ABSTRACT
The exact mechanism of miR-15a-5p shuttled by human umbilical cord-mesenchymal stem cell-derived exosomes (hUC-MSCs-Exo) in Wilms tumor (WT) was estimated. WT tissues were collected clinically. miR-15a-5p and septin 2 (SEPT2) expression levels were examined in tissues . hUC-MSCs-Exo were transfected with miR-15a-5p-related oligonucleotides and co-cultured with WT cells (G-401). In addition, SEPT2 loss-of-function was performed in G-401 cells. The biological functions of G-401 cells after treatments were evaluated. Moreover, tumor formation tests further assessed the role of exosomal miR-15a-5p in WT. The miR-15a-5p level was lower and the SEPT2 level was higher in WT. hUC-MSCs-Exo impaired the biological functions of G-401 cells. hUC-MSCs-Exo carried upregulated miR-15a-5p into G-401 cells, thereby lessening the tumorigenic properties of G-401 cells. Inhibition of SEPT2 suppressed the biological function of WT cells and upregulated SEPT2 reversed hUC-MSCs-Exo-mediated inhibition of G-401 cell growth. The tumorigenicity of G-401 cells in mice was impaired by hUC-MSCs-Exo overexpressing miR-15a-5p. The data prove that miR-15a-5p shuttled by hUC-MSCs-Exo negatively regulates SEPT2 expression, and disrupts WT cell growth in vivo and in vitro.
KEYWORDS: Wilms tumor, human umbilical cord-mesenchymal stem cells-derived exosomes, MicroRNA-15a-5p, septin 2
Graphical Abstract
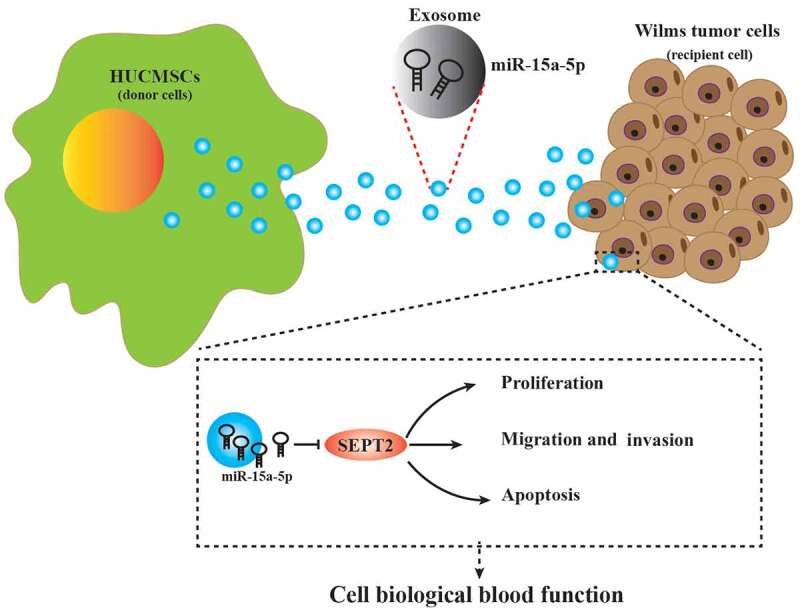
Introduction
Wilms tumor (WT) is an embryonic tumor derived from metanephros, and the most prevalent kidney tumor in childhood [1]. WT tends to grow into the vena cava and even invades the atrium, which increases the difficulty of surgery and elicits surgical complications [2]. WT is staged according to tumor overflow or rupture, lymph node, tumor expansion, peritoneal or blood-borne spread [3]. WT metastasis is often observed at abdominal lymph nodes, asymptomatic solid lung lesions and liver nodules [4]. Currently, treatment is mainly to reduce the risk of treatment-related sequelae in the medium and long term of low-risk WT and to determine and improve the outcome of WT patients [5]. Also, discovery of novel biomarkers suggests great significance in treating WT.
Mesenchymal stem cells (MSCs) have a low immunogenicity and the ability to migrate to tumors [6]. Exosomes (Exo) produced by MSCs act as paracrine mediators by transferring signal molecules to regulate tumor growth and metastasis [7]. Human umbilical cord (hUC)-MSCs-Exo serve as the promising paradigm to manage diseases including cancers [8]. The interplay between hUC-MSCs-Exo and microRNAs (miRNAs) is proved in the regulation of cancers. For example, huc-MSCs-Exo could transport upregulated miR-148b-3p to breast cancer (BCa) cells and then delay cancer development [9]. Indicated by another research, miR-375-loaded huc-MSCs-Exo could postpone the malignant progression of esophageal squamous cell carcinoma [10]. It is recorded that elevated miR-15a expression in WT cells could strain cellular aggressiveness [11]. Widely discussed, miR-15a-5p serves repressively for the development of endometrial cancer [12], lung cancer [13] and neuroblastoma [14]. However, whether miR-15a-5p could cooperate with huc-MSCs-Exo to regulate the biological progression of WT cells remains elusive. Through the prediction of the bioinformatics software, we have known that septin 2 (SEPT2) could be targeted by miR-15a-5p, thus we picked SEPT2 as the downstream effector to explore exosomal miR-15a-5p-mediated mechanism in WT. SEPT2 has often been regarded as the oncogenic factor in BCa [15] and hepatocellular carcinoma (HCC) [16]. Functionally, targeted regulation of SEPT2 could be an applicable approach to control cancers, as proved in glioblastoma [17]. On the basis of previous research, we hypothesized that miR-15a-5p shuttled by huc-MSCs-Exo could frustrate the biological activities of WT cells through regulating SEPT2 and hoped to translate the molecular mechanism related to miR-15a-5p in WT.
Methods and materials
Ethics statement
Before the study, the informed consent of patients was obtained. All animal experiments were conducted following ‘Guidelines for the Care and Use of Laboratory Animals’ (National Academy of Sciences Press, revised in 2010) and the ethics guidelines of The First Affiliated Hospital of Fujian Medical University.
Tissues samples
A total of 87 pairs of WT tissues and normal tissues were collected from the First Affiliated Hospital of Fujian Medical University. No radiotherapy or chemotherapy was administered before surgery.
Cell culture
Fresh hUCs from parturients (25–27 years old) after cesarean delivery in the First Affiliated Hospital of Fujian Medical University were collected. WT cell line G-401 was kept in McCoy’s 5A medium (Sigma-Aldrich, CA, USA) containing 10% fetal bovine serum (FBS), while HK-2 cells in 10% FBS-Dulbecco’s modified Eagle’s medium (DMEM) containing 1% penicillin/streptomycin (Gibco, NY, USA)[18].
