylation and the competing endogenous RNA (ceRNA) network was identified using MethSurv, Starbase3.0, miRNet2.0 and experimental analyses. Then, the co-expression profiles of ADAM12 were determined and subjected to enrichment analysis using the LinkedOmics database. The protein-protein interaction network and the docking model of ADAM12 were constructed using the GeneMANIA, STRING, and HDOCK webservers. The role of ADAM12 in tumor metastasis and immune infiltration was investigated using in vitro assays and TIMER database exploration. It was found that ADAM12 was overexpressed and was correlated with a poor prognosis of GC patients. In addition, the aberrant DNA methylation status and ceRNA regulation may contribute to the upregulation of ADAM12 in GC. Moreover, the enrichment analysis revealed that ADAM12 is involved in multiple vital biological functions and pathways, such as 'macrophage activation', 'extracellular matrix binding' and 'ECM-receptor interaction'. Subsequently, the protein-protein interaction network and molecular docking model demonstrated that follistatin like 3 (FSTL3) is a potential binding partner of ADAM12. Finally, it was demonstrated that ADAM12 promotes tumor metastasis, immune infiltration and M2 macrophage polarization in GC. In summary, these results highlight the potential of ADAM12 to be used as a therapeutic target for GC.
Keywords: gastric cancer, ADAM12, metastasis, FSTL3, immune infiltration
Introduction
Gastric cancer (GC) is characterized by insidious onset, easy metastasis, and generally poor prognosis; the morbidity and mortality of GC have been ranked fifth and third in the world, respectively (1,2). Although intensive interventions including radical resection and drug therapy are available for GC patients, the overall 5-year survival rates of GC patients is still only 25–30% (3). GC has been identified as one of the most severe malignant tumors that endanger human health. The fundamental reason for the poor prognosis includes tumor metastasis, recurrence and drug resistance. It is well known that the occurrence and development of GC is a multifactorial, multistage, and multistep process involving the activation of tumor-promoting factors and the inactivation of tumor-suppressing factors. GC cells gradually adjust their biological characteristics in an unfavorable environment through complex molecular regulation mechanisms to promote tumor proliferation and metastasis. Therefore, targeted drugs are the key to effectively cure or improve long-term survival. However, the effect of existing targeted agents, such as bevacizumab (4), cetuximab (5), and trastuzumab (6), are not completely satisfactory for GC treatment. Therefore, finding pivotal molecular targets and developing effective targeted drugs is an effective way to improve current GC treatments.
The a disintegrin and metalloprotease (ADAM) protein family, as important multi-domain transmembrane metalloproteinases, plays important roles in activating Notch, epidermal growth factor receptor (EGFR) and other signaling pathways which are related to tumor progression by catalyzing the cleavage of cell surface proteins (7). ADAM12, an important member of the ADAM protein family, has been shown to actively participate in various biological processes such as signal transduction, cytoskeleton depolymerization, and micro- environmental regulation by binding and activating targeted proteins (8). The cellular effect mediated by ADAM12 may be a key event in multiple biological and pathological processes. Cumulated evidence indicates that ADAM12 can promote epithelial-mesenchymal transition (EMT) (9), metastasis (10), drug resistance (11) and cancer stemness maintenance (12) in a variety of malignant tumors. However, the biological role of ADAM12 in GC has not yet been elucidated.
In the present study, bioinformatics methods and experimental analyses were utilized to comprehensively investigate the expression level, prognostic value, regulatory mechanism and biological functions of ADAM12 in GC. Collectively, our results identified ADAM12 as a tumor promoter, thus supporting its use as a novel prognostic biomarker and therapeutic target in GC.
Materials and methods
Clinical tissue specimens
The tissues from 63 GC patients (mean age, 59.3±11.6 years; range, 35–82 years; 19 women and 44 men; stage I-II, 44 patients; stage III-IV, 19 patients) used for experiments were obtained from the Affiliated Provincial Hospital of Anhui Medical University between January 2016 to December 2017. This study was approved by the Academic Committee of the Affiliated Provincial Hospital of Anhui Medical University (certification no. 2019KY32) and all patients provided written informed consent.
Immunohistochemistry (IHC) staining analysis
Collected tissue specimens for IHC staining were performed as previously described (13). The final IHC scores were determined according to immunostaining intensity and positive cell percentage. In detail, scores from 0 to 7 represented low expression, and scores from 8 to 12 were regarded as high expression. The staining procedure and results were independently evaluated by two pathologists.
Public database analyses
Gene expression Profiling Interactive Analysis (GEPIA) (14) and the GSE19826 dataset (15) obtained from the GEO database were used to determine the expression level of ADAM12 in GC. Moreover, the prognostic value of ADAM12 in GC was also confirmed by GEPIA database (Group cutoff for separating patients into ADAM12 high and low expression groups was set as median).
To better understand the reasons for ADAM12 overexpression, we firstly used the MethSurv database (16) to evaluate the DNA methylation status of ADAM12 in GC. Subsequently, starBase3.0 (17,18) was used to predict the targeted miRNAs of ADAM12 using PITA (19), miRanda (20), and TargetScan (21) analyses. Thereafter, we analyzed the correlation between ADAM12 expression and targeted miRNAs to screen for miRNAs that were most suited to competing endogenous RNA (ceRNA) conditions. In addition, we predicted the targeted long non-coding RNAs (lncRNAs) of the microRNAs (miRNAs), which were obtained from the previous analysis using miRNet2.0 (22) and starBase 3.0 (17,18). Sankey graph was constructed to exhibit the ceRNA network (lncRNA-miRNA-mRNA) of ADAM12 using d3Network package of R software version 4.0.2 (R Core Team, www.r-project.org/).
The ADAM12 co-expression profiles were obtained from the LinkedOmics database and the results were visualized as a volcano plot and heatmaps (23). These co-expressed genes of ADAM12 were then used to perform Gene Ontology (GO) function and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways enrichment analysis. The GeneMANIA (24) and STRING (25) databases were mined to construct the protein-protein interaction network of ADAM12 to identify the potential interaction-partner of ADAM12.
The TIMER database is a user-friendly database that can be used to comprehensively analyze tumor immune infiltration (26). In our study, the 'gene modules' were used to explore the relationship between ADAM12 and immune cell infiltration levels in GC. In addition, we used the 'somatic copy number alteration module' of the TIMER tool to determine the correlation between the genetic copy number variation (CNV) of ADAM12 and the relative abundance of tumor-infiltrating cells. The 'survival module' was used to detect the association between clinical outcomes and immune cell infiltration in GC. The 'correlation module' was used to explore correlations between ADAM12 expression and the expression of immune-infiltration cell markers with tumor purity adjustment.
Cell culture and transfection
The human gastric mucosal cell line GES1 (control cell line) and gastric cancer cell lines AGS, MKN28, MKN45, HGC27 and MKN1 were obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China) and cultured in corresponding medium with fetal bovine serum (FBS) [Biological Industries (BI), Israel]. Short tandem repeat analysis was performed to authenticate the cell lines before the experiments. The stable GC cells with ADAM12 knockdown or overexpression and the corresponding control cells were constructed by Tsingke Co., Ltd. and named as sh-NC, sh-ADAM12, LV-Control or LV-ADAM12, respectively. In brief, the lentiviral constructs for ADAM12 knockdown (shRNA-ADAM12) and scramble control (sh-NC) were constructed into pLKO.1 neo (Addgene #13425). All the plasmids were co-transfected with psPAX2 and pMD2.G into 293T cells using EndoFectin™ transfection reagent (GeneCopoeia), per the manufacturer's recommendations. The supernatant was collected after culturing for 48 h. Subsequently, the lentivirus supernatant was concentrated and the cells were transfected with polybrene (8 µg/ml, GeneChem, Inc.). After a 48-h infection, the transfected cells were cultured in medium containing G418 (600 ug/ml) to select stable cells. The transfection efficiency of the cells was verified by western blotting. In addition, the miRNA mimics and negative control (miR-NC) were purchased from GenePharma, Inc., and the transfection procedures were performed using Lipofectamine 3000 (Invitrogen/Thermo Fisher Scientific, Inc.) in accordance with the manufacturer's instructions. All the corresponding sequences are listed in Table SI.
