Abstract
Study Objectives:
Consumer home sleep trackers provide a great opportunity for longitudinal objective sleep monitoring. Nonwearable sleep devices cause little to no disruption in the daily life routine and need little maintenance. However, their validity needs further investigation. This study aims to evaluate the accuracy of sleep outcomes of EMFIT Quantified Sleep (QS), an unobtrusive nonwearable sleep tracker based on ballistocardiography, against polysomnography.
Methods:
62 sleep-lab patients underwent a single clinical polysomnography with measures simultaneously collected through polysomnography and EMFIT QS. Resting heart rate, total sleep time, wake after sleep onset, sleep onset latency, and duration in sleep stages, collected from the 2 devices, were compared using paired t-tests and their agreement analyzed using Bland-Altman plots. Additionally, continuous heart rate and sleep stages in 30-seconds epochs were evaluated.
Results:
EMFIT QS data loss occurred in 47% of participants. In the remaining 33 participants (15 women, with mean age of 53.7 ± 16.5 years), EMFIT QS overestimated total sleep time by 177.5 ± 119.4 minutes (<0.001) and underestimated wake after sleep onset by 44.74 ± 68.81 minutes (P < .001). It accurately measured average resting heart rate and was able to distinguish sleep onset latency with some accuracy. However, the agreement between EMFIT QS and polysomnography on sleep-wake detection was low (kappa = 0.13, P < .001), EMFIT QS failed to distinguish sleep stages.
Conclusions:
A consensus between polysomnography and EMFIT QS was found in sleep onset latency and average heart rate. There was significant discrepancy and lack of consensus in other sleep outcomes. These findings indicated that further development is necessary before using EMFIT QS in clinical and research settings.
Clinical Trial Registration:
Registry: Australian New Zealand Clinical Trials Registry; Name: Sleep parameter validation of a consumer home sleep monitoring device, EMFIT Quantified Sleep (QS), against Polysomnography; URL: https://www.anzctr.org.au/ACTRN12621000600842.aspx; Identifier: ACTRN12621000600842.
Citation:
Kholghi M, Szollosi I, Hollamby M, Bradford D, Zhang Q. A validation study of a ballistocardiograph sleep tracker against polysomnography. J Clin Sleep Med. 2022;18(4):1203–1210.
Keywords: EMFIT QS, validation, polysomnography, sleep measurements
INTRODUCTION
The increasing interest in monitoring and improving sleep behaviors has urged the development of consumer-grade devices in recent years.1,2 These devices can be categorized into 2 main groups of wearables and nonwearables. They provide objective measures of sleep, which have been shown to be less biased than self-reported measures,3 and cheaper and less labor-intensive to obtain compared to the current gold standard, polysomnography (PSG). In research and clinical settings, there is a growing need to measure sleep longitudinally in the natural life environment, as it facilitates exploring the impact of sleep changes on health and disease.4,5 This is not quite feasible in the lab environment using PSG. While consumer-grade devices provide a great opportunity for longitudinal monitoring of sleep, validity of their measurements has not been fully investigated in all population groups.
Wearable sleep trackers have been shown to estimate sleep onset latency (SOL) within 10 minutes (level of agreement between −15.1 min to +23.2 min) and have a > 90% sensitivity in detecting sleep in adolescent cohorts, healthy adults, and in sleep-disordered patients.6–9 This group, however, has some limitations for longitudinal monitoring, including limited battery life and the requirement to wear it continuously, which might reduce its acceptability among some population groups.
Nonwearables, on the other hand, have been developed with the aim of minimizing disruptions in daily life routines and natural sleep behaviors. Some examples are Doppler radar-based S+ from ResMed and mattress-based Beddit and EMFIT QS (Quantified Sleep). In an evaluation study including 27 healthy adults, S+ achieved about 87% accuracy in detecting sleep and wake compared to PSG, overestimating total sleep time (TST) by at least 20 minutes.10 However, it showed limited agreement of 65% on sleep stages estimation. Among mattress-based devices, Beddit was found to be an unreliable device to track sleep when compared to PSG within a cohort of 10 healthy young adults.11 It showed poor agreement (kappa = 0.101) with PSG on estimating sleep stages and also significantly underestimated wake after sleep onset and overestimated total sleep time with mean differences of 32.6 minutes and 43.5 minutes, respectively.