Isolation and identification of hUC-MSCs
Isolation of hUC-MSCs: the hUC was rinsed with 75% ethanol, as well as with DMEM (Thermo Fisher Scientific, MA, USA) containing 1% glutamine, 10% FBS (Thermo Fisher Scientific) and 100 μg/mL streptomycin and penicillin. Subsequently, the hUC was cut into small pieces (3–5mm) and incubated. Then, hUC-MSCs at 80–90% confluence were isolated and passaged using trypsin. After that the cells were observed under an optical microscope (Olympus, Tokyo, Japan) [19].
Identification of immunophenotype: hUC-MSCs at 80% confluence were centrifuged and suspended in 1% bovine serum albumin (BSA). Then, the cell suspension was reacted with PE or fluorescein isothiocyanate (FITC)-labeled monoclonal antibodies CD34, CD44, CD73 and CD90 (BD Company, NJ, USA). Finally, hUC-MSCs were suspended in 1 × phosphate-buffered saline (PBS) for detection of flow cytometry [20].
Adipogenic induction: hUC-MSCs of passage 3 were treated with 0.25% trypsin and placed in a48-well plate for 14 d. Next, hUC-MSCs were fixed with 4% neutral formaldehyde and stained with oil red Odye . Finally, supplemented with PBS , hUC-MSCs were observed under an inverted microscope [20].
Osteogenic induction: hUC-MSCs were treated in the same way of adipogenic induction, and stained with Alizarin Red [21].
hUC-MSCs transfection
When transfecting hUC-MSCs, miR-15a-5p mimic, mimic NC, miR-15a-5p inhibitor and inhibitor NC (GenePharma, Shanghai, China) were adopted. Transfection of hUC-MSCs (60% confluence) was performed via Lipofectamine 3000 (Invitrogen) [22].
Isolation of hUC-MSCs-Exo
hUC-MSCs-Exo were purified by differential centrifugation. Then, the supernatant was filtered through a 0.22-μm filter and centrifuged at 100,000 g twice (70 min each). hUC-MSCs-Exo were suspended in sterile PBS (200 µL) [23].
Identification of hUC-MSCs-Exo
Nanoparticle Tracking Analysis (NTA): A NanoSight LM10 instrument (Malvern Instruments, Malvern, UK) equipped with an sCMOS camera was applied to determine the size of hUC-MSCs-Exo. Data were analyzed by NTA software version 3.1.54 [24].
Transmission electron microscope (TEM): hUC-MSCs-Exo were fixed with 1 % glutaraldehyde in PBS (pH 7.4) . The specific steps were conducted as previously described [25].
Uptake of hUC-MSCs-Exo by cells
hUC-MSCs-Exo were marked by 10−6 M PKH26 (Sigma-Aldrich). Quenched with PBS containing 10% BSA, PKH26-labeled hUC-MSCs-Exo were ultra-centrifuged at 100,000 g and co-cultured with G-401 cells. After 12 h, G-401 cells were fixed with 4% paraformaldehyde (pH = 7.4), treated with immunofluorescence staining and viewed by a fluorescence microscope (LSM880, Zeiss, Germany) [26,27].
Cell culture
G-401 cells were seeded into six-well plates at 2 × 105 cells per well and transfected with overexpression negative control (oe-NC), oe-SEPT2, sh-NC, sh-SEPT2 (GeneChem, Shanghai, China) using Lipofectamine 3000 (Invitrogen). For exosome treatment, 2 × 105 G-401 cells were incubated with 2 µg hUC-MSCs-Exo (or hUC-MSCs-Exo transfected with miR-15a-5p mimic, miR-15a-5p inhibitor and corresponding NCs). The cells were collected and treated for 48 h for subsequent experiments [28].
Reverse transcription quantitative polymerase chain reaction (RT-qPCR)
Cellular RNA extracted by Trizol (Invitrogen, CA, USA) method, and reverse-transcribed into complementary DNA. The steps were performed as previously mentioned [29]. Supplementary Table 1 shows the primers used in PCR.
Western blot assay
The protein extracted by radio-immunoprecipitation assay lysis buffer (containing protease inhibitor) was quantified by bicinchoninic acid kit (Takara), separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis, and transferred to a polyvinylidene fluoride membrane (Bio-Rad, CA, USA). The blot was blocked in BSA and immunostained with primary antibodies HSP70 (1:1000), CD9 (1:1000), SEPT2 (1:1000Abcam, MA, USA), CD81(1:500; Santa Cruz Biotechnology, CA, USA), glyceraldehyde-3-phosphate dehydrogenase (GAPDH, 1:1000; CST, MA, USA) and a secondary antibody . Signals were detected by chemiluminescence [16,30].
Flow cytometry
G-401 cells were resuspended in 500 µL binding buffer, incubated with 5 µL Annexin V-FITC and propidium iodide and analyzed on a flow cytometer (BD Biosciences, NJ, USA). The Annexin V-FITC Apoptosis Detection Kit (KeyGEN, Nanjing, China) was adopted in the assay [31].
Colony formation assay
G-401 cells were centrifuged and resuspended. The cell suspension was diluted to 1 × 103 cell/mL was placed in a 6-well plate and incubated for 2–3 w to form visible colonies. The cell colonies were fixed with methanol, stained with crystal violet solution and counted to calculate the colony rate = (number of colonies/number of seeded cells) × 100% .
Cell counting kit (CCK)-8 assay
After 24, 48, 72 and 96 h of seeding in the 96-well plate, cells (2 × 103 cells/well) were supplemented with CCK-8 reagent (10 μL). Optical density of each well was measured at 450 nm using a microplate reader (Bio-Rad). CCK-8 kit (Beyotime, Shanghai, China) was utilized in the assay [22].
Transwell assay
Invasion assay: Serum-free medium was mingled with Matrigel to reach 1 mg/mL, and 50 μL of the diluted medium was placed in the upper chamber. A complete medium with 15% FBS was put into the lower chamber. After cells (5 × 104 cells/well) incubating in the upper chamber for 24 h, cells were fixed with 95% absolute ethanol, processed by 0.1% crystal violet and viewed under an optical microscope.
Migration assay: The lower chamber contained 15% FBS-McCoy’s 5A medium, and cell migration was detected in the same way as invasion assay (Matrigel was not used) [32].
