Abstract
Study Objectives:
While insomnia and sleepiness symptoms are common in shift workers, 20%–30% experience more severe symptoms and meet the criteria for shift work disorder (SWD). SWD can lead to impairments in cognitive function, physical and mental health, and reduced productivity and increased risk of workplace injury. The aim of this study was to deliver and evaluate a shift work individual management coaching program, focusing on sleep education, promoting good sleep hygiene, and providing individualized behavioral strategies to cope with shift schedules.
Methods:
A clustered randomized controlled trial of sleep education and sleep disorders screening was undertaken, based on hospital wards at a tertiary hospital in Melbourne, Australia. Participants identified as high risk for SWD underwent one of two 8-week programs, a shift work individualized management program, or an active control. The primary outcome was ward-based sick leave. Secondary outcomes were SWD risk, sleep hygiene, insomnia, depression, and anxiety. A total of 149 nurses, across 16 wards (96% female, 34.66 ± 11.99 years) completed both baseline and follow-up questionnaires (23.9% were high risk SWD).
Results:
There was no significant reduction in sick leave between intervention and control wards (mean difference = 1.2 days, P = .063). Improvements were seen in insomnia (P < .0001) and depression (intervention, P ≤ .0001, control, P = .023) in both groups, but were not significantly different between programs. Anxiety (P = .001. control P = .079) and Functional Outcomes of Sleep Questionnaire 10 (P = .001 control P = .056) improved only for the intervention.
Conclusions:
This SWD intervention trial did not reduce sick leave compared to the active control but there was an improvement. Improvements in sleep hygiene, insomnia, depression, and anxiety severity were seen for both groups. Future intervention trials should consider including both sleep and mental health interventions, strategies to avoid between group contamination and the duration of programs for optimal behavioral modification.
Clinical Trial Registration:
Registry: Australian New Zealand Clinical Trials Registry; Name: Sleep Health Management for Healthcare Workers; URL: https://anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12616000369426; Identifier: ACTRN12616000369426.
Citation:
Booker LA, Sletten TL, Barnes M, et al. The effectiveness of an individualized sleep and shift work education and coaching program to manage shift work disorder in nurses: a randomized controlled trial. J Clin Sleep Med. 2022;18(4):1035–1045.
Keywords: sleep, shift work disorder, insomnia, anxiety, depression, nurses, circadian rhythm
BRIEF SUMMARY
Current Knowledge/Study Rationale: Treatment options to improve alertness and sleep for shift work disorder include sleep hygiene, light exposure, light avoidance, napping, melatonin, exercise, and caffeine consumption. These strategies, however, are usually applied generically in isolation. Combating the effects of shift work needs to be personalized and multifaceted.
Study Impact: This study implemented an individualized intervention program encompassing a range of different sleep and shift work strategies to treat nurses at high risk for shift work disorder. This approach was successful at reducing insomnia, depression, and anxiety but was not better than an active control. Future research needs to consider both sleep and mental health strategies as well as the duration of the intervention to see optimal behavioral modification effects.
INTRODUCTION
With the increased global demand for 24/7 operations, approximately 25% of the global workforce engage in nonstandard work hours.1,2 Shift work disrupts sleep by requiring people to be awake at night and try and sleep in the day, against the natural circadian cycle. This conflict causes episodes of prolonged wake, insomnia, shorter sleep duration, and/or sleepiness.2 While most people experience insomnia or sleepiness during shift work, some experience prolonged symptoms, with approximately 20%–30% of the shift worker population meeting the criteria for shift work disorder (SWD), a circadian rhythm sleep-wake disorder that is defined as persistent sleep disruption (excessive sleepiness and/or insomnia) due to a conflict between an individual’s internal sleep-wake system and work schedules for at least 1 month.3–5 Those with SWD are at high risk of poor mental health, with depression and anxiety being more severe in shift workers who are at high risk of SWD compared to those at low risk.6–8 Furthermore, compared to other shift workers, those with SWD have a greater reduction in productivity, an increased risk of motor vehicle accidents, and a higher chance of occupational incidents and medical errors.9,10
The underlying basis of individual adaptability to shift work is not well understood, and there is considerable interindividual variability. Work-related factors such as work demand and shift work schedules can have a negative effect on the ability to achieve sufficient sleep duration and quality.11,12 Biological, social, and lifestyle factors, including older age, morning-type chronotype, being married, having children, and caffeine intake, also influence the risk of SWD.13 Consequently, individualized, multifaceted approaches in the treatment of SWD are needed.2,14
Current treatment options designed to increase alertness and improve sleep quality and duration for SWD include good sleep hygiene, bright light exposure, light avoidance, scheduled napping, melatonin, exercise, and caffeine consumption, which have all been shown to be valuable in combating and improving individual responses to shift work.2,8,15,16 These strategies, however, are usually applied as single strategies and generically across the same work shifts/worker and are not individualized to specific circumstances and behavior.14,15,17 These generic approaches might not be effective with varying shift schedules, work environments, and individual circumstances; therefore, it has been suggested that combating the effects of shift work may need to be personalized and multifaceted.14,17 The concept of an individualized sleep modification program has been recommended in the past,2,15,17 although to our knowledge none has encompassed all the strategies simultaneously, tailored it to the individual and current roster schedule, and used a validated SWD screening tool. The aim of this project was to deliver and evaluate a shift work individual management coaching program (SWIM), focusing on sleep education, promoting good sleep hygiene, and providing individualized behavioral strategies to cope with shift schedules. It was hypothesized that, compared to a control program, shift workers who received the intervention program would have a greater reduction in sick leave, SWD risk, depression, anxiety, insomnia, and poor sleep hygiene behavior.
METHODS
Design and participants
The trial was a clustered randomized controlled trial based at Austin Health in Melbourne, Australia (ACTRN 12616000369426). Wards were randomized into either the intervention or active-control program. Participants from these wards were then recruited. Data were collected between January 2015 and December 2017. Participation was voluntary, and individuals provided written informed consent prior to participation. Eligible participants recruited from the wards were aged over 18 years, employed on either regular rotating or permanent night shifts, worked a minimum of 15 hours a week, and had not previously received treatment for a sleep disorder. Where possible, all eligible staff were approached to participate. The trial protocol was approved by the Austin Health and Monash University Human Research Ethics Committees (HREC Ref No: HREC/15/Austin/162). The protocol followed the NHMRC National Statement on ethical Conduct in Human Research (2007) and the Note for Guidance on Good Clinical Practice (CPMP/ICH-135/95) and conformed to the standards set by the latest revision of the Declaration of Helsinki.