Western blot analysis
After lysing cells using RIPA buffer, the protein concentration was determined using the BCA Kit (Beyotime Institute of Biotechnology). Then, 20 µg protein from the samples was separated by 10% SDS-PAGE gel followed by transferring to 0.45-µm PVDF membranes (Millipore, USA). Subsequently, the PVDF membranes were incubated in blocking buffer (5% non-fat milk) for 1 h at room temperature and then incubated with the primary antibody overnight at 4°C, and the corresponding secondary antibody was hybridized for 2 h at room temperature. The blotted proteins was visualized with the help of the ECL detection system (Pierce Biotech, USA), scanned with a Chemi-Doc System (Bio-Rad Laboratories, Inc.) and analyzed using ImageJ software (https://imagej.net). Information regarding the primary antibodies is listed in Table SII.
Reverse transcription-quantitative PCR (RT-qPCR)
The RNAeasy™ animal RNA extraction kit (cat. no. R0026, Beyotime Institute of Biotechnology) was used to extract total intracellular RNA. mRNA and miRNA were reverse transcribed into cDNA using a mRNA reverse transcription kit (Thermo Fisher Scientific, Inc.) and miRNA reverse transcription kit (Vazyme Biotech Co., Ltd.). SYBR-Green PCR Master Mix (Thermo Fisher Scientific, Inc.) was used for RT-qPCR. Levels of miRNA were normalized using small nuclear RNA U6, whereas ADAM12 levels were normalized by GAPDH. The 2-ΔΔCq method was used to calculate the difference in gene expression (27). The primers were synthesized by Tsingke Co., Ltd. The specific sequences of the primers are shown in Table SIII.
Molecular docking analysis
The structure of ADAM12 and FSTL3 were obtained from AlphaFold prediction (https://alphafold.ebi.ac.uk/). Docking study of the binding modes between ADAM12 and FSTL3 was conducted by using HDOCK server (28–33). According to the docking score provided by the HDOCK server, the best predicted binding mode was selected to analyze the detailed interaction network between these two proteins. The best predicted binding mode was visualized, analyzed and mapped by using the PyMOL program version 2.4.0 (https://www.schrodinger.com/pymol).
Wound healing assay
The indicated HGC27 and AGS cells were seeded at a density of 5×105 cells/well into 6-well plates and cultured to achieve over 80% confluence. Subsequently, we created scratches by using a 200-µl pipette tip to scrape longitudinally in the center of the bottom of the well. After washing twice with PBS, serum-free medium was added to the wells. Images were captured with an optical microscope (magnification, x40; Olympus, Japan) at 0 and 48 h after the scratch appeared. Migration distance was calculated as: Migration distance=scratch width observed at 0 h-scratch width observed at 48 h).
Transwell assay
The Transwell chamber was purchased from BD Biosciences, USA. Approximately 5×104 indicated HGC27 and AGS cells were seeded in the upper chamber and 250 µl serum-free medium was added, while the lower chamber was filled with 750 µl complete medium. After 24 h of incubation, the upper chamber was taken out and fixed with methanol for 10 min, and then stained with 0.1% crystal violet at room temperature. The number of transmembrane cells were counted under a microscope (magnification, ×100; Olympus, Japan).
Statistical methods
Statistical analyses were performed using SPSS 21.0 software (IBM Corp.). The Student's t-test was used to analyze differences between two variables, and differences among multiple groups were analyzed using one-way ANOVA followed by Tukey's post hoc test. Survival curves were constructed using the Kaplan-Meier methods, and the P-value was obtained from the log-rank test. Univariate and multivariate Cox regression analyses were performed to evaluate risk factors for overall survival. A P-value of <0.05 was considered to indicate a statistically significant difference.
Results
ADAM12 expression and its prognostic value in GC
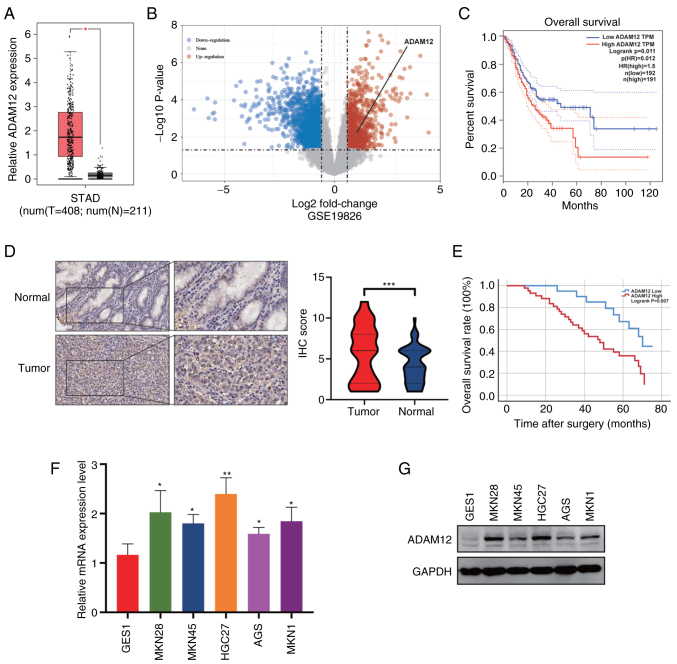
To identify the role of ADAM12 in GC, we performed analysis to determine the expression level of ADAM12 in GC using the GEPIA and GSE19826 dataset. As shown in Fig. 1A and B, the expression of ADAM12 was remarkedly increased in tumor tissues than that in normal tissues. Moreover, the elevated expression of ADAM12 was strongly associated with a poor prognosis in GC patients (Fig. 1C). To further determine the expression level and prognostic value of ADAM12 in GC, IHC staining was performed on a tissue microarray containing 63 pairs of GC tissue and peri-tumor tissue (Fig. S1). In addition, higher protein levels of ADAM12 were observed in tumor tissues in comparison to peri-tumor tissues (Fig. 1D).
Figure 1.
Expression level and prognostic value of ADAM12 in GC tissues and cell lines. (A) The relative expression level of ADAM12 between tumor and normal tissues in GC based on GEPIA database analysis (*P<0.05). (B) The ADAM12 relative expression level in the GSE19826 dataset. (C) The correlation of ADAM12 expression and overall survival (OS) duration in GC patients. (D) The representative immunohistochemical (IHC) staining images (left panel) and score (right panel) of ADAM12 in GC and adjacent non-tumor tissues from a tissue microarray containing 63 paired GC tissue samples (***P<0.001). (E) Kaplan-Meier survival curve of OS for the high and low ADAM12 expression groups based on IHC score from a tissue microarray. (F and G) mRNA and protein expression of ADAM12 in the human gastric mucosal cell line GES1 (control cell line) and five GC cell lines AGS, MKN28, MKN45, HGC27 and MKN1 (*P<0.05; **P<0.01). ADAM12, a disintegrin and metalloprotease 12; GC, gastric cancer; STAD, stomach adenocarcinoma.
