Abstract
Objectives
The spousal relationship is one of the most important social contexts in old age, and the loss of a spouse/partner is associated with stress and cognitive decline. In the present study, we examined whether social relationships can buffer potential negative effects of spousal loss on cognition. We examined the role of social network, social activities, and perceived deficiencies in social relationships (loneliness).
Method
We used longitudinal data between 1998 and 2012 from 2,074 participants of the Health and Retirement Study, who had experienced spousal loss during the study period. Multilevel modeling was used to examine how time-varying indicators of social network, social activities, and loneliness were related to age-related trajectories of episodic memory prior to and after spousal loss. Analyses controlled for gender, race/ethnicity, education, time-varying functional health, and being repartnered/remarried.
Results
Having children living within 10 miles and providing help to others buffered negative effects of widowhood on episodic memory. In addition, within-person increase in providing help to others buffered against decline in episodic memory after spousal loss. Having friends in the neighborhood, more frequent social visits, providing help to others, volunteering, and lack of loneliness were related to higher episodic memory, while having relatives in the neighborhood was related to lower episodic memory.
Discussion
Our findings suggest that social networks, social activities, and loneliness are related to levels of cognitive function at the time of spousal loss and that social relationships can buffer negative effects of spousal loss on cognitive function. Implications for future research are discussed.
Keywords: Episodic memory, Health and Retirement Study, Longitudinal, Social relationships, Spousal loss, Widowhood
Theoretical perspectives and empirical evidence suggest that close social relationships become more important as people age (Carstensen et al., 1999). For older adults in the United States, living with a spouse is the most common living arrangement (Stepler, 2016). As such, the spousal relationship is one of the most important social contexts in old age. Research has widely documented that spousal loss is a devastatingly stressful experience for older adults that substantially affects longitudinal trajectories of health, well-being, and mortality (Luhmann et al., 2012; Nakagawa & Hülür, 2021; Stroebe & Stroebe, 1987). Accumulating evidence suggests that spousal loss is also associated with cognitive decline (Biddle et al., 2020; Liu et al., 2019; Shin et al., 2018; Zhang et al., 2019). Social relationships can provide direct positive effects and/or buffer adverse effects of stressful experiences (Cohen & Wills, 1985). The goal of the present study was to examine whether social relationships can buffer potential adverse effects of spousal loss on cognition. We focused on three aspects of social relationships, including social networks, social activities, and perceived deficiencies in social relationships as indicated by loneliness.
Spousal Loss and Cognitive Function
Widowhood is a major life transition typically experienced in old age and is associated with adverse outcomes in several domains, including well-being and life satisfaction (Luhmann et al., 2012; Nakagawa & Hülür, 2021; Rossi et al., 2007), physical health (Infurna & Luthar, 2017; Stroebe & Stroebe, 1987), mental health (Ong et al., 2010), and mortality (Moon et al., 2011; Stroebe & Stroebe, 1987). Accumulating evidence from longitudinal studies suggests that spousal loss is also associated with adverse effects on cognitive function, including the domains of memory and executive function as well as clinical outcomes (Aartsen et al., 2005; Biddle et al., 2020; van Gelder et al., 2006; Liu et al., 2019; Shin et al., 2018; Zhang et al., 2019). For example, Shin et al. (2018) reported that older adults who were widowed showed more decline in episodic memory compared with older adults who were not widowed. In addition, they found a linear association between time since spousal loss and cognitive decline.
Possible explanations for the effects of spousal loss on cognitive function center around two major mechanisms. First, spousal loss is a major stressor, as evidenced by its substantial detrimental effects on well-being and health outcomes (Luhmann et al., 2012; Stroebe & Stroebe, 1987). Adverse effects of stress on cognitive function are well documented in the literature (Marin et al., 2011). Second, older adults experiencing spousal loss may be at risk for social isolation, which is associated with a lack of cognitive stimulation (Cacioppo & Hawkley, 2009; Evans et al., 2018). Social isolation is associated with adverse health outcomes in multiple domains (Steptoe et al., 2013), including cognition (Evans et al., 2019; Shankar et al., 2013).
According to the “use-it-or-lose-it” hypothesis of cognitive aging, social activities provide cognitive stimulation and are considered beneficial to cognitive aging (Hertzog et al., 2008). Similarly, according to the theory of cognitive reserve (Stern, 2009), it has been argued that social activities help build cognitive reserve (Wang et al., 2002).
Spousal Loss and Cognitive Function: The Buffering Role of Social Relationships
According to the buffering hypothesis, social relationships can buffer adverse effects of stressful experiences (Cohen & Wills, 1985). A large body of research examined the role of social relationships in adaptation to spousal loss and widowhood. Most of this research focused on adaptation in terms of subjective well-being. With regard to social networks, the time period after spousal loss is associated with an increase in contact with children (Roan & Raley, 1996) and an increase in extended family (Anderson, 1984) and friend (Zettel & Rook, 2004) relationships. Based on these results, it has been argued that bereaved individuals turn to these contacts for social support, which can in turn reduce the stress associated with spousal loss. With regard to social activities, informal social activities are also higher among widowed (vs. married) older adults (Utz et al., 2002), and greater frequency of social contact is associated with fewer depressive symptoms among bereaved individuals (Ha & Ingersoll-Dayton, 2011). Similarly, in a study with bereaved individuals, helping others buffered effects of widowhood on depressive symptoms (Brown et al., 2008), and volunteering was also associated with fewer depressive symptoms in bereaved individuals (Li, 2007). Spousal loss is associated with an increase in loneliness (Dykstra et al., 2005). Prior research has linked loneliness with cognitive decline in middle-aged and older adults (Lara et al., 2019).
It is less widely studied whether social relationships show direct or buffering effects for the impact of spousal loss on cognitive outcomes. Social relationships may provide bereaved individuals opportunities for social interaction and cognitive stimulation. With regard to social network factors, research has shown that widowed individuals without children had higher risk of dementia than widowed parents (Sundström et al., 2014). Also, having at least one living sibling buffered the effect of widowhood on cognitive function (Shin et al., 2018). Research has shown that engaging in mentally stimulating activities had a stronger effect on cognitive function in widowed older adults compared with those who were married (Lee et al., 2019). Although social activities were related to higher levels of cognitive function, there was no interaction with marital status, indicating that effects were similar for widowed and married participants. However, this study only examined social activities at one point in time. Another study found that a global indicator of social engagement was associated with lower risk of cognitive impairment in single and widowed older adults (Feng et al., 2014). This study did not examine whether the effects of social engagement differed depending on marital status. Taken together, it is less clear which aspects of social relationships and changes therein are related to trajectories of cognitive function surrounding spousal loss.
