ABSTRACT
Osteoarthritis (OA) affects approximately 12% of the aging Western population. The sirtuin/forkhead box O (SIRT/FOXO) signaling pathway plays essential roles in various biological processes. Despite it has been demonstrated that ubiquitin-specific protease 3 (USP3) inhibits chondrocyte apoptosis induced by interleukin (IL)-1β, the role of USP3/SIRT3/FOXO3 in the senescence of chondrocytes in OA is unclear. This study initially isolated articular chondrocytes and investigated the role of USP3 in IL-1β-induced senescence of chondrocytes. After USP3 was overexpressed or silenced by lentivirus, expressions of genes and proteins were detected using quantitative polymerase chain reaction and immunoblotting, respectively. Cell cycle analysis was performed using flow cytometry. Reactive oxygen species (ROS) levels and senescence were analyzed. Then, SIRT3 was inhibited or overexpressed to explore the underlying mechanism. We found that overexpression of USP3 hindered IL-1β-mediated cell cycle arrest, ROS generation, and chondrocyte senescence. The inhibition of SIRT3 blocked the protective effect of USP3 on cell senescence, whereas the overexpression of SIRT3 abolished USP3-silencing-induced cell senescence. Furthermore, SIRT3 attenuated cell senescence, probably by deacetylating FOXO3. USP3 upregulated SIRT3 to deacetylate FOXO3 and attenuated IL-1β-induced chondrocyte senescence. This study demonstrated that USP3 probably attenuated IL-1β-mediated chondrocyte senescence by deacetylating FOXO3 via SIRT3.
KEYWORDS: Osteoarthritis, senescence, ubiquitination, deacetylation, FOXO3, SIRT3
Introduction
Osteoarthritis (OA), a non-inflammatory disease resulting in articular cartilage deterioration, is characterized by joint pain, loss of mobility, joint deformity, and dysfunction[1]. OA affects approximately 12% of the aging population[2]. The most common pathological OA change is the degeneration or loss of articular cartilage, due to the aging process in chondrocytes. The matrix interleukin 1 beta (IL-1β) has been implicated as a key factor regulating OA [3–6]. Some research has revealed that IL-1β contributes to OA development by decreasing the synthesis of the key structural proteins, stimulating the synthesis of other cytokines like tumor necrosis factor-alpha (TNF-α) and IL-6, and promoting the secretion of tissue inflammatory effectors cyclooxygenase-2 and phospholipase A2 [7–9]. Furthermore, studies have shown that chondrocytes subjected to IL-1β treatment tend to age more rapidly and induce apoptosis [10,11].
Oxidative stress is a situation in which cellular levels of reactive oxygen species (ROS) exceed its antioxidant capacities, and accumulation of damage inflicted by ROS is currently widely accepted as a cause of aging [12,13]. High concentrations of ROS directly induce deoxyribonucleic acid (DNA) damage, contributing to aging[14]. Dysregulation of p16 and p21 gene expression is implicated in oxidative stress-induced cell senescence and aging [15,16]. The activation of p21 gene leads to cell cycle arrest [17,18].
The sirtuin (SIRT) family plays essential roles in various biological processes including gene silencing, anti-stress responses, apoptosis, aging, and inflammation [19–21]. A study has demonstrated that SIRT1 depletion can promote senescence of endothelial cells and podocytes[22], whereas the upregulation of SIRT1 can deacetylate p53 and then inhibit the senescence of endplate chondrocytes[23]. Moreover, overexpression of SIRT6 is demonstrated to inhibit chondrocyte aging and suppress nuclear factor kappa B (NF-κB)-mediated inflammation in OA[24]. The overexpression of SIRT3 suppresses hyperglycemia-induced senescence of human diploid fibroblasts by deacetylating FOXO1 and increasing catalase and manganese superoxide dismutase (MnSOD) in WI-38 cell and is implicated as a potential target to treat diseases of senescence[25]. In addition, studies have shown that SIRT3 can protect the mitochondria against oxidative damage by regulating FOXO3 deacetylation, and the mitochondrial oxidative stress and mtDNA damage in osteoblasts can be induced by inhibiting the SIRT1-FoxO3a-MnSOD pathway in osteoporosis [26–28]. Despite the importance of SIRT3/FOXO3 signaling has been well-recognized, its role in chondrocyte aging needs to be elucidated.
Our previous studies have shown that USP3 suppresses chondrocyte apoptosis induced by IL-1β through the de-ubiquitination of TNF receptor-associated factor 6 (TRAF6)[29]. Since the pathological OA change is correlated to the degeneration or loss of articular cartilage, the upregulation of SIRT1 can inhibit the senescence of endplate chondrocytes. In addition, SIRT3 protects the mitochondria against oxidative damage. It is hypothesized that the USP3 can inhibit the IL-1β induced chondrocytes senescence via SIRT3. However, the function of USP3 in the senescence of chondrocytes in IL-1β-induced OA is to be fully explored. Therefore, this study aims at investigating the potential roles of USP3/SIRT3/FOXO3 in IL-1β-induced chondrocyte senescence.
