Abstract
Anti-β2-glycoprotein I (anti-β2GPI) is an anti-phospholipid antibody that specifically binds to β2GPI. There is growing evidence that this autoantibody is closely linked to specific thrombotic conditions. Cerebral infarction (CI) is a form of thrombosis associated with high rates of morbidity and mortality. In the present study, it was determined that patients with CI exhibited significantly increased serum anti-β2GPI levels as well as increased NLR family pyrin domain containing 3 (NLRP3) expression within neutrophils, suggesting a potential role for inflammatory cell death in this pathological context. Specifically, it was determined that anti-β2GPI/β2GPI is able to induce neutrophil pyroptosis, thereby driving these cells to release IL-1β via a pathway regulated by cell surface Toll-like receptor 4 expression. At the mechanistic level, the double-stranded RNA-dependent protein kinase/p38MAPK/NLRP3 pathway was indicated to govern anti-β2GPI/β2GPI-induced neutrophil pyroptosis. These pyroptotic neutrophils were also observed to release large amounts of high mobility group box protein 1, which, together with IL-1β, promoted IL-8 and intercellular cell adhesion molecule-1 upregulation in endothelial cells. In summary, these data suggest that inhibiting neutrophil pyroptosis may represent a viable approach to treating anti-β2GPI anti- body-associated CI.
Keywords: anti-β2GPI, β2GPI, neutrophils, pyroptosis, endothelial cells
Introduction
Thrombotic diseases and events such as cerebral infarction (CI) are among the most common causes of global morbidity and mortality. Endothelial cell activation, particularly in response to inflammation-associated damage, is thought to be a key driver of these thrombotic processes. Antiphospholipid syndrome (APS) is a rare form of autoimmunity that may increase the risk of thrombotic events and eclampsia in affected individuals (1). Patients with APS exhibit persistent upregulation of autoantibodies including anti-phospholipid antibody, lupus anticoagulant, anti-cardiolipin and anti-β2-glycoprotein I (anti-β2GPI). Anti-β2GPI is thought to have a particularly critical role in the context of thrombus formation (2,3), yet its precise mechanistic pro-thrombotic function remains to be fully elucidated.
β2GPI is a five-domain phospholipid-bound protein and the primary target antigen for anti-β2GPI. Circulating anti-β2GPI/β2GPI immune complexes (ICs) in patients with APS are associated with thrombotic events (4,5). A previous study by our group indicated that these anti-β2GPI/β2GPI ICs may activate platelets and thereby induce thrombosis (6), while also contributing to the neutrophil-mediated release of pro-thrombotic neutrophil extracellular traps (NETs) (7). Neutrophils are the most common leukocytes in the circulation and function as key mediators of innate immune and inflammatory responses (8). In the present study, it was hypothesized that neutrophil-related inflammation may be involved in endothelial cell activation and thrombosis in patients with APS.
Pyroptosis is a form of programmed cell death that was first detected in Shigella flexneri-infected macrophages in 1992 (9), before ultimately being named by Cookson and Brennan (10) in 2001. Pyroptotic cell death is characterized by nucleotide-binding oligomerization domain-like receptor pyrin domain containing 3 (NLRP3) inflammasome activation, cell membrane pore formation and the release of mature interleukin-1β (IL-1β) through these pores. Caspase-1 is an integral mediator of this process, functioning to directly cleave the pro-form of IL-1β in order to facilitate cytokine maturation while also promoting the activation of gasdermin D (GSDMD) (11,12). GSDMD, in turn, forms pores in the cell membrane that are 10-15 nm in diameter, thereby triggering pyroptosis. The activation of the NLRP3 inflammasome is closely associated with CI and other thrombotic diseases (13,14). Double-stranded RNA-dependent protein kinase (PKR) is a serine/threonine protein kinase that regulates inflammatory responses in mammalian cells. Upon activation, PKR is able to bind the NLRP3 inflammasome, thus triggering caspase-1 activation and IL-1β release (15). Yim et al (16) also reported that PKR-induced eukaryotic initiation factor 2α (eIF2α) is able to suppress inflammasome activation and associated inflammatory responses. PKR may regulate inflammatory immune responses through the p38MAPK pathway (17). In addition, PKR may induce apoptotic cell death via p38MAPK (18). Anti-β2GPI/β2GPI is able to activate p38MAPK signaling via Toll-like receptor 4 (TLR4), thus inducing neutrophil-derived NET release (7). The present study thus posits that anti-β2GPI/β2GPI ICs may promote neutrophil pyroptosis and associated IL-1β release in a TLR4-dependent manner.
High mobility group box 1 protein (HMGB1) is a eukaryotic non-histone chromosome-binding protein that is primarily present within the nucleus. However, in response to certain stimuli or stressors, HMGB1 may be released into the extracellular matrix, wherein it may promote inflammatory immune responses (19). The hepatitis B virus X protein promotes hepatocyte NLRP3 inflammasome activation, resulting in IL-1β, IL-18 and HMGB1 release from these cells (20). Of note, both IL-1β and HMGB1 may alter inflammation and cell growth, and stimulate leukocyte activation by binding to specific cell surface receptors, thus shaping immune responses (21,22).
In the present study, it was determined that serum anti-β2GPI levels and neutrophil NLRP3 expression were significantly increased in patients with CI compared to healthy controls. Through a series of in vitro analyses, it was further determined that anti-β2GPI/β2GPI-induced neutrophil pyroptosis may activate the NLRP3 inflammasome via the TLR4/PKR/p38MAPK signaling axis, leading to the release of HMGB1 and IL-1β from these neutrophils. These inflammatory factors, in turn, enhance intercellular cell adhesion molecule-1 (ICAM-1) and IL-8 expression in endothelial cells.
Materials and methods
Patients
A total of 52 patients with acute CI (ACI; 33 males, 19 females) treated at The Second Affiliated Hospital of Harbin Medical University (Harbin, China) between August 2017 and December 2020 were selected for the present study. Patients were excluded if they had a history of autoimmunity, diabetes, malignant tumors, prior CI, coronary heart disease, acute infections, severe renal insufficiency (serum creatinine >3 mg/dl), nervous system diseases and/or were undergoing immunosuppressive therapy. In addition, 29 healthy individuals (18 males, 11 females) undergoing physical examinations at The Second Affiliated Hospital of Harbin Medical University (Harbin, China) during this same time period were recruited as healthy controls. Control patients were free of CI, acute or chronic infections and major comorbidities other than autoimmune diseases. The patients' characteristics are detailed in Table I. Samples were collected from patients with the approval of the Institutional Ethics Committee of Harbin Medical University (Harbin, China) and informed consent was obtained from the patients in accordance with the Declaration of Helsinki.
Table I.
Patient characteristics.
| Variable | Acute cerebral infarction group (n=52) | Control group (n=29) | Normal ranges | P-value |
|---|---|---|---|---|
| Sex | 0.901 | |||
| Male | 33 (63.5) | 18 (62.1) | ||
| Female | 19 (36.5) | 11 (37.9) | ||
| Mean age, years | 52.69±8.78 | 51.44±6.93 | 0.513 | |
| Glu, mmol/l | 5.15±0.61 | 5.29±0.62 | 3.90-6.10 | 0.348 |
| UA, mmol/l | 311.68±83.30 | 324.76±54.84 | 150.0-440.0 | 0.453 |
| TC, mmol/l | 4.85±1.00 | 4.67±0.89 | 1.8-5.17 | 0.424 |
| TG, mmol/l | 1.59 (1.17-2.47) | 1.41 (1.14-1.71) | 0.56-1.70 | 0.319 |
| HDL, mmol/l | 1.30 (1.14-1.57) | 1.42 (1.17-1.60) | 1.04-1.70 | 0.267 |
| LDL, mmol/l | 2.77 (2.31-3.36) | 2.43 (2.01-3.02) | 0.45-3.15 | 0.084 |
Count data are expressed as n (%). For continuous variables, normally distributed data are expressed as the mean ± standard error of the mean, while non-normally distributed data are represented as the median (interquartile range). L/HDL, low/high-density lipoprotein; TG, triglyceride; TC, total cholesterol; UA, uric acid; Glu, glucose.
