Abstract
We present a patient with chronic insomnia resistant to traditional pharmacologic (eg, sedative-hypnotics) and nonpharmacologic (eg, cognitive behavioral therapy for insomnia) therapy. A finding of elevated serum homocysteine triggered a whole-genome sequencing analysis which revealed a homozygous methylenetetrahydrofolate reductase (MTHFR) gene polymorphism (C677T/C677T; dbSNP rs1801133). Interventions targeting her polymorphism-dependent loss of function successfully resolved her insomnia. This case demonstrates a genomic approach for insomnia whereby successful treatment was focused on optimizing the patient’s metabolome, which was altered as a result of a missense single-nucleotide polymorphism.
Citation:
Kapoor V, Watson NF, Ball L. Chronic insomnia in the setting of MTHFR polymorphism. J Clin Sleep Med. 2022;18(4):1215–1218.
Keywords: melatonin, gene reversal, MTHFR single-nucleotide polymorphism, whole-genome sequencing, metabolome, treatment-resistant insomnia, genomic approach, in-born error of metabolism
INTRODUCTION
Chronic insomnia affects 10% of the population.1 Despite the high prevalence, the root cause of insomnia is often uncertain. Although recently discovered genetic polymorphisms in genes such as ADAMTS14, PAX8, ESRRG, PIN1, and UPP, among others, are associated with various sleep traits and insomnia symptoms,2 none are recognized as having treatment-related implications. To our knowledge, this is the first case of chronic insomnia being successfully diagnosed and treated using a genomic approach targeting a missense single-nucleotide methylenetetrahydrofolate reductase (MTHFR) polymorphism associated with sleep disruption.
REPORT OF CASE
A 48-year-old woman with a family history of metabolic, cardiovascular, and neurological disorders and a personal medical history of infertility, hypothyroidism, and treatment-refractory chronic insomnia was evaluated in a sleep medicine clinic. Previously she was trialed on various medications including sedative-hypnotics, some of which (eg, zolpidem) caused persistent rebound insomnia. At the time of evaluation, she was on a nightly combination of the following prescription and nonprescription sleep aids: trazodone, temazepam, valerian root, tincture of hops, melatonin, and suvorexant—often requiring multiple nightly doses of these treatments. Despite these medications and supplements, her average nightly total sleep time remained low at 4–5 hours despite adequate time in bed (average time in bed 7.6 hours).
Upon initial evaluation in the sleep clinic, her Insomnia Severity Index score was 28 (indicating severe insomnia) and Epworth Sleepiness Scale score was 0, indicating no subjective sleepiness. She had no symptomology or risk factors for sleep-disordered breathing. On her initial cognitive behavioral therapy for insomnia evaluation she had a sleep efficiency of 71% with the use of the above-mentioned sleep aids and supplements. She diligently followed cognitive behavioral therapy for insomnia recommendations over a course of 6 months, after which she was successful in discontinuing certain sleep aids such as trazodone and temazepam. However, her sleep efficiency remained unimproved with an ongoing short average total sleep time of 4–5 hours due to her ongoing insomnia (Table 1, top).
Table 1.
Sleep diary.
| Day of the Week | |||||||
|---|---|---|---|---|---|---|---|
| Wed | Thu | Fri | Sat | Sun | Mon | Tue | |
| Last night I took | 20 mg Bel×2, VR, TH | 21 mg Bel×2, VR, TH | 20 mg Bel, VR, TH, trazodone | 20 mg Bel, VR, TH, trazodone | 20 mg Bel, VR, TH, trazodone | 20 mg Bel, VR, TH, trazodone | 20 mg Bel, VR, TH, trazodone |
| Last night I got in my bed at | 11:00 pm | 12:10 am | 12:00 am | 12:30 am | 11:30 pm | 10:30 pm | 11:00 pm |
| Last night I turned off the lights and attempted to fall asleep at | 11:15 pm | 12:15 am | 12:10 am | 12:35 am | 11:50 pm | 10:50 pm | 12:00 am |
| Minutes to fall asleep after turning off the lights (11.5 min average) | 5 | 10 | 20 | 10 | 10 | 10 | 15 |
| Today I woke up at | 3:40 am | 4:40 am | 5:20 am | 6:50 am | 7:10 am | 5:00 am | 6:15 am |
| Today I got out of bed for the day at | 5:50 am | 7:00 am | 7:30 am | 8:00 am | 9:00 am | 6:45 am | 6:50 am |