Materials and methods
Cell isolation and culture
Knee joints of Sprague-Dawley (SD) rats were prepared and articular chondrocytes were subsequently isolated and identified as previously described[29]. The collected cells were cultured in DMEM/F12 (Sigma-Aldrich, China) with 10% fetal bovine serum (Invitrogen, China) and 1× Pen–Strep (Beyotime, China). The experiments of the current research gained approval from the Ethics Committee of the First Affiliated Hospital of Soochow University.
Plasmid construction and cell transfection
SIRT3 coding sequence was synthesized employing primers containing EcoR I and BanH I restriction enzyme cutting sites and subsequently incorporated to pLVX-Puro to improve the expression of SIRT3, including SIRT3-F: 5′-CGGAATTCATGGTGGGGGCTGGCATC-3′, and SIRT3-R: 5′-CGGGATCCTTATCCGTCCTGTCCATCCAG-3′. Overexpression (oeUSP3) or silence (siUSP3) of USP3 was performed as previously described[29].
Quantitative polymerase chain reaction (qPCR)
Ribonucleic acid (RNA) samples were obtained from treated cells by supplying TRIzol reagent and subsequent reverse transcription was performed to complementary DNA (cDNA) using Superscript II (Invitrogen, China). cDNA was amplified based on the following primers employing SYBR Green Master Mix (Thermo Fisher Scientific, China) (Table 1). Relative fold changes were quantified using the 2−ΔΔCt formula[30].
Table 1.
PCR primer sequences
| Gene | Sequence (5′–3′) |
|---|---|
| USP3 (NM_001025424.1) | CCTGACCATGATTCATCTAC |
| GTTACTACCACTGACCAAAC | |
| SIRT3 (NM_001106313.2) | CAGTATGACATCCCGTACCC |
| TGTGTAGAGCCGCAGAAG | |
| GAPDH (NM_017008.4) | GGAGTCTACTGGCGTCTTCAC |
| ATGAGCCCTTCCACGATGC |
Immunoblotting and co-immunoprecipitation assays
Proteins were extracted by adding Immunoprecipitation (IP) Lysis Buffer (20 mM Tris-HCl, pH 7.6; 150 mM NaCl; 1 mM EDTA; 0.5% NP-40; 10% glycerol; 1 mM PMSF; protease inhibitor cocktail), incubated with anti-USP3 (ab80597, Abcam) and anti-SIRT3 antibodies (#5490, CST), and followed by overnight incubation with Protein A/G beads. Rabbit immunoglobulin G (IgG) (Santa Cruz Biotechnology, USA) was used as a control. The beads were washed, re-suspended in a loading buffer, and boiled.
For immunoblotting, proteins were isolated using RIPA lysis solution (Thermo Fisher Scientific, China) and resolved following the addition of SDS-PAGE. The proteins were subsequently transferred to a polyvinylidene fluoride membrane, sealed in 5% skimmed milk, and incubated with optimally diluted primary and secondary antibodies sequentially. After development with ECL luminous fluid (WBKLS0100, Millipore) for 5 min, protein bands were assessed using an imager (Bio-Rad, China)[31]. Antibody information was as follows: anti-USP3 (1:500, Ab82935), anti-SIRT2 (1:1 000, Ab19388), anti-SIRT3 (1:500, Ab189860), anti-SIRT6 (1:5 000, Ab191385), anti-P21 (1:1 000, Ab109199), anti-FOXO3 (1:3 000, Ab17026), anti-Ac-FOXO3 (1:500, Ab47285), anti-catalase (1:1 000, Ab52477), and anti-MnSOD (1:3 000, Ab13533). All were purchased from Abcam. Anti-SIRT1 (1:1 000, 13,161-1-AP) and anti-P16 (1:600, 10,883-1-AP) were purchased from Proteintech. Anti-Cyclin E1 (1:1 000, #20,808), anti-CDK2 (1:1 000, #2546), and anti-β-actin (1:1 000, #4970) were purchased from CST.
ROS detection
ROS levels were detected using an Active Oxygen Detection Kit (S0033, Beyotime, China). Cells were treated, collected, and re-suspended supplemented with 1 mL of cooled PBS containing 10 µM DCFH-DA at 37°C in the dark for 20 min. The cells were tossed and mixed with the solution every 5 min. Following three cycles of washing with serum-free medium, ROS levels were determined using a flow cytometer (BD, Franklin Lakes, NJ)[32].
Cell cycle profile analysis
Forty-eight hours after treatment, cells were fixed in 70% ice-cold ethanol and then incubated with ribonuclease A (0.1 mg/mL, Sigma) and propidium (PI) (0.05 mg/mL, Sigma) at 25°C for 30 min. DNA content was analyzed using the BD flow cytometer (Franklin Lakes, NJ)[33].
Senescence-associated β-galactosidase (SA-β-gal) assay
Chondrocytes were stained using a Senescence β-gal Staining Kit (C0602, Beyotime, China) following the manufacturer’s instructions. Briefly, the cells were initially fixed and incubated with mixed staining solution in a CO2-free dry incubator at 37°C for 12 h. Subsequently, positive (blue) cell ratios of five random fields were counted in all assays using a 100× magnification bright-field microscope [34]. For each group, three pictures were taken and the positive cells dyed blue were quantified by Image J software (National Institutes of Health, Bethesda, MD).