Cell isolation and culture
Neutrophils were isolated from healthy donor peripheral blood at The Second Affiliated Hospital of Harbin Medical University (Harbin, China) from individuals who had provided informed consent. Blood was collected using EDTA as an anticoagulant and was treated for 45 min at 4°C via Dextran T-500 (Beijing Solarbio Science & Technology Co., Ltd.) sedimentation to remove erythrocytes. Neutrophil-containing supernatants were then transferred to a lymphocyte separation solution (Tianjin Hao Yang Biological Manufacture Co., Ltd.) and centrifuged for 15 min at 500 × g at room temperature. Neutrophils were isolated from the precipitate and treated with Red Blood Cell Lysis buffer (Beijing Solarbio Science & Technology Co., Ltd.). After isolation, these neutrophils were rinsed with PBS and resuspended in RPMI-1640 medium (Cellgro; Corning, Inc.) with or without 10% fetal bovine serum (FBS; Biological Industries) (7).
Human umbilical vein endothelial cells (HUVECs) were obtained from the Shanghai Institute of Biochemistry and Cell Biology and cultured in RPMI-1640 containing 10% FBS in an incubator containing a humidified atmosphere with 5% CO2 at 37°C. These cells were passaged using trypsin and replated at a dilution of 1:2, with culture media being replaced every 1-2 days.
Reverse transcription-quantitative (RT-q)PCR
Neutrophils or HUVECs were plated in 12-well plates (1×106 cells/ml). Neutrophils were treated with PBS, M-IgG (cat. no. sc-8432; 10 mg/ml; Santa Cruz Biotechnology, Inc.)/BSA (100 mg/ml; MilliporeSigma), anti-β2GPI (cat. no. 11221-MM06; 10 μg/ml; SinoBiological)/β2GPI (100 μg/ml; MilliporeSigma) or lipopolysaccharide (LPS; 100 ng/ml; MilliporeSigma) as experimentally appropriate, while HUVECs were treated for 24 h with recombinant HMGB1 (4 ng/ml; Prospec-Tany TechnoGene, Ltd.) or recombinant IL-1β (2 ng/ml; SinoBiological). In certain experiments, neutrophils were pretreated for 30 min with 1 μM of the TLR4 inhibitor TAK-242 [MedChemExpress (MCE)]. TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.) was used to extract RNA from these cells after treatment and cDNA was prepared with a Transcriptor First Strand cDNA Synthesis Kit (Roche Diagnostics). RT of RNA to cDNA required a 60-min incubation at 50°C, followed by 5 min of incubation at 85°C. Subsequently qPCR analyses for NLRP3, caspase-1, pro-IL-1β, TLR4, HMGB1, IL-8 and ICAM-1 mRNA levels were performed with SYBR Green I dye (Roche Diagnostics) with double distilled water, cDNA template and upstream/downstream primer using the following primers: NLRP3 forward, 5′-CTACACACGACTGCGTCTCATCAA-3′; and reverse, 5′-CGGGGTCAAACAGCAACTCCAT-3′; caspase-1 forward, 5′-TGGAAGAGCAGAAAGCGATAA-3′ and reverse, 5′-TTTGAAGGACAAACCGAAGGT-3′; pro-IL-1β forward, 5′-TCCAGGGACAGGATATGGAG-3′ and reverse, 5′-TCTTTCAACACGCAGGACAG-3′; TLR4 forward, 5′-GCCCCTACTCAATCTCTCTT-3′ and reverse, 5′-GGACTTCTAAACCAGCCA-3′; HMGB1 forward, 5′-GATCCCAATGCACCCAAGAG-3′ and reverse, 5′-TCGCAACATCACCAATGGAC-3′; IL-8 forward, 5′-CAGCCTTCCTGATTTCTGC-3′ and reverse, 5′-GGGTGGAAAGGTTTGGAGTA-3′; ICAM-1 forward, 5′-AGCTTCGTGTCCTGTATGGC-3′ and reverse, 5′-TTTTCTGGCCACGTCCAGTT-3′; β-actin forward, 5′-CTACCTCATGAAGATCCTCACCGA-3′ and reverse, 5′-TTCTCCTTAATGTCACGCACGATT-3′. Shanghai Generay Biotech Corporation synthesized all primers for the present study. Amplifications were performed on a CFX96 real-time PCR detection system (Bio-Rad Laboratories, Inc.) for 40 cycles with a melting temperature of 30 sec, an annealing temperature of 58°C for 30 sec and an extension temperature of 72°C for 30 sec. Quantification cycle (Cq) counts were determined for each sample and relative mRNA expression was calculated using the 2−ΔΔCq method (23).
Western blot analysis
Neutrophils (5×106 cells/ml) were treated for 1 h with PBS, M-IgG (10 mg/ml)/BSA (100 mg/ml), anti-β2GPI (10 μg/ml)/β2GPI (100 μg/ml) or LPS (100 ng/ml), while HUVECs were treated for 24 h with recombinant HMGB1 (4 ng/ml; Prospec-Tany TechnoGene, Ltd.) or recombinant IL-1β (2 ng/ml; SinoBiological). In certain experiments, neutrophils were pretreated for 30 min with 1 μM of the TLR4 inhibitor TAK-242, 10 μM of the PKR inhibitor GC17925 (GLPBIO), 10 μM of the p38MAPK inhibitor SB203580 (MCE) or 1 μM of the NLRP3 inhibitor MCC950 (MCE). Cells were then lysed on ice for 30 min with RIPA buffer (Beyotime Institute of Biotechnology) containing PMSF (1 mM) and a phosphatase inhibitor cocktail (1 mM; Roche Diagnostics). Protein levels in these lysates were measured with a BCA Protein Assay kit (Beyotime Institute of Biotechnology), after which equal amounts (30 μg) of protein per sample were separated via 10% SDS-PAGE and transferred onto PVDF membranes (Cytiva). Blots were then blocked [5% w/v nonfat dry milk in Tris-buffered saline containing Tween-20) for 30 min at room temperature and stained at 4°C overnight with primary antibodies specific for NLRP3 (cat. no. WL02635; 1:1,000 dilution), caspase-1 (cat. no. WL02996a; 1:500 dilution), pro-IL-1β (cat. no. WL02257; 1:1,000 dilution), IL-8 (cat. no. WL03074; 1:500 dilution), ICAM-1 (cat. no. WL02268; 1:500 dilution; all from Wanleibio Co., Ltd.), phosphorylated (p)-p38MAPK (cat. no. 4511), p38MAPK (cat. no. 8690; both 1:1,000 dilution; both from Cell Signaling Technology, Inc.), p-PKR (cat. no. bs-3335R; 1:500 dilution), PKR (cat. no. bs-1493R; 1:1,000 dilution), HMGB1 (cat. no. bs-55098R; 1:1,000 dilution; all from Bioss) or β-actin (cat. no. TA-09; 1:1,000 dilution; OriGene Technologies, Inc.). After probing for 1 h at room temperature with an HRP-conjugated secondary antibody (cat. no. ZB-2301/ZB-2305; 1:5,000 dilution; OriGene Technologies, Inc.), protein bands were detected using a Fluorescence/Chemiluminescence Imaging System (CLINX Science Instruments).
Immunofluorescence staining
GSDMD and HMGB1 expression in neutrophils and ICAM-1 and IL-8 expression in HUVECs was assessed via immunofluorescent staining. In brief, cells were fixed for 20 min with 4% paraformaldehyde and permeabilized for 30 min with 0.2% Triton X-100 at room temperature, then blocked with 50% goat serum (Beyotime Institute of Biotechnology) in PBS for 30 min at 37°C and incubated overnight with anti-GSDMD (cat. no. 20770-1-AP; 1:100 dilution; Proteintech Group, Inc.), anti-HMGB1 (cat. no. bs-55098R; 1:50 dilution; Bioss) or anti-ICAM-1 (cat. no. WL02268) or anti-IL-8 (cat. no. WL03074; both 1:50 dilution; both from Wanleibio Co., Ltd.) at 4°C. Cells were then stained with an AF488-conjugated secondary antibody (cat. no. R37118; 1:1,000 dilution; Invitrogen; Thermo Fisher Scientific, Inc.) for 2 h and DAPI (1 μg/ml; Invitrogen; Thermo Fisher Scientific, Inc.) was applied for nuclear staining for 10 min at room temperature. Cells were rinsed with PBS and imaged via a fluorescence microscope (ECLIPSE Ti; Nikon Corporation).
