Abstract
Background
Mild parkinsonian signs (MPS), highly prevalent in older adults, predict disability. It is unknown whether energy decline, a predictor of mobility disability, is also associated with MPS. We hypothesized that those with MPS had greater decline in self-reported energy level (SEL) than those without MPS, and that SEL decline and MPS share neural substrates.
Method
Using data from the Health, Aging and Body Composition Study, we analyzed 293 Parkinson’s disease-free participants (83 ± 3 years old, 39% Black, 58% women) with neuroimaging data, MPS evaluation by Unified Parkinson Disease Rating Scale in 2006–2008, and ≥3 measures of SEL since 1999–2000. Individual SEL slopes were computed via linear mixed models. Associations of SEL slopes with MPS were tested using logistic regression models. Associations of SEL slope with volume of striatum, sensorimotor, and cognitive regions were examined using linear regression models adjusted for normalized total gray matter volume. Models were adjusted for baseline SEL, mobility, demographics, and comorbidities.
Results
Compared to those without MPS (n = 165), those with MPS (n = 128) had 37% greater SEL decline in the prior 8 years (p = .001). Greater SEL decline was associated with smaller right striatal volume (adjusted standardized β = 0.126, p = .029). SEL decline was not associated with volumes in other regions. The association of SEL decline with MPS remained similar after adjustment for right striatal volume (adjusted odds ratio = 2.03, 95% CI: 1.16–3.54).
Conclusion
SEL decline may be faster in those with MPS. Striatal atrophy may be important for declining energy but does not explain the association with MPS.
Keywords: Disablement process, Epidemiology, Neuroimaging, Normative aging
Mild parkinsonian signs (MPS), defined by the presence of bradykinesia, rigidity, tremor, and/or postural instability (1,2), are common in the general older adult population without overt neurologic disorders, with prevalence estimates ranging from 15% to 52% (1,3,4). Presence of MPS in older adults is clinically important as MPS progress over time and predict disability and dementia (5–7). While increasing literature explores MPS as a predictor for various neurodegenerative disorders, evidence on predictors of MPS is limited and its etiology is not well understood. MPS may reflect underlying neuropathology, such as vascular burden and/or neurodegenerative changes (3,8,9). Advanced understanding of risk factors for MPS may provide insight into strategies to delay or prevent these disabling signs.
Low perceived energy, commonly reported in older adults (10,11), predicts mobility disability, difficulties in performing activities of daily living, and social isolation (12–14). We and others have shown that perceived energy is correlated with objective measures of energy expenditure and physical activity (10,11). Notably, low perceived energy is distinguishable from related constructs such as fatigue, depression, sleep impairment, and apathy.
In this study, we aimed to examine the relationship of change in perceived energy with MPS in older adults without overt neurological disorders and to examine neural substrates underlying energy decline and MPS. Specifically, we hypothesized that decline in perceived energy would predict MPS and this relationship would be independent of other chronic conditions and risk factors for MPS. We further hypothesized that there would be shared neural substrates underlying MPS and energy regulation (15–19).
Method
Study Population
The Health, Aging and Body Composition Study (Health ABC) is a longitudinal cohort study that enrolled 3 075 community-dwelling Black and White adults aged 70–79 between March 1997 and July 1998 (20). Participants had to be free of reported difficulty walking ¼ mile or climbing 10 steps at Year 1 (1997–1998) (20). Data collection occurred at yearly clinic examinations from Year 1 to Year 16 with phone calls every 6 months to update participants’ functional and health status (20). Additional examinations took place at Years 8 and 10, with continued phone call updates to collect incident major health events and hospitalizations every 6 months. Health ABC had exceptional retention, accounting for 99% of participants at the end of follow-up (20).
Self-reported energy level (SEL) was first assessed at Year 2 (1999–2000), which was considered the “baseline” of this analysis. We identified 293 Parkinson’s disease (PD)-free participants who had concurrent data on brain magnetic resonance imaging (MRI) and MPS at Year 10/11 (2006–2007) and at least 3 assessments of SEL between baseline and the time of brain MRI and MPS (average interval = 8 years, SD = 0.3). PD diagnosis was based on consensus review by 2 movement disorder specialists as previously described (21). Briefly, PD cases were identified if potential cases had 2 or more sources of PD identification without contradictory evidence or only one source of identification with clear internal consistency and no evidence against PD (21). Sources of PD identification included participant self-reported medication use or report of physician diagnosis (21).
The Health ABC study protocol was approved by the University of Pittsburgh institutional review board. All participants provided written informed consent at each visit.
Self-Reported Energy Levels
SEL was collected between 1999–2000 and 2006–2007. Participants were asked about their energy levels during the past month on a 0–10 scale, with 0 representing having no energy at all and 10 representing having the most energy one has ever had. SEL has been validated against objective measures of energy expenditure, including active energy expenditure, intensity (metabolic equivalents), duration (minutes of being active), and step counts (11).