Statistical analysis
All data were analyzed using Prism 7.0 (GraphPad, San Diego, CA) and presented as mean ± standard deviation. Student’s t-tests or one-way analysis of variance were performed for comparison using Tukey’s post-hoc tests. P values less than 0.05 were considered statistical significance.
Results
Multiple methods and technologies were performed to investigate the role of USP3 in IL-1β-induced senescence of isolated articular chondrocytes. The gene and protein expressions were detected using quantitative polymerase chain reaction and immunoblotting when USP3 was overexpressed or silenced by lentivirus in chondrocytes. The cell cycle profile was analyzed using a flow cytometer. Reactive oxygen species (ROS) levels were measured. The overexpression of USP3 was expected to hinder IL-1β-mediated cell cycle arrest, ROS generation, and chondrocyte senescence. The underlying mechanism and vital role of USP3 in chondrocyte senescence were explored.
Overexpression of USP3 attenuates IL-1β-mediated cell senescence
To investigate whether USP3 could affect chondrocyte senescence, USP3 was overexpressed in rat primary chondrocytes using lentivirus. Transfected cells were treated with 10 ng/mL recombinant IL-1β protein. The results revealed that IL-1β markedly arrested the progression of cell cycles at the G1 phase, which was potently abolished by USP3 overexpression (Figure 1a). In addition, the overexpression of USP3 abolished IL-1β-induced ROS production (Figure 1b) and cell senescence (Figure 1c). Western blotting indicated that IL-1β largely decreased USP3, Cyclin E1, and CDK2 while increased p16 and p21, and all were blocked following overexpression of USP3 (Figure 1d). The results indicated that overexpressing USP3 attenuated IL-1β-caused chondrocyte senescence.
Figure 1.
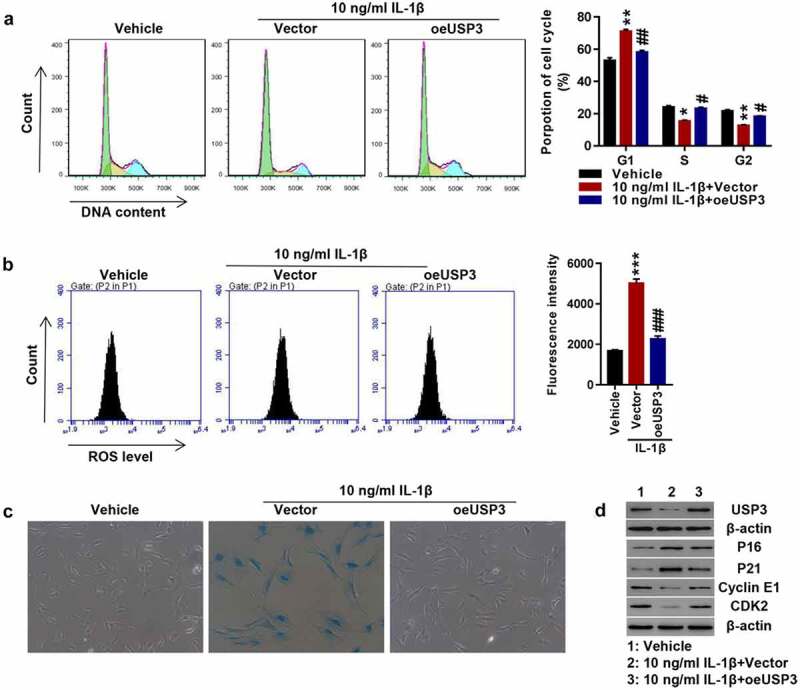
Overexpression of USP3 attenuated IL-1β-mediated chondrocyte senescence.
The primary chondrocytes were transfected with USP3 over expression lentivirus or control vector virus (Vector) in the presence of recombinant IL-1β protein or vehicle. (A) Cell cycle detection using flow cytometry. (B) ROS level detection using flow cytometry. (C) Cell senescence shown by SA-β-Ga1 staining. (D) Levels of USP3, p16, p21, Cyclin E1, and CDK2 detected by Western blotting. Each data shows mean ± standard deviation from three independent biological experiments. Compared with vehicle, *p < 0.05; **p < 0.01; ***p < 0.001. And compared with IL-1β + vector, #p < 0.05; ##p < 0.01; ###p < 0.001.
USP3 regulates SIRT3 expression by inhibiting SIRT3 ubiquitination
As SIRT1, SIRT2, SIRT3, and SIRT6 are family members of SIRT, we detected the expressions of the mentioned anti-aging members and found that recombinant IL-1β proteins significantly inhibited SIRT1, SIRT2, SIRT3, and SIRT6 protein expressions. USP3 overexpression significantly increased SIRT3 at the protein level (Figure 2a) but it did not affect SIRT3 mRNA levels (Figure 2b). Silencing USP3 significantly reduced SIRT3 proteins but did not have a significant effect on SIRT3 mRNA (Figure 2c–2d). The findings indicated that USP3 regulated SIRT3 at the protein level. Co-IP showed that USP3 interacted with SIRT3 (Figure 2e), and the overexpression of USP3 inhibited SIRT3 ubiquitination (figure 2f). This suggested that USP3 alleviated IL-1β-induced senescence of rat chondrocytes by inhibiting SIRT3 ubiquitination, which was consistent with the finding that USP3 expression was positively correlated with SIRT3 expression in OA cartilage tissue (Figure 2g).
