Abstract
Background
The umbilical cord is a structure made of blood vessels and connective tissue that connects the baby and placenta in utero. The umbilical cord is cut after birth, which separates the mother and her baby both physically and symbolically. Omphalitis is defined as infection of the umbilical cord stump. Tracking of bacteria along the umbilical vessels may lead to septicaemia that can result in neonatal morbidity and mortality, especially in developing countries.
Objectives
To determine the effect of application of antimicrobials on newborn's umbilical cord versus routine care for prevention of morbidity and mortality in hospital and community settings.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (1 October 2012). In addition, we also searched LILACS (1982 to 11 October 2012) and HERDIN NeON (October 2012)
Selection criteria
We included randomized, cluster‐randomized and quasi‐randomized controlled trials of topical cord care compared with no topical care, and comparisons between different forms of care.
Data collection and analysis
Two review authors independently assessed trials for inclusion, trial quality and subsequently extracted data. Data were checked for accuracy.
Main results
The search identified 77 trials. We included 34 trials in the review involving 69,338 babies, five studies are awaiting classification and there are two ongoing community trials. Included studies were conducted in both developed and developing countries. Among the 34 included trials, three were large, cluster‐randomized trials conducted in community settings in developing countries and 31 studies were conducted in hospital settings mostly in developed countries. Data for community and hospital studies were analyzed separately. The three trials conducted in community settings contributed 78% of the total number of children included in this review. Of the trials conducted in hospital settings, the majority had small sample sizes. There were 22 different interventions studied across the included trials and the most commonly studied antiseptics were 70% alcohol, triple dye and chlorhexidine.
Only one antiseptic, chlorhexidine was studied in community settings for umbilical cord care. Three community trials reported data on all‐cause mortality that comprised 1325 deaths in 54,624 participants and combined results showed a reduction of 23% (average risk ratio (RR) 0.77, 95% confidence interval (CI) 0.63 to 0.94, random‐effects, T² = 0.02, I² = 50%) in the chlorhexidine group compared with control. The reduction in omphalitis ranged from 27% to 56% depending on the severity of infection. Cord separation time was increased by 1.7 days in the chlorhexidine group compared with dry cord care (mean difference (MD) 1.75 days, 95% CI 0.44 to 3.05, random‐effects, T² = 0.88, I² = 100%). Washing of umbilical cord with soap and water was not advantageous compared with dry cord care in community settings.
Among studies conducted in hospital settings, no study reported data for mortality or tetanus. No antiseptic was advantageous to reduce the incidence of omphalitis compared with dry cord care in hospital settings. Topical triple dye application reduced bacterial colonization with Staphylococcus aureus compared with dry cord care (average RR 0.15, 95% CI 0.10 to 0.22, four studies, n = 1319, random‐effects, T² = 0.04, I² = 24%) or alcohol application (average RR 0.45, 95% CI 0.25 to 0.80, two studies, n = 487, random‐effects, T² = 0.00, I² = 0%). There was no advantage of application of alcohol and triple dye for reduction of colonization with streptococcus. Topical alcohol application was advantageous in reduction of colonization with Enterococcus coli compared with dry cord care (average RR 0.73, 95% CI 0.58 to 0.92, two studies, n = 432, random‐effects, T² = 0.00, I² = 0%) and in a separate analysis, triple dye increased the risk of colonization compared with alcohol (RR 3.44, 95% CI 2.10 to 5.64, one study, n = 373). Cord separation time was significantly increased with topical application of alcohol (MD 1.76 days, 95% CI 0.03 to 3.48, nine studies, n = 2921, random‐effects, T² = 6.54, I² = 97%) and triple dye (MD 4.10 days, 95% CI 3.07 to 5.13, one study, n = 372) compared with dry cord care in hospital settings. The number of studies was insufficient to make any inference about the efficacy of other antiseptics.
Authors' conclusions
There is significant evidence to suggest that topical application of chlorhexidine to umbilical cord reduces neonatal mortality and omphalitis in community and primary care settings in developing countries. It may increase cord separation time however, there is no evidence that it increases risk of subsequent morbidity or infection.
There is insufficient evidence to support the application of an antiseptic to umbilical cord in hospital settings compared with dry cord care in developed countries.
Keywords: Humans; Infant, Newborn; Umbilical Cord; Umbilical Cord/microbiology; Anti‐Infective Agents, Local; Anti‐Infective Agents, Local/administration & dosage; Chlorhexidine; Chlorhexidine/administration & dosage; Inflammation; Inflammation/mortality; Inflammation/prevention & control; Randomized Controlled Trials as Topic; Sepsis; Sepsis/mortality; Sepsis/prevention & control
Plain language summary
Umbilical cord antiseptics for preventing sepsis and death among newborns
The umbilical cord connects the baby and mother during pregnancy. The cord is cut after birth. The cord stump then dries and falls off, generally within five to 15 days. Infection of the umbilical cord stump (omphalitis), caused by skin bacteria, is a significant cause of illness and death in newborn babies in developing countries. This review evaluated all studies that assessed antiseptics applied topically to the umbilical cord to determine if they reduce the risk of cord infection and death. Thirty‐four randomised controlled studies were included involving 69,338 babies. There were 22 different interventions studied. The most commonly studied antiseptics in the included studies were 70% alcohol, triple dye and chlorhexidine. Three studies were conducted in community settings in developing countries; the remainder were conducted in hospital settings, mostly in developed countries. Studies conducted in community settings were large and contributed about 78% of all the participants included in this review. Hospital‐based studies were small and had limitations.
Studies conducted in community settings evaluated the effectiveness of topical application of chlorhexidine and combined results showed that chlorhexidine reduced risk of death by 23% and the risk of cord infection ranging from 27% to 56%, depending on the severity of infection. Topical application of chlorhexidine may increase cord separation time by about 1.7 days, however, this does not increase subsequent risk of cord infection or death.
None of the studies conducted in hospital settings reported data for risk of death or tetanus. No antiseptic was found to be advantageous for the prevention of cord infection compared with dry cord care in hospital settings. Topical triple dye application reduced bacterial colonization with Staphylococcus aureus compared to both dry cord care and alcohol application. There was no advantage of application of alcohol and triple dye for reduction of colonization with streptococcus. Topical alcohol application was advantageous in the reduction of colonization with Enterococcus coli compared with dry cord care and triple dye application. Cord separation time was increased with topical application of alcohol and triple dye compared with dry cord care in hospital settings. There were insufficient studies to determine the efficacy of other antiseptics.
Background
Description of the condition
The umbilical cord is a structure made of blood vessels and connective tissue that connects the baby and placenta in utero. Its outer surface is a membrane that is bathed in amniotic fluid. The umbilical cord is cut after birth, which separates the mother and her baby, both physically and symbolically. The cord stump then dries, falls off and the wound heals. The natural process of the umbilical cord falling off involves the formation of an area of separation between the drying cord and the abdominal wall in which polymorphonuclear leucocytes (a type of white blood cells) are present (Oudesluys‐Murphy 1990). During this process, material may collect at this junction that sometimes looks like pus and is often wrongly identified as an infection. The cord usually separates between five and 15 days after birth (Oudesluys‐Murphy 1987). Before the separation, the remaining stump can be considered to be a healing wound and thus a possible route for infection through the vessels into the baby’s blood stream.
Infection of an umbilical cord may be clinically obvious, but is also sometimes not apparent. In case of frank infections the cord may be swollen, the surrounding skin inflamed, or the cord may be ’smelly’ if infected with anaerobic bacteria (Mullany 2006a). Tracking of bacteria along the umbilical vessels is not obvious to the eye, but can cause septicaemia, or result in other focal infections as a result of blood‐borne spread such as septic arthritis (Forshall 1957). In such cases, affected babies may also present with fever, lethargy or poor feeding, collectively called sepsis, in the neonatal period.
Description of the intervention
As described above, the umbilical cord stump can be the potential source of entry of pathogenic microorganisms causing morbidity and mortality. There are two important considerations in this regard; first, is the colonization of newborn skin and umbilical cord stump with potential pathogenic microorganisms and second is the application of harmful substances to the umbilical cord. It is well known that the skin of the newborn, including the umbilical stump, is colonized by microorganisms soon after birth (Mir 2011; Mullany 2012). These microorganisms include both pathogenic and non‐pathogenic species. The profile of organisms colonizing the cord stump varies according to hygenic conditions at the time of birth and immediate postpartum period. In high‐resource settings, the likely organisms are gram positive ones while in low resource, community settings, gram negative organisms seem more prevalent. A study from US showed that 210 of 211 (99.7%) infants studied were found positive for Staphylococcus aureus at least once in the first six days of life (Fairchild 1958). A recent study from community settings from Bangladesh showed that predominant flora that colonize the newborn umbilical stump were gram negative (Escherichia coli, Klebsiella pneumoniae, and Pseudomonas spp) (Mullany 2012). Pathogenic bacteria such as Escherichia coli, Klebsiella pneumoniae, and Pseudomonas spp and streptococci can track up the umbilical stump causing infection. It is therefore essential to keep the cord clean.
The practice of cord cutting at birth and care of the umbilical stump afterwards varies according to local practice and culture (Elhassani 1984; Mullany 2006; Mullany 2007). In many parts of the world, deliveries occur at home and the cord is cut with unsterile tools such as used razors or scissors after which various substances are applied including mustard oil, turmeric, charcoal, grease, cow dung or dried banana to speed up cord separation (Mir 2011; Mullany 2007; Mullany 2009; Smith 2009). This combination of unhygienic cutting of cord and application of potential harmful substances is an important sources of bacterial infection and neonatal tetanus (Bennett 1997; Mullany 2007; Mullany 2009). Up to this point, there is a general agreement about the ’clean’ technique for cutting the cord using a sterile cutting instrument (blade or scissors) and clean hands to avoid infection (Blencowe 2011), however, there is less agreement on what is the best care of the cord stump (Blencowe 2011; Zupan 2004).
The most frequent modern practice of umbilical cord care is applying antimicrobials to the cord stump. These include antiseptics (such as alcohol, silver sulphadiazine, iodine, chlorhexidine (CHX); and dyes such as triple dye, gentian violet, acriflavine and eosin) and/or topical application of antibiotics (for example, bacitracin, neomycin, nitrofurazone, or tetracycline, or moisture absorbing powders). These substances may be used as solutions in water, alcohol, detergent or ointments. Another approach is to keep the cord clean and dry without applying anything and this is recommended by the World Health Oraganization (WHO 1999). A previous approach was to bath the baby soon after birth with an antimicrobial solution such as hexachlorophene, however, hexachlorophene is no longer recommended in newborn babies as it is absorbed through the skin and is neurotoxic (WHO 1999). One potential side effect of topical antimicrobials is the delay in cord separation time, which can potentially increase the risk of bacterial entry (Novack 1988). However, it has been shown that it does not increase the risk of mortality (Mullany 2006b).
How the intervention might work
Studies from developed countries have shown that use of an antiseptic on umbilical cord stump in hospital nurseries significantly reduced umbilical colonization rates (Barrett 1979; Pezzati 2002; Speck 1977). Seventy per cent alcohol has been used since the 1900s for routine cord care along with antimicrobial solutions such as triple dye, tincture of iodine, iodophors, antibiotic ointments, silver sulphadiazine and CHX. Soaking the umbilical stump in 70% alcohol rapidly kills gram positive and gram negative bacteria. However, it has been shown in several hospital studies to be less effective in controlling umbilical colonization than other antimicrobials such as triple dye or CHX (Panyavudhikrai 2002; Pezzati 2002). Despite its limitations, its low cost and availability have led to widespread use, especially in low‐resource settings (WHO 1999).
Recently, CHX has been studied for cord care in community settings (Arifeen 2012; Mullany 2006). CHX is a broad spectrum antiseptic that is extensively used in dental, obstetric and surgical scrub. It has also been used in obstetrics, peripartum, perineal and vaginal washes in concentrations as high as 4% (McClure 2007). Safety studies in newborn infants exposed to CHX washes in various concentrations found no evidence of toxicity even in babies in which percutaneous absorption may have taken place (Aggett 1981; Johnsson 1987). CHX is currently included in WHO’s Essential Drugs List (WHO 2011).
Why it is important to do this review
According to a recent estimate about 40.3% (3.1 million) of all deaths in children less than five years occurred in the neonatal period (Liu 2012). Most of these deaths occurred in developing countries and infections, along with complications of prematurity are the most important cause of mortality in the neonatal period (Liu 2012). In populations with high neonatal mortality rates, infections account for approximately half of all newborn deaths (Lawn 2005). Infection of the cord stump, called omphalitis, is a significant cause of mortality and morbidity in developing countries (Agrawal 2012; Lehmann 1999; Mir 2011; Mullany 2007; Mullany 2009; Sawardekar 2004; Thaver 2009). The infection typically presents as a superficial cellulitis that may progress to involve the abdominal wall and eventually to necrotizing fasciitis, myonecrosis, or systemic disease (Gallagher 2010). The risk is greatest in situations where deliveries take place at home, often with unskilled traditional birth attendants who do not employ clean delivery practices (Darmstadt 2009; Mullany 2009). Omphalitis is relatively rare in developed countries with an overall incidence rate which varies from 0.2% to 0.7% (McKenna 1977). Incidence of omphalitis in developing countries in community settings may range up to 21% (Mir 2011). In these settings, the mortality rate among all infants with omphalitis, including those who develop complications, is estimated at up to 46% (Mullany 2009). The mortality rate is significantly higher (about 71%) after the development of necrotizing fasciitis or myonecrosis (Sawin 1994). Suggested risk factors for poor prognosis include male sex, prematurity or being small‐for‐gestational age, and septic delivery (Faridi 1993; Gallagher 2010; Mullany 2007).
The WHO and American Academy of Pediatrics recommend good hygiene at delivery, and promote dry cord care practice after birth (AAP 2003; WHO 1999). These recommendations however, are based on insufficient evidence in favour of or against an antiseptic (McClure 2007; Zupan 2004). The aim of this review is to provide data useful for identifying good practice in both high‐ and low‐income countries.
Objectives
To determine the effect of application of antimicrobials on the umbilical cord of newborns versus routine care for prevention of morbidity and mortality in hospital and community settings.
Methods
Criteria for considering studies for this review
Types of studies
Randomized, cluster‐randomized and quasi‐randomized controlled trials.
Types of participants
Live newborns born to mothers with or without risk factors for the development of infection (for example, chorioamnionitis, preterm rupture of membranes, urinary tract infection), regardless of place of delivery (home, hospital, non‐institutional birth, etc) and gestational age and birthweight.
Types of interventions
We evaluated the following interventions.
Antiseptic versus no antiseptic or placebo/dry cord care.
Antibiotics versus no antibiotic.
Antiseptic versus antibiotic.
Antiseptic versus antiseptic.
Single versus multiple application.
Washing umbilical cord with soap/water versus dry cord care.
Those studies were excluded where a combination of antiseptic and antibiotic was used. Studies that evaluated hexachlorophene were excluded as the antiseptic had been removed from the market because of central nervous toxicity.
Types of outcome measures
Primary outcomes
All‐cause mortality
Confirmed or suspected sepsis
Omphalitis
Tetanus
Confirmed sepsis is defined as clinical signs and symptoms consistent with infection and microbiologically proven with a positive blood culture, cerebrospinal fluid culture, urine culture or culture from a normally sterile site (e.g. pleural fluid, peritoneal fluid or autopsy specimens) for bacteria or fungi.
Suspected sepsis is defined as clinical signs and symptoms consistent with sepsis without isolation of a causative organism.
Tetanus is defined as trismus (spasm of the muscles involved in opening of the jaws) and severe generalized muscular spasms not attributable to other causes (i.e. hypocalcaemia, phenothiazine reaction, strychnine poisoning) (AAP 2003).
Omphalitis is defined as clinical signs and symptoms of umbilical stump infection which include the following.
Localized infection:
purulent or malodorous discharge from the umbilical stump;
periumbilical erythema;
oedema;
tenderness.
Three case definitions have been described by Mullany 2006a to describe the severity of omphalitis and had been used wherever data were available. The three definitions are described below.
Algorithm 1: Moderate or severe redness.
Algorithm 2: Moderate redness with pus, or severe redness (without regard to pus).
Algorithm 3: Severe redness with pus.
Extensive local disease that includes conditions such as necrotizing fasciitis or myonecrosis, which are typically found in a periumbilical location but may spread across the abdominal wall, onto the flanks and back, and into the scrotum. These signs may also suggest infection by both aerobic and anaerobic organisms and include the following:
ecchymoses, violaceous discolorations;
bullae;
peau d'orange appearance (the skin looks like orange peel);
crepitus;
petechiae;
progression of cellulitis despite antimicrobial therapy (Gallagher 2010).
Secondary outcomes
Bacterial colonization
Time to cord separation
Search methods for identification of studies
Electronic searches
We contacted the Trials Search Co‐ordinator to search the Cochrane Pregnancy and Childbirth Group’s Trials Register (1 October 2012).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of EMBASE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and EMBASE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
In addition, we searched LILACS (1982 to 11 October 2012) using the search strategy detailed in Appendix 1, and HERDIN NeON, the Philippine database of local science journals (October 2012).
We did not apply any language restrictions.
Data collection and analysis
Selection of studies
Two review authors independently assessed for inclusion potential studies identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted a third person.
Data extraction and management
For eligible studies, at least two review authors extracted the data. We resolved discrepancies through discussion or, if required, we consulted a third person. We entered data into Review Manager software (RevMan 2011) and checked for accuracy.
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal the allocation sequence and determine whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomization; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3) Blinding (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding could not have affected the results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel;
low, high or unclear risk of bias for outcome assessors.
(4) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomized participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we included the missing data in the analyses.
We assessed methods as:
low risk of bias (e.g. less than 10% missing data);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomization);
unclear risk of bias.
If missing data could not be supplied by the trial authors, we carried out subgroup analysis for groups with sufficient outcome data.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found. We assessed the methods as:
low risk of bias (where it was clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review had been reported);
high risk of bias (where not all the study’s pre‐specified outcomes had been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest were reported incompletely and so could not be used; study failed to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other sources of bias
We described for each included study any important concerns we had about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias (e.g. study design, imbalance in baseline data):
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it likely to impact on the findings. We explored the impact of the level of bias through sensitivity analyses ‐ seeSensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals. For the three included cluster‐randomized trials, data were entered using the generic inverse variance option in RevMan 2011 to allow adjustment of the variance for cluster effect (Higgins 2011).
Continuous data
For continuous data, we used the mean difference with 95% confidence intervals. For studies with multiple groups, comparison groups were combined into a single pair‐wise comparison. Standard deviations were adjusted using the formula in the Cochrane Handbook (Higgins 2011).
Unit of analysis issues
We included cluster‐randomized trials in the analyses along with individual‐randomized trials. We used cluster‐adjusted values from the trials, irrespective of the method used. In case a trial was not adjusted for cluster design, results were adjusting by inflating the standard error of the effect size by quare root of design effect given in the study.
Dealing with missing data
For included studies, we took note of levels of attrition. We explored the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we included all participants randomized to each group in the analyses, and analyzed all participants in the group to which they were allocated, regardless of whether or not they received the allocated intervention.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the T², I² and Chi² statistics. We considered heterogeneity as substantial if an I² was greater than 50% and either the T² was greater than zero, or the P value was less than 0.10 in the Chi² test for heterogeneity.
Assessment of reporting biases
We planned that, if there were 10 or more studies in the meta‐analysis, we would investigate reporting biases (such as publication bias) using funnel plots. We intended to assess funnel plot asymmetry visually. If asymmetry was suggested by a visual assessment, we planned to perform exploratory analyses to investigate it.
Data synthesis
We performed statistical analysis using the Review Manager software (RevMan 2011). We used the random‐effects analysis to produce an overall summary of the average treatment effect across trials considering the different interventions across different economic settings. We have presented the results as the average treatment effect with its 95% confidence interval, and the estimates of T² and I².
Subgroup analysis and investigation of heterogeneity
We planned to the following subgroup analyses:
preterm (gestational age less than 37 weeks) versus term (gestational age 37 weeks or more);
hospital setting; community‐based studies; settings mixed or undefined;
trials carried out in Europe‐Americas; Western Pacific; Eastern Mediterranean; South‐East Asia; Africa.
Gestational age and birthweight are relevant subgroups since the incidence of sepsis is high among low birthweight and preterm infants. Causative organisms, corresponding susceptibility to antiseptics and severity of disease, may differ in hospital versus community settings. We therefore analyzed hospital and community‐based studies separately. Baseline neonatal mortality rate varies with region: Europe‐America (11‐12/1000 livebirths); Western Pacific (19/1000 livebirths); Eastern Mediterranean (40/1000 livebirths); Southeast Asia (38/1000 livebirths); Africa (44/1000 livebirths) (WHO 2006).
We included all primary outcomes in the subgroup analysis (mortality, confirmed or suspected sepsis, tetanus and omphalitis).
Sensitivity analysis
We carried out sensitivity analysis removing studies of low quality.
We also carried out sensitivity analyses to investigate the effect of missing data:
less than 5% missing data;
5% to 10% missing data;
10% to 20% missing data;
20% or more missing data.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies.
Results of the search
The search identified 77 trials; we included 34 studies in the review, involving 69,338 babies (See Characteristics of included studies). Thirty‐six studies were excluded (see Characteristics of excluded studies). Five studies are awaiting classification (see Characteristics of studies awaiting classification) while there are two ongoing community trials (see Ongoing studies).
Included studies
Thirty‐four trials reported in 46 papers met the inclusion criteria. More than one report was available for six (17%) trials (Arifeen 2012; Evens 2004; Mullany 2006; Speck 1977; Soofi 2012; Suliman 2010). When results of an included trial were reported in more than one publication, we extracted data from all reports but counted it as one trial. All included trials reported data that could be included in a meta‐analysis.
There were three large, cluster‐randomized trials conducted in community settings (Arifeen 2012; Mullany 2006; Soofi 2012). These three trials contributed 78% of the total number of children included in this review. The two ongoing studies are also community trials that assess effect of topical application of chlorhexidine to umbilical cord. One study is being conducted in Zambia (Hamer 2010) and other in Pemba (Sazawal 2012).
Of the trials conducted in hospital settings, the majority had small sample sizes. Sample size of these 31 (91%) trials ranged between 71 and 2241.
Seventeen (50%) of the included studies had a 'dry cord care' group and 16 (47%) studies comprised more than two study groups.
The studies were conducted in both developing and developed countries. All three community studies were conducted in developing countries and most of the hospital‐based studies were conducted in developed countries. There were eight studies conducted in USA (Barrett 1979; Evens 2004; Gladstone 1988; Golombek 2002; Rosenfeld 1989; Schuman 1985; Speck 1977; Suliman 2010), four in Canada (Dore 1998; Janssen 2003; Medves 1997; Rush 1986), three in Taiwan (Hsu 1999; Hsu 2010; Huang 2001), two each in Iran (Ahmadpour‐Kacho 2006; Nourian 2009), Norway (Meberg 1985; Meberg 1990), Italy (Pezzati 2002; Pezzati 2003), and Pakistan (Shafique 2006; Soofi 2012) and one each in Saudi Arabia (Al‐Binali 2006), Palestine (Arad 1981), Bangladesh (Arifeen 2012), Scotland (Bain 1994), Peru (Davila 2007), Germany (Kapellen 2009), England (Mugford 1986), Nepal (Mullany 2006), Japan (Oishi 2004), Thailand (Panyavudhikrai 2002), and Spain (Perapoch 1993).
There were 18 (52%) studies that included full‐term babies, five (15%) included preterm babies (Bain 1994; Gladstone 1988; Pezzati 2002; Pezzati 2003; Rosenfeld 1989), seven (20%) included both term and preterm babies (Ahmadpour‐Kacho 2006; Arifeen 2012; Davila 2007; Janssen 2003; Mullany 2006; Oishi 2004; Soofi 2012) and gestational age was not mentioned in four (12%) studies (Al‐Binali 2006; Barrett 1979; Mugford 1986; Schuman 1985).
There were 22 different interventions studied across the included trials. These included topical antiseptics, antibiotics and measures such as washing the cord with soap and water. There were 20 studies (58%) that evaluated topical alcohol application, 10 (29%) triple dye, nine (26%) chlorhexidine, five (14%) Silver Sulfadiazine, three (8%) Povidine and two (5%) salicylic powder. There was one study each for Beniktol, neomycin, bismuth, breastmilk, bacitracin, benzine, hydrophobic gauze, mercurochrome, green clay powder, katoxin, fuschine, and citrane.
Of the primary outcomes considered, three trials (Arifeen 2012; Mullany 2006; Soofi 2012) evaluated the effect of cord care on mortality. Thirteen studies reported data on omphalitis. One study reported data on sepsis (Pezzati 2003) and one study reported sepsis‐related mortality (Mullany 2006). No study reported tetanus.