Mild Parkinsonian Signs
MPS were determined at Year 10/11 visit between 2006 and 2007 using scores on the Unified Parkinson Disease Rating Scale (UPDRS) Part III: Motor Examination, which included subdomains of bradykinesia, tremor, and gait disturbances. The distribution of UPDRS score in this sample was left skewed with a median of 0 (interquartile range = 2). MPS was defined as either (i) 2 or more items with a score of 1, indicating mild symptoms, (ii) 1 item with a score of at least 2, indicating moderate-to-severe symptoms, or (iii) a rest tremor score of 1 without meeting diagnostic criteria for PD (8). Bradykinesia was considered present if slowing or hesitation was detected in either right or left extremities during finger tapping, fist clench, pronation–supination, or heel tapping. Gait disturbances were considered present if an abnormality was detected for any one of the following: arising from a chair with arms folded across the chest, postural stability test, posture, or gait. Tremor of the upper extremities was considered present if either facial or hand tremor was observed at rest or during an action (22).
Brain MRI Imaging Acquisition and Processing
The MRI scanning used a Siemens 12-channel head coil and was performed on a 3 T Siemens Tim Trio MR scanner in 2007–2008 (16). Magnetization-prepared rapid gradient-echo T1-weighted images were collected in the axial plane: repetition time (TR) = 2 300 ms; echo time (TE) = 3.43 ms; inversion time (TI) = 900 ms; flip angle (FA) = 9; slice thickness = 1 mm; field of view (FOV) = 256*224 mm; voxel size = 1 mm*1 mm; matrix size = 256*224; and number of slices = 176. Fluid-attenuated inversion recovery (FLAIR) T2-weighted images were collected in the axial plane: TR = 9 160 ms; TE = 89 ms; TI = 2 500 ms; FA = 150; FOV = 256*212 mm; slice thickness = 3 mm; matrix size = 256*240; number of slices = 48 slices; and voxel size = 1 mm*1 mm (16). Total intracranial volume was calculated as the volume within the inner skull using the brain extraction tool (23).
Gray matter volumes were estimated by segmenting the skull-stripped T1-weighted image in native anatomical space using the FAST-FMRIB Automated Segmentation Tool (16). Total brain gray matter volume was estimated in cubic millimeters by summing all voxels classified as gray matter tissue (16). White matter hyperintensity volumes were extracted from T2-FLAIR as previously described (24,25). Neuroanatomic boundaries of regions of interest (ROIs) were identified using the automated anatomical labeling neuroanatomical atlas, for the right and left hemispheres separately; for the entorhinal cortex, Brodmann’s area (BA) parcellations were used (BA 28 and 34) (16). The gray matter volume of the brain was normalized by the intracranial volume and referred to as normalized total gray matter volume.
Neuroimaging ROIs
We identified ROIs based on the literature on neuroimaging correlates of MPS and energy (or fatigue) (Supplementary Table 1). Specifically, we included regions of the nigrostriatal dopaminergic network because of their importance for motor control and relationship with MPS. We also included the main sensorimotor area (precentral and postcentral gyri), globus pallidus, thalamus, executive control function area (middle frontal gyrus), and limbic areas (hippocampus, parahippocampal gyrus, entorhinal cortex, amygdala, and olfactory cortex) connected to the striatum (putamen and caudate). In this analysis, we examined gray matter volumes for the right and left hemispheres separately.
Covariates of Interest
Covariates included baseline SEL and mobility, demographic attributes, comorbidities, and fatigue at the time of MPS evaluation. Demographics included chronological age, sex, race, and years of education (all collected via self-report at baseline). Comorbidities associated with MPS included cardiovascular diseases, stroke, myocardial infarction, diabetes, and small vessel disease indicated by white matter hyperintensities normalized to total brain volume (26–28). These comorbidities were ascertained by participants’ interviews and confirmed by medical record. Mobility was assessed using Short Physical Performance Battery at Year 1.
Self-reported fatigue was determined using the question “I felt that everything I did was an effort” over the past week from the Center for Epidemiologic Studies Depression Scale (CES-D) (29). Higher fatigue was classified as “some or a little of the time” (1–2 days), “occasionally or a moderate amount of time (3–4 days),” or “all of the time (5–7 days).” Lower fatigue was classified as “rarely or none of the time (<1 day)” (29).
Statistical Analysis
Bivariate associations of MPS with participants’ characteristics were examined using independent t tests for continuous variables or chi-squared tests for categorical variables. Individual SEL slopes were estimated using a linear mixed-effects model based on at least 3 available measures between 1999–2000 and 2006–2007. Bivariate associations of SEL slopes with participants’ characteristics were examined using Pearson’s correlation for continuous variables or independent t tests for categorical variables. Associations of SEL slopes with brain ROIs were examined using partial correlation coefficients, controlling for normalized total gray matter volume. Neuroimaging markers of interest that were associated with SEL slopes at p <.05 were further examined in multivariable regression analyses.
We used logistic regression models to examine the association of SEL slopes with MPS, initially adjusting for age, fatigue at the time of MPS, and baseline SEL, and further adjusted for comorbidities and baseline mobility. In exploratory analyses, we examined the associations of SEL slopes with gait disturbances and bradykinesia using logistic regression.