ELISA
Serum anti-β2GPI was detected using an Anti-β2GPI antibody ELISA kit (cat. no. EA1632-9601P; K-BIOanalytica Rayan Company). Neutrophils (5×106 cells/ml) were treated with PBS, anti-β2GPI (10 μg/ml)/β2GPI (100 μg/ml) or LPS (100 ng/ml) for 3 h following pretreatment for 30 min with different inhibitors (1 μM TAK-242, 10 μM GC1725 or 10 μM SB203580). Supernatants were then collected and IL-1β concentrations therein were assessed via a Human IL-1β ELISA Kit (cat. no. JL13662; J&L Biological) according to the manufacturer's instructions.
Statistical analysis
Count data (sex distribution) were assessed via χ2 test and expressed as n (%). For continuous variables, normally distributed data were tested via Student's t-test and expressed as the mean ± standard error of the mean, while non-normally distributed data were assessed via Mann-Whitney U tests and represented as the median (interquartile range). Three or more groups of data were tested via one-way ANOVA with Dunnet's post-hoc test. These data were analyzed using GraphPad Prism 7 (GraphPad Software, Inc.). P<0.05 was considered to indicate a statistically significant difference.
Results
Patients with CI exhibit elevated serum anti-β2GPI levels and increased neutrophil NLRP3 expression
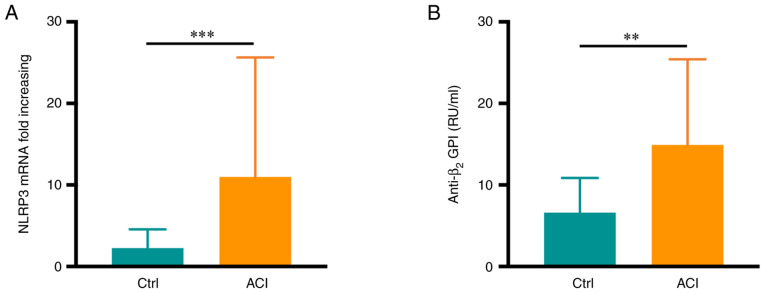
The patients with ACI (age, 31-69 years) included in the present study exhibited no significant differences in age or sex compared with the healthy controls (age, 35-68 years) (P>0.05; Table I), nor were there any differences in serum glucose, uric acid, triglyceride, total cholesterol or low/high-density lipoprotein levels between these groups (P>0.05). Of note, increased neutrophil NLRP3 mRNA expression was observed in patients with ACI relative to healthy controls (P<0.001; Fig. 1A). Serum anti-β2GPI levels in these patients were detected via ELISA, revealing significantly higher levels of this autoantibody in samples from patients with ACI than in the healthy controls (P<0.01; Fig. 1B). Specifically, 9 patients in the ACI group (17.3%) were positive for anti-β2GPI autoantibodies.
Figure 1.
Serum anti-β2GPI and neutrophil NLRP3 expression are increased in patients with CI. (A) NLRP3 levels in neutrophils were elevated in the acute CI group (n=52) relative to the healthy controls (n=29). (B) Serum anti-β2GPI levels were elevated in the acute CI group (n=52) relative to the healthy controls (n=29). Values are expressed as the mean ± standard error of the mean. **P<0.01, ***P<0.001. NLRP3, NLR family pyrin domain containing 3; anti-β2GPI, an ti-β2-glycoprotein I; ACI, acute cerebral infarction; Ctrl, control.
Anti-β2GPI/β2GPI treatment triggers neutrophil pyroptosis
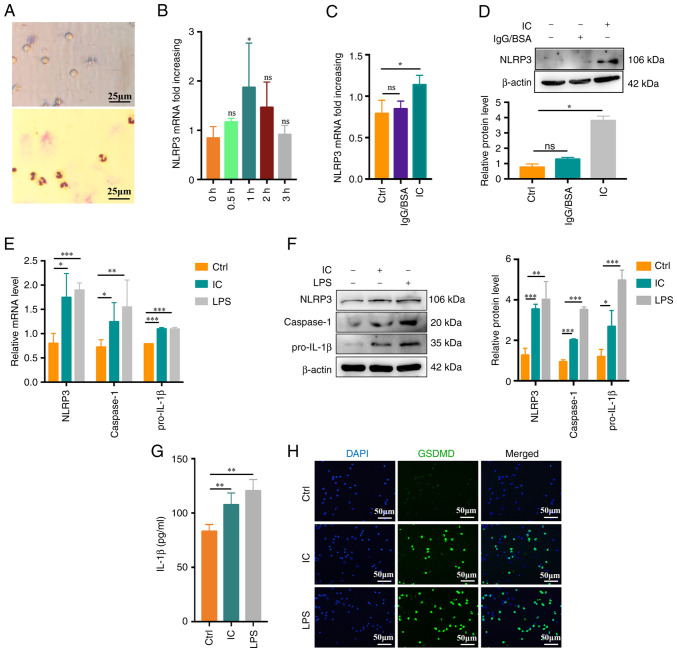
In previous studies, it was demonstrated that β2GPI or anti-β2GPI alone cannot effectively activate neutrophils (7). In light of the above clinical findings, the potential mechanistic role of β2GPI in the context of CI was then examined in vitro. First, neutrophils were extracted from healthy subjects and their purity and viability were confirmed (Fig. 2A). To evaluate neutrophil pyroptosis, neutrophils were treated with PBS, anti-β2GPI (10 μg/ml)/β2GPI (100 μg/ml) or LPS (100 ng/ml; positive control) for up to 3 h. The NLRP3 inflammasome is an important mediator of pyroptosis (24). Anti-β2GPI/β2GPI IC but not IgG/BSA treatment enhanced NLRP3 mRNA expression at 1 h of treatment relative to control treatment or other incubation times (P<0.05; Fig. 2B-D). Caspase-1 and pro-IL-1β mRNA expression was also enhanced by anti-β2GPI/β2GPI treatment, with comparable increases in NLRP3/caspase-1/pro-IL-1β protein levels (Fig. 2E and F). During pyroptosis, more cell fragmentation was associated with the release of more inflammatory factors into the cell supernatant. Expression of IL-1β was present in the cell supernatant at 3 h. Supernatant IL-1β levels also rose significantly following exposure to anti-β2GPI/β2GPI (P<0.01; Fig. 2G). GSDMD mediates membrane pore formation during pyroptosis (25). To more fully characterize neutrophil pyroptosis, these cells were immunostained for GSDMD, revealing significant increases in GSDMD-positive cell frequencies among anti-β2GPI/β2GPI-treated neutrophils (Fig. 2H). Together, these data indicated that anti-β2GPI/β2GPI treatment was sufficient to stimulate neutrophil pyroptosis and IL-1β release.
Figure 2.
