Abstract
Together, the fomA and fomB genes in the fosfomycin biosynthetic gene cluster of Streptomyces wedmorensis confer high-level fosfomycin resistance on Escherichia coli. To elucidate their functions, the fomA and fomB genes were overexpressed in E. coli and the gene products were characterized. The recombinant FomA protein converted fosfomycin to fosfomycin monophosphate, which was inactive on E. coli, in the presence of a magnesium ion and ATP. On the other hand, the recombinant FomB protein did not inactivate fosfomycin. However, a reaction mixture containing FomA and FomB proteins converted fosfomycin to fosfomycin monophosphate and fosfomycin diphosphate in the presence of ATP and a magnesium ion, indicating that FomA and FomB catalyzed phosphorylations of fosfomycin and fosfomycin monophosphate, respectively. These results suggest that the self-resistance mechanism of the fosfomycin-producing organism S. wedmorensis is mono- and diphosphorylation of the phosphonate function of fosfomycin catalyzed by FomA and FomB.
Fosfomycin is a medically important antibiotic produced by various species of Streptomyces (4), by Pseudomonas syringae (14), and by Pseudomonas viridiflava (9). It possesses unique structural features, including a carbon-phosphorus bond and an epoxide. As an analog of phosphoenolpyruvate (PEP), this compound irreversibly inhibits PEP UDP-N-acetylglucosamine-3-O-enolpyruvyltransferase (enolpyruvyltransferase), which catalyzes the first step of peptidoglycan biosynthesis (8). Fosfomycin does not inhibit, however, other enzymes using PEP and shows almost no toxicity in humans (1, 7).
Several clinical isolates resistant to fosfomycin had been found to have fosfomycin resistance genes on plasmids, such as fosA from Serratia marcescens (12) and fosB from Staphylococcus epidermidis (15). Both gene products catalyzed the irreversible addition of glutathione to fosfomycin (12). The deduced amino acid sequences of these gene products exhibit 36.6% identity to each other. On the other hand, to the best of our knowledge, the only precedent for self-resistance genes in the fosfomycin-producing organisms was fosC, found in P. syringae PB-5123 by Garcia et al. (2). They showed that its product inactivated fosfomycin in the presence of ATP. The inactivated products, however, were not well characterized. They also demonstrated that this inactivation was reversed by subsequent treatment with alkaline phosphatase.
Members of our group have cloned the fosfomycin biosynthetic gene cluster of Streptomyces wedmorensis (11) and identified four fosfomycin biosynthetic genes, fom1 to fom4 (6). In addition to these genes, encoding the enzymes necessary for fosfomycin biosynthesis, six open reading frames (ORFs), orfA to orfF, with unknown functions were found on a sequenced fragment including the fosfomycin gene cluster. Among these ORFs, orfA and orfB, contained together in a 2.9-kb fragment, gave Escherichia coli a high-level of resistance to fosfomycin, and fosfomycin was converted to inactivated forms in the presence of ATP by a crude extract from this transformant (10). Subsequently we isolated the inactivated compounds and determined their structures to be fosfomycin monophosphate and fosfomycin diphosphate. The genes orfA and orfB were thus renamed fomA and fomB, respectively. However, individual functions of the fomA and fomB gene products remained to be studied.
We show here that the fomA gene confers fosfomycin resistance on E. coli and that the fomA and fomB gene products catalyze phosphorylations of fosfomycin and fosfomycin monophosphate, respectively. We also describe detailed characterization of the purified recombinant fomA and fomB gene products overexpressed in E. coli.
MATERIALS AND METHODS
Growth media.
Luria-Bertani (LB) medium containing 50 μg of ampicillin per ml was used for pregrowth and growth of E. coli HB101 harboring pFBG1204, pFBG2204, pFBG1216, pFBG1221, or pUC118. The pregrowth medium for E. coli K-12 strain HW8235 was nutrient broth (Eiken, Tokyo, Japan). All E. coli strains were grown at 37°C. Strains and plasmids used are listed in Table 1.
TABLE 1.
