Abstract
Background
Growth and differentiation factor (GDF)-11 controls embryonic development and has been proposed as an antiaging factor. GDF-8 (myostatin) inhibits skeletal muscle growth. Difficulties in accurately measuring circulating GDF-11 and GDF-8 have generated controversy.
Methods
We developed a liquid chromatography–tandem mass spectrometry (LC–MS/MS) method for simultaneous measurement of circulating GDF-8 and GDF-11 that employs denaturation, reduction, and alkylation; cation-exchange solid-phase extraction; tryptic digestion; followed by separation and quantification using 2 signature peptides for multiple reaction monitoring and C-terminal [13C615N4]-Arg peptides as internal standards. We evaluated age trends in serum GDF-11 and GDF-8 concentrations in community-dwelling healthy men, 19 years or older, and determined the effects of graded testosterone doses on GDF-8 and GDF-11 concentrations in healthy men in a randomized trial.
Results
The assay demonstrated linearity over a wide range, lower limit of quantitation 0.5 ng/mL for both proteins, and excellent precision, accuracy, and specificity (no detectable cross-reactivity of GDF-8 in GDF-11 assay or of GDF-11 in GDF-8 assay). Mean ± SD (median ± 1QR) GDF-8 and GDF-11 levels in healthy community-dwelling men, 19 years and older, were 7.2 ± 1.9 (6.8 ± 1.4) ng/mL. Neither GDF-8 nor GDF-11 levels were related to age or body composition. Testosterone treatment significantly increased serum GDF-8 but not GDF-11 levels.
Conclusions
The LC–MS/MS method for the simultaneous measurement of circulating total GDF-8 and GDF-11 demonstrates the characteristics of a valid assay. Testosterone treatment increased GDF-8 levels, but not GDF-11. Increase in GDF-8 levels by testosterone treatment, which increased muscle mass, suggests that GDF-8 acts as a chalone to restrain muscle growth.
Keywords: Aging, Androgen, Antigeronic factors, BMP-11, Myostatin
Growth and differentiation factor-11 (GDF-11), a member of the transforming growth factor-beta superfamily, was identified in parabiosis experiments as a potentially antigeronic factor and subsequently shown to be important in mammalian embryonic development, anterior/posterior patterning, olfactory neurogenesis, as well as organogenesis (1). Additionally, GDF-11 has been proposed as an endocrine regulator of aging and implicated in aging-related cardiac hypertrophy (2,3) and brain vascularization (4,5). GDF-11 has high structural homology with growth and differentiation factor-8 (GDF-8, myostatin), a negative regulator of postnatal muscle growth (1). Disruption of the myostatin gene as well as the inhibition of myostatin signaling through the activin type 2 receptor (ActRII) type B receptor increases skeletal muscle mass in preclinical models as well as in humans (6–8). Substantial pharmaceutical efforts have been directed at developing myostatin inhibitors for the treatment of muscle loss associated with aging or chronic disease (9). Both GDF-8 and GDF-11 bind ActRII and ALK4/5/7 receptors, inducing the Smad 2/3 pathway (10,11). However, it remains uncertain whether GDF11 levels increase or decrease with advancing age and whether GDF-11 and GDF-8 are differentially regulated (12–15,16,17). The lack of specificity of the extant GDF-11 assays has been cited as an important contributor to the apparently conflicting findings on age-related changes in GDF-11 levels in various reports (12–15,16,17). The enzyme-linked immunosorbent assays and the aptamer-based assays for GDF11 have suffered from cross-reactivity of the closely related GDF-8 (1,6,7,14). The proteomic platform LS–MS assays may not be optimized for precise quantitation of low abundance proteins or peptides (17). The immunoaffinity purification can enhance specificity, but the recovery of the peptide of interest during the immunoaffinity purification may be affected by the binding affinity of the antibody/antibodies relative to the endogenous binding proteins (16). These differences among assays, including the cross-reactivity of myostatin and immunoglobulins in the extant immunoassays and aptamer-based assays (14), could partially explain the divergence among studies in the age-related changes in circulating GDF-11 concentrations, as well as the conflicting associations of GDF-11 levels with aging phenotypes.
We have shown that testosterone promotes myogenic differentiation of muscle progenitor cells by activating the androgen receptor/β-catenin pathway and inducing the expression of follistatin (18,19), which blocks the effects of a number of TGF-β family members, including GDF-8 (20); therefore, testosterone treatment would be expected to increase circulating GDF-8 concentrations. It is unknown, however, how the increase in skeletal muscle mass induced by testosterone administration affects GDF-11 levels in humans and whether testosterone-induced changes in skeletal muscle mass are associated with coordinate changes in circulating GDF-8 and GDF-11 levels. Accordingly, we developed and validated a highly specific liquid chromatography–tandem mass spectrometry (LC–MS/MS) assay that uses solid-phase extraction, proteolytic digestion to generate GDF-11-specific peptides that are monitored using multiple reaction monitoring (MRM). Using this validated assay, we studied the age distribution of GDF-11 and GDF-8 levels in a prospective cross-sectional sample of community-dwelling healthy men, 19 years or older. Additionally, we evaluated the effects of graded doses of testosterone on GDF-8 and GDF-11 levels in healthy young men. Because of the uncertainty surrounding the accuracy and specificity of GDF-11 and GDF-8 assays, we provide detailed information on the validation of the novel LC–MS/MS assay used in this study.