We used stepwise linear regression models to examine the association of SEL slopes with neuroimaging markers of interest, initially adjusting for age and normalized total gray matter volume, further adjusting for baseline SEL and white matter hyperintensities, and additionally adjusting for comorbidities at the time of MPS. We also examined associations between neuroimaging markers of interest at Year 10/11 and baseline SEL at Year 2.
To examine whether neuroimaging measures would affect the relationship of SEL slopes with MPS, we further adjusted for the identified neuroimaging marker in logistic regression of SEL slopes with MPS. Models were adjusted for age, normalized total gray matter volume, baseline SEL, fatigue, normalized white matter hyperintensities, comorbidities at the time of MPS, and Short Physical Performance Battery at Year 1.
In additional sensitivity analyses, we repeated analyses by further excluding 15 participants with dementia, thus yielding a sample of 278. Dementia diagnosis was based on longitudinal data collected since 1999–2000 including dementia medication use, hospital records, or a change in Modified Mini-Mental State Examination scores (30).
All analyses were performed using SAS v9.5 (Cary, NC). Because analyses of SEL with neuroimaging markers are exploratory, statistical significance was set at p <.05. A trend was considered at .05 < p <.10.
Results
MPS was significantly associated with older age (p < .001) and with higher fatigue (p = .027) but not with sex, race, education, or prevalence of cardiovascular disease, stroke, myocardial infarction, or diabetes (Table 1). Among the neuroimaging markers of interest, compared to those without MPS, those with MPS had higher normalized white matter hyperintensities and smaller volumes of bilateral precentral and postcentral gyri and bilateral dorsolateral prefrontal cortex (Table 1).
Table 1.
Participants’ Characteristics and Neuroimaging Markers of Interest at the Time of Brain MRI and Their Bivariate Associations With the Slope of Energy and Mild Parkinsonian Signs (n = 293)
| Mean (SD) Are Reported Unless Otherwise Noted | Correlations With SEL Slopes, r (p value) or Mean Difference ± SD (p value) | Correlations With MPS, Mean Difference ± SD (p value), Chi square or t Test p-value, or r (p Value) | |
|---|---|---|---|
| Demographics | |||
| Age, y | 83.0 (2.8) | −0.029 (.620) | 1.2 ± 2.8 (<.001) |
| Women, N (%) | 171 (58) | −0.006 ± 0.05 (.318) | 0.999 |
| Black, N (%) | 114 (39) | 0.010 ± 0.05 (.069) | 0.630 |
| Postsecondary education | 151 (52) | −0.002 ± 0.05 (.684) | 0.195 |
| Body mass index, kg/m2 | 27.4 (4.4) | −0.090 (.124) | 0.6 ± 4.4 (.280) |
| Energy | |||
| Baseline SEL at Year 2 | 6.8 (1.5) | 0.049 (.399) | −0.2 ± 1.5 (.260) |
| SEL slopes, change per year | −0.06 (0.05) | — | −0.019 ± 0.049 (.001) |
| Mobility performance at Year 1 | |||
| Short Physical Performance Battery (0–12) | 10.4 (1.3) | 0.224 (<.001) | −0.48 ± 1.23 (.001) |
| Chair stand per second | 0.39 (0.11) | 0.194 (<.001) | −0.05 ± 0.11 (<.001) |
| Gait speed over 3, 4, or 6 m, m/s | 1.27 (0.24) | 0.118 (.043) | −0.07 ± 0.24 (.012) |
| Standing balance, s (0–90) | 72.9 (20.8) | 0.119 (.042) | −6.6 ± 20.6 (.008) |
| Mild parkinsonian signs (UPDRS ≥ 1), N (%) | 128 (44) | −0.019 ± 0.049 (.001) | |
| Fatigue, N (%) | 85 (29) | −0.030 ± 0.048 (<.001) | 0.027 |
| Prevalence of cardiovascular disease | 84 (29) | −0.005 ± 0.050 (.405) | 0.364 |
| Prevalence of stroke | 24 (8) | −0.005 ± 0.050 (.648) | 0.391 |
| Prevalence of diabetes | 74 (25) | −0.017 ± 0.050 (.014) | 0.685 |
| Prevalence of myocardial infarction | 49 (17) | −0.011 ± 0.050 (.164) | 0.999 |
| Normalized white matter hyperintensities | 0.0058 (0.0074) | −0.018 (.760) | 0.002 ± 0.007 (.007) |
| Striatum, cm3 | 11.6 (3.7) | 0.112 (.056) | −0.005 (.927) |
| Left striatum | 5.5 (2.0) | 0.073 (.217) | −0.015 (.800) |
| Right striatum | 6.1 (1.9) | 0.140 (.017) | 0.005 (.937) |
| Globus pallidus, cm3 | 0.53 (0.49) | 0.040 (.498) | 0.018 (.757) |
| Left pallidus | 0.25 (0.24) | 0.024 (.680) | 0.007 (.902) |
| Right pallidus | 0.29 (0.28) | 0.049 (.401) | 0.026 (.660) |
| Thalamus, cm3 | 1.8 (0.4) | −0.046 (.438) | −0.002 (.970) |
| Left thalamus | 0.91 (0.23) | −0.052 (.380) | −0.026 (.657) |
| Right thalamus | 0.93 (0.24) | −0.035 (.555) | 0.021 (.727) |
