Abstract
Inositol phosphorylceramide (IPC) synthase is an enzyme common to fungi and plants that catalyzes the transfer of phosphoinositol from phosphatidylinositol to ceramide to form IPC. The reaction is a key step in fungal sphingolipid biosynthesis and the target of the antibiotics galbonolide A, aureobasidin A, and khafrefungin. As a first step toward understanding the antifungal spectrum of IPC synthase inhibitors, we examined the sensitivity of IPC synthase to aureobasidin A in membrane preparations of Candida species (Candida albicans, C. glabrata, C. tropicalis, C. parapsilosis, and C. krusei) and Aspergillus species (Aspergillus fumigatus, A. flavus, A. niger, and A. terreus). As expected, preparations from the five Candida species, all exquisitely susceptible to aureobasidin A (MICs, <2 μg/ml), had IPC synthase activity (specific activity, 50 to 400 pmol/min/mg of protein) sensitive to aureobasidin A (50% inhibitory concentrations [IC50s], 2 to 4 ng/ml). Surprisingly, preparations from the four Aspergillus species, including A. fumigatus and A. flavus, which are intrinsically resistant to aureobasidin A (MICs, >50 μg/ml), had IPC synthase activity (specific activity, 1 to 3 pmol/min/mg of protein) also sensitive to aureobasidin A (IC50s, 3 to 5 ng/ml). The mammalian multidrug resistance modulators verapamil, chlorpromazine, and trifluoperazine lowered the MIC of aureobasidin A for A. fumigatus from >50 μg/ml to 2 to 3 μg/ml, suggesting that the resistance of this major fungal pathogen is the result of increased efflux.
The emergence of serious, often life-threatening fungal infections in the past decade, particularly in immunocompromised individuals such as human immunodeficiency virus, cancer, and organ transplant patients, has presented a tremendous medical challenge (1). Currently, there are only two antifungal classes available for the treatment of deep-seated fungal infections, the azoles and the polyenes (5). Azoles interfere with ergosterol biosynthesis at the C-14 demethylation step, cause accumulation of aberrant sterols, and thereby impair membrane functions (7). However, they are fungistatic and prone to resistance development, which limits their utility in severely immunocompromised patients (8). Polyenes, of which only amphotericin B has found wide clinical use. Bind to ergosterol in the plasma membrane and thereby disrupt membrane integrity, causing leakage of cytoplasmic contents and cell death (2). Amphotericin B, discovered in the 1950s, remains the broadest-spectrum fungicidal antibiotic and the “gold standard” for the treatment of systemic fungal infections despite its severe host toxicity (5).
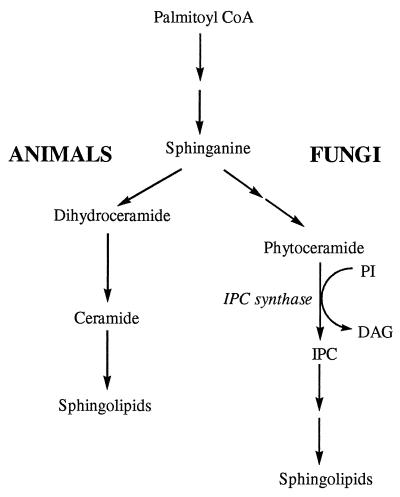
There is thus urgent medical need for novel fungicidal agents with high efficacy, lack of cross-resistance with existing agents, and low host toxicity. Compounds that target enzymes essential in fungi but absent in the mammalian host are particularly attractive. Such an enzyme is inositol phosphorylceramide (IPC) synthase of the fungal sphingolipid biosynthetic pathway (Fig. 1). It transfers the phosphoinositol group from phosphatidylinositol (PI) to the 1-hydroxy group of phytoceramide to form IPC (3). Recent studies have shown that IPC synthase is essential for fungal growth and is the target of aureobasidin A (11).
FIG. 1.
Sphingolipid biosynthesis in animals and fungi and location of IPC synthase within the fungal sphingolipid pathway. DAG, diacylglycerol; CoA, coenzyme A.