Figure 2.
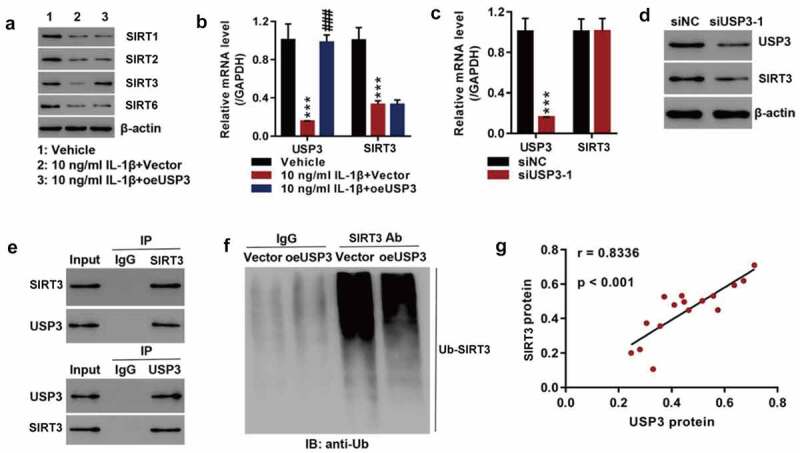
USP3 regulated SIRT3 expression probably by inhibiting SIRT3 ubiquitination.
The primary chondrocytes were transfected with USP3 over expression lentivirus or control vector virus (Vector) in the presence of recombinant IL-1β protein or vehicle. (A) Levels of SIRT1, SIRT2, SIRT3, and SIRT6 were detected using Western blotting. (B) mRNA levels of USP3 and SIRT3 were measured using qPCR. (C) mRNA levels of USP3 and SIRT3 were detected using qPCR after silencing of USP3. (D) Protein levels of USP3 and SIRT3 were determined by Western blotting after silencing of USP3. (E) Co-IP assay of the interaction between USP3 and SIRT3. (F) SIRT3 ubiquitination assay. (G) Correlation analysis between USP3 and SIRT3 in 16 OA cartilage samples (r = 0.8336, P < 0.001). Each data shows mean ± standard deviation from three independent biological experiments. ***p < 0.001 vs vehicle or siNC; ###p < 0.001 vs. IL-1β + vector.
Inhibition of SIRT3 suppresses the effect of USP3 overexpression in cell senescence
To investigate the role of SIRT3 in IL-1β-induced chondrocyte senescence, the USP3-overexpressing cells were treated with IL-1β protein along with SIRT3 inhibitor 3-TYP. USP3 overexpression abolished IL-1β-induced cell cycle arrest. The inhibition of SIRT3 blocked the effects of USP3 overexpression on cell cycles (Figure 3a). Furthermore, the inhibition of SIRT3 abolished the suppressive effect of USP3 overexpression on IL-1β-induced ROS production (Figure 3b) and cell senescence (Figure 3c). The administration of SIRT3 inhibitor 3-TYP reversed the effects of USP3 on IL-1β-induced decrease in USP3, Cyclin E1, and CDK2 and increase in p16 and p21 (Figure 3d). These data suggested that SIRT3 inhibition suppressed the protection of USP3 overexpression on IL-1β-induced chondrocyte senescence.
Figure 3.
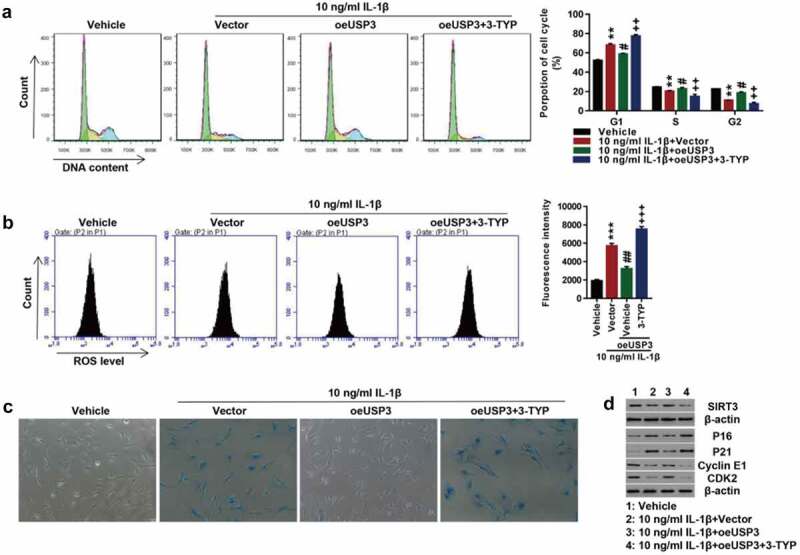
Inhibition of SIRT3 suppressed the effect of USP3 overexpression on IL-1β-mediated cell senescence.