Of the secondary outcomes, 26 studies reported cord separation time, 12 reported bacterial colonization.
Excluded studies
Thirty‐six studies were excluded (See Characteristics of excluded studies). Reasons for exclusion were: 1) not a randomized controlled trial, 2) not a cord care study or, 3) effect of individual intervention was not established due to co‐interventions.
Risk of bias in included studies
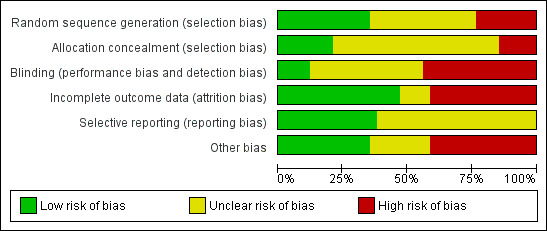
See Figure 1 for summary of assessment of risk of bias.
1.
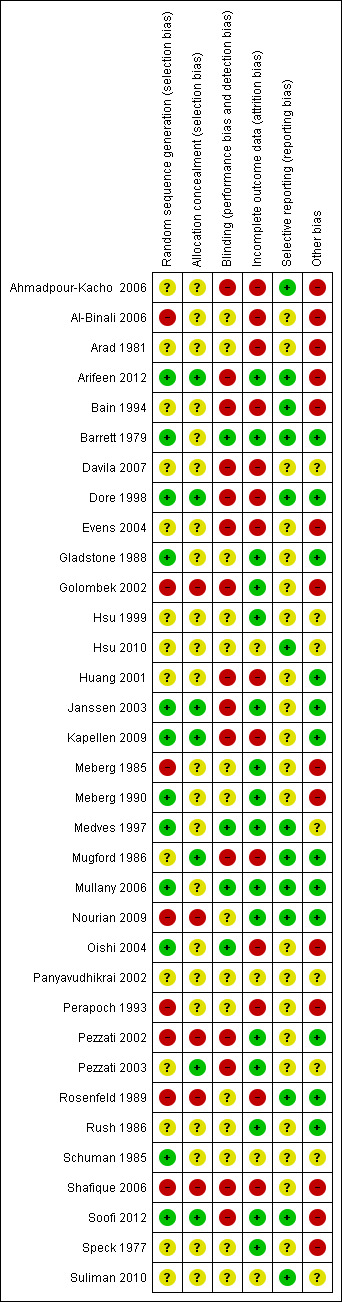
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Twelve (35%) studies had adequate methods of sequence generation, eight (24%) had inadequate methods or were quasi‐randomized trials while in 14 (41%) studies, methods were inadequately described to make a complete assessment.
Allocation was adequately concealed in seven (21%) studies while in 22 (65%) studies it was not mentioned or unclear. Five (15%) studies had inadequate methods of allocation concealment.
Blinding
Method of blinding was not done, not feasible, or not mentioned in 30 (88%) studies. Three studies had "adequate" methods of blinding. The study of Mullany 2006 mentioned that investigators, field workers and participants were masked to chlorhexidine and soap and water but not to the dry cord care.
Incomplete outcome data
Seventeen (50%) studies reported data on attrition and exclusion while 14 studies (41%) did not describe enough details on follow‐up. There was insufficient information in three (9%) studies to make a complete assessment.
Selective reporting
It is difficult to assess true selective reporting bias when protocols of the included studies are not available. Review authors had access to the protocols of three studies to make an assessment (Arifeen 2012; Mullany 2006; Soofi 2012) and they were considered to be adequate. In the rest of the studies, the judgment was made based on variables mentioned in the methods section compared to those reported in the results section of the study. Based on this assessment, 13 studies had adequate descriptions of outcomes in the results section for the outcomes mentioned in the methods section (Ahmadpour‐Kacho 2006; Arifeen 2012; Bain 1994; Barrett 1979; Dore 1998; Hsu 2010; Medves 1997; Mugford 1986; Mullany 2006; Nourian 2009; Rosenfeld 1989; Soofi 2012; Suliman 2010).
Other potential sources of bias
In the study of (Ahmadpour‐Kacho 2006), baseline characteristics showed more vaginal deliveries in the dry care, alcohol and silver groups compared with the breastmilk group. Arad 1981 included the outcome of sepsis but no bacterial cultures were obtained to prove the condition. The community trial from Bangladesh (Arifeen 2012), showed that there was no effect of multiple cleansing with chlorhexidine on neonatal mortality. This may be due to the fact that the study was not powered enough to detect a significant difference in this arm, as pointed out by authors (Arifeen 2012). In the study of Bain 1994, there was a higher rate of rupture of membranes in the dry care group. In Davila 2007, there was no table of baseline characteristics. In the study of Evens 2004, there was a higher rate of vaginal delivery in the alcohol group. The studies by Golombek 2002 and Shafique 2006 gathered data on the incidence of omphalitis by telephone calls and no home visits were made to make an assessment. This could have resulted in false positives or negatives as symptoms were reported by mothers. Kapellen 2009 had no table of baseline characteristics but the text mentioned that the groups were comparable in terms of sex, ethnicity, birthweight, birth length, gestational age, model of delivery and Apgar score. No description of baseline characteristics of study participants was given in five studies (Al‐Binali 2006; Meberg 1985; Meberg 1990; Perapoch 1993; Speck 1977). The study of Medves 1997 had no table of baseline characteristics. Mugford 1986 mentioned in the text that the groups were comparable in terms of sex, birthweight and mode of delivery. The study of Oishi 2004 states that the babies in the alcohol group had low birthweights but the proportion was not mentioned. The desired sample size in Soofi 2012was not achieved due to a security situation in the study area.
Effects of interventions
Antiseptics versus dry cord care/placebo
There were 18 studies that had a comparison group between 'dry cord care' and 'an antiseptic'. Fifteen of these studies were conducted in hospital settings while three were conducted in community settings (Arifeen 2012; Mullany 2006; Soofi 2012).
The antiseptics included in this comparison were: alcohol, triple dye, chlorhexidine, salicylic sugar powder, green clay powder, silver sulphadiazine, benzine, katoxin powder, fuschine, zinc powder and breastmilk.
Community studies
Primary outcomes
Mortality
Only one antiseptic, i.e. chlorhexidine was studied in community settings for umbilical cord care. Three community trials reported data on all‐cause mortality (Arifeen 2012; Mullany 2006; Soofi 2012). There were 1325 deaths in 54,624 participants of three studies and combined results showed a reduction of 23% in the intervention group compared with control (average risk ratio (RR) 0.77, 95% confidence interval (CI) 0.63 to 0.94; random‐effects, T² = 0.02, I² = 50% (Analysis 1.1)).
1.1. Analysis.
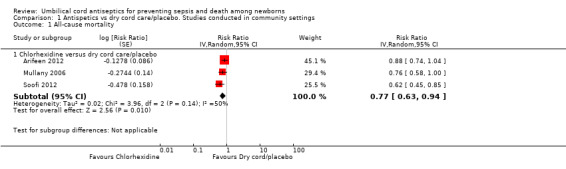
Comparison 1 Antispetics vs dry cord care/placebo. Studies conducted in community settings, Outcome 1 All‐cause mortality.
Sepsis
No community‐based study reported data on the incidence of sepsis, however, one trial (Mullany 2006) from Nepal reported data on sepsis‐specific mortality and showed a 31% reduction in the chlorhexidine group compared with dry cord care but the results were not statistically significant (RR 0.69, 95% CI 0.40 to 1.18).
Omphalitis
Antiseptics were associated with a significant reduction in omphalitis ranging from 27% to 56% depending on the severity of infection: redness extending to skin: average RR 0.73, 95% CI 0.64 to 0.83; three studies, random‐effects, T² = 0.00, I² = 34% (Analysis 1.2); redness with pus or severe redness: average RR 0.69, 95% CI 0.60 to 0.79; three studies, random‐effects, T² = 0.00, I² = 0% (Analysis 1.3); severe redness with pus: average RR 0.44, 95% CI 0.28 to 0.69; three studies, random‐effects, T² = 0.03, I² = 19% (Analysis 1.4), with the most significant reduction in severe cases.
1.2. Analysis.
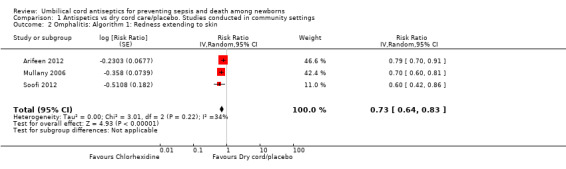
Comparison 1 Antispetics vs dry cord care/placebo. Studies conducted in community settings, Outcome 2 Omphalitis: Algorithm 1: Redness extending to skin.
1.3. Analysis.
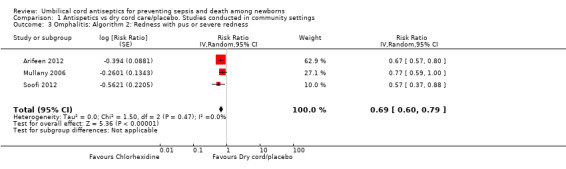
Comparison 1 Antispetics vs dry cord care/placebo. Studies conducted in community settings, Outcome 3 Omphalitis: Algorithm 2: Redness with pus or severe redness.
1.4. Analysis.
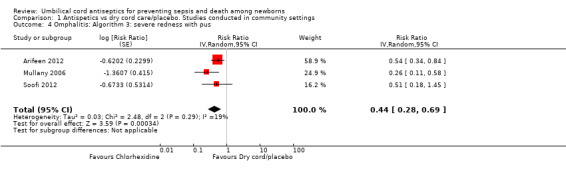
Comparison 1 Antispetics vs dry cord care/placebo. Studies conducted in community settings, Outcome 4 Omphalitis: Algorithm 3: severe redness with pus.
Secondary outcomes
Bacterial colonization
One study (Arifeen 2012) reported data on bacterial colonization and showed a significant reduction in bacterial colonization of Staphylococcus aureus (RR 0.27, 95% CI 0.23 to 0.31 (Analysis 1.5)); Enterococcus coli (RR 0.50, 95% CI 0.46 to 0.54 (Analysis 1.6)) and streptococci (RR 0.28, 95% CI 0.22 to 0.37 (Analysis 1.7)) with topical chlorhexidine application compared with control.
1.5. Analysis.
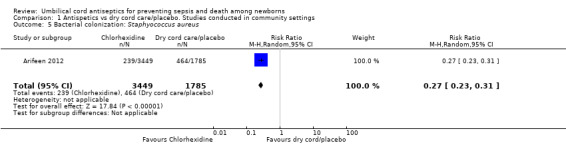
Comparison 1 Antispetics vs dry cord care/placebo. Studies conducted in community settings, Outcome 5 Bacterial colonization: Staphyococcus aureus.
1.6. Analysis.
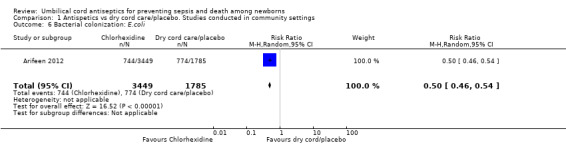
Comparison 1 Antispetics vs dry cord care/placebo. Studies conducted in community settings, Outcome 6 Bacterial colonization: E.coli.
1.7. Analysis.
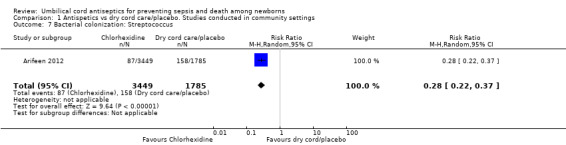
Comparison 1 Antispetics vs dry cord care/placebo. Studies conducted in community settings, Outcome 7 Bacterial colonization: Streptococcus.
Cord separation time
Data for cord separation time was available from two studies (Arifeen 2012; Mullany 2006), carried out in Bangladesh and Nepal respectively. In the study by Mullany 2006, cord separation time was longer in the chlorhexidine group (5.32 ± 2.4 days) compared with the dry cord care group (4.24 ± 1.6 days). There was no increased risk of mortality in children who had increased cord separation time (Mullany 2006). In Arifeen 2012, separation time in the combined chlorhexidine group (7.20 ± 3.0 days) was 2.40 (95% CI 2.17 to 2.64) days longer than among babies not exposed to chlorhexidine (4.79 ± 1.8 days) (Unpublished data). The combined data for both of these studies showed that cord separation time was 1.7 days longer in the chlorhexidine group compared with the non‐chlorhexidine group (mean difference (MD) 1.75, 95% CI 0.44, to 3.05, random‐effects, T² = 0.88, I² = 100% (Analysis 1.8)).
1.8. Analysis.
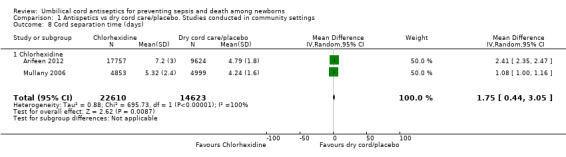
Comparison 1 Antispetics vs dry cord care/placebo. Studies conducted in community settings, Outcome 8 Cord separation time (days).
Hospital studies
Primary outcomes
Mortality/sepsis/tetanus
Among studies conducted in hospital settings, no study reported data on mortality, sepsis or tetanus for the comparison of antiseptics versus dry cord care/placebo.
Omphalitis
Five studies reported data for incidence of omphalitis (Bain 1994; Janssen 2003; Kapellen 2009; Nourian 2009; Pezzati 2002). There was no significant difference for the incidence of omphalitis when alcohol (three studies) (average RR 0.92, 95% CI 0.62 to 1.39), triple dye (two studies) (average RR 0.71, 95% CI 0.13 to 3.73), chlorhexidine (one study) (RR 0.28, 95% CI 0.06 to 1.35), salicylic sugar powder (one study) (RR 0.21, 95% CI 0.01 to 4.38) and green clay powder (one study) (RR 0.48, 95% CI 0.04 to 5.26) were compared with dry cord care/placebo (Analysis 2.1).
2.1. Analysis.
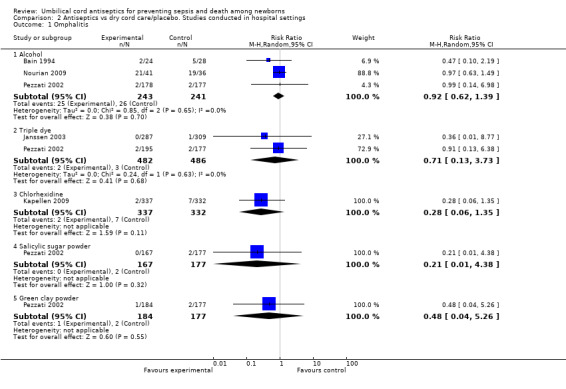
Comparison 2 Antiseptics vs dry cord care/placebo. Studies conducted in hospital settings, Outcome 1 Omphalitis.
Secondary outcomes
Bacterial colonization
Compared with dry cord care/placebo, bacterial colonization of umbilical cord with Staphylococcus aureus was significantly reduced with application of triple dye (four studies) (average RR 0.15, 95% CI 0.10 to 0.22, random‐effects, T² = 0.04, I² = 24%), silver sulphadiazine (two studies) (average RR 0.72, 95% CI 0.60 to 0.87, random‐effects, T² = 0.00, I² = 0%), chlorhexidine (one study) (RR 0.65, 95% CI 0.55 to 0.77), salicylic sugar powder (one study) (RR 0.32, 95% CI 0.17 to 0.58), green clay powder (one study) (RR 0.51, 95% CI 0.31 to 0.82) and fuschine (one study) (RR 0.52, 95% CI 0.32 to 0.84). For the same control group, there was no significant difference for alcohol (two studies) (average RR 0.61, 95% CI 0.11 to 3.36), benzine (one study) (RR 0.99, 95% CI 0.90 to 1.09). Compared with dry cord care/placebo, there was a significantly increased risk of bacterial colonization of umbilical cord with Staphylococcus aureus associated with use of katoxin (one study) (RR 1.43, 95% CI 1.02 to 2.00). See Analysis 2.2.
2.2. Analysis.
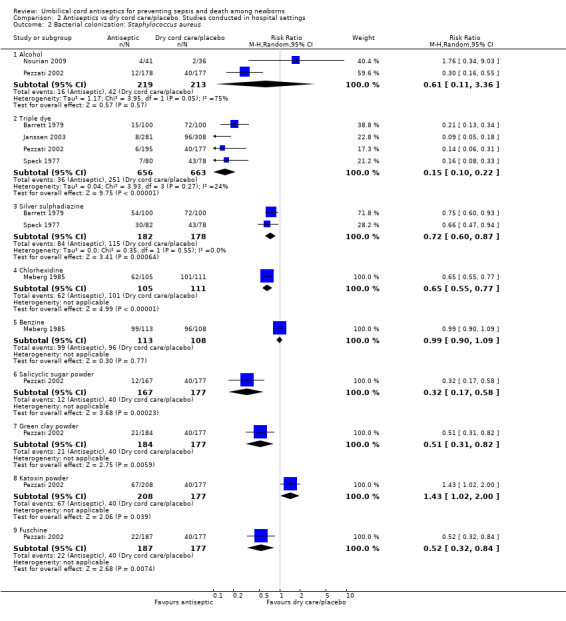
Comparison 2 Antiseptics vs dry cord care/placebo. Studies conducted in hospital settings, Outcome 2 Bacterial colonization: Staphylococcus aureus.
For the outcome of colonization with streptococci, there was a significant reduction with application of silver sulphadiazine (two studies) (average RR 0.62, 95% CI 0.43 to 0.89, random‐effects, T² = 0.00, I² = 0%) and fuschine (one study) (RR 0.19, 95% CI 0.04 to 0.85) compared with dry cord care/placebo. For the same comparison group, there was no difference in colonization of streptococci with the application of alcohol (two studies) (RR 0.42, 95% CI 0.15 to 1.19, random‐effects, T² = 0.33, I² = 50%), triple dye (three studies) (average RR 0.57, 95% CI 0.28 to 1.18, random‐effects, T² = 0.31, I² = 79%), chlorhexidine (one study) (RR 0.53, 95% CI 0.27 to 1.04), salicylic sugar powder (one study) (RR 0.74, 95% CI 0.29 to 1.90). However, there was an increased risk of streptococcal bacterial colonization associated with the use of green clay powder (one study) (RR 4.62, 95% CI 2.41 to 8.84) and katoxin powder (one study) (RR 5.87, 95% CI 3.12 to 11.05). See Analysis 2.3.
2.3. Analysis.
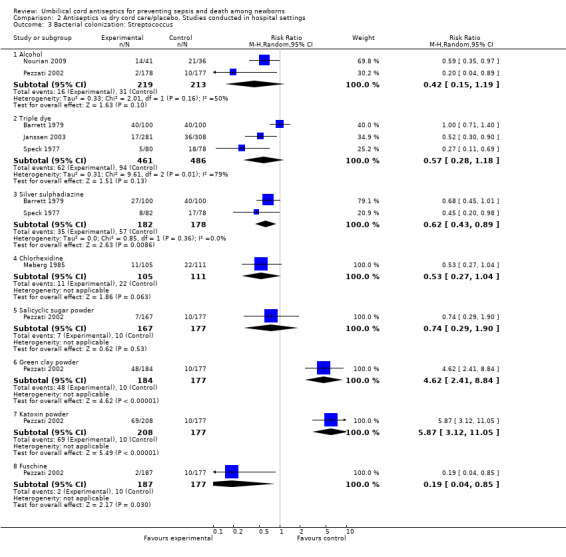
Comparison 2 Antiseptics vs dry cord care/placebo. Studies conducted in hospital settings, Outcome 3 Bacterial colonization: Streptococcus.
Compared with dry cord care/placebo, there was a significant reduction in the colonization of Enterococcus coli with topical application of alcohol (two studies) (average RR 0.73, 95% CI 0.58 to 0.92, random‐effects, T² = 0.00, I² = 0%), silver sulphadiazine (one study) (RR 0.70, 95% CI 0.53 to 0.93) and chlorhexidine (one study) (RR 0.59, 95% CI 0.39 to 0.90). In contrast, there was no significant difference with application of triple dye (two studies) (average RR 0.79, 95% CI 0.53 to 1.17, random‐effects, T² = 0.06, I² = 79%), salicylic sugar powder (one study) (RR 0.59, 95% CI 0.32 to 1.10), green clay powder (one study) (RR 1.27, 95% CI 0.79 to 2.05) and katoxin powder (one study) (RR 1.12, 95% CI 0.70 to 1.81). There was increased risk of Enterococcus coli colonization associated with application of fuschine (one study) (RR 2.04, 95% CI 1.33 to 3.13). See Analysis 2.4.
2.4. Analysis.
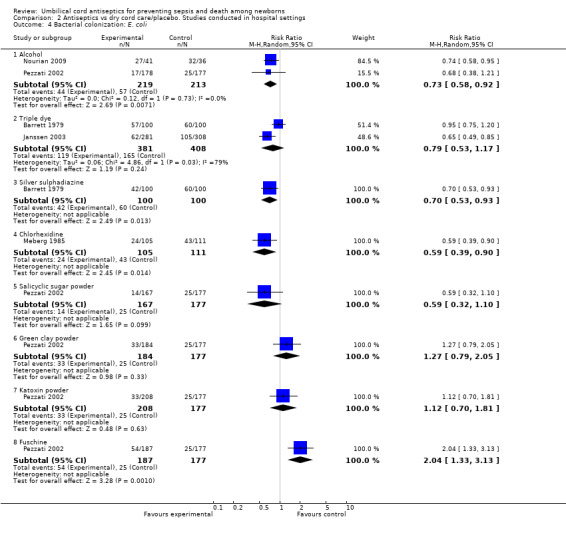
Comparison 2 Antiseptics vs dry cord care/placebo. Studies conducted in hospital settings, Outcome 4 Bacterial colonization: E. coli.
Cord separation time
There was a significant increase in cord separation time when alcohol was applied to umbilical cord compared with dry cord care/placebo (nine studies) (MD 1.76 days, 95% CI 0.03 to 3.48, random‐effects, T² = 6.54, I² = 97%). There was one study each for triple dye (MD 4.10 days, 95% CI 3.07 to 5.13), katoxin powder (MD 0.80 days, 95% CI 0.18 to 1.42), fuschine (MD 2.80 days, 95% CI 2.01 to 3.59) and silver sulphadiazine (MD 3.60 days, 95% CI 2.66 to 4.54) and these antiseptics were associated with a significant increase in cord separation time compared with dry cord care/placebo. One study each for zinc powder (MD ‐1.82 days, 95% CI ‐2.23 to ‐1.41), salicylic sugar powder (MD ‐1.90 days, 95% CI ‐2.47 to ‐1.33), green clay powder (MD ‐0.80 days, 95% CI ‐1.36 to ‐0.24), breastmilk (MD ‐1.69 days, 95% CI ‐2.31 to ‐1.07) and chlorhexidine (MD ‐0.80 days, 95% CI ‐1.21 to ‐0.39) were associated with decreased cord separation time compared with dry cord care. See Analysis 2.6.
2.6. Analysis.
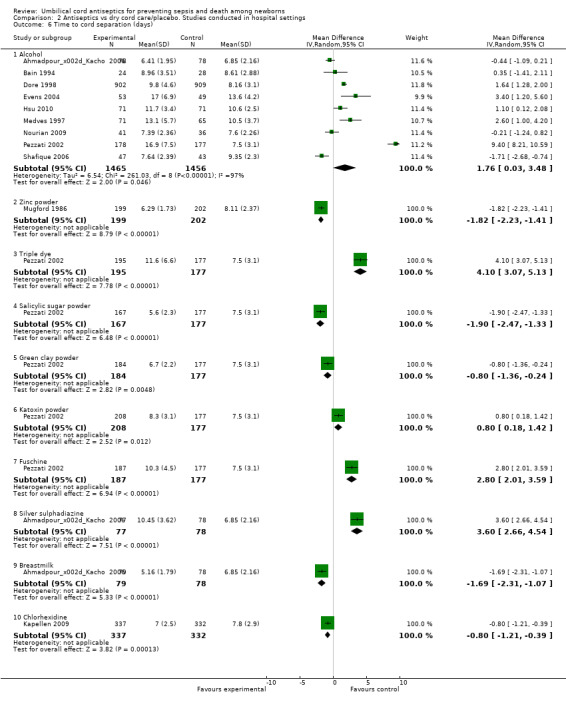
Comparison 2 Antiseptics vs dry cord care/placebo. Studies conducted in hospital settings, Outcome 6 Time to cord separation (days).
Antibiotics versus no antibiotic
There were no studies in community or hospital settings that investigated this comparison.