Similar to Beddit, EMFIT QS12 is a mattress-based sleep tracker, developed and manufactured by Emfit, Finland. The EMFIT QS is a pressure sensor built from electromechanical film, which measures mechanical chest wall movements from heartbeat and respiration, from which sleep stage and time are inferred. It also includes a cloud-based analysis platform that provides overnight sleep summary and sleep stage estimation. The heart rate (HR) and respiration rate of the EMFIT QS were validated against references derived from the simultaneous electrocardiogram and respiratory inductive plethysmography, as part of clinical overnight polysomnography. The analysis of data from 33 patients showed that 95% limits of agreement for HR were −4.4 and 4.4 beats/min, whereas for the respiration rate, these limits were −2.5 and 2.2 respirations/min.13 There is no validation study available for the EMFIT QS sleep stages and duration.
In this study, we evaluated the accuracy of the EMFIT QS in estimating the sleep outcomes and distinguishing sleep stages against PSG. TST, wake after sleep onset (WASO), SOL, sleep stages’ duration and time, and average resting and continuous HR were assessed. Additionally, we investigated the effect of other factors such as arousals and demographic factors on the discrepancies between PSG-derived and EMFIT QS-derived measures.
METHODS
Participants and data collection
Sixty-two adult patients (> 18 years old) were recruited from the Sleep Disorders Centre of The Prince Charles Hospital in Brisbane, Australia. The study was approved by The Prince Charles Hospital Human Research Ethics Committee, and a written informed consent was obtained prior to the study.
All participants underwent standard overnight PSG as per American Academy of Sleep Medicine 2020 guidelines (Compumedics Profusion PSG4, Abbotsford, Australia). The following channels were recorded: electroencephalogram (C4-M1, F4-M1, O2-M1), left and right electro-oculogram, electrocardiogram, submental electromyogram, diaphragmatic electromyogram, left and right anterior tibialis electromyogram, body position, oronasal airflow via thermocouple (Ambu A/S, Balllerup, Denmark), nasal pressure (Salter Labs, El Paso, TX), thoracic and abdominal movement via respiratory inductance plethysmography (Compumedics, Abbotsford, Australia), pulse oximetry (Massimo Radical, Irvine, CA) and sound level (Tecpel 332, New Taipei City, Taiwan).
PSG analysis was performed manually, as per American Academy of Sleep Medicine 2020 Guidelines with hypopnea scoring according to definition 1A (≥ 30% drop in baseline with ≥ 3% desaturation or arousal). In addition to standard report metrics, data were exported in 30-second epochs to allow epoch-by-epoch (EBE) analysis. EBE data included epoch number, sleep stage, and average heart rate. Average epoch heart rate was derived from the R to R interval of the electrocardiogram channel.
The EMFIT QS’s thin strip was placed under the mattress or mattress topper to simultaneously collect heart (ballistocardiograph), respiration, and gross body movements. The HR and respiration rate were calculated in real-time with a short dynamic window that automatically discards artifacts. The vital values were transferred to a cloud-based analysis platform in 4 second resolution to derive overnight activity summary and sleep stage estimation.
The following measures of EMFIT QS and PSG equivalent were evaluated in this study:
Average (avg) HR: whole night (presence period) average of heart rate, beats per minute;
Minimum (min) HR: minimum 3 min average heart rate from whole night, beats per minute;
Maximum (max) HR: maximum 3 min average heart rate from whole night, beats per minute;
TST: the sum of minutes spent in any stage of sleep (light, deep, rapid eye movement [REM]);
SOL: time from presence-in-bed period start to fall asleep in minutes;
WASO: the sum of minutes spent awake after sleep onset;
Light: the sum of minutes spent in stage N1 and N2 sleep;
Deep: the sum of minutes spent in stage N3 sleep;
HR and sleep stages in 30-second epochs.