Rat primary chondrocytes following USP3 overexpression were treated with IL-1β protein (10 ng/mL) along with SIRT3 inhibitor 3-TYP (50 μM). (A) Cell cycle detection by flow cytometry. (B) ROS levels by flow cytometry. (C) Cell senescence shown by SA-β-Ga1 staining. (D) Expressions of USP3, p21, p16, Cyclin E1, and CDK2 detection using Western blotting. Each data shows mean ± standard deviation from three independent biological experiments. **p < 0.01; ***p < 0.001 compared with vehicle; #p < 0.05; ##p < 0.01 compared with IL-1β + vector; ++p < 0.01; +++p < 0.001 compared with IL-1β + vector.
Overexpression of SIRT3 attenuates USP3 interfere-induced cell senescence
To further investigate SIRT3 action on IL-1β-induced senescence, SIRT3 was successfully overexpressed at both mRNA and protein levels in primary chondrocytes of the rats (Figure 4a-b). Efficiency of USP3-silencing was shown in Figure 2c-D. Data showed that USP3 silencing significantly arrested cell cycle progression at phase G1, abolished following SIRT3 overexpression (Figure 4c). Furthermore, SIRT3 overexpression abolished USP3-silencing-induced ROS production (Figure 4d) and cell senescence (Figure 4e). In addition, USP3 silencing significantly decreased SIRT3, Cyclin E1, CDK2, catalase, and Mn-SOD and increased p16, p21, and Ac-FOXO3, which were blocked by SIRT3 overexpression (figure 4f). The results indicated that the overexpression of SIRT3 attenuated USP interfere-induced cell senescence.
Figure 4.

Overexpression of SIRT3 attenuated USP interfere-induced cell senescence.
SIRT3 was overexpressed in USP3-silencing rat primary chondrocytes. (A–B) Confirmation of SIRT3 overexpression using qPCR (A) and Western blotting (B) ***p < 0.001 vs. vector. (C) Cell cycle detection by flow cytometry. (D) ROS levels measured by flow cytometry. (E) Cell senescence shown by SA-β-Ga1 staining. (F) Expressions of SIRT3, p16, p21, Cyclin E1, CDK2, FOXO3, Ac-FOXO3, catalase, and Mn-SOD detected using Western blotting. Each data shows mean ± standard deviation from three independent biological experiments. *p < 0.05; **p < 0.01; ***p < 0.001 compared with siNC; #p < 0.05; ##p < 0.01; ###p < 0.001 compared with siUSP3-1 + vector.
Overexpression of SIRT3 attenuates chondrocyte senescence probably by deacetylating FOXO3
To study how SIRT3 regulates cell senescence, IL-1β was used to treat rat SIRT3-overexpressing primary chondrocytes. The overexpression of SIRT3 potently reversed IL-1β recombinant protein-arrested cell cycle progression (Figure 5a). In addition, IL-1β-induced ROS production (Figure 5b) and cell senescence (Figure 5c) were blocked by SIRT3 overexpression. Furthermore, IL-1β treatment caused significant downregulation of SIRT3, FOXO3, catalase, Mn-SOD, Cyclin E1, and CDK2 and upregulation of Ac-FOXO3, p16, and p21, which were blocked by SIRT3 overexpression (Figure 5d).
Figure 5.
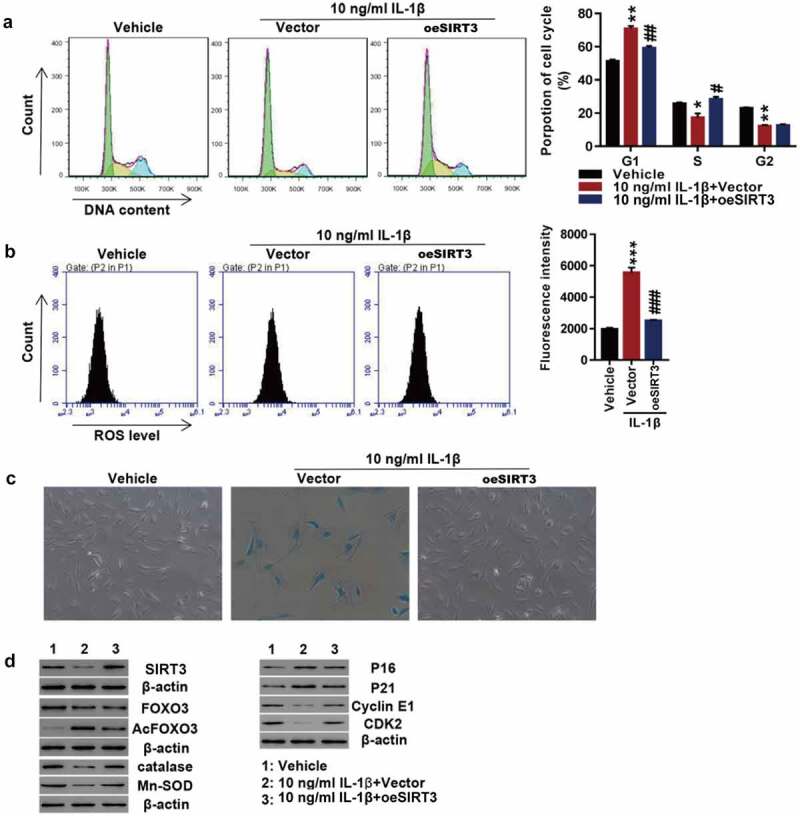
Overexpression of SIRT3 attenuated cell senescence probably by deacetylating FOXO3.