We then analyzed the relationship between ADAM12 expression and clinicopathological factors. There was no significant difference in sex, age, BMI, alcohol consumption, venous invasion, operation time, TNM stage and postoperative complications (P>0.05) between the ADAM12 high expression group and ADAM12 low expression group (Table I). The Kaplan-Meier survival curve demonstrated that the overall survival (OS) of patients with elevated levels of ADAM12 expression was significantly shorter than that of patients with low levels of ADAM12 expression (Fig. 1E). In regards to the disease-free survival (DFS), the GC patients with high ADAM12 expression showed a clear trend of adverse prognosis, although the difference was insufficient to reach statistical significance (Fig. S2). Moreover, our results demonstrated that ADAM12 expression level is an independent risk factor for OS based on the results of the multivariate analysis (Table II). The results from RT-qPCR and western blotting showed that ADAM12 was significantly overexpressed in five GC cell lines (MKN28, MKN45, HGC27, AGS and MKN1) compared to the GES1 cell line. (Fig. 1F and G).
Table I.
Associations between ADAM12 protein expression (immunohistochemical staining) in gastric cancer and various clinicopathological variables.
| Variables | Total | ADAM12 expression
|
|||
|---|---|---|---|---|---|
| Low (n=20) | High (n=43) | χ2 | P-value | ||
| Sex | |||||
| Female | 19 | 7 | 12 | 0.326 | 0.568 |
| Male | 44 | 13 | 31 | ||
| Age (years) | |||||
| ≤60 | 25 | 8 | 17 | 0.001 | 0.972 |
| >60 | 38 | 12 | 26 | ||
| BMI (kg/m2) | |||||
| Normal | 34 | 9 | 25 | 0.949 | 0.330 |
| Abnormal | 29 | 11 | 18 | ||
| Alcohol consumption | |||||
| No | 37 | 14 | 23 | 1.535 | 0.215 |
| Yes | 26 | 6 | 20 | ||
| Venous invasion | |||||
| No | 33 | 10 | 23 | 0.067 | 0.796 |
| Yes | 30 | 10 | 20 | ||
| Operation time | |||||
| ≤3 h | 33 | 13 | 20 | 1.871 | 0.171 |
| >3 | 30 | 7 | 23 | ||
| TNM stage | |||||
| I–II | 44 | 16 | 28 | 1.436 | 0.231 |
| III–IV | 19 | 4 | 15 | ||
| Postoperative complications | |||||
| No | 33 | 14 | 19 | 3.647 | 0.056 |
| Yes | 30 | 6 | 24 | ||
ADAM12, a disintegrin and metalloprotease 12; BMI, body mass index.
Table II.
Univariate and multivariate analysis of the correlation between clinicopathological parameters and overall survival of the patients with gastric cancer.
| Variables | Univariate analysis
|
Multivariate analysis
|
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Sex | 1.440 | 0.735-2.825 | 0.288 | |||
| Age (years) | 1.826 | 0.836-3.989 | 0.131 | |||
| BMI (kg/m2) | 1.112 | 0.582-2.123 | 0.748 | |||
| Consumption of alcohol | 0.864 | 0.447-1.672 | 0.665 | |||
| Operation time | 0.985 | 0.516-1.879 | 0.962 | |||
| Venous invasion | 3.509 | 1.705-7.221 | 0.001 | 3.877 | 1.853-8.112 | <0.001 |
| TNM stage | 2.639 | 1.375-5.063 | 0.004 | 2.441 | 1.252-4.757 | 0.009 |
| Postoperative complications | 2.287 | 1.187-4.406 | 0.013 | 2.374 | 1.187-4.749 | 0.015 |
| ADAM12 expression | 2.720 | 1.270-5.827 | 0.010 | 2.315 | 1.076-4.984 | 0.032 |
ADAM12, a disintegrin and metalloprotease 12; BMI, body mass index; HR, hazard ratio; CI, confidence interval.
ADAM12 DNA methylation status and ceRNA regulatory network in GC
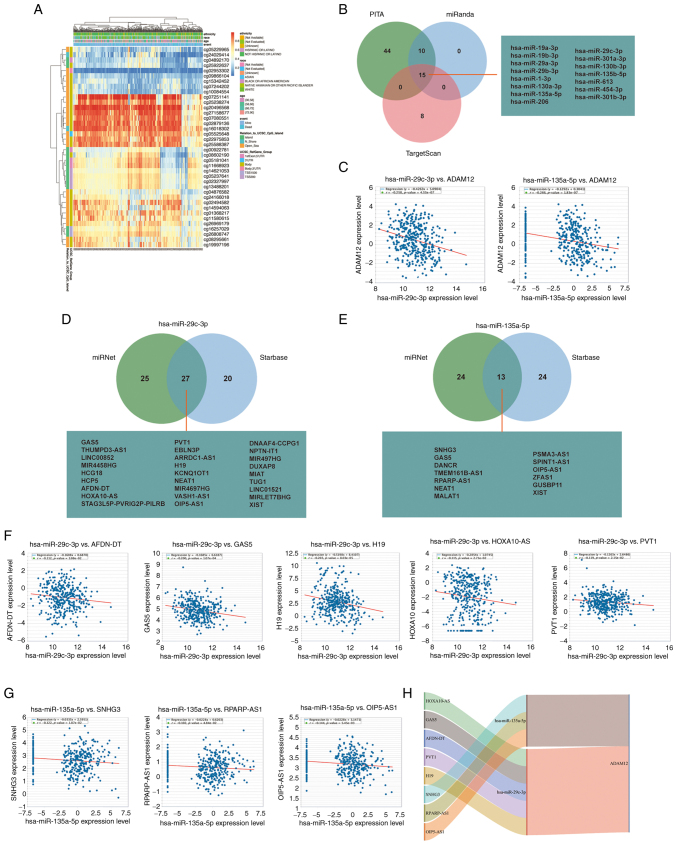
Aberrant DNA methylation level and pattern resulted in abnormal activation of proto-oncogenes (34). Therefore, we attempted to investigate the DNA methylation status of ADAM12 in GC. As shown in Fig. 2A, we determined that cg05229965, cg24029414, cg04892170, cg25922637, cg02953302, cg09866104, cg15342452, cg07244202, and cg10084554 sites exist with lower levels of DNA methylation based on the data obtained from TCGA-STAD. More importantly, cg04892170 and cg02953302 are located in the region of the 1st exon and 5'UTR. Hypomethylation in these regions may reveal the cause of the abnormally elevated ADAM12 expression.
Figure 2.
Regulatory mechanisms of ADAM12 overexpression in GC. (A) Waterfall plot of the methylation status in the ADAM12 gene. (B) The predicted microRNAs of ADAM12 targets in PITA, miRanda, and TargetScan databases. (C) Scatter plots of predicted miRNAs (hsa-miR-29c-3p and hsa-miR-135a-5p) and ADAM12 expression showing a negative correlation. (D and E) Venn diagrams which show the target lncRNAs of hsa-miR-29c-3p and hsa-miR-135a-5p. (F and G) Scatter plots of the predicted lncRNA and miRNA (hsa-miR-29c-3p and hsa-miR-135a-5p) expression showing a negative correlation. (H) The Sankey diagram which shows the lncRNA-miRNA-mRNA (ADAM12) regulatory network in line with the ceRNA hypothesis. ADAM12, a disintegrin and metalloprotease 12; GC, gastric cancer; ceRNAs, competing endogenous RNAs; lncRNAs, long non-coding RNAs.