The Present Study
In this study, we examined trajectories of age-related change in episodic memory following spousal loss. We hypothesized that spousal loss would be associated with a decline in episodic memory and with a steeper decline in episodic memory in the period following spousal loss. Furthermore, we expected social relationships to buffer the effects of spousal loss on episodic memory and to have a stronger effect on cognitive function in the period following spousal loss compared with the preloss period. We focused on three different aspects of social relationships, including social network, social activities, and perceived deficiencies in social relationships. We controlled for variables that may affect both the predictor (social relationships) and the outcome (episodic memory). The control variables included gender, education, race/ethnicity, functional health, and being remarried or repartnered after spousal loss. Being remarried or repartnered was included as a control variable because it may both increase an individual’s social network and activities and reduce feelings of loneliness and because it may counteract lack of cognitive stimulation in previously widowed individuals.
Method
Procedure and Participants
We used data from the Health and Retirement Study (HRS), a national population-based study of households in the United States that include at least one member who is 50 years old or older. Spouses/partners living in the same household were invited to participate regardless of age. Data collection started in 1992 and is performed every 2 years. Currently, the HRS includes data from more than 40,000 individuals. Detailed information about the procedure and participants is provided in previous work (Sonnega et al., 2014). Below, we present information relevant to the present study.
To examine the role of social relationships in episodic memory change with spousal loss, we used data from all participants who experienced the loss of a spouse between 1998 and 2012. Data were used from 1998 onward because (a) memory performance was assessed differently in the 1992 and 1994 waves of the HRS and (b) older cohorts joined the HRS in 1998 for the first time. Data from 2014 and 2016 were not used because some study variables (having friends and relatives in the neighborhood, frequency of social visits) were measured differently. We used data from participants who had (a) at least two data points with complete data, one before spousal loss and one after spousal loss, and (b) valid data on control variables. Data from 11 participants who experienced spousal loss multiple times in our study were excluded. Our analyses focused on episodic memory, because other cognitive measures in the HRS were not administered at all waves or were part of screening instruments and showed skewed distributions with very high rates of correct responses (McArdle et al., 2007). In summary, we used data from 2,074 participants, who contributed 12,932 longitudinal observations. On average, participants contributed 6.2 longitudinal observations (SD = 1.5, range = 2–8).
Measurements
Time metrics
The time metric age indicated the chronological age in years at each time point. The timing of widowhood was determined based on variables in the RAND HRS longitudinal file (Bugliari et al., 2019) indicating widowhood status at each wave. Eleven participants who experienced widowhood multiple times over the observation period were excluded from the analysis. We created a binary variable, coded 0 for all observations prior to spousal loss and coded 1 for all observations after spousal loss.
Episodic memory
Episodic memory was measured as the sum of immediate and delayed recall scores for a 10-item word list and ranged from 0 to 20 (Ofstedal et al., 2005). Participants were presented with a 10-item word list and asked to recall all words immediately and after a delay of approximately 5 min at each measurement occasion.
Social relationships
Social relationship variables included various indicators of social network, social activities, and perceived deficiencies in social relationships at each measurement occasion. Social network was measured with three indicators. Children residing within 10 miles was a binary variable (“yes” = 1; “no” = 0) based on participants’ responses to questions whether they had (a) any children, (b) coresident children, and (c) children living within 10 miles. Having relatives or friends in one’s neighborhood was indicated by two binary variables (“yes” = 1; “no” = 0). Social activities were measured by three variables. Frequency of social visits was coded into a 6-point scale (daily or more frequent = 5, at least weekly = 4, at least every 2 weeks = 3, at least monthly = 2, at least yearly = 1, almost never = 0). Providing help was a binary variable with any amount of help coded as 1 and not providing help coded as 0. Volunteering in the past 12 months was a binary variable (“yes” = 1; “no” = 0). Perceived deficiencies in social relationships were indicated by loneliness as assessed with a single item from the Center for Epidemiologic Studies Depression scale (Steffick, 2000). Participants reported whether they felt lonely in the previous week (“yes” = 1; “no” = 0).
Covariates
Control variables included gender (women = 1; men = 0), years of education, race/ethnicity (non-Hispanic White = 1; Other = 0), functional limitations with activities of daily living (ADLs; range = 0–6; including number of impairments in walking, bathing, eating, toileting, dressing, and transferring in and out of bed), and being repartnered or remarried after spousal loss (“yes” = 1; “no” = 0).
Statistical Analyses
We used multilevel models to examine age-related trajectories of episodic memory surrounding spousal loss (Singer et al., 2003). As a first step, we specified an unconditional model (Model 1) including the effects of the two time metrics, age and widowhood, and no other predictors. Age was centered at 73 years, the average age at widowhood in the present sample. At the within-person level (Level 1), we specified Model 1 as:
| (1) |
where Episodic memoryti, person i’s episodic memory score at occasion t, is a function of an individual-specific intercept parameter, β 0i that indicates memory performance at the time of and average age of spousal loss; individual-specific slope parameters, β 1i, capturing linear change per year of age, and β 2i capturing the acceleration of change per year of age; the difference in memory performance between the time periods before and after spousal loss, β 3i; the difference in age-related memory change between the time periods before and after spousal loss, β 4i; and residual error, eti.
Individual-specific parameters (Level 2) were modeled as
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
where the γ parameters represent sample-level averages and the u parameters represent individual-specific deviations from these sample-level averages. Individual-specific deviations were not modeled for βs 2 through 4 to achieve model convergence.
Models 2 examined the effects of social relationship variables. Following recommendations in the literature (Bolger & Laurenceau, 2013; Schwartz & Stone, 1998), the effects of time-varying variables were separated into between-person (BP) and within-person (WP) components (see also Elayoubi et al., 2021; Hülür, 2021). Between-person means were calculated by taking the average of a variable across all available observations for each individual. For example, the between-person mean of social visits indicated the average frequency of social visits across an individual’s time series. Between-person means were centered at sample means. Within-person change was calculated as within-person deviation from the between-person average on each occasion. For example, the within-person change variable indicated changes in the frequency of social visits from the between-person mean at each time point.
At the within-person level (Level 1), models were specified as:
| (7) |
where the additional parameter β 5i indicates the effect of within-person change in a social relationship variable from its between-person average on memory performance and the additional parameter β 6i indicates whether this effect differs across the time periods before and after spousal loss.
Individual-specific parameters (Level 2) were modeled as
| (8) |
| (9) |
| (10) |
| (11) |
| (12) |
| (13) |
| (14) |
where the γ parameters represent sample-level averages and sample-level associations (γ 01, the effect of the between-person mean of a predictor variable on memory function at spousal loss; γ 11, the effect of the between-person mean of a predictor variable on linear change over time [effects on quadratic change were omitted for parsimony]; γ 31, indicating the effect of the between-person mean of a predictor variable on memory change with spousal loss), and the u parameters represent individual-specific deviations from these sample-level parameters. Some u parameters were omitted to achieve model convergence. In addition, we controlled for effects of gender, education, race/ethnicity, and being repartnered/remarried centered at sample means. Limitations in ADLs were separated into a between-person mean (centered at the sample mean) and within-person change component and entered as control variables. Follow-up analyses examined the role of living alone after spousal loss.