The primary chondrocytes were transfected with SIRT3 over expression lentivirus or control vector virus (Vector) in the presence of recombinant IL-1β protein or vehicle. (A) Cell cycle detection by flow cytometry. (B) ROS levels measured by flow cytometry. (C) Cell senescence shown by SA-β-Ga1 staining. (D) Levels of SIRT3, FOXO3, Ac-FOXO3, catalase, Mn-SOD, p16, p21, Cyclin E1, and CDK2 detected by Western blotting. Each data shows mean ± standard deviation from three independent biological experiments. *p < 0.05; **p < 0.01; ***p < 0.001 compared with vehicle; #p < 0.05; ##p < 0.01; ###p < 0.001 compared with IL-1β + vector.
Discussion
It has been reported that IL-1β has involved in the occurrence and progression of OA[35]. Studies have highlighted that this inflammatory cytokine was significantly increased in both chondrocytes and synovial cells from patients with OA [36,37]. Chondrocytes, expressing the receptor of IL-1β, are the major cellular targets for this cytokine. Therefore, IL-1β was chosen for chondrocyte treatment in this study. IL-1β processed chondrocytes could arrest the progression of cell cycles in the G1 phase, induce ROS production, and promote chondrocyte senescence. These findings have further confirmed that IL-1β plays a central role in OA.
As one of the five types of deubiquitinases (DUBs), USP3 trims ubiquitin chains from substrate-distal ends and rescues proteins from being degraded[38]. USPs have been implicated in various pathological processes including rheumatoid arthritis and autophagy [38,39]. Ogrunc et al. have shown that USP1 regulated cellular senescence by controlling genomic integrity[40]. Fukuura et al. have shown that USP17 prevented cell senescence by stabilizing SET domain-containing protein 8 and transcriptionally repressing p21[41]. More importantly, we have shown that USP3 deubiquitinates TRAF6 to mediate IL-1β-induced chondrocyte apoptosis[29]. Here, we further showed that USP3 increased SIRT3 expression levels by inhibiting SIRT3 ubiquitination and rescuing SIRT3 from degradation. Furthermore, inhibition of SIRT3 suppressed the protective effect of USP3 overexpression on IL-1β-induced senescence, and the overexpression of SIRT3 attenuated USP interfere-induced cell senescence. These findings indicate that USP3 upregulates SIRT3 levels to suppress chondrocyte senescence. The result is in agreement with a previous finding revealing that SIRT3 deficiency has been implicated in OA development, and SIRT3 activation suppresses chondrocyte degeneration[31].
SIRT3 serves as an NAD+-dependent protein deacetylase, deacetylates FOXO3, and regulates expressions of various genes being responsible for survival, apoptosis, autophagy, and metabolism[27]. The present study suggested that overexpression of SIRT3 attenuated IL-1β-induced chondrocyte senescence by deacetylating FOXO3. A study has shown that SIRT3 suppresses hyperglycemia-induced senescence of human diploid fibroblasts by deacetylating FOXO1[25]. Akasaki et al. have reported that FOXO3 is significantly decreased with joint aging[42]. Another study has indicated that overexpression of FOXO1 in chondrocytes decreases inflammatory cytokines and abolishes IL-1β effects[43]. Despite numerous studies have reported SIRT and FOXO proteins, studies on SIRT3/FOXO3 signaling in pathological processes are relatively scarce. The role of the SIRT3/FOXO3 axis in the senescence of chondrocytes is not fully understood. This study initiated experiments to demonstrate that overexpression of SIRT3 reduced the level of Ac-FOXO3 and attenuated IL-1β-induced chondrocyte senescence using various molecular biology techniques.
Conclusions
Taken together, the present research revealed that USP3 served as a deubiquitinating enzyme to upregulate the expression of SIRT3. The upregulation of SIRT3 promoted FOXO3 deacetylation to inhibit IL-1β-mediated ROS production and cell senescence. The findings indicated a pivotal role of USP3/SIRT3/FOXO3 in OA, allowing for a better understanding of regulating chondrocyte senescence in OA. Although further investigation of the underlying mechanisms is necessary, the current study provides us with a feasible therapeutic approach for OA.
Funding Statement
The author(s) reported there is no funding associated with the work featured in this article.
Highlights
Overexpression of USP3 inhibits IL-1β-mediated cell cycle arrest, ROS generation, and chondrocyte senescence.
SIRT3 inhibition blocks the protective effect of USP3 on cell senescence.
SIRT3 attenuates cell senescence by deacetylating FOXO3 in IL-1β-mediated chondrocyte senescence
Disclosure statement
No potential conflict of interest was reported by the author(s).