Antiseptics versus antibiotics
Community studies
There was no study that was conducted in community settings for this comparison.
Hospital studies
Primary outcomes
Mortality/sepsis/tetanus/omphalitis
Among studies conducted in hospital settings, no data were reported on mortality, sepsis, tetanus or omphalitis for this comparison.
Secondary outcomes
Bacterial colonization
One study compared triple dye (RR 0.65, 95% CI 0.03 to 12.87), silver sulphadiazine (RR 2.18, 95% CI 0.42 to 11.33) and povidone (RR 2.18, 95% CI 0.42 to 11.33) with bacitracin and there were no differences in bacterial colonization rates for Staphylococcus aureus (Analysis 3.1). No data were reported for colonization with streptococcus and enterococcus.
3.1. Analysis.
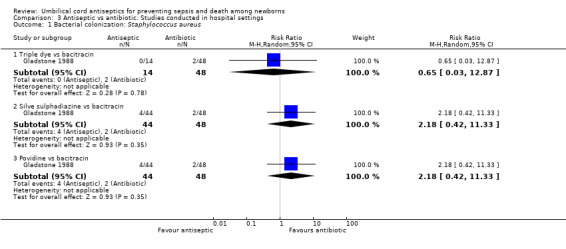
Comparison 3 Antiseptic vs antibiotic. Studies conducted in hospital settings, Outcome 1 Bacterial colonization: Staphylococcus aureus.
Cord separation time
Cord separation time was significantly reduced when umbilical cord was treated with triple dye compared with bacitracin (one study) (MD ‐5.60 days, 95% CI ‐9.36 to ‐1.84) and neomycin (one study) (MD ‐4.30, 95% CI ‐6.27 to ‐2.33). There was no difference in cord separation time when silver sulphadiazine was compared with neomycin (one study) (MD ‐1.40 days, 95% CI ‐3.65 to 0.85). Cord separation time was significantly reduced when umbilical cord was treated with povidone versus bacitracin (one study) (MD ‐2.00 days, 95% CI ‐3.67 to ‐0.33). Similarly, alcohol was associated with a significantly reduced cord separation time compared with beniktol (MD ‐2.33 days, 95% CI ‐3.77 to ‐0.89). See Analysis 3.2.
3.2. Analysis.
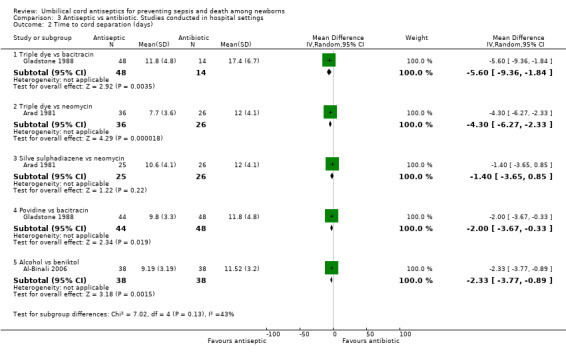
Comparison 3 Antiseptic vs antibiotic. Studies conducted in hospital settings, Outcome 2 Time to cord separation (days).
Antiseptics versus antiseptics
Community studies
There was no study that was conducted in community settings for this comparison.
Hospital studies
Primary outcomes
Sepsis and mortality
Among studies conducted in hospital settings, no study reported data on mortality or tetanus. One study compared chlorhexidine with salicylic acid powder and reported no difference in the incidence of sepsis between the two groups (RR 1.11, 95% CI 0.07 to 17.50 (Analysis 4.1)).
4.1. Analysis.
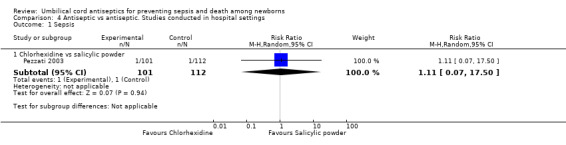
Comparison 4 Antiseptic vs antiseptic. Studies conducted in hospital settings, Outcome 1 Sepsis.
Omphalitis
Three studies evaluated triple dye versus alcohol and reported no significant reduction in the incidence of omphalitis in the triple dye group compared with the alcohol‐treated group (average RR 0.48, 95% CI 0.14 to 1.63, random‐effects, T² = 0.54, I² = 41%). There was no difference when triple dye was compared with salicylic sugar powder (one study) (RR 4.29, 95% CI 0.21 to 88.65) and katoxin (one study) (RR 2.13, 95% CI 0.19 to 23.34).
Similarly, there was no difference in the incidence of omphalitis for the comparisons of alcohol versus povidone (RR 0.84, 95% CI 0.62 to 1.16, one study), alcohol versus salicylic powder (RR 4.69, 95% CI 0.23 to 97.03, one study), alcohol versus green clay powder (RR 2.07, 95% CI 0.19 to 22.60, one study), alcohol versus katoxin (RR 2.10, 95% CI 0.19 to 22.97, one study), alcohol versus fuschine (RR 2.10, 95% CI 0.19 to 22.97, one study) and alcohol versus chlorhexidine (RR 2.77, 95% CI 0.12 to 66.49, one study). One study also compared chlorhexidine with hydrophobic gauze and reported no significant difference in the incidence of omphalitis (RR 1.36, 95% CI 0.55 to 3.36).
In contrast, triple dye was associated with a significant reduction in the incidence of omphalitis compared with povidone‐iodine (RR 0.15, 95% CI 0.07 to 0.32, one study). See Analysis 4.2.
4.2. Analysis.
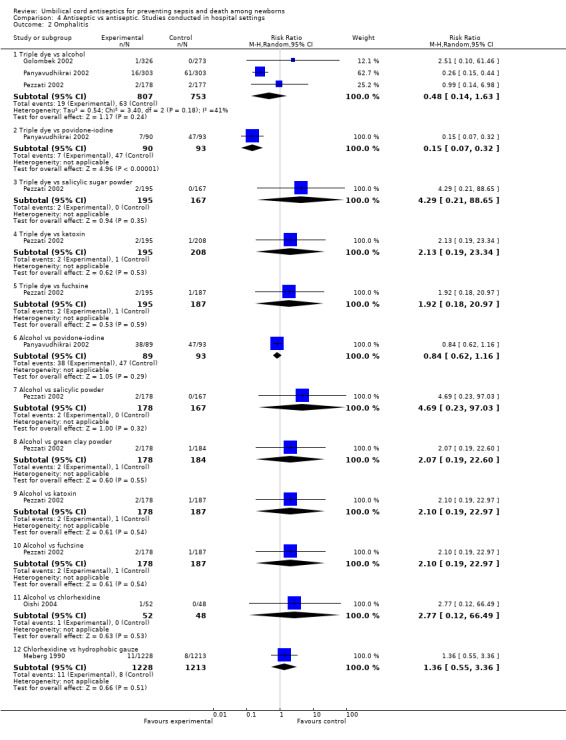
Comparison 4 Antiseptic vs antiseptic. Studies conducted in hospital settings, Outcome 2 Omphalitis.
Secondary outcomes
Bacterial colonization
Bacterial colonization with Staphylococcus aureus was significantly reduced when umbilical cord was treated with triple dye compared with alcohol (two studies) (average RR 0.45, 95% CI 0.25 to 0.80, random‐effects, T² = 0.00, I² = 0%), silver sulphadiazine (three studies) (average RR 0.36, 95% CI 0.25 to 0.50, random‐effects, T² = 0.00, I² = 0%), green clay powder (one study) (RR 0.27, 95% CI 0.11 to 0.65) and katoxin powder (one study) (RR 0.26, 95% CI 0.11 to 0.63) (Analysis 4.3). There was no significant difference in Staphylococcus aureus colonization rates for comparisons of triple dye versus povidone (RR 0.65, 95% CI 0.03 to 12.87), triple dye versus salicylic sugar powder (one study) (RR 0.43, 95% CI 0.16 to 1.12) or triple dye versus fuschine (one study) (RR 1.92, 95% CI 0.18 to 20.97). One trial (Pezzati 2002) studied alcohol in comparison with green clay powder (RR 0.59, 95% CI 0.30 to 1.16), fuschine (RR 0.57, 95% CI 0.29 to 1.12), salicylic powder (RR 0.94, 95% CI 0.43 to 2.03) and povidone (RR 0.84, 95% CI 0.62 to 1.16) and found no significant difference in the colonization of umbilical cord with Staphylococcus aureus.
4.3. Analysis.
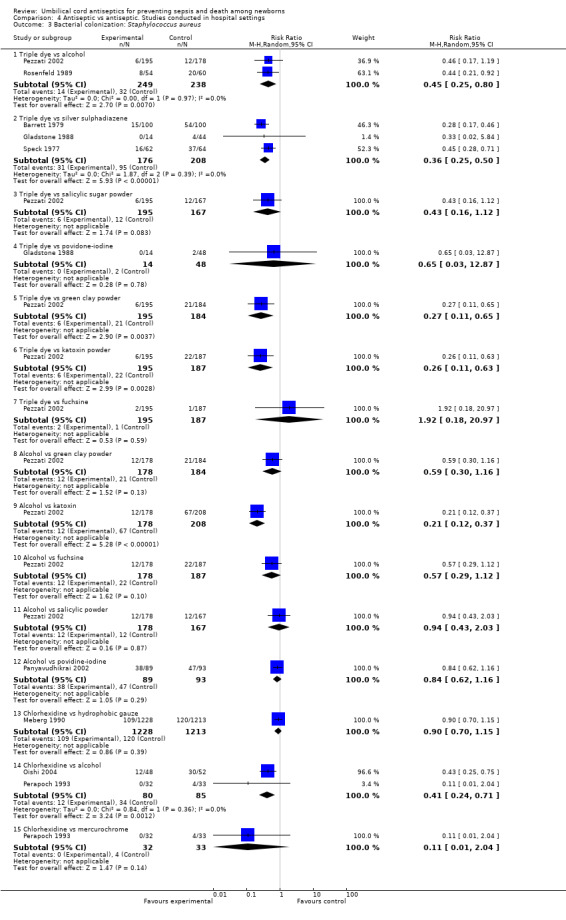
Comparison 4 Antiseptic vs antiseptic. Studies conducted in hospital settings, Outcome 3 Bacterial colonization: Staphylococcus aureus.
However, treating the umbilical cord with alcohol was associated with a significant reduction in bacterial colonization with Staphylococcus aureus compared with the use of katoxin powder (RR 0.21, 95% CI 0.12 to 0.37, one study). One study each evaluated chlorhexidine in comparison with hydrophobic gauze (RR 0.90, 95% CI 0.70 to 1.15) and mercurochrome (RR 0.11, 95% CI 0.01 to 2.04) and found no significant difference in colonization rates for Staphylococcus aureus. There was however, a significant difference when chlorhexidine was compared with alcohol (two studies) (RR 0.41, 95% CI 0.24 to 0.71). See (Analysis 4.3).
One study each compared triple dye with alcohol (RR 0.46, 95% CI 0.04 to 4.94), silver sulphadiazine (RR 1.26, 95% CI 0.71 to 2.25), and fuschine (RR 0.48, 95% CI 0.04 to 5.24) and reported no significant difference between the groups for colonization of streptococcus (Analysis 4.4). However, for the same outcome, there was a significant effect of triple dye compared with green clay powder (one study) (RR 0.02, 95% CI 0.00 to 0.14), salicylic sugar powder (one study) (RR 0.12, 95% CI 0.02 to 0.98) and katoxin powder (one study) (RR 0.02, 95% CI 0.00 to 0.11). Application of alcohol to the umbilical cord was also associated with a reduction in colonization of streptococcus compared with green clay powder (one study) (RR 0.04, 95% CI 0.01 to 0.17) and katoxin powder (one study) (RR 0.03, 95% CI 0.01 to 0.14) but there was no difference when alcohol was compared with fuschine (one study) (RR 1.05, 95% CI 0.15 to 7.38) or salicylic powder (one study) (RR 0.94, 95% CI 0.43 to 2.03). See Analysis 4.4.
4.4. Analysis.
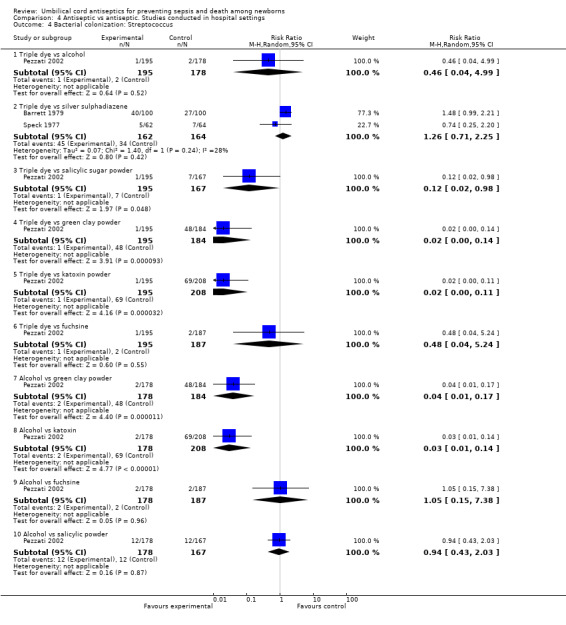
Comparison 4 Antiseptic vs antiseptic. Studies conducted in hospital settings, Outcome 4 Bacterial colonization: Streptococcus.
Triple dye led to an increased risk of colonization of Enterococcus coli compared with alcohol (one study) (RR 3.44, 95% CI 2.10 to 5.64), silver sulphadiazine (one study) (RR 1.36, 95% CI 1.02 to 1.81), green clay powder (one study) (RR 1.83, 95% CI 1.27 to 2.65) and salicylic sugar powder (one study) (RR 3.92, 95% CI 2.28 to 6.72) and katoxin powder (one study) (RR 2.07, 95% CI 1.43 to 3.00) (Analysis 4.5). For the same outcomes, alcohol had a significant preventive effect compared with green clay powder (RR 0.53, 95% CI 0.31 to 0.92) and fuschine (RR 0.33, 95% CI 0.20 to 0.55). However, there was no difference in colonization rates of Enterococcus coli for the comparison of alcohol with salicylic powder (one study) (RR 1.14, 95% CI 0.58 to 2.4) and katoxin powder (one study) (RR 0.60, 95% CI 0.35 to 1.04). Similarly, there were no differences in colonization rates when comparing chlorhexidine with hydrophobic gauze (one study) (RR 0.79, 95% CI 0.31 to 2.00) or mercurochrome (one study) (RR 0.21, 95% CI 0.01 to 4.13). See Analysis 4.5.
4.5. Analysis.
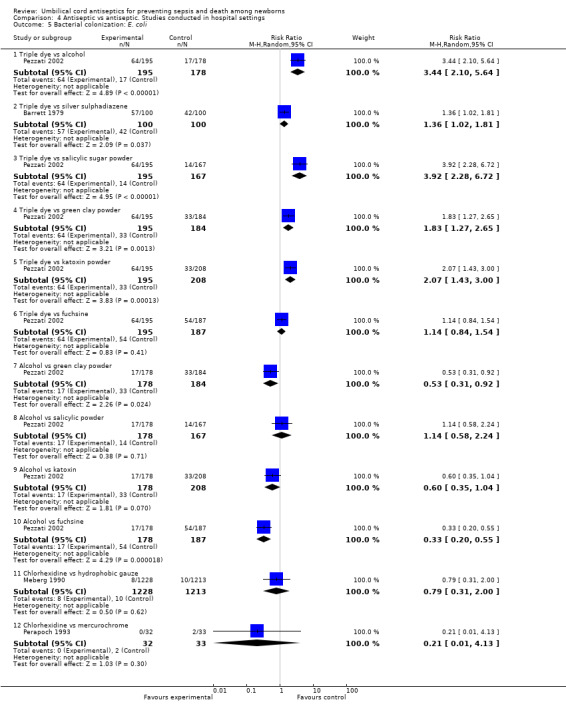
Comparison 4 Antiseptic vs antiseptic. Studies conducted in hospital settings, Outcome 5 Bacterial colonization: E. coli.
Cord separation time
Cord separation time was not significantly different when triple dye was compared with alcohol (two studies) (MD 0.43 days, 95% CI ‐8.49 to 9.35, random‐effects, T² = 40.92, I² = 99%) or when triple dye was compared with silver sulphadiazine (two studies) (MD 0.15 days, 95% CI ‐6.20 to 6.51, random‐effects, T² = 18.82, I² = 89%). Cord separation time was increased when triple dye was compared with salicylic sugar powder (one study) (MD 6.00 days, 95% CI 5.01 to 6.99), povidone‐iodine (one study) (MD 7.60 days, 95% CI 3.96 to 11.24), green clay powder (one study) (MD 4.90 days, 95% CI 3.92 to 5.88), katoxin powder (one study) (MD 3.30 days, 95% CI 2.28 to 4.32) and fuschine (one study) (MD 1.30 days, 95% CI 0.17 to 2.43) (Analysis 4.6). There was also a significant increase in cord separation time when the umbilical cord was treated with alcohol compared with green clay powder (one study) (MD 10.20 days, 95% CI 9.05 to 11.35), katoxin powder (one study) (MD 8.60 days, 95% CI 7.42 to 9.78), salicylic powder (one study) (MD 11.30 days, 95% CI 10.14 to 12.46) or fuschine (one study) (MD 6.60 days, 95% CI 5.32 to 7.88) (Analysis 4.6). Chlorhexidine decreased cord separation time compared with hydrophobic gauze (one study) (MD ‐0.40 days, 95% CI ‐0.57 to ‐0.23), however, chlorhexidine significantly increased cord separation time compared with either salicylic powder (one study) (MD 3.00 days, 95% CI 2.46 to 3.54) or mercurochrome (one study) (MD 6.40 days, 95% CI 5.25 to 7.55) (Analysis 4.6). One study showed that application of povidone resulted in a shorter cord separation time compared with silver sulphadiazine (MD ‐4.00 days, 95% CI ‐5.53 to ‐2.47). However, there was no significant difference in cord separation time when a combination of triple dye and alcohol was compared with triple dye alone (MD 1.00 day, 95% CI ‐0.45 to 2.45). See Analysis 4.6.
4.6. Analysis.
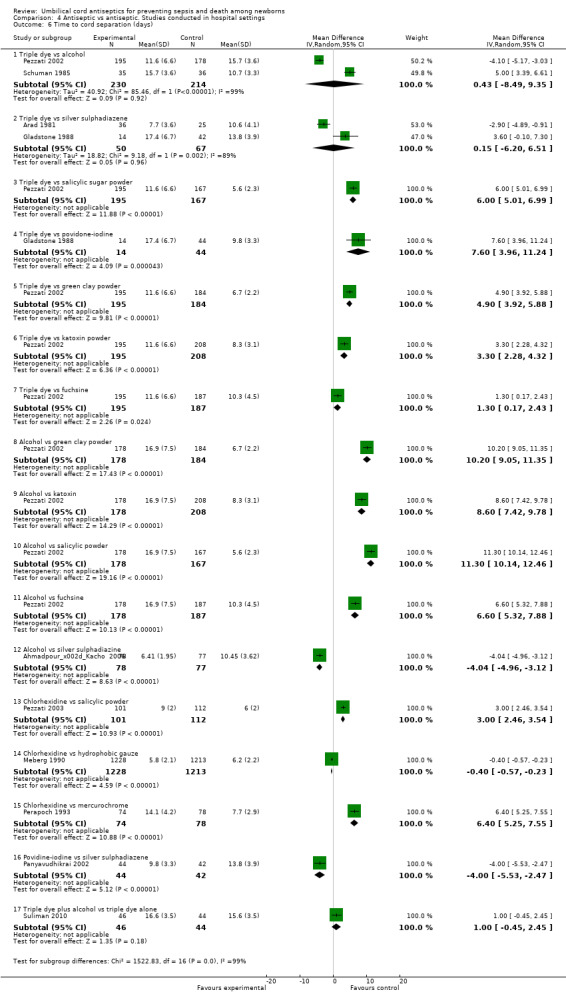
Comparison 4 Antiseptic vs antiseptic. Studies conducted in hospital settings, Outcome 6 Time to cord separation (days).
Single versus multiple application of antiseptic
Community studies
There was one study from community settings that compared single versus multiple application of chlorhexidine and reported all‐cause mortality, incidence of omphalitis and bacterial colonization (Arifeen 2012). No study reported sepsis and tetanus for this comparison.
Primary outcomes
Mortality
One study (Arifeen 2012) reported data on mortality and there was no significant difference in all‐cause mortality between the two groups (RR 0.85, 95% CI 0.70 to 1.03] (Analysis 5.1).
5.1. Analysis.
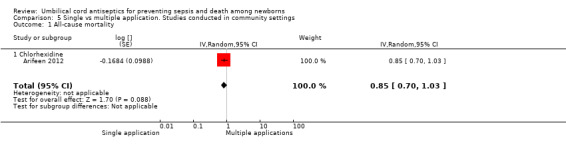
Comparison 5 Single vs multiple application. Studies conducted in community settings, Outcome 1 All‐cause mortality.
Omphalitis
The effect on the incidence of omphalitis varied according to the severity of omphalitis. Single application was associated with an increased incidence of moderate (RR 1.53, 95% CI 1.22 to 1.92) and severe episodes (RR 2.12, 95% CI 1.10 to 4.11) of omphalitis (Analysis 5.3; Analysis 5.4) compared with multiple applications. There was no difference between single application and multiple application groups for mild omphalitis (RR 1.14, 95% CI 0.97 to 1.34 (Analysis 5.2)).
5.3. Analysis.
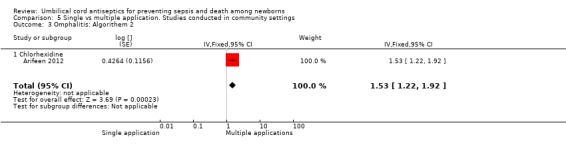
Comparison 5 Single vs multiple application. Studies conducted in community settings, Outcome 3 Omphalitis: Algorithem 2.
5.4. Analysis.
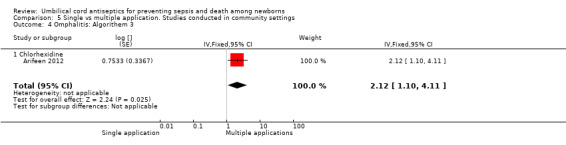
Comparison 5 Single vs multiple application. Studies conducted in community settings, Outcome 4 Omphalitis: Algorithem 3.
5.2. Analysis.
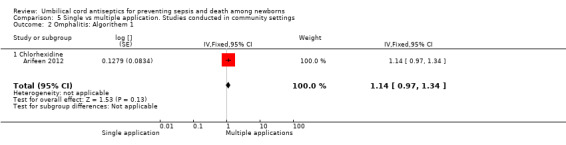
Comparison 5 Single vs multiple application. Studies conducted in community settings, Outcome 2 Omphalitis: Algorithem 1.
Secondary outcomes
Bacterial colonization
Compared with multiple applications, single application of chlorhexidine increased the risk of bacterial colonization with Staphylococcus aureus in one study (RR 3.63, 95% CI 2.74 to 4.82 (Analysis 5.5)) and Enterococcus coli (RR 1.17, 95% CI 1.03 to 1.32 (Analysis 5.7)). For bacterial colonization with streptococcus, there was no difference between single and multiple application of chlorhexidine groups in one study (RR 1.01, 95% CI 0.67 to 1.53 (Analysis 5.6)).
5.5. Analysis.
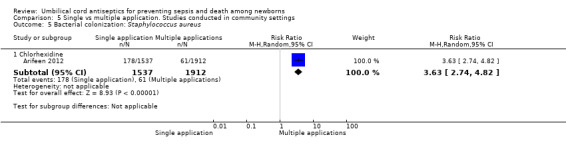
Comparison 5 Single vs multiple application. Studies conducted in community settings, Outcome 5 Bacterial colonization: Staphylococcus aureus.
5.7. Analysis.
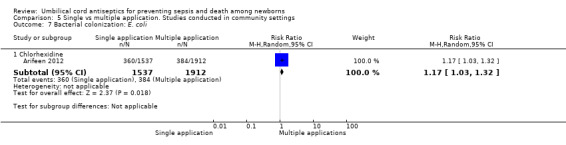
Comparison 5 Single vs multiple application. Studies conducted in community settings, Outcome 7 Bacterial colonization: E. coli.
5.6. Analysis.
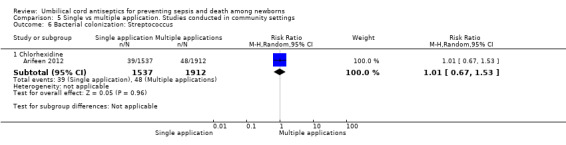
Comparison 5 Single vs multiple application. Studies conducted in community settings, Outcome 6 Bacterial colonization: Streptococcus.