Procedure
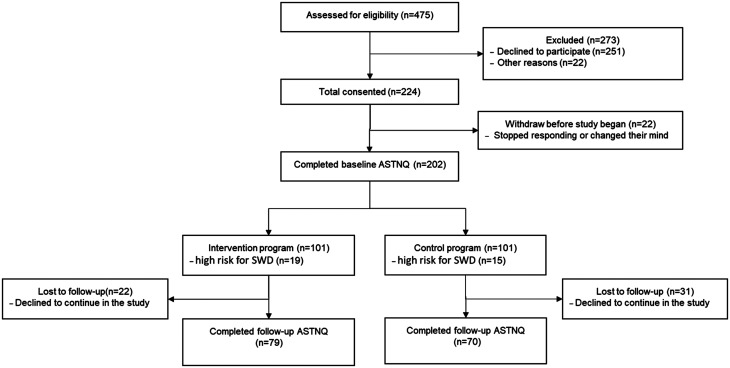
Sixteen wards were recruited, matched 1:1 by ward type (medical or surgical) with approximate number of staff. One from each pair of wards was then randomly allocated into either the intervention or control program using a computerized program. Delivery of the program could not be blinded as the researchers were also delivering the education and programs. Ward management was told that they would receive 1 of 2 intervention programs but blinded as to which program was the control program. Each program presented ward-based education sessions to all staff on multiple occasions. Education sessions were based on which program their ward was randomized to (control or intervention). The intervention wards were educated on sleep and the consequences of shift work, while the control wards were given information on the benefits of a low glycemic index (GI) diet. Recruitment was undertaken at these education sessions as well as approaching staff face-to-face on the wards and distributing flyers. Out of the 475 participants approached, 224 consented to the trial (47.2% response rate); 202 completed the required protocol questionnaire at baseline, and 149 participants (control n = 70, intervention n = 79) completed the full protocol at the follow-up questionnaire (Figure 1). The programs were delivered during participants’ work hours.
Figure 1. The SWIM project process CONSORT flow chart.
ASTNQ = Australasian Sleep Trials Network Questionnaire, SWD = shift work disorder.
All participants who provided consent completed the online baseline Australasian Sleep Trials Network Questionnaire (ASTNQ) independently without researchers present. Results from the ASTNQ were discussed with the participant afterward in confidence and any questions answered about the outcomes. Those at high risk for SWD were invited to participate in 1 of the 2 coaching programs, depending on what their ward was randomized to receive. The programs comprised 4 fortnightly one-on-one coaching sessions with a researcher during work hours over 8 weeks, followed by completion of a sleep/work/driving diaries for 2 additional weeks (10 weeks in all). All participants completed the follow-up ASTNQ (Figure 1). Both the education and one-on-one coaching sessions were delivered by the same research team, who had backgrounds in psychology and undertook training in SWD management via psychology clinics. Participants were blinded as to whether their ward was allocated to the intervention or control program but both programs were active programs. Compliance to recommendations was monitored by completion of home-based activities in the fortnightly coaching sessions
Intervention coaching program
The program was composed of the following:
-
-
Information was presented at each fortnightly meeting on the biology of sleep, stages of sleep, myths of sleep, and the importance of good sleep hygiene. The researcher focused on educating the participant on how to reduce the harmful effects of shift work by improving sleep and alertness.
-
-
Home-based activities were developed every meeting (based on participants forthcoming roster provided by the employee). This was undertaken between the researcher and employee, so they gained education to implement strategies themselves going forward. This information was presented in the form of a scheduling document that listed each day and a 24-hour time range. Recommendation strategies were individualized dependent on personal circumstances and work schedules. Strategies included good sleep hygiene principles, light exposure and avoidance, scheduled sleep and napping, melatonin, exercise, and strategic caffeine consumption. Each strategy had a symbol and was marked on the scheduling document for the participant to follow. The participant then filled out underneath each day what they were able to implement and what they did not. To measure compliance, comparisons were made between what was recommended and what the participants actually did.
These strategies were dependent on the individual circumstances and included the following.
Napping and scheduled sleep
Prior to the first night shift, nurses were recommended to nap in the afternoon, approximately 8 to 9 hours after wake time, as a countermeasure to reduce the duration of wakefulness prior to shift and improve alertness and performance.18,19 The nap duration was recommended to be approximately 2 hours (or as long as possible) or longer if the morning awakening was earlier. For subsequent night shifts, naps were encouraged in the late afternoon to early evening for between 20 and 90 minutes.20,21 In addition, scheduled sleep episodes of between 7 and 9 hours between shifts were recommended.
High-intensity light exposure during work hours
The intervention wards were provided with desk lamps that were placed above a couple of computer monitors to help facilitate individuals with bright light exposure during their shift. These lamps were Prism Broadwing Adjustable LED (LTI Pty Ltd, Victoria, Australia) had 3 different settings (Cool: 2,700 K, 1,290 lux; Warm: 4,000 K, 1,100 lux; Bright: 6,500 K, 1,650 lux) and emitted a light at around 1,200–1,950 lux (based on manufacturer’s reports on lux meter readings from sitting under the lamp while working at a desk computer). Lamps were strategically placed in high-use staff areas above the computer monitors shinning downward, such as at reception or computers, avoiding areas that would affect patients. Bright light exposure was recommended for 20 minutes at a time while writing notes, while on breaks, or before driving home, as intermittent exposure to artificial bright light can increase alertness during shift work.22,23 Depending on individual conditions and chronotype, it was recommended to use light exposure and light avoidance during other times to increase alertness and assist with adjusting circadian phase. For example, individuals with excessive sleepiness early in the morning and features of delayed circadian phase, were prescribed bright light before they started morning shifts.
Caffeine consumption (excluding those who did not consume caffeine already)
It was recommended that 60 mg of caffeine (approximately 1 250-ml cup of instant coffee or 1 55-ml latte/cappuccino) was consumed every 2 hours until 6 hours before planned sleep time when working night shifts and 9 hours before planned sleep on day shifts.24,25
Good sleep hygiene
Sleep hygiene advice included reducing light exposure and screen time 1 hour before bedtime, improving the sleep environment, including reducing noise and disturbances as well as having a comfortable bed and cool room. Ear plugs and eye masks were provided to participants to aid them in optimizing sleep.26,27 This information was delivered via brochures handed to participants during the coaching sessions as well as verbally via the researcher.
Melatonin
Halfway through the program, after the 3rd coaching session, if participants’ insomnia, sleep duration and sleepiness were not improving there was an option of sending a letter to their local General Practitioner for a prescription of melatonin (1–3 mg short acting melatonin, which was recommended to be taken 30 minutes prior to bedtime).28
Fortnightly control program
The active control program was composed of the following:
-
-
Education focused on the benefits of a low GI diet.