Tumor xenografts in nude mice
Specific pathogen-free BALB/c nude mice (6 weeks old and 18–22 g) (5 mice/group) were subcutaneously injected with WT cells (1 × 106 cells). A week later, hUC-MSCs-Exo after transfection were also injected into the mice. The size of the tumors was measured every 7 d. The volume = (length × width2)/2. All the mice were euthanized 5 w after injection, and the tumors were harvested and weighed [33,34].
Dual luciferase reporter gene assay
The target relationship between miR-15a-5p and SEPT2 was predicted through the bioinformatics software http://starbase.sysu.edu.cn/. The SEPT2 3ʹUTR containing miR-15a-5p binding sites was composed to construct the wild-type plasmid (SEPT2-WT), from which a mutant plasmid (SEPT2-MUT) was mutated. G-401 cells were co-transfected with the sequenced SEPT2-WT or SEPT2-MUT plasmids with NC or miR-15a-5p mimic. During transfection, Lipofectamine 3000 reagent was applied. After a transfection of 48 h, luciferase activity was verified by a luciferase detection kit [21].
Statistical analysis
After analysis at Graphad Prism 6.0 (Graphpad Software, USA)和SPSS 21.0 (SPSS,©Inc., Chicago,IL, USA), data were expressed as mean ± standard deviation. The comparisons of data were evaluated by t-test or analysis of variance, followed by Tukey’s post-test. Pearson correlation analysis was performed to determine the correlation between two variables. Survival analysis was determined using the Kaplan-Meiersurvival curve and log-rank test. Statistical significance was found at P < 0.05.
Results
Topic: To explore the therapeutic effect of miR-15a-5p in hUC-MSCs-Exo on WT by targeting SEPT2 expression.
Goals and hypothesis: To verify that miR-15a-5p in hUC-MSCs-Exo play a role in the treatment of WT by targeting SEPT2 expression, provide a theoretical basis for the treatment of WT by hUC-MSCs-Exo and explore the molecular mechanism of the treatment.
What was done: WT cells and hUC-MSCs-Exo were obtained. HUC-MSCs-Exo were co-cultured with G-401 cells, and the effects of Exo, miR-15a-5p or SEPT2 on the proliferation, apoptosis, migration and invasion ability of G-401 cells and tumor growth in vivo were detected; HUC-MSCs-Exo could inhibit G-401 cell proliferation, migration and invasion, in vivo tumor growth, and induce cell apoptosis. HUC-MSCs-Exo overexpressing miR-15a-5p can further inhibit the biological function of G-401 cells. It was determined that miR-15a-5p targeted and negatively regulated the expression of SEPT2. Downregulation of SEPT2 suppressed the biological function of G-401 cells.
miR-15a-5p is downregulated in WT
miR-15a-5p is a tumor suppressor that is downregulated in osteosarcoma [35]. To clarify its expression in WT, we utilized RT-qPCR to test miR-15a-5p in WT tissues and normal tissues and finally discovered that its level was reduced in WT tissues (Figure 1a). Also, the same trend of miR-15a-5p expression was found in the WT cell line (G-401) versus normal renal tubular epithelial cell line (HK-2) (Figure 1b). The finding indicated that miR-15a-5p was lowly expressed in WT.
Figure 1.
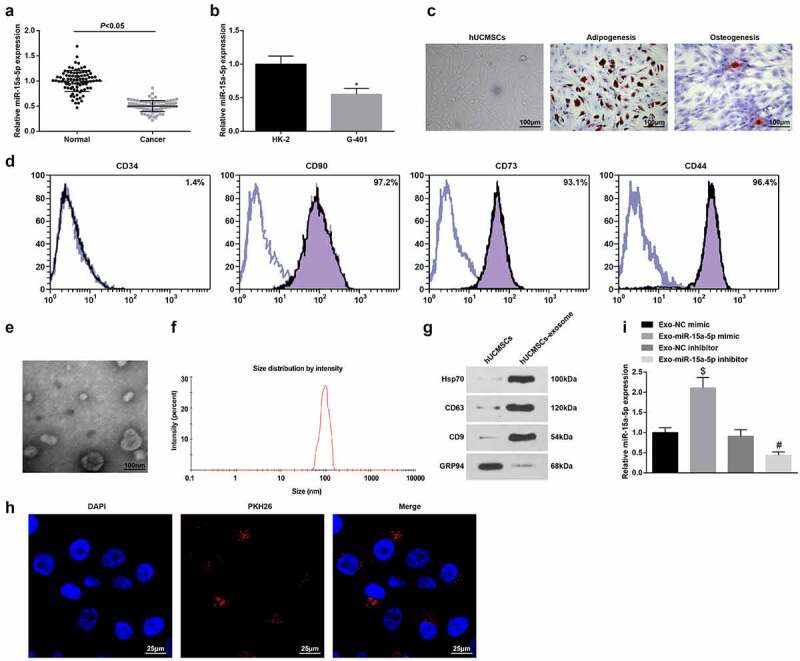
miR-15a-5p is downregulated in WT. A. RT-qPCR tested miR-15a-5p expression in WT tissues and normal tissues; B. RT-qPCR tested miR-15a-5p expression in normal renal tubular epithelial HK-2 cell line and human WT G-401 cells; C. Microscopic observation of hUC-MSCs, and staining of adipocytes and osteoblasts; D. Flow cytometry tested hUC-MSCs surface markers; E. TEM observation of hUC-MSCs-Exo; F. NTA detection of hUC-MSCs-Exo; G. Western blot tested HSP70, CD81, CD9 and GRP94 in hUC-MSCs-Exo; H. Internalization of PKH26-labeled exosomes (red) in G-401 cells; I. RT-qPCR tested miR-15a-5p expression in hUC-MSCs-Exo. Measurement data were shown by the mean ± standard deviation.
hUC-MSCs-Exo carry miR-15a-5p into G-401 cells
MSCs-Exo have been reported to protect against acute tubular injury [36] and could promote proximal tubular epithelial cell proliferation [37]. Therefore, the possible mechanism of hUC-MSCs-Exo involved in the biological functions of WT was explored.
Under a light microscope, elongated or spindle-shaped hUC-MSCs grew in groups and were closely arranged in a radial or parallel fashion, having strong osteogenic and adipogenic abilities (Figure 1c). Flow cytometry detecting the surface antigens of hUC-MSCs demonstrated that in hUC-MSCs of passage 3, CD44, CD73 and CD90 were positively expressed, while CD34 was negatively expressed (Figure 1d). Thus, MSCs were successfully isolated.
hUC-MSCs-Exo collected by differential centrifugation were verified TEM, Western blot and NTA. TEM showed that Exo were round and cup-shaped vesicles with a diameter of about 100 nm (Figure 1e). In addition, NTA traced that the average diameter of Exo was about 100 nm (Figure 1f). Western blot presented higher HSP70, CD81 and CD9 and lower GRP94 expression levels in hUC-MSCs-Exo (Figure 1g). It was proved that hUC-MSCs-Exo were successfully isolated.