Method
Study Design and Participants
The study protocols were approved by the institutional review board of Brigham and Women’s Hospital. All participants gave written informed consent. Community-dwelling healthy men, 19 years or older, with body mass index (BMI) more than 19 and less than 40 kg/m2 were recruited prospectively by advertising in local newspapers and internet sites and from lists of volunteers who had expressed interest in or had participated in previous studies, as part of a National Institutes of Health funded study to generate reference ranges for free testosterone levels. Participants were excluded if they had a history of illness within the last 6 weeks; any chronic illness except treated hypertension, hypothyroidism, and hyperlipidemia; history of diabetes mellitus, taking diabetes medication, fasting glucose more than 126 mg/dL or A1C more than 6.4%; aspartate aminotransferase or alanine aminotransferase more than 1.5 times the upper limit of normal, serum creatinine more than 1.5 mg/dL; current use of opioid analgesics, antibiotics, anticancer drugs, and biologic agents; use of testosterone, anabolic steroids, systemic glucocorticoids, steroid 5α-reductase (5aR) inhibitor, spironolactone, or dietary supplements containing any androgen including DHEA and androstenedione currently or in the last 3 months; or diseases of the hypothalamus, pituitary, or the testes. Within 8 weeks of the screening visit, in which eligibility criteria were verified by medical history, physical exam, and laboratory tests, eligible participants had serum samples collected after at least an 8-hour fast before 11:00 am. Serum was aliquoted and stored at −80oC and not thawed until the assay.
The 5aR trial
The design and primary findings of the 5aR trial are published (21) (NCT00493987). Briefly, the participants in the 5aR trial (21) were healthy men, 18–50 years, with normal testosterone levels, who were recruited using advertisements in the local newspapers and from lists of volunteers who had expressed interest in or had participated in previous studies. Men who had a history of prostate cancer, lower urinary tract symptom score more than 20 on the International Prostate Symptom Scale, body weight ≥135 kg, uncontrolled hypertension, hematocrit ≥51%, prostate-specific antigen level ≥4 ng/mL, aspartate aminotransferase or alanine aminotransferase ≥1.5 times the upper limit of normal, creatinine ≥2 mg/dL, and those receiving glucocorticoids, growth hormone, androgens, or 5aR inhibitors were excluded.
Participants received monthly injections of 7.5-mg leuprolide acetate to suppress endogenous testosterone production and weekly injections of one of the 4 graded doses of testosterone enanthate (TE; 50, 125, 300, or 600 mg/week) plus either placebo or 2.5 mg dutasteride daily for 20 weeks (21). In this study, we used serum samples from participants in the placebo group to avoid the potential effects of dutasteride treatment on serum GDF-11 and GDF-8 levels. Serum samples collected at baseline and Weeks 4 and 20 of treatment were used for the measurement of GDF-11 and GDF-8. Body composition was measured at baseline and during Week 19 using dual-energy X-ray absorptiometry (Hologic QDR 4500A), calibrated using a soft-tissue phantom (22) before each scan. Serum total testosterone was measured using LC–MS/MS with a sensitivity of 2 ng/dL (23). Free testosterone was calculated (24).
GDF-11 and GDF-8 quantification
Materials
—Recombinant human GDF-11 (≥98% purity) was purchased from PeproTech (Rocky Hill, NJ). Recombinant GDF-8 was purchased from R&D Systems (Minneapolis, MN) and was derived from a mouse myeloma cell line (NS0). Internal standard peptides IPGMVVD^R and NLGLDCDEHSSES^R with C-terminal [13C615N4]-Arg were synthesized by New England Peptide Inc. (Gardner, MA).
Calibration standards
—Stock solutions were prepared with 100 µL of 10 µg/mL protein standards containing 300 µg/mL bovine serum albumin; 1 mL of pooled serum was added as a matrix. The calibration standards were prepared in the range 1–100 ng/mL in 0.1% FA solution, and 100 µL of each calibration standard was mixed with 290 µL of matrix solution. Additional quality control samples were prepared by spiking different amounts of GDF-8 and GDF-11 into the pooled serum.
Sample denaturation, reduction, and alkylation, and solid-phase extraction.