Anti-β2GPI/β2GPI treatment triggers neutrophil pyroptosis. (A) Representative images of neutrophil viability (upper) and purity (lower) as assessed via trypan blue dye exclusion assays and Giemsa staining, respectively (scale bar, 25 μm). (B) Following treatment with anti-β2GPI (10 μg/ml)/β2GPI (100 μg/ml), neutrophils exhibited significantly increased NLRP3 mRNA upregulation at 1 vs. 0 h. (C and D) Anti-β2GPI/β2GPI treatment enhanced the expression of NLRP3 more efficiently than IgG/BSA treatment as measured via (C) reverse transcription-quantitative PCR and (D) western blot analysis. (E) Relative NLRP3, caspase-1 and pro-IL-1β mRNA levels rose in neutrophils following 1 h of treatment with anti-β2GPI/β2GPI. (F) NLRP3, caspase-1 and pro-IL-1β protein levels were increased in neutrophils following 1 h of treatment with anti-β2GPI/β2GPI, as measured via western blot analysis. (G) IL-1β levels increased significantly in neutrophils treated for 3 h with anti-β2GPI/β2GPI as measured by ELISA. (H) GSDMD-positive cells (green) following treatment with anti-β2GPI/β2GPI (IC). DAPI (blue) was used for nuclear staining (scale bar, 50 μm). Values are expressed as the mean ± standard error of the mean (n=3 or n=4 in G). *P<0.05, **P<0.01, ***P<0.001 as indicated or vs. 0 h. NLRP3, NLR family pyrin domain containing 3; anti-β2GPI, anti-β2-glycoprotein I; LPS, lipopolysaccharide; Ctrl, control; ns, no significance; IC, anti-β2GPI/β2GPI immune complex; GSDMD, gasdermin D.
Anti-β2GPI/β2GPI IC treatment induces neutrophil pyroptosis in a TLR4-dependent manner
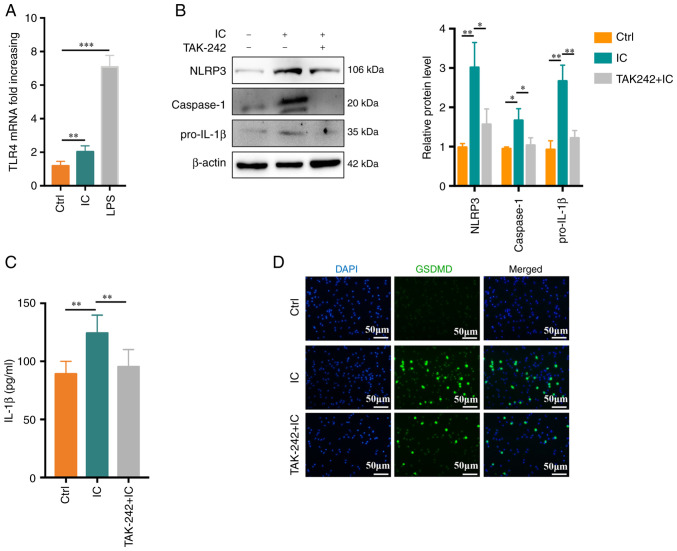
A previous study by our group suggested that TLR4 has a role in anti-β2GPI/β2GPI-induced NETs formation (7). In the present study, TLR4 activation was therefore assessed and its functional importance in the context of anti-β2GPI/β2GPI IC-induced neutrophil pyroptosis was explored. Anti-β2GPI/β2GPI treatment was associated with an increase in TLR4 mRNA expression in treated neutrophils (Fig. 3A). When these cells were pretreated with the TLR4 inhibitor TAK-242, significant reductions in anti-β2GPI/β2GPI-induced NLRP3/caspase-1/pro-IL-1β protein expression we observed (Fig. 3B). Similarly, TAK-242 treatment markedly suppressed neutrophil IL-1β secretion (P<0.01; Fig. 3C) and decreased the frequency of GSDMD-positive cells, as determined via immunofluorescent staining, relative to the control treatment (Fig. 3D).
Figure 3.
Anti-β2GPI/β2GPI treatment induces neutrophil pyroptosis in a TLR4-dependent manner. (A) Relative TLR4 mRNA levels rose significantly in neutrophils following 1 h of treatment with anti-β2GPI/β2GPI (n=5). (B) Following pretreatment for 30 min with TAK-242 (1 μM), NLRP3, caspase-1 and pro-IL-1β protein levels in neutrophils were reduced (n=3). (C) IL-1β production was suppressed by TAK-242, as measured via ELISA (n=6). (D) TAK-242 suppressed the number of GSDMD-positive cells (scale bar, 50 μm). Values are expressed as the mean ± standard error of the mean. *P<0.05, **P<0.01, ***P<0.001. TLR4, Toll-like receptor 4; NLRP3, NLR family pyrin domain containing 3; anti-β2GPI, anti-β2-glycoprotein I; LPS, lipopolysaccharide; Ctrl, control; IC, anti-β2GPI/β2GPI immune complex; GSDMD, gasdermin D.
Anti-β2GPI/β2GPI-induced neutrophil pyroptosis is associated with the PKR/p38MAPK axis
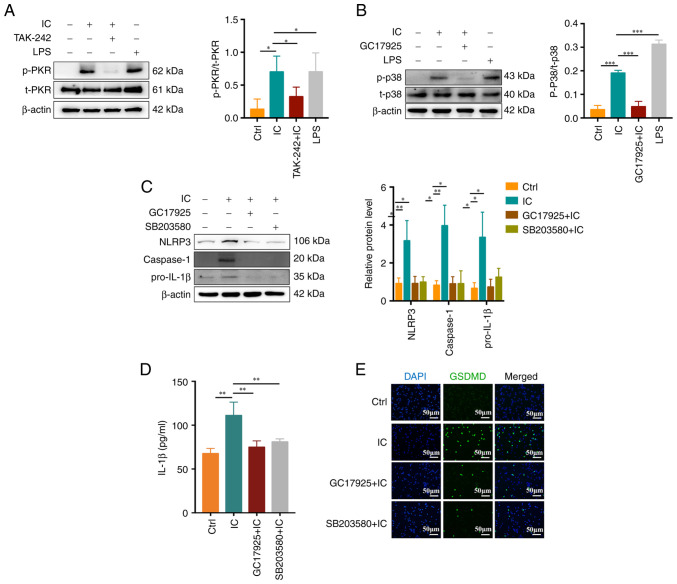
PKR has previously been identified as a key regulator of inflammasome activation, while PKR-induced eIF2α is able to inhibit such inflammasome activity (16). A recent study suggested that p38MAPK is involved in PKR activation and consequent human chondrocyte apoptosis (18). Thus, the role of PKR/p38MAPK in the context of anti-β2GPI/β2GPI-induced neutrophil pyroptosis was explored in the present study. After pretreatment for 30 min with 1 μM TAK-242, 10 μM GC17925 (a PKR inhibitor) or 10 μM SB203580 (a p38MAPK inhibitor), neutrophils were stimulated for 1 h with anti-β2GPI (10 μg/ml)/β2GPI (100 μg/ml). It was indicated that anti-β2GPI/β2GPI treatment was able to stimulate PKR (Fig. 4A) and p38MAPK (Fig. 4B) phosphorylation, whereas TAK-242 and GC17925 suppressed the activation of these proteins. GC17925 and SB203580 also suppressed NLRP3/caspase-1/pro-IL-1β expression (Fig. 4C) and inhibited IL-1β release (P<0.01; Fig. 4D) and GSDMD activation (Fig. 4E) in neutrophils. These results suggested that the PKR/p38MAPK signaling pathway has an important role in anti-β2GPI/β2GPI IC-induced neutrophil pyroptosis.
Figure 4.
Association between the PKR/p38MAPK signaling and anti-β2GPI/β2GPI complex-induced neutrophil pyroptosis. (A and B) Anti-β2GPI/β2GPI induced (A) PKR and (B) p38MAPK phosphorylation, which was respectively inhibited by TAK-242 and GC17925 treatment. (C) NLRP3, caspase-1 and pro-IL-1β protein levels were reduced by GC17925 or SB203580 treatment. (D) IL-1β secretion was decreased by GC17925 or SB203580 treatment. (E) GSDMD fluorescent staining demonstrated inhibition of anti-β2GPI/β2GPI-induced neutrophil pyroptosis mediated by GC17925 or SB203580 (scale bar, 50 μm). Values are expressed as the mean ± standard error of the mean (n=3 and n=4 in D). *P<0.05, **P<0.01, ***P<0.001. NLRP3, NLR family pyrin domain containing 3; anti-β2GPI, anti-β2-glycoprotein I; LPS, lipopolysaccharide; Ctrl, control; IC, anti-β2GPI/β2GPI immune complex; GSDMD, gasdermin D; p-/t-PKR, phosphorylated/total double-stranded RNA-dependent protein kinase.