PKH26-labeled exosomes were incubated with G-401 cells, and the uptake of exosomes was observed after co-culture for 12 h.T Through fluorescence microscopy, red fluorescence was detected in G-401 cells (Figure 1h) RT-qPCR found that miR-15a-5p was upregulated or downregulated in hUC-MSCs-Exo that had been transfected with miR-15a-5p mimic or inhibitor (Figure 1i).
hUC-MSCs-Exo impair the biological functions of G-401 cells
The above results indicated that hUC-MSCs-Exo delivered miR-15a-5p to G-401 cells and miR-15a-5p was downregulated in WT. To study the effects of hUC-MSCs-Exo on the biological functions of WT cells, hUC-MSCs-Exo were co-cultured with G-401 cells. Then, RT-qPCR revealed that hUC-MSCs-Exo could elevate miR-15a-5p expression in G-401 cells (Figure 2a). Next, the findings obtained from CCK-8 and colony formation assays, Transwell assay and flow cytometry implied that hUC-MSCs-Exo inhibited the proliferation, migration and invasion and induced apoptosis of G-401 cells (Figure 2b-f). It was believed that hUC-MSCs-Exo destructed the biological functions of WT cells.
Figure 2.
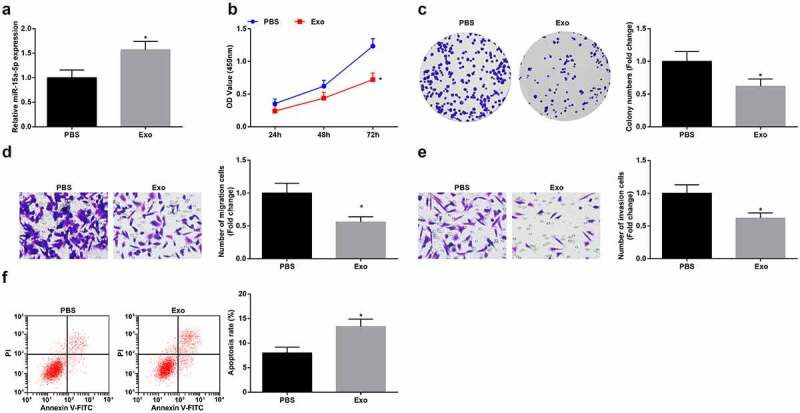
MSC-Exo impair the biological functions of G-401 cells. A. RT-qPCR tested miR-15a-5p in G-401 cells after co-culture with hUC-MSCs-EV; B. CCK-8 tested G-401 cell proliferation; C. Colony formation assay tested G-401 cell colony formation ability; D. Transwell assay tested G-401 cell migration; E. Transwell assay tested G-401 cell invasion; F. Flow cytometry tested G-401 cell apoptosis. Measurement data were shown by the mean ± standard deviation, * P < 0.05 vs the PBS group.
hUC-MSCs-Exo carrying miR-15a-5p lessen the tumorigenic properties of G-401 cells
To testify the effect of miR-15a-5p delivered by hUC-MSCs-Exo, we co-cultured G-401 cells with hUC-MSCs-Exo transfected with miR-15a-5p mimic or inhibitor. Subsequently, RT-qPCR measured that miR-15a-5p expression was raised or decreased in G-401 cells (Figure 3a). Next, cellular experiments exhibited that as to G-401 cells co-cultured with hUC-MSCs-Exo overexpressing miR-15a-5p, cell biological functions were impaired. hUC-MSCs-Exo inhibiting miR-15a-5p had the opposite effects on G-401 cell fate (Figure 3b-f). In summary, the delivery of miR-15a-5p by hUC-MSCs-Exo inhibited the growth of G-401 cells.
Figure 3.
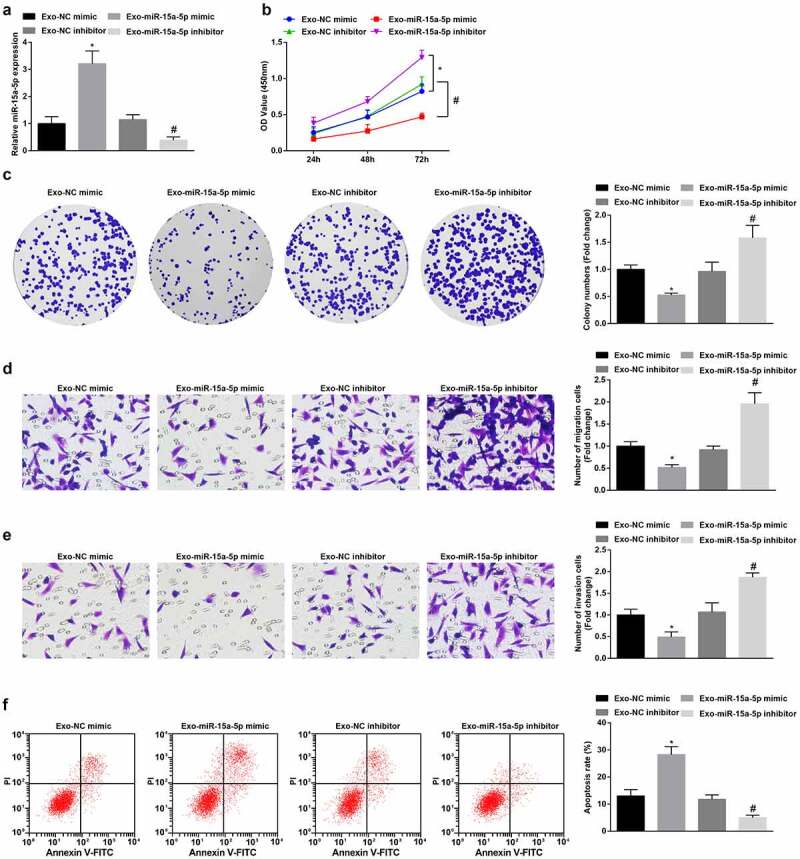
MSC-Exo carrying miR-15a-5p lessen the tumorigenic properties of G-401 cells. A. RT-qPCR tested miR-15a-5p in G-401 cells after co-culture with hUC-MSCs-Exo; B. CCK-8 tested G-401 cell proliferation; C. Colony formation assay tested G-401 cell colony formation ability; D. Transwell assay tested G-401 cell migration; E. Transwell assay tested G-401 cell invasion; F. Flow cytometry tested G-401 cell apoptosis. Measurement data were shown by the mean ± standard deviation, * P < 0.05 vs the Exo-NC mimic group; # P < 0.05 vs the Exo-NC inhibitor group.
miR-15a-5p targets SEPT2
Downregulation of SEPT2 activates the peroxisome proliferator-activated receptor γ to limit cell growth in liver cancer [17]. We tested SEPT2 at clinical and cellular levels by RT-qPCR and Western blot, and found that it was upregulated in WT tissues and cells (Figure 4a, b).