Properties of bacterial strains and plasmids
| Strain or plasmid | Relevant characteristicsa | Source or reference |
|---|---|---|
| E. coli strains | ||
| HB101 | supE44 hsdS20 (rB− mB−) recA13 ara-14 proA2 lacY1 galK2 rpsL20 xyl-5 mtl-1 leuB6 thi-1 | Takara Shuzo |
| JM109 | recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 Δ(lac-proAB)/F′ (traD36 proAB+ lacIqZΔM15) | Takara Shuzo |
| M15 | lac ara gal mtl F−recA+ uvr+ | Qiagen |
| Plasmids | ||
| pFBG1204 | pUC118ΩBamHI-BglII 2.9 kb; Ampr | This study (Fig. 1) |
| pFBG2204 | pUC119ΩBamHI-BglII 2.9 kb; Ampr | This study (Fig. 1) |
| pFBG1216 | Deleted plasmid derived from pFBG1204; Ampr | This study (Fig. 1) |
| pFBG1221 | Deleted plasmid derived from pFBG1204; Ampr | This study (Fig. 1) |
| pUC118 | Vector; Ampr | Takara Shuzo |
| pUC119 | Vector; Ampr | Takara Shuzo |
| pREP4 | neo lac1; Kmr | Qiagen |
| pQE30 | Vector; Ampr | Qiagen |
| pQEFA | pQE30ΩfomA; Ampr | This study (see text) |
| pQEFB | pQE30ΩfomB; Ampr | This study (see text) |
Ampr, ampicillin resistant; Kmr, kanamycin resistant.
Identification of fosfomycin resistance gene(s).
In order to identify the resistance gene(s), DNA fragments derived from the 12.1-kb fragment containing fosfomycin biosynthetic genes were inserted downstream of the lacZ promoter in pUC118 or pUC119 and then introduced into E. coli HB101 as described by Hanahan (3). Each transformant mixture was spread on LB agar plates containing 50 μg of ampicillin per ml and 400 μg of fosfomycin per ml and then incubated at 37°C for 24 h.
Plasmid constructions.
Deletion plasmids, pFBG1216 and pFBG1221, derived from pFBG1204 (which includes the fomA and fomB genes) (Fig. 1) were constructed by using exonuclease III and mung bean nucleases as described by Henikoff (5). These deletion plasmids were introduced into E. coli HB101 as described by Hanahan (3) for further experiments.
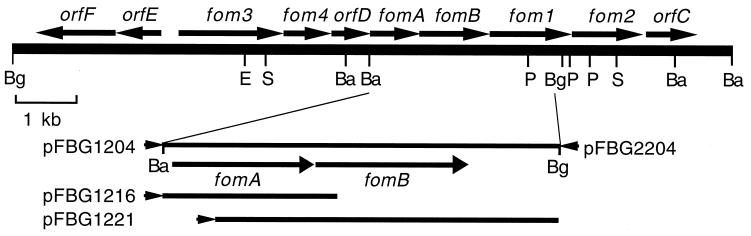
FIG. 1.
Fosfomycin biosynthetic gene cluster and deletion plasmids used to determine the ORFs necessary to confer fosfomycin resistance on E. coli HB101. Abbreviations for restriction sites: Ba, BamHI; Bg, BglII; E, EcoRI; P, PstI; S, SphI. Arrows indicate the direction of transcription from the lac promoter of pUC118 or pUC119 with respect to the inserted fragments.
Determination of MICs of fosfomycin.
E. coli HB101 harboring one of the plasmids mentioned above (pFBG1204, pFBG2204, pFBG1216, pFBG1221, and pUC118) was grown in LB medium containing 50 μg of ampicillin per ml with or without 6.25 to 800 μg/ml of fosfomycin at 37°C. Growth of each strain was measured by optical density at 660 nm.
DNA sequencing.
DNA sequencing was carried out using a DNA sequencer (model 4000L; Licor, Lincoln, Nebr.). Sequencing reactions were made with a Thermo Sequenase cycle sequencing kit (U.S. Biochemical Corporation, Cleveland, Ohio) using infrared-dye-labeled M13 forward (−29) primer (Nisshinbo, Tokyo, Japan) or infrared-dye-labeled M13 reverse primer (Nisshinbo).