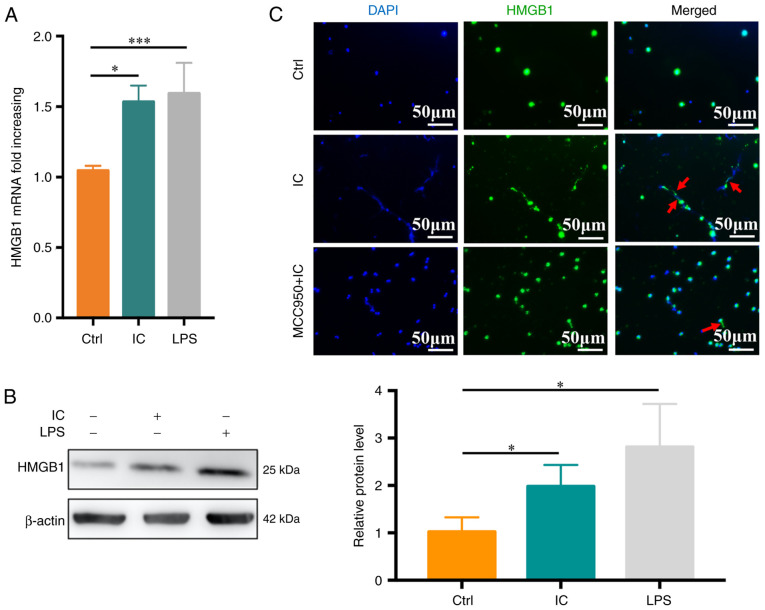
Anti-β2GPI/β2GPI ICs induce neutrophil pyroptosis-mediated HMGB1 release
HMGB1 is an inflammatory protein that is present in most cell types and that is released extracellularly in response to specific stimuli or stress conditions. To explore its relevance in the experimental system of the present study, neutrophils were treated with PBS, anti-β2GPI (10 μg/ml)/β2GPI (100 μg/ml) or LPS (100 ng/ml), and HMGB1 expression was then monitored. Anti-β2GPI/β2GP treatment was sufficient to significantly enhance HMGB1 mRNA levels relative to the control treatment (P<0.05; Fig. 5A), with similar changes being observed at the protein level (Fig. 5B). To assess whether anti-β2GPI/β2GP-stimulated HMGB1 release was dependent on pyroptosis, immunofluorescent staining was performed, which revealed that HMGB1 release was significantly reduced in the anti-β2GPI/β2GP-MCC950-treated group relative to the anti-β2GPI/β2GP-treated group (Fig. 5C).
Figure 5.
Anti-β2GPI/β2GPI-induced neutrophil pyroptosis promotes HMGB1 upregulation and release. (A) HMGB1 mRNA levels in neutrophils were assessed following 1 h of treatment with anti-β2GPI/β2GPI (n=5). (B) HMBG1 protein levels in neutrophils were measured, with β-actin used for normalization (n=3). (C) HMGB1 release (red arrow) was visualized via fluorescence microscopy following treatment for 3 h with anti-β2GPI/β2GPI (scale bar, 50 μm). Values are expressed as the mean ± standard error of the mean. *P<0.05, ***P<0.001. Anti-β2GPI, anti-β2-glycoprotein I; Ctrl, control; LPS, lipopolysaccharide; IC, anti-β2GPI/β2GPI immune complex; HMGB1, high mobility group box protein 1.
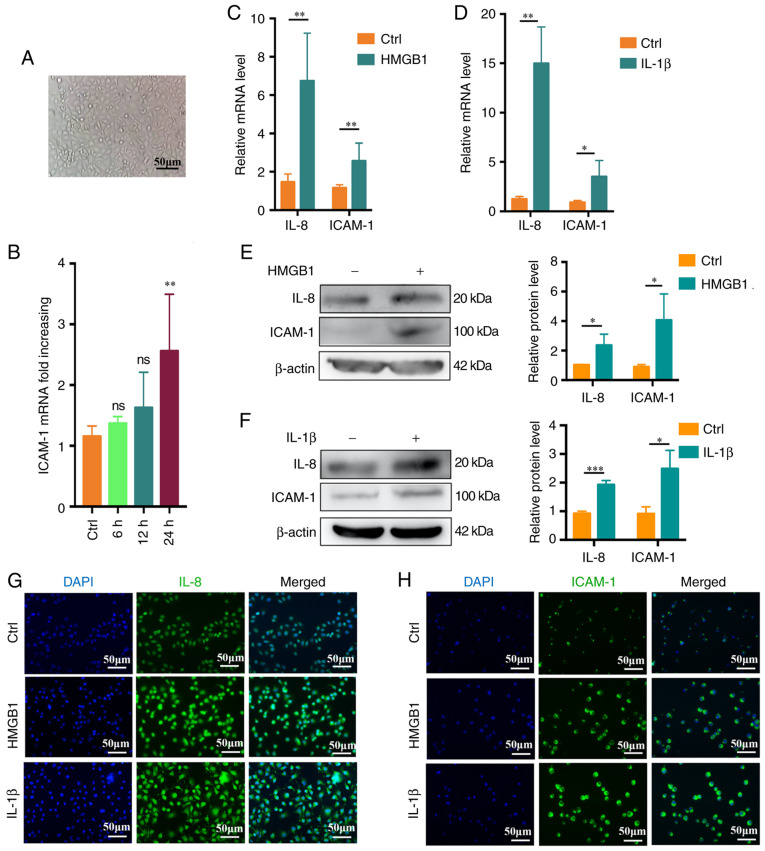
HMGB1 and IL-1β promote the activation of endothelial cells
To determine the optimal stimulation time, microscopy was used to confirm that the endothelial cells were in good condition (Fig. 6A). The cells were then treated with recombinant human HMGB1 (4 ng/ml) for 0-24 h. Maximal ICAM-1 upregulation relative to the baseline was observed at 24 h via RT-qPCR (P<0.05; Fig. 6B). HUVECs were then cultured for 24 h with recombinant human HMGB1 (4 ng/ml) or recombinant human IL-1β (2 ng/ml) and when using this incubation time, both IL-8 and ICAM-1 were upregulated at the mRNA level relative to control cells (Fig. 6C and D), with consistent increases in IL-8 and ICAM-1 protein levels following stimulation (Fig. 6E and F). Fluorescence microscopy further confirmed these results (Fig. 6G and H).
Figure 6.
HMGB1 and IL-1β promote in vitro endothelial cell activation. (A) Representative microscopic image of endothelial cell morphology (scale bar, 50 μm). (B) Changes in endothelial cell ICAM-1 expression over time (0-24 h) (n=3). (C) IL-8 and ICAM-1 expression levels in endothelial cells following treatment for 24 h with HMGB1 (4 ng/ml), with β-actin being used for normalization (n=4). (D) IL-8 and ICAM-1 expression levels in endothelial cells stimulated for 24 h with IL-1β (2 ng/ml), with β-actin being used for normalization (n=4). (E and F) IL-8 and ICAM-1 protein levels following (E) HMGB1 or (F) IL-1β stimulation (n=3). (G) IL-8 expression as assessed via fluorescence microscopy following a 24 h stimulation with HMGB1 (4 ng/ml) or IL-1β (2 ng/ml). (H) ICAM-1 expression was assessed via fluorescence microscopy following a 24 h stimulation with HMGB1 (4 ng/ml) or IL-1β (2 ng/ml) (scale bar, 50 μm). Values are expressed as the mean ± standard error of the mean. *P<0.05, **P<0.01, ***P<0.001 as indicated or vs. Ctrl. Ctrl, control; ns, no significance; HMGB1, high mobility group box protein 1; ICAM-1, intercellular adhesion molecule-1.