Emerging evidence highlights that aberrant ceRNA networks are important tumor promoters in human cancers. Therefore, we attempted to construct the ceRNA network of ADAM12 in GC. Based on Starbase3.0 database analysis, a series of target miRNAs of ADAM12 were predicted. As shown in Fig. 2B, a total of 15 target miRNAs were jointly predicted by PITA, miRanda, and TargetScan databases. Among them, the expression of hsa-miR-29c-3p (R=-0.258, P<0.0001) and hsa-miR-135a-5p (R=−0.266, P<0.0001) were negatively correlated with the expression level of ADAM12 (Fig. 2C). To clarify the relationship of hsa-miR-29c-3p and hsa-miR-135a-5p with ADAM12, the gain of hsa-miR-29c-3p and hsa-miR-135a-5p were performed in HGC27 cells. RT-qPCR experiment confirmed the successful transfection efficiency of hsa-miR-29c-3p and hsa-miR-135a-5p mimics (Fig. S3A). The western blotting experiments demonstrated that hsa-miR-29c-3p and hsa-miR-135a-5p can indeed inhibit the expression of ADAM12 in HGC27 cells (Fig. S3B).
Subsequently, we used the miRNet and starBase online databases to further predict the lncRNAs that could bind to the two target miRNAs (hsa-miR-29c-3p and hsa-miR-135a-5p) (Fig. 2D and E). The ceRNA network hypothesis indicated that there was a negative correlation between lncRNAs and miRNAs. As shown in Fig. 2F and G, the expression levels of the 5 lncRNAs (AFDN-DT, GAS5, H19, HOXA10-AS and PVT1) were negatively correlated with hsa-miR-29c-3p, while 3 lncRNAs (SNHG3, RPARP-AS1 and OIP5-AS1) were negatively correlated with the expression level of hsa-miR-135a-5p. Therefore, we could construct 8 pairs of ceRNA networks based on the results of the correlation analysis (Fig. 2H).
Enrichment analysis of the ADAM12 gene co-expression profiles in GC
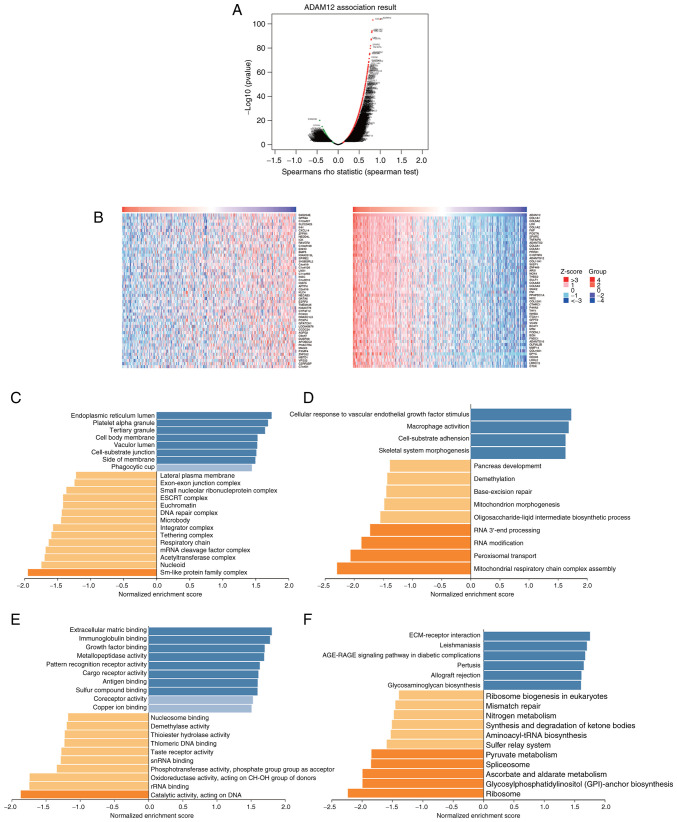
To better understand the biological function of ADAM12 in GC, LinkedOmics was applied to perform a co-expression gene profile analysis. As shown in Fig. 3A, a total of 9,307 genes were found to be significantly positively correlated with ADAM12 (red dots), while 10,919 genes showed significantly negative correlations (green dots). The top 50 genes that were significantly positively or negatively correlated with ADAM12 are shown on a heat map (Fig. 3B).
Figure 3.
Co-expressed profiles and enriched analyses of ADAM12 in GC. (A) The volcano plot of ADAM12 co-expressed genes according to LinkedOmics database analysis. (B) The heatmap of the top 50 genes significantly positively or negatively correlated with ADAM12 expression. (C) Biological process enrichment analysis for ADAM12 co-expressed genes by LinkedOmics using GSEA methods. (D) Molecular function enrichment analysis for ADAM12 co-expressed genes by LinkedOmics using GSEA methods; (E) Cellular component enrichment analysis for ADAM12 co-expressed genes by LinkedOmics using GSEA methods. (F) KEGG pathway analysis for ADAM12 co-expressed genes by LinkedOmics using GSEA methods visualized as bar chart. ADAM12, a disintegrin and metalloprotease 12; GC, gastric cancer.
Next, enrichment analysis was performed based on GO functions and KEGG pathways using GSEA methods. The main biological process was identified as 'endoplasmic reticulum lumen', 'platelet alpha granule', while the most enriched molecular function was 'cellular response to vascular endothelial growth factor stimulus' and 'macrophage activation' (Fig. 3C and D). The top two cellular component terms were 'extracellular matrix binding' and 'immunoglobulin binding' (Fig. 3E). KEGG pathway analysis revealed that the most enriched pathways were 'ECM-receptor interaction' and 'Leishmaniasis' (Fig. 3F).
The protein-protein interaction network of ADAM12
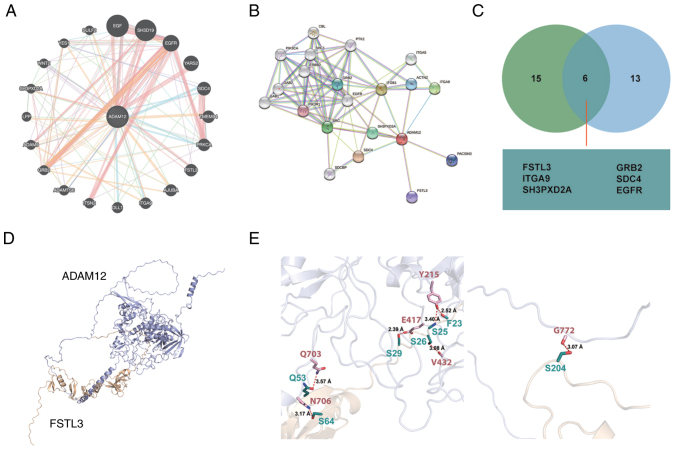
The interactions between proteins is the foundation of many biological processes (35). To identify the potential binding partner of ADAM12, the GeneMANIA and STRING data- bases were used to construct the protein-protein interaction network of ADAM12 (Fig. 4A and B). Proteins, including FSTL3, GRB2, ITGA9, SDC4, SH3PXD2A and EGFR, were identified as potential common binding partners of ADAM12 through the combined results obtained from the GeneMANIA and STRING databases (Fig. 4C).
Figure 4.