Availability of Data and Materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Author contributions
Conceptualization, Y.Z.X. and Q.R.Q. Methodology, Q.Z. Software, W.W. Validation, J.W. Formal analysis, S.Q. Investigation, S.Y. Data curation, P.L.F. Writing (original draft preparation), Q.Z. Writing (review and editing), Y.Z.X. and Q.R.Q. Supervision, Q.R.Q. Project administration, Y.Z.X.
Ethics approval and consent to participate
All experiments conducted in this study were approved by the Ethics Committee of the First Affiliated Hospital of Soochow University.
References
- [1].Mandl LA. Osteoarthritis year in review 2018: clinical. Osteoarthritis Cartilage. 2019;27:359–364. [DOI] [PubMed] [Google Scholar]
- [2].Neogi T. The epidemiology and impact of pain in osteoarthritis. Osteoarthritis Cartilage. 2013;21:1145–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Rahmati M, Nalesso G, Mobasheri A, et al. Aging and osteoarthritis: Central role of the extracellular matrix. Ageing Res Rev. 2017;40:20–30. [DOI] [PubMed] [Google Scholar]
- [4].Chen D, Shen J, Zhao W, et al. Osteoarthritis: toward a comprehensive understanding of pathological mechanism. Bone Res. 2017;5:16044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Nasi S, Ea HK, So A, et al. Revisiting the role of interleukin-1 pathway in osteoarthritis: Interleukin-1α and −1β, and NLRP3 inflammasome are not involved in the pathological features of the murine menisectomy model of osteoarthritis. Front Pharmacol. 2017;8:282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Mei X, Din H, Zhao J, et al. Transcription factor Krüppel-like factor 5-regulated N-myc downstream-regulated gene 2 reduces IL-1β-induced chondrocyte inflammatory injury and extracellular matrix degradation. Bioengineered. 2021;12:7020–7032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wang X, Fan J, Ding X, et al. Tanshinone I inhibits IL-1β-induced apoptosis, inflammation and extracellular matrix degradation in chondrocytes CHON-001 cells and attenuates murine osteoarthritis. Drug Des Devel Ther. 2019;13:3559–3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chen X, Zhang C, Wang X, et al. Juglanin inhibits IL-1β-induced inflammation in human chondrocytes. Artif Cells Nanomed Biotechnol. 2019;47:3614–3620. [DOI] [PubMed] [Google Scholar]
- [9].Wojdasiewicz P, Poniatowski ŁA, Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm . 2014;2014:561459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chen J, Gu YT, Xie JJ, et al. Gastrodin reduces IL-1β-induced apoptosis, inflammation, and matrix catabolism in osteoarthritis chondrocytes and attenuates rat cartilage degeneration in vivo. Biomed Pharmacothe. 2018;97:642–651. [DOI] [PubMed] [Google Scholar]
- [11].Li Z, Wang J, Ma Y. Montelukast attenuates interleukin IL-1β-induced oxidative stress and apoptosis in chondrocytes by inhibiting CYSLTR1 (Cysteinyl leukotriene receptor 1) and activating KLF2 (Kruppel like factor 2). Bioengineered. 2021;12:8476–8484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol. 2014;24:R453–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gladyshev VN. The free radical theory of aging is dead. Long live the damage theory! Antioxid Redox Signal. 2014;20:727–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Srinivas US, Tan BWQ, Vellayappan BA, et al. ROS and the DNA damage response in cancer. Redox Biol. 2019;25:101084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Li J, Han S, Cousin W, et al. Age-specific functional epigenetic changes in p21 and p16 in injury-activated satellite cells. Stem Cells. 2015;33:951–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Muteliefu G, Shimizu H, Enomoto A, et al. Indoxyl sulfate promotes vascular smooth muscle cell senescence with upregulation of p53, p21, and prelamin A through oxidative stress. Am J Physiol Cell Physiol. 2012;303:C126–34. [DOI] [PubMed] [Google Scholar]
- [17].Koyano T, Namba M, Kobayashi T, et al. The p21 dependent G2 arrest of the cell cycle in epithelial tubular cells links to the early stage of renal fibrosis. Sci Rep. 2019;9:12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hoeferlin LA, Oleinik NV, Krupenko NI, et al. Activation of p21-Dependent G1/G2 arrest in the absence of DNA damage as an antiapoptotic response to metabolic stress. Genes Cancer. 2011;2:889–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kida Y, Goligorsky MS. Sirtuins, cell senescence, and vascular aging. Can J Cardiol. 2016;32:634–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kincaid B, Bossy-Wetzel E. Forever young: SIRT3 a shield against mitochondrial meltdown, aging, and neurodegeneration. Front Aging Neurosci. 2013;5:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zhao J, He X, Zuo M, et al. Anagliptin prevented interleukin 1β (IL-1β)-induced cellular senescence in vascular smooth muscle cells through increasing the expression of sirtuin1 (SIRT1). Bioengineered. 