| Quality of sleep rating (5.7 average)* | 5 | 3 | 5 | 7 | 8 | 6 | 6 |
| How well rested did you feel? (4.9 average)* | 4 | 2 | 4 | 6 | 6 | 5 | 7 |
| TIB in minutes (456 average) | 410 | 410 | 450 | 450 | 570 | 435 | 470 |
| TST in minutes (323 average) | 260 | 255 | 290 | 365 | 430 | 300 | 360 |
| Sleep efficiency (71% average) | 63% | 62% | 64% | 81% | 75% | 69% | 77% |
| Day of the Week | |||||||
| Wed | Thu | Fri | Sat | Sun | Mon | Tue | |
| Last night I took | 3 mg Mel and 20 mg Bel | 3 mg Mel and 20 mg Bel | 3 mg Mel and 20 mg Bel | 3 mg Mel and 20 mg Bel | 3 mg Mel and 20 mg Bel | 3 mg Mel and 20 mg Bel | 3 mg Mel and 20 mg Bel |
| Last night I got in my bed at | 10:15 pm | 11:20 pm | 11:20 pm | 10:45 pm | 11:05 pm | 10:30 pm | 10:50 pm |
| Last night I turned off the lights and attempted to fall asleep at | 10:40 pm | 11:40 pm | 11:40 pm | 11:10 pm | 11:20 pm | 11:05 pm | 11:30 pm |
| Minutes to fall asleep after turning off the lights (8.5 min average) | 10 | 5 | 10 | 10 | 10 | 5 | 10 |
| Today I woke up at | 6:40 am | 7:40 am | 6:40 am | 6:50 am | 4:30 am | 6:30 am | 7:30 am |
| Today I got out of bed for the day at | 7:30 am | 8:15 am | 7:45 am | 8:45 am | 6:00 am | 7:30 am | 8:15 am |
| Quality of sleep rating (8.1 average)* | 9 | 9 | 9 | 8 | 6 | 9 | 7 |
| How well rested did you feel? (8.1 average)* | 8 | 8 | 9 | 9 | 7 | 9 | 7 |
| TIB in minutes (522 average) | 555 | 535 | 505 | 540 | 415 | 540 | 565 |
| TST in minutes (436 average) | 500 | 480 | 420 | 450 | 370 | 450 | 380 |
| Sleep efficiency (84% average) | 90% | 90% | 83% | 83% | 89% | 83% | 67% |
(Top) Sleep diary at initial cognitive behavioral therapy for insomnia evaluation. (Bottom) Sleep diary after vitamin B12 and folate supplementation. *Quality and rested values ranged 1–10. Bel = Belsomra, Mel = melatonin, TH = tincture of hop, TIB = time in bed, TST = total sleep time, VR = valerian root.
She continued to have difficulties with insomnia, resulting in short sleep times. Due to the recalcitrant nature of her insomnia and its detrimental effects on her quality of life, we decided to pursue a more thorough work-up for other possible etiologies of her sleep disturbances. Further testing revealed normal folate (14.3 ng/mL) and B12 (471 pg/mL) levels and elevated homocysteine (20.2 μmol/L) levels. Genomic DNA was subsequently obtained for whole-genome sequencing as described herein: https://nebula.org/whole-genome-sequencing-dna-test/.
This whole-genome sequencing analysis revealed that the patient was homozygous for a polymorphism in the MTHFR gene (C677T; dbSNP rs18011333). As such, the patient underwent a treatment protocol with methylcobalamin (vitamin B12) and folic acid addressing the polymorphism-dependent loss of function. Within 4 weeks of treatment initiation the patient had improved ability to maintain sleep, with the Insomnia Severity Index reducing from 28 (severe insomnia) to 6 (no clinically significant insomnia). The patient also endorsed longer and more restful sleep in the setting of fewer sleep aids. Specifically, the patient reported a shorter sleep latency, higher-quality and more restful sleep, a longer total sleep time, and an increase in sleep efficiency of 13% (Table 1, bottom); per the patient’s last visit, which was greater than 3 years after treatment onset, these improvements in her sleep persisted.
DISCUSSION
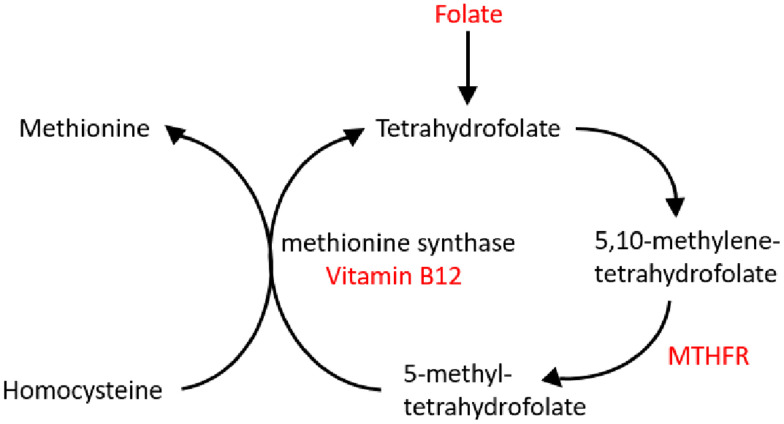
A recent genome-wide association study revealed several distinct single-nucleotide polymorphisms associated with sleep traits such as sleep duration, insomnia symptoms, and sleep latency.2 The MTHFR gene product converts homocysteine into methionine using B vitamin precursors (Figure 1). In this case, the patient—with chronic insomnia, fragmented sleep, and short sleep duration—was a homozygous carrier for the MTHFR C677T polymorphism, causing mildly elevated homocysteine levels. This polymorphism compromises biochemical methylation reactions crucial to healthy human physiology. Previously, elevated homocysteine levels have been associated with short sleep duration.3
Figure 1. Biochemical functionality of MTHFR.