All analyses were conducted in SAS version 9.4 with PROC Mixed (Littell et al., 1996) with incomplete data treated as missing at random (Little & Rubin, 1987). Statistical significance was assessed at p < .05 (two-sided).
Results
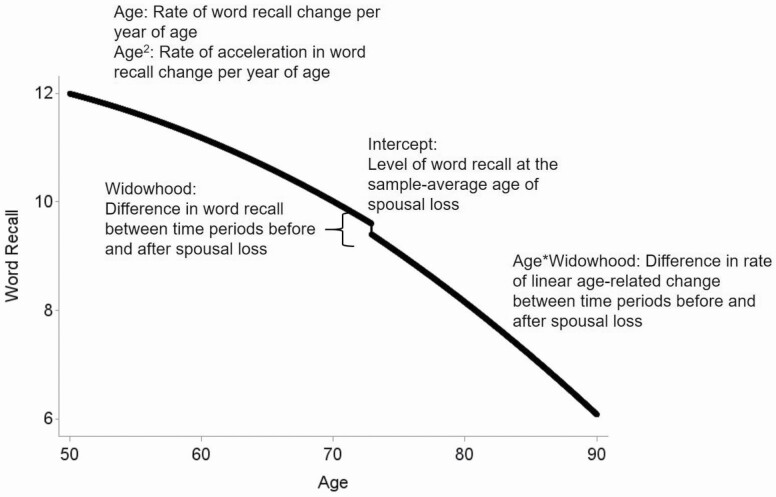
Table 1 presents descriptive statistics and intercorrelations for study variables taken at the earliest observation after spousal loss. Table 2 presents the results of the unconditional growth curve model examining age-related changes in episodic memory with spousal loss. Results of this model are illustrated in Figure 1. The fixed effect of the intercept indicates that people on average recalled approximately 10 words out of 20 at baseline, that is, at the time of spousal loss and at the age of 73 years, the average age at spousal loss in the present sample (γ = 9.60, SE = 0.07, p < .01). The fixed effect of linear age indicates that episodic memory declined at a rate of 0.14 words per year of age (γ = −0.14, SE = 0.01, p < .01). The quadratic effect of age indicated accelerated decline in episodic memory with age (γ = −0.002, SE < 0.01, p < .01). In line with our hypothesis, participants recalled 0.2 fewer words in the period after spousal loss compared with the period before spousal loss (γ = −0.20, SE = 0.06, p < .01). In addition, the rate of age-related change in episodic memory was faster in the period after spousal loss (γ = −0.02, SE = 0.01, p < .01). Random effects showed that participants differed in levels of episodic memory at baseline (u = 5.07, SE = 0.20, p < .01) and in the rate of age-related change in episodic memory (u = 0.01, SE < 0.01, p< .01).
Table 1.
Baseline Descriptive Characteristics and Intercorrelations of Study Variables
| Variables | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Age at spousal loss | ||||||||||||||
| 2. Gender (women) | −0.15* | |||||||||||||
| 3. Race/ethnicity (non-Hispanic White) | 0.18* | −0.04 | ||||||||||||
| 4. Education | 0.00 | −0.01 | 0.36* | |||||||||||
| 5. Functional limitations | 0.16* | −0.03 | −0.08* | −0.14* | ||||||||||
| 6. Repartnered/remarried | −0.20* | −0.20* | 0.02 | 0.07* | −0.03 | |||||||||
| 7. SN: Close children | −0.04 | 0.03 | −0.07* | −0.12* | 0.02 | −0.07* | ||||||||
| 8. SN: Relatives | 0.01 | 0.02 | 0.02 | −0.07* | 0.03 | −0.03 | 0.10* | |||||||
| 9. SN: Friends | 0.08* | 0.01 | 0.05* | 0.00 | −0.08* | 0.01 | −0.08* | 0.06* | ||||||
| 10. SA: Social visits | 0.07* | −0.03 | 0.07* | 0.02 | −0.07* | −0.01 | −0.11* | 0.02 | 0.44* | |||||
| 11. SA: Providing help | −0.18* | 0.01 | 0.10* | 0.19* | −0.20* | 0.06* | −0.06* | 0.00 | 0.14* | 0.16* | ||||
| 12. SA: Volunteering | −0.07* | 0.09* | 0.06* | 0.22* | −0.16* | 0.01 | −0.05* | 0.00 | 0.12* | 0.10* | 0.31* | |||
| 13. PD: Loneliness | 0.05* | −0.03 | 0.01 | −0.09* | 0.10* | −0.12* | −0.02 | 0.01 | −0.02 | −0.02 | −0.09* | −0.09* | ||
| 14. Word recall | −0.37* | 0.20* | 0.12* | 0.30* | −0.24* | 0.06* | −0.03 | −0.06* | 0.02 | 0.01 | 0.25* | 0.21* | −0.11* | |
| M | 72.90 | 0.74 | 0.76 | 11.85 | 0.36 | 0.09 | 0.68 | 0.27 | 0.68 | 2.76 | 0.39 | 0.30 | 0.36 | 9.04 |
| SD | 9.87 | 3.20 | 0.90 | 1.88 | 3.60 |
Notes: N = 2,074. M = mean; SD = standard deviation; SN = social network; SA = social activity; PD = perceived deficiencies in social relationships. Close children: presence of children residing within 10 miles (0 = no, 1 = yes). Repartnered: married or cohabitating with partner after spousal loss (0 = no, 1 = yes). Friends: presence of friends living in the neighborhood (0 = no, 1 = yes). Relatives: presence of relatives living in the neighborhood (0 = no, 1 = yes). Loneliness: measured with the perceived loneliness item from the Center for Epidemiologic Studies—Depression scale (0 = no, 1 = yes). Providing help: unpaid help to friends or family in the neighborhood (0 = no, 1 = yes). Social visits: frequency of social visits with friends or relatives in the neighborhood (on a scale from 0 to 5, higher values indicate higher frequency). Volunteering: volunteering in the community (0 = no, 1 = yes).
*p < .05.
Table 2.
Results From Multilevel Model Examining Trajectories of Word Recall Surrounding Spousal Loss
| Variable | Estimate | SE |
|---|---|---|
| Fixed effects | ||
| Intercept | 9.60* | 0.07 |
| Age | −0.14* | 0.01 |
| Age2 | −0.002* | <0.01 |
| Widowhood | −0.20* | 0.06 |
| Age × Widowhood | −0.02* | 0.01 |
| Random effects | ||
| Variance intercept | 5.07* | 0.20 |
| Variance age | 0.01* | <0.01 |
| Covariance intercept and age | −0.02* | 0.01 |
| Residual variance | 5.44* | 0.08 |
Notes: N = 2,074. SE = standard error. Age is scaled in years and centered at 73 years, the average age at spousal loss in the present sample. Widowhood is coded 0 for all observations before spousal loss and 1 for all observations after spousal loss.