Hospital‐based studies
Among studies conducted in hospitals settings, no study reported data for mortality, sepsis, tetanus, omphalitis or bacterial colonization.
Secondary outcomes
Cord separation time
Four studies reported data for cord separation time. Among these studies, three studied triple dye and reported a significant reduction with single application compared with multiple application (MD ‐4.24 days, 95% CI ‐4.41 to ‐4.07, random‐effects, T² = 0.00, I² = 0% (Analysis 6.1). One study evaluated zinc powder and showed no difference in cord separation time for single application compared with multiple application (MD ‐0.02 days, 95% CI ‐0.31 to 0.27 (Analysis 6.1)).
6.1. Analysis.
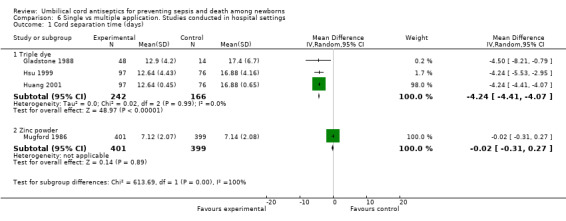
Comparison 6 Single vs multiple application. Studies conducted in hospital settings, Outcome 1 Cord separation time (days).
Washing umbilical cord versus dry cord care
Community studies
One large cluster‐randomized community trial (Mullany 2006) evaluated washing the umbilical cord with soap and water compared with dry cord care. No study reported data on the incidence of sepsis or tetanus.
Primary outcomes
Mortality
There was no significant difference in all‐cause mortality between the group washing the umbilical cord with soap and water and the dry cord care group (RR 1.00, 95% CI 0.76 to 1.32 (Analysis 7.1)).
7.1. Analysis.
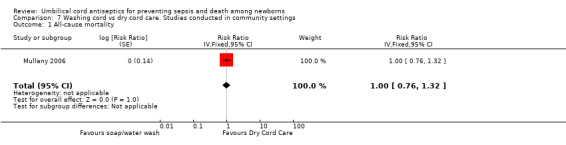
Comparison 7 Washing cord vs dry cord care. Studies conducted in community settings, Outcome 1 All‐cause mortality.
Omphalitis
There was no differential effect of cord washing with soap/water compared with dry cord care for the incidence of mild (RR 1.03, 95% CI 0.87 to 1.22 (Analysis 7.2)), moderate (RR 0.88, 95% CI 0.69 to 1.12 (Analysis 7.3)) and severe omphalitis (RR 1.01, 95% CI 0.58 to 1.76 (Analysis 7.4)).
7.2. Analysis.
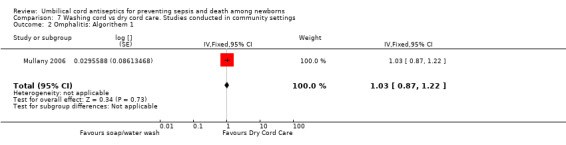
Comparison 7 Washing cord vs dry cord care. Studies conducted in community settings, Outcome 2 Omphalitis: Algorithem 1.
7.3. Analysis.
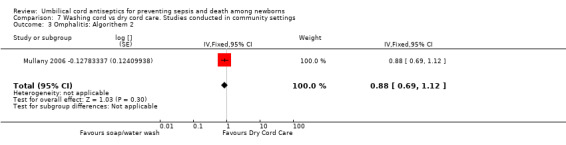
Comparison 7 Washing cord vs dry cord care. Studies conducted in community settings, Outcome 3 Omphalitis: Algorithem 2.
7.4. Analysis.
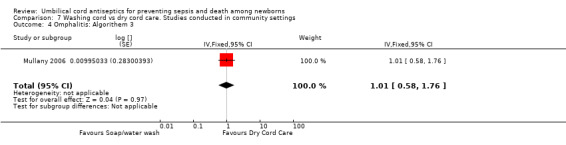
Comparison 7 Washing cord vs dry cord care. Studies conducted in community settings, Outcome 4 Omphalitis: Algorithem 3.
Secondary outcomes
Cord separation time
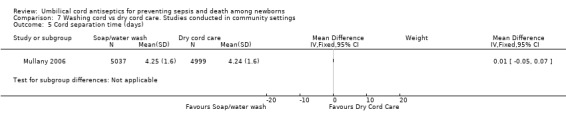
Cord separation times were similar between the group washing the umbilical cord with soap and water and the dry cord care group (MD 0.01 day, 95% CI ‐0.05 to 0.07 (Analysis 7.5)).
7.5. Analysis.
Comparison 7 Washing cord vs dry cord care. Studies conducted in community settings, Outcome 5 Cord separation time (days).
Hospital‐based studies
No study reported data for mortality, sepsis, tetanus, omphalitis and cord separation time.
Secondary outcomes
Bacterial colonization
One study (Rush 1986), was conducted in hospital settings and reported no significant difference in colonization rates of Staphylococcus aureus between the cord washing and dry cord care groups (RR 0.93, 95% CI 0.65 to 1.34 (Analysis 8.1)).
8.1. Analysis.
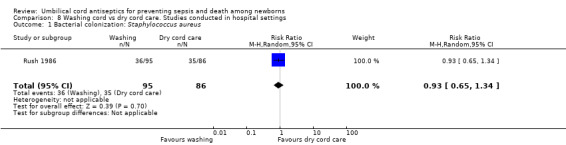
Comparison 8 Washing cord vs dry cord care. Studies conducted in hospital settings, Outcome 1 Bacterial colonization: Staphylococcus aureus.
Discussion
Summary of main results
This review included 34 studies, involving 69,338 babies. Five further studies are awaiting classification and there are two ongoing community trials. Included studies were conducted in both developed and developing countries. Among the 34 included trials, three were large, cluster‐randomized trials conducted in community settings in developing countries and 31 studies were conducted in hospital settings mostly in developed countries. Data for community and hospital studies were analyzed separately. There were 22 different interventions studied across the included trials; 70% alcohol, triple dye and chlorhexidine were the most commonly studied antiseptics in included studies.
Studies conducted in community settings
Combined results of three large, community‐based, cluster‐randomized trials showed that topical application of chlorhexidine to umbilical cord stump reduced neonatal mortality and incidence of omphalitis (Arifeen 2012; Mullany 2006; Soofi 2012). One study carried out in community‐based settings reported microbiologic data and showed that topical application of chlorhexidine reduces colonization of common pathologic bacteria, which correlates with a reduction in mortality and omphalitis (Arifeen 2012). There was no difference in mortality reduction with single versus multiple application of chlorhexidine to umbilical cord, however, multiple application seems advantageous for the reduction of omphalitis and bacterial colonization compared with single application.Topical application of chlorhexidine may increase cord separation time by about 1.7 days based on the combined results of two trials from community settings (Arifeen 2012; Mullany 2006). There was no beneficial effect of washing the umbilical cord with soap and water compared with dry cord care for reduction of mortality, omphalitis and cord separation time (Mullany 2006). Results of one trial showed that there was no advantage of the promotion of handwashing among caretakers for prevention of omphalitis and mortality in community settings compared with control (Soofi 2012).
Studies conducted in hospital settings
Studies conducted in hospital setting were small and had limitations. No study reported data for mortality or tetanus. Triple dye and alcohol were the most commonly studied antiseptics in hospital settings. When compared with dry cord care, no antiseptic was convincingly advantageous to reduce the incidence of omphalitis. Topical triple dye application reduced bacterial colonization with Staphylococcus aureus compared with dry cord care and that of alcohol application (two separate analyses). There was no advantage of application of alcohol and triple dye for reduction of colonization with streptococcus. Topical alcohol application was advantageous for the reduction of colonization with Enterococcus coli compared with dry cord care and triple dye application (two separate analyses). Topical application of alcohol and triple dye increased cord separation time compared with dry cord care in hospital settings. When triple dye was compared with alcohol, no significant difference was noticed in the incidence of omphalitis and cord separation time. Single application of triple dye was associated with a decrease in cord separation time compared with multiple application. Washing the cord was not advantageous compared with dry cord care. The number of studies was insufficient to make an inference about the efficacy of other antiseptics.
Overall completeness and applicability of evidence
Studies conducted in community settings
Three large, well‐conducted, cluster‐randomized, community trials reported data on the effectiveness of topical application of chlorhexidine for prevention of mortality and omphalitis. The aggregated sample size of these trials involved 78% of the 69,338 babies included in this review. Pooled results for all‐cause mortality showed a significant reduction of 23% in the intervention group compared with control. The statistical heterogeneity for this comparison was significant (I2 = 50%). The likely reasons for this statistical heterogeneity could be the difference in mortality rates in control populations of included studies and the diversity in topical application of materials other than antiseptics such as ash, surma (a lead‐based preparation Soofi 2012), mud, mustard oil and even cow dung. The mortality in the control group was 36.1/1000 in Soofi 2012, 28.3/1000 in Arifeen 2012 and 19.3/1000 in Mullany 2006. The reduction in the incidence of omphalitis was also significant and was more prominent for severe cases. Data on reduction of bacterial colonization reported by one trial (Arifeen 2012) correlated with the reduction in mortality and omphalitis. Cord separation time may be slightly increased with application of chlorhexidine, however, there was no increased risk of mortality or omphalitis as discussed by authors in the study by Mullany 2006.These findings suggest that there is significant evidence to recommend topical application of chlorhexidine in community settings for prevention of mortality and omphalitis.
Studies conducted in hospital settings
Most of the studies conducted in hospital settings were small and had limitations. The sample size ranged between 71 and 2241. There were no studies conducted in hospital settings that reported mortality or tetanus. Compared with dry cord care, no antiseptic was advantageous to prevent omphalitis. The most commonly studied antiseptics were alcohol and triple dye. Both of them reduced bacterial colonization but there are no data to show that this decrease in bacterial colonization converts into prevention of clinical outcomes of mortality, sepsis, tetanus or omphalitis. Cord separation time may increase with application of alcohol and triple dye. In summary, there is no convincing evidence to recommend an antiseptic in hospital settings compared with dry cord care. Most of the hospital‐based studies were conducted in developed countries. The lack of protective effect of antiseptics may be correlated with better hygiene at the time of birth and later care of babies in newborn nurseries. It is also likely that most of the hospital‐based deliveries were planned and mothers were getting regular prenatal care. The chances of getting therapeutic care for perinatal infections are also greater with hospital‐based deliveries. It may thus be correlated that the overall risk of infection is low in hospital‐based deliveries in developed countries compared with community‐based deliveries in developing countries and thus the apparent protective effect of chlorhexidine in community studies.
Quality of the evidence
Figure 1 summarizes the risk of bias in the studies. Most of the studies included in this review had a moderate risk of bias. The overall risk of bias is summarized in Figure 2.
2.
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Studies conducted in community settings were large and were at low risk of bias. Pooled data showed a moderate amount of statistical heterogeneity that could be expected because of the baseline difference in study settings, topical application of other substances and baseline mortality.
Studies conducted in hospital settings were small and had outcomes that did not have clinical significance. No study reported data on mortality or tetanus.
Potential biases in the review process
This review used clearly specified inclusion and exclusion criteria and a comprehensive search strategy for the identification of relevant studies. The post‐hoc decision to include studies on all antiseptics for umbilical cord care is noted in Differences between protocol and review. This decision was made to include all available evidence on umbilical cord care. Studies conducted in hospital and community studies were analysed separately because the risk factors for umbilical cord infection may differ between hospital and community settings. The comprehensive search strategy was devised to minimize publication bias by searching for both published and unpublished studies. While studies with positive results are more likely to be published than studies with negative results, studies large enough to make a difference in this review are very likely to be published. Pre‐specified subgroup analyses for gestational age and geographic location were not performed because there were insufficient studies to make a conclusive comparison.
The three community trials involved 10 individual study groups that tested different frequencies and durations of chlorhexidine application along with other interventions such as washing the cord with soap and water (Mullany 2006) and promotion of handwashing among caretakers (Soofi 2012). There was no differential effect of interventions other than topical chlorhexidine application. In order to examine whether chlorhexidine has any protective effect, all the chlorhexidine groups were combined and compared with non‐chlorhexidine groups. This combination of study groups is not expected to bias the results as the soap/water group in Mullany 2006 had an effect size very similar to control, i.e. dry cord care. Soofi 2012 was a factorial design trial and we included the factorial analyses in which handwashing groups were balanced between the two study groups (chlorhexidine + handwashing plus chlorhexidine only versus handwashing only plus dry cord care). Arifeen 2012 had two chlorhexidine groups, i.e. seven‐day and one‐day application. These were combined to include chlorhexidine groups in one arm and compared it with dry cord care.
Random‐effects models were used for all meta‐analyses. There are no comprehensive rules on when to use random‐effects or fixed‐effect models for meta‐analysis (Higgins 2011). The difference between two models is that a fixed‐effect model assumes that observed differences between results of trials is due to sampling variation of individual studies only whereas a random‐effects model assumes that outcomes of trials might differ both because of sampling variation of individual studies and true diversity in effects. Both models can be appropriately applied for pooling data but a random‐effects model is usually preferred with heterogeneity. We used random‐effects models because there was substantial heterogeneity across studies in study design, settings, and package of interventions and/or intensity of delivery of those interventions.
Agreements and disagreements with other studies or reviews
The only comprehensive review on umbilical cord care was by Zupan et al that was first published in 1998. The most updated version (Zupan 2004) came to conclusion that there are not enough data to recommend in favour of or against an antiseptic for umbilical cord care. Most of the included studies were small and conducted in hospital settings. Results of our review are in agreement with those of Zupan 2004 for studies conducted in hospital settings with the addition of the effectiveness of chlorhexidine to reduce mortality and morbidity in community settings. We have not only included studies from community settings but have also included more studies from hospital settings. The updated analyses did not change the results for hospital‐based studies.
Authors' conclusions
Implications for practice.
There is significant evidence to suggest that topical application of chlorhexidine to umbilical cord reduces neonatal mortality and omphalitis in community and primary care settings in developing countries. It may increase cord separation time however there is no evidence that it increases subsequent risk of mortality or infection.
There is not sufficient evidence to support the application of an antiseptic to umbilical cord in hospital settings compared with dry cord care in developed countries.
Implications for research.
More studies are needed to explore the effects of antiseptics in hospital settings with clinically important outcomes of mortality, sepsis, tetanus and omphalitis. Studies should be undertaken in a range of settings and should account for differences in outcomes among different gestational ages.
Three community trials were conducted in South Asia. Further trials should be conducted in other parts of the world to replicate the results. Further evaluation of the effectiveness of chlorhexidine should be performed in the context of interventions packaged to facilitate their delivery by health systems. Such packages may include a wide range of interventions to reduce perinatal and neonatal mortality, such as clean delivery practices, breastfeeding, mother/infant skin‐to‐skin care, and delayed bathing.
Feedback
Aydin, 27 July 2013
Summary
Chlorhexidine gluconate is a widely used topical antiseptic that is recommended by the Center for Disease Control and Prevention for skin cleansing before central venous catheter insertion in adults and children. But, because of limited safety data, infection prevention guidelines do not recommend its use in babies who are less than two months old. There are many reports on the potential toxicity of chlorhexidine gluconate, for example genotoxic and cytotoxic effects (1). Chlorhexidine gluconate contact may cause corneal damage, ototoxicity, and neurotoxicity (2). In one study chlorhexidine gluconate was detected in the blood of preterm infants receiving chlorhexidine gluconate skin antisepsis for peripherally central catheter insertion (3). Further investigation is required to determine the safety of this use for newborn infants, especially preterm infants.
References
1. Arabaci T, et al. Assessment of cytogenetic and cytotoxic effects of chlorhexidine digluconate on cultured human lymphocytes. Acta Odontol Scand 2013 71: 1255‐60.
2. Zinn J, Jenkins JB,et al. Intraoperative patient skin prep agents: is there a difference. AORN J 2012 92: 662‐74.
3. Chapman AK, et al. Absorption and tolerability of aqueous chlorhexidine gluconate used for skin antisepsis prior to catheter insertion in preterm neonates. J Perinatol 2013 33):768‐71. doi: 10.1038/jp.2013.61. Epub 2013 May 23.
Comment submitted by Mustafa Aydin, July 2013
Reply
Cleansing of the umbilical cord with chlorhexidine is considered safe [1]. Since its synthesis in the 1950s, it has been used extensively in dental, obstetric and surgical scrubs. In obstetrics, it is used for peri‐partum, perineal and vaginal washes in concentrations as high as 4% [2]. Chlorhexidine is also included in WHO’s Essential Drugs List for children [3].
Chlorhexidine has a benign adverse reaction profile. Topical application can lead to occasional contact dermatitis, photosensitivity and in case of umbilical cord cleansing, prolonged cord separation time [1, 4, 5]. Rare and serious side effects include systemic anaphylactic reaction, neurotoxicity, ototoxicity and corneal damage[1]. These later side effects are more hypothetical than based on clinical data from human studies [6‐8], however. The safety of topical chlorhexidine application for term and preterm infants has been studied in both hospital and community settings, and no significant adverse event has been reported except occasional local dermatitis and relatively prolonged cord separation time [1, 4, 5, 9‐12]. Neither of these side effects increase any significant risk of morbidity or mortality in neonates [4, 5, 9, 10, 12].
Chlorhexidine application has also been studied in preterm babies admitted to neonatal intensive care units, where chlorhexidine was compared with Povidine for prevention of central line infection in sick preterm neonates[6]. Side effects of chlorhexidine application included contact dermatitis, that was reported in 5% of preterm (< 28 weeks' gestation) extremely low‐birth‐weight (< 1000 g) infants after long‐term (> 7 days) placement of chlorhexidine‐impregnated dressings for central venous catheters. This effect might have been caused by the occlusive placement of the dressing rather than the chlorhexidine itself. In the same study, no infants receiving a pre‐placement scrub with 0.5% chlorhexidine developed dermatitis. Based on these studies, we believe that application of chlorhexidine to the umbilical cord is safe, and the benefits for mortality reduction clearly outweigh the risks associated with its topical application.
Topical application is safe and does not lead to significant systemic absorption. In one study no chlorhexidine was found in blood samples from 41 full term infants who were bathed 1−3 times with a 0.4% chlorhexidine solution [9]. Another study followed 50 full‐term infants bathed daily for 3 days with 4% solution and no chlorhexidine was noticed in sample taken from heel pricks[10]. Babies included in this study were followed for 1 year, and no long‐term adverse effects of the bathing were recorded. A hospital based study in South Africa showed that chlorhexidine was detected in sera of 30% (3/10) and 10% (1/10) of infants receiving a single bath with 1% or 2% chlorhexidine, respectively [11].
A study of the safety of chlorhexidine application to the umbilical cord stump[12] included 133 neonates and found out that skin cleansing with chlorhexidine had no adverse effects on skin condition, and resulted in minimal reduction (mean 0.5 degrees C) in body temperature compared with placebo [12]. Another study conducted in Nepal studied 32 neonates pre and post chlorhexidine body washes and found out that body temperature of newborns decreased an average of 0.4 C during the procedure, however there was no evidence of skin aggravation, injury or removal of vernix, and mothers expressed satisfaction with the procedure[13].
Chlorhexidine application has also been studied in preterm babies admitted to neonatal intensive care unit, where chlorhexidine was compared with povidine for prevention of central line infection [4]. This study also compared the adverse reactions of the two antispetics. No significant side effects were reported for chlorhexidine except contact dermatitis in extremely low birth weight infants (< 1000 g) after long‐term (> 7 days) placement of chlorhexidine‐impregnated dressings for central venous catheters. This effect might have been caused by the occlusive placement of the dressing rather than the chlorhexidine itself. In the same study, no infants receiving a pre‐placement scrub with 0.5% chlorhexidine developed dermatitis.
Based on the studies discussed above, we believe that application of chlorhexidine to the umbilical cord is safe, and the benefits for mortality reduction clearly outweigh the risks associated with its topical application.
References:
Mullany LC, Darmstadt GL, Tielsch JM: Safety and impact of chlorhexidine antisepsis interventions for improving neonatal health in developing countries. Pediatr Infect Dis J 2006, 25(8):665‐675.
McClure EM, Goldenberg RL, Brandes N, Darmstadt GL, Wright LL, Armbruster D, Biggar R, Carpenter J, Free MJ, Mattison Det al: The use of chlorhexidine to reduce maternal and neonatal mortality and morbidity in low‐resource settings. Int J Gynaecol Obstet 2007, 97(2):89‐94.
WHO Model Lists of Essential Medicines for children, 4th Edition (April 2013) ‐‐ Rev. Oct.2013 [http://apps.who.int/iris/bitstream/10665/93143/1/EMLc_4_eng.pdf]
Garland JS, Alex CP, Mueller CD, Otten D, Shivpuri C, Harris MC, Naples M, Pellegrini J, Buck RK, McAuliffe TLet al: A randomized trial comparing povidone‐iodine to a chlorhexidine gluconate‐impregnated dressing for prevention of central venous catheter infections in neonates. Pediatrics 2001, 107(6):1431‐1436.
Mullany LC, Darmstadt GL, Khatry SK, LeClerq SC, Katz J, Tielsch JM: Impact of umbilical cord cleansing with 4.0% chlorhexidine on time to cord separation among newborns in southern Nepal: a cluster‐randomized, community‐based trial. Pediatrics 2006, 118(5):1864‐1871.
Gillespie WA, Corner BD, Burman D, Alder VG: Absorption of hexachlorophane from dusting powder on newborn infant's skin. J Hyg (Lond) 1974, 73(2):311‐315.
Gongwer LE, Hubben K, Lenkiewicz RS, Hart ER, Cockrell BY: The effects of daily bathing of neonatal rhesus monkeys with an antimicrobial skin cleanser containing chlorhexidine gluconate. Toxicol Appl Pharmacol 1980, 52(2):255‐261.
Olson L, Bjorklund H, Henschen A, Palmer M, Hoffer B: Some toxic effects of lead, other metals and antibacterial agents on the nervous system‐‐animal experiment models. Acta Neurol Scand Suppl 1984, 100:77‐87.
O'Neill J, Hosmer M, Challop R, et al. : Percutaneous absorption potential of chlorhexidine in neonates. Curr Ther Res 1982, 31:485‐489.
O'Brien CA, Blumer JL, Speck WT, et al: Effect of bathing with a 4 per cent chlorhexidine gluconate solution on neonatal bacterial colonization. J Hosp Infect 1984, 5(Suppl 1):141.
Wilson CM, Gray G, Read JS, Mwatha A, Lala S, Johnson S, Violari A, Sibiya PM, Fleming TR, Koonce Aet al: Tolerance and safety of different concentrations of chlorhexidine for peripartum vaginal and infant washes: HIVNET 025. J Acquir Immune Defic Syndr 2004, 35(2):138‐143.
Darmstadt GL, Hossain MM, Choi Y, Shirin M, Mullany LC, Islam M, Saha SK: Safety and effect of chlorhexidine skin cleansing on skin flora of neonates in Bangladesh. Pediatr Infect Dis J 2007, 26(6):492‐495.
Mullany LC, Darmstadt GL, Khatry SK, LeClerq SC, Tielsch JM: Safety of neonatal skin cleansing in rural Nepal. Indian Pediatr 2006, 43(2):117‐124.
Contributors
Response from Aamer Imdad and Zulfiqar A Bhutta, December 2013
What's new
| Date | Event | Description |
|---|---|---|
| 10 April 2014 | Feedback has been incorporated | Response to Feedback 1 from Aamer Imdad and Zulfiqar A Bhutta. |
History
Protocol first published: Issue 8, 2010 Review first published: Issue 5, 2013
| Date | Event | Description |
|---|---|---|
| 27 July 2013 | Feedback has been incorporated | Feedback 1 received from Mustafa Aydin. |
Acknowledgements
We would like to acknowledge Professor Lourdes Amarillo and Professor Cynthia Cordero for their statistical contributions.
We also thank Sara Roden‐Scott for her translation of Davila 2007.
We would like to give special thanks to Sonja Henderson from Pregnancy and Childbirth Cochrane Review Group for her continuous support and co‐ordination during the conduct of this review.
The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Pregnancy and Childbirth Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health.