Discussion
Anti-β2GPI antibodies are closely linked to increased rates of coagulation and thrombosis in patients with APS (3). β2GPI is a single-stranded 50-kDa glycoprotein produced by liver cells that forms a complex with phospholipids and thereby adopts an open conformation that facilitates anti-β2GPI binding, such that a single antibody is able to bind to two β2GPI molecules (26). The resultant anti-β2GPI/β2GPI complexes are associated with an increased risk of thrombotic events (5). Zhou et al (27) determined that anti-β2GPI/β2GPI ICs, rather than IgG/β2GPI or anti-β2GPI/BSA complexes, were responsible for in vitro THP-1 cell activation. A previous study by our group also indicated that an anti-β2GPI to β2GPI ratio of 1:10 is able to facilitate efficient IC formation in vitro (6). The resultant complexes may promote neutrophil-mediated NETs release, thereby driving thrombosis (7). In the present study, increased anti-β2GPI levels and neutrophil NLRP3 expression were observed in patients with CI.
Neutrophils function as critical mediators of innate immune responses against a wide array of pathogens, releasing IL-1β in an NLRP3-dependent manner in the context of bacterial infection or inflammation (28). Anti-β2GPI may induce trophoblast-derived IL-1β release via promoting NLRP3 activation, thereby contributing to the incidence of morbid pregnancy (29). There is robust evidence that NLRP3 participates in thrombosis via the regulation of inflammatory responses (30). In the present study, it was determined that anti-β2GPI/β2GPI treatment resulted in increased NLRP3 and caspase-1 expression in neutrophils. During pyroptosis, activated NLRP3 recruits apoptosis-associated speck-like protein containing CARD and subsequently activates caspase-1, thereby forming the multi-protein NLRP3 inflammasome complex (24). Activated caspase-1 additionally cleaves pro-IL-1β to yield activated IL-1β, which may be secreted from cells (31). However, data reported by Karmakar et al (32) suggest that, while neutrophils release IL-1β in an NLRP3-dependent manner in certain inflammatory contexts with concomitant caspase-1 activation, this is not indicative of ongoing neutrophil pyroptosis. Indeed, pyroptosis is characterized by the presence of small pores in the cell membrane that are detectable via electron microscopy, ultimately leading to cellular swelling, rupture and the release of the contents therein. GSDMD has been indicated to bind to and form pores in the cell membrane following caspase-1 activation in the context of pyroptosis. While the loss of GSDMD has no impact on caspase-1-mediated IL-1β processing, it does nonetheless suppress the secretion of this cytokine (33). In the present study, it was determined that anti-β2GPI/β2GPI ICss are able to trigger neutrophil pyroptosis and consequent IL-1β via an NLRP3-caspase-1 pathway with concomitantly increased GSDMD expression, and in addition, control for the potential interference of IgG/BSA was provided. The above results confirmed that non-pathogenic immune complexes did not have any effect on neutrophil pyroptosis. MCC950 is a selective NLRP3 inhibitor that also inhibits inflammatory responses (34). MCC950 or related inhibitors may thus be effective tools for preventing APS-related thrombosis.
Anti-β2GPI/β2GPI ICs have previously been indicated to bind to TLR4 on the surface of neutrophils, thereby triggering NET release (7). TLR4 is an important inducer of both innate and adaptive immunity (35). Tumor-derived autophagosomes may activate TLRs, thereby promoting NLRP3 inflammasome-dependent innate immune responses (36). The present study suggested that anti-β2GPI/β2GPI IC-induced neutrophil pyroptosis was dependent upon TLR4 activation, as such activity was blunted by treatment with the TLR4 inhibitor TAK-242. Consistently, TAK-242 was recently reported to inhibit GSDMD-mediated pyroptosis in tubular cells in the context of diabetic kidney disease (37). PKR controls translation and inflammatory signaling in the context of antiviral immune responses. Bat-Erdene et al (38) posited that breast cancer cell glycosaminoglycans may induce a proliferation-inducing ligand secretion through a mechanism dependent on neutrophil TLR4 and PKR. In the present study, the ability of anti-β2GPI/β2GPI IC treatment to induce PKR phosphorylation in neutrophils was established, and TAK-242 treatment inhibited this process. Previous studies have indicated that PKR is able to regulate inflammatory responses through the JNK, p38MAPK, interferon regulatory factor 3 and NF-κB pathways (17,39,40). Consistently, the present study suggested that anti-β2GPI/β2GPI ICs were able to induce p38MAPK phosphorylation, while treatment with the PKR inhibitor GC17925 prevented this process. Yim et al (16) determined that PKR is able to suppress inflammasome activity. Ma et al (18) further indicated that PKR promotes apoptosis via p38MAPK signaling. However, Lu et al (15) proposed that PKR promotes inflammasome activation. The present study confirmed that inhibitors of PKR (GC17925) or p38MAPK (SB203580) were able to suppress NLRP3 inflammasome activation and associated IL-1β secretion, while also inhibiting GSDMD expression in treated neutrophils. It was thus demonstrated that the PKR/p38MAPK signaling pathway has an essential role in anti-β2GPI/β2GPI-induced neutrophil pyroptosis.
In addition to IL-1β, pyroptosis also induces the release of the nuclear protein HMGB1 (15). Barlan et al (41) confirmed that macrophages are able to recognize adenovirus 5 upon infection via a TLR9-dependent mechanism that induces NLRP3 and caspase-1 expression and consequent reactive oxygen species production, leading to NLRP3 inflammasome activation and the release of IL-1β and HMGB1. MCC950 is able to selectively suppress NLRP3 and thereby inhibit inflammatory responses (35). Adamiak et al (42) determined that murine hematopoietic stem/progenitor cells exhibited reductions in peripheral blood ATP stimulation-induced IL-1β expression when MCC950 was applied to inhibit the NLRP3 inflammasome. In the present study, it was determined that neutrophils were able to release IL-1β via pyroptosis following anti-β2GPI/β2GPI stimulation. Such IC exposure also induced neutrophil-mediated HMGB1 release, whereas MCC950 inhibited this process. MCC950 or related inhibitors may thus be effective tools for preventing APS-related thrombosis. In addition, PKR controls the release of IL-1β and the HMGB1, and may thus be a relevant target in this pathological context (15).
Circulating IL-1β and HMGB1 have previously been indicated to participate in a range of pathological processes, including inflammation, thrombosis and tumor metastasis (43,44). For instance, Schulze et al (45) found plasma HMGB1 levels to be correlated with stroke severity, while Yoshida et al (46) identified IL-1β as a mediator of extra-intestinal thrombosis in a model of experimental colitis. Endothelial-cell activation has a central role in thrombosis, with endothelial cell upregulation of the inflammatory marker proteins ICAM-1 and IL-8 being closely linked to the induction of thrombotic diseases (47,48). The present data revealed that HUVECs stimulated with recombinant IL-1β and HMGB1 upregulated both IL-8 and ICAM-1 in vitro. However, a limitation of the present study is the lack of in vivo experiments to verify neutrophil pyroptosis and rule out other cellular interference. TLR4 is also expressed on vascular endothelial cells and the anti-β2GPI/β2GPI IC may also directly activate endothelial cells (49). Xia et al (50) indicated that anti-β2GPI/β2GPI ICs induced thrombosis by TLR4-induced monocyte activation.
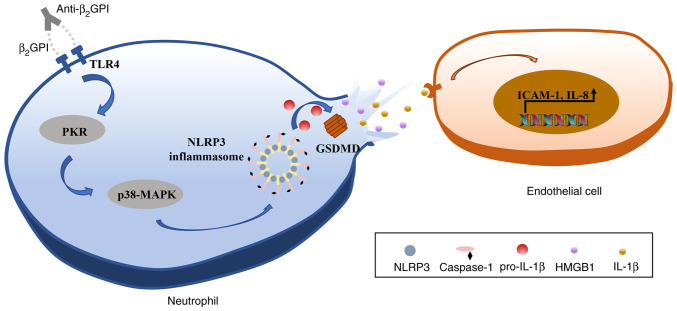
In conclusion, the results of the present study indicated that anti-β2GPI/β2GPI is able to induce pyroptotic neutrophil-mediated IL-1β and HMGB1 release, in turn activating endothelial cells and promoting their upregulation of IL-8 and ICAM-1 expression in vitro. It was further established that these effects were mediated via TLR4 through an intracellular PKR/p38MAPK/NLRP3 signaling pathway (Fig. 7). Together, these data suggest that neutrophil pyroptosis may represent a key mechanism driving thrombosis, thereby offering new insight into the potential pathogenic etiology underlying CI and related thrombotic diseases in patients with APS.