Data analysis
PSG and equivalent EMFIT QS sleep outcomes were compared using paired t-tests or the Wilcoxon signed-rank test, depending on the distribution of the sleep outcome assessed by Shapiro-Wilk test. In both cases, P < .05 indicated a statistically significant difference between the 2 devices’ estimation. The overall agreement between PSG and equivalent EMFIT QS sleep outcomes was analyzed using Bland-Altman plots. Bland-Altman plot biases (EMFIT QS mean differences in sleep outcomes), standard deviation (SD) and ± 95% confidence interval (CI) of the biases, and lower and upper agreement limits (mean difference ± 1.96 SD) were calculated. A positive and negative bias indicate that EMFIT QS underestimated and overestimated the PSG sleep outcome, respectively. EBE analysis included a comparison of 30-second EBE HR of PSG and EMFIT QS. A 4-stage classification (awake, light, deep, and REM) was used to evaluate the sleep stages estimation. EMFIT outcome measures (as predicted labels) were compared against the one from PSG (as true labels) using an error matrix. We also derived sensitivity , specificity , and accuracy from the overall error matrix, where TP = true positive, TN = true negative, FP = false positive, FN = false negative. Additionally, linear regression models were built to analyze the effect of total arousals, arousal index, apnea-hypopnea index (AHI), and periodic limb movements (PLM) arousal index as independent variables (x), on the discrepancies between PSG-derived and EMFIT QS-derived measures (), as a dependent variable. Discrepancy in TST, WASO, light, deep, and REM were investigated and age, sex, body mass index (BMI), weight, and sleep study type (diagnostic vs continuous positive airway pressure) were used as covariates.
RESULTS
Sample demographics
EMFIT QS failed to capture data from 29 out of 62 participants (47% data loss). The sensor’s analysis platform requires bed entry and exit times to provide sleep summary outcomes for each episode, and most of the data loss was due to failure in identifying the bed exit time.
Sample demographics of the entire study population (n = 62) and those whose data were correctly collected by EMFIT QS (n = 33) are provided in Table 1.
Table 1.
Sample characteristics.
| Consented | EMFIT Data Received | |
|---|---|---|
| Females/males, n/n | 27/35 | 15/18 |
| Age (years): mean ± SD [range] | 56.2 ± 15.2 [18–81] | 53.7 ± 16.5 [18–80] |
| Weight (kg): mean ± SD [range] | 97 ± 20 [60.5–138] | 96.4 ± 21.3 [60.5–138] |
| BMI (kg/m2): mean ± SD [range] | 33.9 ± 7.4 [19.9–48.4] | 33.8 ± 8.3 [21.4–46.6] |
| Diagnostic/CPAP, n/n | 31/31 | 17/16 |
| AHI (events/h) | 9.6 ± 15.2 [0–74.8] | 7.6 ± 12.5 [0–62.1] |
| PLM arousal index (events/h) | 2 ± 4.9 [0–32.3] | 1.7 ± 3.7 [0–19.1] |
| Arousal index (events/h) | 20.5 ± 15 [4.3–72.8] | 18.2 ± 12.5 [5.2–59.1] |
| Polysomnographic diagnosis (ratio by 100) | 24% normal | 30% normal |
| 19% OSA–mild | 21% OSA–mild | |
| 19% OSA–moderate | 21% OSA–moderate | |
| 34% OSA–severe | 21% OSA–severe | |
| 10% PLMa | 6% PLMb |
aThere were 2 PLM cases, 1 with OSA–mild and 1 with OSA–moderate. bThere were 6 PLM cases, 1 with OSA–mild, 2 with OSA–moderate, and 3 with OSA–severe. AHI = apnea-hypopnea index, BMI = body mass index, CPAP = continuous positive airway pressure, OSA = obstructive sleep apnea, PLM = periodic limb movements, SD = standard deviation.
PSG and EMFIT QS sleep summary outcomes
A pairwise comparison of PSG and EMFIT QS sleep outcomes are provided in Table 2. Bland-Altman plots for the main sleep outcomes are provided in Figure 1, and biases, SD, 95% CI of the biases, and upper and lower agreement limits are provided in Table 3.
Table 2.
