Abstract
Background
Perceived physical fatigability is highly prevalent in older adults and associated with mobility decline and other health consequences. We examined the prognostic value of perceived physical fatigability as an independent predictor of risk of death among older adults.
Methods
Participants (N = 2 906), mean age 73.5 [SD, 10.4] years, 54.2% women, 99.7% white enrolled in the Long Life Family Study, were assessed at Visit 2 (2014–2017) with 2.7 [SD, 1.0] years follow-up. The Pittsburgh Fatigability Scale (PFS), a 10-item, self-administered validated questionnaire (score range 0–50, higher = greater fatigability) measured perceived physical fatigability at Visit 2. Deaths post-Visit 2 through December 31, 2019 were identified by family members notifying field centers, reporting during another family member’s annual phone follow-up, an obituary, or Civil Registration System (Denmark). We censored all other participants at their last contact. Cox proportional hazard models predicted mortality by fatigability severity, adjusted for family relatedness and other covariates.
Results
Age-adjusted PFS Physical scores were higher for those who died (19.1 [SE, 0.8]) compared with alive (12.2, [SE, 0.4]) overall, as well as across age strata (p < .001), except for those 60–69 years (p = .79). Participants with the most severe fatigability (PFS Physical scores ≥ 25) were over twice as likely to die (hazard ratio, 2.33 [95% CI, 1.65–3.28]) compared with those who had less severe fatigability (PFS Physical scores < 25) after adjustment.
Conclusions
Our work underscores the utility of the PFS as a novel patient-reported prognostic indicator of phenotypic aging that captures both overt and underlying disease burden that predicts death.
Keywords: Death, Epidemiology, Family study, Fatigue
Perceived physical fatigability, whole-body tiredness anchored to quantifiable activities/tasks of fixed intensity and duration, provides a sensitive patient-reported assessment of the degree to which an individual is physically limited by fatigue.1–6 Perceived physical fatigability is a highly prevalent characteristic reported by older adults, ranging from 22.5% to 89.5%,5,7–9 and is greater with advancing age,5 higher in women than men,5 predicts physical and cognitive functional decline,8,10 and associated with cardiovascular risk,11 and depressive symptomology.5 In older adults, perceived physical fatigability is clinically useful because it provides a holistic indicator of an individual’s vulnerability to fatigability by capturing what an individual thinks they can do as well as how much effort it takes to perform standard activities. Furthermore, perceived physical fatigability reflects energetic capacity and fitness levels (i.e., VO2 peak),2,12–14 functional decline,8 and underlying disease burden,1 which in turn, all strongly predict mortality.15–18
The Pittsburgh Fatigability Scale (PFS), the only validated questionnaire to measure perceived physical fatigability, can be used as a patient-centered clinical tool when objective measures of fatigability, function, or fitness are unavailable.2 For example, although cardiopulmonary exercise testing is the gold-standard measure of fitness,19 it is rarely conducted in epidemiologic research and general clinical practice for pragmatic reasons including the need to have trained staff, specialized equipment/space, and safety concerns for those with mobility impairment. Highlighting the utility of the PFS as a novel sensitive marker of effort/capacity, Qiao et al.6 recently reported clinically meaningful reductions in perceived physical fatigability concurrent with increased activity levels after a short-term personalized physical activity intervention.
Over the past decade, perceived fatigability has emerged as a key marker of phenotypic aging,3 yet no study has assessed whether this patient-centered measure predicts all-cause mortality. Therefore, we evaluated the prognostic value of perceived physical fatigability among older adults enrolled in the Long Life Family Study (LLFS) to address whether PFS Physical score severity independently predicted risk of death.
Method
Study Population
LLFS is an international, multicenter, prospective family study of exceptional longevity. Enrollment of 2 generations, probands (oldest) and their offspring plus respective spouse controls in both generations (Visit 1, 2006–2009, N = 4 953 from 539 families), was described elsewhere.20,21 Annual telephone follow-up continued until a second in-person assessment (Visit 2, 2014–2016, N = 2 906 from 498 families, mean family size 4.5 [SD, 4.6]) and thereafter. Of the 2 906 participants who completed Visit 2, we excluded 567 (n = 312 <60 years; n = 255 no PFS Physical score/unable to impute). The sample was limited to complete data for physical activity, health conditions, and smoking; final analytic sample N = 2 258 (includes n = 137 imputed PFS Physical scores).