Protein expression and purification of recombinant FomA and FomB.
On the basis of the entire nucleotide sequences of fomA and fomB genes from S. wedmorensis, two pairs of oligonucleotide primers, 5′-GGGGGATCCACGCCCGATTTCTTGGCC-3′ (5′ of the fomA gene) plus 5′-GGGGGATCCCGCAGAAGCAGTCGTGGTG-3′ (3′ of the fomA gene) and 5′-GGGGGATCCCTGGAAAACCTCACGATCCGC-3′ (5′ of the fomB gene) plus 5′-GGGGGATCCTTCGGCAAGCTGCTTGAGCGTC-3′ (3′ of the fomB gene), including BamHI restriction sites (underlined), were synthesized (Amersham Pharmacia, Uppsala, Sweden). Then, these pairs of primers were used with pFBG1204 to amplify the fomA or fomB gene. By using Taq DNA polymerase (Boehringer, Mannheim, Germany) and a standard PCR protocol, an 813-bp fragment for the fomA gene or a 1,009-bp fragment for the fomB gene was amplified. These PCR fragments were cleaved with BamHI and cloned into pUC118 (Takara Shuzo, Kyoto, Japan). The sequences of the cloned DNA fragments were confirmed with a Licor DNA sequencer. The coding region of the fomA or fomB gene was cloned into the expression vector pQE30 (Qiagen, Hilden, Germany) to yield pQEFA or pQEFB, respectively.
E. coli M15 containing pREP4 (Qiagen) was used as a host for expression of FomA. E. coli (pREP4) harboring pQEFA was cultured at 37°C in 100 ml of LB medium containing 25 μg of kanamycin per ml and 200 μg of ampicillin per ml for 5 h with 2 mM isopropyl-β-d-thiogalactopyranoside. Upon reaching an optical density at 660 nm of 0.8, cells were harvested by centrifugation and resuspended in 100 mM Tris-HCl (pH 7.5). After 5 min of sonication, the lysate was centrifuged at 10,000 × g for 20 min and the supernatant was collected. The supernatant was applied to a nickel-nitrilotriacetic acid (Ni-NTA)-agarose resin (Qiagen) column (7.5 by 10 mm). After a washing with 100 mM Tris-HCl buffer (pH 7.5) containing 50 mM imidazole, the protein which had bound to the Ni-NTA agarose resin was eluted with 100 mM Tris-HCl buffer (pH 7.5) containing 100 mM imidazole. The eluate was used as purified recombinant FomA in subsequent experiments. Expression and purification of recombinant FomB were done in a way similar to that for recombinant FomA, with replacement of pQEFA by pQEFB.
Identification of reaction products synthesized by the fomA or fomB gene product.
A reaction mixture containing the recombinant FomA protein, 5.4 mM ATP, 10 mM MgCl2, 6 mM KCl, and 5.5 mM fosfomycin was made up to 5 ml and incubated at 30°C for 3 h. One equivalent of methanol was added to the reaction mixture to stop the reaction, followed by centrifugation (15,000 × g, 10 min) to remove insoluble materials. The amount of fosfomycin remaining in the supernatant was quantitatively determined by bioassay against E. coli K-12 strain HW8235 (Meiji Seika Kaisha Ltd., Tokyo, Japan).
The supernatant (10 ml) was passed through an activated carbon column (15 by 27 mm) and, after dilution to 20 ml with H2O, subjected to Dowex 1-X8 (Cl− type, 15 by 55 mm) chromatography with elution with 2.0% NaCl (50 ml). The eluate was diluted twofold with water and lyophilized. After being dissolved in H2O, the sample was analyzed by 31P nuclear magnetic resonance (NMR) (JEOL [Tokyo, Japan] A-500 instrument).
Phosphorylation of fosfomycin monophosphate by FomB was done in a way similar to that for phosphorylation by FomA, with replacement of fosfomycin by 5 mM fosfomycin monophosphate.
Assay for FomA and FomB activities.