Pairwise comparison of PSG and EMFIT QS sleep measures.
| Variable | PSG | EMFIT QS | t/w | P | ||
|---|---|---|---|---|---|---|
| Mean ± SD | 95% CI | Mean ± SD | 95% CI | |||
| Avg HR | 64.3 ± 13.9 | 59.5, 69.2 | 62.8 ± 7.3 | 60.3, 65.3 | 209 | .45 |
| Min HR | 50.3 ± 12.4 | 46.03, 54.6 | 45.6 ± 3.8 | 44.2, 46.9 | 147 | 0.08 |
| Max HR | 79.3 ± 21.2 | 72, 86.7 | 102.5 ± 17.4 | 96.6, 108.5 | 40.5 | 7.8e-05* |
| TST | 303 ± 95.1 | 270.6, 335.5 | 480.5 ± 97.9 | 447.1, 513.9 | 34 | 1.1e-05* |
| SOL | 33.6 ± 43.8 | 18.7, 48.5 | 28 ± 11.9 | 23.9, 32 | 201 | .16 |
| WASO | 102.2 ± 66.1 | 79.6, 124.7 | 57.4 ± 19.2 | 50.9, 64 | 3.7 | .0007* |
| Light sleep | 184.9 ± 74.8 | 159.3, 210.4 | 279.1 ± 59.5 | 258.8, 299.1 | −5.98 | 1.1e-06* |
| Deep sleep | 77.3 ± 44 | 62.3, 92.4 | 85.6 ± 27.4 | 76.3, 95 | 197.5 | .13 |
| REM | 44.5 ± 26.5 | 28.2, 60.95 | 106.5 ± 32.1 | 86.6, 126.4 | −10.5 | 6.2e-12* |
All times are in minutes. *Represents statistical significance. Avg = average, CI = confidence interval, HR = heart rate, Max = maximum, Min = minimum, QS = quantified sleep, REM = rapid eye movement, SD = standard deviation, SOL = sleep onset latency, t = paired t-test, TST = total sleep time, w = Wilcoxon signed-rank, WASO = wake after sleep onset.
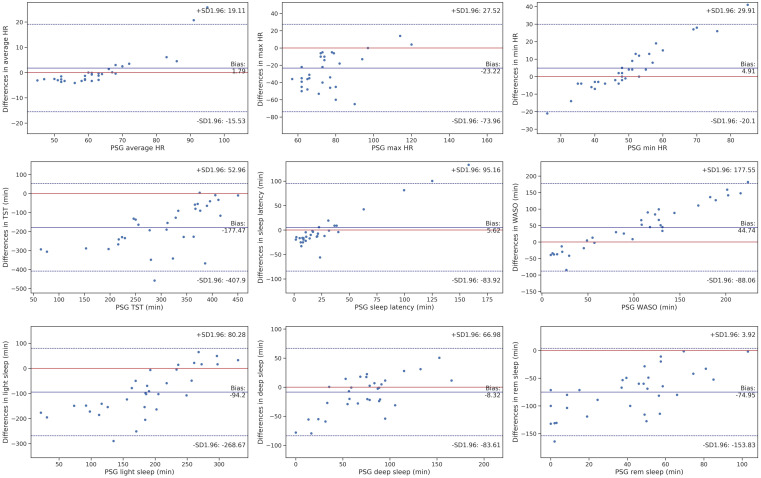
Figure 1. Bland-Altman plots for HR and main sleep outcomes.
PSG-EMFIT QS discrepancies for sleep outcomes (y-axis) are plotted as a function of the PSG outcomes (x-axis) for each individual. Circles represent individuals. Biases are marked as a solid blue line. The blue dotted lines refer to the upper and lower Bland-Altman plots agreement limits, and the red solid line indicates no bias. HR = heart rate, max = maximum, min = minimum, PSG = polysomnography, QS = quantified sleep, rem = rapid eye movement, SD = standard deviation. TST = total sleep time, WASO = wake after sleep onset.
Table 3.