Figure 4.
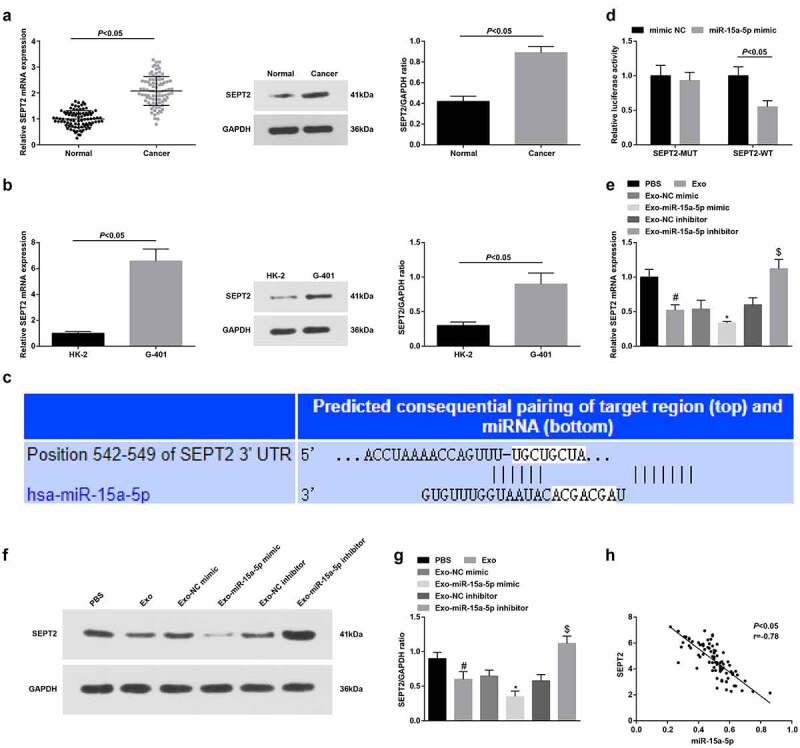
miR-15a-5p targets SEPT2. A-B. RT-qPCR and Western blot tested SEPT2 expression in tissues and cells; C. Targeting site between miR-15a-5p and SEPT2; D. Dual luciferase reporter gene assay tested the targeting of miR-15a-5p and SEPT2; E. Western blot tested SEPT2 expression in G-401 cells after co-culture with hUC-MSCs-Exo; F. Pearson analyzed the correlation between miR-15a-5p and SEPT2. Measurement data were shown by the mean ± standard deviation, # P < 0.05 vs the PBS group; * P < 0.05 vs the Exo-NC mimic group; $ P < 0.05 vs the Exo-NC inhibitor group.
The predicted data of TargetScan 7.2 suggested the target relationship between miR-15a-5p and SEPT2 (Figure 4c). Also, the dual luciferase reporter gene experiment verified that miR-15a-5p mimic transfection inhibited the luciferase activity of SEPT2-WT (Figure 4d).
Western blot indicated that hUC-MSCs-Exo could reduce SEPT2 levels in G-401 cells. Moreover, hUC-MSCs-Exo overexpressing miR-15a-5p or suppressing miR-15a-5p could lower or augment SEPT2 levels in G-401 cells (Figure 4e). Pearson correlation analysis further surveyed that miR-15a-5p was negatively correlated with SEPT2 in WT tissues (Figure 4f).
Inhibition of SEPT2 represses the biological function of WT cells
To study the effect of SEPT2 on WT cells, SEPT2 loss-of-function was performed by transfecting sh-SEPT2 into G-401 cells (Figure 5a), leading to the reduction of proliferation (Figure 5b, c), migration and invasion (Figure 5d, e) and induction of apoptosis (Figure 5f). The above results indicated that inhibition of SEPT2 reduces the biological function of WT cells.
Figure 5.
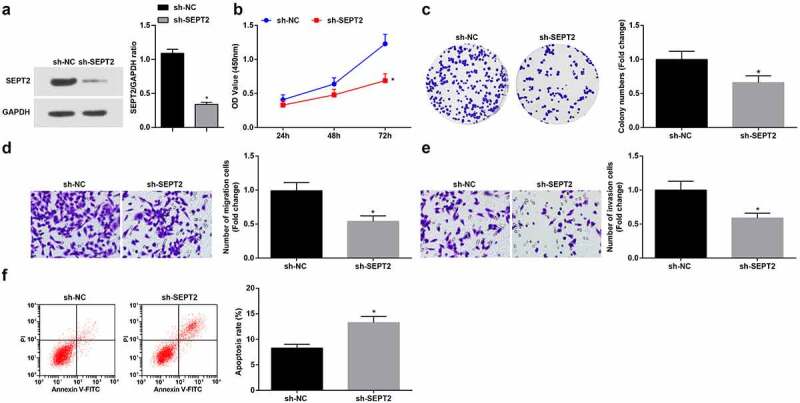
Inhibition of SEPT2 represses the biological function of WT cells. A. Western blot tested SEPT2 expression in G-401 cells transfected with sh-SEPT2; B. CCK-8 tested G-401 cell proliferation; C. Colony formation assay tested G-401 cell colony formation ability; D. Transwell assay tested G-401 cell migration; E. Transwell assay tested G-401 cell invasion; F. Flow cytometry tested G-401 cell apoptosis. Measurement data were shown by the mean ± standard deviation, * P < 0.05 vs the sh-NC group.
Upregulated SEPT2 reverses hUC-MSCs-Exo-mediated inhibition on G-401 cell growth
To further unravel the regulation of SEPT2 in hUC-MSCs-Exo on the biological functions of WT cells, we co-cultured G-401 cells with hUC-MSCs-Exo transfected with oe-SEPT2. The findings suggested that upregulated SEPT2 reverses hUC-MSCs-Exo mediated inhibition on G-401 cell growth (Figure 6a-f).
Figure 6.