—Control and serum or plasma samples were denatured and reduced in 6 M urea and 20 mM dithiothreitol at 60ºC for 40 minutes in deep well plates (Eppendorf 96/2mL Protein LoBind) and alkylated with 35 mM iodoacetamide at 37ºC in the dark for 30 minutes. The standards, controls, and serum samples were acidified using 1.5 mL 0.1% FA and loaded into conditioned ion-exchange strong cation exchange cartridge for solid phase extraction (SCX-SPE) 96/2 mL well plates (Strata—XL-C 100µm, PN: 8E-S044-TGB; Phenomenex Inc., Torrance, CA) using 1 mL of methanol, followed by 1 mL of 0.1% FA. Samples were washed by applying 1 mL of 0.1% FA followed by 1 mL of 10 mM bis-Tris, pH 5.8 buffer. Bound proteins were eluted in 1.5 mL of buffer solution containing 10% methanol in 50 mM Tris at pH 10.5.
Tryptic digestion.
—Eluents from SCX–SPE were adjusted to pH 8 using 0.1–1 M HCl and mixed with 50 µL of 100 µg/mL trypsin in 50 mM Tris buffer at pH 8. The solution was incubated at 37ºC overnight (18 hours) and proteolysis stopped with 50 µL of 10% FA. 50 µL of 5 ng/mL isotope-labeled peptides prepared in 0.1% FA was added as internal standards. The digested solution was loaded into a Strata-X 33µm SPE system (PN:8E-S100-AGB; Phenomenex Inc.) that was conditioned using 1 mL methanol followed by 1 mL of deionized water. Samples were eluted with 1 mL methanol after washing with 1 mL deionized water and evaporated to dryness. Residues were reconstituted with 50 µL of 5% methanol, 0.1% FA in deionized water for LC–MS/MS analysis.
LC–MS/MS analysis
A Shimadzu UPLC system consisting of LC-20 ADXR Binary Pump and SIL-20 ACXR Autosampler (Framingham, MA) was used for liquid chromatographic separation. An ABSciex (Framingham, MA) QTRAP 5500 hybrid triple quadrupole/linear ion trap mass spectrometer equipped with a Turbo V ion source was used for detection. Instrument control, data acquisition, and processing were carried out using Analyst Software 1.6.1. version (ABSciex).
Chromatographic separation was achieved using Aeris Peptide 3.6 µm XB—C18 phase from Phenomenex Inc. Column and autosampler temperatures were set at 45ºC and 10ºC, respectively. The mobile phase consisted of 0.1% FA in deionized water (A) and 0.1% FA in methanol (B).
The mass spectrometer was operated in positive electrospray ionization mode using an ion-spray voltage of 5500 V. The Turbo V ion source was operated at 650°C and nitrogen flow set at 55 psi. The unique peptides and their MRM transitions were identified by peptide mapping and optimized by the autotune function of Analyst Software by infusion of a desalted tryptic digest of GDF-11 and GDF-8 proteins. Ion source optimization was performed by flow injection using 0.1% FA in deionized water/methanol at 70/30 (v/v) at 600 µL/minute or 200 µL/minute depending on the columns used.
The calibration curves were prepared using pooled human serum and spiked with graded concentrations of GDF-11 (0, 0.5, 1, 5, 10, 25, 50 ng/mL) or GDF-8 (0, 0.5, 1, 5, 10, 25, 50, and 100 ng/mL). Isotope-labeled IPGMVVD^R and NLGLDEHSSES^R peptides were added as internal standards to a final concentration of 5 ng/mL after pH-based fractionation by SCX–SPE and tryptic digestion. The calibration curve was constructed by weighted least-squares linear regression.
Method Validation
The method validation was carried out according to the United States Food and Drug Administration bioanalytical method validation guidance (25). The accuracy of the assay was determined in pooled human plasma spiked with one of the 3 concentration levels of GDF-11 and GDF-8 (5, 10, and 50 ng/mL) by running these validation samples on different days. Inter- and intraassay coefficients of variation were determined as the relative standard deviation (RSD%) in quality control pools in multiple assays. The accuracy of the assay was determined as intra- and interassay percent recovery with the acceptance range set at 80%–120% at the lower limit of quantitation. The specificity of the assay was evaluated in duplicates by spiking 100 µL of human serum with 25, 50, or 100 ng/mL IgG1, GDF-11, and GDF-8. We evaluated 3 matrices: human serum and plasma, and DMEM tissue culture medium.
Statistical Analysis
Baseline characteristics of participants across testosterone arms and overall are presented as means and standard deviations and changes from baseline for GDF-11 and GDF-8 are expressed as point estimates along with 95% confidence intervals and p values. Distributional properties of analyzed outcomes were inspected graphically. The mean change from baseline in the GDF-11 and GDF-8 levels over the 20-week intervention was assessed using mixed-effects regression models including dose group factor, controlling for baseline values and allowing for unstructured correlation between participants’ serial measurements at Weeks 4 and 20. Associations between mean change from baseline of GDF-11 and GDF-8 and mean change from baseline of fat-free mass, fat mass, total and free testosterone at Week 20 were investigated using simple linear regression models. The associations between baseline values of GDF-8, GDF-11, age, BMI, hormones, and body composition measures were analyzed in a similar manner. The magnitude of these relationships was assessed using R-squared metrics and corresponding p values were provided. Sensitivity analyses using log-transformed GDF-8 and GDF-11 values were performed for all regression models. The 2-sided type I error was set at 0.05 for all hypotheses testing. Statistical analyses were conducted using SAS 9.4 (SAS Institute, Inc., Cary, NC) and R software version 3.2.5 (R Foundation).