FomA and FomB activities were measured by a spectrophotometric method. The reaction mixture for the fosfomycin phosphorylation by FomA contained 100 mM sodium phosphate buffer (pH 7.0), 10 mM MgCl2, 10 mM dithiothreitol, 0.5 mM NADH, 1 mM PEP, 4.2 U of pyruvate kinase, 6.6 U of lactate dehydrogenase, and several concentrations of fosfomycin and ATP. The final volume of the reaction mixture was 200 μl. The FomA enzyme assay was conducted at 37°C on a Benchmark microplate reader (Bio-Rad). The background rate of NADH oxidation was measured for the reaction mixture without fosfomycin or ATP. The reaction rate was measured for 10 min. One unit of FomA protein was defined as the amount of protein required to inactivate 1 μmol of fosfomycin per min.
The effect of temperature on the enzymatic activity was investigated over the range of 20 to 50°C. The effect of pH on the enzymatic activity was investigated over the ranges of pH 5.5 to 7.0 with morpholineethanesulfonic acid (MES)-NaOH buffer, pH 6.8 to 7.8 with sodium phosphate buffer, and pH 7.0 to 9.0 with Tris-HCl buffer.
The Km for fosfomycin was determined using an enzyme assay system containing 4 mM Na2ATP2−, fosfomycin concentrations between 20 and 200 μM, and 10 μg of purified recombinant FomA. Data were analyzed on a Lineweaver-Burk plot, and the slope and x intercept were determined using a linear-regression computer program.
The Km for Na2ATP2− was determined using an enzyme assay system containing 4 mM fosfomycin, Na2ATP2− concentrations between 20 and 200 μM, and 10 μg of purified recombinant FomA. Data were analyzed as stated above for fosfomycin.
The reaction mixture for the FomB activity assay contained 100 mM sodium phosphate buffer (pH 7.0), 10 mM MgCl2, 10 mM dithiothreitol, 0.5 mM NADH, 1 mM PEP, 4.2 U of pyruvate kinase, 6.6 U of lactate dehydrogenase, and several concentrations of fosfomycin monophosphate (40 to 1,000 μM) and ATP (40 to 1,000 μM). The final volume of the reaction mixture was 200 μl. Enzyme assays were conducted in the same way as for FomA. The background rate of NADH oxidation was measured for the reaction mixture without fosfomycin monophosphate or ATP. The reaction rate was measured for 10 min. One unit of FomB protein was defined as the amount of protein required to produce 1 μmol of fosfomycin diphosphate per min.
The Km for fosfomycin monophosphate was determined using an enzyme assay system containing 4 mM Na2ATP2−, fosfomycin monophosphate concentrations between 40 and 1,000 μM, and 5 μg of purified recombinant FomB. Data were analyzed as stated above for FomA.
The Km for Na2ATP2− was determined using an enzyme assay system containing 2 mM fosfomycin, Na2ATP2− concentrations between 40 and 1,000 μM, and 5 μg of purified recombinant FomB. Data were analyzed as for fosfomycin monophosphate.
Nucleotide sequence accession number.
The DNA sequence of the fomA gene has been corrected (one nucleotide). The updated version is available in DDBJ/EMBL/GenBank under both the original accession number (D38561) and a new one (AB016934).
RESULTS
Identification of the fosfomycin resistance gene in the biosynthetic gene cluster.
Among tested plasmids, only pFBG1204 conferred fosfomycin resistance (>800 μg/ml) on E. coli. On the other hand, pFBG2204, with the same 2.9-kb BamHI-BglII fragment as pFBG1204 inserted in the opposite orientation relative to the direction of transcription of the lacZ promoter, did not provide fosfomycin resistance. Thus, fomA and/or fomB was judged to confer fosfomycin resistance on the host under the control of the lacZ promoter.
To determine the minimum region essential for fosfomycin resistance, deletion plasmids derived from pFBG1204 were constructed and then introduced into E. coli HB101. The resulting transformants were spread on LB agar plates containing various concentrations of fosfomycin. The MICs of fosfomycin for E. coli carrying pFBG1204, pFBG2204, pFBG1216, pFBG1221, and pUC118 were >800, <6.25, >800, <6.25, and <6.25 μg/ml, respectively. As can be seen from these results, a slight loss of fomA (pFBG1221) caused the complete disappearance of resistance, showing that fomA is essential for resistance. On the other hand, the loss of fomB (pFBG1216) did not influence resistance.