Bland-Altman plots biases, SD and 95% CI of the biases, and upper and lower agreement limits for PSG and equivalent EMFIT QS.
| Variable | Bias ± SD | 95% CI of the Bias | Lower Agreement Limit | Upper Agreement Limit |
|---|---|---|---|---|
| Avg HR | 1.79 ± 8.98 | −3.8, 7.4 | −15.53 | 19.11 |
| Min HR | 4.9 ± 12.96 | −3.1, 12.9 | −20.1 | 29.91 |
| Max HR | −23.22 ± 26.3 | −39.5, −6.9 | −73.96 | 27.52 |
| TST | −177.5 ± 119.4 | −251.5, −103.5 | −407.9 | 52.96 |
| SOL | 5.62 ± 46.39 | −23.1, 34.4 | −83.92 | 95.16 |
| WASO | 44.74 ± 68.81 | 2.1, 87.4 | −88.06 | 177.55 |
| Light sleep | −94.2 ± 90.4 | −150.2, −38.2 | −268.67 | −80.28 |
| Deep sleep | −8.3 ± 39 | −32.5, 15.9 | −83.61 | 66.98 |
| REM | −74.95 ± 40.87 | −100.3, −49.6 | −153.83 | 3.92 |
Avg = average, CI = confidence interval, HR = heart rate, Max = maximum, Min = minimum, QS = quantified sleep, REM = rapid eye movement, SD = standard deviation, SOL = sleep onset latency, TST = total sleep time, WASO = wake after sleep onset.
Among the three measures of HR, only maximum HR was significantly (P = 7.8e-05) overestimated by 23.22 ± 26.3 beats/min. Biases for minimum and average HR were small (1.79 and 4.9, respectively, in Table 3) and nonsignificant (P > 0.05 in Table 2).
EMFIT QS significantly (P = 9.3e-10 in Table 2) overestimated TST by 177.5 ± 119.4 minutes and significantly (P = 0.0007) underestimated WASO by 44.74 ± 68.81 minutes. However, there was no significant difference between the 2 devices’ estimation of SOL, which was slightly underestimated by 5.62 minutes. There was 1 participant whose TST and WASO estimation exceeded the agreement limits, and 3 participants with almost perfect estimation (the red solid line as no bias) (Figure 1). Also, a considerable number of participants had perfect estimation of SOL, with only 2 exceeding the agreement limits.
Light sleep and REM were significantly (P = 1.13e-06 and 6.2e-12) overestimated (around 94 and 75 minutes) by EMFIT QS. However, EMFIT QS slightly overestimated deep sleep time by 8.3 minutes, which was not statistically significant (P = 0.13), and the estimation for all participants were in the agreement limit.
EBE analysis outcomes
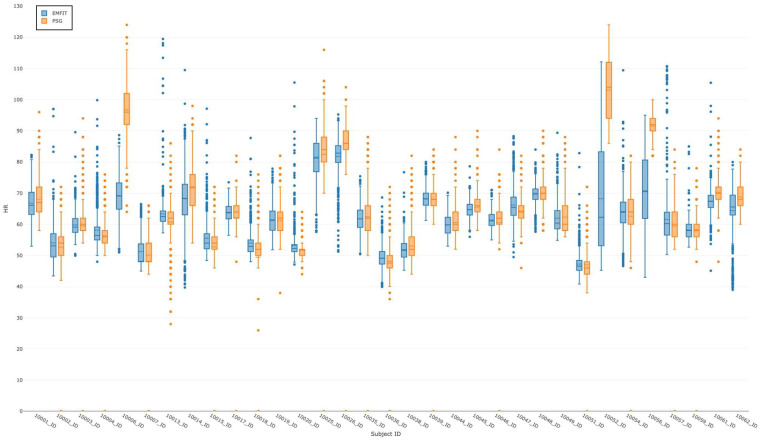
PSG and EMFIT EBE HR are summarized as 2 box plots per individual, as depicted in Figure 2. There was a significant difference in measuring EBE HR between 2 devices for 3 out of 33 participants (ID: 10006, 10052, 10056).
Figure 2. Epoch-by-epoch HR.
A comparison of the summarized PSG (orange) and EMFIT QS (blue) epoch-by-epoch HR (heart rate in the vertical axis) per participant (participant identification in the horizontal axis). HR = heart rate, PSG = polysomnography, QS = quantified sleep.
The error matrix in Table 4 and the classification evaluation measures in Table 5 allow assessment of the performance of EMFIT QS in discriminating sleep stages. EMFIT QS misclassified awake, deep sleep, and REM as light sleep in 53%, 49%, and 46% of the epochs, respectively. These errors were also reflected in the evaluation measures in Table 5. EMFIT QS had the lowest specificity (0.49) and accuracy (0.55) in detecting light sleep. Light sleep had the highest rate of TP cases (63%), which led to the highest sensitivity (0.63). While the specificity and accuracy of 3 other states of awake, deep, and REM are reasonable, their sensitivity is low as they were mostly misclassified as light sleep.
