Abstract
Pseudomonas aeruginosa expresses a low level of the MexAB-OprM efflux pump and shows natural resistance to many structurally and functionally diverse antibiotics. The mutation that has been referred to previously as nfxC expresses an additional efflux pump, MexEF-OprN, exhibiting resistance to fluoroquinolones, imipenem, and chloramphenicol and hypersusceptibility to β-lactam antibiotics. To address the antibiotic specificity of the MexEF-OprN efflux pump, we introduced a plasmid carrying the mexEF-oprN operon into P. aeruginosa lacking the mexAB-oprM operon. The transformants exhibited resistance to fluoroquinolones, trimethoprim, and chloramphenicol but, unlike most nfxC-type mutants, did not show β-lactam hypersusceptibility. The transformants exhibited additional resistance to tetracycline. In the next experiment, we analyzed the MexEF-OprN pump subunit(s) responsible for substrate selectivity by expressing MexE, MexF, OprN, and MexEF in strains lacking MexA, MexB, OprM, and MexAB, respectively. The MexEF-OprM/ΔMexAB transformants exhibited MexEF-OprN-type pump function that rendered the strains resistant to fluoroquinolones and chloramphenicol but did not change susceptibility to β-lactam antibiotics compared with the host strain. The MexAB-OprN/ΔOprM, MexAF-OprM/ΔMexB, and MexEB-OprM/ΔMexA mutants exhibited antibiotic susceptibility indistinguishable from that in the mutant lacking both types of efflux pumps. The results imply that the MexEF-OprM pump selects substrates by a MexEF functional unit. Interestingly, OprN did not link functionally with the MexAB complex, despite the fact that OprM interacted functionally with MexEF.
Infections by Pseudomonas aeruginosa in patients with low immune activity are a major problem in hospitals, in part because this organism exhibits natural (inherent) as well as acquired resistance to a broad spectrum of antibiotics. The naturally occurring antibiotic resistance of this organism is attributable mainly to the interplay of tight outer membrane permeability and low-level expression of the MexAB-OprM efflux pump (13, 14, 18). All nalB mutants previously reported overexpress the MexAB-OprM pump and become highly resistant to a wide variety of antimicrobial agents, including most β-lactam antibiotics, fluoroquinolones, tetracycline, chloramphenicol, and others (13, 18, 21, 28). In contrast, mutations in the nfxB (7, 15) and nfxC (3) loci located near the ilvB (0 min) and catA (46 min) genes, respectively, of the P. aeruginosa chromosome render the bacterium hypersusceptible to β-lactam antibiotics and resistant to fluoroquinolones, chloramphenicol, and other antibiotics. The resistance is mainly attributable to the expression of the MexAB-OprM, MexCD-OprJ, and MexEF-OprN efflux pumps, respectively (8, 13, 17–19). Among these efflux pump systems, only the MexAB-OprM pump is expressed in most, if not all, strains of P. aeruginosa so far tested, including laboratory and clinical strains (8, 17), and thus most, if not all, nfxB and nfxC mutations occur in strains producing at least low levels of MexAB-OprM pump. Therefore, the antibiotic resistance profile of the nfxB and nfxC mutants might be a consequence of the expression of the MexCD-OprJ and MexEF-OprN systems, respectively, plus a low level of MexAB-OprM pump expression.
MexB and its homologues span the cytoplasmic membrane 12 times (6, 16) and have been assumed to function as the substrate-exporting subunit across the cytoplasmic membrane. MexA and its homologues are membrane fusion proteins associated with the cytoplasmic membrane via the fatty acid residue, and the peptide moiety extends almost to the periplasmic space (20, 30). OprM and its homologues are outer membrane proteins that are assumed to form a channel to facilitate the exit of substrates through the outer membrane (10).
Since the subunit proteins of the three efflux pumps are similar to each other, it has been assumed that a subunit of one pump system could be substituted for the homologous subunit of another pump system. Experiments exchanging the subunits of MexAB-OprM and MexCD-OprJ have been carried out and revealed that replacement of OprM with OprJ or vice versa partially complemented the pump function (5, 26, 27); however, replacing the inner membrane subunits totally abolished the pump function. These experiments still left the following important questions unanswered. (i) What antibiotics are the substrates of the MexEF-OprN pump? (ii) Does expression of the MexEF-OprN pump suppress OprD production and confer carbapenem resistance? (iii) Can nfxC-type β-lactam hypersusceptibility be surmounted by overexpression of the MexEF-OprN pump without nfxC mutation?