Individual function of the fomA and fomB gene products.
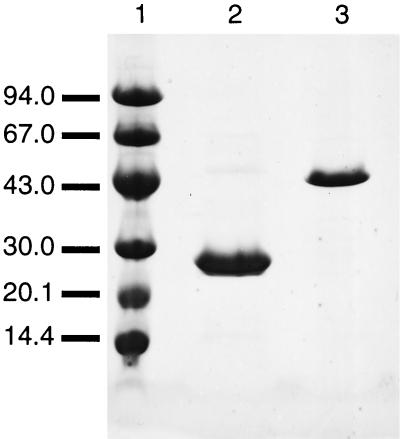
To elucidate the fomA and fomB gene functions, we constructed plasmids for the overexpression of the gene products followed by a one-step purification of the recombinant enzyme by Ni-NTA-agarose resin. Based on the total sequence of the fomA gene deposited in DDBJ/EMBL/GenBank under accession number D38561, we first constructed a FomA overexpression system. The overexpressed protein, however, did not inactivate fosfomycin under the conditions described previously (10). We then carried out resequencing of the fomA gene around the region corresponding to the N terminus and found an error, an omission of one base (see Materials and Methods). We constructed the FomA overexpression system again using primers described in Materials and Methods. The purified recombinant FomA and FomB proteins gave homogeneous protein bands on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with subunit sizes of 29 and 50 kDa, respectively (Fig. 2).
FIG. 2.
SDS-PAGE of purified FomA and FomB. Purified enzymes were analyzed by SDS-PAGE with 8 to 25% polyacrylamide gels. Lanes: 1, molecular mass standard; 2, SDS-treated FomA (1.7 μg); 3, SDS-treated FomB (0.5 μg). Proteins were stained with Coomassie brilliant blue R-250.
We had previously reported isolation and structural determination of the inactivated fosfomycin derivatives fosfomycin monophosphate and fosfomycin diphosphate, formed by a crude extract of E. coli carrying pFBG1204 containing both the fomA and fomB genes in the presence of ATP (10). To elucidate the function of FomA, we used a purified recombinant FomA protein instead of the crude extract of E. coli carrying pFBG1204. The reaction was carried out under the same condition as for pFBG1204. 31P NMR analysis revealed formation of fosfomycin monophosphate in the reaction mixture. Since fosfomycin diphosphate was not produced by the fomA gene product, the fomB product must convert fosfomycin monophosphate into fosfomycin diphosphate.
To confirm this hypothesis, purified recombinant FomA and FomB were mixed for fosfomycin inactivation experiments. Both fosfomycin monophosphate and fosfomycin diphosphate were detected in the reaction mixture after incubation. In addition, fosfomycin was not inactivated by the recombinant FomB protein alone under the same conditions. Attempts to characterize the reaction product of fosfomycin monophosphate and the FomB protein, however, were hampered by the unstable nature of fosfomycin monophosphate, which prevented preparation of the sample in a quantity large enough for NMR analysis.
Characterization of the fomA and fomB gene products.
The enzymatic properties of purified recombinant FomA are summarized along with those of FomB in Table 2. The results of SDS-PAGE and gel filtration suggested that FomA and FomB are a tetramer and a dimer, respectively.
TABLE 2.
Kinetic properties of the fomA and fomB gene products
| Parameter | Value for:
|
|
|---|---|---|
| FomA | FomB | |
| Molecular mass (kDa) as determined by: | ||
| Deduced amino acid sequence | 29 | 37 |
| SDS-PAGE | 29 | 50 |
| Gel filtration | 110 | 75 |
| Km (μM) for: | ||
| Fosfomycin | 150 | |
| Fosfomycin monophosphate | 680 | |
| ATP | 120 | 560 |
| Vmax (μmol/s) | 0.050 | 0.12 |
| Optimum temp (°C) | 45 | 37 |
| Optimum pH | 7.2 | 7.5 |
DISCUSSION
We have shown in this study that the fomA and fomB gene products phosphorylated fosfomycin to fosfomycin monophosphate and fosfomycin monophosphate to fosfomycin diphosphate, respectively. These results raise two questions about the self-resistance mechanism of the fosfomycin-producing organism. (i) How does the fosfomycin-producing organism convert phosphorylated fosfomycin into active fosfomycin and export it into the fermentation medium? (ii) Why does the fomB gene product catalyze the additional phosphorylation of biologically inactive fosfomycin monophosphate?