-
-
Home-based activities involved 1) the completion of a food diary for a fortnight; 2) then, based on the habits of the participant, information was provided on low GI foods; 3) goal setting was undertaken between the participant and researcher each fortnight to try and introduce/substitute high GI for low GI alternative foods.
The active-control group mirrored all aspects of the intervention program including the amount of contact time with the researcher and the amount of coaching, education, and home activities; however, it did not include sleep and shift work management education. A low GI diet was chosen as it would still engage participants but not have any side effects or have an impact on sleep. It was important that the control group be an active control to achieve face validity and avoid participants believing it was a placebo/control program. Information on what is a low GI diet and a list of food were sourced from Queensland Health29 and Glycemic Index Foundation.30
Measurements
Primary outcome
Monthly archival ward-based sick leave data were obtained from Human Resource Department records at Austin Health. Total sick leave utilization was calculated by obtaining the total amount of sick leave hours taken each month by each ward and dividing this by the total contracted hours for all staff on the ward. Each ward was involved for a 6-month period (January–June or July–December), with sick leave data calculated for 6 months preprogram and postprogram delivery to ensure that sick leave data were compared at the same time of year for that ward. Therefore, any differences seen were not due to seasonal variation. The project also planned to include medical error data as a primary outcome but could not obtain the required data, and thus the protocol was revised to have only 1 primary outcome.
Secondary outcomes
The ASTNQ comprised of a collection of general and validated surveys was used to collect secondary outcome measures at baseline and follow-up time points. The online questionnaire was developed by members of the Cooperative Research Centre for Alertness, Safety and Productivity, with the support of the ASTN. The ASTNQ includes general demographic, social and lifestyle questions including age, sex, body mass index, number of children, smoking status, caffeinate and alcoholic intake, as well as work- related questions including the number and type (day/evening/night) of shifts worked in the prior month, total hours worked over a typical week and month, and years of shift work experience.
Furthermore, the ASTNQ included the following validated surveys; The Shift Work Disorder Questionnaire,31 4 items that are used to assess an individual’s risk of SWD. Each item is scored on a scale between 0 and 4. The questionnaire has 89% positive predictive value and 62% negative predictive value (sensitivity = .74; specificity = .82)31 and has good internal consistency and test–retest stability.32 The Sleep Hygiene Index was used to measure sleep hygiene behavior among participants.26 Total scores range on a continuous scale from 13 to 65, with higher scores indicative of poorer sleep hygiene. The Horne–Östberg Morningness-Eveningness Questionnaire was used to measure diurnal preference.33 The Morningness-Eveningness Questionnaire ranges on a continuous scale from 16 to 86, with lower scores indicating greater eveningness chronotype. The Insomnia Severity Index (ISI) was used to examine the severity of insomnia symptoms.34 Scores range from 0 to 28, higher scores represent more severe insomnia symptoms. The ISI has been validated in a range of populations, including shift workers.34,35 The Functional Outcomes of Sleep Questionnaire 10 (FOSQ-10) was used to measure the impact of sleepiness on daily living and activities.36 Scores range from 5 to 20, with higher scores for better quality of life. Mental health was assessed with the Patient Health Questionnaire-9 (PHQ-9)37 and the General Anxiety Disorder-7 (GAD-7).38 The PHQ-9 is a 9-item, 4-point Likert scale, with an overall score range from 0 to 27. The PHQ-9 has been validated to assess mental health in hospital-employed nurses and other health care workers.39,40 The GAD-7 is a 7-item, 4-point Likert scale, with an overall score range from 0 to 24. The GAD-7 has been validated in primary care patients41 and the general public.42 Higher scores suggest more severe symptoms of mental health problems.
Statistical analysis
SPSS Statistics 25 for Microsoft Windows (SPSS Inc., Chicago, IL) was used for the statistical analysis. An alpha of < 0.05 (2-tailed) was considered significant for all analyses. Mixed model analysis, controlling for wards (clusters), was performed on the average amount of sick leave hours taken for each ward over the 6 months preprogram and post when the program was delivered on the ward. Paired t-tests were used to compare within-group differences between baseline and follow-up questionnaires for Sleep Hygiene Index, ISI, FOSQ-10, PHQ-9 and GAD-7. Mixed model analysis was then completed to see if there was an effect on program × time point interaction, controlling for clusters. Analysis was undertaken for all staff recruited on each ward as the primary outcome and then separately just for those who undertook the intervention program.
RESULTS
Sick leave utilization changes
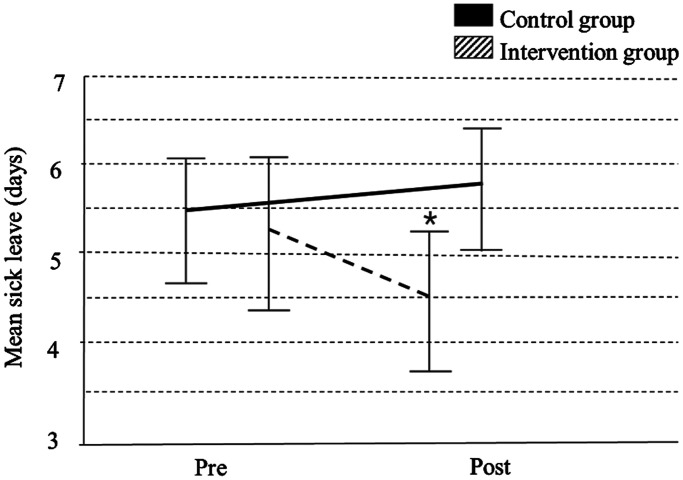
Approximately 35 permanent staff were employed on each ward, working an average was 29 ± 8.82 hours per week. The average staff tenure was 10.28 ± 8.44 years. The intervention wards had a reduction in average sick leave taken during the 6 months pre-post program (mean [M] = 4.46 days, confidence interval [CI] = 3.62–5.29 vs M = 5.26 days, CI = 4.35–6.17), while the active-control wards had an increase in sick leave (M = 5.38 days, CI = 4.58–6.17 vs M = 5.74 days, CI = 5.01–6.47); however, mixed model analysis showed that there was no significant program × time point interaction when controlling for cluster (P = .063) (Figure 2).
Figure 2. Mixed model analysis comparing sick leave (6 months pre- and post-program) for the control vs intervention groups (P = .063).
*P < .05 effect of time in intervention group (2-tailed).