Predictor Variable—Perceived Physical Fatigability
The PFS, a 10-item, self-administered questionnaire validated for those age ≥60 years to ascertain both physical and mental fatigability,2,22 was included at Visit 2. In this article, we focused on physical fatigability, which captures capacity/effort, rather than mental fatigability, which captures cognitive and mood-related domains.22 Participants rated their tiredness/exhaustion from 0 (“no fatigue”) to 5 (“extreme fatigue”) for how they expected or imagined they would feel after completing activities ranging in type and intensity. The PFS activities included: leisurely walk for 30 minutes, brisk or fast walk for 1 hour, light household activity for 1 hour, heavy gardening or outdoor work for 1 hour, watching television for 2 hours, sitting quietly for 1 hour, moderate- to high-intensity strength training for 30 minutes, participating in a social activity for 1 hour, hosting a social event for 1 hour, and high-intensity activity for 30 minutes. Ratings were summed (range 0–50, higher scores = greater perceived physical fatigability).2 We imputed the PFS Physical scores when 1–3 items were missing.23 Scores were categorized into established PFS Physical score severity strata (0–4, 5–9, 10–14, 15–19, 20–24, and ≥25) that were previously associated with clinically meaningful decline in physical function.3,8
Outcome Measure—Vital Status
LLFS deaths that occurred post-Visit 2 and by December 31, 2019 were identified at the U.S. field centers by (1) family members notifying field centers, (2) reporting during another family member’s annual phone follow-up, and/or (3) finding an obituary when unable to reach a participant at the time of their annual follow-up. We obtained date of death for the Danish participants in LLFS through the Civil Registration System. For all others, we censored participants at their most recent contact date when confirmed alive.
Covariates
At Visit 1, we queried sex, race, and validated date of birth.20 We collected all other covariates, unless otherwise stated, at Visit 2. Covariates and potential confounders for model building were chosen based on the fatigability literature in epidemiology and aging.5,8,11 The Framingham Physical Activity Index captured rest and activity in 5 domains for a typical day over the past year (MET-h/d); due to skewness (right), we dichotomized at the median for the analyses. We asked self-reported doctor diagnosis of heart disease, stroke, kidney disease, lung disease, peripheral arterial disease, and cancer (excluding skin) at each visit and annually during telephone follow-ups. We defined hypertension as systolic ≥ 130 mmHg and/or diastolic ≥ 80 mmHg or taking blood pressure medication according to the American College of Cardiology/American Heart Association (ACC/AHA) 2017 guidelines. Hemoglobin A1c ≥ 6.5%, fasting glucose ≥ 126 mg/dL, or self-reported doctor diagnosis defined diabetes according to the 2018 criteria from the American Diabetes Association. Smoking history was self-reported former, current or nonsmoker cigarette/cigar use. We assessed depressive symptomatology with the 10-item (30 point) Center for Epidemiologic Studies–Depression Scale (CES-D) score.