In this study, we addressed these issues by expressing the MexEF-OprN pump in a strain lacking the MexAB-OprM pump. Moreover, we assigned the subunit protein(s) of the MexEF-OprN pump responsible for substrate recognition based on the results of the subunit swapping experiment.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
Bacterial strains and plasmids used are listed in Table 1. Escherichia coli XL10 Gold was the host in the DNA manipulation.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid(s) | Relevant properties | Source (reference) |
|---|---|---|
| P. aeruginosa | ||
| PAO4009 | leu-9018 nir-9006; FP5+ | H. Matsumoto (7) |
| KH4014a | nfxC mutant of PAO4009 | H. Fukuda (3) |
| PAO4290 | leu-10 argF10 aph-9004; FP− | H. Matsumoto (28) |
| TNP070 | ΔmexA; derivative of PAO4290 | H. Yoneyama (28) |
| TNP071 | ΔmexB; derivative of PAO4290 | H. Yoneyama (28) |
| TNP072 | ΔoprM; derivative of PAO4290 | H. Yoneyama (28) |
| TNP073 | ΔmexA ΔmexB; derivative of PAO4290 | H. Yoneyama (28) |
| TNP077 | ΔmexA ΔmexB ΔoprM; derivative of PAO4290 | This study |
| TNP0701 | TNP070 derivative harboring pMEXA1 | H. Yoneyama (27) |
| TNP0705 | TNP070 derivative harboring pMEXE1 | This study |
| TNP0703 | TNP070 derivative harboring pMMB67HE | H. Yoneyama (27) |
| TNP0711 | TNP071 derivative harboring pMEXB1 | H. Yoneyama (27) |
| TNP0715 | TNP071 derivative harboring pMEXF1 | This study |
| TNP0713 | TNP071 derivative harboring pMMB67EH | H. Yoneyama (27) |
| TNP0721 | TNP072 derivative harboring pOPRM1 | H. Yoneyama (27) |
| TNP0725 | TNP072 derivative harboring pOPRN1 | This study |
| TNP0723 | TNP072 derivative harboring pMMB67EH | H. Yoneyama (27) |
| TNP0731 | TNP073 derivative harboring pMEXAB1 | This study |
| TNP0735 | TNP073 derivative harboring pMEXEF1 | This study |
| TNP0733 | TNP073 derivative harboring pMMB67EH | H. Yoneyama (27) |
| TNP0771 | TNP077 derivative harboring pMEXAB-OPRM1 | This study |
| TNP0775 | TNP077 derivative harboring pMEXEF-OPRN1 | This study |
| TNP0773 | TNP077 derivative harboring pMMB67EH | This study |
| TNP0776 | TNP077 derivative harboring pVLT33 | This study |
| TNP0777 | TNP077 derivative harboring pMEXEF-OPRN-KM1 | This study |
| E. coli | ||
| XL10 Gold | E. coli strain for transformation | Stratagene |
| S17-1 | Mobilizer strain | R. Simon (25) |
| Plasmids | ||
| pUC19 | Cloning vector; Ampr | Toyobo |
| pNOT19 | pUC19 derivative with NotI site; Ampr | H. P. Schweizer (24) |
| pKF18K | pUC18 derivative cloning vector; Ampr | Toyobo |
| pBluescript II SK(+) | Cloning vector; Ampr | Stratagene |
| pMOB3 | pHSS21 derivative carrying MOB cassette; Cmr Kmr | H. P. Schweizer (24) |
| pQE30, pQE31 | Expression vectors carrying a six-His affinity tag; Ampr | Qiagen, Inc. |
| pMMB67EH/HE | Broad-host-range vector; Ampr IncQ | Fürste (4) |
| pVLT33 | Broad-host-range vector; Kmr IncQ | V. de Lorenzo (2) |
| pMEXA1 | pMMB67EH derivative carrying mexA gene | H. Yoneyama (27) |
| pMEXB1 | pMMB67EH derivative carrying mexB gene | H. Yoneyama (27) |
| pOPRM1 | pMMB67EH derivative carrying oprM gene | H. Yoneyama (27) |
| pMEXE1 | pMMB67EH derivative carrying mexE gene | This study |
| pMEXF1 | pMMB67EH derivative carrying mexF gene | This study |
| pOPRN1 | pMMB67EH derivative carrying oprN gene | This study |
| pMEXAB1 | pMMB67EH derivative carrying mexA and mexB genes | This study |
| pMEXEF1 | pMMB67EH derivative carrying mexE and mexF genes | This study |
| pMEXAB-OPRM1 | pMMB67EH derivative carrying mexA, mexB, and oprM genes | This study |
| pMEXEF-OPRN1 | pMMB67EH derivative carrying mexE, mexF, and oprN genes | This study |
| pMEXEF-OPRN-KM1 | pVLT33 derivative carrying mexE, mexF, and oprN genes | This study |
| pΔMexA-B-OprM | pNOT19 derivative carrying defective mexA, mexB, and oprM; Ampr Kmr Cmr | This study |
Recombinant DNA techniques.
We manipulated recombinant DNA by the standard procedures described previously (22). Chromosomal DNA from the P. aeruginosa cells was isolated by the procedure described by Ausubel et al. (1). For Southern blotting, DNA fragments were blotted onto Hybond-N+ (Amersham Life Science, Arlington Heights, Ill.) by the capillary method and visualized with a digoxigenin-labeled probe (Boehringer Mannheim, Laval, Quebec, Canada) prepared according to the manufacturer's instructions. Nucleotide sequencing of the recombinant DNA was conducted by the dideoxy chain-termination method (23). PCR amplification of chromosomal DNA was carried out using the LA Taq kit (Takara Shuzo, Osaka, Japan) according to the manufacturer's instructions. The primers used were as follows: mexE1 (5′-GCGGTACCGACTGGCGGAGTCAAGCA-3′) and mexF2 (5′-CGAAGCTTGCGCGTGAATCAT-3′) for mexEF and oprN1 (5′-GTGGTACCCTGCCAGAGGTGCATGCAT-3′) and oprN2 (5′-AACAAGCTTCAGGCGCTGGGTTGCCAG-3′) for oprN. The sizes of the amplified DNA fragments obtained using these primers were about 4.5 and 1.4 kbp, respectively.
Preparation of rabbit antisera against MexE, MexF, and OprN.