Questionnaire outcomes Between control vs intervention groups
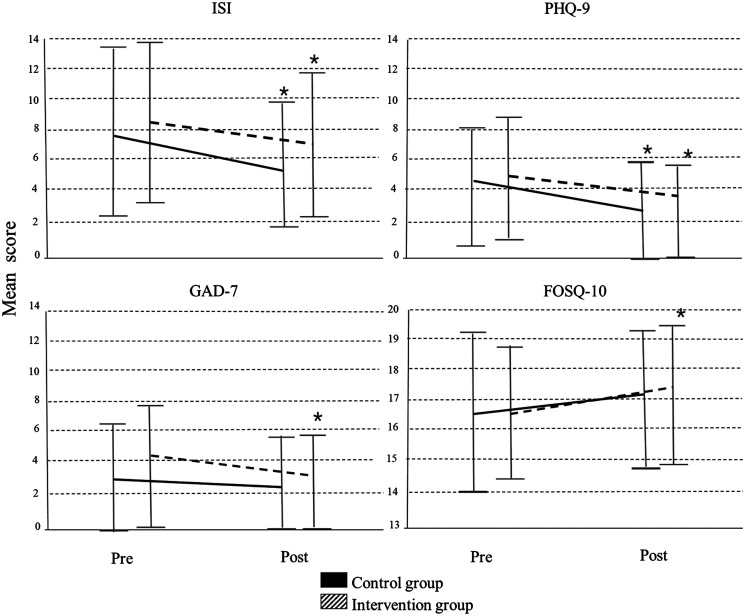
A total of 149 participants completed both the baseline and follow-up ASTNQ (control, n = 70 vs intervention, n = 79). Overall, 96% were women, aged 34.66 ± 11.99 years, with an average of 10 years of shift work experience (Table 1). At baseline, there were no significant differences between the control and intervention groups in demographic and work-related variables, except for the number of evening shifts worked in the past month, which was slightly higher in the control group (Table 1). In addition, both groups had similar scores for the Sleep Hygiene Index, Morningness-Eveningness Questionnaire, ISI, FOSQ-10, and PHQ-9 questionnaires at baseline, except the GAD-7, which was significantly higher for the intervention group (Table 1). Results from paired t-tests showed significant within group improvements from baseline to follow-up for the ISI and PHQ-9. The intervention group also improved significantly in the GAD-7 and FOSQ-10 (Figure 3); however, mixed model analysis showed no between-group differences in programs between program × time point interaction for any outcome measure (Table 2).
Table 1.
Baseline descriptive statistics comparing participant and shift schedule data between groups.
| Total (n = 149) | Control (n = 70) | Intervention (n = 79) | P | |
|---|---|---|---|---|
| SWD risk (% high) | 34 (23.9%) | 15 (21.4%) | 19 (24.1%) | .428a |
| Sex (% female) | 142 (96%) | 67 (95.7%) | 75 (94.9%) | .567a |
| Age | 34.66 ± 11.99 | 36.06 ± 12.99 | 33.42 ± 10.96 | .181b |
| BMI | 25.75 ± 5.46 | 26.20 ± 5.46 | 25.36 ± 5.48 | .352b |
| No. of day shifts (past month) | 9.04 ± 6.79 | 9.49 ± 8.68 | 8.65 ± 4.53 | .461b |
| No. of evening shifts (past month) | 7.56 ± 4.91 | 8.43 ± 5.93 | 6.77 ± 3.62 | .044*,b |
| No. of night shifts (past month) | 5.47 ± 3.35 | 6.04 ± 2.32 | 5.00 ± 3.98 | .228b |
| Years of shift work experience | 10.28 ± 8.44 | 10.39 ± 9.35 | 10.19 ± 7.61 | .888b |
| SHI | 32.59 ± 6.97 | 32.19 ± 7.83 | 32.95 ± 6.13 | .506a |
| ISI | 8.28 ± 5.50 | 7.97 ± 5.65 | 8.56 ± 5.39 | .519a |
| MEQ | 37.13 ± 6.22 | 37.27 ± 6.88 | 37.00 ± 5.62 | .791a |
| FOSQ-10 | 16.62 ± 2.36 | 16.63 ± 2.59 | 16.59 ± 2.15 | .923a |
| PHQ-9 | 4.57 ± 3.62 | 4.33 ± 3.69 | 4.78 ± 3.57 | .445a |
| GAD-7 | 3.52 ± 3.58 | 2.86 ± 3.26 | 4.10 ± 3.76 | .034*,a |
Values represent mean (standard deviation) or %. aChi-square, bt-test. *Significance P < .05 level (2-tailed). BMI = body mass index, FOSQ-10 = Functional Outcomes of Sleep Questionnaire 10, GAD-7 = General Anxiety Disorder Questionnaire, ISI = Insomnia Severity Scale, MEQ = Morningness-Eveningness Questionnaire, PHQ-9 = Patient Health Questionnaire, SHI = Sleep Hygiene Index, SWD = shift work disorder.
Figure 3. Analysis of the change from baseline and follow-up using paired sample t-tests to measure within group differences (mean ± standard deviation).
*Significance pre/post change P < .05 level (2-tailed). FOSQ-10 = Functional Outcomes of Sleep Questionnaire 10, GAD-7 = General Anxiety Disorder Questionnaire, ISI = Insomnia Severity Scale, PHQ-9 = Patient Health Questionnaire.
Table 2.
Mixed model analysis impact of time, program, and program × time interaction on insomnia, sleep hygiene, morning-eveningness, depression, anxiety, and SWD risk (n = 149).
| SHI | ISI | MEQ | FOSQ-10 | PHQ-9 | GAD-7 | SWD risk % | |
|---|---|---|---|---|---|---|---|
| Time Point | |||||||
| Baseline | 32.58 (31.10–34.06) | 8.13 (−7.01–9.25) | 37.50 (35.97–39.03) | 16.65 (16.14–17.15) | 4.56 (3.83–5.30) | 3.44 (2.75–4.13) | .25 (0.12–0.38) |
| Follow-up | 31.87 (30.40–33.36) | 6.27 (5.15–7.40) | 38.55 (37.02–40.08) | 17.15 (16.64–17.65) | 3.45 (2.71–4.18) | 2.51 (1.82–3.20) | .24 (0.11–0.36) |
| P | .356 | .001* | .143 | .055 | .006* | .013* | .784 |
| Program | |||||||
| Control | 31.75 (29.93–33.57) | 6.90 (−5.53–8.28) | 38.51 (36.61–40.42) | 16.85 (16.22–17.47) | 3.72 (2.84–4.61) | 2.56 (1.73–3.40) | 0.26 (0.10–0.43) |
| Intervention | 32.71 (30.75–34.67) | 7.50 (6.03–8.97) | 37.54 (35.48–39.60) | 16.95 (16.28–17.61) | 4.28 (3.34–5.22) | 3.39 (2.50–4.28) | 0.23 (0.04–0.41) |
| P | .441 | .531 | .462 | .809 | .359 | .163 | .722 |
| Program × Timepoint Interaction | |||||||
| Control- pre | 32.09 (30.03–34.16) | 8.03 (6.47–9.59) | 37.84 (35.72–39.95) | 16.64 (15.93–17.35) | 4.41 (3.38–5.44) | 2.88 (1.91–3.85) | 0.26 (0.03–0.44) |
| Control- post | 31.41 (29.35–33.47) | 5.77 (4.22–7.34) | 39.19 (37.08–41.31) | 17.05 (16.34–17.76 | 3.04 (2.01–4.07) | 2.25 (1.28–3.22) | 0.26 (0.09–0.44). |
| Intervention- pre | 33.07 (29.35–33.47) | 8.23 (6.62–9.84) | 37.20 (34.95–39.38) | 16.65 (15.92–17.38) | 4.71 (3.67–5.76) | 4.01 (3.02–4.99) | 0.24 (0.05–0.43) |
| Intervention- post | 32.35 (30.22–34.48) | 6.77 (5.16–8.38) | 37.91 (35.70–40.12) | 17.24 (16.51–17.97) | 3.85 (2.81–4.90) | 2.77 (1.78–3.76) | 0.21 (0.03–0.40) |
| P | .981 | .477 | .670 | .728 | .530 | .416 | .784 |
Values are presented as marginal mean (95% CI). *Significance P < .05 level (2-tailed). FOSQ-10 = Functional Outcomes of Sleep Questionnaire 10, GAD-7 = General Anxiety Disorder Questionnaire, ISI = Insomnia Severity Index, MEQ = Morningness-Eveningness Questionnaire, PHQ-9 = Patient Health Questionnaire, SHI = Sleep Hygiene Index.