Results
Signature Peptides Selection and LC–MS/MS Analysis of GDF-8 and GDF-11
We tested several peptide fragments and selected peptides NLGLDCDEHSSESR and IPGMVVDR for GDF-11 and DFGLDCDEHSTESR and IPAMVVDR for GDF-8 because of their specificity and higher signal intensity. The MRM transitions of these unique peptides from GDF-11 and GDF-8 identified after tryptic digestion are displayed in Supplementary Figure 1.
Performance of the GDF-8 and GDF-11 LC–MS/MS Assay
The assay’s key performance characteristics are summarized in Table 1. The standard curve was linear from 0 to 50 ng/mL concentration range for GDF-11 and from 0 to 100 ng/mL range for GDF-8 using any of the 2 signature peptides of each protein (Table 1). The intraassay and interassay coefficient of variations at various concentrations of GDF-8 and GDF-11 are given in Table 1. IgG1 had no significant cross-reactivity in either the GDF-8 or the GDF-11 assay. Similarly, the addition of up to 100 ng/mL of GDF-11 did not significantly affect GDF-8 measurement and the addition of up to 100 ng/mL GDF-8 did not affect GDF-11 measurement. The lower limit of quantitation was 0.5 ng/mL for GDF-8 as well as for GDF-11.
Table 1.
Attributes of the LC–MS/MS Assay for the Simultaneous Measurement of Circulating GDF-11 and GDF-8 Levels
| Assay Attributes | GDF-11 | GDF-8 | ||||
|---|---|---|---|---|---|---|
| 1. Peptides | NLGLDEHSSESR | IPGMVVDR | DFGLDEHSTESR | IPAMVVDR | ||
| 2. MRM | 540.5/697.0 | 448.5/676.4 | 556.9/703.8 | 450.8/787.4 | ||
| 3. Method linearity R2 | >0.999 | >0.999 | >0.999 | >0.999 | ||
| 4. Lower limit of quantitation | 0.5 ng/mL | 0.5 ng/mL | ||||
| 5. Accuracy (recovery %, n = 14) | ||||||
| 5 ng/mL | 92.1–103 | 80.1–116 | 95.94–105 | 105–111 | ||
| 10 ng/mL | 103–108 | 92.0–113 | 76.9–94.8 | 94.8–103 | ||
| 50 ng/mL | 88.8– 103 | 85.8–102 | 81.7–84.8 | 80.9–98.3 | ||
| 6. Precision (intraassay and interassay coefficients of variation at 5 different concentration levels) in 14 assays | ||||||
| GDF-11 | GDF-8 | |||||
| Concentration (ng/mL) | Intraassay CV % | Interassay CV % | Concentration (ng/mL) | Intraassay CV % | Interassay CV % | |
| Level 1 | 3.42 | 8.01 | 8.71 | 8.71 | 15.1 | 15.1 |
| Level 2 | 7.43 | 13.0 | 13.0 | 14.1 | 12.5 | 12.5 |
| Level 3 | 12.5 | 10.9 | 14.2 | 17.3 | 11.5 | 16.4 |
| Level 4 | 52.0 | 8.89 | 12.8 | 51.1 | 11.3 | 12.0 |
| Level 5 | 104.7 | 12.9 | 17.1 | 97.6 | 7.43 | 11.5 |
| 7. Cross-reactivity of IgG, GDF8, and GDF11 | ||||||
| IgG | No detectable cross-reactivity | No detectable cross-reactivity | No detectable cross-reactivity | No detectable cross-reactivity | ||
| GDF-8 | No detectable cross-reactivity | No detectable cross-reactivity | N/A | N/A | ||
| GDF-11 | N/A | N/A | No detectable cross-reactivity | No detectable cross-reactivity | ||
Notes: GDF-11 = growth and differentiation factor-11; GDF-8 = growth and differentiation factor-8; MRM = multiple reaction monitoring transitions used for detection and quantitation; CV = coefficient of variation; N/A = not applicable; LC–MS/MS = liquid chromatography–tandem mass spectrometry. The major attributes of the LC–MS/MS assay for the measurement of GDF-11 and GDF-8 are summarized.
The matrix effect ratios of analyte to the internal standard were similar at different levels of concentrations after extraction using SCX–SPE, enabling accurate measurement of targeted compounds in serum, plasma, and tissue culture medium.