Figure 7.
Schematic overview of the proposed mechanism whereby anti-β2GPI/β2GPI interactions drive neutrophil pyroptosis and associated IL-1β and HMGB1 release, thereby causing endothelial cell activation. TLR4, Toll-like receptor 4; NLRP3, NLR family pyrin domain containing 3; anti-β2GPI, anti-β2-glycoprotein I; GSDMD, gasdermin D; PKR, double-stranded RNA-dependent protein kinase; HMGB1, high mobility group box protein 1; ICAM-1, intercellular adhesion molecule-1.
Acknowledgments
Not applicable.
Funding Statement
This work was supported by the National Natural Science Foundation of China (grant no. 81974108 to YL).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
JL, MZ and YL designed this study; JL, MZ and ZW performed the experiments; ZW and LY were involved in data curation and formal analyses; JL and MZ wrote the manuscript; YL critically revised the manuscript for important intellectual content, approved the final manuscript version to be published and agreed to be accountable for all aspects of the work. JL and MZ checked and approved the authenticity of all the raw data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Samples were collected from patients with the approval of the Institutional Ethics Committee of Harbin Medical University (Harbin, China) and informed consent was obtained from the patients in accordance with the Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Tektonidou MG. Antiphospholipid syndrome nephropathy: From pathogenesis to treatment. Front Immunol. 2018;9:1181. doi: 10.3389/fimmu.2018.01181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giannakopoulos B, Passam F, Rahgozar S, Krilis SA. Current concepts on the pathogenesis of the antiphospholipid syndrome. Blood. 2007;109:422–430. doi: 10.1182/blood-2006-04-001206. [DOI] [PubMed] [Google Scholar]
- 3.Pierangeli SS, Chen PP, Raschi E, Scurati S, Grossi C, Borghi MO, Palomo I, Harris EN, Meroni PL. Antiphospholipid antibodies and the antiphospholipid syndrome: Pathogenic mechanisms. Semin Thromb Hemost. 2008;34:236–250. doi: 10.1055/s-0028-1082267. [DOI] [PubMed] [Google Scholar]
- 4.McDonnell T, Wincup C, Buchholz I, Pericleous C, Giles I, Ripoll V, Cohen H, Delcea M, Rahman A. The role of beta-2-glycoprotein I in health and disease associating structure with function: More than just APS. Blood Rev. 2020;39:100610. doi: 10.1016/j.blre.2019.100610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martínez-Flores JA, Serrano M, Pérez D, Cámara A G, Lora D, Morillas L, Ayala R, Paz-Artal E, Morales JM, Serrano A. Circulating immune complexes of IgA bound to beta 2 glycoprotein are strongly associated with the occurrence of acute thrombotic events. J Atheroscler Thromb. 2016;23:1242–1253. doi: 10.5551/jat.34488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang W, Gao F, Lu D, Sun N, Yin X, Jin M, Liu Y. Anti-β2 glycoprotein I antibodies in complex with β2 glycoprotein I induce platelet activation via two receptors: Apolipoprotein E receptor 2' and glycoprotein I bα. Front Med. 2016;10:76–84. doi: 10.1007/s11684-015-0426-7. [DOI] [PubMed] [Google Scholar]
- 7.Zha C, Zhang W, Gao F, Xu J, Jia R, Cai J, Liu Y. Anti-β2GPI/β2GPI induces neutrophil extracellular traps formation to promote thrombogenesis via the TLR4/MyD88/MAPKs axis activation. Neuropharmacology. 2018;138:140–150. doi: 10.1016/j.neuropharm.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Segel GB, Halterman MW, Lichtman MA. The paradox of the neutrophil's role in tissue injury. J Leukoc Biol. 2011;89:359–372. doi: 10.1189/jlb.0910538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zychlinsky A, Prevost MC, Sansonetti PJ. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992;358:167–169. doi: 10.1038/358167a0. [DOI] [PubMed] [Google Scholar]
- 10.Cookson BT, Brennan MA. Pro-inflammatory programmed cell death. Trends Microbiol. 2011;9:113–114. doi: 10.1016/S0966-842X(00)01936-3. [DOI] [PubMed] [Google Scholar]
- 11.Miao EA, Rajan JV, Aderem A. Caspase-1-induced pyroptotic cell death. Immunol Rev. 2011;243:206–214. doi: 10.1111/j.1600-065X.2011.01044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu X, Zhang Z, Ruan J, Pan Y, Magupalli VG, Wu H, Lieberman J. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535:153–158. doi: 10.1038/nature18629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mezzaroma E, Toldo S, Farkas D, Seropian IM, Van Tassell BW, Salloum FN, Kannan HR, Menna AC, Voelkel NF, Abbate A. The inflammasome promotes adverse cardiac remodeling following acute myocardial infarction in the mouse. Proc Natl Acad Sci USA. 2011;108:19725–19730. doi: 10.1073/pnas.1108586108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inoue Y, Shirasuna K, Kimura H, Usui F, Kawashima A, Karasawa T, Tago K, Dezaki K, Nishimura S, Sagara J, et al. NLRP3 regulates neutrophil functions and contributes to hepatic ischemia-reperfusion injury independently of inflammasomes. J Immunol. 2014;192:4342–4351. doi: 10.4049/jimmunol.1302039. [DOI] [PubMed] [Google Scholar]
- 15.Lu B, Nakamura T, Inouye K, Li J, Tang Y, Lundbäck P, Valdes-Ferrer SI, Olofsson PS, Kalb T, Roth J, et al. Novel role of PKR in inflammasome activation and HMGB1 release. Nature. 2012;488:670–674. doi: 10.1038/nature11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yim HC, Wang D, Yu L, White CL, Faber PW, Williams BR, Sadler AJ. The kinase activity of PKR represses inflammasome activity. Cell Res. 2016;26:367–379. doi: 10.1038/cr.2016.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goh KC, deVeer MJ, Williams BR. The protein kinase PKR is required for p38 MAPK activation and the innate immune response to bacterial endotoxin. EMBO J. 2000;19:4292–4297. doi: 10.1093/emboj/19.16.4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma CH, Wu CH, Jou IM, Tu YK, Hung CH, Chou WC, Chang YC, Hsieh PL, Tsai KL. PKR promotes oxidative stress and apoptosis of human articular chondrocytes by causing mitochondrial dysfunction through p38 MAPK activation-PKR activation causes apoptosis in human chondrocytes. Antioxidants (Basel) 2019;8:370. doi: 10.3390/antiox8090370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang R, Chen R, Zhang Q, Hou W, Wu S, Cao L, Huang J, Yu Y, Fan XG, Yan Z, et al. HMGB1 in health and disease. Mol Aspects Med. 2014;40:1–116. doi: 10.1016/j.mam.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie WH, Ding J, Xie XX, Yang XH, Wu XF, Chen ZX, Guo QL, Gao WY, Wang XZ, Li D. Hepatitis B virus X protein promotes liver cell pyroptosis under oxidative stress through NLRP3 inflammasome activation. Inflamm Res. 2020;69:683–696. doi: 10.1007/s00011-020-01351-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee PH, Yamamoto TN, Gurusamy D, Sukumar M, Yu Z, Hu-Li J, Kawabe T, Gangaplara A, Kishton RJ, Henning AN, et al. Host conditioning with IL-1β improves the antitumor function of adoptively transferred T cells. J Exp Med. 2019;216:2619–2634. doi: 10.1084/jem.20181218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huebener P, Pradere JP, Hernandez C, Gwak GY, Caviglia JM, Mu X, Loike JD, Schwabe RF. The HMGB1/RAGE axis triggers neutrophil-mediated injury amplification following necrosis. J Clin Invest. 2015;125:539–550. doi: 10.1172/JCI76887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu X, Zhang H, Qi W, Zhang Y, Li J, Li Z, Lin Y, Bai X, Liu X, Chen X, et al. Nicotine promotes atherosclerosis via ROS-NLRP3-mediated endothelial cell pyroptosis. Cell Death Dis. 2018;9:171. doi: 10.1038/s41419-017-0257-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takahashi M. NLRP3 inflammasome as a novel player in myocardial infarction. Int Heart J. 2014;55:101–105. doi: 10.1536/ihj.13-388. [DOI] [PubMed] [Google Scholar]