Using the PCR technique, we amplified 1.1-, 0.8-, and 1.3-kbp fragments encoding deduced amino acid sequences from positions 42 to 413, 47 to 317, and 27 to 472 of MexE, MexF, and OprN, respectively. The following primer pairs were used: mexE3 (5′-GCGGTACCGCCGAAGTCATCGAACAAC-3′) and mexE2 (5′-GCAAGCTTCGGTTCTTCCTATCGCCGC-3′) for MexE, mexF3 (5′-GCGGTACCGGTCCGCGCCAACTTCCC-3′) and mexF4 (5′-CGAAGCTTTCAGCTCGGCCATCTTCTC-3′) for MexF, and oprN3 (5′-AAGGATCCACGGTGGGTCCGGACTAC-3′) and oprN2 (see above) for OprN. The amplified fragments were subcloned into the pQN30 or pQN31 (Qiagen, Inc., Chatsworth, Calif.) expression vector carrying a sequence coding for the polyhistidine affinity tag, to which the N terminus of the desired DNA fragment was fused. By nucleotide sequencing, we confirmed that the reading frames of all these genes were correctly maintained. Fully grown E. coli cells, which harbored an appropriate plasmid, were diluted 50-fold with fresh medium and incubated in the presence of appropriate concentrations of antibiotics.
At an A600 of 0.5, 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) was added and the culture was continued for an additional 5 h under vigorous shaking. The hybrid protein was purified by affinity chromatography using an Ni-nitrilotriacetic acid column (Qiagen, Inc.) according to the manufacturer's manual. The protein was subjected to preparative sodium dodecyl sulfate-polyacrylamide gel electrophoresis (3-mm thickness) and stained, and then the desired protein band was excised and eluted electrophoretically. Rabbits were immunized, and antisera were obtained.
Deletion of chromosomal mexAB-oprM.
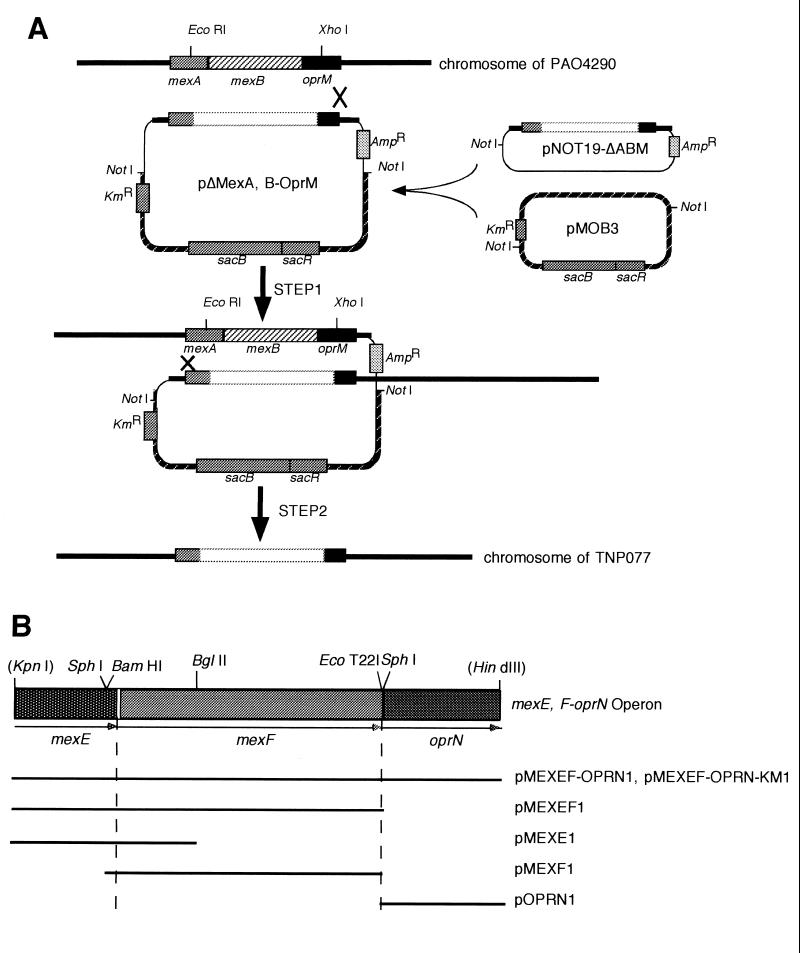
We subcloned a 6.5-kbp fragment containing the mexAB-oprM genes from the previously cloned 26-kbp fragment (13) on pNOT19-ΔRI yielding pMex(sac/Hind)/ΔRI-pNOT19. To disrupt the chromosomal mexAB-oprM, pMex(sac/Hind)ΔRI-pNOT19 was treated with EcoRI and XhoI and then self-ligated after blunt-ending with T4 DNA polymerase. The resulting plasmid, pNOT19-ΔMexABM, and pMOB3 were ligated at the NotI site and transferred into the mobilizer strain E. coli S17-1. The resulting plasmid pΔMexA-B-OprM was transferred to P. aeruginosa PAO4290 by conjugation as reported earlier (as shown in Fig. 1A) (29). The deletion was confirmed by PCR (data not shown). MexA, MexB, and OprM proteins were undetectable in the mutant TNP077, tested by an immunoblot assay using polyclonal antisera (Fig. 2). Determination of MICs of antibiotic agents revealed that the MexAB-OprM deletion mutant TNP077 was hypersusceptible to fluoroquinolones, chloramphenicol, and β-lactams, except for imipenem, as reported previously (Table 2) (28).
FIG. 1.
Schematic representation of the procedure for deleting the chromosomal mexAB-oprM genes and subcloning DNA fragments into shuttle vector pMMB67EH. (A) Deletion of chromosomal mexAB-oprM. NotI-treated pNOT19-ΔABM was ligated to the NotI site of pMOB3, and the resulting pΔMexA,B-OprM was inserted into chromosomal mexAB-oprM by homologous recombination. The transconjugant was Kmr and sucrose-sensitive and contained an unwanted DNA fragment, which was excised by selecting for sucrose resistance (29). (B) Physical mapping of the restriction fragments subcloned into the shuttle vector. Solid lines represent the restriction fragments cloned into pMMB67EH (pMEXE1, pMEXF1, pOPRN1, pMEXEF1, and pMEXEF-OPRN1) and pVLT33 (pMEXEF-OPRN-KM1). Physical distances of the lines to the mexEF-oprN genes are arbitrary.