*p < .05.
Figure 1.
Average trajectory of word recall surrounding spousal loss.
Table 3 presents results from models examining the role of social relationship variables on age-related trajectories of episodic memory surrounding spousal loss while controlling for relevant covariates (gender, education, race/ethnicity, time-varying functional limitations with ADLs, and being remarried/repartnered after spousal loss). Having friends in the neighborhood (γ = 0.42, SE = 0.17, p = .01), more frequent social visits (γ = 0.09, SE = 0.04, p = .03), providing help to others (γ = 0.49, SE = 0.18, p = .01), volunteering (γ = 0.80, SE = 0.16, p < .01), and lack of loneliness (γ = −1.06, SE = 0.20, p < .01) were related to better episodic memory at baseline. Having relatives in the neighborhood was related to worse episodic memory at baseline (γ = −0.34, SE = 0.16, p = .03).
Table 3.
Associations Between Social Relationships and Trajectories of Memory Change With Spousal Loss: Findings From Models Including Control Variables (Gender, Education, Race/Ethnicity, Time-Varying Functional Health, and Repartnered/Remarried)
| Social network | Social activities | Perceived deficiencies | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Children within 10 miles | Relatives in the neighborhood | Friends in the neighborhood | Frequency of social visits | Providing help | Volunteering | Loneliness | |||||||
| Fixed effects | γ | SE | γ | SE | γ | SE | γ | SE | γ | SE | γ | SE | γ | SE |
| Intercept | 9.60* | 0.06 | 9.60* | 0.06 | 9.59* | 0.06 | 9.60* | 0.06 | 9.60* | 0.06 | 9.59* | 0.06 | 9.60* | 0.06 |
| Age | −0.14* | 0.01 | −0.14* | 0.01 | −0.14* | 0.01 | −0.14* | 0.01 | −0.14* | 0.01 | −0.14* | 0.01 | −0.14* | 0.01 |
| Age2 | −0.002* | <0.01 | −0.002* | <0.01 | −0.002* | <0.01 | −0.002* | <0.01 | −0.002* | <0.01 | −0.002* | <0.01 | −0.002* | <0.01 |
| Widowhood | −0.22* | 0.06 | −0.23* | 0.06 | −0.20* | 0.06 | −0.22* | 0.06 | −0.25* | 0.06 | −0.22* | 0.06 | −0.24* | 0.06 |
| Widowhood × Age | −0.01 | 0.01 | −0.01 | 0.01 | −0.01* | 0.01 | −0.01 | 0.01 | −0.01 | 0.01 | −0.01* | 0.01 | −0.02 | 0.01 |
| BP mean | −0.27 | 0.13 | −0.34* | 0.16 | 0.42* | 0.17 | 0.09* | 0.04 | 0.49* | 0.18 | 0.80* | 0.16 | −1.06* | 0.20 |
| BP mean × Age | −0.01 | 0.01 | −0.02 | 0.02 | −0.02 | 0.02 | −0.01 | <0.01 | −0.03 | 0.02 | 0.00 | 0.02 | 0.01 | 0.02 |
| BP mean × Widowhood | 0.39* | 0.14 | 0.03 | 0.17 | −0.02 | 0.17 | −0.07 | 0.04 | 0.47* | 0.19 | 0.32 | 0.17 | −0.29 | 0.21 |
| BP mean × Age × Widowhood | 0.01 | 0.01 | 0.00 | 0.02 | −0.01 | 0.02 | 0.00 | <0.01 | −0.01 | 0.02 | 0.00 | 0.02 | 0.03 | 0.02 |
| WP change | 0.07 | 0.11 | 0.06 | 0.09 | 0.04 | 0.08 | 0.04* | 0.02 | 0.07 | 0.07 | 0.17 | 0.09 | −0.04 | 0.08 |
| WP change × Widowhood | −0.16 | 0.18 | −0.11 | 0.14 | 0.04 | 0.13 | 0.00 | 0.03 | 0.29* | 0.12 | 0.10 | 0.15 | 0.25 | 0.13 |
| Random effects | u | SE | u | SE | u | SE | u | SE | u | SE | u | SE | u | SE |
| Var (intercept) | 3.18* | 0.14 | 3.18* | 0.14 | 3.18* | 0.14 | 3.19* | 0.14 | 3.12* | 0.14 | 3.09* | 0.14 | 3.11* | 0.14 |
| Var (age) | 0.005* | <0.01 | 0.005* | <0.01 | 0.005* | <0.01 | 0.005* | <0.01 | 0.005* | <0.01 | 0.005* | <0.01 | 0.005* | <0.01 |
| Covar (intercept, age) | −0.01 | 0.01 | −0.01 | 0.01 | −0.01 | 0.01 | −0.01 | 0.01 | −0.01 | 0.01 | −0.01 | 0.01 | −0.01 | 0.01 |
| Residual variance (eti) | 5.43* | 0.08 | 5.42* | 0.08 | 5.43* | 0.08 | 5.42* | 0.08 | 5.42* | 0.08 | 5.42* | 0.08 | 5.43* | 0.08 |
Notes: N = 2,074. SE = standard error; BP = between-person; WP = within-person; Var = variance; Covar = covariance. Age is scaled in years and centered at 73 years, the average age at spousal loss in the present sample. Widowhood is coded 0 for all observations before spousal loss and 1 for all observations after spousal loss.
*p < .05.
In line with our hypothesis that social relationships buffer negative effects of widowhood, the between-person averages of having children living within 10 miles (γ = 0.39, SE = 0.14, p = .01) and providing help to others (γ = 0.47, SE = 0.19, p = .01) buffered the between-person effects of spousal loss on levels of episodic memory.
Within-person increase in the frequency of social visits was related to better episodic memory (γ = 0.04, SE = 0.02, p = .02). In other words, engaging in more social visits than usual at a given occasion was related to better episodic memory than usual.
Finally, interaction effects between spousal loss and within-person change in providing help (γ = 0.29, SE = 0.12, p = .02) indicated that episodic memory was higher on occasions after spousal loss when providing help increased. This finding is in line with our hypothesis that effects of social relationships as a buffer against the negative effects of widowhood would be more protective in the time period following spousal loss.
Table 4 presents the associations of control variables with trajectories of episodic memory. Women (γ = 1.24, SE = 0.12, p < .01), participants with higher levels of education (γ = 0.29, SE = 0.02, p < .01), who identified as non-Hispanic White (γ = 1.19, SE = 0.14, p < .01), and those with fewer functional limitations than others (γ = −0.44, SE = 0.09, p < .01) showed higher levels of episodic memory performance. The effect of spousal loss on level of episodic memory was stronger for participants identifying as non-Hispanic White (γ = −0.41, SE = 0.14, p < .01). Participants showed lower levels of episodic memory performance than usual on occasions when they reported more functional limitations than their own average (γ = −0.17, SE = 0.06, p < .01). This effect was stronger in the time period after spousal loss (γ = −0.22, SE = 0.09, p = .01).