Statistical Analyses
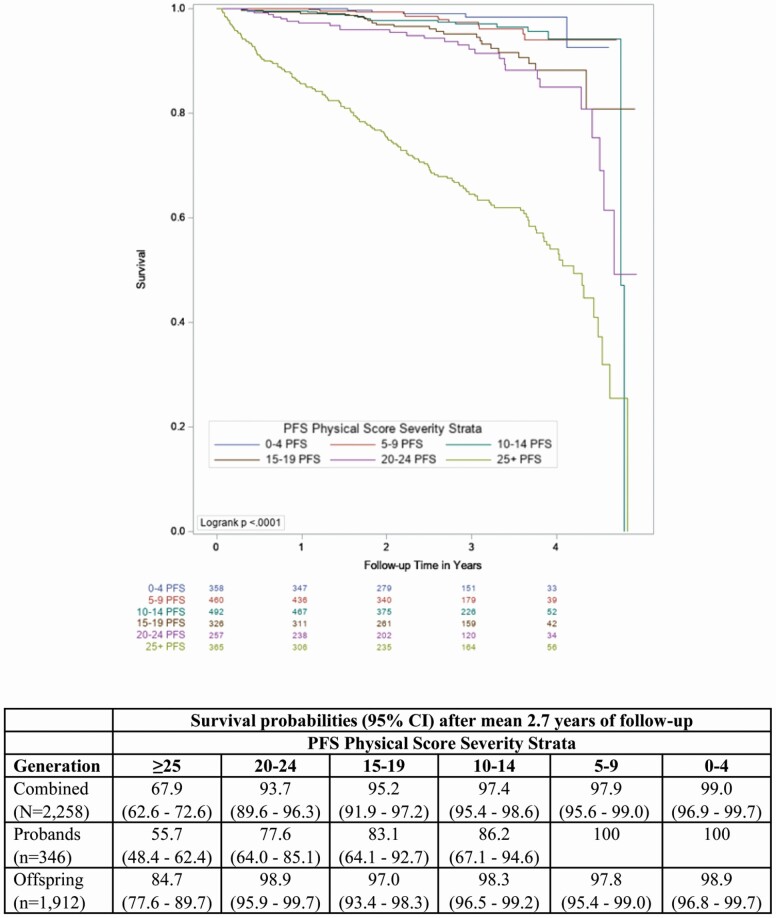
First, we plotted Kaplan–Meier survival curves for time to death by PFS Physical score severity strata (Figure 1). Visual inspection indicated a clear delineation between the most severe fatigability stratum (PFS Physical scores ≥ 25) and the lower 5 severity strata. Next, we compared participant characteristics by the most severe fatigability stratum versus the combined less severe strata using t-tests or χ 2 tests, as appropriate. Then, we constructed a Cox proportional hazard model for the prediction of mortality by the most severe fatigability stratum versus the combined less severity strata, accounting for family relatedness (R package “coxme” using a genetic kinship matrix) and adjusted for field center (Model 1). We additionally adjusted Model 2 for age and sex; Models 3 and 4 added physical activity and health conditions, respectively. Our final analytic model included all prior variables plus smoking status (Model 5). We evaluated the sensitivity of perceived physical fatigability compared to a traditional global fatigue measure by repeating the analysis for Model 5 using the single-item global fatigue measure “I felt everything I did was an effort” from the CES-D instead of the PFS Physical scores. Higher fatigue was classified as those answering “some,” “moderate,” or “most of the time,” whereas those answering “rarely or none of the time” were classified with lower fatigue.5
Figure 1.
Kaplan–Meir survival curves across Pittsburgh Fatigability Scale (PFS) Physical Score Severity Strata (N = 2 258).
We used Schoenfeld residuals to examine the proportional hazards assumption. Because missing data for depressive symptomatology reduced our sample by 73 (including 33 deaths), we evaluated this measure as a potential confounder via conducting a sensitivity analysis by adding depressive symptomatology to Model 5.
Except when stated, we conducted our analyses using SAS v9.4 (SAS Institute Inc., Cary, NC).
Results
Participants (mean age 73.5 [SD, 10.4] years, range 60–108) who completed Visit 2 were predominantly white (99.5%) and women (58.4%; Table 1). Post-Visit 2, 10.2% (n = 230) of the sample died during 2.7 [SD, 1.0] years follow-up. Age-adjusted PFS Physical scores were higher for those who died (19.1 [SE, 0.8]) compared with alive (12.2 [SE, 0.4]) overall, as well as across age strata (p < .001), except for the 60–69 year old stratum (p < .001, Supplementary Table 1 and Supplementary Figure 1). Overall, participants with incomplete data excluded from analysis were more likely to be older (p < .001) and had a higher proportion of deaths (p < .001), heart disease (p = .02), hypertension (p < .001), and stroke (p = .001; Supplementary Table 2).
Table 1.