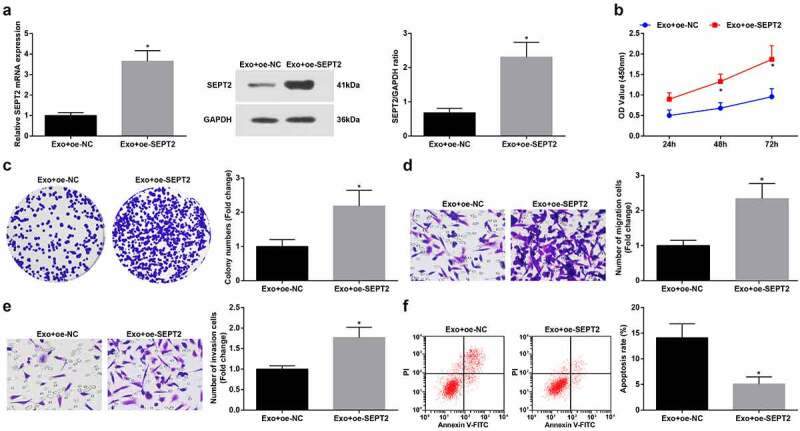
Upregulated SEPT2 reverses hUC-MSCs-Exo-mediated inhibition on G-401 cell growth. A. Western blot tested SEPT2 expression after co-culture of G-401 cells with hUC-MSCs-Exo; B. CCK-8 tested G-401 cell proliferation; C. Colony formation assay tested G-401 cell colony formation ability; D. Transwell assay tested G-401 cell migration; E. Transwell assay tested G-401 cell invasion; F. Flow cytometry tested G-401 cell apoptosis. Measurement data were shown by the mean ± standard deviation, * P < 0.05 vs the Exo+oe-NC group.
hUC-MSCs-Exo carrying miR-15a-5p weakens tumorigenicity of G-401 cells in vivo
Finally, tumor formation in vivo was carried out to decipher the mechanism of miR-15a-5p delivered by hUC-MSCs-Exo in WT. After 5 w for tumor growth, the tumor volume was increased in mice. It was found that the tumor volume and weight were reduced and apoptosis was increased in mice treated with hUC-MSCs-Exo. Furthermore, injection of hUC-MSCs-Exo overexpressing miR-15a-5p into mice further caused tumor growth suppression(Figure 7a, b).
Figure 7.
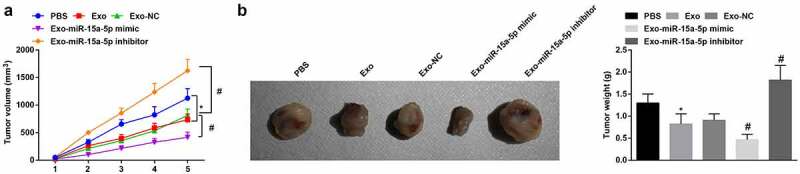
MSC-EV carrying miR-15a-5p weakens tumorigenicity of G-401 cells in vivo. A. Tumor volume change in nude mice; B. Tumors and tumor weight. Measurement data were shown by the mean ± standard deviation, * P < 0.05 vs the PBS group; # P < 0.05 vs the Exo group.
Discussion
WT stands for a neoplastic disease in children, second only to neuroblastoma in extracranial solid tumors [38]. Treatment with hUC-MSCs-Exo and miRNAs in combination has emerged to control cancer development. Thus, the reciprocal between hUC-MSCs-Exo and miR-15a-5p attracted our attention, and the underlying mechanism in WT was interpreted in part from exosomal miR-15a-5p/SEPT2 axis. The major outcome of the research outlined that miR-15a-5p encapsulated by hUC-MSCs-Exo curbed the malignant progression of WT, which was assisted by targeted suppression of SEPT2.
First, we examined miR-15a-5p expression in WT, finding a decreased trend of it in both WT tissues and cells. Showing the consistency with our study finding, prior studies have confirmed the reduction of miR-15a-5p expression in cancers, including lung cancer [13] and prostate cancer [31]. Next, we determined that hUC-MSCs-Exo themselves had the substantial abilities to hamper WT cell proliferation, invasion, migration and excite apoptosis, and we further clarified that when hUC-MSCs-Exo carrying elevated miR-15a-5p were introduced into WT cells, the repressive effects of hUC-MSCs-Exo on WT were aggrandized. In compliance with the experimental outcomes, a later paper has described that hUC-MSCs-Exo exert harshly as to the aggressive behaviors of BCa cells in vitro and tumor formation in vivo through transmitting miR-148b-3p [9]. In a similar manner, hUC-MSCs-Exo are applicable to encumber the tumorigenic capacities of esophageal squamous cell carcinoma cells via delivering elevated miR-375 [10]. Moreover, as implied by the current report, the introduction of hUC-MSCs-Exo overexpressing miR-145-5p to pancreatic ductal adenocarcinoma cells imposes the burden for cancer cells to proliferate and invade [39]. In addition to that, hUC-MSCs-Exo could raise miR-451a expression in HCC cells, thus to retard cell cycle transition, as well as other malignant phenotypes [40]. Suggested in an update article, hUC-MSCs-Exo establish a bridge for the communication of miR-139-5p and bladder cancer cells, and then benefit to ameliorate the tumorigenic characteristics of cancer cells [19]. All the listed studies have validated the contributory effects of hUC-MSCs-Exo carrying miRNAs in the retarded development of cancers, which further support our experimental discoveries to some extent.
As to the functional impacts of miR-15a-5p, research studies on cancers have figured out that miR-15a-5p may work as a tumor inhibitor. In Vitamin D3-treated liver cancer cells, the elevation of miR-15a-5p silences E2F transcription factor 3, so that the proliferation of cancer cells is impaired [41]. In HCC, if artificially raising miR-15a-5p expression in cancer cells, cellular proliferation would be suppressed and cell cycle arrest would be induced [42]. Mechanistically, in response to the knockdown of small nucleolar RNA host gene 12 in BCa cells, miR-15a-5p expression is enhanced which then functions to lower Sal-like 4 expression and to retard cell biological activities [43]. Similar to this, lncRNA-mediated upregulation of miR-15a-5p in papillary thyroid cancer is also capable of impairing tumorigenesis and progression [44], and the same mechanism concerning the elevation of miR-15a has been proved in WT [11]. These papers regarding miR-15a-5p have further confirmed the theory that miR-15a-5p suppresses carcinogenesis in various cancer types, including WT.