Table 4.
Trajectories of Memory Change With Spousal Loss: The Effects of Control Variables
| Variable | Estimate | SE |
|---|---|---|
| Fixed effects | ||
| Intercept | 9.61* | 0.06 |
| Age | −0.14* | 0.01 |
| Age2 | −0.002* | <0.01 |
| Widowhood | −0.23* | 0.06 |
| Age × Widowhood | −0.01 | 0.01 |
| Women | 1.24* | 0.12 |
| Women × Age | −0.01 | 0.01 |
| Women × Widowhood | −0.03 | 0.13 |
| Women × Widowhood × Age | −0.01 | 0.01 |
| Non-Hispanic White | 1.19* | 0.14 |
| Non-Hispanic White × Age | 0.02 | 0.01 |
| Non-Hispanic White × Widowhood | −0.41* | 0.14 |
| Non-Hispanic White × Widowhood × Age | −0.01 | 0.01 |
| Education | 0.29* | 0.02 |
| Education × Age | 0.00 | <0.01 |
| Education × Widowhood | 0.01 | 0.02 |
| Education × Widowhood × Age | 0.00 | <0.01 |
| BP mean FL | −0.44* | 0.09 |
| BP mean FL × Age | 0.00 | 0.01 |
| BP mean FL × Widowhood | −0.15 | 0.09 |
| BP mean FL × Widowhood × Age | 0.00 | <0.01 |
| WP change in FL | −0.17* | 0.06 |
| WP change in FL × Widowhood | −0.22* | 0.09 |
| Repartnered | −0.05 | 0.23 |
| Repartnered × Age | −0.02 | 0.02 |
| Repartnered × Widowhood | −0.03 | 0.22 |
| Repartnered × Widowhood × Age | 0.02 | 0.02 |
| Random effects | ||
| Variance intercept | 3.19* | 0.14 |
| Variance age | 0.005* | <0.01 |
| Covariance intercept and age | −0.01 | 0.01 |
| Residual variance | 5.43* | 0.08 |
Notes: N = 2,074. SE = standard error; FL = functional limitations; BP = between-person; WP = within-person. Age is scaled in years and centered at 73 years, the average age at spousal loss in the present sample. Widowhood is coded 0 for all observations before spousal loss and 1 for all observations after spousal loss.
*p < .05.
Follow-Up Analyses
Follow-up analyses examined the role of living alone after spousal loss, which can be considered another indicator of an individual’s social network. Fifty-eight percent of participants lived alone after spousal loss. Participants who lived alone after spousal loss had higher levels of episodic memory performance than others (γ = 0.63, SE = 0.13, p < .01). Living alone after spousal loss was unrelated to change in episodic memory with spousal loss (γ = −0.02, SE = 0.12, p = .85), to the rate of age-related decline in episodic memory (γ = 0.01, SE = 0.01, p = .23), and to the rate of age-related decline in episodic memory following spousal loss (γ = 0.00, SE = 0.01, p = .86).
Discussion
The present study aimed to examine the role of social relationships in age-related trajectories of episodic memory surrounding spousal loss focusing on three different aspects of social relationships, including social network, social activities, and perceived deficiencies in social relationships (loneliness). To do so, we used 14-year longitudinal data from participants of the HRS who experienced spousal loss during the study. Our findings showed that spousal loss was associated with lower levels of episodic memory and with a higher rate of age-related episodic memory decline. In line with our hypothesis that social relationships would buffer negative effects of spousal loss on episodic memory, having children living within 10 miles and providing help to others were associated with less negative effects of widowhood on episodic memory. In line with our second hypothesis that social relationships would show stronger effects with episodic memory after spousal loss, within-person change in helping others was associated with within-person change in episodic memory after spousal loss. Taken together, these findings support the buffering hypothesis of social relationships (Cohen & Wills, 1985) by showing that one’s social network and social activities can buffer negative effects of spousal loss on cognition during the transition to widowhood. Having children living nearby can buffer the adverse effects of spousal loss through several mechanisms. Adult children can provide social support to parents (Silverstein et al., 1996), which may buffer adverse effects of spousal loss on well-being (Silverstein & Bengtson, 1994) which may in turn affect memory function (Gerstorf et al., 2007; Hittner et al., 2020). Also, interacting with children (and potentially grandchildren) can be a cognitively stimulating activity (Sneed & Schulz, 2019). Likewise, helping others may promote cognitive reserve (Stern, 2009) and serve as a cognitively enriching activity (Hertzog et al., 2008).
Our findings showed several other direct associations between social relationships and episodic memory. Participants with higher average levels of having friends in the neighborhood, frequency of social visits, volunteering, and providing help, and lower average levels of loneliness showed higher levels of memory performance than others. The finding that people with larger networks, who are more socially active, and feel less lonely show higher levels of memory performance is consistent with earlier research (Evans et al., 2019; James et al., 2011; Marioni et al., 2015). However, participants who had relatives living in the neighborhood had overall lower levels of episodic memory performance than others and participants who lived alone following spousal loss had overall higher levels of episodic memory performance. Taken together, these findings indicate that participants with higher levels of cognitive function may be able to live independently and may not need to live with or in close proximity of relatives. Having relatives in the neighborhood or living alone was unrelated to adjustment to spousal loss.
Associations with covariates were in line with previous research, with women, participants with higher levels of education, and better average functional health showing higher levels of memory performance (Hülür et al., 2018; Nelson et al., 2020). Within-person change from average levels of functional health was related to within-person change in episodic memory before spousal loss and the association became stronger after spousal loss. This finding indicates that the spousal relationship may have buffered negative effects of functional limitations on episodic memory prior to widowhood. The lack of a significant gender by widowhood interaction effect for cognitive decline was interesting, because this effect is commonly reported in studies of widowhood and mortality, with men showing increased mortality after the death of a spouse and women showing no impact or improvement in mortality (Moon et al., 2011; Stahl et al., 2016). Finally, participants who identified as non-Hispanic White showed higher levels of episodic memory performance (Sharifian et al., 2019), but more decline in episodic memory with spousal loss. Research has shown that non-Hispanic White individuals in the United States draw a stronger line between nuclear versus extended family and have weaker ties to extended family than Hispanic or Black non-Hispanic individuals (Comeau, 2012). Therefore, for non-Hispanic White individuals, the effect of spousal loss may be less likely to be buffered by social support received from other sources.
Spouses are caregivers for 38.6% of community-dwelling older adults at the end of life in the United States (Ornstein et al., 2017). End-of-life care is perceived as highly stressful (Ornstein et al., 2017), and negative effects of stress on cognition are well documented (Marin et al., 2011). Therefore, it is an open question for future research on how the caregiving context affects changes in cognition surrounding spousal loss.