Aureobasidin A is a cyclic nonadepsipeptide produced by Aureobasidium pullulans (9). It has strong fungicidal activity against many pathogenic fungi, including Candida spp., Cryptococcus neoformans, and some Aspergillus spp., but not Aspergillus fumigatus, a major fungal pathogen (17). The AUR1 (aureobasidin A resistance) gene that encodes IPC synthase, however, has been detected in both Candida albicans and A. fumigatus (10). Therefore, it was important to elucidate the cause of the observed resistance of A. fumigatus to aureobasidin A. In this report, we describe the detection of IPC synthase activity in several Candida and Aspergillus species, its inhibition by aureobasidin A, and the effect of some mammalian multidrug resistance (MDR) modulators on fungal susceptibility to aureobasidin A.
MATERIALS AND METHODS
Materials.
C. albicans (ATCC 90028 and ATCC 24433), C. krusei (ATCC 14243), C. glabrata (ATCC 90030), C. parapsilosis (ATCC 22019), C. tropicalis (ATCC 750), A. fumigatus (ATCC 1022), A. flavus (ATCC 9643), A. niger (ATCC 9642), and A. terreus (ATCC 1012) were purchased form the American Type Culture Collection, Manassas, Va.) 6-[N-(7-Nitro-2,1,3-benzoxadiazol-4-yl)amino]hexanoyl ceramide (C6-NBD-cer) was obtained from Matreya Inc. (Pleasant Gap, Pa.); PI was from Avanti Polar Lipids (Alabaster, Ala.); aureobasidin A was from PanVera Corp. (Madison, Wis.); quinidine and reserpine were from Sigma Chemical Co. (St. Louis, Mo.); and chlorpromazine, trifluoroperazine, cyclosporin A, forskolin, nifedipine, and verapamil were from Calbiochem (San Diego, Calif.). Bradford reagent was from Pierce Chemical Co. (Rockford, Ill.); acetic acid (CH3COOH) and acetonitrile (CH3CN) (high-pressure liquid chromatography [HPLC] grade) were from J. T. Baker (Phillipsburg, N.J.). All other reagents were of the highest grade available commercially.
HPLC measurements were performed with a Waters 2690 Alliance System (Waters Corp., Milford, Mass.) using a C18 reversed-phase column (ZOBAX; 25 cm by 4.6 mm [inside diameter]; 5 μm; Hewlett-Packard, Wilmington, Del.).
Preparation of IPC synthase microsomal membrane from Candida and Aspergillus species.
Microsomal membranes from Candida species were prepared in accordance with a published procedure (4). Microsomal membranes from Aspergillus species were prepared as follows. Aspergillus cells from a frozen glycerol culture were streaked onto a potato agar slant and incubated at 35°C for 7 days. One milliliter of 0.85% saline was added to the slant, and the colony was gently scraped with a pipette tip. After the filaments settled down, the supernatant containing the conidia was transferred to another tube and about 50 μl of Tween 20 was added. The cell suspension was then added to 50 ml of Sabouraud liquid medium (2% dextrose, 1% peptone) and grown at 35°C for 24 h. A 20-ml volume of this culture was used to inoculate 1 liter of fresh Sabouraud medium, which was incubated at 35°C for 24 h. Cells were harvested by filtration through a sterile filter unit and resuspended in buffer (20 ml/liter of culture) containing 0.1 M NaCl, 0.05 M Tris-HCl (pH 8.0), 1 mM EDTA, 1 mM EGTA, 1 mM phenylmethylsulfonyl fluoride, 1 mM benzamidine, 1.5 μg of leupeptin per ml, and 3 μg of pepstatin A per ml. Cells were lysed using a Bead-beater (BioSpec Products, Bartlesville, Okla.) as follows. The cell suspension was poured into an ice-chilled chamber filled with 0.5 volume of 0.5-mm glass beads (BioSpec Products) and vortexed for 5 × 30 s with 30-s intervals between operations. After the beads settled down, the cell suspension was centrifuged at 2,000 × g for 15 min (4°C) to remove cell debris and nuclei. The supernatant was collected and further centrifuged at 20,000 × g for 15 min. The resulting supernatant was centrifuged at 100,000 × g for 1 h. The 100,000 × g pellet was resuspended in membrane storage buffer (0.25 M sucrose, 50 mM Tris-HCl [pH 7.0], 10% glycerol, 1 mM dithiothreitol) and, if not used immediately, stored at −80°C. Under these conditions, IPC synthase activity was stable for at least 3 months.