2021;12:3968–3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Chuang PY, Cai W, Li X, et al. Reduction in podocyte SIRT1 accelerates kidney injury in aging mice. Am J Physiol Renal Physiol. 2017;313:F621–f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zhou N, Lin X, Dong W, et al. SIRT1 alleviates senescence of degenerative human intervertebral disc cartilage endo-plate cells via the p53/p21 pathway. Sci Rep. 2016;6:22628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wu Y, Chen L, Wang Y, et al. Overexpression of Sirtuin 6 suppresses cellular senescence and NF-κB mediated inflammatory responses in osteoarthritis development. Sci Rep. 2015;5:17602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zhang B, Cui S, Bai X, et al. SIRT3 overexpression antagonizes high glucose accelerated cellular senescence in human diploid fibroblasts via the SIRT3-FOXO1 signaling pathway. Age (Dordr). 2013;35:2237–2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Tseng AH, Wu LH, Shieh SS, et al. SIRT3 interactions with FOXO3 acetylation, phosphorylation and ubiquitinylation mediate endothelial cell responses to hypoxia. Biochem J. 2014;464:157–168. [DOI] [PubMed] [Google Scholar]
- [27].Tseng AH, Shieh SS, Wang DL. SIRT3 deacetylates FOXO3 to protect mitochondria against oxidative damage. Free Radic Biol Med. 2013;63:222–234. [DOI] [PubMed] [Google Scholar]
- [28].Li Y, Shen G, Yu C, et al. Angiotensin II induces mitochondrial oxidative stress and mtDNA damage in osteoblasts by inhibiting SIRT1–FOXO3A–MnSOD pathway. Biochem Biophys Res Commun. 2014;455:113–118. [DOI] [PubMed] [Google Scholar]
- [29].Zhou Q, Xiao Z, Zhou R, et al. Ubiquitin-specific protease 3 targets TRAF6 for deubiquitination and suppresses IL-1β induced chondrocyte apoptosis. Biochem Biophys Res Commun. 2019;514:482–489. [DOI] [PubMed] [Google Scholar]
- [30].Zeng L, Tan J, Xue M, et al. An engineering probiotic producing defensin-5 ameliorating dextran sodium sulfate-induced mice colitis via Inhibiting NF-kB pathway. J Transl Med. 2020;18:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wang J, Wang K, Huang C, et al. SIRT3 activation by dihydromyricetin suppresses chondrocytes degeneration via maintaining mitochondrial homeostasis. Int J Biol Sci. 2018;14:1873–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wang H, Zheng Z, Han W, et al. Metformin promotes axon regeneration after spinal cord injury through inhibiting oxidative stress and stabilizing microtubule. Oxid Med Cell Longev. 2020;2020:9741369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zhu P, Wang Z, Sun Z, et al. Recombinant platelet-derived growth factor-BB alleviates osteoarthritis in a rat model by decreasing chondrocyte apoptosis in vitro and in vivo. J Cell Mol Med. 2021;25:7472–7484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Yan L, Liu G, Wu X. The umbilical cord mesenchymal stem cell-derived exosomal lncRNA H19 improves osteochondral activity through miR-29b-3p/FoxO3 axis. Clin Transl Med. 2021;11:e255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Vincent TL. IL-1 in osteoarthritis: time for a critical review of the literature. F1000Res. 2019;8. doi: 10.12688/f1000research.18831.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].van Dalen SC, Blom AB, Slöetjes AW, et al. Interleukin-1 is not involved in synovial inflammation and cartilage destruction in collagenase-induced osteoarthritis. Osteoarthritis Cartilage. 2017;25:385–396. [DOI] [PubMed] [Google Scholar]
- [37].Benito MJ, Veale DJ, FitzGerald O, et al. Synovial tissue inflammation in early and late osteoarthritis. Ann Rheum Dis. 2005;64:1263–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Min Y, Lee S, Kim MJ, et al. Ubiquitin-specific protease 14 negatively regulates toll-like receptor 4-mediated signaling and autophagy induction by inhibiting ubiquitination of TAK1-binding protein 2 and beclin 1. Front Immunol. 2017;8:1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Luo XB, Xi JC. Proinflammatory effects of ubiquitin-specific protease 5 (USP5) in rheumatoid arthritis fibroblast-like synoviocytes. Mediators Inflamm. 2020;2020:8295149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ogrunc M, Martinez-Zamudio RI, Sadoun PB, et al. USP1 regulates cellular senescence by controlling genomic integrity. Cell Rep. 2016;15:1401–1411. [DOI] [PubMed] [Google Scholar]
- [41].Fukuura K, Inoue Y. The ubiquitin-specific protease USP17 prevents cellular senescence by stabilizing the methyltransferase SET8 and transcriptionally repressing p21. J Biol Chem. 2019;294:16429–16439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Akasaki Y, Hasegawa A, Saito M, et al. Dysregulated FOXO transcription factors in articular cartilage in aging and osteoarthritis. Osteoarthritis Cartilage. 2014;22:162–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Matsuzaki T, Alvarez-Garcia O. FoxO transcription factors modulate autophagy and proteoglycan 4 in cartilage homeostasis and osteoarthritis Sci Transl Med . 2018 Feb 14;102: eaan0746. doi: 10.1126/scitranslmed.aan0746 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