Visit 2 Participant Characteristics by Most Severe (PFS Physical Scores ≥ 25) Versus Less Severe (PFS Physical Scores < 25) Perceived Physical Fatigability (N = 2 258)
| All (N = 2 258) | Most Severe Fatigability (n = 365) | Less Severe Fatigability (n = 1 893) | p-Value | |
|---|---|---|---|---|
| Number of deaths | 230 (10.2) | 147 (40.3) | 83 (4.4) | <.0001 |
| Age, y | 73.5 ± 10.4 | 85.4 ± 12.6 | 71.2 ± 8.2 | <.0001 |
| Sex, women | 1 235 (54.7) | 236 (64.7) | 999 (52.7) | <.0001 |
| Physical activity, MET-h/d* | 36.4 ±7.0 | 30.4 ± 5.6 | 37.5 ± 6.7 | <.0001 |
| Heart disease† | 143 (6.3) | 50 (13.7) | 93 (4.9) | <.0001 |
| Hypertension‡ | 1 354 (60.0) | 232 (63.6) | 1 122 (59.3) | .13 |
| Stroke† | 115 (5.1) | 52 (14.3) | 63 (3.3) | <.0001 |
| Kidney disease† | 75 (3.3) | 28 (7.7) | 47 (2.5) | <.0001 |
| Diabetes§ | 252 (11.2) | 67 (18.4) | 185 (9.8) | <.0001 |
| Peripheral arterial disease† | 53 (2.4) | 32 (8.7) | 21 (1.1) | <.0001 |
| Liver disease† | 65 (2.9) | 7 (1.9) | 58 (3.1) | .23 |
| Lung disease† | 306 (13.6) | 68 (18.6) | 238 (12.6) | .002 |
| Cancer (excluding skin)† | 563 (25.0) | 132 (36.2) | 431 (22.8) | <.0001 |
| Depressive symptomatology, 0–30‖ | 3.2 ± 3.5 | 5.5 ± 4.6 | 2.8 ± 3.1 | <.0001 |
| Smoking status | ||||
| Former smoker | 877 (38.8) | 142 (38.8) | 735 (38.8) | .99 |
| Current smoker | 87 (3.9) | 7 (1.9) | 80 (4.2) | .04 |
| Nonsmoker | 1 294 (57.3) | 216 (59.8) | 1 078 (57.0) | .43 |
| Follow-up time, y | 2.7 ± 1.0 | 2.6 ± 1.3 | 2.8 ± 1.0 | .007 |
Notes: PFS = Pittsburgh Fatigability Scale. All reported in mean ± SD or n (%).
*Framingham Physical Activity Index score.
†Self-reported doctor diagnosis (prevalence/history).
‡Hypertension defined as systolic ≥ 130 mm Hg and/or diastolic ≥ 80 mm Hg or taking blood pressure medication.
§Hemoglobin A1c ≥ 6.5%, fasting glucose ≥ 126 mg/dL, or self-reported doctor diagnosis defined diabetes.
‖Center for Epidemiological Studies—Depression Scale.
Survival probabilities by PFS Physical score severity strata were lowest in the most severe (PFS ≥ 25) category, overall (67.9% [95% CI, 62.6–72.6]) and for both the proband (55.7% [95% CI, 48.4–62.4]) and offspring (84.7% [95% CI, 77.6–89.7]) generations (Figure 1). When we dichotomized PFS Physical scores versus <25, participants with the most severe perceived physical fatigability were over twice as likely to die (hazard ratio [HR], 2.33 [95% CI, 1.65–3.28]) compared with those who had less severe fatigability after adjustment (Table 2, Model 5). Results remained significant with slight attenuation in the sensitivity analysis with additional adjustment for depressive symptoms (HR, 2.00 [95% CI, 1.38–2.92]). When substituting the global fatigue measure for fatigability, higher fatigue did not predict mortality (HR: 1.33, [95% CI, 0.97–1.85]) in the fully adjusted model.
Table 2.