| Primary motor cortex, cm3 | 16.8 (2.7) | 0.054 (.357) | −0.183 (.002) |
| Left precentral gyrus | 8.8 (1.5) | 0.002 (.974) | −0.165 (.005) |
| Right precentral gyrus | 8.1 (1.4) | 0.099 (.090) | −0.169 (.004) |
| Primary sensory cortex, cm3 | 19.1 (3.2) | 0.084 (.150) | −0.161 (.006) |
| Left postcentral gyrus | 10.0 (1.8) | 0.080 (.171) | −0.151 (.010) |
| Right postcentral gyrus | 9.1 (1.7) | 0.074 (.203) | −0.145 (.013) |
| Limbic area, cm3 | 29.3 (3.9) | −0.009 (.877) | −0.065 (.265) |
| Left limbic area | 14.5 (2.1) | 0.016 (.783) | −0.055 (.347) |
| Right limbic area | 14.8 (2.0) | −0.034 (.568) | −0.068 (.245) |
| Dorsolateral prefrontal cortex, cm3 | 2.7 (3.2) | 0.012 (.832) | −0.123 (.036) |
| Left middle frontal gyrus | 13.2 (1.7) | 0.013 (.823) | −0.114 (.053) |
| Right middle frontal gyrus | 13.8 (1.8) | 0.009 (.873) | −0.108 (.066) |
Notes: MPS = mild parkinsonian signs; SEL = self-reported energy level; UPDRS = Unified Parkinson Disease Rating Scale. Correlations with regional neuroimaging markers were adjusted for normalized total gray matter volume, ie, ratio of total gray matter volume by intracranial volume. The bold number reflects correlations at p <.05. Normalized white matter hyperintensities: ratio of total white matter hyperintensities by total brain volume.
On average, SEL declined by 0.06 points per year between 1999–2000 and 2006–2007. SEL decline was 37% greater in those with MPS at Year 10/11 than those without (p = .001) (Table 1). SEL decline was also higher in those with diabetes or who reported fatigue at the time of MPS (Table 1). SEL decline was not significantly associated with age, sex, race, education, or prevalence of cardiovascular disease, stroke, or myocardial infarction (Table 1).
Among the neuroimaging markers of interest, greater SEL decline was associated with smaller right striatal volume (Table 1). This association remained largely unchanged after adjustment for age, normalized total gray matter volume, baseline SEL, normalized white matter hyperintensities, and comorbidities (standardized β [p value]: 0.137 [.017], 0.138 [.017], 0.142 [.013], and 0.126 [.029], respectively). Associations with SEL decline were similar for the following striatal subregions: right putamen and right caudate (not shown). There was also a trend towards smaller right precentral gyrus being associated with greater SEL decline (Table 1). SEL decline was not significantly associated with other neuroimaging markers of interest (Table 1). Baseline SEL was not statistically significantly associated with any neuroimaging marker of interest at Year 10/11 (all p > .05).
Greater decline in SEL was associated with higher odds of MPS after adjustment for age, fatigue, and baseline SEL (odds ratio = 1.96, 95% CI: 1.18–3.26) (Table 2, Model 4; Figure 1). These associations remained largely unchanged after further adjustment for right striatal volume, comorbidities, and Short Physical Performance Battery at Year 1 (Table 2, Model 5–8). In exploratory analyses, greater decline in SEL was associated with higher odds of gait disturbance but was not associated with bradykinesia (not shown).
Table 2.
Associations of the Slope of Energy With the Presence of MPS in this Parkinson’s Disease-Free Sample (n = 293)
| Model 1: Unadjusted | Model 2: Adjusted for Age | Model 3: Model 2 + Fatigue | Model 4: Model 3 + Baseline SEL | Model 5: Model 4 + WMH | Model 6: Model 5 + Right Striatum + Normalized Total Gray Matter Volume | Model 7: Model 6 + Disease Conditions Affecting MPS | Model 8: Model 7 + SPPB a Year 1 | |
|---|---|---|---|---|---|---|---|---|
| Unadjusted OR (95% CI) | Adjusted OR (95% CI) | |||||||
| SEL slopes | 2.20 (1.34, 3.63) | 2.19 (1.33, 3.61) | 1.98 (1.18, 3.30) | 1.96 (1.18, 3.26) | 2.00 (1.19, 3.37) | 2.20 (1.29, 3.77) | 2.26 (1.31, 3.91) | 2.03 (1.16, 3.54) |
Notes: MPS = mild parkinsonian signs; SEL = self-reported energy level; SPPB = Short Physical Performance Battery; WMH = white matter hyperintensities. Model 2: Model 8 adjusted for covariates that were bivariately associated with MPS (ie, age; refer to Table 1). In Model 7, diseases included the prevalence of cardiovascular disease, stroke, myocardial infarction, and diabetes. Due to small values, the slope of energy was multiplied by 10 for interpretation purposes. The sign of slope was reversed with higher values indicating a greater decline in SEL.