Practical implications of these findings include the importance of social networks and social activities for maintaining memory function in the transition to widowhood. Thus, interventions should aim to increase social activity, especially in individuals who may not have a support network nearby (e.g., children living within 10 miles). Support groups may also be beneficial for cognitive functioning for bereaved individuals by providing an outlet for social engagement, such as providing and receiving help.
Limitations and Outlook
In closing, we note several limitations of the present study. Because the assessments were taken at 2-year intervals, it was not possible to examine immediate effects of spousal loss. Over the observation period (1998–2012), available measures of social relationships were limited and did not take several important functional aspects of social relationships into account, such as relationship quality (Seeman et al., 2001), grief (Carnelley et al., 2006), social support (La Fleur & Salthouse, 2017), or social strain (Tun et al., 2013). For example, research has shown that social support helped to buffer negative effects of widowhood on various indicators of well-being (Silverstein & Bengtson, 1994). Our study focused on a single domain of cognitive function, episodic memory. It is an open question whether findings can be generalized to other cognitive domains. The timing of widowhood was determined based on information from married participants. Therefore, our analyses excluded participants who experienced the loss of a partner in a cohabiting relationship. Finally, the data were collected in a single country (United States). Data from other countries with different family and social structures (e.g., nuclear family- vs. extended family-centered, friend- vs. family-centered) would allow examination of the generalizability of these findings.
Conclusion
The present study adds to previous research by examining the buffering hypothesis in the context of age-related memory change during the widowhood transition. The findings show that having children nearby and helping others buffer the effects of spousal loss on episodic memory, even after controlling for sociodemographic factors (gender, race/ethnicity, education, being remarried/repartnered) and time-varying effects of functional health. Furthermore, within-person change in helping others is related to within-person change in episodic memory in the time period after spousal loss. Taken together, these findings suggest that social relationships play an important role in episodic memory in the transition to widowhood. More research is needed to understand underlying mechanisms.
Funding
This study is based on data from the publicly available Health and Retirement Study data set. The Health and Retirement Study was supported by a cooperative agreement (grant U01AG09740) between the National Institute on Aging and the University of Michigan (data and materials available at https://hrs.isr.umich.edu/). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Conflict of Interest
None declared.
Data Availability
The findings were not previously disseminated elsewhere. This study was not preregistered.
References
- Aartsen, M. J., Van Tilburg, T., Smits, C. H., Comijs, H. C., & Knipscheer, K. C. (2005). Does widowhood affect memory performance of older persons? Psychological Medicine, 35(2), 217–226. doi: 10.1017/s0033291704002831 [DOI] [PubMed] [Google Scholar]
- Anderson, T. B. (1984). Widowhood as a life transition: Its impact on kinship ties. Journal of Marriage and Family, 46(1), 105–114. doi: 10.2307/351869 [DOI] [Google Scholar]
- Biddle, K. D., Jacobs, H. I. L., d’Oleire Uquillas, F., Zide, B. S., Kirn, D. R., Properzi, M. R., Rentz, D. M., Johnson, K. A., Sperling, R. A., & Donovan, N. J. (2020). Associations of widowhood and β-amyloid with cognitive decline in cognitively unimpaired older adults. JAMA Network Open, 3(2), e200121. doi: 10.1001/jamanetworkopen.2020.0121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger, N., & Laurenceau, J.-P. (2013). Intensive longitudinal methods: An introduction to diary and experience sampling research. Guilford Press. [Google Scholar]
- Brown, S. L., Brown, R. M., House, J. S., & Smith, D. M. (2008). Coping with spousal loss: Potential buffering effects of self-reported helping behavior. Personality & Social Psychology Bulletin, 34(6), 849–861. doi: 10.1177/0146167208314972 [DOI] [PubMed] [Google Scholar]
- Bugliari, D., Campbell, N., Chan, C., Hayden, O., Hayes, J., Hurd, M., Karabatakis, A., Main, R., Mallett, J., & McCullough, C. (2019). RAND HRS longitudinal file 2016 (V1) documentation. RAND Center for the Study of Aging. [Google Scholar]
- Cacioppo, J. T., & Hawkley, L. C. (2009). Perceived social isolation and cognition. Trends in Cognitive Sciences, 13(10), 447–454. doi: 10.1016/j.tics.2009.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnelley, K. B., Wortman, C. B., Bolger, N., & Burke, C. T. (2006). The time course of grief reactions to spousal loss: Evidence from a national probability sample. Journal of Personality and Social Psychology, 91(3), 476–492. doi: 10.1037/0022-3514.91.3.476 [DOI] [PubMed] [Google Scholar]
- Carstensen, L. L., Isaacowitz, D. M., & Charles, S. T. (1999). Taking time seriously: A theory of socioemotional selectivity. American Psychologist, 54(3), 165–181. doi: 10.1037/0003-066X.54.3.165 [DOI] [PubMed] [Google Scholar]
- Cohen, S., & Wills, T. A. (1985). Stress, social support, and the buffering hypothesis. Psychological Bulletin, 98(2), 310–357. doi: 10.1037/0033-2909.98.2.310 [DOI] [PubMed] [Google Scholar]
- Comeau, J. A. (2012). Race/ethnicity and family contact: Toward a behavioral measure of familialism. Hispanic Journal of Behavioral Sciences, 34(2), 251–268. doi: 10.1177/0739986311435899 [DOI] [Google Scholar]
- Dykstra, P. A., van Tilburg, T. G., & Gierveld, J. de J.(2005). Changes in older adult loneliness: Results from a seven-year longitudinal study. Research on Aging, 27(6), 725–747. doi: 10.1177/0164027505279712 [DOI] [Google Scholar]
- Elayoubi, J., Nelson, M. E., Haley, W. E., & Hueluer, G.(2021). The role of social connection/engagement in episodic memory change in stroke. The Gerontologist. doi: 10.1093/geront/gnab095 [DOI] [PubMed] [Google Scholar]