External Validation of the Assay
The Central Laboratory for the Comprehensive Evaluation of Aging-Related Clinical Outcomes and Geroproteins (CARGO) Project at the University of Vermont’s Laboratory for Clinical Biochemistry Research, as a part of its assay evaluation for the NIA-funded CARGO Project, prepared 2 external validation experiments. In Experiment 1, 21 blinded, anonymized, quality control serum samples were spiked with 2 levels of both GDF-8 and GDF-11 (R&D Systems) and assayed for both analytes. Amounts added were as per the manufacturer’s quantitation. Level 1 (L1) was spiked with 4 ng/mL of GDF-8 and 1 ng/mL of GDF-11. Level 2 (L2) was spiked with 10 ng/mL of GDF-8 and 5 ng/mL of GDF-11. The measured GDF-8 and GDF-11 concentrations were 9.9 and 3.5 ng/mL, respectively, in the unspiked serum samples. The mean recoveries for GDF-8 and GDF-11, respectively, were L1 107% and 98%, L2 108% and 118%, suggesting appropriate accuracy and specificity (Supplementary Table 1).
To pursue this further, in Experiment 2, 17 blinded, anonymized, quality control serum samples were spiked with either 10 ng /mL GDF-8 (L3) or 5 ng /mL GDF-11 (L4) and assayed for both analytes. The measured GDF-8 and GDF-11 concentrations were 8.3 and 3.0 ng/mL, respectively, in the unspiked serum samples. The mean recoveries for GDF-8 and GDF-11, respectively, were L3 102% and 0.4%, L4 4.4%, 101%, confirming both accuracy and specificity.
The Age Trends in GDF-11 and GDF-8 Concentrations in Healthy Community-Dwelling Men
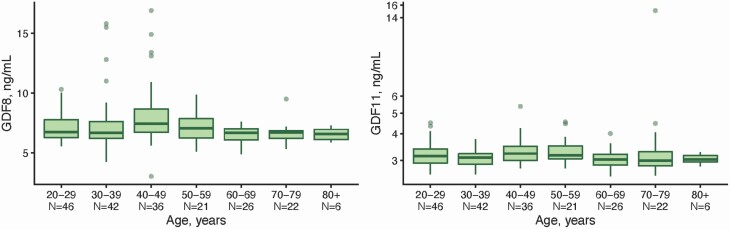
The baseline characteristics of the 145 community-dwelling healthy adults included in the study are given in Supplementary Table 2. Their median age was 47 years (range 20–90 years) and mean BMI was 25.6 kg/m2 (range 19.2–36.1 kg/m2). Mean ± SD serum GDF-11 concentration was 3.2 ± 1.1 ng/mL, while mean serum GDF-8 concentration was 6.6 ± 0.7 ng/mL. The distribution of GDF-8 and GDF-11 levels in men is given in Table 2. There was no association between age and GDF-11 levels (p = .24, R2 = 0.010) or GDF-8 (p = .94, R2 < 0.001; Figure 1) and between BMI and GDF-11 (p = .73, R2 < 0.001) or GDF-8 levels (p = .34, R2 < 0.001).
Table 2.
Distribution of Serum GDF-8 and GDF-11 Concentrations in Community-Dwelling Healthy Men, 19 Years or Older (N = 199)
| GDF-8 (ng/mL) | GDF-11 (ng/mL) | |
|---|---|---|
| Mean | 7.22 | 3.24 |
| Median | 6.79 | 3.11 |
| Standard deviation | 1.86 | 0.94 |
| Quartile range | 1.38 (6.22–7.60) | 0.48 (2.90–3.38) |
| 2.5th percentile | 5.09 | 2.59 |
| 5th percentile | 5.36 | 2.70 |
| 95th percentile | 10.3 | 4.06 |
| 97.5th percentile | 13.4 | 4.48 |
Notes: GDF-11 = growth and differentiation factor-11; GDF-8 = growth and differentiation factor-8. The distribution of serum GDF-11 and GDF-8 concentrations (ng/mL) in healthy men, 19 years or older.
Figure 1.
The box and whisker plots for age trends in serum GDF-8 (A) and GDF-8 (B) levels in community-dwelling healthy men and 5aR participants, 19–100 years, by decades of age. GDF = growth and differentiation factor; 5aR = 5α-reductase. Box-and-whisker plot: Box shows the range between first and third quartile and horizontal line inside the box is the median. The upper whisker indicates largest value smaller than 1.5 interquartile range (IQR) above third quartile and the lower whisker indicates smallest value larger than 1.5 IQR below the first quartile. Dots show values outside ranges specified by whiskers.
The Effects of Testosterone Treatment on Serum GDF-8 and GDF-11 Levels
The baseline characteristics of the 54 participants included in the 5aR study are given in Supplementary Table 3. Their median age was 40 years (range 24–50 years) and mean body mass index was 26.3 kg/m2 (range 19.8–36.9 kg/m2). The mean baseline GDF-8 concentration in healthy young men, 21–50 years of age, was 8.8 ± 2.8 ng/mL. The age at baseline was not associated with serum GDF-8 (p = .92, R2 = 0.001) or with GDF-11 (p = .11, R2 = 0.048) concentrations. Baseline fat-free mass was not associated with baseline serum GDF-8 (p = .14, R2 = 0.042) or with GDF-11 (p = .96, R2 = 0.001) concentrations. GDF-8 and GDF-11 levels were not associated with body mass index (p = .41, R2 = 0.013 and p = .88, R2 < 0.001, respectively). These relationships remained statistically not significant in the models adjusted to age. Similarly, baseline fat mass was not associated with serum GDF-8 (p = .39, R2 = 0.015) or with GDF-11 (p = .88, R2 = 0.001) concentrations.