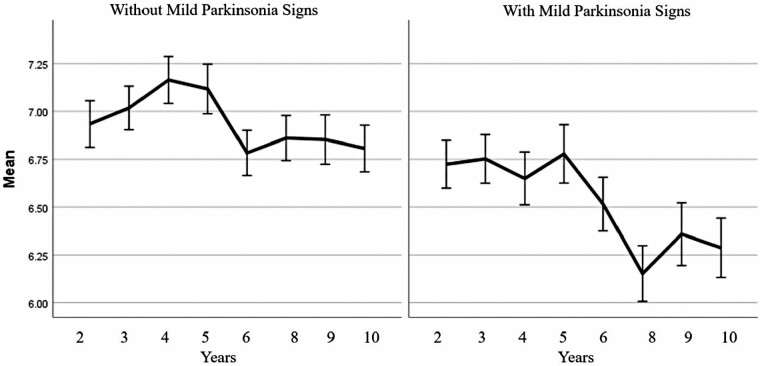
Figure 1.
Previous self-reported energy levels in those with (right) and without mild parkinsonian signs assessed in Year 10 (left). Those with lower mean self-reported energy levels over time had higher odds of mild parkinsonian signs.
All results remained similar after excluding 15 participants with dementia (Supplementary Table 2).
Discussion
In this study of community-dwelling older adults, we find that those with MPS had greater decline in SEL in the prior 8 years compared to those without MPS, and this relationship was independent of comorbidities and other locomotor risk factors. While smaller right striatal volume was associated with greater decline in SEL, it did not explain the relationship of SEL decline with MPS.
Our study examined perceived energy as the primary predictor of MPS, separately from measures of fatigue or tiredness. While feelings of low energy and high fatigue are both commonly reported in older adults, they are rarely measured or analyzed separately (31). Studies on the neurobiology of energy and fatigue in healthy adults without neurological diseases have suggested distinct brain networks and neurotransmitter signaling systems (32). Evidence indicates that energy may be regulated by the dopaminergic signaling system, whereas fatigue may be primarily regulated by the serotoninergic system (33–35). Dopaminergic neuromodulation is strongly implicated in motivation, cognition, and mobility; therefore, dopamine decreases associated with aging may lead to decreased motivation or perceived energy (36). Dopamine may also serve as a mechanism to balance energy expenditure with the amount of energy available; the presence of dopamine may signal energy availability and regulates energy expended in an activity (37). Energy and fatigue also may have played different evolutionary roles, with energy driving proactive behaviors (hunting, gathering) and fatigue regulating aversive behaviors aimed at preserving energy, especially during an illness (32). Our study did not have data on dopaminergic or serotoninergic neurotransmitters, thus we cannot assess whether the association of striatal volume and decline in SEL reflects serotoninergic or dopaminergic signaling.
We identified neural substrates of SEL decline among community-dwelling older adults, while previous neuroimaging studies have only examined correlates with fatigue. Our results share consistencies with previous neuroimaging findings on fatigue. Both our study and previous research have demonstrated an important role of the striatum in energy or fatigue (18). Other functional MRI studies have suggested that impairments of the cortico-striatal network may lead to increased fatigue (38,39). Some previous work has also shown that fatigue is associated with low cortical thickness, primarily in frontal and temporal regions, and with smaller hippocampal volume (40). The fact that we did not find associations of volumes in these areas with SEL decline may support the notion that energy and fatigue are relevant but distinct constructs.
Contrary to our hypotheses, we did not find shared neural substrates underlying both SEL decline and MPS. Our results indicate that neural correlates of energy decline are localized in the right striatum, whereas neural correlates of MPS are localized in the primary sensorimotor cortex and dorsolateral prefrontal cortex. The mechanisms underlying energy decline and MPS remain unclear. It is worth noting that most evidence for the neurobiological substrates of low energy or high fatigue is from clinical populations with neurodegenerative diseases, including PD and multiple sclerosis (10). Previous studies in clinical populations underscore an important role of structural integrity and functional connectivity of the striatum in energy perception (41,42). Future studies are warranted to understand mechanisms underlying energy decline and MPS among community-dwelling older adults without overt neurological disorders.
One potential explanation to support the SEL–MPS relationship could be that SEL is related to bioenergetic resources. Prior literature suggests that poor mobility may develop once a threshold of energy loss is met (43,44). Maximal energy expenditure, maximum oxygen consumption, and resting metabolic rate all decline with increasing age (43). With less aerobic capacity and less energy available in later life, there is a higher deficit of available energy to perform physical and mental daily tasks (43). Evidence to date is from animal models, patient populations, or studies focusing on measures of skeletal muscle mitochondrial function (43,45). Studies using self-reported measures of energy levels are sparse. Assessing energy levels by self-report may be clinically accessible and cost-effective. We have previously shown in this cohort that SEL was associated with both objective measures of energy expenditure, and mobility (19). Studies should assess whether SEL may reflect biological measures of energy metabolism, such as mitochondrial function. If this were the case, assessing SEL could be critical to identify older adults with lower bioenergetics profiles.