Control vs intervention program comparison in high SWD risk group only
A total of 34 nurses scored at high-risk for SWD and undertook 1 of the 2 coaching programs (control n = 15 vs intervention n = 19). There were no significant differences between groups at baseline. Mixed model analysis showed no significant differences in outcomes between time point, programs, and program × time point interaction (Table 3). A greater proportion of participants in the intervention program improved compared to the control program, with 10 participants (52.6%) from the intervention group improving from high risk at baseline to low risk at follow-up, compared to 5 (33%) from the control group; however, this was not significantly different (P = .64) (Table 4).
Table 3.
Mixed model analysis comparing only those at high risk of SWD (program vs time point).
| Control (n = 15) | Intervention (n = 19) | Change | |||||
|---|---|---|---|---|---|---|---|
| Pre | Post | Significance | Pre | Post | p | p | |
| SHI | 35.35 | 31.54 | .343 | 37.06 | 32.44 | .277 | 0.736 |
| (31.93–38.78) | (29.48–33.60) | (33.83–40.28) | (30.26–34.62) | ||||
| ISI | 12.95 | 5.79 | .621 | 13.16 | 6.93 | .696 | 0.592 |
| (10.51–15.38) | (4.37–7.21) | (10.88–15.45) | (5.46–8.40) | ||||
| MEQ | 34.11 | 38.99 | .519 | 36.28 | 37.78 | .664 | 0.166 |
| (30.68–37.54) | (36.96–41.04) | (33.05–39.51) | (35.64–39.92) | ||||
| FOSQ | 15.25 | 17.04 | .405 | 14.75 | 17.2 | .659 | 0.447 |
| (14.10–16.40) | (16.45–17.62) | (13.72–15.79) | (16.61–17.78) | ||||
| PHQ-9 | 6.45 | 3 | .374 | 7.4 | 3.89 | .226 | 0.966 |
| (4.62–8.28) | (2.01–3.99) | (5.72–9.07) | (2.87–4.90) | ||||
| GAD-7 | 4.74 | 2.22 | .152 | 6.34 | 2.83 | .939 | 0.411 |
| (3.06–6.42) | (1.24–3.20) | (4.76–7.91) | (1.81–3.85) | ||||
FOSQ-10 = Functional Outcomes of Sleep Questionnaire 10, GAD-7 = General Anxiety Disorder Questionnaire, ISI = Insomnia Severity Index, MEQ = Morningness-Eveningness Questionnaire, p = Significance at .05 level, PHQ-9 = Patient Health Questionnaire, SHI = Sleep Hygiene Index.
Table 4.
Proportion of participants at high risk for SWD that improved from baseline to follow-up (n = 34).
| SHI | ISI | ESS | FOSQ-10 | PHQ-9 | GAD-7 | ||
|---|---|---|---|---|---|---|---|
| Intervention (n = 19) | % | 79 | 95 | 58 | 89 | 89 | 74 |
| Control (n = 15) | % | 60 | 67 | N/A | 73 | 53 | 47 |
| P | .229 | .075 | — | .750 | .790 | .106 |
P = chi-square. FOSQ-10 = Functional Outcomes of Sleep Questionnaire 10, GAD-7 = General Anxiety Disorder Questionnaire, ISI = Insomnia Severity Index, PHQ-9 = Patient Health Questionnaire, SHI = Sleep Hygiene Index.
DISCUSSION
This study evaluated the effectiveness of an educational and individualized intervention program to manage SWD among health care shift workers. An SWD and sleep education and screening program, including an individualized coaching program for those at high risk of SWD, did not significantly improve sick leave, sleep, or mental health outcomes in comparison to an active control group. There was, however, a nonsignificant trend in sick leave reduction, with a between-group difference in the change in average sick leave utilization of more than 1 day per participant over 6 months.
The estimated total cost associated with sick leave due to sleep impairment and/or depression, is around $96.4AUD million per year in Australia,10,43,44 hence a reduction in sick leave can realize a large cost benefit from an organizational perspective. Sick leave utilization decreased by 15% for the 6 months after the program in the intervention group, whereas the control group increased by 6%, a mean difference in change of sick leave of 1.2 days over 6 months, which is clinically significant. In Australia shift workers take, on average, 11.5 days of sick leave a year compared to 8–9 days for nonshift workers10,45,46 and, compared to other industries, the health care industry utilizes even more sick leave, with an average 13.6 days per year of sick leave.9,45–47 In this study, however, sick leave was already lower than the average rate for health care workers at baseline, with an average of 10.6 days of sick leave over a 12-month period (M = 5.3 days × 6 months), therefore there was less opportunity for further reduction. From an organizational perspective, reducing sick leave is important, first to reduce burnout, improve retention rates, decrease workplace accidents and medical errors, and improve staff retention and productivity9,10 and, second, for the financial benefits for the organization from reduced costs from staff taking sick leave. Further exploration into successful interventions to reduce the impact of shift work on sick leave is needed in the health care sector. As sick leave analysis in this study was ward based, analysis could not be undertaken to compare individual differences in shift work schedules of individuals and whether this contributed to sick leave utilized. Although this project did not find a significant difference, it was clinically important and provides helpful data that can be used to help underpin a larger trial.