Appendices
Appendix 1. LILACS search strategy
((Pt randomized controlled trial OR Pt controlled clinical trial OR Mh r andomized controlled trials OR Mh random allocation OR Mh double‐blind method OR Mh single‐blind method) AND NOT (Ct animal AND NOT (Ct human and Ct animal)) OR (Pt clinical trial OR Ex E05.318.760.535$ OR (Tw clin$ AND (Tw trial$ OR Tw ensa$ OR Tw estud$ OR Tw experim$ OR Tw investiga$)) OR ((Tw singl$ OR Tw simple$ OR Tw doubl$ OR Tw doble$ OR Tw duplo$ OR Tw trebl$ OR Tw trip$) AND (Tw blind$ OR Tw cego$ OR Tw ciego$ OR Tw mask$ OR Tw mascar$)) OR Mh placebos OR Tw placebo$ OR (Tw random$ OR Tw randon$ OR Tw casual$ OR Tw acaso$ OR Tw azar OR Tw aleator$) OR Mh research design) AND NOT (Ct animal AND NOT (Ct human and Ct animal)) OR (Ct comparative study OR Ex E05.337$ OR Mh follow‐up studies OR Mh prospective studies OR Tw control$ OR Tw prospectiv$ OR Tw volunt$ OR Tw volunteer$) AND NOT (Ct animal AND NOT (Ct human and Ct animal))) AND (umbilical OR umbilicus) AND (cord OR cordón OR care OR hygiene OR higiene OR separate OR separation OR chlorhexidine OR clorhexidina OR iodin$ OR alcohol OR clean OR cleanse OR cleansing OR Mh Anti‐Infective Agents OR regime OR regimen OR topical OR topically OR “triple dye” OR antiseptic$ OR anti‐septic$ or antiséptic$ OR antimicrob$ OR omphalitis)
Data and analyses
Comparison 1. Antispetics vs dry cord care/placebo. Studies conducted in community settings.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 3 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 1.1 Chlorhexidine versus dry cord care/placebo | 3 | Risk Ratio (Random, 95% CI) | 0.77 [0.63, 0.94] | |
| 2 Omphalitis: Algorithm 1: Redness extending to skin | 3 | Risk Ratio (Random, 95% CI) | 0.73 [0.64, 0.83] | |
| 3 Omphalitis: Algorithm 2: Redness with pus or severe redness | 3 | Risk Ratio (Random, 95% CI) | 0.69 [0.60, 0.79] | |
| 4 Omphalitis: Algorithm 3: severe redness with pus | 3 | Risk Ratio (Random, 95% CI) | 0.44 [0.28, 0.69] | |
| 5 Bacterial colonization: Staphyococcus aureus | 1 | 5234 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.23, 0.31] |
| 6 Bacterial colonization: E.coli | 1 | 5234 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.46, 0.54] |
| 7 Bacterial colonization: Streptococcus | 1 | 5234 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.22, 0.37] |
| 8 Cord separation time (days) | 2 | 37233 | Mean Difference (IV, Random, 95% CI) | 1.75 [0.44, 3.05] |
| 8.1 Chlorhexidine | 2 | 37233 | Mean Difference (IV, Random, 95% CI) | 1.75 [0.44, 3.05] |
Comparison 2. Antiseptics vs dry cord care/placebo. Studies conducted in hospital settings.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Omphalitis | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Alcohol | 3 | 484 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.62, 1.39] |
| 1.2 Triple dye | 2 | 968 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.13, 3.73] |
| 1.3 Chlorhexidine | 1 | 669 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.06, 1.35] |
| 1.4 Salicylic sugar powder | 1 | 344 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.01, 4.38] |
| 1.5 Green clay powder | 1 | 361 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.04, 5.26] |
| 2 Bacterial colonization: Staphylococcus aureus | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Alcohol | 2 | 432 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.11, 3.36] |
| 2.2 Triple dye | 4 | 1319 | Risk Ratio (M‐H, Random, 95% CI) | 0.15 [0.10, 0.22] |
| 2.3 Silver sulphadiazine | 2 | 360 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.60, 0.87] |
| 2.4 Chlorhexidine | 1 | 216 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.55, 0.77] |
| 2.5 Benzine | 1 | 221 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.90, 1.09] |
| 2.6 Salicyclic sugar powder | 1 | 344 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.17, 0.58] |
| 2.7 Green clay powder | 1 | 361 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.31, 0.82] |
| 2.8 Katoxin powder | 1 | 385 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [1.02, 2.00] |
| 2.9 Fuschine | 1 | 364 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.32, 0.84] |
| 3 Bacterial colonization: Streptococcus | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Alcohol | 2 | 432 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.15, 1.19] |
| 3.2 Triple dye | 3 | 947 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.28, 1.18] |
| 3.3 Silver sulphadiazine | 2 | 360 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.43, 0.89] |
| 3.4 Chlorhexidine | 1 | 216 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.27, 1.04] |
| 3.5 Salicyclic sugar powder | 1 | 344 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.29, 1.90] |
| 3.6 Green clay powder | 1 | 361 | Risk Ratio (M‐H, Random, 95% CI) | 4.62 [2.41, 8.84] |
| 3.7 Katoxin powder | 1 | 385 | Risk Ratio (M‐H, Random, 95% CI) | 5.87 [3.12, 11.05] |
| 3.8 Fuschine | 1 | 364 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.04, 0.85] |
| 4 Bacterial colonization: E. coli | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Alcohol | 2 | 432 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.58, 0.92] |
| 4.2 Triple dye | 2 | 789 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.53, 1.17] |
| 4.3 Silver sulphadiazine | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 0.7 [0.53, 0.93] |
| 4.4 Chlorhexidine | 1 | 216 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.39, 0.90] |
| 4.5 Salicyclic sugar powder | 1 | 344 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.32, 1.10] |
| 4.6 Green clay powder | 1 | 361 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.79, 2.05] |
| 4.7 Katoxin powder | 1 | 385 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.70, 1.81] |
| 4.8 Fuschine | 1 | 364 | Risk Ratio (M‐H, Random, 95% CI) | 2.04 [1.33, 3.13] |
| 5 Parental satisfaction | 1 | 355 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.45, 0.66] |
| 6 Time to cord separation (days) | 11 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Alcohol | 9 | 2921 | Mean Difference (IV, Random, 95% CI) | 1.76 [0.03, 3.48] |
| 6.2 Zinc powder | 1 | 401 | Mean Difference (IV, Random, 95% CI) | ‐1.82 [‐2.23, ‐1.41] |
| 6.3 Triple dye | 1 | 372 | Mean Difference (IV, Random, 95% CI) | 4.1 [3.07, 5.13] |
| 6.4 Salicylic sugar powder | 1 | 344 | Mean Difference (IV, Random, 95% CI) | ‐1.90 [‐2.47, ‐1.33] |
| 6.5 Green clay powder | 1 | 361 | Mean Difference (IV, Random, 95% CI) | ‐0.80 [‐1.36, ‐0.24] |
| 6.6 Katoxin powder | 1 | 385 | Mean Difference (IV, Random, 95% CI) | 0.80 [0.18, 1.42] |
| 6.7 Fuschine | 1 | 364 | Mean Difference (IV, Random, 95% CI) | 2.80 [2.01, 3.59] |
| 6.8 Silver sulphadiazine | 1 | 155 | Mean Difference (IV, Random, 95% CI) | 3.60 [2.66, 4.54] |
| 6.9 Breastmilk | 1 | 157 | Mean Difference (IV, Random, 95% CI) | ‐1.69 [‐2.31, ‐1.07] |
| 6.10 Chlorhexidine | 1 | 669 | Mean Difference (IV, Random, 95% CI) | ‐0.80 [‐1.21, ‐0.39] |
2.5. Analysis.
Comparison 2 Antiseptics vs dry cord care/placebo. Studies conducted in hospital settings, Outcome 5 Parental satisfaction.
Comparison 3. Antiseptic vs antibiotic. Studies conducted in hospital settings.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Bacterial colonization: Staphylococcus aureus | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Triple dye vs bacitracin | 1 | 62 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.03, 12.87] |
| 1.2 Silve sulphadiazine vs bacitracin | 1 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 2.18 [0.42, 11.33] |
| 1.3 Povidine vs bacitracin | 1 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 2.18 [0.42, 11.33] |
| 2 Time to cord separation (days) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Triple dye vs bacitracin | 1 | 62 | Mean Difference (IV, Random, 95% CI) | ‐5.60 [‐9.36, ‐1.84] |
| 2.2 Triple dye vs neomycin | 1 | 62 | Mean Difference (IV, Random, 95% CI) | ‐4.3 [‐6.27, ‐2.33] |
| 2.3 Silve sulphadiazene vs neomycin | 1 | 51 | Mean Difference (IV, Random, 95% CI) | ‐1.40 [‐3.65, 0.85] |
| 2.4 Povidine vs bacitracin | 1 | 92 | Mean Difference (IV, Random, 95% CI) | ‐2.0 [‐3.67, ‐0.33] |
| 2.5 Alcohol vs beniktol | 1 | 76 | Mean Difference (IV, Random, 95% CI) | ‐2.33 [‐3.77, ‐0.89] |
Comparison 4. Antiseptic vs antiseptic. Studies conducted in hospital settings.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sepsis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Chlorhexidine vs salicylic powder | 1 | 213 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.07, 17.50] |
| 2 Omphalitis | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Triple dye vs alcohol | 3 | 1560 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.14, 1.63] |
| 2.2 Triple dye vs povidone‐iodine | 1 | 183 | Risk Ratio (M‐H, Random, 95% CI) | 0.15 [0.07, 0.32] |
| 2.3 Triple dye vs salicylic sugar powder | 1 | 362 | Risk Ratio (M‐H, Random, 95% CI) | 4.29 [0.21, 88.65] |
| 2.4 Triple dye vs katoxin | 1 | 403 | Risk Ratio (M‐H, Random, 95% CI) | 2.13 [0.19, 23.34] |
| 2.5 Triple dye vs fuchsine | 1 | 382 | Risk Ratio (M‐H, Random, 95% CI) | 1.92 [0.18, 20.97] |
| 2.6 Alcohol vs povidone‐iodine | 1 | 182 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.62, 1.16] |
| 2.7 Alcohol vs salicylic powder | 1 | 345 | Risk Ratio (M‐H, Random, 95% CI) | 4.69 [0.23, 97.03] |
| 2.8 Alcohol vs green clay powder | 1 | 362 | Risk Ratio (M‐H, Random, 95% CI) | 2.07 [0.19, 22.60] |
| 2.9 Alcohol vs katoxin | 1 | 365 | Risk Ratio (M‐H, Random, 95% CI) | 2.10 [0.19, 22.97] |
| 2.10 Alcohol vs fuchsine | 1 | 365 | Risk Ratio (M‐H, Random, 95% CI) | 2.10 [0.19, 22.97] |
| 2.11 Alcohol vs chlorhexidine | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 2.77 [0.12, 66.49] |
| 2.12 Chlorhexidine vs hydrophobic gauze | 1 | 2441 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [0.55, 3.36] |
| 3 Bacterial colonization: Staphylococcus aureus | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Triple dye vs alcohol | 2 | 487 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.25, 0.80] |
| 3.2 Triple dye vs silver sulphadiazene | 3 | 384 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.25, 0.50] |
| 3.3 Triple dye vs salicylic sugar powder | 1 | 362 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.16, 1.12] |
| 3.4 Triple dye vs povidone‐iodine | 1 | 62 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.03, 12.87] |
| 3.5 Triple dye vs green clay powder | 1 | 379 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.11, 0.65] |
| 3.6 Triple dye vs katoxin powder | 1 | 382 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.11, 0.63] |
| 3.7 Triple dye vs fuchsine | 1 | 382 | Risk Ratio (M‐H, Random, 95% CI) | 1.92 [0.18, 20.97] |
| 3.8 Alcohol vs green clay powder | 1 | 362 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.30, 1.16] |
| 3.9 Alcohol vs katoxin | 1 | 386 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.12, 0.37] |
| 3.10 Alcohol vs fuchsine | 1 | 365 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.29, 1.12] |
| 3.11 Alcohol vs salicylic powder | 1 | 345 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.43, 2.03] |
| 3.12 Alcohol vs povidine‐iodine | 1 | 182 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.62, 1.16] |
| 3.13 Chlorhexidine vs hydrophobic gauze | 1 | 2441 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.70, 1.15] |
| 3.14 Chlorhexidine vs alcohol | 2 | 165 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.24, 0.71] |
| 3.15 Chlorhexidine vs mercurochrome | 1 | 65 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.01, 2.04] |
| 4 Bacterial colonization: Streptococcus | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Triple dye vs alcohol | 1 | 373 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.04, 4.99] |
| 4.2 Triple dye vs silver sulphadiazene | 2 | 326 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.71, 2.25] |
| 4.3 Triple dye vs salicylic sugar powder | 1 | 362 | Risk Ratio (M‐H, Random, 95% CI) | 0.12 [0.02, 0.98] |
| 4.4 Triple dye vs green clay powder | 1 | 379 | Risk Ratio (M‐H, Random, 95% CI) | 0.02 [0.00, 0.14] |
| 4.5 Triple dye vs katoxin powder | 1 | 403 | Risk Ratio (M‐H, Random, 95% CI) | 0.02 [0.00, 0.11] |
| 4.6 Triple dye vs fuchsine | 1 | 382 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.04, 5.24] |
| 4.7 Alcohol vs green clay powder | 1 | 362 | Risk Ratio (M‐H, Random, 95% CI) | 0.04 [0.01, 0.17] |
| 4.8 Alcohol vs katoxin | 1 | 386 | Risk Ratio (M‐H, Random, 95% CI) | 0.03 [0.01, 0.14] |
| 4.9 Alcohol vs fuchsine | 1 | 365 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.15, 7.38] |
| 4.10 Alcohol vs salicylic powder | 1 | 345 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.43, 2.03] |
| 5 Bacterial colonization: E. coli | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Triple dye vs alcohol | 1 | 373 | Risk Ratio (M‐H, Random, 95% CI) | 3.44 [2.10, 5.64] |
| 5.2 Triple dye vs silver sulphadiazene | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [1.02, 1.81] |
| 5.3 Triple dye vs salicylic sugar powder | 1 | 362 | Risk Ratio (M‐H, Random, 95% CI) | 3.92 [2.28, 6.72] |
| 5.4 Triple dye vs green clay powder | 1 | 379 | Risk Ratio (M‐H, Random, 95% CI) | 1.83 [1.27, 2.65] |
| 5.5 Triple dye vs katoxin powder | 1 | 403 | Risk Ratio (M‐H, Random, 95% CI) | 2.07 [1.43, 3.00] |
| 5.6 Triple dye vs fuchsine | 1 | 382 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.84, 1.54] |
| 5.7 Alcohol vs green clay powder | 1 | 362 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.31, 0.92] |
| 5.8 Alcohol vs salicylic powder | 1 | 345 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.58, 2.24] |
| 5.9 Alcohol vs katoxin | 1 | 386 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.35, 1.04] |
| 5.10 Alcohol vs fuchsine | 1 | 365 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.20, 0.55] |
| 5.11 Chlorhexidine vs hydrophobic gauze | 1 | 2441 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.31, 2.00] |
| 5.12 Chlorhexidine vs mercurochrome | 1 | 65 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.01, 4.13] |
| 6 Time to cord separation (days) | 10 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Triple dye vs alcohol | 2 | 444 | Mean Difference (IV, Random, 95% CI) | 0.43 [‐8.49, 9.35] |
| 6.2 Triple dye vs silver sulphadiazene | 2 | 117 | Mean Difference (IV, Random, 95% CI) | 0.15 [‐6.20, 6.51] |
| 6.3 Triple dye vs salicylic sugar powder | 1 | 362 | Mean Difference (IV, Random, 95% CI) | 6.0 [5.01, 6.99] |
| 6.4 Triple dye vs povidone‐iodine | 1 | 58 | Mean Difference (IV, Random, 95% CI) | 7.60 [3.96, 11.24] |
| 6.5 Triple dye vs green clay powder | 1 | 379 | Mean Difference (IV, Random, 95% CI) | 4.90 [3.92, 5.88] |
| 6.6 Triple dye vs katoxin powder | 1 | 403 | Mean Difference (IV, Random, 95% CI) | 3.30 [2.28, 4.32] |
| 6.7 Triple dye vs fuchsine | 1 | 382 | Mean Difference (IV, Random, 95% CI) | 1.30 [0.17, 2.43] |
| 6.8 Alcohol vs green clay powder | 1 | 362 | Mean Difference (IV, Random, 95% CI) | 10.2 [9.05, 11.35] |
| 6.9 Alcohol vs katoxin | 1 | 386 | Mean Difference (IV, Random, 95% CI) | 8.60 [7.42, 9.78] |
| 6.10 Alcohol vs salicylic powder | 1 | 345 | Mean Difference (IV, Random, 95% CI) | 11.30 [10.14, 12.46] |
| 6.11 Alcohol vs fuchsine | 1 | 365 | Mean Difference (IV, Random, 95% CI) | 6.60 [5.32, 7.88] |
| 6.12 Alcohol vs silver sulphadiazine | 1 | 155 | Mean Difference (IV, Random, 95% CI) | ‐4.04 [‐4.96, ‐3.12] |
| 6.13 Chlorhexidine vs salicylic powder | 1 | 213 | Mean Difference (IV, Random, 95% CI) | 3.0 [2.46, 3.54] |
| 6.14 Chlorhexidine vs hydrophobic gauze | 1 | 2441 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐0.57, ‐0.23] |
| 6.15 Chlorhexidine vs mercurochrome | 1 | 152 | Mean Difference (IV, Random, 95% CI) | 6.40 [5.25, 7.55] |
| 6.16 Povidine‐iodine vs silver sulphadiazene | 1 | 86 | Mean Difference (IV, Random, 95% CI) | ‐4.0 [‐5.53, ‐2.47] |
| 6.17 Triple dye plus alcohol vs triple dye alone | 1 | 90 | Mean Difference (IV, Random, 95% CI) | 1.00 [‐0.45, 2.45] |
Comparison 5. Single vs multiple application. Studies conducted in community settings.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 1 | (Random, 95% CI) | 0.85 [0.70, 1.03] | |
| 1.1 Chlorhexidine | 1 | (Random, 95% CI) | 0.85 [0.70, 1.03] | |
| 2 Omphalitis: Algorithem 1 | 1 | (Fixed, 95% CI) | 1.14 [0.97, 1.34] | |
| 2.1 Chlorhexidine | 1 | (Fixed, 95% CI) | 1.14 [0.97, 1.34] | |
| 3 Omphalitis: Algorithem 2 | 1 | (Fixed, 95% CI) | 1.53 [1.22, 1.92] | |
| 3.1 Chlorhexidine | 1 | (Fixed, 95% CI) | 1.53 [1.22, 1.92] | |
| 4 Omphalitis: Algorithem 3 | 1 | (Fixed, 95% CI) | 2.12 [1.10, 4.11] | |
| 4.1 Chlorhexidine | 1 | (Fixed, 95% CI) | 2.12 [1.10, 4.11] | |
| 5 Bacterial colonization: Staphylococcus aureus | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Chlorhexidine | 1 | 3449 | Risk Ratio (M‐H, Random, 95% CI) | 3.63 [2.74, 4.82] |
| 6 Bacterial colonization: Streptococcus | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Chlorhexidine | 1 | 3449 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.67, 1.53] |
| 7 Bacterial colonization: E. coli | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Chlorhexidine | 1 | 3449 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [1.03, 1.32] |
Comparison 6. Single vs multiple application. Studies conducted in hospital settings.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cord separation time (days) | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Triple dye | 3 | 408 | Mean Difference (IV, Random, 95% CI) | ‐4.24 [‐4.41, ‐4.07] |
| 1.2 Zinc powder | 1 | 800 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.31, 0.27] |
Comparison 7. Washing cord vs dry cord care. Studies conducted in community settings.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 1 | Risk Ratio (Fixed, 95% CI) | 1.0 [0.76, 1.32] | |
| 2 Omphalitis: Algorithem 1 | 1 | (Fixed, 95% CI) | 1.03 [0.87, 1.22] | |
| 3 Omphalitis: Algorithem 2 | 1 | (Fixed, 95% CI) | 0.88 [0.69, 1.12] | |
| 4 Omphalitis: Algorithem 3 | 1 | (Fixed, 95% CI) | 1.01 [0.58, 1.76] | |
| 5 Cord separation time (days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
Comparison 8. Washing cord vs dry cord care. Studies conducted in hospital settings.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Bacterial colonization: Staphylococcus aureus | 1 | 181 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.65, 1.34] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ahmadpour‐Kacho 2006.
| Methods | Randomized, non‐blinded, conducted in hospital settings. | |
| Participants | Infants 36‐42 weeks, appropriate for gestational age, no disease/congenital anomalies, mother without significant complication (N = 312). | |
| Interventions | (1) Dry care (n = 78). (2) Breastmilk (n = 79). (3) 95% ethyl alcohol (n = 78). (4) Silver sulphadiazine (n = 77). |
|
| Outcomes | Time to cord separation. | |
| Notes | Preterm and term. Non‐low birthweight. Hospital setting. Eastern Mediterranean (Iran). All fed with own mother's milk. All roomed in. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not mentioned. |
| Allocation concealment (selection bias) | Unclear risk | Method not mentioned. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not feasible. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No ITT analysis. Outcome of 61 (19.6%) neonates (16 in dry care, 15 in breastmilk, 15 in alcohol, 15 in silver sulphadiazine) were excluded because of concurrent use of 2 topical agents for cord care, the need for admission in the neonatal ward for treatment with antibiotics and for not reporting time/date of cord separation. |
| Selective reporting (reporting bias) | Low risk | Outcomes were reported as mentioned in the method's section. |
| Other bias | High risk | Baseline characteristics showed more cesarean deliveries in the dry care, alcohol and silver groups compared with the breastmilk group. |
Al‐Binali 2006.
| Methods | Randomized study conducted in Saudi Arbia in hospital setting. | |
| Participants | Healthy newborn admitted to nursery. Total sample size was 76 in which there were 38 babies in the alcohol group and 38 in Beniktol spray group. | |
| Interventions | 1) 70% alcohol. 2) Beniktol spray (Nebacetin with Neomycin sulphate and Bacitracin). |
|
| Outcomes | Cord separation time, omphalitis. | |
| Notes | No particular definition was used for omphalitis. Gestational ages of the newborns were not mentioned in the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Comment, "Sequence generation was based on month of delivery". |
| Allocation concealment (selection bias) | Unclear risk | Method not mentioned. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Method not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition and exclusion were not described. |
| Selective reporting (reporting bias) | Unclear risk | Insufficent information to make an assessment. |
| Other bias | High risk | Baseline characteristics were not compared at the baseline. |
Arad 1981.
| Methods | Randomized but method not mentioned, conducted in hospital settings at Jerusalem. | |
| Participants | Healthy, appropriate‐for‐gestational age, term infants admitted to nursery. Total sample size 121. | |
| Interventions | Initial bath. Daily application during hospital stay. 1. Triple dye (n = 36). 2. Neomycin ointment (n = 26). 3. Sulphadiazine ointment (n = 25). 4. Bismuth powder (n = 34). Daily alcohol applied at home. | |
| Outcomes | Cord separation time, sepsis. | |
| Notes | No control group with dry cord care. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "..... were each randomly assigned to one of four treatment regimens." Comment: Probably not done. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No account of attrition or exclusion was given in the text or tables. |
| Selective reporting (reporting bias) | Unclear risk | Insufficent information to permit a judgement. |
| Other bias | High risk | Bacteriological data were not obtained. |
Arifeen 2012.
| Methods | Cluster‐randomized trial conducted in community settings in Bangladesh. | |
| Participants | Neonates whose parents were enrolled in study. Babies whose parents were not enrolled in parent trial and who were first visited after 48 hours of life, were excluded. Total sample size 29, 760. |
|
| Interventions | 1. Dry care (n = 10,008). 2. 4.0% chlorhexidine single application (n = 9423). 3.4.0% chlorhexidine multiple application (n = 10329). |
|
| Outcomes | Omphalitis and all‐cause mortality. | |
| Notes | Single and multiple application CHX groups were combined for the analysis mortality and omphalitis analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A random allocation sequence for each stratum was done using a computer‐generated procedure". |
| Allocation concealment (selection bias) | Low risk | Comment: As this was a cluster‐randomized trial allocation concealment is not an issue. |
| Blinding (performance bias and detection bias) All outcomes | High risk | This study was not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition and exclusion was adequately addressed. |
| Selective reporting (reporting bias) | Low risk | Study protocol was available to make an assessment and authors reported the outcomes mentioned in the protocol. |
| Other bias | High risk | There was no effect of multiple cleansing with CHX on neonatal mortality. This may be due to the fact that the study was not powered enough to detect a significant difference in this arm, as pointed out by authors. |
Bain 1994.