Determination of specific activity and IC50 of aureobasidin A with IPC synthase from Candida and Aspergillus species.
Activity of IPC synthase from different species was measured by a fluorometric HPLC assay using C6-NBD-cer as the substrate (18). Assay mixtures contained 0.1 mM C6-NBD-cer, 2 mM PI, and 1.0 mg of microsomal membranes per ml in a total volume of 50 μl. For the inhibition studies, aureobasidin A (50 to 1.6 ng/ml, corresponding to 50 to 1.6 nM aureobasidin A; stock solution, 1 mM in dimethyl sulfoxide) was preincubated with microsomal membranes for 5 min prior to substrate addition. Fifty percent inhibitory concentrations (IC50s) were defined as the concentrations that inhibited IPC synthase activity by 50%.
Susceptibility testing of Candida and Aspergillus species with aureobasidin A.
The MICs of aureobasidin A for Candida and Aspergillus species were obtained by broth microdilution following the National Committee for Clinical Laboratory Standards standard (12, 13). Final concentrations of aureobasidin A ranged from 0.05 to 50 μg/ml. The Candida inoculum was adjusted to concentrations of 5 × 102 to 2.5 × 103 cells per ml in RPMI 1640 medium (GIBCO Bethesda Research Laboratories, Rockville, Md.), and an aliquot of 0.1 ml was added to microtiter wells containing 0.1 ml of aureobasidin A solution in RPMI 1640 medium. The microtiter plate was then incubated at 35°C for 24 h, and the MIC of aureobasidin A was determined as the lowest concentration that completely inhibited growth. The Aspergillus inoculum was also adjusted in RPMI 1640 medium to concentrations of 0.4 × 104 to 5 × 104 CFU/ml. A 0.1-ml aliquot of this mixture was added to a microtiter well containing 0.1 ml of aureobasidin A solution in RPMI 1640 medium. The plate was incubated at 35°C for 48 h, and the aureobasidin A MIC was determined as the lowest concentration that completely inhibited growth.
RESULTS AND DISCUSSION
IPC synthase activity determination.
IPC synthase activity was determined using a recently developed fluorometric HPLC assay, and the results are summarized in Table 1. The activities observed for Candida species were comparable to those reported for Saccharamyces cerevisiae (11), while Aspergillus species showed far lower activity. This is the first report of the existence of a functional IPC synthase in Aspergillus spp. and complements the finding of an IPC synthase (AUR1) gene in A. fumigatus (10).
TABLE 1.
Specific activity of IPC synthase, its inhibition by aureobasidin A, and susceptibility to aureobasidin A in the presence and absence of verapamil in Candida and Aspergillus spp.
| Fungal species | IPC sp. act. (pmol/min/mg of protein) | Aureobasidin A
|
||
|---|---|---|---|---|
| IC50 (ng/ml) | MIC (μg/ml)
|
|||
| Minus verapamil | Plus verapamil (0.2 mM) | |||
| C. albicans | 134 | 2.1 | 0.8 | 0.2 |
| C. glabrata | 211 | 2.5 | 1.6 | NDa |
| C. tropicalis | 333 | 3.4 | 1.6 | ND |
| C. parapsilosis | 81 | 3.2 | 1.6 | ND |
| C. krusei | 42 | 3.5 | 1.6 | ND |
| A. fumigatus | 3.0 | 5.4 | >50 | 3.0 |
| A. flavus | 6.1 | 2.6 | >50 | >50 |
| A. terreus | 4.1 | 4.2 | 1.6 | 1.6 |
| A. niger | 1.0 | 4.2 | 0.8 | 0.8 |
ND, not determined.