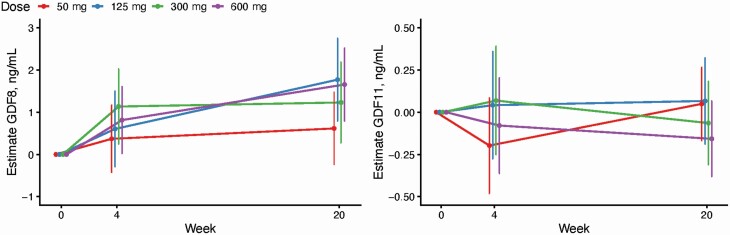
Testosterone treatment was associated with a significant increase in GDF-8 levels compared to baseline in men allocated to weekly administration of 125 mg (p = .001), 300 mg (p = .013), and 600 mg (p < .001) of TE, but not in those receiving 50 mg (p = .16; Figure 2). The mean ± SD percent changes in GDF-8 levels from baseline in men treated with 50, 125, 300, and 600 mg dose of TE were 15.6 ± 22.7%, 24.1 ± 39.4%, 26.7 ± 53.0%, and 29.1 ± 37.3%, respectively. There was no association between changes from baseline in GDF-8 levels with changes in fat-free mass (p = .53, R2 = 0.008), changes in fat mass (p = .12, R2 = 0.048), or with changes in total (p = .26, R2 = 0.026) or free (p = .29, R2 = 0.023) testosterone levels.
Figure 2.
Estimated mean changes from baseline in (A) serum GDF-8 levels and (B) serum GDF-11 levels for each testosterone dose group (50, 125, 300, or 600 mg/week) at Weeks 4 and 20 of the intervention period. The error bars, extracted from the mixed-model framework, indicate 95% confidence intervals. GDF = growth and differentiation factor.
The mean ± SD baseline GDF-11 concentration in healthy young men was 3.5 ± 0.5 ng/mL. The baseline GDF-11 concentrations were not significantly related to baseline fat-free mass or fat mass. Testosterone treatment had no significant effect on serum GDF-11 levels at any of the doses administered, and there was no dose-related effect of TE treatment on GDF-11 levels (p = .49; Figure 2). The mean ± SD percent changes in GDF-11 levels from baseline in men treated with 50, 125, 300, and 600 mg dose of TE were 1.5 ± 15%, −0.3 ± 13.7%, 3.1 ± 12.0%, and −3.4 ± 12.9%, respectively. Additionally, there was no association between changes from baseline in GDF-11 levels with changes in fat-free mass (p = .78, R2 = 0.002), changes in fat mass (p = .46, R2 = 0.011), or with changes in total (p = .30, R2 = 0.022) or free (p = .20, R2 = 0.032) testosterone.
Sensitivity analyses rerun for log-transformed GDF-8 and GDF-11 levels yielded similar findings for all regression models.
Discussion
Accurate and precise measurement of circulating levels of GDF-11 and GDF-8 has been challenging due to the high homology between these 2 proteins and their low concentration in the blood (1,16). Various assays have reported divergent values of circulating GDF-11 levels and it remains unclear whether GDF-11 levels are higher or lower in older adults (16,17). Here, we describe the development of an accurate and specific LC–MS/MS method for the simultaneous measurement of total GDF-8 and GDF-11 levels in human serum and plasma. The LC–MS/MS assay had sufficient sensitivity to be able to measure circulating myostatin concentrations in all participants, excellent linearity over the range of GDF-8 and GDF-11 concentrations that are prevalent in community-dwelling adults, and excellent recovery, specificity, and intra- and interassay precision even at low concentrations. Neither IgG nor GDF-8 had detectable cross-reactivity in the GDF-11 assay; this is particularly important because the nonspecificity of some of the published assays has been attributed to cross-reactivity of GDF-8 and immunoglobulin G. A blinded external evaluation of the assay provided additional independent verification of assay’s specificity and accuracy.
The validated LC–MS/MS method uses SCX–SPE for sample preparation, which generates “cleaner” samples for LC–MS/MS analysis, reducing the interference from other macromolecules present in the matrix, improving accuracy and precision, and providing consistent recoveries. The immunoassays for GDF-11 have reported excellent precision, but have suffered from cross-reactivity of GDF-8 and possibly immunoglobulins. By using MRM transitions for peptide fragments that are unique to each protein, a high degree of specificity was achieved. The aptamer-based multiplex platform assays provide the advantage of multiplexing several analytes, but are limited in their ability to provide precise quantitative information on GDF-11 concentrations. Immunoaffinity-linked LC–MS/MS assays have reported excellent precision and specificity (16,17); it is unclear whether competition between the antibody used for immunoaffinity purification and the endogenous binding proteins could affect recovery and the measured concentrations.