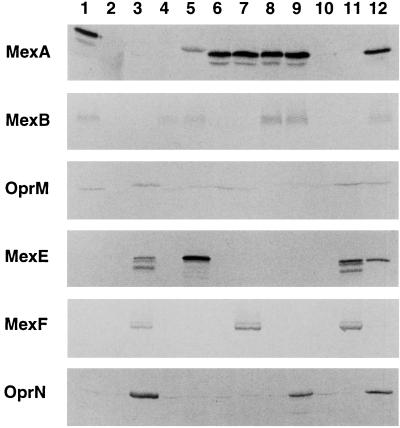
FIG. 2.
Immunoblotting visualization of MexAB-OprM and MexEF-OprN proteins expressed in the constructs. Total cell lysate was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and the protein bands were visualized by the immunoblotting method. The amount of protein applied per lane was 8 μg for MexA, about 20 μg each for MexE, MexF, and OprN, and about 40 μg each for MexB and OprM. The antibody used to visualize a protein(s) is marked at the far left as the membrane protein. Lanes: 1, PAO4290(pMMB67EH); 2, TNP0773; 3, TNP0775; 4, TNP0703; 5, TNP0705; 6, TNP0713; 7, TNP0715; 8, TNP0723; 9, TNP0725; 10, TNP0733; 11, TNP0735; 12, KH4014a. Unexpected protein bands that appeared, for example, MexA in lane 5, where the MexE protein was stained by the anti-MexA protein, were most likely due to cross-reactivity of the antibody with highly homologous protein(s).
TABLE 2.
Antibiotic susceptibility of the strains constructed to test the substrate selectivity of the MexEF-OprN pumpa
| Strain | Plasmid | Pump component(s) expressed | MIC (μg/ml)
|
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NFLX | OFLX | CP | TET | GM | KM | EM | NOV | CAZ | CPZ | CPR | CZOP | CAR | AZT | MPM | IPM | TMP | |||
| PAO4290 | ABM | 0.78 | 0.78 | 100 | 25 | 3.13 | 3.13 | 400 | 1,600 | 1.56 | 6.25 | 3.13 | 0.78 | 25 | 3.13 | 0.39 | 1.56 | 200 | |
| TNP077 | 0.1 | 0.05 | 3.13 | 0.39 | 0.39 | 3.13 | 25 | 100 | 0.39 | 1.56 | 1.56 | 0.39 | 3.13 | 0.2 | 0.2 | 1.56 | 12.5 | ||
| TNP0773 | pMMB67EH | 0.1 | 0.05 | 3.13 | 0.39 | 0.39 | 3.13 | 12.5 | 100 | 0.78 | 25 | 3.13 | 0.39 | >800 | 0.2 | 0.2 | 1.56 | 12.5 | |
| TNP0771 | pMEXAB-OPRM1 | ABM | 3.13 | 3.13 | 800 | 50 | 3.13 | 3.13 | 400 | 1600 | 3.13 | 25 | 6.25 | 1.56 | >800 | 25 | 1.56 | 1.56 | >1,600 |
| TNP0775 | pMEXEF-OPRN1 | EFN | 3.13 | 3.13 | 1,600 | 3.13 | 0.39 | 3.13 | 12.5 | 100 | 0.39 | 25 | 1.56 | 0.39 | >800 | 0.2 | 0.2 | 1.56 | >1,600 |
| TNP0776 | pVLT33 | 0.1 | 0.1 | 3.13 | 0.39 | 0.39 | 400 | 12.5 | 100 | 0.39 | 3.13 | 3.13 | 0.39 | 6.25 | 0.2 | 0.39 | 1.56 | 12.5 | |
| TNP0777 | pMEXEF-OPRN-KM1 | EFN | 6.25 | 3.13 | 1,600 | 1.56 | 0.39 | 400 | 12.5 | 100 | 0.39 | 3.13 | 1.56 | 0.39 | 3.13 | 0.2 | 0.2 | 1.56 | >1,600 |
| PAO4009 | ABM | 0.39 | 0.78 | 50 | 25 | 3.13 | 100 | 400 | 800 | 0.78 | 6.25 | 3.13 | 0.78 | 25 | 3.13 | 0.78 | 0.78 | 200 | |
| KH4014a | ABM, EFN | 12.5 | 12.5 | 1,600 | 25 | 1.56 | 25 | 200 | 400 | 0.78 | 3.13 | 0.78 | 0.39 | 12.5 | 1.56 | 0.78 | 3.13 | >1,600 | |
Mueller-Hinton agar contained 2 mM IPTG. Abbreviations: NFLX, norfloxacin; OFLX, ofloxacin; CP, chloramphenicol; TET, tetracycline; GM, gentamicin; KM, kanamycin; EM, erythromycin; NOV, novobiocin; CAZ, ceftazidime; CPZ, cefoperazone; CPR, cefpirome; CZOP, cefozopran; CAR, carbenicillin; AZT, aztreonam; MPM, meropenem; IPM, imipenem; TMP, trimethoprim. Pump components expressed are shown as varying combinations of the following subunits: A, MexA; B, MexB; E, MexE; F, MexF; M, OprM; and N, OprN.
Cloning of the mexEF-oprN genes.