| Methods | Randomized but method not mentioned, non‐blinded, non‐ITT, conducted in hospital settings. | |
| Participants | Newborn with weight > 1 kg, gestational age < 37 weeks, no umbilical lines, no abdominal surgery (N = 102). | |
| Interventions | (1) Dry care (no treatment; "cleaning only with water when necessary") (n = 28). (2) 0.33% Hexachlorophene + 3% zinc oxide powder (Sterzac) (n = 24). (3) 70% isopropyl alcohol (Steret) (n = 24). (4) 70% isopropyl alcohol (Steret) + 0.33% hexachlorophene + 3% zinc oxide powder (Sterzac) (n = 26). |
|
| Outcomes | Time to cord separation. | |
| Notes | Preterm only. Low birthweight. Hospital setting. Europe (Scotland). None roomed in. Group 2 and 4 was excluded from the analysis as it included hexachlorophene, which is neurotoxic. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomization method." |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not feasible. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No ITT analysis. 18 babies (17.6%) were subsequently excluded due to the insertion of umbilical lines or transferred back to their mothers before completion of trial. |
| Selective reporting (reporting bias) | Low risk | This study seems to report the outcomes mentioned in the methods section. |
| Other bias | High risk | Higher rate of rupture of membranes > 24 hours in dry care. |
Barrett 1979.
| Methods | Randomized study conducted in USA. | |
| Participants | Infants were assigned to the normal newborn nursery, received routine general care. Total sample size was 300. Infants who received antibiotics or developed complications precluding their assignment to the normal newborn nursery, or who were subsequently transferred from this nursery, were excluded. |
|
| Interventions | 1. Silver sulphadiazine, single application (n = 100). 2. Triple dye, single application (n = 100). 3. Dry cord care (n = 100). | |
| Outcomes | Colonization of periumbilical area and anterior nares at 48 hours. | |
| Notes | Cultures of the periumbilical area and the anterior nares were obtained at 48 hours of age. Gestational ages of newborns were not described in the study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Cord‐care regimens were assigned by a computer‐generated table of random numbers." |
| Allocation concealment (selection bias) | Unclear risk | Insufficent information to permit a judgement. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Laboratory personnel were not aware of the cord‐care regimen the subjects had received." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A complete account of attrition and exclusion was given in the result's section. |
| Selective reporting (reporting bias) | Low risk | The study reported the outcomes mentioned in the methods section. |
| Other bias | Low risk | No other form of bias was found. |
Davila 2007.
| Methods | Randomized but method not mentioned (unclear), non‐blinded, non‐ITT, conducted in hospital settings. | |
| Participants | Healthy newborns, birthweight > 2000 g and < 4000 g, gestational age 34‐41 weeks, Apgar > or equal to 7, vaginal or abdominal delivery (N = 162). | |
| Interventions | (1) 70% alcohol (n = 57). (2) 5% povidone iodine (n = 55). (3) 4% chlorhexidine (n = 50). |
|
| Outcomes | Time to cord separation, bacterial colonization. | |
| Notes | Preterm and term. Low birthweight and non‐low birthweight. Latin Americas (Peru). Paper in Spanish. Unable to include data on time to cord separation as no SDs were reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not mentioned. |
| Allocation concealment (selection bias) | Unclear risk | Method not mentioned. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Method not mentioned, blinding not feasible. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No ITT analysis. Some mothers refused for samples to be taken because they were discharged outside of hospital hours. |
| Selective reporting (reporting bias) | Unclear risk | Insufficent information to make an assessment. |
| Other bias | Unclear risk | No table of baseline characteristics. |
Dore 1998.
| Methods | Randomized, non‐blinded, non‐ITT, conducted in hospital settings. | |
| Participants | Admitted at postpartum unit, > or equal to 36 weeks' gestation, not receiving antibiotics, fewer than 8 hours of age at randomizations, spent no time in NICU, mother spoke and read English, mother had telephone, mother planned to keep newborn (N = 1811). | |
| Interventions | (1) Dry care; "natural drying" (n = 909). (2) 70% isopropyl alcohol (n = 902). |
|
| Outcomes | Omphalitis; time to cord separation. | |
| Notes | Term. Non‐low birthweight. Hospital setting. Americas (Canada). Sponge bath 52.9% vs 52.8%, tub bath 44% vs 44%. Intention to breastfeed 87% vs 86.4%. All roomed in ("admitted to postpartum unit"). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers. |
| Allocation concealment (selection bias) | Low risk | Opaque envelopes. |
| Blinding (performance bias and detection bias) All outcomes | High risk | "A coloured dot for the newborn's bassinette indicates group assignment." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No ITT analysis. 65 newborns (3.6%) did not complete the study (27 were admitted to the NICU, 25 families could not be reached for telephone interview, 7 families changed their mind, 6 gave mixed reason for not participating). |
| Selective reporting (reporting bias) | Low risk | Study reported all the outcomes mentioned in the methods section. |
| Other bias | Low risk | Baseline characteristics comparable. |
Evens 2004.
| Methods | Randomized, non‐blinded, non‐ITT, conducted in hospital settings. | |
| Participants | Infants < 34 weeks, no umbilical vessel catheterization, no umbilical cord anomalies (N = 103). | |
| Interventions | (1) Dry care, "natural drying" (n = 50). (2) 70% isopropyl alcohol (n = 53). |
|
| Outcomes | Omphalitis; cord separation time; bacterial colonization. | |
| Notes | Preterm. Low birthweight. Hospital setting. Americas (USA). Initial bath at > 12 hours of life. None roomed in. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficent information to make an assessment. |
| Allocation concealment (selection bias) | Unclear risk | Insufficent information to make an assessment. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not feasible. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No ITT analysis. 7 infants (6.4%) were excluded from the final analyses because of incomplete data due to transfer or need for umbilical vessel catheterization. (It was mentioned from which group were the excluded.) |
| Selective reporting (reporting bias) | Unclear risk | Insufficent information to make an assessment. |
| Other bias | High risk | Higher rate of cesarean delivery in alcohol group (83% vs 69%). |
Gladstone 1988.
| Methods | Randomized study conducted in hospital settings in USA. | |
| Participants | Inborn healthy term infants > 2500 g. A total of 249 participants were included (53, 48, 44, 42, 14, and 48 in each group). | |
| Interventions | 6 study groups 1. Triple dye once daily until separation. 2. Triple dye once then alcohol until separation. 3. Triple dye once only. 4. Povidone iodine daily until separation. 5. Silver sulphadiazine daily until separation. 6. Bacitracin ointment until cord separation. | |
| Outcomes | Colonization at discharge from hospital. Separation time. Maternal satisfaction. Local or other infections. Nursing staff satisfaction. |
|
| Notes | Infants were excluded from the study for any of the following reasons: birthweight less than 2500 g, gestational age less than 37 weeks, refusal to give consent, non‐routine treatments such as phototherapy or systemic antibiotics, or follow‐up care planned at a different medical facility. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "by use of a table of random numbers". |
| Allocation concealment (selection bias) | Unclear risk | Insufficent information to make an assessment. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Insufficent information to make an assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition and excluded participants were adequately described in the result's section. |
| Selective reporting (reporting bias) | Unclear risk | Insufficent information to make an assessment. |
| Other bias | Low risk | Baseline characteristics comparable. |
Golombek 2002.
| Methods | Randomized trial conducted in hospital settings in USA. | |
| Participants | Infants admitted to the well‐baby nursery. A total of 634 infants were enrolled and 599 completed the study. | |
| Interventions | 1. Alcohol (n = 292). 2. Triple dye (n = 342). | |
| Outcomes | Cord infection. Cord separation. Nursing staff satisfaction. | |
| Notes | Data for cord separation time were not included as means and SDs were not given. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Comment: Triple dye was applied to infants admitted to newborn nursery during month of January and March while alcohol was applied during months of December and February. |
| Allocation concealment (selection bias) | High risk | Comment: Methods of randomizations were not adequate and no effort was made to conceal the allocation. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Comment: Intervention was known to house staff, parents and assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition and exclusion was adequately described in the result's section. |
| Selective reporting (reporting bias) | Unclear risk | Insufficent information to make an assessment. |
| Other bias | High risk | Data on incidence of omphalitis were gathered by telephone calls. No home visits were made to make an assessment. |
Hsu 1999.
| Methods | A randomized study conducted in hospital settings in Taiwan. | |
| Participants | Healthy term newborn, 101 in experimental group and 79 in control group. | |
| Interventions | 1. Triple dye, single application.
2. Triple dye, daily application. Single application group was later treated with 70% alcohol until discharge from hospital while the second group was continued to be treated with triple dye. |
|
| Outcomes | Cord separation time. Omphalitis. |
|
| Notes | Daily whole body wash with soap for both the groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization methods were not described. |
| Allocation concealment (selection bias) | Unclear risk | Randomization methods were not described. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information was provided about blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Exclusion and attrition was described. |
| Selective reporting (reporting bias) | Unclear risk | Insufficent information to make an assessment. |
| Other bias | Unclear risk | Single application group was treated with another antiseptic(i.e. 70% alcohol) that may interfere with cord separation time. |
Hsu 2010.
| Methods | Randomized trial. | |
| Participants | Newborn delivered at Tri‐Service Heneral Hospital, Taiwan. Total sample size 142. | |
| Interventions | 1) Dry cord care (n = 71). 2) 95% isopropyl alcohol (n = 71). |
|
| Outcomes | Cord separation time | |
| Notes | Following newborns were excluded: 1) low birthweight < 2500 g; 2) gestational age < 36 weeks; 3) those receiving phototherapy; 4) being treated with systemic antibiotics; 5) those with umbilical catheters; 6) diagnosed with any disease before discharge; 7) who transgressed study protocols; 8) or were lost to follow‐up. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "They were then randomly assigned to one of two groups for cord management". No more details were provided for sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Insufficent information to make an assessment. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Insufficent information to make an assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Reason for exclusion and attrition were given. |
| Selective reporting (reporting bias) | Low risk | Study reported all the outcomes mentioned in the methods section. |
| Other bias | Unclear risk | None noticed. |
Huang 2001.
| Methods | "Randomly assigned" but method not mentioned (unclear), open label, non‐ITT, conducted in hospital settings. | |
| Participants | Healthy term newborns (N = 150). | |
| Interventions | (1) Dry care (n = 75). (2) 95% alcohol (n = 75). |
|
| Outcomes | Omphalitis and umbilical cord separation time. | |
| Notes | Term. Non‐low birthweight. Hospital setting. Western Pacific (Taiwan). Daily bath with ordinary soap. None roomed in. Only abstract available for evaluation. No data were included as the means and SDs were not given. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not mentioned. |
| Allocation concealment (selection bias) | Unclear risk | Method not mentioned. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not mentioned; not feasible. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No ITT. 8 neonates (5.3%) were excluded: dry care (3 protocol breach and 1 lost to follow‐up) and alcohol (4 lost to follow‐up). |
| Selective reporting (reporting bias) | Unclear risk | Insufficent information to make an assessment. |
| Other bias | Low risk | Comparable baseline characteristics. |
Janssen 2003.
| Methods | Randomized, non‐blinded, ITT conducted in hospital settings. | |
| Participants | Hospital born, no antibiotics since birth, Vancouver address, fluent in English, Cantonese or Mandarin, not admitted to level III nursery (N = 766). | |
| Interventions | (1) Dry care (n = 382). (2) Triple dye on day of birth then alcohol until cord separation (n = 384). |
|
| Outcomes | Omphalitis; bacterial colonization. | |
| Notes | No mention of gestational age or birthweight. Hospital setting with community follow‐up. Americas (Canada). Daily bath with mild soap. Breastfeeding at discharge 95% vs 95.6%. Exclusive breastfeeding at home visit 69.9% vs 72.4%. Mixed feeding at home visit 24.9% vs 24.2%. "Omphalitis was defined as erythema (redness, swelling and/or warmth) of the abdominal skin in the periumbilical region, extending beyond 5mm from the umbilicus." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was stratified on the clinical area; consecutively numbered opaque envelopes. |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not mentioned; not feasible. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | Insufficent information to make an assessment. |
| Other bias | Low risk | Baseline characteristics comparable. |
Kapellen 2009.
| Methods | Randomized, non‐blinded, non‐ITT, conducted in hospital settings. | |
| Participants | Healthy newborn within the 1st 36 hours of life, 37‐42 weeks' gestation, birthweight > 2500 g, with consent (N = 669). | |
| Interventions | (1) Dry care (n = 332). (2) Chlorhexidine powder (n = 337). |
|
| Outcomes | Omphalitis, time to cord separation. | |
| Notes | Term. Non‐low birthweight. Hospital setting. Europe (Germany). Admitted to nursery then roomed in. Omphalitis was defined the classification of (Mason 1989). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized by sealed envelope, randomizations was stratified in neonates delivered normally or by caesarean section because of the expected differences in time to cord detachment. |
| Allocation concealment (selection bias) | Low risk | Sealed envelope. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not mentioned; not feasible. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No ITT analysis. 669 neonates were enrolled but cord separation time was only documented in 578 neonates (13.6% missing); no mention of breakdown of dropouts. |
| Selective reporting (reporting bias) | Unclear risk | Insufficent information to make an assessment. |
| Other bias | Low risk | "Neonates randomized to either group did not differ in sex, ethnicity, birthweight or length, gestational age, mode of delivery or Apgar score." (No table of baseline characteristics.) |
Meberg 1985.
| Methods | Hospital‐based randomized study conducted in Norway. | |
| Participants | Healthy term newborn admitted to nursery. A total of 547 infants were included. | |
| Interventions | 1. Benzine daily (n = 113). 2. Chlorhexidine (0.05%) daily (n = 217). 3. Total body wash with soap (n = 217). | |
| Outcomes | Colonization of stump at discharge. Infection (umbilical and severe) within 6 weeks. | |
| Notes | Phase 1: 1982; Phase 2: 1983.
Roomed in with mothers. Data were give for all kind of infection (pemphigus, conjunctivitis, paronychia, and umbilical infection) and no separate data were available for omphalitis (cord infection). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "infants were consecutively and randomly selected to 1 of the following groups". There was no description of exact methods of sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Insufficent information to permit a judgement. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Insufficent information to permit a judgement. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition and exclusion were adequately described in result's section. |
| Selective reporting (reporting bias) | Unclear risk | Insufficent information to make an assessment. |
| Other bias | High risk | No description of baseline characteristics of study participants was given. |
Meberg 1990.
| Methods | Prospective randomized study conducted in hospital setting in Norway. | |
| Participants | Healthy term infants admitted to newborn nursery. A total of 2441 infants were registered. | |
| Interventions | 1. Hydrophobic gauze material bandage, applied daily (n = 1213). 2. Chlorhexidine in alcohol, applied daily (n = 1228). | |
| Outcomes | Infections of skin, cord, eyes during stay and at 6 weeks. Cord separation time. |
|
| Notes | Methods of statistical analyses were not described in adequate details. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Births were consecutively numbered, and infants with even and uneven numbers enlisted into the two groups respectively." |
| Allocation concealment (selection bias) | Unclear risk | Insufficent information to permit a judgement. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Insufficent information to permit a judgement. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition and exclusion were adequately described in results section. |
| Selective reporting (reporting bias) | Unclear risk | Insufficent information to permit a judgement. |
| Other bias | High risk | No description of baseline characteristics of study participants were given. |
Medves 1997.
| Methods | Randomized, non‐blinded, ITT, conducted in hospital settings. | |
| Participants | Term infants (except 1 born 36 weeks and 5 days), born at a tertiary care hospital in Western Canada (N = 148). | |
| Interventions | (1) Alcohol (n = 74). (2) Sterile water (n = 74). |
|
| Outcomes | Bacterial colonization; time to cord separation. | |
| Notes | Preterm (1 participant), term. No mention of birthweight. Hospital setting. Americas (Canada). Initial bath with chlorhexidine gluconate 2% within first 6 hours of life. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocked randomizations. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Only outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis for bacterial colonization. "Infants remained in the treatment group to which they were assigned, even if the parents did not follow the treatment". |
| Selective reporting (reporting bias) | Low risk | Study reported the outcomes mentioned in the method's section. |
| Other bias | Unclear risk | No table of baseline characteristics. |
Mugford 1986.
| Methods | Randomized, blinded, non‐ITT, conducted in hospital settings. | |
| Participants | Babies born in the labour ward, normal postnatal care, singleton, mother resident of West Berkshire District. (N = 815). | |
| Interventions | It was a factorial design trial that compared different forms of antiseptics, cleansing method and frequency of application. The trial studied powders (zinc and baby talcum powder = 203, Sterzac = 204, Cordocel = 202, no powder = 203), Cleansing method (water = 272, spirit = 271, and no routine cleansing = 272) and frequency of application (daily = 407 and once only = 408). | |
| Outcomes | Time to cord separation. | |
| Notes | Hospital setting. Europe (England). All rooming in ("admitted to postnatal ward"). There was no mention of gestational age or birthweight of the babies. Data for group 5 and 6 were excluded. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomized". Method not mentioned. |
| Allocation concealment (selection bias) | Low risk | Sealed envelope. Use of "blind" packaging so that it was not possible to guess the allocation before entry of a baby to the trial. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not feasible. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No ITT analysis. |
| Selective reporting (reporting bias) | Low risk | Study reported the outcomes mentioned in the method's section. |
| Other bias | Low risk | "Groups are comparable in respect in distributions of sex, birthweight and mode of delivery." Cannot extrapolate data from included table of results. |
Mullany 2006.
| Methods | Cluster‐randomized, double‐blind (for chlorhexidine and soap and water), study conducted in community settings. | |
| Participants | All infants born after November 17, 2002, in 413 actively monitored enters in Southern Nepal, with consent, alive at first visit, first visit within first 10 days of life. | |
| Interventions | (1) Dry care (n = 5082). (2) 4% chlorhexidine (n = 4924). (3) Soap and water (n = 5107). |
|
| Outcomes | Time to cord separation; neonatal mortality and omphalitis. | |
| Notes | No mention of gestational age. Low birthweight and non‐low birthweight. Community‐based. South‐East Asia (Nepal). Initial cleansing full body wipe with 0.25% chlorhexidine or placebo. No breastfeeding < 1% all groups. Breastfeeding initiated at < 11.9 hours 46% vs 52% vs 47%. Facility birth 8% vs 8% vs 9%. Data on omphalitis have been taken from table 3 for the category of "moderate or sever redness". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence. |
| Allocation concealment (selection bias) | Unclear risk | Method not mentioned. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Investigators, field workers, and participants were masked with respect to the chlorhexidine and soap/water treatment groups". Not blinded to dry care. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No ITT analysis. A total of 15,113 infants were enrolled in the trial but analyses were restricted to 14,887 (98.4%) of those infants, for whom specific information on time to cord separation was collected (1.5% missing). |
| Selective reporting (reporting bias) | Low risk | This study appears to be free of selective reporting. Outcomes are described in 2 different papers. |
| Other bias | Low risk | Comparable baseline characteristics. |
Nourian 2009.
| Methods | A quasi‐randomized study conducted in Iran. | |
| Participants | Healthy term Newborn, 36 in group 1 and 41 in group 2. | |
| Interventions | 1) Dry cord care. 2) 70 alcohol. |
|
| Outcomes | Omphalitis. Bacterial colonization. Cord separation time. |
|
| Notes | Hospital‐based study Newborns with gestational age < 37 weeks, birthweight < 2500 g, Apgar score < 8 and the twins were excluded and so were infants receiving antibiotics or developing any complications requiring hospitalization. SDs for mean cord separation time were not given in the study. They were derived from other studies with similar settings and sample size. Omphalitis data were taken from table 2. Redness, exudates, foul odor, tenderness and inflammation were taken as signs of omphalitis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | A quasi‐randomized study. |
| Allocation concealment (selection bias) | High risk | A quasi‐randomized study. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding methods were not described in the study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition was described in the results section. |
| Selective reporting (reporting bias) | Low risk | Study reports all the outcomes mentioned in the method's section. |
| Other bias | Low risk | None noticed. |
Oishi 2004.
| Methods | Randomized, double‐blind, no ITT, conducted in hospital settings. | |
| Participants | Neonates born at National Tokyo Medical Centre from March to May 2000, nursed at a maternity ward from birth to discharge, with consent (N = 100). | |
| Interventions | (1) 80% ethanol (n = 52). (2) 80% ethanol with 0.5% chlorhexidine (n = 48). |
|
| Outcomes | Omphalitis, bacterial colonization. | |
| Notes | Preterm, term. Low birthweight, non‐low birthweight. Hospital setting. Western Pacific (Japan). Daily bath. All roomed in ("admitted to maternity ward from birth"). Reports omphalitis but does not state which group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Method not mentioned. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The mothers of the neonates and the nursing staff were unaware as to which disinfectant was being used. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | For colonization, complete outcomes reported for all participants for omphalitis, no ITT analysis. 1 case of omphalitis but not stated in which group; no loss of participants. |
| Selective reporting (reporting bias) | Unclear risk | Insufficent information to make an assessment. |
| Other bias | High risk | Alcohol group had low birthweight babies but the proportion is not specified. |
Panyavudhikrai 2002.
| Methods | Randomized trial, conducted in hospital settings. | |
| Participants | Neonates born at department of obstetrics and gynaecology, Siriraj hospital, Thialand. Mean gestational age of included babies was 38 weeks. Total n = 272. | |
| Interventions | 1) Povidine‐iodine (n = 93). 2) Triple dye (n = 90). 3) 70% alcohol (n = 89). |
|
| Outcomes | Incidence of omphalitis, cord separation time. | |
| Notes | Data have been included for 70% alcohol vs triple dye only, as the data for Povidine group were incomplete. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Simple randomisation was used", No further description of the sequence generation procedure was described. |
| Allocation concealment (selection bias) | Unclear risk | Insufficent information to permit judgement. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Insufficent information to permit judgement. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficent information to permit judgement. |
| Selective reporting (reporting bias) | Unclear risk | Insufficent information to permit judgement. |
| Other bias | Unclear risk | Insufficent information to permit judgement. |
Perapoch 1993.
| Methods | Randomized study conducted in hospital settings in Spain. | |
| Participants | Healthy term newborns admitted to nursery. A total of 311 infants were included in the study. | |
| Interventions | 1. Alcohol (70%) (n = 75). 2. Alcohol + mercurochrome (n = 78). 3. Mercurochrome (n = 84). 4. Chlorhexidine (1%) (n = 74). | |
| Outcomes | Cord infection. Cord separation. Bacterial colonization. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "They were randomly assigned to one of the four assessed methods". No further details of methods of randomization were given. |
| Allocation concealment (selection bias) | Unclear risk | Insufficent information to permit judgement. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Insufficent information to permit judgement. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No details of exclusion or attrition were given. |
| Selective reporting (reporting bias) | Unclear risk | Insufficent information to permit judgement. |
| Other bias | High risk | Baseline characteristics of study participants were not given. |
Pezzati 2002.
| Methods | Randomized study conducted in hospital settings | |
| Participants | Healthy term newborns admitted to nursery. A total of 1470 infants were included. | |
| Interventions | 1. Salicylic sugar powder (n = 167). 2. Green clay powder (n = 184). 3. Natural drying (n = 177). 4. Katoxin (n = 208). 5. Cicatrene (n = 174). 6. 1% basic fuschine (n = 187). 7. Triple dye (n = 195). 8. 70% alcohol (n = 178). | |
| Outcomes | Sepsis. Death. Cord infection. Cord separation. Cord bleeding. Compliance. Parental satisfaction. Bacterial colonization. | |
| Notes | Preterm (gestational age < 37 weeks) and low birthweight (< 2500 g) infants were excluded from the study, as well as infants who were receiving phototherapy, antibiotics or who developed complications of any kind requiring hospitalization in our neonatal pathology unit. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: ''It was deemed unnecessary to make use of a stricter randomization since the type of treatment was recognizable both by the nursery staff and parents." |
| Allocation concealment (selection bias) | High risk | Quote: ''It was deemed unnecessary to make use of a stricter randomization since the type of treatment was recognizable both by the nursery staff and parents." |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: ''It was deemed unnecessary to make use of a stricter randomization since the type of treatment was recognizable both by the nursery staff and parents." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition and exclusion were adequately described in result's section. |
| Selective reporting (reporting bias) | Unclear risk | Insufficent information to permit judgement. |
| Other bias | Low risk | Baseline characteristics were comparable at the baseline. |
Pezzati 2003.
| Methods | A prospective randomized trial conducted in Italy in hospital settings. | |
| Participants | Premature neonates. Total sample size was 213. | |
| Interventions | 1) 4% Cchlorhexidine solution (n = 101). 2) Salicylic sugar powder (n = 112). |
|
| Outcomes | Mortality. Sepsis. Cord separation time. Bacterial colonization. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A prospective, randomised study was conducted on all premature infants" Exact methods of sequence generation were not described. |
| Allocation concealment (selection bias) | Low risk | Quote: "The patients were selected to enter either of the 2 groups randomly by means of the sealed envelope technique". |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "The caregivers were not blinded as to allocation because the cord stumps looked different depending on which treatment group the infant was in". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Exlusion and attrition was described in the result section. |
| Selective reporting (reporting bias) | Unclear risk | Insufficent information to permit judgement. |
| Other bias | Unclear risk | None noticed. |
Rosenfeld 1989.