Another potential explanation for the link between SEL decline and MPS is that lower SEL may reflect depressive symptoms and/or sleep disturbances. Both are known predictors of MPS (46). We have recently shown strong cross-sectional associations between SEL and depressive symptoms measured by CES-D (19). However, in the present analysis, the associations between SEL and MPS were robust to the adjustment for 1 subitem from CES-D (fatigue). Notably, the range of depressive symptoms and/or sleep disturbances is small in this sample with few participants showing severe manifestations. A cohort with a wider range of depressive symptoms and sleep disturbances would be more appropriate to explore their contribution to the SEL decline–MPS relationship.
This study has several strengths. First, the longitudinal assessment of SEL allowed us to capture individual trajectories of perceived energy, while much of the previous literature primarily examined energy at one time (11). Furthermore, this well-characterized sample of community-dwelling older adults allowed us to quantify the relationship between SEL decline and MPS while accounting for comorbidities and other locomotor risk factors at the time of MPS as well as mobility performance at baseline. Our results may be more generalizable to the majority of older adults free of conditions such as multiple sclerosis, sleep apnea, PD, and other pathologies in which energy/fatigue is more closely studied (39,47,48).
Limitations of this study include modest sample size, healthier than the entire Health ABC cohort. There was a competing risk of death by the time of MRI evaluation in 2006–2008, as some participants may have died earlier in the study who would otherwise have been eligible for MRI. Although SEL was measured longitudinally, both MPS and brain MRI were measured at one time. Although we controlled for baseline mobility as a surrogate indicator of baseline MPS, causality between SEL decline and MPS cannot be assumed without MPS assessment at baseline. Future analyses should investigate the relationship between brain structure and the development of MPS over time.
Conclusion
Our results suggest SEL decline may be faster in those with MPS among older adults without overt neurological disorders. This is of particular clinical relevance because SEL on a 0–10 scale can be more easily assessed in routine clinic visits relative to objective measures of energy. Furthermore, SEL may be a modifiable risk factor that can be targeted to reduce incident MPS. While more research is required to determine the full clinical utility and implications of SEL decline, it may be possible to incorporate SEL as part of a screening test or risk stratification algorithm for MPS. Further exploration of neuropathophysiological mechanisms underlying perceived energy loss and MPS should include multimodal neuroimaging protocols and network analysis.
Supplementary Material
Funding
This work was supported in part by the National Institutes of Health, the National Institute on Aging (NIA), Intramural Research Program, Baltimore, MD. This research was supported by the NIA Contracts N01-AG-2101, N01-AG-6-2103, and NIA grant R01-AG028050, and National Institute of Nursing Research grant R01-NR012459.
Conflict of Interest
None declared.
Author Contributions
All authors discussed the results and contributed to writing and editing of the manuscript per ICMJE criteria for authorship. R.E., Q.T., C.R., and X.Z.: study planning and design. Q.T. and X.Z.: coding and analysis. R.E., Q.T., N.W.G., L.M.C., J.H., and C.R: interpretation of the results and preparation of the manuscript.
References
- 1. Louis ED, Bennett DA. Mild parkinsonian signs: an overview of an emerging concept. Mov Disord. 2007;22(12):1681–1688. doi:10.1002/mds.21433 [DOI] [PubMed] [Google Scholar]
- 2. Homayoun H. Parkinson disease. Ann Intern Med. 2018;169(5):ITC33–ITC48. doi:10.7326/AITC201809040 [DOI] [PubMed] [Google Scholar]
- 3. Kishi M, Wada-Isoe K, Hanajima R, Nakashima K. Predictors for incident mild parkinsonian signs in older Japanese. Yonago Acta Med. 2020;63(1):1–7. doi:10.33160/yam.2020.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bennett DA, Beckett LA, Murray AM, et al. Prevalence of parkinsonian signs and associated mortality in a community population of older people. N Engl J Med. 1996;334(2):71–76. doi:10.1056/NEJM199601113340202 [DOI] [PubMed] [Google Scholar]
- 5. Louis ED, Tang MX, Schupf N. Mild parkinsonian signs are associated with increased risk of dementia in a prospective, population-based study of elders. Mov Disord. 2010;25(2):172–178. doi:10.1002/mds.22943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Louis ED, Schupf N, Manly J, Marder K, Tang MX, Mayeux R. Association between mild parkinsonian signs and mild cognitive impairment in a community. Neurology. 2005;64(7):1157–1161. doi:10.1212/01.WNL.0000156157.97411.5E [DOI] [PubMed] [Google Scholar]