- Evans, I. E. M., Llewellyn, D. J., Matthews, F. E., Woods, R. T., Brayne, C., & Clare, L.; CFAS-Wales Research Team . (2018). Social isolation, cognitive reserve, and cognition in healthy older people. PLoS One, 13(8), e0201008. doi: 10.1371/journal.pone.0201008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans, I. E. M., Martyr, A., Collins, R., Brayne, C., & Clare, L. (2019). Social isolation and cognitive function in later life: A systematic review and meta-analysis. Journal of Alzheimer’s Disease, 70(s1), S119–S144. doi: 10.3233/JAD-180501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng, L., Ng, X. T., Yap, P., Li, J., Lee, T. S., Håkansson, K., Kua, E. H., & Ng, T. P. (2014). Marital status and cognitive impairment among community-dwelling Chinese older adults: The role of gender and social engagement. Dementia and Geriatric Cognitive Disorders Extra, 4(3), 375–384. doi: 10.1159/000358584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gelder, B. M., Tijhuis, M., Kalmijn, S., Giampaoli, S., Nissinen, A., & Kromhout, D. (2006). Marital status and living situation during a 5-year period are associated with a subsequent 10-year cognitive decline in older men: The FINE Study. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 61(4), 213–219. doi: 10.1093/geronb/61.4.p213 [DOI] [PubMed] [Google Scholar]
- Gerstorf, D., Lövdén, M., Röcke, C., Smith, J., & Lindenberger, U. (2007). Well-being affects changes in perceptual speed in advanced old age: Longitudinal evidence for a dynamic link. Developmental Psychology, 43(3), 705–718. doi: 10.1037/0012-1649.43.3.705 [DOI] [PubMed] [Google Scholar]
- Ha, J. H., & Ingersoll-Dayton, B. (2011). Moderators in the relationship between social contact and psychological distress among widowed adults. Aging & Mental Health, 15(3), 354–363. doi: 10.1080/13607863.2010.519325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzog, C., Kramer, A. F., Wilson, R. S., & Lindenberger, U. (2008). Enrichment effects on adult cognitive development: Can the functional capacity of older adults be preserved and enhanced? Psychological Science in the Public Interest, 9(1), 1–65. doi: 10.1111/j.1539-6053.2009.01034.x [DOI] [PubMed] [Google Scholar]
- Hittner, E. F., Stephens, J. E., Turiano, N. A., Gerstorf, D., Lachman, M. E., & Haase, C. M. (2020). Positive affect is associated with less memory decline: Evidence from a 9-year longitudinal study. Psychological Science, 31(11), 1386–1395. doi: 10.1177/0956797620953883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hülür, G. (2021). Structural and functional aspects of social relationships and episodic memory: Between-person and within-person associations in middle-aged and older adults. Gerontology. doi: 10.1159/000514949 [DOI] [PubMed] [Google Scholar]
- Hülür, G., Willis, S. L., Hertzog, C., Schaie, K. W., & Gerstorf, D. (2018). Is subjective memory specific for memory performance or general across cognitive domains? Findings from the Seattle Longitudinal Study. Psychology and Aging, 33(3), 448–460. doi: 10.1037/pag0000243 [DOI] [PubMed] [Google Scholar]
- Infurna, F. J., & Luthar, S. S. (2017). The multidimensional nature of resilience to spousal loss. Journal of Personality and Social Psychology, 112(6), 926–947. doi: 10.1037/pspp0000095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James, B. D., Wilson, R. S., Barnes, L. L., & Bennett, D. A. (2011). Late-life social activity and cognitive decline in old age. Journal of the International Neuropsychological Society, 17(6), 998–1005. doi: 10.1017/S1355617711000531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Fleur, C. G., & Salthouse, T. A. (2017). Which aspects of social support are associated with which cognitive abilities for which people? The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 72(6), 1006–1016. doi: 10.1093/geronb/gbv119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara, E., Caballero, F. F., Rico-Uribe, L. A., Olaya, B., Haro, J. M., Ayuso-Mateos, J. L., & Miret, M. (2019). Are loneliness and social isolation associated with cognitive decline? International Journal of Geriatric Psychiatry, 34(11), 1613–1622. doi: 10.1002/gps.5174 [DOI] [PubMed] [Google Scholar]
- Lee, Y., Chi, I., & A Palinkas, L. (2019). Widowhood, leisure activity engagement, and cognitive function among older adults. Aging & Mental Health, 23(6), 771–780. doi: 10.1080/13607863.2018.1450837 [DOI] [PubMed] [Google Scholar]
- Li, Y. (2007). Recovering from spousal bereavement in later life: Does volunteer participation play a role? The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 62(4), 257–266. doi: 10.1093/geronb/62.4.s257 [DOI] [PubMed] [Google Scholar]
- Littell, R. C., Milliken, G. A., Stroup, W. W., Wolfinger, R. D., & Schabenberger, O. (1996). SAS system for mixed models (Vol. 633). SAS Institute. [Google Scholar]
- Little, R. J. A., & Rubin, D. B. (1987). Statistical analysis with missing data. Wiley. [Google Scholar]
- Liu, H., Zhang, Y., Burgard, S. A., & Needham, B. L. (2019). Marital status and cognitive impairment in the United States: Evidence from the National Health and Aging Trends Study. Annals of Epidemiology, 38, 28–34.e2. doi: 10.1016/j.annepidem.2019.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luhmann, M., Hofmann, W., Eid, M., & Lucas, R. E. (2012). Subjective well-being and adaptation to life events: A meta-analysis. Journal of Personality and Social Psychology, 102(3), 592–615. doi: 10.1037/a0025948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin, M. F., Lord, C., Andrews, J., Juster, R. P., Sindi, S., Arsenault-Lapierre, G., Fiocco, A. J., & Lupien, S. J. (2011). Chronic stress, cognitive functioning and mental health. Neurobiology of Learning and Memory, 96(4), 583–595. doi: 10.1016/j.nlm.2011.02.016 [DOI] [PubMed] [Google Scholar]
- Marioni, R. E., Proust-Lima, C., Amieva, H., Brayne, C., Matthews, F. E., Dartigues, J. F., & Jacqmin-Gadda, H. (2015). Social activity, cognitive decline and dementia risk: A 20-year prospective cohort study. BMC Public Health, 15, 1089. doi: 10.1186/s12889-015-2426-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle, J. J., Fisher, G. G., & Kadlec, K. M. (2007). Latent variable analyses of age trends of cognition in the Health and Retirement Study, 1992–2004. Psychology and Aging, 22(3), 525–545. doi: 10.1037/0882-7974.22.3.525 [DOI] [PubMed] [Google Scholar]
- Moon, J. R., Kondo, N., Glymour, M. M., & Subramanian, S. V. (2011). Widowhood and mortality: A meta-analysis. PLoS One, 6(8), e23465. doi: 10.1371/journal.pone.0023465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa, T., & Hülür, G. (2021). Life satisfaction during the transition to widowhood among Japanese older adults. Gerontology, 67(3), 338–349. doi: 10.1159/000512859 [DOI] [PubMed] [Google Scholar]
- Nelson, N. A., Jacobucci, R., Grimm, K. J., & Zelinski, E. M. (2020). The bidirectional relationship between physical health and memory. Psychology and Aging, 35(8), 1140–1153. doi: 10.1037/pag0000579 [DOI] [PubMed] [Google Scholar]
- Ofstedal, M. B., Fisher, G. G., & Herzog, A. R.(2005). Documentation of cognitive functioning measures in the Health and Retirement Study. University of Michigan. [Google Scholar]