Also, in this paper, it was revealed that SEPT2 could be targeted by miR-15a-5p and upregulated SEPT2 reversed hUC-MSCs-Exo-mediated inhibition on G-401 cell growth, indicating the pro-tumor actions of SEPT2 in WT. As a matter of fact, in BCa migration and invasion processes, SEPT2 is required whilst depleting SEPT2 critically diminishes the aggressive functions of BCa cells [45]. The raised expression of SEPT2 has been detected in biliary tract cancer, and SEPT2 downregulated by miR-140-5p lessens cell proliferation [46]. In the case of colorectal cancer, the restoration of miR-744-5p directly reduces SEPT2 expression, after which cell proliferation would be disturbed, while apoptosis be accelerated [47]. Although non-coding RNAs could interact with SEPT2, many reports have explained other pathways involved in the interaction with SEPT2. SEPT2 affects GMB progression by controlling MEK/ERK activation and p53/p21 expression [17]. SEPT2 binds to the p85 subunit of PI3K, which subsequently recruits PI3K to the cell membrane and activates the PI3K/Akt signaling pathway [48]. Anyway, repression of SEPT2 in cancer cells contributes to a delay in cancer development, including WT as proved in the present work.
Conclusion
The data collected from the experiments prove that miR-15a-5p-loaded hUC-MSCs-Exo could repress WT progression through decreasing SEPT2. This research may create a niche in treating WT from the novel axis of exosomal miR-15a-5p/SEPT2. Due to the limitations of the current experimental conditions, the related signaling pathways were not discussed. In the future, if the experimental conditions allow, the pathway research will be carried out.
Supplementary Material
Funding Statement
The author(s) reported there is no funding associated with the work featured in this article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed here
References
- [1].Millar AJW, Cox S, Davidson A.. Management of bilateral Wilms tumours. Pediatr Surg Int. 2017;33(7):737–745. [DOI] [PubMed] [Google Scholar]
- [2].Xu S, Sun N, Zhang W-P, et al. Management of Wilms tumor with intravenous thrombus in children: a single center experience. World J Pediatr. 2019;15(5):476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Pater L, Melchior P, Rübe C, et al. Wilms tumor. Pediatr Blood Cancer. 2021;68(Suppl 2):e28257. [DOI] [PubMed] [Google Scholar]
- [4].Friedman AD. Wilms tumor. Pediatr Rev. 2013;34(7):328–330. discussion 330. [DOI] [PubMed] [Google Scholar]
- [5].Parsons LN. Wilms tumor: challenges and newcomers in prognosis. Surg Pathol Clin. 2020;13(4):683–693. [DOI] [PubMed] [Google Scholar]
- [6].Bianco P, Cao X, Frenette PS, et al. The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med. 2013;19(1):35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Vakhshiteh F, Atyabi F, Ostad SN. Mesenchymal stem cell exosomes: a two-edged sword in cancer therapy. Int J Nanomedicine. 2019;14:2847–2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Abbaszadeh H, Ghorbani F, Derakhshani M, et al. Human umbilical cord mesenchymal stem cell-derived extracellular vesicles: a novel therapeutic paradigm. J Cell Physiol. 2020;235(2):706–717. [DOI] [PubMed] [Google Scholar]
- [9].Yuan L, Liu Y, Qu Y, et al. Exosomes derived from MicroRNA-148b-3p-overexpressing human umbilical cord mesenchymal stem cells restrain breast cancer progression. Front Oncol. 2019;9:1076. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [10].He Z, Li W, Zheng T, et al. Human umbilical cord mesenchymal stem cells-derived exosomes deliver microRNA-375 to downregulate ENAH and thus retard esophageal squamous cell carcinoma progression. J Exp Clin Cancer Res. 2020;39(1):140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Su L, Wu A, Zhang W, et al. Silencing long non-coding RNA SNHG6 restrains proliferation, migration and invasion of Wilms’ tumour cell lines by regulating miR-15a. Artif Cells Nanomed Biotechnol. 2019;47(1):2670–2677. [DOI] [PubMed] [Google Scholar]
- [12].Wang ZM, Wan X-H, Sang G-Y, et al. miR-15a-5p suppresses endometrial cancer cell growth via Wnt/beta-catenin signaling pathway by inhibiting WNT3A. Eur Rev Med Pharmacol Sci. 2017;21(21):4810–4818. [PubMed] [Google Scholar]
- [13].Ni Y, Yang Y, Ran J, et al. miR-15a-5p inhibits metastasis and lipid metabolism by suppressing histone acetylation in lung cancer. Free Radic Biol Med. 2020;161:150–162. [DOI] [PubMed] [Google Scholar]
- [14].Chava S, Reynolds CP, Pathania AS, et al. miR-15a-5p, miR-15b-5p, and miR-16-5p inhibit tumor progression by directly targeting MYCN in neuroblastoma. Mol Oncol. 2020;14(1):180–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Liu F, Gu L, Cao Y, et al. Aberrant overexpression of EZH2 and H3K27me3 serves as poor prognostic biomarker for esophageal squamous cell carcinoma patients. Biomarkers. 2016;21(1):80–90. [DOI] [PubMed] [Google Scholar]
- [16].Xu C, Zhang W, Zhang X, et al. Coupling function of cyclin-dependent kinase 2 and Septin2 in the promotion of hepatocellular carcinoma. Cancer Sci. 2019;110(2):540–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Xu D, Liu A, Wang X, et al. Repression of Septin9 and Septin2 suppresses tumor growth of human glioblastoma cells. Cell Death Dis. 2018;9(5):514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Luo X, Dong J, He X, et al. MiR-155-5p exerts tumor-suppressing functions in Wilms tumor by targeting IGF2 via the PI3K signaling pathway. Biomed Pharmacother. 2020;125:109880. [DOI] [PubMed] [Google Scholar]
- [19].Jia Y, Ding X, Zhou L, et al. Mesenchymal stem cells-derived exosomal microRNA-139-5p restrains tumorigenesis in bladder cancer by targeting PRC1. Oncogene. 2021;40(2):246–261. [DOI] [PubMed] [Google Scholar]
- [20].Lee WJ, Jeon R-H, Jang S-J, et al. Selection of reference genes for quantitative gene expression in porcine mesenchymal stem cells derived from various sources along with differentiation into multilineages. Stem Cells Int. 2015;2015:235192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wu H, Mu X, Liu L, et al. Bone marrow mesenchymal stem cells-derived exosomal microRNA-193a reduces cisplatin resistance of non-small cell lung cancer cells via targeting LRRC1. Cell Death Dis. 2020;11(9):801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Tian Q, Yan X, Yang L, et al. lncRNA NORAD promotes hepatocellular carcinoma progression via regulating miR-144-3p/SEPT2. Am J Transl Res. 2020;12(5):2257–2266. [PMC free article] [PubMed] [Google Scholar]