Using this validated assay and a well-characterized cohort of healthy men over a wide range, we did not find a significant association of GDF-11 or GDF-8 levels with age. However, testosterone treatment increased serum levels of GDF-8 but not of GDF-11, suggesting that the expression of these 2 highly homologous TGF-β family members is differentially regulated by testosterone. Myostatin levels increased in response to testosterone administration. These observations support the hypothesis that myostatin acts as a chalone—a counter-regulatory hormone—to restrain skeletal muscle growth in response to an anabolic stimulus (26,27).
This study’s findings should be viewed in the context of its limitations. The measurement of GDF-8 and GDF-11 was not prespecified outcomes of the 5aR trial, and only participants enrolled in the placebo group were included in these exploratory analyses, reducing the number of participants evaluated, which might have reduced power. The reference ranges were generated in a carefully selected, sample of community-dwelling, healthy men; men with acute or chronic health conditions were excluded. The sample should not be viewed as representative of the general population. The participants were all men, because the samples were derived from studies that were designed to test hypotheses that were specific to men. We used highly denaturing conditions for sample extraction to ensure dissociation of GDF-8 and GDF-11 from their cognate binding proteins; thus, the assay was designed to accurately measure total GDF-8 and GDF-11 levels; we do not know how binding of these proteins to plasma binding proteins affects their bioavailability and activity. Also, the long-term stability of these peptides in stored serum or plasma at −80°C remains unknown.
In conclusion, the novel LC–MS/MS method for quantification of GDF-8 and GDF-11 offers an accurate tool to evaluate the physiologic regulation of GDF-8 and GDF-11 in clinical studies of aging and age-related conditions. GDF-8 and GDF-11 are differentially regulated by testosterone, which increases serum GDF-8 levels as well as lean body mass, suggesting that GDF-8 acts as a chalone to restrain muscle growth in response to an anabolic stimulus such as testosterone.
Funding
This project was funded by a grant from the National Institute on Aging (NIA) (R56AG052972) to S.B. The prospective collection of samples from healthy men, 19 years or older, was supported by a Small Business Innovation Research (SBIR) grant (R44G045011) awarded by the NIA to R.J. External validation was supported by an NIA grant R01 AG052964 (R.P.T., S. Cummings MPIs). The funding agencies played no role in the analyses, design, writing, or decision to publish.
Conflict of Interest
S.B. reports receiving research grants from NIA, NINR, NICHD-NCMRR, FNIH, Alivegen, AbbVie, and Metro International Biotechnology and consultation fees from OPKO. These grants are managed by the Brigham and Women’s Hospital. Other authors report no conflicts.
Supplementary Material
References
- 1. Walker RG, Poggioli T, Katsimpardi L, et al. Biochemistry and biology of GDF11 and myostatin: similarities, differences, and questions for future investigation. Circ Res. 2016;118:1125–41; discussion 1142. doi:10.1161/CIRCRESAHA.116.308391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhou X, Wang JL, Lu J, et al. Reversal of cancer cachexia and muscle wasting by ActRIIB antagonism leads to prolonged survival. Cell. 2010;142:531–543. doi:10.1016/j.cell.2010.07.011 [DOI] [PubMed] [Google Scholar]
- 3. Smith RC, Lin BK. Myostatin inhibitors as therapies for muscle wasting associated with cancer and other disorders. Curr Opin Support Palliat Care. 2013;7:352–360. doi:10.1097/SPC.0000000000000013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ho DM, Yeo CY, Whitman M. The role and regulation of GDF11 in Smad2 activation during tailbud formation in the Xenopus embryo. Mech Dev. 2010;127:485–495. doi:10.1016/j.mod.2010.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Trendelenburg AU, Meyer A, Rohner D, Boyle J, Hatakeyama S, Glass DJ. Myostatin reduces Akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size. Am J Physiol Cell Physiol. 2009;296:C1258–C1270. doi:10.1152/ajpcell.00105.2009 [DOI] [PubMed] [Google Scholar]
- 6. Loffredo FS, Steinhauser ML, Jay SM, et al. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell. 2013;153:828–839. doi:10.1016/j.cell.2013.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sinha M, Jang YC, Oh J, et al. Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle. Science. 2014;344:649–652. doi:10.1126/science.1251152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Katsimpardi L, Litterman NK, Schein PA, et al. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science. 2014;344:630–634. doi:10.1126/science.1251141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang W, Guo Y, Li B, et al. GDF11 rejuvenates cerebrovascular structure and function in an animal model of Alzheimer’s disease. J Alzheimers Dis. 2018;62:807–819. doi:10.3233/JAD-170474 [DOI] [PubMed] [Google Scholar]