Several clues to answer these questions may be found in the following observations: while a large amount of fosfomycin accumulated in the fermentation medium, no phosphorylated fosfomycin was detected in S. wedmorensis cells (data not shown). In addition, phosphorylation and diphosphorylation of the phosphonate function in the fosfomycin molecule had proved to be reversible (10). A simple model for the export of fosfomycin may thus be speculated as follows. Fosfomycin biosynthesized in the cytosol is rapidly converted to biologically inactive fosfomycin monophosphate by FomA to confer self-resistance on the producing organism. Next, fosfomycin monophosphate is converted by FomB to fosfomycin diphosphate, which is an energy-rich molecule. Finally, fosfomycin diphosphate is exported into the fermentation medium as its free form, fosfomycin, by utilization of free energy liberated in the hydrolysis of the two phosphate bonds in the fosfomycin diphosphate molecule. In this model, the role of the first phosphorylation by FomA is inactivation of fosfomycin for self-resistance and that of the second phosphorylation by FomB is accumulation of free energy required to export fosfomycin diphosphate outside of the cell. This reversible self-resistance mechanism together with the efflux could be reasonable for fosfomycin-producing organisms. We are now trying to identify the phosphatase involved in the hydrolysis of fosfomycin diphosphate.
It has been reported that the fosC gene product of another fosfomycin-producing organism, P. syringae PB-5123, converted fosfomycin into an inactive compound by using ATP and that the inactive compound was reactivated by treatment with alkaline phosphatase. These results suggest that like the fomA gene product, the fosC gene product converts fosfomycin into fosfomycin monophosphate. Therefore, the fosC gene product, which seems to be fosfomycin phosphotransferase, should show similarity to the fomA gene product in amino acid sequence. However, the amino acid sequence of the fomA gene product, as corrected in this study, has only 25.8% identity to the fosC gene product. This low identity may suggest that the two fosfomycin-producing organisms, taxonomically quite different from each other, independently acquired the self-resistance mechanism against fosfomycin during evolution. On the other hand, no FomB homolog has yet been found in P. syringae PB-5123.
Suarez and Mendoza had proposed that the fosfomycin resistance genes, fosA and fosB, on plasmids isolated from clinical isolates were transferred horizontally from fosfomycin-producing organisms such as Streptomyces (13). The hypothesis was based on the following two findings. (i) The GC content ratios of the fosA and fosB genes are higher than those of the other genes on the chromosome and as high as those of actinomycetes. (ii) Most antibiotic-producing organisms have self-resistance genes.
Our finding that the self-resistance mechanism of the fosfomycin-producing S. wedmorensis is quite different from those of the clinical isolates, however, strongly indicates that clinical isolates independently acquired the fosfomycin resistance mechanism. In addition, irreversible inactivation of fosfomycin by addition of glutathione is not reasonable as the self-resistance mechanism, even for fosfomycin-producing organisms with high GC content ratios other than S. wedmorensis. The resistance mechanism of the fosfomycin-producing organisms may be found in clinical isolates in the future.
ACKNOWLEDGMENTS
This work was supported in part by a Grant-in-Aid for Encouragement of Young Scientists from The Ministry of Education, Science, Sports and Culture, Japan (09760114 to T.K.), and by Research Fellowships of the Japan Society for the Promotion of Science for Young Scientists (JSPS) to S.K.