Cox Proportional Hazards Models Examining the Association of Most Severe (PFS Physical Scores ≥ 25) Versus Less Severe (PFS Physical Scores < 25) Perceived Physical Fatigability on 2.7-Year Survival* (N = 2 258)
| Models | Hazard Ratio (95% Confidence Interval) | p-Value | |
|---|---|---|---|
| Unadjusted | 9.34 (7.13–12.24) | <.0001 | |
| 1 | Accounting for family relatedness and adjusted for field center | 10.20 (7.64–13.66) | <.0001 |
| 2 | Model 1 plus age and sex | 2.93 (2.10–4.10) | <.0001 |
| 3 | Model 2 plus physical activity score† | 2.60 (1.85–3.66) | <.0001 |
| 4 | Model 3 plus health conditions‡ | 2.29 (1.63–3.21) | <.0001 |
| 5 | Model 4 plus smoking history§ | 2.33 (1.65–3.28) | <.0001 |
Note: PFS = Pittsburgh Fatigability Scale.
*Mean 2.7 ± 1.0 years follow-up.
†Framingham Physical Activity Index score dichotomized at the median=35.4 MET-h/d.
‡Heart disease, stroke, kidney disease, peripheral artery disease, liver disease, lung disease, and cancer (not including skin) all self-report prevalence/history; hypertension defined as systolic ≥ 130 mm Hg and/or diastolic ≥80 mm Hg or taking blood pressure medication; hemoglobin A1c ≥ 6.5%, fasting glucose ≥ 126 mg/dL, or self-reported doctor diagnosis defined diabetes.
§Former, current smoker, or nonsmoker.
All covariates in the final model met the proportional hazards assumption based on a Bonferroni-adjusted significance level as well as a global test (p = .51). To evaluate reverse causality, as well as exclude participants with preexisting or underlying terminal illness, deaths within 6 months (n = 37) from their Visit 2 were removed from Model 5; results remained significant (HR, 1.89 [95% CI, 1.32–2.72]).
Discussion
Our work is the first to establish that perceived physical fatigability is a robust independent indicator of mortality in older adults. We found that most severe perceived physical fatigability (PFS Physical scores ≥ 25) was a 2.3-fold higher risk indicator of death over 2.7 years follow-up compared with less fatigability (PFS Physical scores < 25). This relationship persisted despite a 71% attenuation of the HR after adjustment for age and sex, two strong independent predictors of mortality.24 Our findings extend previous work showing that global fatigue, a less-sensitive measure of one’s perception of fatigue as it does not contextualize fatigue to activity,3,4 predicted excess mortality over 7–20 years.24,25 Our shorter follow-up time, and robust HR to predict mortality, demonstrates the enhanced ability of the PFS to differentiate severity of perceived physical fatigability that is lacking from commonly used fatigue measures. Findings also remained significant in the sensitivity analyses when excluding any deaths occurring within 6 months post-Visit 2 follow-up, as well as when adjusted for depressive symptomology. Furthermore, as expected, perceived physical fatigability, but not the global fatigue measure, predicted risk of death confirming the sensitivity of the Pittsburgh Fatigability Scale.
Whereas traditional global fatigue measures have been limited in their ability to detect expected associations with age and for other clinical outcomes and interventions,4–6 the PFS improves upon this with increased range and sensitivity due to the anchoring of fatigue items to activities with standardized intensity and duration.1–6 This work also revealed a strong, positive stepwise gradient with higher PFS Physical scores across age strata for both those alive and deceased. Interestingly, from age 70 and older, LLFS participants who died during the follow-up period had markedly higher PFS Physical scores as well as concurrently reported more multimorbidity than those participants still alive (Supplementary Table 1), indicating that the PFS captured their overall disease burden.3 However, PFS Physical scores in the youngest age strata (60–69 years) were lower than observed in a similarly aged healthy sample.9 We postulate that the lower PFS Physical scores, and lack of difference between those alive and deceased for the youngest age strata, may be a result of reduced power due to the few (n = 16) deaths in this age group. It is also plausible that those <70 years old in this cohort may have died from causes not related to fatigability (e.g., accidents). Due to the limited number of deaths and since adjudication of cause of death remains ongoing in the LLFS, future work will specifically evaluate the prognostic value of the PFS for younger ages.