- 10. O’Connell KE, Guo W, Serra C, et al. The effects of an ActRIIb receptor Fc fusion protein ligand trap in juvenile simian immunodeficiency virus-infected rhesus macaques. FASEB J. 2015;29:1165–1175. doi:10.1096/fj.14-257543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schuelke M, Wagner KR, Stolz LE, et al. Myostatin mutation associated with gross muscle hypertrophy in a child. N Engl J Med. 2004;350:2682–2688. doi:10.1056/NEJMoa040933 [DOI] [PubMed] [Google Scholar]
- 12. Smith SC, Zhang X, Zhang X, et al. GDF11 does not rescue aging-related pathological hypertrophy. Circ Res. 2015;117:926–932. doi:10.1161/CIRCRESAHA.115.307527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hinken AC, Powers JM, Luo G, Holt JA, Billin AN, Russell AJ. Lack of evidence for GDF11 as a rejuvenator of aged skeletal muscle satellite cells. Aging Cell. 2016;15:582–584. doi:10.1111/acel.12475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Egerman MA, Cadena SM, Gilbert JA, et al. GDF11 Increases with age and inhibits skeletal muscle regeneration. Cell Metab. 2015;22:164–174. doi:10.1016/j.cmet.2015.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zimmers TA, Jiang Y, Wang M, et al. Erratum to: exogenous GDF11 induces cardiac and skeletal muscle dysfunction and wasting. Basic Res Cardiol. 2017;112:53. doi:10.1007/s00395-017-0642-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schafer MJ, Atkinson EJ, Vanderboom PM, et al. Quantification of GDF11 and myostatin in human aging and cardiovascular disease. Cell Metab. 2016;23:1207–1215. doi:10.1016/j.cmet.2016.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Semba RD, Zhang P, Zhu M, et al. Relationship of circulating growth and differentiation factors 8 and 11 and their antagonists as measured using liquid chromatography–tandem mass spectrometry with age and skeletal muscle strength in healthy adults. J Gerontol A Biol Sci Med Sci. 2019;74:129–136. doi:10.1093/gerona/gly255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Singh R, Bhasin S, Braga M, et al. Regulation of myogenic differentiation by androgens: cross talk between androgen receptor/ beta-catenin and follistatin/transforming growth factor-beta signaling pathways. Endocrinology. 2009;150:1259–1268. doi:10.1210/en.2008-0858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Braga M, Bhasin S, Jasuja R, Pervin S, Singh R. Testosterone inhibits transforming growth factor-β signaling during myogenic differentiation and proliferation of mouse satellite cells: potential role of follistatin in mediating testosterone action. Mol Cell Endocrinol. 2012;350:39–52. doi:10.1016/j.mce.2011.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Amthor H, Nicholas G, McKinnell I, et al. Follistatin complexes myostatin and antagonises myostatin-mediated inhibition of myogenesis. Dev Biol. 2004;270:19–30. doi:10.1016/j.ydbio.2004.01.046 [DOI] [PubMed] [Google Scholar]
- 21. Bhasin S, Travison TG, Storer TW, et al. Effect of testosterone supplementation with and without a dual 5α-reductase inhibitor on fat-free mass in men with suppressed testosterone production: a randomized controlled trial. JAMA. 2012;307:931–939. doi:10.1001/jama.2012.227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim J, Wang Z, Heymsfield SB, Baumgartner RN, Gallagher D. Total-body skeletal muscle mass: estimation by a new dual-energy X-ray absorptiometry method. Am J Clin Nutr. 2002;76:378–383. doi:10.1093/ajcn/76.2.378 [DOI] [PubMed] [Google Scholar]
- 23. Shiraishi S, Lee PW, Leung A, Goh VH, Swerdloff RS, Wang C. Simultaneous measurement of serum testosterone and dihydrotestosterone by liquid chromatography–tandem mass spectrometry. Clin Chem. 2008;54:1855–1863. doi:10.1373/clinchem.2008.103846 [DOI] [PubMed] [Google Scholar]
- 24. Mazer NA. A novel spreadsheet method for calculating the free serum concentrations of testosterone, dihydrotestosterone, estradiol, estrone and cortisol: with illustrative examples from male and female populations. Steroids. 2009;74:512–519. doi:10.1016/j.steroids.2009.01.008 [DOI] [PubMed] [Google Scholar]
- 25. U.S. Food and Drug Administration. Guidance for industry: bioanalytical method validation. 2001. https://www.fda.gov/media/70858/download
- 26. Lee SJ. Regulation of muscle mass by myostatin. Annu Rev Cell Dev Biol. 2004;20:61–86. doi:10.1146/annurev.cellbio.20.012103.135836 [DOI] [PubMed] [Google Scholar]
- 27. Gaussin V, Depre C. Myostatin, the cardiac chalone of insulin-like growth factor-1. Cardiovasc Res. 2005;68:347–349. doi:10.1016/j.cardiores.2005.09.007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.