In vitro inhibition of IPC synthase activity from Candida and Aspergillus species by aureobasidin A.
The IC50s of aureobasidin A were next determined for IPC synthase from several Candida and Aspergillus species (Table 1). In all of the species tested, including A. fumigatus and A. flavus, that were previously reported to be resistant to this compound (17), aureobasidin A strongly inhibited IPC synthase, with IC50s in the nanomolar range. This contrasts with the wide variation in susceptibility to aureobasidin A in this genus, ranging from 0.8 μg/ml for A. niger to greater than 50 μg/ml for A. fumigatus and A. flavus. The aureobasidin A resistance observed in the last two species may thus result from factors unrelated to the target, such as altered membrane transport.
Effects of MDR modulators on aureobasidin A MICs.
Aureobasidin A-resistant mutants of S. cerevisiae have been isolated. One of them expresses the ABC transporter gene YOR1 at high levels (14). Similar, transporter-mediated resistance has been reported for other antifungal agents, such as azoles (16). To investigate the possibility of increased efflux as the cause of the elevated MICs for A. fumigatus (15), the MIC of aureobasidin A was determined in the presence of various known mammalian MDR modulators (6) (Table 2). Of the compounds tested, only three potentiated aureobasidin A activity: verapamil, chlorpromazine, and trifluoroperazine. Verapamil, at 200 μM, lowered the MIC of aureobasidin A from >50 μg/ml to 3 μg/ml. The minimum verapamil concentration causing this effect was 50 μM. However, the compound had no effect on A. flavus (Table 1). Chlorpromazine at 25 μM and trifluoperazine at 10 μM also lowered the MIC of aureobasidin A from >50 μg/ml to 1.6 μg/ml. This finding strongly suggests that the resistance observed in A. fumigatus is due to increased efflux of aureobasidin A by some transporter(s), whose identity remains to be elucidated in future studies. Such studies would include (i) measurement of radiolabeled aureobasidin A accumulation in the presence and absence of verapamil to confirm that efflux is indeed involved and (ii) identification and deletion of a YOR1 homolog in A. fumigatus to confirm its involvement in the natural aureobasidin A resistance of this organism. Nonetheless, the present study firmly expands the potential antifungal spectrum of aureobasidin A, and possibly other IPC synthase inhibitors, to include Aspergillus pathogens.
TABLE 2.
Effects of mammalian MDR modulators on susceptibility of A. fumigatus to aureobasidin A
| Compound | Concn | MIC of aureobasidin A (μg/ml) |
|---|---|---|
| None | >50 | |
| Chlorpromazinea | 0.025 mM | 1.6 |
| Trifluoperazinea | 0.010 mM | 1.6 |
| Verapamil | 0.2 mM | 3 |
| Nifedipine | 0.2 mM | >50 |
| Cyclosporin A | 0.2 mM | >50 |
| Quinidine | 0.2 mM | >50 |
| Reserpine | 0.2 mM | >50 |
| Forskolin | 0.2 mM | >50 |
| Triton X-100 | 1.0% | >50 |
| Tween 20 | 1.0% | >50 |
Displayed antifungal activity at 0.2 mM.
ACKNOWLEDGMENTS
We thank Andy Slee and David Pompliano for their interest in and support of this work.