Protein-protein interaction network of ADAM12 and the binding mode of FSTL3 on ADAM12 as predicted by docking. (A) Interaction network of ADAM12 constructed by GeneMANIA. (B) Interaction network of ADAM12 constructed by STRING. (C) Venn diagram showing the commonly interactive proteins with ADAM12 based on GeneMANIA and STRING databases. (D) Overall structure of FSTL3 bound to ADAM12 in cartoon view. ADAM12 and FSTL3 are colored in silver white and light yellow respectively. (E) Detailed interaction network between ADAM12 and FSTL3 hexamer. Key residues of ADAM12 (pink) and fstl3 (deep teal) are displayed as sticks. H-bonds are displayed in red dash lines and the distances (acceptor to donor heavy atom) of H-bonds are labeled. ADAM12, a disintegrin and metalloprotease 12; FSTL3, follistatin like 3.
Previous studies suggest the interaction of ADAM12 with certain proteins, such as GRB2 (36), SH3PXD2A (37,38) and EGFR (39,40). However, the relationship between ADAM12 and follistatin like 3 (FSTL3) has not been reported until now. Due to the similarity in cell location and function of ADAM12 and FSTL3, we performed docking experiment to investigate the binding mode between ADAM12 and FSTL3. As shown in Fig. 4D, the F23 and S29 of FSTL3 were able to form hydro-bonding interactions with Y215 and E417 of ADAM12, respectively. Moreover, the distances between the acceptor and donor heavy atom of the two hydrogen bonds were both less than 3 Å, which indicates that these two hydrogen bonds are quite stable (Fig. 4E, left panel). Moreover, the Y215 and V432 of ADAM12 were also able to form hydrogen-bonding interactions with S25 and S26 of FSTL3, respectively. Each of the two residues, Q53 and S64 of FSTL3, were able to form hydrogen-bonding interaction with the transmembrane helical region of ADAM12. A hydrogen-bonding interaction was also found between the S204 of FSTL3 and G772 of ADAM12 (Fig. 4E, right panel).
Key role of ADAM12 in GC cell migration, invasion and EMT-like phenotype
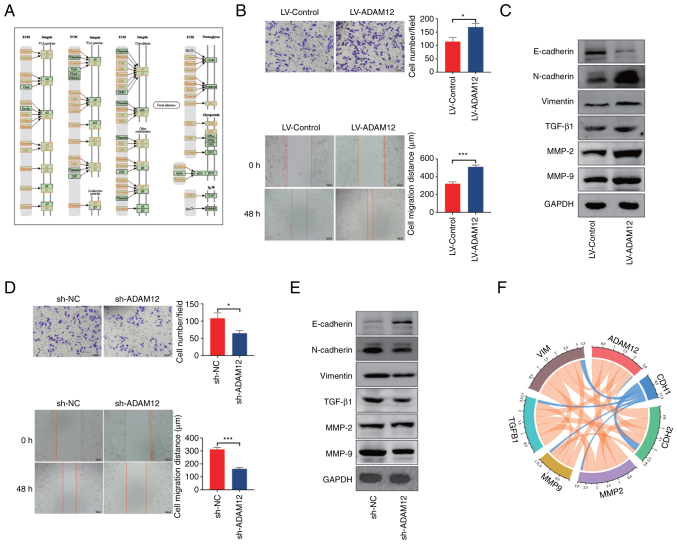
According to the results of our enrichment analysis, ADAM12 may participate in the 'ECM-receptor interaction' signaling pathway. As shown in Fig. 5A, most of the molecules in this signaling pathway have a significant relationship with the EMT phenotype. The analysis of the interaction network indicated that ADAM12 may interact with FSTL3 to potentially promote the metastasis and EMT phenotype of tumors (41,42). Therefore, we constructed stable ADAM12 overexpression AGS cells and knockdown HGC27 cells (Fig. S4). The in vitro assay demonstrated that ADAM12 overexpression significantly promoted the migration and invasion abilities of AGS cells (Fig. 5B), while ADAM12 knockdown significantly reduced the migration and invasion ability of HGC27 cells (Fig. 5D). In addition, ADAM12 overexpression significantly downregulated the expression of E-cadherin and upregulated the expression of TGF-β1, matrix metalloproteinase (MMP)-2, MMP-9, N-cadherin and vimentin in AGS cells (Fig. 5C). In contrast, knockdown of ADAM12 markedly downregulated TGF-β1, MMP-2, MMP-9, N-cadherin, and vimentin expression but promoted the expression of E-cadherin protein in HGC27 cells (Fig. 5E).
Figure 5.
Effect of ADAM12 on migration, invasion and the EMT-like phenotype in GC cells. (A) The diagram of ECM-receptor interaction signaling pathway. (B) ADAM12 overexpression enhances the migration and invasion ability in AGS cells (*P<0.05, ***P<0.001). (C) ADAM12 overexpression promotes the EMT-like phenotype in AGS cells. (D) ADAM12 knockdown interferes with the migration and invasion ability in HGC27 cells (*P<0.05, ***P<0.001). (E) ADAM12 knockdown inhibits the EMT-like phenotype in HGC27 cells. (F) Correlation coefficient circles for ADAM12 and EMT-related genes from the TIMER database. Pink lines represent positive correlations and blue lines represent negative correlations. ADAM12, a disintegrin and metalloprotease 12; GC, gastric cancer; EMT, epithelial-mesenchymal transition.
In addition, the correlation analysis conducted using the TIMER database demonstrated that ADAM12 expression was associated with CDH2 (R=0.492, P=1.58e-24), MMP-2 (R= 0.628, P=5.58e-43), MMP-9 (R= 0.407, P=1.59e-16), TGF-β1 (R=0.450, P=2.83e-20), and vimentin expression (R=0.554, P=7.21e-32) but negatively correlated with CDH1 expression (R=-0.061, P=2.23e-01), which is consistent with the result of the in vitro experiments (Fig. 5F). Based on the above results, it was suggested that ADAM12 may regulate the ability of metastasis and EMT in GC cells in vitro.
Correlation between ADAM12 and tumor immune infiltration
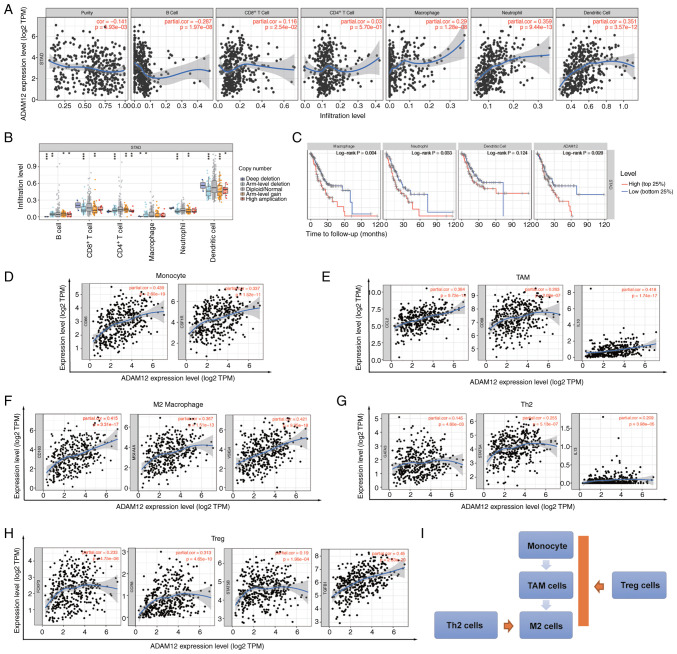
Crosstalk between tumor cells and tumor-infiltrating immune cells is extremely important for cancer development and affects treatment outcomes (43). Our results indicated that ADAM12 is involved in interactions between cells and the extracellular matrix, as well as between tumor cells. Therefore, we aimed to explore the role of ADAM12 in the GC tumor immune environment. Based on the results of the TIMER database analysis, we observed a positive correlation between ADAM12 expression and infiltrating levels of CD8+ T cells (R=0.116, P=2.54e-02), macrophages (R=0.29, P=1.28e-08), neutrophils (R=0.359, P=9.44e-13), and dendritic cells (R=0.351, P=3.57e-12) in GC (Fig. 6A). We also analyzed the effect of ADAM12 copy number alternation (CNA) on the infiltration level of six immune cells. As shown in Fig. 6B, the deep deletion, arm-level deletion and arm-level gain of ADAM12 significantly affected the level of infiltration level of B cells, CD8+ T cells, CD4+ T cells, neutrophils, macrophages, and dendritic cells in GC. In addition, macrophages, neutrophils, dendritic cells, and ADAM12 were identified as factors involved with the cumulative overall survival rate of GC patients (Fig. 6C).