Table 4.
Error matrix.
| PSG Stage | EMFIT QS Stage | |||
|---|---|---|---|---|
| Awake | Light Sleep | Deep Sleep | REM | |
| Awake | 10% | 53% | 11% | 26% |
| Light sleep | 1% | 63% | 19% | 17% |
| Deep sleep | 0% | 49% | 39% | 12% |
| REM | 0% | 46% | 8% | 46% |
Each cell indicates the percentage of epochs that EMFIT QS correctly classified (bold) or misclassified when compared to PSG. QS = quantified sleep, REM = rapid eye movement.
Table 5.
Sleep stage classification outcome.
| Sensitivity | Specificity | Accuracy | |
|---|---|---|---|
| Awake | 0.104 | 0.991 | 0.711 |
| Light sleep | 0.631 | 0.493 | 0.55 |
| Deep sleep | 0.389 | 0.858 | 0.775 |
| REM | 0.457 | 0.808 | 0.776 |
REM = rapid eye movement.
Effect of arousals, AHI, and PLM on EMFIT QS and PSG discrepancies
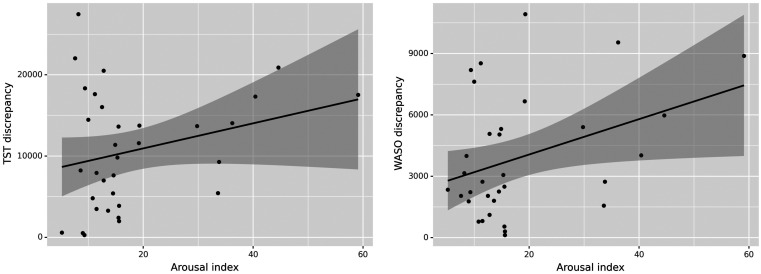
There was no association between total arousals and the discrepancy in sleep outcome measures. Arousal index, however, had a significant direct effect on WASO discrepancy (R2 = 0.318, P = 0.006) and TST discrepancy (R2 = 0.377, P = 0.036), as shown in Figure 3. Since high AHI and PLM arousal are 2 contributing factors to high arousal index,14,15 we investigated their effect on sleep outcomes discrepancy, and found that AHI is directly correlated with WASO (R2 = 0.265, P = 0.02) and TST (R2 = 0.379, P = 0.049) discrepancies, but PLM arousal index was found to have no effect. We also found that TST discrepancy was significantly higher in continuous positive airway pressure studies compared to diagnostics (R2 = 0.379, P = 0.03). Among other covariates, BMI (coef = −463.6, P = 0.005) and weight (coef = −154.6, P = 0.007) was inversely associated with TST discrepancy, and no association was found between age, sex, and sleep measures discrepancy.
Figure 3. The association of arousal index with TST and WASO discrepancy.
Sleep measure (TST and WASO) discrepancy is the absolute difference of the measure obtained from PSG and EMFIT QS. Circles represent individuals. PSG = polysomnography, QS = quantified sleep, TST = total sleep time, WASO = wake after sleep onset.
DISCUSSION
This study investigated the reliability of EMFIT QS against PSG in measuring summary outcomes of HR and sleep and also evaluated the EMFIT QS continuous HR in 30-second epochs and sleep stage classification performance.