- 25.Kovacs SB, Miao EA. Gasdermins: Effectors of pyroptosis. Trends Cell Biol. 2017;27:673–684. doi: 10.1016/j.tcb.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agar C, van Os GM, Mörgelin M, Sprenger RR, Marquart JA, Urbanus RT, Derksen RH, Meijers JC, de Groot PG. Beta2-glycoprotein I can exist in 2 conformations: Implications for our understanding of the antiphospholipid syndrome. Blood. 2010;116:1336–1343. doi: 10.1182/blood-2009-12-260976. [DOI] [PubMed] [Google Scholar]
- 27.Zhou H, Sheng L, Wang H, Xie H, Mu Y, Wang T, Yan J. Anti-β2GPI/β2GPI stimulates activation of THP-1 cells through TLR4/MD-2/MyD88 and NF-κB signaling pathways. Thromb Res. 2013;132:742–749. doi: 10.1016/j.thromres.2013.09.039. [DOI] [PubMed] [Google Scholar]
- 28.Cho JS, Guo Y, Ramos RI, Hebroni F, Plaisier SB, Xuan C, Granick JL, Matsushima H, Takashima A, Iwakura Y, et al. Neutrophil-derived IL-1β is sufficient for abscess formation in immunity against staphylococcus aureus in mice. PLoS Pathog. 2012;8:e1003047. doi: 10.1371/journal.ppat.1003047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mulla MJ, Salmon JE, Chamley LW, Brosens JJ, Boeras CM, Kavathas PB, Abrahams VM. A role for uric acid and the Nalp3 inflammasome in antiphospholipid antibody-induced IL-1β production by human first trimester trophoblast. PLoS One. 2013;8:e65237. doi: 10.1371/journal.pone.0065237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo Z, Yu S, Chen X, Ye R, Zhu W, Liu X. NLRP3 is involved in ischemia/reperfusion injury. CNS Neurol Disord Drug Targets. 2016;15:699–712. doi: 10.2174/1871527315666160321111829. [DOI] [PubMed] [Google Scholar]
- 31.Wang W, Zhang T. Caspase-1-mediated pyroptosis of the predominance for driving CD4[Formula: See text] T cells death: A nonlocal spatial mathematical model. Bull Math Biol. 2018;80:540–582. doi: 10.1007/s11538-017-0389-8. [DOI] [PubMed] [Google Scholar]
- 32.Karmakar M, Katsnelson M, Malak HA, Greene NG, Howell SJ, Hise AG, Camilli A, Kadioglu A, Dubyak GR, Pearlman E. Neutrophil IL-1β processing induced by pneumolysin is mediated by the NLRP3/ASC inflammasome and caspase-1 activation and is dependent on K+ efflux. J Immunol. 2015;194:1763–1775. doi: 10.4049/jimmunol.1401624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, Shao F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 34.Ismael S, Zhao L, Nasoohi S, Ishrat T. Inhibition of the NLRP3-inflammasome as a potential approach for neuroprotection after stroke. Sci Rep. 2018;8:5971. doi: 10.1038/s41598-018-24350-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.An H, Qian C, Cao X. Regulation of Toll-like receptor signaling in the innate immunity. Sci China Life Sci. 2010;53:34–43. doi: 10.1007/s11427-010-0011-x. [DOI] [PubMed] [Google Scholar]
- 36.Xing Y, Cao R, Hu HM. TLR and NLRP3 inflammasome-dependent innate immune responses to tumor-derived autophagosomes (DRibbles) Cell Death Dis. 2016;7:e2322. doi: 10.1038/cddis.2016.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y, Zhu X, Yuan S, Wen S, Liu X, Wang C, Qu Z, Li J, Liu H, Sun L, Liu F. TLR4/NF-κB signaling induces GSDMD-Related pyroptosis in tubular cells in diabetic kidney disease. Front Endocrinol (Lausanne) 2019;10:603. doi: 10.3389/fendo.2019.00603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bat-Erdene U, Quan E, Chan K, Lee BM, Matook W, Lee KY, Rosales JL. Neutrophil TLR4 and PKR are targets of breast cancer cell glycosaminoglycans and effectors of glycosaminoglycan-induced APRIL secretion. Oncogenesis. 2018;7:45. doi: 10.1038/s41389-018-0058-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bonnet MC, Weil R, Dam E, Hovanessian AG, Meurs EF. PKR stimulates NF-kappaB irrespective of its kinase function by interacting with the IkappaB kinase complex. Mol Cell Biol. 2000;20:4532–4542. doi: 10.1128/MCB.20.13.4532-4542.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang P, Samuel CE. Induction of protein kinase PKR-dependent activation of interferon regulatory factor 3 by vaccinia virus occurs through adapter IPS-1 signaling. J Biol Chem. 2008;283:34580–34587. doi: 10.1074/jbc.M807029200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barlan AU, Griffin TM, McGuire KA, Wiethoff CM. Adenovirus membrane penetration activates the NLRP3 inflammasome. J Virol. 2011;85:146–155. doi: 10.1128/JVI.01265-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adamiak M, Ciechanowicz A, Skoda M, Cymer M, Tracz M, Xu B, Ratajczak MZ. Novel Evidence that purinergic signaling-Nlrp3 inflammasome axis regulates circadian rhythm of hematopoietic stem/progenitor cells circulation in peripheral blood. Stem Cell Rev Rep. 2020;16:335–343. doi: 10.1007/s12015-020-09953-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dyer MR, Chen Q, Haldeman S, Yazdani H, Hoffman R, Loughran P, Tsung A, Zuckerbraun BS, Simmons RL, Neal MD. Deep vein thrombosis in mice is regulated by platelet HMGB1 through release of neutrophil-extracellular traps and DNA. Sci Rep. 2018;8:2068. doi: 10.1038/s41598-018-20479-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tulotta C, Ottewell P. The role of IL-1B in breast cancer bone metastasis. Endocr Relat Cancer. 2018;25:R421–R434. doi: 10.1530/ERC-17-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schulze J, Zierath D, Tanzi P, Cain K, Shibata D, Dressel A, Becker K. Severe stroke induces long-lasting alterations of high-mobility group box 1. Stroke. 2013;44:246–248. doi: 10.1161/STROKEAHA.112.676072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoshida H, Russell J, Senchenkova EY, Almeida Paula LD, Granger DN. Interleukin-1beta mediates the extra-intestinal thrombosis associated with experimental colitis. Am J Pathol. 2010;177:2774–2781. doi: 10.2353/ajpath.2010.100205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khodabandehlou K, Masehi-Lano JJ, Poon C, Wang J, Chung EJ. Targeting cell adhesion molecules with nanoparticles using in vivo and flow-based in vitro models of atherosclerosis. Exp Biol Med (Maywood) 2017;242:799–812. doi: 10.1177/1535370217693116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hosseinkhani B, Kuypers S, van den Akker NMS, Molin DGM, Michiels L. Extracellular vesicles work as a functional inflammatory mediator between vascular endothelial cells and immune cells. Front Immunol. 2018;9:1789. doi: 10.3389/fimmu.2018.01789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raschi E, Chighizola CB, Grossi C, Ronda N, Gatti R, Meroni PL, Borghi MO. β2-glycoprotein I, lipopolysaccharide and endothelial TLR4: Three players in the two hit theory for anti-phospholipid-mediated thrombosis. J Autoimmun. 2014;55:42–50. doi: 10.1016/j.jaut.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 50.Xia L, Zhou H, Wang T, Xie Y, Wang T, Wang X, Yan J. Activation of mTOR is involved in anti-β2GPI/β2GPI-induced expression of tissue factor and IL-8 in monocytes. Thromb Res. 2017;157:103–110. doi: 10.1016/j.thromres.2017.05.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.