Comparisons between the control and intervention groups showed a substantial improvement, with a large effect size observed for insomnia (Cohen’s d = −1.05 and −1.20) and depression (Cohen’s d = −0.63 and −0.75), while anxiety severity decreased only in the intervention group (Cohen’s d = −0.57). The decrease in ISI scores for both groups was comparable to other intervention studies that implemented cognitive behavioral therapy for insomnia for 9 weeks and saw a large change (Cohen’s d = −1.21).48 While improvements in the PHQ-9 were seen in both groups and for anxiety in the intervention group, which had a significantly higher severity of anxiety at baseline, there was no group effect. Improvements by both groups could have been due to the Hawthorne effect or a genuine impact on these outcomes in both groups, raising the possibility that education, screening shift workers for sleep disorders, providing feedback on results and support for their health (sleep or low GI diet) may improve their insomnia symptoms and mental health. In addition, some individuals shifted from high to low risk for SWD at the end of the program, demonstrating the potential to impact SWD through individual behavioral interventions shift workers.
SWD was common in this study (23.9%). Those at high risk of SWD did not significantly improve in any of the outcome measures, leaving room for further investigation into appropriate strategies to help this subgroup of shift workers.
The intervention program in this study was designed to target SWD symptoms (insomnia and sleepiness) as well as improve sleep hygiene behavior, but not mental health. Poor sleep hygiene behavior has been shown to substantially increases SWD risk in shift workers49,50 and hence was considered a potential intervention target for SWD. Sleep hygiene knowledge (good sleep environment, reducing television/screen time, reducing worry/anger before bedtime) alone, however, may not be effective in combating the scheduling demands of shift workers.51 Given the high severity of depression and anxiety found among nurses with SWD,6 targeted mental health treatment along with sleep and shift work management should be considered for future trials. There were significantly higher levels of depression and anxiety among those at high risk of SWD in this study. Past research shows that insomnia, sleepiness, and SWD are strongly related to depression and anxiety2,6,7,40 and that treating poor sleep can help to improvement mental health.52 The direction of this relationship, however, is not clear: does mental health contribute to the development of SWD or develop from the effects of longer term shift work (or both)? Future SWD management intervention programs would benefit from directly addressing mental health as well as sleep. Strategies such as incorporating standard cognitive behavioral therapy or cognitive behavioral therapy for insomnia to treat both, coping skills to reduce anxiety, or, in severe cases, referring to a psychologist to address the underlying mental health issues would be valuable.
There was a high prevalence of SWD risk among nurses in this current trial (23.9% at high risk of SWD), which is comparable to other shift work populations. Factors that related to SWD risk at follow-up included insomnia, depression, anxiety, and SWD risk at baseline. This is similar to other studies that have shown strong associations between insomnia, mental health,7,53 and a longitudinal study that found SWD at baseline to be the biggest predictor of SWD risk in nurses after 2 years.8 Although proportionally more of those in the SWIM program improved across a range of domains including sleep hygiene, insomnia, and mental health (Table 4), the change was not significant. This might be because there was inadequate power given the small sample size. Also, these findings suggest that screening and ward-based education alone might be effective at decreasing SWD symptoms by improving sleep hygiene, mental health, and insomnia. The compliance rate for both groups were low, however, with participants missing meetings or not undertaking the home-based activities before the meetings. It is recommended that future projects look at techniques to improve the level of compliance of intervention recommendations.
Several limitations should be acknowledged in this current trial. First, while the trial carefully recruited comparable wards, rotation of some casual staff meant that cross-contamination of programs could have occurred. To reduce this risk, wards on the same level of the hospital who shared facilities such as kitchens and bathrooms were recruited together into the same program. Disclosure of questionnaire scores at baseline may have generated awareness and insight into SWD risk, insomnia, sleep hygiene behavior, and mental health, resulting in a modification (consciously or not) to behavior in the control group. Also, due to the Morningness-Eveningness Questionnaire measuring a biological trait, this was unlikely to change in the given time period. Mechanisms to objectively measure and achieve higher compliance is recommended for future research. The trial measured ward-based sick leave utilization rather than individual data, hence it was not possible to ascertain changes in sick leave in the high-risk SWD staff participating in the coaching program and match shift types, with the control group having undertaken a significantly higher number of evening shifts. There was inadequate power to identify an effect on anxiety or sick leave specifically in the high-risk SWD group, and there was a dropout rate of approximately 25%. A larger, multicentered project would be required to address these 2 limitations. Furthermore, disclosure of baseline results from the questionnaire and the dietary program may have had an impact on the improvement of the outcomes, such as sleep or mood. Future trials should look at implementing an inactive control group and not disclose questionnaire scores bias to avoid this information influencing outcomes. Finally, it is unknown whether the duration of the intervention program was optimal to achieve behavior modification of participants.
This is a unique, randomized control trial to implement and evaluate a multifaceted shift work individualized management program using a validated SWD screening tool. Although this trial did not demonstrate a benefit compared to an active control, the information and lessons learned from this trial provide an important foundation for future study design, both within the health care sector and other shift work industries. Future trial designs should consider cluster randomization by hospital sites to avoid cross-contamination between programs and intervention programs, including both sleep and mental health strategies in treatment programs, consideration as to the duration of the program for optimal behavioral modification, and consideration of an inactive control. Understanding the prevalence and impact SWD will assist with workforce planning and staff health and safety by assisting with the awareness of SWD symptoms, implementing screening tools to monitor the development of SWD, and directing appropriate education and training into shift work management for shift workers as part of induction programs or university courses to help mitigate the effects of SWD before it develops.
DISCLOSURE STATEMENT
All authors have seen and approved this manuscript. Work for this study was performed at Austin Hospital, Melbourne, Australia. This study was primarily funded by the Cooperative Research Centre (CRC) for Alertness, Safety and Productivity (Melbourne, Australia), in conjunction with Austin Health and the Institute for Breathing and Sleep (Melbourne, Australia). Dr Booker had a scholarship with the CRC for Alertness, Safety and Productivity. Dr Sletten served as a Project Leader in the CRC for Alertness, Safety and Productivity. Prof. Lockley was a Program Leader for the CRC for Alertness, Safety and Productivity, which funded this work. Prof. Rajaratnam was also a Program Leader for the CRC for Alertness, Safety and Productivity, which funded this work. Assoc./Prof. Howard served as a Theme Leader in the CRC for Alertness, Safety and Productivity, which funded this work; and had received grants from Prevention Express and TEVA, which are not related to the work reported in this paper. The other authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors thank all the staff at the hospital for their in-kind contributions to the project and allowing the project to roll-out across multiple wards and Monash University Honors students Casandra Fong and Aqsa Naqvi for helping with recruitment and data management. Staff were recruited during work hours with permission and support of unit management and Austin Health Executive. Their support in the implementation of this trial was invaluable, and participating staff were generous with their time. This work was supported by the Cooperative Research Centre for Alertness, Safety and Productivity, Australia.