In the absence of MTHFR, a methyl group is not generated to convert homocysteine to methionine, resulting in a buildup of homocysteine contributing to human diseases such as insomnia. Supplementation with folate and vitamin B12 can help compensate for mutation related reduction in MTHFR enzymatic activity. MTHFR = methylenetetrahydrofolate reductase.
While 20–40% of individuals carry MTHFR polymorphisms in North America, Europe, and Australia, only 8–20% are homozygous for the MTHFR C677T polymorphism.4 These individuals express only 30% of normal enzyme MTHFR function.4 Data from the 1000 Genomes Project Phase 3 reports that this allele is most frequently found in populations with Native American admixture (44–54%), followed by Europeans and European-American populations (27–47%) and East Asians (13–47%). This allele was least frequently reported in South Asian or Indo-Americans (8–15%) and African and African-American populations (7–14%). When deficient in necessary vitamin cofactors (B12, B6, or folate) their homocysteine is not efficiently recycled to methionine, causing homocysteine accumulation in the blood (Figure 1). The clinical implications of MTHFR polymorphisms are many including disorders of the cardiovascular, immune, reproductive, metabolic, and neurological systems.5 This patient and her family members had a history of disorders presenting in all of these systems. The patient’s homozygosity indicates she received the same polymorphism from both her maternal and paternal genomes.
Melatonin is crucial to sleep–wake and circadian health and its production is reliant on the methylation of serotonin.6 MTHFR plays a large role in the methylation cycle and the production of methyl groups for reactions such as these. As such, polymorphism-related functional reductions in MTHFR enzyme activity (as seen with the homozygous C677T polymorphism in this patient) can compromise melatonin production with untoward implications for sleep and circadian health. As such, 3 mg of melatonin at bedtime was added to the patient’s initial insomnia treatment regimen after lower doses were proven not efficacious. Importantly, after balancing the patient’s metabolome with sufficient methyl donors and B-vitamin interventions, the patient was able to successfully discontinue melatonin use without any adverse effects on her sleep.
Given the low cost of gene sequencing, the high prevalence of MTHFR polymorphisms, and the increased risks in cardiovascular, reproductive, metabolic, cerebrovascular, and neurological events associated with this polymorphism, wide-scale testing within certain populations for these single-nucleotide polymorphisms may be warranted for both disease prevention and treatment. Herein, we describe a patient who initially failed to respond to multiple sedative-hypnotic medications, supplements, and cognitive behavioral therapy for insomnia, the current gold standard for insomnia intervention. A whole-genome sequencing analysis was then undertaken which revealed a highly penetrable in-born error of metabolism involving the MTHFR enzyme. To our knowledge, this is the first case of chronic insomnia being successfully diagnosed and treated using a genomic approach targeting a missense single-nucleotide MTHFR polymorphism associated with sleep disruption. Importantly, case reports alone obviate definitive conclusions and clearly should not be the sole reason for adoption of new clinical care paradigms or practice. Hopefully this case will stimulate further observational studies regarding the association between MTHFR polymorphisms and insomnia and perhaps motivate clinical trials of supplemental B12 and folate in affected individuals. At the very least, this case report indicates a need for a deeper exploration of the role of MTHFR polymorphisms in chronic insomnia and circadian health.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. The authors report no conflicts of interest.
REFERENCES
- 1. Roth T . Insomnia: definition, prevalence, etiology, and consequences . J Clin Sleep Med. 2007. ; 3 ( 5 Suppl ): S7 – S10 . [PMC free article] [PubMed] [Google Scholar]
- 2. Bragantini D , Sivertsen B , Gehrman P , Lydersen S , Güzey IC . Genetic polymorphisms associated with sleep-related phenotypes; relationships with individual nocturnal symptoms of insomnia in the HUNT study . BMC Med Genet. 2019. ; 20 (1 ): 179 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen T-Y , Winkelman JW , Mao W-C , et al . Short sleep duration is associated with increased serum homocysteine: insights from a national survey . J Clin Sleep Med. 2019. ; 15 ( 1 ): 139 – 148 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moll S , Varga EA . Homocysteine and MTHFR mutations . Circulation 2015. ; 132 ( 1 ): e6 – e9 . [DOI] [PubMed] [Google Scholar]
- 5. Liew S-C , Gupta ED . Methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism: epidemiology, metabolism and the associated diseases . Eur J Med Genet. 2015. ; 58 ( 1 ): 1 – 10 . [DOI] [PubMed] [Google Scholar]
- 6. Ball LJ , Palesh O , Kriegsfeld LJ . The pathophysiologic role of disrupted circadian and neuroendocrine rhythms in breast carcinogenesis . Endocr Rev. 2016. ; 37 ( 5 ): 450 – 466 . [DOI] [PMC free article] [PubMed] [Google Scholar]