The mexEF-oprN genes were obtained by PCR and colony hybridization techniques. A 4.5-kbp DNA fragment encoded by the mexEF genes and a 1.4-kbp DNA fragment encoded by the oprN gene were amplified with primer sets mexE1 and mexF2 and oprN1 and oprN2, respectively, as mentioned above, by using the LA Taq kit. The 4.5- and 1.4-kbp fragments were cloned onto pNOT19 and pKF18, respectively, to yield pUC19-mexEF and pKF18-oprN. Nucleotide sequencing of these fragments revealed that the 4.5-kbp fragment contained two incorrect nucleotides. To obtain the correct 4.5-kbp DNA fragment, the 3.4-kbp SphI-SphI fragment from the genomic DNA encoded by a part of mexEF was cloned on pBluescript II SK(+) as follows. Total DNA prepared from P. aeruginosa PAO1 was digested with SphI and separated by gel electrophoresis. Fragments of around 3.4 kbp were collected; this was followed by ligation with SphI-treated pBluescript II SK(+). The ligation mixture was used for transformation of E. coli XL10 Gold. One clone that harbored recombinant pBluescript II SK(+) with a SphI-SphI fragment was selected from 684 Ampr clones using the labeled pUC19-mexEF as a probe in the colony hybridization method. Next, we replaced the 3.4-kbp SphI-SphI region on pUC19-mexEF with this newly cloned fragment on pBluescript II SK(+) and constructed the pUC19-mexEF plasmid with the correct mexEF. Finally, to join mexEF with oprN, the new pUC19-mexEF was digested with EcoT22I and HindIII and ligated to a 1.4-kbp oprN gene fragment from pKF18-oprN, resulting in pUC19-mexEFN. The nucleotide sequence of cloned mexEF-oprN was identical to that of the strain PAO1 gene.
Construction of recombinant plasmids.
To construct pMEXEF1, a KpnI-HindIII fragment of about 4.5 kbp from pUC19-mexEF was inserted into pMMB67EH. This plasmid DNA was digested with KpnI and BamHI, followed by blunt-ending with T4 DNA polymerase and self-ligating to yield the pMEXF1 (Fig. 1B). The 2.2-kbp KpnI-BglII fragment from pUC19-mexEF was inserted at KpnI and BamHI sites of the pMMB67EH to construct pMEXE1. A KpnI-HindIII fragment of about 6 kbp from pUC19-mexEFN was ligated to pMMB67EH and pVLT33 and then digested with KpnI and HindIII to construct pMEXEF-ORRN1 and pMEXEF-OPRN-KM1, respectively. A 1.4-kbp KpnI-HindIII fragment from pKF18-oprN was inserted into pMMB67EH to construct pOPRN1. To construct pMEXAB-OPRM1, pMex(sac/Hind)/Δ RI-pNOT19 was partially digested and a SacI-HindIII fragment of about 6.5 kbp was separated by agarose gel electrophoresis and inserted into pMMB67EH.
Other techniques.
Western blot analysis has been described before (28). The MICs of the antibiotics were determined by the agar dilution method using Mueller-Hinton agar (Becton Dickinson Microbiology Systems, Cockeysville, Md.). Protein was quantified by the method of Lowry et al. (9).
RESULTS
Expression of MexEF-OprN in the mutant lacking MexAB-OprM and examination of antibiotic susceptibilities.
To express the mexE, mexF, and oprN genes under the control of the tac promoter in P. aeruginosa, the DNA fragment containing the mexE, mexF, and oprN genes was excised from pUC19-mexEFN. This fragment was subcloned into the broad-host-range vector, pMMB67EH, yielding pMEXEF-OPRN1 (Fig. 1B). Introduction of pMEXEF-OPRN1 into TNP077 led to expression of substantial amounts of MexE, MexF, and OprN (Fig. 2, lane 3) and an undetectable level of MexAB-OprM. Similarly, strain TNP0771 carrying pMEXAB-OPRM produced substantial levels of MexAB-OprM proteins, but MexEF-OprN was undetectable (data not shown).
To elucidate which antibiotic might be a valid substrate of the MexEF-OprN pump, the antibiotic susceptibilities of these strains were examined. Strain TNP0773 harboring the pMMB67EH plasmid barely restored resistance to any of the antibiotics except for carbenicillin and cefoperazone. This resistance is most likely due to the selective Ampr marker. On the other hand, TNP0775 harboring pMEXEF-OPRN restored resistance to norfloxacin, ofloxacin, chloramphenicol, and tetracycline (Table 2), suggesting that these antibiotics are substrates of the MexEF-OprN efflux pump. Interestingly, TNP0775 did not show resistance to gentamicin, kanamycin, erythromycin, novobiocin, ceftazidime, cefpirome, cefozopran, aztreonam, or meropenem, clearly indicating that these antibiotics are not substrates of the MexEF-OprN pump. Importantly, TNP0775 did not show resistance to imipenem. These findings showed that this carbapenem is not a substrate of the MexEF-OprN pump and suggested that carbapenem resistance in the nfxC mutant is not due to expression of MexEF-OprN. Consequently, it became clear that low-level expression of OprD is not linked to the expression of MexEF-OprN. Earlier studies showed inconsistent tetracycline susceptibility among the nfxC mutants (3, 11). However, the present study clarified this ambiguity and demonstrated that tetracycline is an excellent substrate of the MexEF-OprN pump.