Figure 6.
Correlation between ADAM12 expression and immune cell infiltration in GC. (A) Correlation of ADAM12 expression with tumor purity and infiltrating levels of B cells, CD8+ T cells, CD4+ T cells, macrophages, neutrophils and dendritic cells in GC. (B) Effect of ADAM12 copy number variation (CNV) on the immune infiltration level of B cells, CD8+ T cells, CD4+ T cells, macrophages, neutrophils and dendritic cells in GC. (C) The survival plot of immune cell infiltration and ADAM12 expression on GC patients. (D) Correlation between ADAM12 expression and markers of monocyte cells. (E) Correlation between ADAM12 expression and markers of tumor-associated macrophage (TAM) cells. (F) Correlation between ADAM12 expression and markers of M2 cells. (G) Correlation between ADAM12 expression and markers of Th2 macrophage cells. (H) Correlation between ADAM12 expression and markers of Treg cells. (I) The schematic diagram for the role of ADAM12 in immune infiltration cells. ADAM12, a disintegrin and metalloprotease 12; GC, gastric cancer.
T cell and macrophage infiltration is strongly associated with the clinical outcome of patients with malignant tumors. Therefore, we decided to explore the correlation between ADAM12 expression and the differentiation status of macrophages and T cell subpopulation. As shown in Fig. 6D-F, ADAM12 was significantly positively correlated with the cell markers CD86 (R=0.439, P=2.60e-19) and CSF1R (R=0.337, P=1.52e-11) in monocytes, and the cell markers CCL2 (R=0.384, P=9.72e-15), CD68 (R=0.263, P=2.08e-07), IL10 (R=0.418, P=1.74e-17) in tumor-associated macrophages (TAMs), as well as the M2 macrophage cell markers CD163 (R=0.415, P=3.31e-17), MS4A4A (R=0.367, P=1.51e-13), and VSIG4 (R=0.421, P=9.35e-18). It is well known that macrophages are polarized into an M2 subpopulation upon Th2 and Treg cell stimulation. Intriguingly, ADAM12 expression was significantly positively associated with the expression of Th2 cell markers, GATA3 (R=0.145, P=4.60e-03), STAT5A (R=0.255, P=5.13e-07), and IL13 (R=0.209, P=3.98e-05), and the Treg cell markers FOXP3 (R=0.233, P=4.75e-06), CCR8 (R=0.313, P=4.65e-10), STAT5B (R=0.19, P=1.96e-04), and TGF- β1 (R=0.45, P=2.63e-20) (Fig. 6G and H). Therefore, we speculated that ADAM12 may further promote the polarization of M2 macrophages by regulating the maturation of monocytes, TAMs, Th2 and Treg cells in the tumor microenvironment (Fig. 6I).
Discussion
Cumulated evidence has demonstrated important roles for ADAM family proteins in tumor formation and progression (44). ADAM12, an important member of the ADAM family, promotes the proliferation and metastasis of tumor cells by participating in biological processes such as enzyme catalysis, cell-cell binding and cell signal transduction (8). Although previous studies have shown that ADAM12 is highly expressed in GC (45), the clinical value and biological function of ADAM12 in GC have not yet been fully elucidated.
In the present study, it was demonstrated that the expression level of ADAM12 was elevated in GC. Moreover, GC patients with high ADAM12 expression demonstrated a poorer prognosis. To determine the potential causes of ADAM12 elevation, the DNA methylation status and ceRNA regulatory network of ADAM12 were investigated, respectively. Intriguingly, two hypomethylation sites were identified in the ADAM12 promoter region, indicating a potential cause of ADAM12 overexpression. In addition, we constructed eight vital ceRNA networks of ADAM12 using bioinformatics analysis. Although previous studies have shown that ADAM12 may be regulated by a ceRNA network (9,46), our newly discovered ceRNA networks need further experimental verification.
To gain further insights into the biological functions of ADAM12 in GC, GO and KEGG enrichment analyses of ADAM12 co-expressed genes in GC were conducted. We found that they were mainly involved in 'macrophage activation', 'extracellular matrix binding', 'immunoglobulin binding' and 'ECM-receptor interaction', indicating the potential role of ADAM12 in tumor metastasis and tumor immune infiltration. Interestingly, the protein-protein interaction network revealed that follistatin like 3 (FSTL3) may be a potential partner of ADAM12. Previous studies have emphasized that FSTL3 can enhance tumor cell metastasis (41,42) and the polarization of macrophages and fibroblasts by forming an inhibitory immune microenvironment (47). Therefore, it is clear that ADAM12 and FSTL3 perform similar functions, which may imply a mutual adjustment relationship between them. The subsequent molecular docking model was used to predict potential binding sites between them to further determine their interactions.
Next, we attempted to determine whether ADAM12 plays a crucial role in GC metastasis and tumor immune invasion. A stable overexpression cell line, LV-ADAM12-AGS, and a knockdown cell line, sh-ADAM12-HGC27 were constructed. As expected, wound healing and Transwell experiments confirmed that ADAM12 could enhance the migration and metastatic abilities of GC cells. In addition, the expression of epithelial-mesenchymal transition (EMT)-related markers were assessed and this demonstrated that ADAM12 plays an important role in regulating EMT. This is similar to the results of a previous study conducted on pituitary tumors (48). Therefore, we speculated that ADAM12 may promote the migration and metastasis of GC cells by inducing EMT.
The role of ADAM12 in GC immune infiltration was another focus of our investigation. In the present study, the expression level of ADAM12 was significantly correlated with multiple immune cell infiltration in GC. Our results based on data mining indicated that ADAM12 expression was significantly positively correlated with M2 macrophages. In addition, a positive correlation was demonstrated between ADAM12 and monocytes, tumor associated macrophages (TAMs), Th2 and Treg cells by analyzing the correlation between immune cell-specific markers and ADAM12 expression levels. It is well known that TAMs derived from circulating monocyte populations can be differentiated into M2 macrophages under the stimulation of Treg and Th2 cells (49,50). Therefore, we speculated that ADAM12, as a ubiquitously expressed transmembrane protein in tumor cells, may participate in the recruitment of various immune cells (including monocytes, TAM, Th2 and Treg cells), which in turn promotes the polarization and maturation of M2 macrophages. M2 macrophages are known to be a necessary factor in favoring tumor metastasis and immunosuppression. These results suggest the promoting role of ADAM12 in tumorigenesis and progression from another perspective, indicating the complex function of ADAM12 in tumors.