Our findings suggested that while there was a consensus between 2 devices in SOL, average HR, and minimum HR, there was a significant discrepancy in the estimation of other summary sleep outcomes. EMFIT QS starts recording a sleep period from the time an individual is present in bed until they leave the bed, whereas PSG uses a manual light-off and light-on approach. While this might affect the time in bed, it cannot explain the significant overestimation of TST and underestimation of WASO by EMFIT QS. Other nonwearables, such as S+10 and Beddit,11 were also found to underestimate WASO and overestimate TST, however, with significantly less bias. It should be noted that these studies evaluated healthy populations, which could mitigate sleep outcome discrepancies and make the bias negligible. We also found that EMFIT QS had difficulty in differentiating awake and sleep with a level of agreement with the gold standard below minimal (kappa = 0.13, P < 0.001). The agreement between the 2 devices on REM and light sleep was also poor. This is aligned with studies demonstrating the inability of these commercial sleep trackers in accurately quantifying and discriminating sleep stages.10,11,16
Further analysis of the effect of arousals on discrepancies between the 2 devices showed that the more fragmented the sleep and the higher the AHI were, the less reliable the EMFIT became in detecting sleep and awake time. According to Table 1, around 70% of the participants whose data were included in our analysis were diagnosed with obstructive sleep apnea (mild, moderate, and severe) and around half of the studies included in our analysis were continuous positive airway pressure. Our findings suggested that sleep-disordered breathing adversely affected the accuracy and reliability of EMFIT in detecting sleep and awake. However, no association was found between PLM arousal index and PSG-EMFIT discrepancies. It should be noted that only a small portion (6%) of the cohort we studied was diagnosed with PLM, indicating further investigation is warranted before conclusions can be drawn.
We also found that the higher the BMI and weight, the less the TST discrepancy. We speculate that EMFIT is better in distinguishing bigger movements generated by heavier individuals rather than smaller movements from lighter individuals. Failure in identifying smaller movements can be happen either when 1) capturing signals, 2) preprocessing and filtering noise from signals, or 3) analyzing signals using their algorithm. To better understand the main reason behind this, access to raw signal and EMFIT algorithm is required. Also, the average BMI and weight in our study was quite high (33.8 kg/m2), and further investigation on a cohort with lower BMI could help to clarify this.
There was a significant difference in maximum HR and some of the EBB HR comparisons. It was not possible to investigate the reason behind this due to lack of access to EMFIT QS raw signals in this study; however, we speculate that the low sampling rate (100 Hz) in high ballistocardiographic frequency band in 6 − 16 Hz could be a potential explanation.
This study has some limitations: 1) the sample size is relatively small, 2) recruitment was only done through sleep lab patients, and 3) study population was not filtered based on history of neurological or psychiatric diagnoses or an online screening with the Pittsburgh Sleep Quality Index.
Overall, EMFIT QS was found to be less sensitive, specific, and accurate than PSG. EMFIT QS performance in estimating summary sleep outcomes and classifying sleep stages was poor. Sleep fragmentation, common in a number of sleep disorders, was found to contribute to unreliable and inaccurate detection of sleep and wake by EMFIT QS, which makes it unsuitable for clinical studies in its current form. While the outcome of this study is not generalizable to all population groups, further development on sleep monitoring and the analysis platform is necessary before using EMFIT QS in clinical and research settings.
EDITOR'S NOTE
The Emerging Technologies section focuses on new tools and techniques of potential utility in the diagnosis and management of any and all sleep disorders. The technologies may not yet be marketed, and indeed may only exist in prototype form. Some preliminary evidence of efficacy must be available, which can consist of small pilot studies or even data from animal studies, but definitive evidence of efficacy will not be required, and the submissions will be reviewed according to this standard. The intent is to alert readers of Journal of Clinical Sleep Medicine of promising technology that is in early stages of development. With this information, the reader may wish to (1) contact the author(s) in order to offer assistance in more definitive studies of the technology; (2) use the ideas underlying the technology to develop novel approaches of their own (with due respect for any patent issues); and (3) focus on subsequent publications involving the technology in order to determine when and if it is suitable for application to their own clinical practice. The Journal of Clinical Sleep Medicine and the American Academy of Sleep Medicine expressly do not endorse or represent that any of the technology described in the Emerging Technologies section has proven efficacy or effectiveness in the treatment of human disease, nor that any required regulatory approval has been obtained.