| Methods | Randomized study conducted in hospital settings in USA. | |
| Participants | Premature babies < 2200 g. A total of 114 babies were included in the study. | |
| Interventions | 1. Triple dye, single application (n = 54). 2. Isopropyl alcohol each diaper change (n = 60). | |
| Outcomes | Colonization on day 4 and at discharge. | |
| Notes | Only Abstract available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Alternate allocation. |
| Allocation concealment (selection bias) | High risk | Inadequate methods of randomization. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Insufficent information to permit judgement. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No details of exclusion or attrition were given. |
| Selective reporting (reporting bias) | Low risk | Study reported the outcomes mentioned in the methods section. |
| Other bias | Low risk | Baseline characteristics were comparable between the 2 groups. |
Rush 1986.
| Methods | Randomized study conducted in hospital settings in Canada. | |
| Participants | Heathy term newborns admitted to nursery. A total of 181 infants were included in the study. | |
| Interventions | 1. Routine daily bath with water and soap (n = 95). 2. Initial bath only(n = 86). | |
| Outcomes | Colonization on day 4 in the nose and umbilicus. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficent information to permit judgement. |
| Allocation concealment (selection bias) | Unclear risk | Insufficent information to permit judgement. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Insufficent information to permit judgement. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Details of exclusion and attrition were adequately described. |
| Selective reporting (reporting bias) | Unclear risk | Insufficent information to permit judgement. |
| Other bias | Low risk | Baseline characteristics were comparable between the 2 groups. |
Schuman 1985.
| Methods | Randomized trial conducted in hospital settings. | |
| Participants | Newborns delivered at USAF hospital, Grand forks, USA (N = 71). | |
| Interventions | 1) Triple dye (n = 35). 2) Isopropyle alcohol (n = 36). |
|
| Outcomes | Cord separation time. | |
| Notes | The following infants were excluded from the analysis: 1) infants receiving phototherapy; 2) had insertion of umbilical catheter; 3) during hospitalizations developed complications. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | ".....infants were randomly assigned by computer to one of two treatment regimens." |
| Allocation concealment (selection bias) | Unclear risk | Insufficent information to permit judgement. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Insufficent information to permit judgement. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficent information to permit judgement. |
| Selective reporting (reporting bias) | Unclear risk | Insufficent information to permit judgement. |
| Other bias | Unclear risk | Insufficent information to permit judgement. |
Shafique 2006.
| Methods | A quasi‐randomized study conducted in Pakistan. | |
| Participants | Full term newborn, 50 in group A and 50 in group B. | |
| Interventions | A) 70% alcohol. B) Dry cord care. |
|
| Outcomes | Cord separation time. | |
| Notes | Study conducted in hospital settings. The exclusion criteria were maternal pyrexia (temperature more than 38°C) during labour, premature rupture of membrane for greater than 18 hours, low birthweight newborns (weight < 2.5 kg), newborns being given oral or systemic antibiotic, admission in nursery within 48 hours after birth and presence of open congenital anomalies in the newborn. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | A quasi‐randomized study. |
| Allocation concealment (selection bias) | High risk | A quasi‐randomized al study. |
| Blinding (performance bias and detection bias) All outcomes | High risk | No methods described for blindings. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No reasons for loss to follow‐up were described. |
| Selective reporting (reporting bias) | Unclear risk | Insufficent information to permit judgement.. |
| Other bias | High risk | Data on umbilical cord infections and sepsis were collected by telephone and no physician examined the child for surveillance. |
Soofi 2012.
| Methods | 2 x 2 cluster‐randomized community trial conducted in Pakistan. | |
| Participants | Newborn whose parents were enrolled in the study (N = 9741). | |
| Interventions | 1) Chlorhexidine (n = 2214). 2) Chlorhexidine + handwashing (n = 2475). 3) Handwashing alone (n = 2653). 4) Control (n = 2399). |
|
| Outcomes | Omphalitis, all‐cause mortality, cord separation time. | |
| Notes | Intervention was delivered by TBAs. Factorial analysis (chlorhexidine vs no chlorhexidine) was included in this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: ".........randomly allocated to one of four groups (groups A to D) with a computer‐generated random number sequence". |
| Allocation concealment (selection bias) | Low risk | Comment: As this was a cluster‐randomized trial allocation concealment is not an issue. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Comment: Particiants were not blinded to the intervention however, Implementation and data collection teams were masked to allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition and exclusion were adequately described. ITT analysis. |
| Selective reporting (reporting bias) | Low risk | This study was free of selective reporting. |
| Other bias | High risk | The desired sample size was not achieved due to security situation in the study area. |
Speck 1977.
| Methods | Randomized trial conducted in hospital settings. | |
| Participants | Term healthy newborn. A total of 240 newborn babies were included in the study. | |
| Interventions | 1. Daily wash with Castile soap (n = 78). 2. Triple dye (n = 80). 3. Silver sulphadiazine (n = 82). Routine daily sponge bath with tap water. After discharge, daily application of isopropanol. | |
| Outcomes | Bacterial culture from the nose day 3, 14. Cord infection. Conjunctivitis. Impetigo. | |
| Notes | Data were taken from table II. Percentages were converted into number of events. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficent information to permit judgement. |
| Allocation concealment (selection bias) | Unclear risk | Insufficent information to permit judgement. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Insufficent information to permit judgement. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition and exclusion were described in the results section. |
| Selective reporting (reporting bias) | Unclear risk | Insufficent information to permit judgement. |
| Other bias | High risk | Baseline characteristics of study participants were not given. |
Suliman 2010.
| Methods | A prospective, randomized clinical trial conducted in US in hospital settings. | |
| Participants | Healthy full term newborn, 90 babies completed the study. | |
| Interventions | 1) Alcohol rub plus triple dye application (n = 46). 2) Triple dye application alone (n = 44). |
|
| Outcomes | Omphalitis. Cord separation time. |
|
| Notes | Data were described for different appearances of cord but no definition was used to define omphalitis. SDs were not given for cord separation time. We used the SDs from similar settings with similar sample size. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: ".......newborns were randomised to receive either no further preventive treatment to their umbilical cord or twice daily rubbing alcohol application". |
| Allocation concealment (selection bias) | Unclear risk | Insufficent information to make an assessment. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Methods of blinding were not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition and exclusion was described. |
| Selective reporting (reporting bias) | Low risk | Study reported all the outcomes mentioned in the method's section. |
| Other bias | Unclear risk | No physical surveillance was done to make an assessment for cord infection. |
CHX: chlorhexidine ITT: Intention‐to‐treat (analysis) NICU: neonatal intensive care unit SD: standard deviation TBAs: traditional birth attendants vs: versus
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alda 2000 | Comparison between hexachlorophene and chlorhexidine. Hexachlorophene not recommended anymore because of central nervous toxicity. |
| Aldar 1980 | Comparison between hexachlorophene, which is not recommended anymore due to neural toxicity, and chlorhexidine. |
| Barclay 1994 | This study is not a randomized trial. 890 babies in comparison between chlorhexidine and no specific treatment to cord. |
| Bhakoo 1969 | Non‐randomized, no alcohol or dry care in treatment, comparison between bathing and non‐bathing. |
| Birenbaum 1990 | Not cord care. |
| Bourke 1989 | This study was not a randomized trial. All babies born in 2 designated wards were entered into study. The treatment groups included dry cord care and alcohol (70%). |
| Bradshaw 1993 | Independent effect of alcohol cannot be assessed; alcohol was administered with hexachlorophene powder.Hexachlorophene not recommended anymore because of central nervous toxicity. |
| Chamnanvanakij 2005 | Triple dye applied to all participants before randomization. |
| Coyer 1975 | Not cord care. |
| Darmstadt 2007 | Not cord care. |
| Erenel 2010 | A quasi‐experimental study. |
| Gezon 1964 | Not cord care. |
| Gluck 1963 | Non‐randomized, total body skin care including cord. |
| Guala 2003 | 6 study groups. Allocation was in groups of 50. No randomization was done. |
| Guinsburg 1991 | 4 study groups. Only abstract available. Non‐randomized. |
| Healey 1991 | Not cord care. |
| Henningsson 1981 | Not cord care. |
| Hnatko 1977 | Intervention included hexachlorophene which is not recommended because of central nervous toxicity. Allocation to groups was according to predetermined schedule. |
| Hodgins 2010 | No dry cord care group, comparison between gel and aqueous chlorhexidine. |
| Jellard 1957 | All received surgical spirit so effect of treatment with antiseptics could not be assessed. |
| Kumar 2008 | Not cord care. |
| Kwong 1973 | Non‐randomized, total body bathing. |
| Olowe 1980 | Not cord care. |
| Pildes 1973 | Non‐randomized, no alcohol or dry care in treatment. |
| Pyati 1977 | Controlled trial in which multiple applications of povidone iodine were compared with a single application. |
| Ronchera‐Oms 1994 | Non‐randomized. |
| Saleem 2007 | Not cord care. |
| Saleem 2010 | Not a cord care study. |
| Shah 2010 | Not a cord care study. |
| Smales 1988 | No alcohol or dry care in treatment. 2 hospitals with different regimens reversed after 2 months. |
| Tielsch 2006 | Not cord care. |
| Tielsch 2007 | Not cord care. |
| Wade 2006 | Not cord care. |
| Wald 1977 | 1 of the treatment arms used Hexachlorophene which is not recommended because of central nervous toxicity. |
| Watkinson 1992 | Comparison between alcohol and hexachlorophane with no antiseptic. Hexachlorophene not recommended because of central nervous toxicity. |
| Wojciechowska 1989 | Study completed but not analyzed. No data available. |
Characteristics of studies awaiting assessment [ordered by study ID]
Covas 2011.
| Methods | Open prospective controlled clinical trial. |
| Participants | Newborns. |
| Interventions |
Study group‐body bath with neutral soap in the first 2 days of life and natural drying of the umbilical cord without special treatment. Control group ‐umbilical cord hygiene with alcohol 70% at each diaper change until its separation and bath 2 days later. |
| Outcomes | Cord separation time. |
| Notes | Need full text for further assessment. |
Nasrallah 2003.
| Methods | Randomized trial. |
| Participants | Newborn. |
| Interventions | Intervention: 70% isopropyl alcohol application. Control: natural drying of the cord. |
| Outcomes | Cord separation time. |
| Notes | Need full text for further assessment. |
Sellares Casas 2002.
| Methods | Experimental study. |
| Participants | Newborn, total sample size 285. |
| Interventions | 1) 1 application of Merbromin. 2) 3 application of Merbromin. |
| Outcomes | Omphalitis. Cord separation time. |
| Notes | Need full text for further assessment. |
Srinivasan 2003.
| Methods | Randomized trial. |
| Participants | Very low birthweight infants admitted to Mount Sinai hospital. |
| Interventions | Intervention: umbilical cord cleaned with alcohol swab, followed by application of triple dye daily. Control: umbilical cord cleaned with alcohol swab. |
| Outcomes | Bacterial colonization. |
| Notes | Need full text for further assessment. |
Taffazoli 2008.
| Methods | Randomized clinical trial conducted in Iran. |
| Participants | Newborn, total sample size 118. |
| Interventions | 1) Mothers milk. 2) Dry cord care. |
| Outcomes | Cord separation time. Bacterial colonization. |
| Notes | Need full text for further assessment. |
Characteristics of ongoing studies [ordered by study ID]
Hamer 2010.
| Trial name or title | Zambia Chlorhexidine Application Trial (ZamCAT). |
| Methods | Cluster‐randomized community trial. |
| Participants | Newborn whose parents are enrolled in the study. |
| Interventions | Chlorhexidine cord care: experimental. Control group: dry cord care. |
| Outcomes | All‐cause neonatal mortality and neonatal omphalitis. |
| Starting date | November 12 2010. |
| Contact information | Principal Investigator: Davidson H Hamer, MD: Boston University Center for Global Health and Development. |
| Notes | Currently the trial is in recruitment phase. |
Sazawal 2012.
| Trial name or title | Chlorhexidine Cordcare for Reduction in Neonatal Mortality and Omphalitis (CHX‐Pemba). |
| Methods | Community‐based controlled trial. |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | 1. Experimental: 4% chlorhexidine cord application for 10 days. 2. Active comparator: control: same liquid as intervention without the chlorhexidine used for cord cleaning for 10 days once daily. 3. No intervention: dry cord care use current recommended keep cord dry. |
| Outcomes | Neonatal mortality. Omphalitis. |
| Starting date | October 2010. |
| Contact information | Contact: Said M Ali, MS. |
| Notes |
ICU: intensive care unit
Differences between protocol and review
It was decided post‐hoc to include studies on all antiseptics rather than just alcohol. This was to include all possible evidence on umbilical cord care.
Comparison of dry cord care with other antiseptics was not performed as there was too much clinical heterogeneity in the comparison group.
It was decided not to pool community and hospital‐based studies together because of significant clinical heterogeneity of population of patients in hospitals and community settings.
Subgroup analyses based on gestational age and that of geographical regions were not performed as the number of studies were not sufficient.
Contributions of authors
Aamer Imdad contributed to the background. Aamer Imdad and Jacinto Blas V Mantaring were primarily responsible for the methods. Jacinto Blas V Mantaring carried out the additional searches listed in the methods section. Aamer Imdad, Jacinto Blas V Mantaring, Resti Ma M Bautista, Ma Esterlita V Uy and Kathlynne Anne Abat‐Senen reviewed citations for inclusion, extracted data and formulated the 'Characteristics of included study' table along with 'Risk of bias' table. Aamer Imdad, Jacinto Blas V Mantaring, Resti Ma M Bautista, Ma Esterlita V Uy and Kathlynne Anne Abat‐Senen entered outcome data into RevMan, analyzed the data and wrote the results. Jacinto Blas V Mantaring, Resti Ma M Bautista, Ma Esterlita V Uy and Kathlynne Anne Abat‐Senen drafted the discussion text, which Aamer Imdad and Zulfiqar Ahmed Bhutta subsequently revised. Zulfiqar Ahmed Bhutta provided supervision and is the guarantor for the review.
Sources of support
Internal sources
Effective Health Care Research Programme Consortium, Philippines.
External sources
SEA‐ORCHID Project (South‐East Asia ‐ Optimising Reproductive and Child Health Outcomes in Developing Countries), Australia.
Declarations of interest
Aamer Imdad and Zulfiqar Ahmed Bhutta were authors for study Soofi 2012. Data for Soofi 2012 were not extracted by these two authors.
Edited (no change to conclusions), comment added to review
References
References to studies included in this review
Ahmadpour‐Kacho 2006 {published data only}
- Ahmadpour‐Kacho M, Zahedpasha Y, Hajian K, Javadi G, Talebian H. The effect of topical application of human milk, ethyl alcohol 96%, and silver sulfadiazine on umbilical cord separation time in newborn infants. Archives of Iranian Medicine 2006;9:33‐8. [PubMed] [Google Scholar]
Al‐Binali 2006 {published data only}
- Al‐Binali AM. Umbilical cord care: comparison between Beniktol spray and alcohol swab. Biomedical Research 2006;17(1):23‐5. [Google Scholar]
Arad 1981 {published data only}
- Arad I, Eyal F, Fainmesser P. Umbilical care and cord separation. Archives of Disease in Childhood 1981;56:887‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Arifeen 2012 {published data only}
- Arifeen SE, Mullany LC, Shah R, Mannan I, Rahman SM, Talukder MRR, et al. Impact of cord cleansing with chlorhexidine on neonatal mortality in rural Bangladesh: a community‐based, cluster‐randomised trial. Lancet 2012;379(9820):1022‐8. [DOI] [PubMed] [Google Scholar]
- Bacqui AH. Impact of umbilical cord cleansing with 4.0% chlorhexidine on neonatal mortality (CHX). ClinicalTrials.gov (http://clinicaltrials.gov/show/NCT00434408) 2010.
- Mullany LC, Arifeen S, Winch PJ, Shah R, Mannan I, Rahman SM, et al. Impact of 4.0% chlorhexidine cleansing of the umbilical cord on mortality and omphalitis among newborns of Sylhet, Bangladesh: design of a community‐based cluster randomized trial. BMC Pediatrics 2009;9:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullany LC, Khatry SK, Sherchand JB, LeClerq SC, Darmstadt GL, Katz J, et al. A randomized controlled trial of the impact of chlorhexidine skin cleansing on bacterial colonization of hospital‐born infants in Nepal. Pediatric Infectious Disease Journal 2008;27(6):505‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullany LC, Saha SK, Shah R, Islam MS, Rahman M, Islam M, et al. Impact of 4.0% chlorhexidine cord cleansing on the bacteriologic profile of the newborn umbilical stump in rural sylhet district, Bangladesh: A community‐based, cluster‐randomized trial. Pediatric Infectious Disease Journal 2012;31(5):444‐50. [DOI] [PubMed] [Google Scholar]
Bain 1994 {published data only}
- Bain J. Midwifery: umbilical cord care in pre‐term babies. Nursing Standard 1994;8(15):32‐6. [DOI] [PubMed] [Google Scholar]
Barrett 1979 {published data only}
- Barrett FF, Mason EO, Fleming D. The effect of three cord‐care regimens on bacterial colonization of normal newborn infants. Journal of Pediatrics 1979;94:796‐800. [Google Scholar]
Davila 2007 {published data only}
- Davila GR, Pomar JV, Meza EQ, Villanueva CQ, Serkovic KR, Jordan FR, et al. Umbilical cord care: effect of three different antiseptic solutions (clorhexidine 4 per cent, alcohol 70 per cent and iodo povidone 5 per cent) on bacterial colonization, infection and cord separation time [Cuidados del cordón umbilical: efecto de tres soluciones antisépticas (gluconato de clorhexidina al 4 por ciento, alcohol al 70 por ciento y yodopovidona al 5 por ciento) sobre la colonización bacteriana, infección y separación del muñón umbilical]. Revista Peruana de Pediatria 2007;60(2):81‐7. [Google Scholar]
Dore 1998 {published data only}
- Dore S, Buchan D, Coulas S, Hamber L, Stewart M, Cowan D, et al. Alcohol versus natural drying for newborn cord care. Journal of Obstetric, Gynecologic and Neonatal Nursing 1998;27(6):621‐7. [DOI] [PubMed] [Google Scholar]
Evens 2004 {published data only}
- Evens K, George J, Angst D, Schweig L. Does umbilical cord care in preterm infants influence cord bacterial colonization or detachment?. Journal of Perinatology 2004;24:100‐4. [DOI] [PubMed] [Google Scholar]
- Evens KH, George J, Angst DB, Schweig L. Does umbilical cord care in preterm infants influence cord colonization and/or detachment. Pediatric Academic Societies Annual Meeting; 2003 May 1‐4; Seattle, Washington, USA. 2003.
Gladstone 1988 {published data only}
- Gladstone IM, Clapper L, Thorp JW, Wright DI. Randomized study of six umbilical cord care regimens. Comparing length of attachment, microbial control, and satisfaction. Clinical Pediatrics 1988;27:127‐9. [DOI] [PubMed] [Google Scholar]
Golombek 2002 {published data only}
- Golombek SG, Brill PE, Salice AL. Randomized trial of alcohol versus triple dye for umbilical cord care. Clinical Pediatrics 2002;41(6):419‐23. [DOI] [PubMed] [Google Scholar]
Hsu 1999 {published data only}
- Hsu CF, Wang CC, Yuh YS, Chen YH, Chu ML. The effectiveness of single and multiple applications of triple dye on umbilical cord separation time. European Journal of Pediatrics 1999;158:144‐6. [DOI] [PubMed] [Google Scholar]
Hsu 2010 {published data only}
Huang 2001 {published data only}
- Huang CF, Yeh LC, Chuang MY, Yuh YS. Umbilical separation time delayed by alcohol application. Journal of Perinatal Medicine 2001;29 Suppl 1(Pt 2):648. [Google Scholar]
Janssen 2003 {published data only}
- Janssen PA, Selwood BL, Dobson SR, Peacock D, Theissen PN. To dye or not to dye: a randomized, clinical trial of a triple dye/alcohol regime versus dry cord care. Pediatrics 2003;111:15‐20. [DOI] [PubMed] [Google Scholar]
Kapellen 2009 {published data only}
- Kapellen TM, Gebauer CM, Brosteanu O, Labitzke B, Vogtmann C, Kiess W. Higher rate of cord‐related adverse events in neonates with dry umbilical cord care compared to chlorhexidine powder. Results of a randomized controlled study to compare efficacy and safety of chlorhexidine powder versus dry care in umbilical cord care of the newborn. Neonatology 2009;96(1):13‐8. [DOI] [PubMed] [Google Scholar]
- Vogtmann C. A randomised controlled study to compare efficacy and safety of chlorhexidine powder versus dry care in umbilical cord care of the newborn. Current Controlled Trials (http://www.controlled‐trials.com/ISRCTN63012285/) (accessed 2010). [DOI] [PubMed]
Meberg 1985 {published data only}
- Meberg A, Schoyen R. Bacterial colonization and neonatal infections. Effects of skin and umbilical disinfection in the nursery. Acta Paediatrica Scandinavica 1985;74:366‐71. [DOI] [PubMed] [Google Scholar]
Meberg 1990 {published data only}
- Meberg A, Schoyen R. Hydrophobic material in routine umbilical cord care and prevention of infections in newborn infants. Scandinavian Journal of Infectious Diseases 1990;22:729‐33. [DOI] [PubMed] [Google Scholar]
Medves 1997 {published data only}
- Medves JM, O'Brien BA. Cleaning solutions and bacterial colonization in promoting healing and early separation of the umbilical cord in healthy newborns. Canadian Journal of Public Health 1997;88(6):380‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mugford 1986 {published data only}
- Mugford M, Somchiwong M, Waterhouse IL. Treatment of umbilical cords: a randomised trial to assess the effect of treatment methods on the work of midwives. MIdwifery 1986;2:177‐86. [DOI] [PubMed] [Google Scholar]
Mullany 2006 {published data only}
- Mullany LC, Darmstadt GL, Khatry SK, Katz J, LeClerq SC, Shrestha S, et al. Topical applications of chlorhexidine to the umbilical cord for prevention of omphalitis and neonatal mortality in southern Nepal: a community‐based, cluster‐randomised trial. Lancet 2006;367(9514):910‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullany LC, Darmstadt GL, Khatry SK, LeClerq SC, Katz J, Tielsch JM. Impact of umbilical cord cleansing with 4.0% chlorhexidine on time to cord separation among newborns in southern Nepal: a cluster‐randomized, community‐based trial. Pediatrics 2006;118(5):1864‐71. [DOI] [PubMed] [Google Scholar]
Nourian 2009 {published data only}
- Nourian M, Allaii F, Heidari A. Comparison of the effect of alcohol 70% versus dry cord care on cord bacterial colonization and cord separation time among newborns. Pakistan Journal of Medical Sciences 2009;25(1):103‐7. [Google Scholar]
Oishi 2004 {published data only}
- Oishi T, Iwata S, Nonoyama M, Tsuji A, Sunakawa K. Double‐blind comparative study on the care of the neonatal umbilical cord using 80% ethanol with or without chlorhexidine. Journal of Hospital Infection 2004;58:34‐7. [DOI] [PubMed] [Google Scholar]
Panyavudhikrai 2002 {published data only}
- Panyavudhikrai S, Danchaivijitr S, Vantanasiri C, Trakulsomboon S, Kolatat T, Dhiraputra C, et al. Antiseptics for preventing omphalitis. Journal of the Medical Association of Thailand 2002;85:229‐34. [PubMed] [Google Scholar]
Perapoch 1993 {published data only}
- Perapoch Lopez J, Salcedo Abizanda S, Gallart Catala A, Peguero Monforte G, Casellas Caro M, Barroso Perez C, et al. Umbilical colonization in normal newborns. A comparative study of 4 methods for umbilical antisepsis [Colonizacion umbilical en recien nacidos normales: Estudio comparativo de cuatro metodos de antisepsia umbilical]. Anales Espanoles de Pediatria 1993;39:195‐8. [PubMed] [Google Scholar]
Pezzati 2002 {published data only}
- Pezzati M, Biagoli EC, Martelli E, Gambi B, Biagiotti R, Rubaltelli FF. Umbilical cord care: the effect of eight different cord‐care regimens on cord separation time and other outcomes. Biology of the Neonate 2002;81:38‐44. [DOI] [PubMed] [Google Scholar]
Pezzati 2003 {published data only}
- Pezzati M, Rossi S, Tronchin M, Dani C, Filippi L, Rubaltelli FF. Umbilical cord care in premature infants: the effect of two different cord‐care regimens (salicylic sugar powder vs chlorhexidine) on cord separation time and other outcomes. Pediatrics 2003;112(4):e275. [DOI] [PubMed] [Google Scholar]
Rosenfeld 1989 {published data only}
- Rosenfeld CR, Laptook AR, Jeffery J. Limited effectiveness of triple dye (TD) in prevention of colonization with methacillin resistant staphylococcus aureus (MRSA) in a special care nursery (SCN). Pediatric Research 1989;25:281A. [DOI] [PubMed] [Google Scholar]
Rush 1986 {published data only}
- Rush J. Does routine newborn bathing reduce staphylococcus aureus colonization rates? A randomized controlled trial. Birth 1986;13:176‐80. [DOI] [PubMed] [Google Scholar]
Schuman 1985 {published data only}
- Schuman AJ, Oksol BA. The effect of isopropyl alcohol and triple dye on umbilical cord separation time. Military Medicine 1985;150:49‐50. [PubMed] [Google Scholar]
Shafique 2006 {published data only}
- Shafique MF, Ali S, Roshan E, Jamal S. Alcohol application versus natural drying of umbilical cord. Rawal Medical Journal 2006;31(2):58‐60. [Google Scholar]
Soofi 2012 {published data only}
- Bhutta Z. Topical application of chlorhexidine to the umbilical cord for prevention of omphalitis and neonatal mortality in rural distract of Pakistan. ClinicalTrials.gov (http://clinicaltrials.gov/ct2/show/NCT00682006) (accessed May 2010).