One limitation of our study is our current inability to identify cause-specific mortality. Furthermore, other fatiguing health conditions may not have been adjusted for due to low prevalence in this cohort as LLFS participants are generally healthier than individuals from non long lived families.20 The overall better health of LLFS participants, coupled with lost to follow-up of the oldest and potentially sickest, may have led to more conservative HRs, as we would expect the association between fatigability and mortality to be even stronger in less healthy people. Strengths include the sizable cohort, number of deaths for analysis, and availability of comprehensive covariate information. The PFS is also an attractive low-cost and easy to administer tool for use in both clinical and research settings. Lastly, the PFS is currently available in 12 languages, rendering it readily accessible for scientists worldwide to include this sensitive patient-centered measure into their protocols.
Our work underscores the utility of measuring perceived physical fatigability using the PFS as a prognostic indicator of phenotypic aging that captures both overt and underlying disease burden that independently predicts death. Future directions include evaluating the discriminatory power of the PFS with longer follow-up and extending our findings to hospitalized patients as well as more racially diverse cohorts.
Supplementary Material
Acknowledgments
Preliminary findings were presented at the Gerontological Society of America 2019 Annual Scientific Meeting, Austin, TX, in November 2019.
Funding
This work was supported by the National Institutes of Health/ National Institute on Aging U01 AG023712, U01 AG023744, U01 AG023746, U01 AG023749, U01 AG023755, P01 AG08761, and U19 AG063893. Additionally, the National Institutes of Health/National Institute on Aging, University of Pittsburgh Claude D. Pepper Older Americans Independence Center, Research Registry and Developmental Pilot Grant P30 AG024827, and the Intramural Research Program, National Institute on Aging supported N.W.G. to develop the Pittsburgh Fatigability Scale. The National Institute on Aging, Epidemiology of Aging training grant at the University of Pittsburgh T32 AG000181 supported T.G. and the National Institutes of Health/National Institute on Aging K01 AG057798 supported S.L.A.
Conflict of Interest
None declared.
References
- 1. Eldadah BA. Fatigue and fatigability in older adults. PMR. 2010;2(5):406–413. doi:10.1016/j.pmrj.2010.03.022 [DOI] [PubMed] [Google Scholar]
- 2. Glynn NW, Santanasto AJ, Simonsick EM, et al. The Pittsburgh Fatigability Scale for older adults: development and validation. J Am Geriatr Soc. 2015;63(1):130–135. doi:10.1111/jgs.13191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schrack JA, Simonsick EM, Glynn NW. Fatigability: a prognostic indicator of phenotypic aging. J Gerontol A Biol Sci Med Sci. 2020;75(9):e63–e66. doi:10.1093/gerona/glaa185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Prochaska MT, Zhang H, Alavi C, Meltzer DO. Fatigability: a new perspective on and patient-centered outcome measure for patients with anemia. Am J Hematol. 2020;95(7):E166–E169. doi:10.1002/ajh.25803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. LaSorda KR, Gmelin T, Kuipers AL, et al. Epidemiology of perceived physical fatigability in older adults: the Long Life Family Study. J Gerontol A Biol Sci Med Sci. 2020;75(9):e81–e88. doi:10.1093/gerona/glz288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Qiao Y, van Londen GJ, Brufsky JW, et al. Perceived physical fatigability improves after an exercise intervention among breast cancer survivors: a randomized clinical trial. Breast Cancer. 2022;29(1):30–37. doi:10.1007/s12282-021-01278-1 [DOI] [PubMed] [Google Scholar]