There are still some limitations to the present study. Firstly, the small sample size resulted in an unclear relationship between ADAM12 expression levels and disease-free survival (DFS) in gastric cancer patients. The collection of GC tissue samples and follow-up data in subsequent studies is required to further analyze the correlation between ADAM12 and DFS. Secondly, the present study was based on public data mining and bioinformatic analysis. The available data was limited and the results still require corresponding experimental verification. Furthermore, sufficient data was lacking to explore the role of ADAM12 in regulating immune cell recruitment, and more sophisticated experimental designs are required to validate these promising results.
In the present study, we demonstrated that ADAM12 expression is frequently upregulated and correlated with a poor prognosis of GC patients. Promoter hypomethylation and aberrant ceRNA network regulation may contribute to the dysregulation of ADAM12 expression. The results of enrichment analysis and protein-protein interaction network indicated that ADAM12 is probably involved in a variety of pivotal biological processes regulating GC metastasis and the immune microenvironment. Through further experimental analysis, we successfully confirmed that ADAM12 significantly enhances the invasion and metastatic abilities of GC cells. More importantly, it was found that ADAM12 potentially plays a crucial role in tumor immune infiltration, especially the polarization of M2 cells. In conclusion, our results highlight that ADAM12 is a vital tumor promoter and a potential therapeutic target for GC.
Supplementary Data
Acknowledgments
Not applicable.
Funding Statement
This work was supported by the Natural Science Foundation of Anhui Province (1908085MH282).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
HZ, WJ and XH designed the study and wrote the manuscript. HZ, HZ, BT and ZZ performed the experiments. WJ, JH and XH performed patient recruitment and sample collection. HZ, WJ, JH and XH analyzed and verified the integrity of the data. All authors contributed to the article and approved the submitted version. All authors read and approved the manuscript and agree to be accountable for all aspects of the research in ensuring that the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethics approval and consent to participate
The studies involving human participants were reviewed and approved by the Medical Ethics Committee of the Affiliated Provincial Hospital of Anhui Medical University (certification no. 2019KY32). The patients/participants provided their written informed consent to participate in this study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Rawla P, Barsouk A. Epidemiology of gastric cancer: Global trends, risk factors and prevention. Prz Gastroenterol. 2019;14:26–38. doi: 10.5114/pg.2018.80001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thrift AP, El-Serag HB. Burden of gastric cancer. Clin Gastroenterol Hepatol. 2020;18:534–542. doi: 10.1016/j.cgh.2019.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shen L, Li J, Xu J, Pan H, Dai G, Qin S, Wang L, Wang J, Yang Z, Shu Y, et al. Bevacizumab plus capecitabine and cisplatin in Chinese patients with inoperable locally advanced or metastatic gastric or gastroesophageal junction cancer: Randomized, double-blind, phase III study (AVATAR study) Gastric Cancer. 2015;18:168–176. doi: 10.1007/s10120-014-0351-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lordick F, Kang YK, Chung HC, Salman P, Oh SC, Bodoky G, Kurteva G, Volovat C, Moiseyenko VM, Gorbunova V, et al. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): A randomised, open-label phase 3 trial. Lancet Oncol. 2013;14:490–499. doi: 10.1016/S1470-2045(13)70102-5. [DOI] [PubMed] [Google Scholar]
- 6.Thuss-Patience PC, Shah MA, Ohtsu A, Van Cutsem E, Ajani JA, Castro H, Mansoor W, Chung HC, Bodoky G, Shitara K, et al. Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): An international randomised, open-label, adaptive, phase 2/3 study. Lancet Oncol. 2017;18:640–653. doi: 10.1016/S1470-2045(17)30111-0. [DOI] [PubMed] [Google Scholar]
- 7.Saha N, Robev D, Himanen JP, Nikolov DB. ADAM proteases: Emerging role and targeting of the non-catalytic domains. Cancer Lett. 2019;467:50–57. doi: 10.1016/j.canlet.2019.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kveiborg M, Albrechtsen R, Couchman JR, Wewer UM. Cellular roles of ADAM12 in health and disease. Int J Biochem Cell Biol. 2008;40:1685–1702. doi: 10.1016/j.biocel.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 9.Huang X, Xie X, Liu P, Yang L, Chen B, Song C, Tang H, Xie X. Adam12 and lnc015192 act as ceRNAs in breast cancer by regulating miR-34a. Oncogene. 2018;37:6316–6326. doi: 10.1038/s41388-018-0410-1. [DOI] [PubMed] [Google Scholar]
- 10.Luo ML, Zhou Z, Sun L, Yu L, Sun L, Liu J, Yang Z, Ran Y, Yao Y, Hu H. An ADAM12 and FAK positive feedback loop amplifies the interaction signal of tumor cells with extracellular matrix to promote esophageal cancer metastasis. Cancer Lett. 2018;422:118–128. doi: 10.1016/j.canlet.2018.02.031. [DOI] [PubMed] [Google Scholar]
- 11.Wang X, Wang Y, Gu J, Zhou D, He Z, Wang X, Ferrone S. ADAM12-L confers acquired 5-fluorouracil resistance in breast cancer cells. Sci Rep. 2017;7:9687. doi: 10.1038/s41598-017-10468-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duhachek-Muggy S, Qi Y, Wise R, Alyahya L, Li H, Hodge J, Zolkiewska A. Metalloprotease-disintegrin ADAM12 actively promotes the stem cell-like phenotype in claudin-low breast cancer. Mol Cancer. 2017;16:32. doi: 10.1186/s12943-017-0599-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu H, Wang G, Zhu H, Xu A. ITGA5 is a prognostic biomarker and correlated with immune infiltration in gastrointestinal tumors. BMC Cancer. 2021;21:269. doi: 10.1186/s12885-021-07996-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: A web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45:W98–W102. doi: 10.1093/nar/gkx247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Q, Wen YG, Li DP, Xia J, Zhou CZ, Yan DW, Tang HM, Peng ZH. Upregulated INHBA expression is associated with poor survival in gastric cancer. Med Oncol. 2012;29:77–83. doi: 10.1007/s12032-010-9766-y. [DOI] [PubMed] [Google Scholar]
- 16.Modhukur V, Iljasenko T, Metsalu T, Lokk K, Laisk-Podar T, Vilo J. MethSurv: A web tool to perform multivariable survival analysis using DNA methylation data. Epigenomics. 2018;10:277–288. doi: 10.2217/epi-2017-0118. [DOI] [PubMed] [Google Scholar]
- 17.Li JH, Liu S, Zhou H, Qu LH, Yang JH. starBase v2.0: Decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 2017;42:D92–D97. doi: 10.1093/nar/gkt1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou KR, Liu S, Cai L, Bin L. The Encyclopedia of RNA Interactomes (ENCORI): The Encyclopedia of RNA Interactomes, 2021 [Google Scholar]
- 19.Kertesz M, Iovino N, Unnerstall U, Gaul U, Segal E. The role of site accessibility in microRNA target recognition. Nat Genet. 2007;39:1278–1284. doi: 10.1038/ng2135. [DOI] [PubMed] [Google Scholar]