- Soofi S, Cousens S, Imdad A, Bhutto N, Ali N, Bhutta ZA. Topical application of chlorhexidine to neonatal umbilical cords for prevention of omphalitis and neonatal mortality in a rural district of Pakistan: a community‐based,cluster‐randomised trial. Lancet 2012;379(9820):1029‐36. [DOI] [PubMed] [Google Scholar]
Speck 1977 {published data only}
- Speck WT, Driscoll JM, O'Neill J, Rosenkranz HS. Effect of antiseptic cord care on bacterial colonization in the newborn infant. Chemotherapy 1980;26:372‐6. [DOI] [PubMed] [Google Scholar]
- Speck WT, Driscoll JM, Polin RA, O'Neill J, Rosenkraz HS. Staphylococcal and streptococcal colonization of the newborn infant: effect of antiseptic cord care. American Journal of Diseases of Children 1977;131:1005‐8. [DOI] [PubMed] [Google Scholar]
- Speck WT, Driscoll JM, Polin RA, Rosenkranz HS. Bacterial colonization in the newborn ‐ effect of cord care. Pediatric Research 1976;10:335. [Google Scholar]
Suliman 2010 {published data only}
- Beiler J. Triple dye plus alcohol versus triple dye alone for newborn umbilical cord care. ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 21 March 2006).
- Suliman AK, Watts H, Beiler J, King TS, Khan S, Carnuccio M, et al. Triple dye plus rubbing alcohol versus triple dye alone for umbilical cord care. Clinical Pediatrics 2010;49(1):45‐8. [DOI] [PubMed] [Google Scholar]
- Suliman AK, Watts HN, Beiler JS, King TS, Khan S, Carnuccio MB, et al. Triple dye plus rubbing alcohol versus triple dye alone in umbilical cord care. Pediatric Academic Societies Annual Meeting; 2007 May 5‐8; Toronto, Canada. 2007.
References to studies excluded from this review
Alda 2000 {published data only}
- Alda ER, Covas MC, Ventura SS, Baeza AM. Umbilical cord bacterial growth in healthy newborn with two different antiseptics. Pediatric Research 2000;47:385A. [Google Scholar]
Aldar 1980 {published data only}
- Alder VG, Burman D, Simpson RA, Fysh J, Gillespie WA. Comparison of hexachlorophane and chlorhexidine powders in prevention of neonatal infection. Archives of Disease in Childhood 1980;55:277‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barclay 1994 {published data only}
- Barclay L, Harrington A, Conroy R, Royal R, LaForgia J. A comparative study of neonates' umbilical cord management. Australian Journal of Advanced Nursing 1994;11:34‐40. [PubMed] [Google Scholar]
Bhakoo 1969 {published data only}
- Bhakoo ON, Lall JC, Agarwal KC. Prevention of hospital infections in neonates: an evaluation of no bath regimen. Indian Pediatrics 1969;6:697‐700. [PubMed] [Google Scholar]
Birenbaum 1990 {published data only}
- Birenbaum HJ, Glorioso L, Rosenberger C, Arshad C, Edwards K. Gowning on a postpartum ward fails to decrease colonization in the newborn infant. American Journal of Diseases of Children 1990;144:1031‐3. [DOI] [PubMed] [Google Scholar]
Bourke 1989 {published data only}
- Bourke E. Cord care: too much or too little. Australian Journal of Advanced Nursing 1990;7(2):19‐22. [PubMed] [Google Scholar]
Bradshaw 1993 {published data only}
- Bradshaw C. An experimental study to compare treatment vs non treatment of the umbilical cord. National Conference on Research in Midwifery; 1993 Sept 14; Birmingham, UK. 1993.
Chamnanvanakij 2005 {published data only}
- Chamnanvanakij S, Decharachakul K, Rasamimaree P, Vanprapar N. A randomised study of 3 umbilical cord care regimens at home in Thai neonates: comparison of time to umbilical cord separation, parental satisfaction and bacterial colonization. Journal of the Medical Association of Thailand 2005;88(7):967‐72. [PubMed] [Google Scholar]
Coyer 1975 {published data only}
- Coyer WF. Neonatal skin care and the prevention of staphylococcus aureus (staph) colonization. Pediatric Research 1975;9:339. [Google Scholar]
Darmstadt 2007 {published data only}
- Darmstadt GL, Hossain MM, Choi Y, Shirin M, Mullany LC, Islam M, et al. Safety and effect of chlorhexidine skin cleansing on skin flora of neonates in Bangladesh. Pediatric Infectious Disease Journal 2007;26(6):492‐5. [DOI] [PubMed] [Google Scholar]
Erenel 2010 {published data only}
- Erenel AS, Vural G, Efe SY, Ozkan S, Ozgen S, Erenoglu R. Comparison of olive oil and dry‐clean keeping methods in umbilical cord care as microbiological. Maternal & Child Health Journal 2010;14(6):999‐1004. [DOI] [PubMed] [Google Scholar]
Gezon 1964 {published data only}
- Gezon HM, Thompson DJ, Rogers KD, Hatch TF, Taylor PM. Hexachlorophene bathing in early infancy. New England Journal of Medicine 1964;270(8):379‐6. [DOI] [PubMed] [Google Scholar]
Gluck 1963 {published data only}
- Gluck L, Wood HF. Staphylococcal colonization in newborn infants with and without antiseptic skin care. A consideration of epidemiologic routes. New England Journal of Medicine 1963;268:1265‐8. [DOI] [PubMed] [Google Scholar]
Guala 2003 {published data only}
- Guala A, Pastore G, Garipoli V, Agosti M, Vitali M, Bona G. The time of umbilical cord separation in healthy full‐term newborns: a controlled clinical trial of different cord care practices. European Journal of Pediatrics 2003;162(5):350‐1. [DOI] [PubMed] [Google Scholar]
Guinsburg 1991 {published data only}
- Guinsburg R, Ikezawa MK, Fogliano RRF, Reichert MCF, Carvalho ES, Cardo DM, et al. Umbilical bacterial colonization (UBC) of normal newborn (NB) infants: effect of four antiseptic regimens. Pediatric Research 1991;29:282A. [Google Scholar]
Healey 1991 {published data only}
- Healey E, Greenish K, Armstrong C, Ayers S. A study of the relationship between the delivery to cord clamping interval and the time of cord separation. Midwifery 1991;7:167‐76. [DOI] [PubMed] [Google Scholar]
Henningsson 1981 {published data only}
- Henningsson A, Nystrom B, Tunnell R. Bathing or washing babies after birth?. Lancet 1981;2:1401‐3. [DOI] [PubMed] [Google Scholar]
Hnatko 1977 {published data only}
- Hnatko SI. Alternatives to hexachlorophene bathing of newborn infants. Canadian Medical Association Journal 1977;117:223‐6. [PMC free article] [PubMed] [Google Scholar]
Hodgins 2010 {published data only}
- Hodgins S, Thapa K, Khanal L, Aryal S, Suvedi BK, Baidya U, et al. Chlorhexidine gel versus aqueous for preventive use on umbilical stump: a randomized noninferiority trial. Pediatric Infectious Disease Journal 2010;29(11):999‐1003. [DOI] [PubMed] [Google Scholar]
Jellard 1957 {published data only}
- Jellard J. Umbilical cord as reservoir of infection in a maternity hospital. BMJ 1977;1(5024):925‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kumar 2008 {published data only}
- Kumar V, Mohanty S, Kumar A, Misra RP, Santosham M, Awasthi S, et al. Effect of community‐based behaviour change management on neonatal mortality in Shivgarh, Uttar Pradesh, India: a cluster‐randomised controlled trial. Lancet 2008;372(9644):1151‐62. [DOI] [PubMed] [Google Scholar]
Kwong 1973 {published data only}
- Kwong MS, Loew AD, Anthony BF, Oh W. The effect of hexachlorophene on staphylococcal colonization rates in the newborn infant: a controlled study using a single‐bath method. Journal of Pediatrics 1973;82:982‐6. [DOI] [PubMed] [Google Scholar]
Olowe 1980 {published data only}
- Olowe SA, Ransome‐Kuti O. The risk of jaundice in glucose‐6‐phosphate dehydrogenase deficient babies exposed to menthol. Acta Paediatrica Scandinavica 1980;69:341‐5. [DOI] [PubMed] [Google Scholar]
Pildes 1973 {published data only}
- Pildes RS, Ramamurthy RS, Vidyasagar D. Effect of triple dye on staphylococcal colonization in the newborn infant. Journal of Pediatrics 1973;82:987‐90. [DOI] [PubMed] [Google Scholar]
Pyati 1977 {published data only}
- Pyati SP, Ramamurthy RS, Krauss MT, Pildes RS. Absorption of iodine in the neonate following topical use of povidone iodine. Journal of Pediatrics 1977;91(5):825‐8. [DOI] [PubMed] [Google Scholar]
Ronchera‐Oms 1994 {published data only}
- Ronchera‐Oms C, Hernandez C, Jimenez NV. Antiseptic cord care reduces bacterial colonisation but delays cord detachment. Archives of Disease in Childhood. Fetal and Neonatal Edition 1994;70:F70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saleem 2007 {published data only}
- Saleem S, Reza T, McClure EM, Pasha O, Moss N, Rouse DJ, et al. Chlorhexidine vaginal and neonatal wipes in home births in Pakistan: a randomized controlled trial. Obstetrics and Gynecology 2007;110(5):977‐85. [DOI] [PubMed] [Google Scholar]
Saleem 2010 {published data only}
- Saleem S, Rouse DJ, McClure EM, Zaidi A, Reza T, Yahya Y, et al. Chlorhexidine vaginal and infant wipes to reduce perinatal mortality and morbidity: a randomized controlled trial. Obstetrics and Gynecology 2010;115(6):1225‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shah 2010 {published data only}
- Shah R, Munos MK, Winch PJ, Mullany LC, Mannan I, Rahman SM, et al. Community‐based health workers achieve high coverage in neonatal intervention trials: a case study from Sylhet, Bangladesh. Journal of Health, Population & Nutrition 2010;28(6):610‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smales 1988 {published data only}
- Smales O. A comparison of umbilical cord treatment in the control of superficial infection. New Zealand Medical Journal 1988;101:453‐5. [PubMed] [Google Scholar]
Tielsch 2006 {published data only}
- Tielsch JM. Community trial of newborn skin and umbilical cord cleansing on neonatal mortality in Nepal. ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 2006) 2006.
Tielsch 2007 {published data only}
- Tielsch JM, Darmstadt GL, Mullany LC, Khatry SK, Katz J, LeClerq SC, et al. Impact of newborn skin‐cleansing with chlorhexidine on neonatal mortality in Southern Nepal: a community‐based, cluster‐randomized trial. Pediatrics 2007;119(2):e330‐e340. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wade 2006 {published data only}
- Wade A, Osrin D, Shrestha BP, Sen A, Morrison J, Tumbahangphe KM, et al. Behaviour change in perinatal care practices among rural women exposed to a women's group intervention in Nepal. BMC Pregnancy and Childbirth 2006;6:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wald 1977 {published data only}
- Wald ER, Snyder MJ, Gutberlet RL. Group B beta‐hemolytic streptococcal colonization. Acquisition, persistence, and effect of umbilical cord treatment with triple dye. American Journal of Diseases of Children 1977;131:178‐80. [DOI] [PubMed] [Google Scholar]
Watkinson 1992 {published data only}
- Watkinson M, Dyas A. Staphylococcus aureus still colonizes the untreated neonatal umbilicus. Journal of Hospital Infection 1992;21:131‐6. [DOI] [PubMed] [Google Scholar]
Wojciechowska 1989 {published data only}
- Wojciechowska L. Trial to assess the effects of different treatments of the umbilical cord in newborn infants. Personal communication 1989.
References to studies awaiting assessment
Covas 2011 {published data only}
- Covas MC, Alda ER, Medina MS, Ventura S, Esandi ME, Pezutti O, et al. Alcohol versus bath and natural drying for term newborns' umbilical cord care: a prospective randomized clinical trial. Pediatric Academic Societies Annual Meeting; 2010 May 1‐4; Vancouver, Canada. 2010. [DOI] [PubMed]
- Covas Mdel C, Alda E, Medina MS, Ventura S, Pezutti O, Paris de Baeza A, et al. Alcohol versus bath and natural drying for term newborns' umbilical cord care: A prospective randomized clinical trial [Spanish]. Archivos Argentinos de Pediatria 2011;109(4):305‐13. [DOI] [PubMed] [Google Scholar]
Nasrallah 2003 {published data only}
- Nasrallah BK, Rox AK, Mazer B, Richards L, Chuachingco J, Naqvi M. Comparison of two methods of umbilical cord care: application of 70% isopropyl alcohol vs. natural drying. Pediatric Academic Societies Annual Meeting; 2003 May 1‐4; Seattle Washington, USA. 2003.
Sellares Casas 2002 {published data only}
- Sellares Casas E, Yanez Juan A, Lopez de Aguileta Ibisate A, Peix Sambola MA, Esteva Nuto N, Domenech Terricabras P. Efficacy of one versus three applications of merbromin in achieving umbilical cord detachment [Spanish] [Efficacia de una aplicacion frente a tres de merbromina en el tiempo de caida del cordon]. Acta Pediatrica Espanola 2002;60(9):521‐5. [Google Scholar]
Srinivasan 2003 {published data only}
- Srinivasan H, Luayon M, Barrios F, Geraldo V, Poladian A, Reyes J, et al. Prospective randomized controlled trial to study the effect of daily application of triple dye to umbilical cord of very low birth weight (VLBW) infants. Pediatric Academic Societies Annual Meeting; 2003 May 1‐4; Seattle, Washington. 2003.
Taffazoli 2008 {published data only}
- Taffazoli M, Farahani LA, Mohammadzadeh A, Esmaeeli H, Ghazvini K. Does topical application of breast milk affect on bacterial colonization in umbilical cord?. Koomesh 2008;10(1):29‐35. [Google Scholar]
References to ongoing studies
Hamer 2010 {published data only}
- Hamer DH. Zambia Chlorhexidine Application Trial (ZamCAT). Clinicaltrials.gov (http://clinicaltrials.gov) (accessed 2010) 2010.
- Herlihy JM, Semrau K, Mazimba A, Yeboah‐Antwi K, Grogan C, Banda B, et al. Chlorhexidine 4% umbilical wash lengthens time to cord separation. Pediatric Academic Societies Annual Meeting 2012 April 29‐May 1; Boston, USA. 2012.
Sazawal 2012 {published data only}
- Sazawal S. Chlorhexidine cordcare for reduction in neonatal mortality and omphalitis (CHX‐Pemba). ClinicalTrials.gov 2012.
Additional references
AAP 2003
- AAP Committee on Infectious Diseases. Red Book: a Report of the Committee on Infectious Diseases. 26th Edition. Elk Grove Village, IL: American Academy of Pediatrics, 2003. [Google Scholar]
Aggett 1981
- Aggett PJ, Cooper LV, Ellis SH, McAinsh J. Percutaneous absorption of chlorhexidine in neonatal cord care. Archives of Disease in Childhood 1981;56(11):878‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Agrawal 2012
- Agrawal PK, Agrawal S, Mullany LC, Darmstadt GL, Kumar V, Kiran U, et al. Clean cord care practices and neonatal mortality: evidence from rural Uttar Pradesh, India. Journal of Epidemiology and Community Health 2012;66(8):755‐8. [DOI] [PubMed] [Google Scholar]
Bennett 1997
- Bennett J, Macia J, Traverso H, Banoagha S, Malooly C, Boring J. Protective effects of topical antimicrobials against neonatal tetanus. International Journal of Epidemiology 1997;26(4):897‐903. [DOI] [PubMed] [Google Scholar]
Blencowe 2011
- Blencowe H, Cousens S, Mullany LC, Lee AC, Kerber K, Wall S, et al. Clean birth and postnatal care practices to reduce neonatal deaths from sepsis and tetanus: a systematic review and Delphi estimation of mortality effect. BMC Public Health 2011;11 Suppl 3:S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Darmstadt 2009
- Darmstadt GL, Lee AC, Cousens S, Sibley L, Bhutta ZA, Donnay F, et al. 60 Million non‐facility births: who can deliver in community settings to reduce intrapartum‐related deaths?. International Journal of Gynaecology and Obstetrics 2009;107(Suppl 1):S89‐S112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elhassani 1984
- Elhassani SB. The umbilical cord: care, anomalies, and diseases. Southern Medical Journal 1984;77(6):730‐6. [DOI] [PubMed] [Google Scholar]
Fairchild 1958
- Fairchild JP, Graber CD, Vogel EH, Jr, Ingersoll RL, Ingersoll RL. Flora of the umbilical stump; 2,479 cultures. Journal of Pediatrics 1958;53(5):538‐46. [DOI] [PubMed] [Google Scholar]
Faridi 1993
- Faridi MM, Rattan A, Ahmad SH. Omphalitis neonatorum. Journal of the Indian Medical Association 1993;91(11):283‐5. [PubMed] [Google Scholar]
Forshall 1957
- Forshall I. Septic umbilical arteritis. Archives of Disease in Childhood 1957;32(161):25‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gallagher 2010
- Gallagher PG. Omphalitis. eMedicine (http://emedicine.medscape.com/) (accessed April 14 2010) 2010.
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Johnsson 1987
- Johnsson J, Seeberg S, Kjellmer I. Blood concentrations of chlorhexidine in neonates undergoing routine cord care with 4% chlorhexidine gluconate solution. Acta Paediatrica Scandinavica 1987;76(4):675‐6. [DOI] [PubMed] [Google Scholar]
Lawn 2005
- Lawn JE, Cousens S, Zupan J. 4 million neonatal deaths: when? where? why?. Lancet 2005;365(9462):891‐900. [DOI] [PubMed] [Google Scholar]
Lehmann 1999
- Lehmann D, Michael A, Omena M, Clegg A, Lupiwa T, Sanders RC, et al. The bacterial and viral etiology of severe infection in children aged less than three months in the highlands of Papua New Guinea. Pediatric Infectious Disease Journal 1999;18(10 Suppl):S42‐S49. [DOI] [PubMed] [Google Scholar]
Liu 2012
- Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since. Lancet 2012;379(9832):2151‐61. [DOI] [PubMed] [Google Scholar]
Mason 1989
- Mason WH, Andrews R, Ross LA, Wright HT Jr. Omphalitis in the newborn infant. Pediatric Infectious Disease Journal 1989;5:521‐5. [DOI] [PubMed] [Google Scholar]
McClure 2007
- McClure EM, Goldenberg RL, Brandes N, Darmstadt GL, Wright LL, Armbruster D, et al. The use of chlorhexidine to reduce maternal and neonatal mortality and morbidity in low‐resource settings. International Journal of Gynaecology and Obstetrics 2007;97(2):89‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
McKenna 1977
- McKenna H, Johnson D. Bacteria in neonatal omphalitis. Pathology 1977;9(2):111‐3. [DOI] [PubMed] [Google Scholar]
Mir 2011
- Mir F, Tikmani SS, Shakoor S, Warraich HJ, Sultana S, Ali SA, et al. Incidence and etiology of omphalitis in Pakistan: a community‐based cohort study. Journal of Infection in Developing Countries 2011;5(12):828‐33. [DOI] [PubMed] [Google Scholar]
Mullany 2006a
- Mullany LC, Darmstadt GL, Katz J, Khatry SK, LeClerq SC, Adhikari RK, et al. Development of clinical sign based algorithms for community based assessment of omphalitis. Archives of Disease in Childhood. Fetal and Neonatal Edition 2006;91(2):F99‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mullany 2006b
- Mullany LC, Darmstadt GL, Khatry SK, LeClerq SC, Katz J, Tielsch JM. Impact of umbilical cord cleansing with 4.0% chlorhexidine on time to cord separation among newborns in southern Nepal: a cluster‐randomized, community‐based trial. Pediatrics 2006;118(5):1864‐71. [DOI] [PubMed] [Google Scholar]
Mullany 2007
- Mullany LC, Darmstadt GL, Katz J, Khatry SK, LeClerq SC, Adhikari RK, et al. Risk factors for umbilical cord infection among newborns of southern Nepal. American Journal of Epidemiology 2007;165(2):203‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mullany 2009
- Mullany LC, Darmstadt GL, Katz J, Khatry SK, Leclerq SC, Adhikari RK, et al. Risk of mortality subsequent to umbilical cord infection among newborns of southern Nepal: cord infection and mortality. Pediatric Infectious Disease Journal 2009;28(1):17‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mullany 2012
- Mullany LC, Saha SK, Shah R, Islam MS, Rahman M, Islam M, et al. chlorhexidine cord cleansing on the bacteriologic profile of the newborn umbilical stump in rural Sylhet District, Bangladesh: a community‐based, cluster‐randomized trial. Pediatric Infectious Disease Journal 2012;31(5):444‐50. [DOI] [PubMed] [Google Scholar]
Novack 1988
- Novack AH, Mueller B, Ochs H. Umbilical separation in the normal newborn. American Journal of Diseases of Children 1988;142(2):220‐3. [DOI] [PubMed] [Google Scholar]
Oudesluys‐Murphy 1987
- Oudesluys‐Murphy AM, Eilers GA, Groot CJ. The time of separation of the umbilical cord. European Journal of Pediatrics 1987;146(4):387‐9. [DOI] [PubMed] [Google Scholar]
Oudesluys‐Murphy 1990
- Oudesluys‐Murphy AM, Hollander JC. Separation of the umbilical cord‐‐histological findings. Biology of the Neonate 1990;58(1):54‐6. [DOI] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Sawardekar 2004
- Sawardekar KP. Changing spectrum of neonatal omphalitis. Pediatric Infectious Disease Journal 2004;23(1):22‐6. [DOI] [PubMed] [Google Scholar]
Sawin 1994
- Sawin RS, Schaller RT, Tapper D, Morgan A, Cahill J. Early recognition of neonatal abdominal wall necrotizing fasciitis. American Journal of Surgery 1994;167(5):481‐4. [DOI] [PubMed] [Google Scholar]
Smith 2009
- Smith CK. Some traditional umbilical cord care practices in developing countries. Midwifery Today With International Midwife 2009;91:12‐3. [PubMed] [Google Scholar]
Thaver 2009
- Thaver D, Zaidi AK. Burden of neonatal infections in developing countries: a review of evidence from community‐based studies. Pediatric Infectious Disease Journal 2009;28(1 Suppl):S3‐S9. [DOI] [PubMed] [Google Scholar]
WHO 1999
- World Health Organization. Care of the umbilical cord: a review of the evidence. WHO, maternal and newborn health safe motherhood. Geneva: WHO, 1999. [Google Scholar]
WHO 2006
- World Health Organization. Neonatal and perinatal mortality: country, regional and global estimates. Geneva: WHO, 2006:47. [Google Scholar]
WHO 2011
- World Health Organization. Essential Medicines List 2011. WHO (http://www.who.int/selection_medicines/list/en/index.html) (accessed 2011) 2011.
References to other published versions of this review
Zupan 2004
- Zupan J, Garner P, Omari AAA. Topical umbilical cord care at birth. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD001057.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