- 7. Davis B, Liu Y-H, Stampley J, et al. The association between poor diet quality, physical fatigability and physical function in the oldest-old from the Geisinger Rural Aging Study. Geriatrics (Basel). 2021;6(2): 1– 15. doi:10.3390/geriatrics6020041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Simonsick EM, Schrack JA, Santanasto AJ, Studenski SA, Ferrucci L, Glynn NW. Pittsburgh Fatigability Scale: one-page predictor of mobility decline in mobility-intact older adults. J Am Geriatr Soc. 2018;66(11):2092–2096. doi:10.1111/jgs.15531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carlozzi NE, Boileau NR, Murphy SL, Braley TJ, Kratz AL. Validation of the Pittsburgh Fatigability Scale in a mixed sample of adults with and without chronic conditions. J Health Psychol. 2021;26(9):1455–1467. doi:10.1177/1359105319877448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Salerno EA, Wanigatunga AA, An Y, et al. Longitudinal association between perceived fatigability and cognitive function in older adults: results from the Baltimore Longitudinal Study of Aging. J Gerontol A Biol Sci Med Sci. 2020;75(9):e67–e73. doi:10.1093/gerona/glz287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Qiao Y, Martinez-Amezcua P, Wanigatunga AA, et al. Association between cardiovascular risk and perceived fatigability in mid-to-late life. J Am Heart Assoc. 2019;8(16):e013049. doi:10.1161/JAHA.119.013049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Santanasto AJ, Glynn NW, Jubrias SA, et al. Skeletal muscle mitochondrial function and fatigability in older adults. J Gerontol A Biol Sci Med Sci. 2015;70(11):1379–1385. doi:10.1093/gerona/glu134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moffit RE, Qiao YS, Moored KD, et al. Estimating cardiorespiratory fitness in older adults using a usual-paced 400-m long-distance corridor walk. J Am Geriatr Soc. 2021;69(11):3328–3330. doi:10.1111/jgs.17360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Murphy SL, Kratz AL, Schepens Niemiec SL. Assessing fatigability in the lab and in daily life in older adults with osteoarthritis using perceived, performance, and ecological measures. J Gerontol A Biol Sci Med Sci. 2017;72(1):115–120. doi:10.1093/gerona/glw173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346(11):793–801. doi:10.1056/NEJMoa011858 [DOI] [PubMed] [Google Scholar]
- 16. Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305(1):50–58. doi:10.1001/jama.2010.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pavasini R, Guralnik J, Brown JC, et al. Short physical performance battery and all-cause mortality: systematic review and meta-analysis. BMC Med. 2016;14(1):215. doi:10.1186/s12916-016-0763-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wei MY, Mukamal KJ. Multimorbidity, mortality, and long-term physical functioning in 3 prospective cohorts of community-dwelling adults. Am J Epidemiol. 2018;187(1):103–112. doi:10.1093/aje/kwx198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huggett DL, Connelly DM, Overend TJ. Maximal aerobic capacity testing of older adults: a critical review. J Gerontol A Biol Sci Med Sci. 2005;60(1):57–66. doi:10.1093/gerona/60.1.57 [DOI] [PubMed] [Google Scholar]
- 20. Newman AB, Glynn NW, Taylor CA, et al. Health and function of participants in the long life family study: a comparison with other cohorts. Aging (Albany NY). 2011;3(1):63–76. doi:10.18632/aging.100242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wojczynski MK, Lin SJ, Sebastiani P, et al. NIA Long Life Family Study: objectives, design, and heritability of cross sectional and longitudinal phenotypes. J Gerontol A Biol Sci Med Sci. 2021: 1– 11. doi:10.1093/gerona/glab333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Renner SW, Bear TM, Brown PJ, et al. Validation of perceived mental fatigability using the Pittsburgh Fatigability Scale. J Am Geriatr Soc. 2021;69(5):1343–1348. doi:10.1111/jgs.17017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cooper R, Popham M, Santanasto AJ, Hardy R, Glynn NW, Kuh D. Are BMI and inflammatory markers independently associated with physical fatigability in old age? Int J Obes (Lond). 2019;43(4):832–841. doi:10.1038/s41366-018-0087-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hardy SE, Studenski SA. Fatigue predicts mortality in older adults. J Am Geriatr Soc. 2008;56(10):1910–1914. doi:10.1111/j.1532-5415.2008.01957.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Knoop V, Cloots B, Costenoble A, et al. ; Gerontopole Brussels Study Group . Fatigue and the prediction of negative health outcomes: a systematic review with meta-analysis. Ageing Res Rev. 2021;67:101261. doi:10.1016/j.arr.2021.101261 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.