A previously described nfxC mutant was hypersusceptible to β-lactam antibiotics (3). In this study, TNP0775 harboring the plasmid containing mexEF-oprN showed susceptibility to β-lactam antibiotics comparable to that of the strain harboring the plasmid only. To ascertain whether or not the MexEF-OprN pump is capable of recognizing and exporting the β-lactam antibiotics, we inserted mexEF-oprN genes into the pVLT33 plasmid with the Kmr marker instead of the Ampr marker (2) and the resulting plasmid was introduced into TNP077. The recombinant strain (TNP0776) showed susceptibility to β-lactam antibiotics equal to that of the strain without pMEXEF-OPRN-KM1, besides demonstrating resistance to the fluoroquinolone antibiotics, chloramphenicol, trimethoprim, and tetracycline. Based on these results, we concluded that the MexEF-OprN pump does not export β-lactam antibiotics. In addition, the result showed that β-lactam hypersusceptibility in the nfxC mutant was not attributable to expression of the MexEF-OprN pump.
Assignment of the subunit protein(s) that recognizes the MexEF-OprN substrate.
Whereas both MexAB-OprM and MexEF-OprN pumps export flluoroquinolone antibiotics, chloramphenicol, trimethoprim, and tetracycline, only the former, not the latter, exports β-lactams, novobiocin, and erythromycin. To determine which subunit of the MexEF-OprN pump recognizes substrates, we constructed an efflux pump consisting of mixed subunit proteins from MexAB-OprM and MexEF-OprN and conducted an assay for antibiotic selectivity. Expression of the desired subunit from two different efflux pump systems was confirmed by immunoblotting assays (Fig. 2). Mutants lacking both pump subunits were hypersusceptible to all antibiotics tested. Strains TNP0701, TNP0711, TNP0721, and TNP0731, which were MexA/ΔMexA, MexB/ΔMexB, OprM/ΔOprM, and MexAB/ΔMexAB recombinants, respectively, produced MexAB-OprM proteins and restored antibiotic resistance.
Next, strains with deletions of only one subunit, TNP070, TNP071, and TNP072, lacking MexA, MexB, and OprM, respectively, were transformed with a plasmid carrying genes encoding MexE, MexF, and OprN, respectively. The antibiotic susceptibility test with these constructs revealed that replacement of MexA with MexE and OprM with OprN resulted in total pump dysfunction. Two important observations were recorded. First, strain TNP0715, which expresses MexAF-OprM, showed a MIC of aztreonam that was two and four times higher, respectively, than those of TNP0713 and TNP071 (Table 3). This result implies that the MexAM complex may recognize β-lactam antibiotics. Second, replacement of OprM with OprN totally abolished the pump function, suggesting that the MexAB unit does not interact functionally with OprN. Replacement of OprN with OprM fully restored the MexEF-OprN-type pump function, in which the strain was resistant to norfloxacin and chloramphenicol but susceptible to novobiocin and aztreonam. This phenotype is different from that of TNP0755 but is distinguishable from the MexAB-OprM-type pump function. The results imply that the MexEF unit may select the substrate for the MexEF-OprN pump.
TABLE 3.
Antibiotic susceptibility of the strains constructed to assign the substrate-selective subunit(s)a
| Strain | Plasmid | Pump component expressed | MIC (μg/ml)
|
|||
|---|---|---|---|---|---|---|
| NFLX | CP | NOV | AZT | |||
| TNP070 | -BM | 0.39 | 12.5 | 100 | 0.2 | |
| TNP0703 | pMMB67EH | -BM | 0.39 | 12.5 | 100 | 0.39 |
| TNP0701 | pMEXA1 | ABM | 0.39 | 100 | 1,600 | 1.56 |
| TNP0705 | pMEXE1 | EBM | 0.39 | 12.5 | 100 | 0.39 |
| TNP071 | A-M | 0.39 | 12.5 | 100 | 0.2 | |
| TNP0713 | pMMB67EH | A-M | 0.39 | 12.5 | 100 | 0.39 |
| TNP0711 | pMEXB1 | ABM | 0.78 | 100 | 1,600 | 3.13 |
| TNP0715 | pMEXF1 | AFM | 0.39 | 12.5 | 50 | 0.78 |
| TNP072 | AB- | 0.1 | 3.13 | 100 | 0.2 | |
| TNP0723 | pMMB67EH | AB- | 0.1 | 3.13 | 100 | 0.39 |
| TNP0721 | pOPRM1 | ABM | 0.78 | 50 | 200 | 3.13 |
| TNP0725 | pOPRN1 | ABN | 0.1 | 3.13 | 50 | 0.2 |
| TNP073 | --M | 0.39 | 12.5 | 100 | 0.2 | |
| TNP0733 | pMMB67EH | --M | 0.39 | 12.5 | 100 | 0.39 |
| TNP0731 | pMEXAB1 | ABM | 0.78 | 200 | 1,600 | 3.13 |
| TNP0735 | pMEXEF1 | EFM | 3.13 | 1,600 | 100 | 0.2 |
Mueller-Hinton agar contained 2 mM IPTG. Abbreviations: NFLX, norfloxacin; CP, chloramphenicol; NOV, novobiocin; AZT, aztreonam. Pump components expressed are shown as various combinations of the following subunits: A, MexA; B, MexB; E, MexE; F, MexF; M, OprM; and N, OprN. A hyphen indicates a missing subunit.
DISCUSSION
This study was conducted to clarify antibiotic selectivity of the MexEF-OprN pump and to assign a subunit protein(s), which filters the substrate antibiotics. The rationale for such a study is as follows. (i) The nfxC mutant expresses the MexEF-OprN efflux pump in the presence of a low level of MexAB-OprM. Therefore, the valid substrate for the MexEF-OprN pump was not clear. (ii) The nfxC mutant exhibits particularly intriguing properties, such as expressing the MexEF-OprN pump and gaining resistance to fluoroquinolone antibiotics, chloramphenicol, and trimethoprim; lacking the OprD protein, resulting in imipenem resistance; and showing hypersusceptibility to β-lactam antibiotics by an unknown mechanism. Therefore, the question of whether β-lactam hypersusceptibility is attributable to expression of MexEF-OprN or the nfxC mutation remains unanswered.