- 20.Betel D, Koppal A, Agius P, Sander C, Leslie C. Comprehensive modeling of microRNA targets predicts functional non-conserved and non-canonical sites. Genome Biol. 2010;11:R90. doi: 10.1186/gb-2010-11-8-r90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grimson A, Farh KK, Johnston WK, Garrett-Engele P, Lim LP, Bartel DP. MicroRNA targeting specificity in mammals: Determinants beyond seed pairing. Mol Cell. 2007;27:91–105. doi: 10.1016/j.molcel.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang L, Zhou G, Soufan O, Xia J. miRNet 2.0: Network-based visual analytics for miRNA functional analysis and systems biology. Nucleic Acids Res. 2020;48:W244–W251. doi: 10.1093/nar/gkaa467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vasaikar SV, Straub P, Wang J, Zhang B. LinkedOmics: Analyzing multi-omics data within and across 32 cancer types. Nucleic Acids Res. 2018;46:D956–D963. doi: 10.1093/nar/gkx1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Warde-Farley D, Donaldson SL, Comes O, Zuberi K, Badrawi R, Chao P, Franz M, Grouios C, Kazi F, Lopes CT, et al. The GeneMANIA prediction server: Biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res. 2010;38:W214–W220. doi: 10.1093/nar/gkq537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, Simonovic M, Doncheva NT, Morris JH, Bork P, Jensen LJ, Mering CV. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47:D607–D613. doi: 10.1093/nar/gky1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li T, Fan J, Wang B, Traugh N, Chen Q, Liu JS, Li B, Liu XS. TIMER: A web server for comprehensive analysis of tumor-infiltrating immune cells. Cancer Res. 2017;77:e108–e110. doi: 10.1158/0008-5472.CAN-17-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 28.Remmert M, Biegert A, Hauser A, Söding J. HHblits: Lightning-fast iterative protein sequence searching by HMM-HMM alignment. Nature Methods. 2011;9:173–175. doi: 10.1038/nmeth.1818. [DOI] [PubMed] [Google Scholar]
- 29.Pearson WR, Lipman DJ. Improved tools for biological sequence comparison. Proc Natl Acad Sci USA. 1988;85:2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Söding J, et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol. 2011;7:539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 32.Martí-Renom MA, Stuart AC, Fiser A, Sánchez R, Melo F, Sali A. Comparative protein structure modeling of genes and genomes. Annu Rev Biophys Biomol Struct. 2000;29:291–325. doi: 10.1146/annurev.biophys.29.1.291. [DOI] [PubMed] [Google Scholar]
- 33.Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE. The protein data bank. Nucleic Acids Res. 2000;28:235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kulis M, Esteller M. DNA methylation and cancer. Adv Genet. 2010;70:27–56. doi: 10.1016/B978-0-12-380866-0.60002-2. [DOI] [PubMed] [Google Scholar]
- 35.Liu X, Salokas K, Tamene F, Jiu Y, Weldatsadik RG, Öhman T, Varjosalo M. An AP-MS- and BioID-compatible MAC-tag enables comprehensive mapping of protein interactions and subcellular localizations. Nat Commun. 2018;9:1188. doi: 10.1038/s41467-018-03523-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suzuki A, Kadota N, Hara T, Nakagami Y, Izumi T, Takenawa T, Sabe H, Endo T. Meltrin alpha cytoplasmic domain interacts with SH3 domains of Src and Grb2 and is phosphorylated by v-Src. Oncogene. 2000;19:5842–5580. doi: 10.1038/sj.onc.1203986. [DOI] [PubMed] [Google Scholar]
- 37.Malinin NL, Wright S, Seubert P, Schenk D, Griswold-Prenner I. Amyloid-beta neurotoxicity is mediated by FISH adapter protein and ADAM12 metalloprotease activity. Proc Natl Acad Sci USA. 2005;102:3058–3063. doi: 10.1073/pnas.0408237102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harold D, Jehu L, Turic D, Hollingworth P, Moore P, Summerhayes P, Moskvina V, Foy C, Archer N, Hamilton BA, et al. Interaction between the ADAM12 and SH3MD1 genes may confer susceptibility to late-onset Alzheimer's disease. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:448–452. doi: 10.1002/ajmg.b.30456. [DOI] [PubMed] [Google Scholar]
- 39.Wang R, Godet I, Yang Y, Salman S, Lu H, Lyu Y, Zuo Q, Wang Y, Zhu Y, Chen C, et al. Hypoxia-inducible factor-dependent ADAM12 expression mediates breast cancer invasion and metastasis. Proc Natl Acad Sci USA. 2021;118:e2020490118. doi: 10.1073/pnas.2020490118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Díaz B, Yuen A, Iizuka S, Higashiyama S, Courtneidge SA. Notch increases the shedding of HB-EGF by ADAM12 to potentiate invadopodia formation in hypoxia. J Cell Biol. 2013;201:279–292. doi: 10.1083/jcb.201209151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Y, Tian M, Liu W, Wang D, Zhou Z, Pei Q, Huang Y, Tan F, Güngör C. Follistatin-Like 3 enhances invasion and metastasis via β-catenin-mediated EMT and aerobic glycolysis in colorectal cancer. Front Cell Dev Biol. 2021;9:660159. doi: 10.3389/fcell.2021.660159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dai ZT, Xiang Y, Zhang XY, Zong QB, Wu QF, Huang Y, Shen C, Li JP, Ponnambalam S, Liao XH. Regulation of follistatin-like 3 expression by miR-486-5p modulates gastric cancer cell proliferation, migration and tumor progression. Aging (Albany NY) 2021;13:20302–20318. doi: 10.18632/aging.203412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang J, Li D, Cang H, Guo B. Crosstalk between cancer and immune cells: Role of tumor-associated macrophages in the tumor microenvironment. Cancer Med. 2019;8:4709–4721. doi: 10.1002/cam4.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duffy MJ, McKiernan E, O'Donovan N, McGowan PM. Role of ADAMs in cancer formation and progression. Clin Cancer Res. 2009;15:1140–1144. doi: 10.1158/1078-0432.CCR-08-1585. [DOI] [PubMed] [Google Scholar]
- 45.Carl-McGrath S, Lendeckel U, Ebert M, Roessner A, Röcken C. The disintegrin-metalloproteinases ADAM9, ADAM12, and ADAM15 are upregulated in gastric cancer. Int J Oncol. 2005;26:17–24. [PubMed] [Google Scholar]
- 46.Wang H, Wu J, Guo W. SP1-Mediated upregulation of lncRNA LINC01614 Functions a ceRNA for miR-383 to facilitate glioma progression through regulation of ADAM12. Onco Targets Ther. 2020;13:4305–4318. doi: 10.2147/OTT.S242854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang C, Cao F, Huang S, Zheng Y. Follistatin-like 3 correlates with lymph node metastasis and serves as a biomarker of extracellular matrix remodeling in colorectal cancer. Front Immunol. 2021;12:717505. doi: 10.3389/fimmu.2021.717505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang J, Zhang Z, Li R, Mao F, Sun W, Chen J, Zhang H, Bartsch JW, Shu K, Lei T. ADAM12 induces EMT and promotes cell migration, invasion and proliferation in pituitary adenomas via EGFR/ERK signaling pathway. Biomed Pharmacother. 2018;97:1066–1077. doi: 10.1016/j.biopha.2017.11.034. [DOI] [PubMed] [Google Scholar]
- 49.Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F, Seifi B, Mohammadi A, Afshari JT, Sahebkar A. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233:6425–6440. doi: 10.1002/jcp.26429. [DOI] [PubMed] [Google Scholar]
- 50.Weirather J, Hofmann UD, Beyersdorf N, Ramos GC, Vogel B, Frey A, Ertl G, Kerkau T, Frantz S. Foxp3+ CD4+ T cells improve healing after myocardial infarction by modulating monocyte/macrophage differentiation. Circ Res. 2014;115:55–67. doi: 10.1161/CIRCRESAHA.115.303895. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.