- 7. Mahlknecht P, Stockner H, Marini K, et al. Midbrain hyperechogenicity, hyposmia, mild parkinsonian signs and risk for incident Parkinson’s disease over 10 years: a prospective population-based study. Parkinsonism Relat Disord. 2020;70:51–54. doi:10.1016/j.parkreldis.2019.12.008 [DOI] [PubMed] [Google Scholar]
- 8. Rosso AL, Bohnen NI, Launer LJ, Aizenstein HJ, Yaffe K, Rosano C. Vascular and dopaminergic contributors to mild parkinsonian signs in older adults. Neurology. 2018;90(3):e223–e229. doi:10.1212/WNL.0000000000004842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Louis ED, Luchsinger JA. History of vascular disease and mild parkinsonian signs in community-dwelling elderly individuals. Arch Neurol. 2006;63(5):717–722. doi:10.1001/archneur.63.5.717 [DOI] [PubMed] [Google Scholar]
- 10. Kluger BM, Krupp LB, Enoka RM. Fatigue and fatigability in neurologic illnesses: proposal for a unified taxonomy. Neurology. 2013;80(4):409–416. doi:10.1212/WNL.0b013e31827f07be [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tian Q, Glynn NW, Ehrenkranz RC, Sprague BN, Rosso AL, Rosano C. Perception of energy and objective measures of physical activity in older adults. J Am Geriatr Soc. 2020:68(8):1876–1878. doi:10.1111/jgs.16577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cheng H, Gurland BJ, Maurer MS. Self-reported lack of energy (anergia) among elders in a multiethnic community. J Gerontol A Biol Sci Med Sci. 2008;63(7):707–714. doi:10.1093/gerona/63.7.707 [DOI] [PubMed] [Google Scholar]
- 13. Avlund K, Damsgaard MT, Schroll M. Tiredness as determinant of subsequent use of health and social services among nondisabled elderly people. J Aging Health. 2001;13(2):267–286. doi:10.1177/089826430101300206 [DOI] [PubMed] [Google Scholar]
- 14. Avlund K. Fatigue in older adults: an early indicator of the aging process? Aging Clin Exp Res. 2010;22(2):100–115. doi:10.1007/BF03324782 [DOI] [PubMed] [Google Scholar]
- 15. Chaudhuri A, Behan PO. Fatigue and basal ganglia. J Neurol Sci. 2000;179(S 1–2):34–42. doi:10.1016/s0022-510x(00)00411-1 [DOI] [PubMed] [Google Scholar]
- 16. Rosano C, Aizenstein HJ, Newman AB, et al. ; Health ABC Study . Neuroimaging differences between older adults with maintained versus declining cognition over a 10-year period. Neuroimage. 2012;62(1):307–313. doi:10.1016/j.neuroimage.2012.04.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Camarda C, Pipia C, Battaglini I, et al. Mild parkinsonian signs in a hospital-based cohort of mild cognitive impairment types: a cross-sectional study. Curr Alzheimer Res. 2019;16(7):633–649. doi:10.2174/1567205016666190726100744 [DOI] [PubMed] [Google Scholar]
- 18. Wasson E, Rosso AL, Santanasto AJ, et al. ; LIFE Study Group . Neural correlates of perceived physical and mental fatigability in older adults: a pilot study. Exp Gerontol. 2019;115:139–147. doi:10.1016/j.exger.2018.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ehrenkranz R, Rosso AL, Sprague BN, et al. Functional correlates of self-reported energy levels in the Health, Aging and Body Composition Study. Aging Clin Exp Res. Published online March 10, 2021. doi:10.1007/s40520-021-01788-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. National Institute on Aging. Introducing the Health ABC Study: The Dynamics of Health, Aging, and Body Composition. https://healthabc.nia.nih.gov/. Accessed June 28, 2021.
- 21. Chen H, Shrestha S, Huang X, et al. ; Health ABC Study . Olfaction and incident Parkinson disease in US white and black older adults. Neurology. 2017;89(14):1441–1447. doi:10.1212/WNL.0000000000004382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rosano C, Bennett DA, Newman AB, et al. Patterns of focal gray matter atrophy are associated with bradykinesia and gait disturbances in older adults. J Gerontol A Biol Sci Med Sci. 2012;67A(9):957–962. doi:10.1093/gerona/glr262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jenkinson M, Pechaud M, Smith S. BET2-MR-Based Estimation of Brain, Skull and Scalp Surfaces. Vol. 17. 2002. http://ftp.nmr.mgh.harvard.edu/pub/dist/freesurfer/tutorial_packages/centos6/fsl_507/doc/wiki/BET.html. Accessed June 19, 2020. [Google Scholar]
- 24. Wu M, Rosano C, Lopez-Garcia P, Carter CS, Aizenstein HJ. Optimum template selection for atlas-based segmentation. Neuroimage. 2007;34(4):1612–1618. doi:10.1016/j.neuroimage.2006.07.050 [DOI] [PubMed] [Google Scholar]
- 25. Wu M, Rosano C, Butters M, et al. A fully automated method for quantifying and localizing white matter hyperintensities on MR images. Psychiatry Res. 2006;148(2–3):133–142. doi:10.1016/j.pscychresns.2006.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Prins ND, Scheltens P. White matter hyperintensities, cognitive impairment and dementia: an update. Nat Rev Neurol. 2015;11(3):157–165. doi:10.1038/nrneurol.2015.10 [DOI] [PubMed] [Google Scholar]