To address the former issue, we expressed the MexEF-OprN pump in the strain lacking MexAB-OprM and, importantly, without an nfxC mutation. Under these conditions, the MICs of the antibiotics appeared to be determined by the MexEF-OprN pump without any influence of the MexAB-OprM pump and the nfxC mutation. The results were that the transformant exhibited resistance to fluoroquinolones, chloramphenicol, trimethoprim, and tetracycline but was susceptible to β-lactam antibiotics, including carbapenem, at the level of the host strain. One may argue that β-lactamase interferes with the efflux pump function. However, this is unlikely because the level of β-lactamase in the nfxC mutant was fully comparable with that in the strain having wild-type nfxC in the presence of carbenicillin (3).
Tetracycline resistance in nfxC mutants has been shown to be strain dependent (3, 8, 11). Our study clearly demonstrated that tetracycline is an excellent substrate of the MexEF-OprN pump. On the other hand, MexEF-OprN pump expression caused neither β-lactam hypersusceptibility nor carbapenem resistance.
To assign the subunit protein responsible for substrate selectivity, we carried out a subunit swapping experiment similar to earlier investigations (5, 26, 27). We found that the MexEF unit acts synergistically with OprM and that the hybrid pump functioned equally to the MexEF-OprN pump. Three independent groups of investigators have carried out experiments to exchange the subunit proteins OprM and OprJ (5, 26, 27). They concluded that OprM fully functions with the MexCD unit and OprJ partially functions with the MexAB unit (5, 26, 27). More recently, interplay between the MexXY pump and OprM in E. coli cells was demonstrated (12). Therefore, OprM seems to be a universal outer membrane subunit for most P. aeruginosa efflux pumps.
To our surprise, OprN showed undetectable collaboration with the MexAB unit. The reason for the dysfunction of the MexAB-OprN hybrid pump is not known at present. We tested the possibility of a dominant-negative phenotype by expressing OprN in the strain expressing the MexAB-OprM but could not find such interaction (data not shown). We assumed until recently that two efflux pump systems, MexAB-OprM and MexEF-OprN, functioned in the nfxC mutant. However, the present study revealed that the nfxC mutant expresses three efflux pumps simultaneously: MexAB-OprM, MexEF-OprN, and an additional pump, MexEF-OprM.
Another interesting finding of the subunit swapping experiment was that strain TNP0725 expressing MexAB-OprM showed a slightly higher aztreonam MIC than either TNP070 or TNP0715, both of which lack MexB. This result implies that the hybrid pump consisting of MexAB-OprM might recognize β-lactam antibiotics. Since the MexF subunit in the MexEF-OprN pump is not involved in the export of β-lactam antibiotics, it is possible that MexA in combination with MexF is involved in β-lactam recognition. If so, it is conceivable that MexA in the MexAB-OprM pump plays a role in β-lactam selectivity. Though the difference is rather small, this result was confirmed by repeated experiments.
ACKNOWLEDGMENTS
This study was supported in part by grants from the Ministry of Education of Japan, Science, Sport and Culture; the Emerging and Reemerging Infectious Diseases Program of the Ministry of Health and Welfare; the Japan Society for Promotion of Science; and Tokai University School of Medicine Project Research.
REFERENCES
- 1.Ausubel F M, Brent R E, Kingston R E, Moore D D, Seidman F G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: Greene and Wiley Interscience; 1995. [Google Scholar]
- 2.de Lorenzo V, Eltis L, Kessler B, Timmis K N. Analysis of Pseudomonas gene products using lacIq/Ptrp-lac plasmids and transposons that confer conditional phenotypes. Gene. 1993;123:17–24. doi: 10.1016/0378-1119(93)90533-9. [DOI] [PubMed] [Google Scholar]
- 3.Fukuda H, Hosaka M, Hirai K, Iyobe S. New norfloxacin resistance gene in Pseudomonas aeruginosa PAO. Antimicrob Agents Chemother. 1990;34:1757–1761. doi: 10.1128/aac.34.9.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fürste J P, Pansegrau W, Frank R, Blöcker H, Scholz P, Bagdasarian M, Lanka E. Molecular cloning of the plasmid RP4 primase region in a multi-host-range tacP expression vector. Gene. 1986;48:119–131. doi: 10.1016/0378-1119(86)90358-6. [DOI] [PubMed] [Google Scholar]
- 5.Gotoh N, Tsujimoto H, Nomura A, Okamoto K, Tsuda M, Nishino T. Functional replacement of OprJ by OprM in the MexCD-OprJ multidrug efflux system of Pseudomonas aeruginosa. FEMS Microbiol Lett. 1998;165:21–27. doi: 10.1111/j.1574-6968.1998.tb13122.x. [DOI] [PubMed] [Google Scholar]
- 6.Guan L, Ehrmann M, Yoneyama H, Nakae T. Membrane topology of the xenobiotic-exporting subunit, MexB, of the MexA,B-OprM extrusion pump in Pseudomonas aeruginosa. J Biol Chem. 1999;274:10517–10522. doi: 10.1074/jbc.274.15.10517. [DOI] [PubMed] [Google Scholar]