REFERENCES
- 1.Christensen B G, Leanza W J, Beattie T R, Patchett A A, Arison B H, Ormond R E, Kuehl F A, Jr, Alders-Schonberg G, Jardetzky O. Phosphonomycin: structure and synthesis. Science. 1969;166:123–125. doi: 10.1126/science.166.3901.123. [DOI] [PubMed] [Google Scholar]
- 2.Garcia P, Arca P, Suarez J E. Product of fosC, a gene from Pseudomonas syringae, mediates fosfomycin resistance by using ATP as cosubstrate. Antimicrob Agents Chemother. 1995;39:1569–1573. doi: 10.1128/aac.39.7.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 4.Hendlin D, Stapley E O, Jackson M, Wallick H, Miller A K, Wolf F J, Miller T W, Chaiet L, Kahan F M, Foltz E L, Woodruff H B, Mata J M, Hernandez S, Mochales S. Phosphonomycin, a new antibiotic produced by strains of Streptomyces. Science. 1969;166:122–123. doi: 10.1126/science.166.3901.122. [DOI] [PubMed] [Google Scholar]
- 5.Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984;28:351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- 6.Hidaka T, Goda M, Kuzuyama T, Takei N, Hidaka M, Seto H. Cloning and nucleotide sequence of fosfomycin biosynthetic genes of Streptomyces wedmorensis. Mol Gen Genet. 1995;249:274–280. doi: 10.1007/BF00290527. [DOI] [PubMed] [Google Scholar]
- 7.Imai S, Seto H, Ogawa H, Satoh A, Otake N. Studies on the biosynthesis of fosfomycin. Conversion of 2-hydroxyethylphosphonic acid and 2-aminoethylphosphonic acid to fosfomycin. Agric Biol Chem. 1985;49:873–874. [Google Scholar]
- 8.Kahan F M, Kahan J S, Cassidy P J, Kropp H. The mechanism of action of fosfomycin. Ann N Y Acad Sci. 1974;235:364–385. doi: 10.1111/j.1749-6632.1974.tb43277.x. [DOI] [PubMed] [Google Scholar]
- 9.Katayama N, Tsubotani S, Nozaki Y, Harada S, Ono H. Fosfadecin and fosfocytocin, new nucleotide antibiotics produced by bacteria. J Antibiot. 1990;43:238–246. doi: 10.7164/antibiotics.43.238. [DOI] [PubMed] [Google Scholar]
- 10.Kuzuyama T, Kobayashi S, O'Hara K, Hidaka T, Seto H. Fosfomycin monophosphate and fosfomycin diphosphate, two inactivated fosfomycin derivatives formed by gene products of fomA and fomB from a fosfomycin producing organism Streptomyces wedmorensis. J Antibiot. 1996;49:502–504. doi: 10.7164/antibiotics.49.502. [DOI] [PubMed] [Google Scholar]
- 11.Kuzuyama T, Hidaka T, Imai S, Seto H. Studies on the biosynthesis of fosfomycin. 5. Cloning of genes for fosfomycin biosynthesis. J Antibiot. 1993;46:1478–1480. doi: 10.7164/antibiotics.46.1478. [DOI] [PubMed] [Google Scholar]
- 12.Mendoza C, Garcia J M, Llaneza J, Mendez F J, Hardisson C, Ortiz J M. Plasmid-determined resistance to fosfomycin in Serratia marcescens. Antimicrob Agents Chemother. 1980;18:215–219. doi: 10.1128/aac.18.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saurez J E, Mendoza M C. Plasmid-encoded fosfomycin resistance. Antimicrob Agents Chemother. 1991;35:791–795. doi: 10.1128/aac.35.5.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shoji J, Kato T, Hinoo H, Hattori T, Hirooka K, Matsumoto K, Tanimoto T, Kondo E. Production of fosfomycin (phosphonomycin) by Pseudomonas syringae. J Antibiot. 1986;39:1011–1012. doi: 10.7164/antibiotics.39.1011. [DOI] [PubMed] [Google Scholar]
- 15.Zilharo R, Courvalin P. Nucleotide sequence of the fosB gene conferring fosfomycin resistance in Staphylococcus epidermidis. FEMS Microbiol Lett. 1990;68:267–272. doi: 10.1016/s0378-1097(05)80052-7. [DOI] [PubMed] [Google Scholar]