- [23].Tsivion-Visbord H, Perets N, Sofer T, et al. Mesenchymal stem cells derived extracellular vesicles improve behavioral and biochemical deficits in a phencyclidine model of schizophrenia. Transl Psychiatry. 2020;10(1):305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zheng J, Lu T, Zhou C, et al. Extracellular vesicles derived from human umbilical cord mesenchymal stem cells protect liver ischemia/reperfusion injury by reducing CD154 expression on CD4+ T Cells via CCT2. Adv Sci (Weinh). 2020;7(18):1903746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Li X, Wang S, and Zhu R, et al. Lung tumor exosomes induce a pro-inflammatory phenotype in mesenchymal stem cells via NFκB-TLR signaling pathway. J Hematol Oncol. 2016;18(9):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hu JL, Wang W, Lan XL, et al. CAFs secreted exosomes promote metastasis and chemotherapy resistance by enhancing cell stemness and epithelial-mesenchymal transition in colorectal cancer. Mol Cancer. 2019;18(1):91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Qu L, Ding J, Chen C, et al. Exosome-transmitted lncARSR promotes sunitinib resistance in renal cancer by acting as a competing endogenous RNA. Cancer Cell. 2016;29(5):653–668. [DOI] [PubMed] [Google Scholar]
- [28].Yang D, Liu K, Fan L, et al. LncRNA RP11-361F15.2 promotes osteosarcoma tumorigenesis by inhibiting M2-Like polarization of tumor-associated macrophages of CPEB4. Cancer Lett. 2020;473:33–49. [DOI] [PubMed] [Google Scholar]
- [29].Xing Z, Li S, Liu Z, et al. The long non-coding RNA LINC00473 contributes to cell proliferation via JAK-STAT3 signaling pathway by regulating miR-195-5p/SEPT2 axis in prostate cancer. Biosci Rep. 2020;40(9). DOI: 10.1042/BSR20191850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Huang S, Xue P, Han X, et al. Exosomal miR-130b-3p targets SIK1 to inhibit medulloblastoma tumorigenesis. Cell Death Dis. 2020;11(6):408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wu H, Tian X, Zhu C. Knockdown of lncRNA PVT1 inhibits prostate cancer progression in vitro and in vivo by the suppression of KIF23 through stimulating miR-15a-5p. Cancer Cell Int. 2020;20(1):283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zong S, Zhao J, Liu L. miR-30d Induced Apoptosis by Targeting Sox4 to Inhibit the Proliferation, Invasion and Migration of Nephroblastoma. Onco Targets Ther. 2020;13:7177–7188. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [33].Zhao W, Qin P, Zhang D, et al. Long non-coding RNA PVT1 encapsulated in bone marrow mesenchymal stem cell-derived exosomes promotes osteosarcoma growth and metastasis by stabilizing ERG and sponging miR-183-5p. Aging (Albany NY). 2019;11(21):9581–9596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zhang K, Dong C, Chen M, et al. Extracellular vesicle-mediated delivery of miR-101 inhibits lung metastasis in osteosarcoma. Theranostics. 2020;10(1):411–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Liu S, Meng X. LINC00662 long non-coding RNA knockdown attenuates the proliferation, migration, and invasion of osteosarcoma cells by regulating the microRNA-15a-5p/Notch2 axis. Onco Targets Ther. 2020;13:7517–7530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Bruno S, Grange C, Deregibus MC, et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol. 2009;20(5):1053–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Tomasoni S, Longaretti L, Rota C, et al. Transfer of growth factor receptor mRNA via exosomes unravels the regenerative effect of mesenchymal stem cells. Stem Cells Dev. 2013;22(5):772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Pietras W. Advances and changes in the treatment of children with nephroblastoma. Adv Clin Exp Med. 2012;21(6):809–820. [PubMed] [Google Scholar]
- [39].Ding Y, Cao F, Sun H, et al. Exosomes derived from human umbilical cord mesenchymal stromal cells deliver exogenous miR-145-5p to inhibit pancreatic ductal adenocarcinoma progression. Cancer Lett. 2019;442:351–361. [DOI] [PubMed] [Google Scholar]
- [40].Xu Y, Lai Y, Cao L, et al. Human umbilical cord mesenchymal stem cells-derived exosomal microRNA-451a represses epithelial-mesenchymal transition of hepatocellular carcinoma cells by inhibiting ADAM10. RNA Biol. 2021;18(10):1408–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Li Y, Lin Q, Chang S, et al. Vitamin D3 mediates miR-15a-5p inhibition of liver cancer cell proliferation via targeting E2F3. Oncol Lett. 2020;20(1):292–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Long J, Jiang C, Liu B, et al. MicroRNA-15a-5p suppresses cancer proliferation and division in human hepatocellular carcinoma by targeting BDNF. Tumour Biol. 2016;37(5):5821–5828. [DOI] [PubMed] [Google Scholar]
- [43].Yuan JH, Li WX, Hu C, et al. Upregulation of SNHG12 accelerates cell proliferation, migration, invasion and restrain cell apoptosis in breast cancer by enhancing regulating SALL4 expression via sponging miR-15a-5p. Neoplasma. 2020;67(4):861–870. [DOI] [PubMed] [Google Scholar]
- [44].Jiang L, Wu Z, Meng X, et al. LncRNA HOXA-AS2 facilitates tumorigenesis and progression of papillary thyroid cancer by modulating the miR-15a-5p/HOXA3 axis. Hum Gene Ther. 2019;30(5):618–631. [DOI] [PubMed] [Google Scholar]
- [45].Zhang N, Liu L, Fan N, et al. The requirement of SEPT2 and SEPT7 for migration and invasion in human breast cancer via MEK/ERK activation. Oncotarget. 2016;7(38):61587–61600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Yu J, Zhang W, Tang H, et al. Septin 2 accelerates the progression of biliary tract cancer and is negatively regulated by mir-140-5p. Gene. 2016;589(1):20–26. [DOI] [PubMed] [Google Scholar]
- [47].Zhang W, Liao K, and Liu D. MicroRNA7445p is downregulated in colorectal cancer and targets SEPT2 to suppress the malignant phenotype. Mol Med Rep. 2021;23(1 54). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Garcia Z, Silio V, Marqués M, et al. A PI3K activity-independent function of p85 regulatory subunit in control of mammalian cytokinesis. EMBO J. 2006;25(20):4740–4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