- Ong, A. D., Fuller-Rowell, T. E., & Bonanno, G. A. (2010). Prospective predictors of positive emotions following spousal loss. Psychology and Aging, 25(3), 653–660. doi: 10.1037/a0018870 [DOI] [PubMed] [Google Scholar]
- Ornstein, K. A., Kelley, A. S., Bollens-Lund, E., & Wolff, J. L. (2017). A national profile of end-of-life caregiving in the United States. Health Affairs (Project Hope), 36(7), 1184–1192. doi: 10.1377/hlthaff.2017.0134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roan, C., & Raley, R. K. (1996). Intergenerational coresidence and contact: A longitudinal analysis of adult children’s response to their mother’s widowhood. Journal of Marriage and Family, 58(3), 708–717. doi: 10.2307/353730 [DOI] [Google Scholar]
- Rossi, N. E., Bisconti, T. L., & Bergeman, C. S. (2007). The role of dispositional resilience in regaining life satisfaction after the loss of a spouse. Death Studies, 31(10), 863–883. doi: 10.1080/07481180701603246 [DOI] [PubMed] [Google Scholar]
- Schwartz, J. E., & Stone, A. A. (1998). Strategies for analyzing ecological momentary assessment data. Health Psychology, 17(1), 6–16. doi: 10.1037//0278-6133.17.1.6 [DOI] [PubMed] [Google Scholar]
- Seeman, T. E., Lusignolo, T. M., Albert, M., & Berkman, L. (2001). Social relationships, social support, and patterns of cognitive aging in healthy, high-functioning older adults: MacArthur studies of successful aging. Health Psychology, 20(4), 243–255. doi: 10.1037//0278-6133.20.4.243 [DOI] [PubMed] [Google Scholar]
- Shankar, A., Hamer, M., McMunn, A., & Steptoe, A. (2013). Social isolation and loneliness: Relationships with cognitive function during 4 years of follow-up in the English Longitudinal Study of Ageing. Psychosomatic Medicine, 75(2), 161–170. doi: 10.1097/PSY.0b013e31827f09cd [DOI] [PubMed] [Google Scholar]
- Sharifian, N., Manly, J. J., Brickman, A. M., & Zahodne, L. B. (2019). Social network characteristics and cognitive functioning in ethnically diverse older adults: The role of network size and composition. Neuropsychology, 33(7), 956–963. doi: 10.1037/neu0000564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin, S. H., Kim, G., & Park, S. (2018). Widowhood status as a risk factor for cognitive decline among older adults. The American Journal of Geriatric Psychiatry, 26(7), 778–787. doi: 10.1016/j.jagp.2018.03.013 [DOI] [PubMed] [Google Scholar]
- Silverstein, M., & Bengtson, V. L. (1994). Does intergenerational social support influence the psychological well-being of older parents? The contingencies of declining health and widowhood. Social Science & Medicine (1982), 38(7), 943–957. doi: 10.1016/0277-9536(94)90427-8 [DOI] [PubMed] [Google Scholar]
- Silverstein, M., Chen, X., & Heller, K. (1996). Too much of a good thing? Intergenerational social support and the psychological well-being of older parents. Journal of Marriage and Family, 58(4), 970–982. doi: 10.2307/353984 [DOI] [Google Scholar]
- Singer, J. D., Willett, J. B., Willett, C. W. E. P. J. B., & Willett, J. B. (2003). Applied longitudinal data analysis: Modeling change and event occurrence. Oxford University Press. [Google Scholar]
- Sneed, R. S., & Schulz, R. (2019). Grandparent caregiving, race, and cognitive functioning in a population-based sample of older adults. Journal of Aging and Health, 31(3), 415–438. doi: 10.1177/0898264317733362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnega, A., Faul, J. D., Ofstedal, M. B., Langa, K. M., Phillips, J. W., & Weir, D. R. (2014). Cohort profile: The Health and Retirement Study (HRS). International Journal of Epidemiology, 43(2), 576–585. doi: 10.1093/ije/dyu067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl, S. T., Arnold, A. M., Chen, J. Y., Anderson, S., & Schulz, R. (2016). Mortality after bereavement: The role of cardiovascular disease and depression. Psychosomatic Medicine, 78(6), 697–703. doi: 10.1097/PSY.0000000000000317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffick, D. E. (2000). Documentation of affective functioning measures in the Health and Retirement Study. University of Michigan. [Google Scholar]
- Stepler, R. (2016, February 18). 2. Living arrangements of older Americans by gender. Pew Research Center’s Social & Demographic Trends Project. https://www.pewresearch.org/social-trends/2016/02/18/2-living-arrangements-of-older-americans-by-gender/ [Google Scholar]
- Steptoe, A., Shankar, A., Demakakos, P., & Wardle, J. (2013). Social isolation, loneliness, and all-cause mortality in older men and women. Proceedings of the National Academy of Sciences of the United States of America, 110(15), 5797–5801. doi: 10.1073/pnas.1219686110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern, Y. (2009). Cognitive reserve. Neuropsychologia, 47(10), 2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroebe, W., & Stroebe, M. S. (1987). Bereavement and health: The psychological and physical consequences of partner loss. Cambridge University Press. [Google Scholar]
- Sundström, A., Westerlund, O., Mousavi-Nasab, H., Adolfsson, R., & Nilsson, L. G. (2014). The relationship between marital and parental status and the risk of dementia. International Psychogeriatrics, 26(5), 749–757. doi: 10.1017/S1041610213002652 [DOI] [PubMed] [Google Scholar]
- Tun, P. A., Miller-Martinez, D., Lachman, M. E., & Seeman, T. (2013). Social strain and executive function across the lifespan: The dark (and light) sides of social engagement. Neuropsychology, Development, and Cognition. Section B, Aging, Neuropsychology and Cognition, 20(3), 320–338. doi: 10.1080/13825585.2012.707173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utz, R. L., Carr, D., Nesse, R., & Wortman, C. B. (2002). The effect of widowhood on older adults’ social participation: An evaluation of activity, disengagement, and continuity theories. The Gerontologist, 42(4), 522–533. doi: 10.1093/geront/42.4.522 [DOI] [PubMed] [Google Scholar]
- Wang, H. X., Karp, A., Winblad, B., & Fratiglioni, L. (2002). Late-life engagement in social and leisure activities is associated with a decreased risk of dementia: A longitudinal study from the Kungsholmen project. American Journal of Epidemiology, 155(12), 1081–1087. doi: 10.1093/aje/155.12.1081 [DOI] [PubMed] [Google Scholar]
- Zettel, L. A., & Rook, K. S. (2004). Substitution and compensation in the social networks of older widowed women. Psychology and Aging, 19(3), 433–443. doi: 10.1037/0882-7974.19.3.433 [DOI] [PubMed] [Google Scholar]
- Zhang, Z., Li, L. W., Xu, H., & Liu, J. (2019). Does widowhood affect cognitive function among Chinese older adults? SSM—Population Health, 7, 100329. doi: 10.1016/j.ssmph.2018.100329 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The findings were not previously disseminated elsewhere. This study was not preregistered.