REFERENCES
- 1.Beck-Sague C, Jarvis W R. Secular trends in the epidemiology of nosocomial fungal infections in the United States, 1980–1990. National Nosocomial Infections Surveillance System. J Infect Dis. 1993;167:1247–1251. doi: 10.1093/infdis/167.5.1247. [DOI] [PubMed] [Google Scholar]
- 2.Bolard J. How do the polyene macrolide antibiotics affect the cellular membrane properties? Biochim Biophys Acta. 1986;864:257–304. doi: 10.1016/0304-4157(86)90002-x. [DOI] [PubMed] [Google Scholar]
- 3.Dickson R C. Sphingolipid functions in Saccharomyces cerevisiae: comparison to mammals. Annu Rev Biochem. 1998;67:27–48. doi: 10.1146/annurev.biochem.67.1.27. [DOI] [PubMed] [Google Scholar]
- 4.Fuanzukoff A, Rothblatt J, Schekman R. Analysis of polypeptide transit through yeast secretory pathway. Methods Enzymol. 1991;194:662–674. doi: 10.1016/0076-6879(91)94048-h. [DOI] [PubMed] [Google Scholar]
- 5.Georgopapadakou N H, Walsh T J. Antifungal agents: chemotherapeutic targets and immunologic strategies. Antimicrob Agents Chemother. 1996;40:279–291. doi: 10.1128/aac.40.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gottesman M M, Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- 7.Hitchcock C A. Cytochrome P-450-dependent 14 alpha-sterol demethylase of Candida albicans and its interaction with azole antifungals. Biochem Soc Trans. 1991;19:782–287. doi: 10.1042/bst0190782. [DOI] [PubMed] [Google Scholar]
- 8.Hitchcock C A. Resistance of Candida albicans to azole antifungal agents. Biochem Soc Trans. 1993;21:1039–1047. doi: 10.1042/bst0211039. [DOI] [PubMed] [Google Scholar]
- 9.Ikai K, Takesako K, Shiomi K, Moriguchi M, Umeda Y, Yamamoto J, Kato I, Naganawa H. Structure of aureobasidin A. J Antibiot (Tokyo) 1991;44:925–933. doi: 10.7164/antibiotics.44.925. [DOI] [PubMed] [Google Scholar]
- 10.Kuroda M, Hashida-Okado T, Yasumoto R, Gomi K, Kato I, Takesako K. An aureobasidin A resistance gene isolated from Aspergillus is a homolog of yeast AUR1, a gene responsible for inositol phosphorylceramide (IPC) synthase activity. Mol Gen Genet. 1999;261:290–296. doi: 10.1007/s004380050969. [DOI] [PubMed] [Google Scholar]
- 11.Nagiec M M, Nagiec E E, Baltisberger J A, Wells G B, Lester R L, Dickson R C. Sphingolipid synthesis as a target for antifungal drugs. Complementation of the inositol phosphorylceramide synthase defect in a mutant strain of Saccharomyces cerevisiae by the AUR1 gene. J Biol Chem. 1997;272:9809–9817. doi: 10.1074/jbc.272.15.9809. [DOI] [PubMed] [Google Scholar]
- 12.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of conidium-forming filamentous fungi. Proposed standard no. M38-P. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1998. [Google Scholar]
- 13.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard no. M27-A. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 14.Ogawa A, Hashida-Okado T, Endo M, Yoshioka H, Tsuruo T, Takesako K, Kato I. Role of ABC transporters in aureobasidin A resistance. Antimicrob Agents Chemother. 1998;42:755–761. doi: 10.1128/aac.42.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peery R B, Skatrud P L. Isolation of MDR-like genes from Aspergillus fumigatus and Aspergillus flavus. J Cell Biochem. 1995;19(Suppl.):159. [Google Scholar]
- 16.Sanglard D, Kuchler K, Ischer F, Pagani J I, Monod M, Bille J. Mechanisms of resistance to azole antifungal agents in Candida albicans isolates from AIDS patients involve specific multidrug transporters. Antimicrob Agents Chemother. 1995;39:2378–2386. doi: 10.1128/aac.39.11.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takesako K, Kuroda H, Inoue T, Haruna F, Yoshikawa Y, Kato I, Uchida K, Hiratani T, Yamaguchi H. Biological properties of aureobasidin A, a cyclic depsipeptide antifungal antibiotic. J Antibiot (Tokyo) 1993;46:1414–1420. doi: 10.7164/antibiotics.46.1414. [DOI] [PubMed] [Google Scholar]
- 18.Zhong W, Murphy D J, Georgopapadakou N H. Inhibition of yeast inositol phosphorylceramide synthase by aureobasidin A measured by a fluorometric assay. FEBS Lett. 1999;463:241–244. doi: 10.1016/s0014-5793(99)01633-6. [DOI] [PubMed] [Google Scholar]