- 7.Hirai K, Suzue S, Irikura T, Iyobe S, Mitsuhashi S. Mutations producing resistance to norfloxacin in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1987;31:582–586. doi: 10.1128/aac.31.4.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Köhler T, Michéa-Hamzehpour M, Henze U, Gotoh N, Curty L K, Pechere J C. Characterization of MexE-MexF-OprN, a positively regulated multidrug efflux system of Pseudomonas aeruginosa. Mol Microbiol. 1997;23:345–354. doi: 10.1046/j.1365-2958.1997.2281594.x. [DOI] [PubMed] [Google Scholar]
- 9.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 10.Ma D, Cook D N, Hearst J E, Nikaido H. Efflux pumps and drug resistance in gram-negative bacteria. Trends Microbiol. 1994;2:489–493. doi: 10.1016/0966-842x(94)90654-8. [DOI] [PubMed] [Google Scholar]
- 11.Masuda N, Sakagawa E, Ohya S. Outer membrane proteins responsible for multiple drug resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1995;39:645–649. doi: 10.1128/AAC.39.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mine T, Morita Y, Kataoka A, Mizushima T, Tsuchiya T. Expression in Escherichia coli of a new multidrug efflux pump, MexXY, from Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1999;43:415–417. doi: 10.1128/aac.43.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morshed S R, Lei Y, Yoneyama H, Nakae T. Expression of genes associated with antibiotic extrusion in Pseudomonas aeruginosa. Biochem Biophys Res Commun. 1995;210:356–362. doi: 10.1006/bbrc.1995.1669. [DOI] [PubMed] [Google Scholar]
- 14.Nikaido H. Outer membrane barrier as a mechanism of antimicrobial resistance. Antimicrob Agents Chemother. 1989;33:1831–1836. doi: 10.1128/aac.33.11.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okazaki T, Hirai K. Cloning and nucleotide sequence of the Pseudomonas aeruginosa nfxB gene, conferring resistance to new quinolones. FEMS Microbiol Lett. 1992;76:197–202. doi: 10.1016/0378-1097(92)90386-3. [DOI] [PubMed] [Google Scholar]
- 16.Paulsen I T, Brown M H, Skurray R A. Proton-dependent multidrug efflux systems. Microbiol Rev. 1996;60:575–608. doi: 10.1128/mr.60.4.575-608.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poole K, Gotoh N, Tsujimoto H, Zhao Q, Wada A, Yamasaki T, Neshat S, Yamagishi J, Li X Z, Nishino T. Overexpression of the mexC-mexD-oprJ efflux operon in nfxB-type multidrug-resistant strains of Pseudomonas aeruginosa. Mol Microbiol. 1996;21:713–724. doi: 10.1046/j.1365-2958.1996.281397.x. [DOI] [PubMed] [Google Scholar]
- 18.Poole K, Krebes K, McNally C, Neshat S. Multiple antibiotic resistance in Pseudomonas aeruginosa: evidence for involvement of an efflux operon. J Bacteriol. 1993;175:7363–7372. doi: 10.1128/jb.175.22.7363-7372.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rella M, Haas D. Resistance of Pseudomonas aeruginosa PAO to nalidixic acid and low levels of beta-lactam antibiotics: mapping of chromosomal genes. Antimicrob Agents Chemother. 1982;22:242–249. doi: 10.1128/aac.22.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saier M H, Jr, Tam R, Reizer A, Reizer J. Two novel families of bacterial membrane proteins concerned with nodulation, cell division and transport. Mol Microbiol. 1994;11:841–847. doi: 10.1111/j.1365-2958.1994.tb00362.x. [DOI] [PubMed] [Google Scholar]
- 21.Saito K, Yoneyama H, Nakae T. nalB-type mutations causing the overexpression of the MexAB-OprM efflux pump are located in the mexR gene of the Pseudomonas aeruginosa chromosome. FEMS Microbiol Lett. 1999;179:67–72. doi: 10.1111/j.1574-6968.1999.tb08709.x. [DOI] [PubMed] [Google Scholar]
- 22.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 23.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schweizer H P. Allelic exchange in Pseudomonas aeruginosa using novel ColE1-type vectors and a family of cassettes containing a portable oriT and the counter-selectable Bacillus subtilis sacB marker. Mol Microbiol. 1992;6:1195–1204. doi: 10.1111/j.1365-2958.1992.tb01558.x. [DOI] [PubMed] [Google Scholar]
- 25.Simon R, O'Connell M, Labes M, Pühler A. Plasmid vector for the genetic analysis and manipulation of rhizobia and other gram-negative bacteria. Methods Enzymol. 1986;118:640–659. doi: 10.1016/0076-6879(86)18106-7. [DOI] [PubMed] [Google Scholar]
- 26.Srikumar R, Li X Z, Poole K. Inner membrane efflux components are responsible for β-lactam specificity of multidrug efflux pumps in Pseudomonas aeruginosa. J Bacteriol. 1997;179:7875–7881. doi: 10.1128/jb.179.24.7875-7881.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoneyama H, Ocaktan A, Gotoh N, Nishino T, Nakae T. Subunit swapping in the Mex-extrusion pumps in Pseudomonas aeruginosa. Biochem Biophys Res Commun. 1998;244:898–902. doi: 10.1006/bbrc.1998.8351. [DOI] [PubMed] [Google Scholar]
- 28.Yoneyama H, Ocaktan A, Tsuda M, Nakae T. The role of mex gene products in antibiotic extrusion in Pseudomonas aeruginosa. Biochem Biophys Res Commun. 1997;233:6111–6118. doi: 10.1006/bbrc.1997.6506. [DOI] [PubMed] [Google Scholar]
- 29.Yoneyama H, Yamano Y, Nakae T. Role of porins in the antibiotic susceptibility of Pseudomonas aeruginosa: construction of mutants with deletions in the multiple porin genes. Biochem Biophys Res Commun. 1995;213:88–95. doi: 10.1006/bbrc.1995.2102. [DOI] [PubMed] [Google Scholar]
- 30.Yoneyama, H., and T. Nakae. J. Biol. Chem., in press.