ABBREVIATIONS
- ASTNQ
Australasian Sleep Trials Network Questionnaire
- CI
confidence interval
- GAD-7
General Anxiety Disorder- 7
- GI
glycemic index
- ISI
Insomnia Severity Scale
- FOSQ-10
Functional Outcomes of Sleep Questionnaire 10
- M
mean
- PHQ-9
Patient Health Questionnaire
- RCT
randomized controlled trial
- SWD
shift work disorder
REFERENCES
- 1.McMenami TM. A time to work: recent trends in shift work and flexible schedules. Monthly Labor Rev. 2007; 130:3. [Google Scholar]
- 2. Wright KP Jr , Bogan RK , Wyatt JK . Shift work and the assessment and management of shift work disorder (SWD) . Sleep Med Rev. 2013. ; 17 ( 1 ): 41 – 54 . [DOI] [PubMed] [Google Scholar]
- 3. Waage S , Moen BE , Pallesen S , et al . Shift work disorder among oil rig workers in the North Sea . Sleep. 2009. ; 32 ( 4 ): 558 – 565 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Di Milia L , Waage S , Pallesen S , Bjorvatn B . Shift work disorder in a random population sample--prevalence and comorbidities . PLoS One. 2013. ; 8 ( 1 ): e55306 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014 [Google Scholar]
- 6. Booker LA , Sletten TL , Alvaro PK , et al . Exploring the associations between shift work disorder, depression, anxiety and sick leave taken amongst nurses . J Sleep Res. 2020. ; 29 ( 3 ): e12872 . [DOI] [PubMed] [Google Scholar]
- 7. Kalmbach DA , Pillai V , Cheng P , Arnedt JT , Drake CL . Shift work disorder, depression, and anxiety in the transition to rotating shifts: the role of sleep reactivity . Sleep Med. 2015. ; 16 ( 12 ): 1532 – 1538 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Waage S , Pallesen S , Moen BE , et al . Predictors of shift work disorder among nurses: a longitudinal study . Sleep Med. 2014. ; 15 ( 12 ): 1449 – 1455 . [DOI] [PubMed] [Google Scholar]
- 9. Rajaratnam SM , Howard ME , Grunstein RR . Sleep loss and circadian disruption in shift work: health burden and management . Med J Aust. 2013. ; 199 ( 8 ): S11 – S15 . [DOI] [PubMed] [Google Scholar]
- 10.Sleep Health Foundation. Asleep on the Job: Costs of Inadequate Sleep in Australia. Australia: Sleep Health Foundation; 2017. [Google Scholar]
- 11. Lammers-van der Holst HM , Van Dongen HPA , Drosopoulos S , Kerkhof GA . Inter-individual differences in sleep response to shift work in novice police officers - A prospective study . Chronobiol Int. 2016. ; 33 ( 6 ): 671 – 677 . [DOI] [PubMed] [Google Scholar]
- 12. Van Dongen HP . Shift work and inter-individual differences in sleep and sleepiness . Chronobiol Int. 2006. ; 23 ( 6 ): 1139 – 1147 . [DOI] [PubMed] [Google Scholar]
- 13. Booker LA , Magee M , Rajaratnam SMW , Sletten TL , Howard ME . Individual vulnerability to insomnia, excessive sleepiness and shift work disorder amongst healthcare shift workers. A systematic review . Sleep Med Rev. 2018. ; 41 : 220 – 233 . [DOI] [PubMed] [Google Scholar]
- 14. Zee PC , Badr MS , Kushida C , et al . Strategic opportunities in sleep and circadian research: report of the Joint Task Force of the Sleep Research Society and American Academy of Sleep Medicine . Sleep. 2014. ; 37 ( 2 ): 219 – 227 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zee PC , Goldstein CA . Treatment of shift work disorder and jet lag . Curr Treat Options Neurol. 2010. ; 12 ( 5 ): 396 – 411 . [DOI] [PubMed] [Google Scholar]
- 16. Barion A , Zee PC . A clinical approach to circadian rhythm sleep disorders . Sleep Med. 2007. ; 8 ( 6 ): 566 – 577 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Roth T . Appropriate therapeutic selection for patients with shift work disorder . Sleep Med. 2012. ; 13 ( 4 ): 335 – 341 . [DOI] [PubMed] [Google Scholar]
- 18. Purnell MT , Feyer AM , Herbison GP . The impact of a nap opportunity during the night shift on the performance and alertness of 12-h shift workers . J Sleep Res. 2002. ; 11 ( 3 ): 219 – 227 . [DOI] [PubMed] [Google Scholar]
- 19. Leger D , Philip P , Jarriault P , Metlaine A , Choudat D . Effects of a combination of napping and bright light pulses on shift workers’ sleepiness at the wheel: a pilot study . J Sleep Res. 2009. ; 18 ( 4 ): 472 – 479 . [DOI] [PubMed] [Google Scholar]
- 20. Härmä M , Knauth P , Ilmarinen J . Daytime napping and its effects on alertness and short-term memory performance in shiftworkers . Int Arch Occup Environ Health. 1989. ; 61 ( 5 ): 341 – 345 . [DOI] [PubMed] [Google Scholar]
- 21. Ruggiero JS , Redeker NS . Effects of napping on sleepiness and sleep-related performance deficits in night-shift workers: a systematic review . Biol Res Nurs. 2014. ; 16 ( 2 ): 134 – 142 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Barger LK , Sullivan JP , Lockley SW , Czeisler CA . Exposure to short wavelength-enriched white light and exercise improves alertness and performance in operational NASA flight controllers working overnight shifts . J Occup Environ Med. 2021. ; 63 ( 2 ): 111 – 118 . [DOI] [PubMed] [Google Scholar]
- 23. Bjorvatn B , Stangenes K , Øyane N , et al . Randomized placebo-controlled field study of the effects of bright light and melatonin in adaptation to night work . Scand J Work Environ Health. 2007. ; 33 ( 3 ): 204 – 214 . [DOI] [PubMed] [Google Scholar]
- 24. Wyatt JK , Cajochen C , Ritz-De Cecco A , Czeisler CA , Dijk DJ . Low-dose repeated caffeine administration for circadian-phase-dependent performance degradation during extended wakefulness . Sleep. 2004. ; 27 ( 3 ): 374 – 381 . [DOI] [PubMed] [Google Scholar]
- 25. Ker K , Edwards PJ , Felix LM , Blackhall K , Roberts I . Caffeine for the prevention of injuries and errors in shift workers . Cochrane Database Syst Rev. 2010. : CD008508 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mastin DF , Bryson J , Corwyn R . Assessment of sleep hygiene using the Sleep Hygiene Index . J Behav Med. 2006. ; 29 ( 3 ): 223 – 227 . [DOI] [PubMed] [Google Scholar]
- 27. Greenwood KM , Rich WJ , James JE . Sleep hygiene practices and sleep duration in rotating- shift shiftworkers . Work Stress. 1995. ; 9 ( 2–3 ): 262 – 271 . [Google Scholar]
- 28. Burgess HJ , Revell VL , Molina TA , Eastman CI . Human phase response curves to three days of daily melatonin: 0.5 mg versus 3.0 mg . J Clin Endocrinol Metab. 2010. ; 95 ( 7 ): 3325 – 3331 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Queensland Government. Glycaemic Index of Foods; 2014; https://www.health.qld.gov.au/__data/assets/pdf_file/0026/143567/paeds_gi.pdf Accessed Dec. 15, 2021.