DISCLOSURE STATEMENT
All authors have seen and approved this manuscript. Work for this study was performed at the Sleep Disorders Centre of The Prince Charles Hospital, Brisbane, QLD, Australia. This study was funded by Prospective Imaging Study of Ageing (PISA) project, which is funded by a National Health and Medical Research Council (NHMRC) Boosting Dementia Research Initiative–Team Grant (APP1095227). The authors report no conflicts of interest.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- BMI
body mass index
- CI
confidence interval
- EBE
epoch-by-epoch
- HR
heart rate
- PLM
periodic limb movements
- PSG
polysomnography
- QS
quantified sleep
- REM
rapid eye movement
- SD
standard deviation
- SOL
sleep onset latency
- TST
total sleep time
- WASO
wake after sleep onset
REFERENCES
- 1. Van de Water ATM , Holmes A , Hurley DA . Objective measurements of sleep for non-laboratory settings as alternatives to polysomnography–a systematic review . J Sleep Res. 2011. ; 20 ( 1 Pt 2 ): 183 – 200 . [DOI] [PubMed] [Google Scholar]
- 2. Kelly JM , Strecker RE , Bianchi MT . Recent developments in home sleep-monitoring devices . ISRN Neurol. 2012. ; 2012 : 768794 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lauderdale DS , Knutson KL , Yan LL , Liu K , Rathouz PJ . Self-reported and measured sleep duration: how similar are they? Epidemiology. 2008. ; 19 ( 6 ): 838 – 845 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dawson D , Reid K . Fatigue, alcohol and performance impairment . Nature. 1997. ; 388 ( 6639 ): 235 – 235 . [DOI] [PubMed] [Google Scholar]
- 5. Bertisch SM , Pollock BD , Mittleman MA , et al . Insomnia with objective short sleep duration and risk of incident cardiovascular disease and all-cause mortality: Sleep Heart Health Study . Sleep. 2018. ; 41 ( 6 ): zsy047 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Scott H , Lack L , Lovato N . A systematic review of the accuracy of sleep wearable devices for estimating sleep onset . Sleep Med Rev. 2020. ; 49 : 101227 . [DOI] [PubMed] [Google Scholar]
- 7. de Zambotti M , Cellini N , Goldstone A , Colrain IM , Baker FC . Wearable sleep technology in clinical and research settings . Med Sci Sports Exerc. 2019. ; 51 ( 7 ): 1538 – 1557 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. de Zambotti M , Baker FC , Willoughby AR , et al . Measures of sleep and cardiac functioning during sleep using a multi-sensory commercially-available wristband in adolescents . Physiol Behav. 2016. ; 158 : 143 – 149 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kang S-G , Kang JM , Ko K-P , Park S-C , Mariani S , Weng J . Validity of a commercial wearable sleep tracker in adult insomnia disorder patients and good sleepers . J Psychosomatic Res. 2017. ; 97 : 38 – 44 . [DOI] [PubMed] [Google Scholar]
- 10. Schade MM , Bauer CE , Murray BR , et al . Sleep validity of a non-contact bedside movement and respiration-sensing device . J Clin Sleep Med. 2019. ; 15 ( 7 ): 1051 – 1061 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tuominen J , Peltola K , Saaresranta T , Valli K . Sleep parameter assessment accuracy of a consumer home sleep monitoring ballistocardiograph Beddit Sleep Tracker: A validation study . J Clin Sleep Med. 2019. ; 15 ( 3 ): 483 – 487 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. EMFIT Sleep Tracking and Monitoring . 2019. . https://www.emfit.com/ . Accessed June 28, 2019.
- 13. Ranta J , Aittokoski T , Tenhunen M , Alasaukko-oja M . EMFIT QS heart rate and respiration rate validation . Biomed Physics Engineering Express. 2019. ; 5 ( 2 ): 025016 . [Google Scholar]
- 14. Loredo JS , Ziegler MG , Ancoli-Israel S , Clausen JL , Dimsdale JE . Relationship of arousals from sleep to sympathetic nervous system activity and BP in obstructive sleep apnea . Chest. 1999. ; 116 ( 3 ): 655 – 659 . [DOI] [PubMed] [Google Scholar]
- 15. Sieminski M , Pyrzowski J , Partinen M . Periodic limb movements in sleep are followed by increases in EEG activity, blood pressure, and heart rate during sleep . Sleep Breath. 2017. ; 21 ( 2 ): 497 – 503 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stone JD , Rentz LE , Forsey J , et al . Evaluations of commercial sleep technologies for objective monitoring during routine sleeping conditions . Nat Sci Sleep. 2020. ; 12 : 821 – 842 . [DOI] [PMC free article] [PubMed] [Google Scholar]