- 27. Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. Br Med J. 2010;341(7767):c3666. doi:10.1136/bmj.c3666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gouw AA, Seewann A, van der Flier WM, et al. Heterogeneity of small vessel disease: a systematic review of MRI and histopathology correlations. J Neurol Neurosurg Psychiatry. 2011;82(2):126–135. doi:10.1136/jnnp.2009.204685 [DOI] [PubMed] [Google Scholar]
- 29. LaSorda KR, Gmelin T, Kuipers AL, et al. Epidemiology of perceived physical fatigability in older adults: the Long Life Family Study. J Gerontol A Biol Sci Med Sci. 2020;75(9):e81–e88. doi:10.1093/gerona/glz288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hong CH, Falvey C, Harris TB, et al. Anemia and risk of dementia in older adults: findings from the Health ABC Study. Neurology. 2013;81(6):528–533. doi:10.1212/WNL.0b013e31829e701d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zengarini E, Ruggiero C, Pérez-Zepeda MU, et al. Fatigue: relevance and implications in the aging population. Exp Gerontol. 2015;70:78–83. doi:10.1016/j.exger.2015.07.011 [DOI] [PubMed] [Google Scholar]
- 32. Meeusen R, Roelands B. Fatigue: is it all neurochemistry? Eur J Sport Sci. 2018;18(1):37–46. doi:10.1080/17461391.2017.1296890 [DOI] [PubMed] [Google Scholar]
- 33. Loy BD, Cameron MH, O’Connor PJ. Perceived fatigue and energy are independent unipolar states: supporting evidence. Med Hypotheses. 2018;113:46–51. doi:10.1016/j.mehy.2018.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Loy BD, O’Connor PJ. The effect of histamine on changes in mental energy and fatigue after a single bout of exercise. Physiol Behav. 2016;153:7–18. doi:10.1016/j.physbeh.2015.10.016 [DOI] [PubMed] [Google Scholar]
- 35. Loy BD, O’Connor PJ, Dishman RK. The effect of a single bout of exercise on energy and fatigue states: a systematic review and meta-analysis. Fatigue Biomed Heal Behav. 2013;1(4):223–242. doi:10.1080/21641846.2013.843266 [Google Scholar]
- 36. Düzel E, Bunzeck N, Guitart-Masip M, Düzel S. NOvelty-related motivation of anticipation and exploration by dopamine (NOMAD): implications for healthy aging. Neurosci Biobehav Rev. 2010;34(5):660–669. doi:10.1016/j.neubiorev.2009.08.006 [DOI] [PubMed] [Google Scholar]
- 37. Beeler JA, Frazier CR, Zhuang X. Putting desire on a budget: dopamine and energy expenditure, reconciling reward and resources. Front Integr Neurosci. 2012;6:49. doi:10.3389/fnint.2012.00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ren P, Anderson AJ, McDermott K, Baran TM, Lin F. Cognitive fatigue and cortical-striatal network in old age. Aging (Albany NY). 2019;11(8):2312–2326. doi:10.18632/aging.101915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dobryakova E, Genova HM, DeLuca J, Wylie GR. The dopamine imbalance hypothesis of fatigue in multiple sclerosis and other neurological disorders. Front Neurol. 2015;6:52. doi:10.3389/fneur.2015.00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Carvalho DZ, St Louis EK, Boeve BF, et al. Excessive daytime sleepiness and fatigue may indicate accelerated brain aging in cognitively normal late middle-aged and older adults. Sleep Med. 2017;32:236–243. doi:10.1016/j.sleep.2016.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jaeger S, Paul F, Scheel M, et al. Multiple sclerosis-related fatigue: altered resting-state functional connectivity of the ventral striatum and dorsolateral prefrontal cortex. Mult Scler. 2019;25(4):554–564. doi:10.1177/1352458518758911 [DOI] [PubMed] [Google Scholar]
- 42. Kluger BM, Zhao Q, Tanner JJ, et al. Structural brain correlates of fatigue in older adults with and without Parkinson’s disease. Neuroimage Clin. 2019;22:101730. doi:10.1016/j.nicl.2019.101730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schrack JA, Simonsick EM, Ferrucci L. The energetic pathway to mobility loss: an emerging new framework for longitudinal studies on aging. J Am Geriatr Soc. 2010;58(suppl. 2):S329. doi:10.1111/j.1532-5415.2010.02913.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Priede IG. Natural selection for energetic efficiency and the relationship between activity level and mortality. Nature. 1977;267(5612):610–611. doi:10.1038/267610a0 [DOI] [PubMed] [Google Scholar]
- 45. Evans WJ, Lambert CP. Physiological basis of fatigue. Am J Phys Med Rehabil. 2007;86(suppl. 1):S29–S46. doi:10.1097/phm.0b013e31802ba53c [DOI] [PubMed] [Google Scholar]
- 46. Prasuhn J, Piskol L, Vollstedt EJ, et al. Non-motor symptoms and quality of life in subjects with mild parkinsonian signs. Acta Neurol Scand. 2017;136(5):495–500. doi:10.1111/ane.12760 [DOI] [PubMed] [Google Scholar]
- 47. Hong S, Dimsdale JE. Physical activity and perception of energy and fatigue in obstructive sleep apnea. Med Sci Sports Exerc. 2003;35(7):1088–1092. doi:10.1249/01.MSS.0000074566.94791.24 [DOI] [PubMed] [Google Scholar]
- 48. Schrag A, Horsfall L, Walters K, Noyce A, Petersen I. Prediagnostic presentations of Parkinson’s disease in primary care: a case–control study. Lancet Neurol. 2015;14(1):57–64. doi:10.1016/S1474-4422(14)70287-X [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.