- 30.Glycemic Index Foundation. Consumer Fact Sheets; 2015; https://www.gisymbol.com/fact-sheets/. Accessed Dec. 15, 2021
- 31.Barger LK, Ogeil RP, Drake CL, O"Brien CS, Ng KT, Rajaratnam SM. Validation of a questionnaire to screen for shift work disorder. Sleep. 2012;35(12):1693–1703. [DOI] [PMC free article] [PubMed]
- 32. Cho S , Kim GS , Lee JH . Psychometric evaluation of the sleep hygiene index: a sample of patients with chronic pain . Health Qual Life Outcomes. 2013. ; 11 ( 1 ): 213 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4(2):97–110. [PubMed] [Google Scholar]
- 34. Bastien CH , Vallières A , Morin CM . Validation of the Insomnia Severity Index as an outcome measure for insomnia research . Sleep Med. 2001. ; 2 ( 4 ): 297 – 307 . [DOI] [PubMed] [Google Scholar]
- 35. Morin CM , Belleville G , Bélanger L , Ivers H . The Insomnia Severity Index: Psychometric indicators to detect insomnia cases and evaluate treatment response . Sleep. 2011. ; 34 ( 5 ): 601 – 608 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chasens ER , Ratcliffe SJ , Weaver TE . Development of the FOSQ-10: A short version of the Functional Outcomes of Sleep Questionnaire . Sleep. 2009. ; 32 ( 7 ): 915 – 919 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kroenke K , Spitzer RL , Williams JBW . The PHQ-9: Validity of a brief depression severity measure . J Gen Intern Med. 2001. ; 16 ( 9 ): 606 – 613 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10): 1092–1097. [DOI] [PubMed] [Google Scholar]
- 39. Letvak S , Ruhm CJ , McCoy T . Depression in hospital-employed nurses . Clin Nurse Spec. 2012. ; 26 ( 3 ): 177 – 182 . [DOI] [PubMed] [Google Scholar]
- 40. Lee HY , Kim MS , Kim O , Lee IH , Kim HK . Association between shift work and severity of depressive symptoms among female nurses: the Korea Nurses’ Health Study . J Nurs Manag. 2016. ; 24 ( 2 ): 192 – 200 . [DOI] [PubMed] [Google Scholar]
- 41. Kroenke K , Spitzer RL , Williams JB , Löwe B . The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review . Gen Hosp Psychiatry. 2010. ; 32 ( 4 ): 345 – 359 . [DOI] [PubMed] [Google Scholar]
- 42. Löwe B , Decker O , Müller S , et al . Validation and standardization of the Generalized Anxiety Disorder Screener (GAD-7) in the general population . Med Care. 2008. ; 46 ( 3 ): 266 – 274 . [DOI] [PubMed] [Google Scholar]
- 43.Harvey S, Joyce S, Modini M, et al. Work and Depression/Anxiety Disorders – A Systematic Review of Reviews. Melbourne: BeyondBlue; 2012. [Google Scholar]
- 44.Merkus SL, van Drongelen A, Holte KA, et al. The association between shift work and sick leave: a systematic review. Occup Environ Med. 2012;69(10): 701–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Australian Bureau of Statistics. Australian Labour Market Statistics 2010. Shift Workers- Working Time Arrangements; 2011; www.abs.gov.au. Accessed Feb. 26, 2016.
- 46.Australian Institute of Health and Welfare. Health system expenditure on disease and injury in Australia. 2000–2001; AIHW cat. no. HWE 26 Canberra: AIHW (Health and Welfare Expenditure Series no. 19); https://www.aihw.gov.au/getmedia/28c32a26-a016-4c0b-a273-f3450c17e776/hsedia00-01.pdf.aspx?inline=true. Accessed Dec. 15, 2021.
- 47.Drake CL, Roehrs T, Richardson G, Walsh JK, Roth T. Shift work sleep disorder: prevalence and consequences beyond that of symptomatic day workers. Sleep. 2004;27(8):1453–1462. [DOI] [PubMed] [Google Scholar]
- 48.Vedaa Ø, Kallestad H, Scott J, et al. Effects of digital cognitive behavioural therapy for insomnia on insomnia severity: a large-scale randomised controlled trial. Lancet Digit Health. 2020;2(8):e397–e406. [DOI] [PubMed] [Google Scholar]
- 49.Booker LA, Barnes M, Alvaro P, et al. The role of sleep hygiene in the risk of Shift Work Disorder in nurses. 2020;43(2):zsz228. [DOI] [PubMed] [Google Scholar]
- 50.Chou TL, Chang LI, Chung MH. The mediating and moderating effects of sleep hygiene practice on anxiety and insomnia in hospital nurses. Int J Nurs Pract. 2015;21( Suppl 2):9–18. [DOI] [PubMed] [Google Scholar]
- 51. Nishinoue N , Takano T , Kaku A , et al . Effects of sleep hygiene education and behavioral therapy on sleep quality of white-collar workers: a randomized controlled trial . Ind Health. 2012. ; 50 ( 2 ): 123 – 131 . [DOI] [PubMed] [Google Scholar]
- 52. Manber R , Edinger JD , Gress JL , San Pedro-Salcedo MG , Kuo TF , Kalista T . Cognitive behavioral therapy for insomnia enhances depression outcome in patients with comorbid major depressive disorder and insomnia . Sleep. 2008. ; 31 ( 4 ): 489 – 495 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Asaoka S , Aritake S , Komada Y , et al . Factors associated with shift work disorder in nurses working with rapid-rotation schedules in Japan: the nurses’ sleep health project . Chronobiol Int. 2013. ; 30 ( 4 ): 628 – 636 . [DOI] [PubMed] [Google Scholar]