Abstract
Study Objectives:
Pulmonary hypertension (PH) is prevalent in obesity hypoventilation syndrome (OHS). However, there is a paucity of data assessing pathogenic factors associated with PH. Our objective is to assess risk factors that may be involved in the pathogenesis of PH in untreated OHS.
Methods:
In a post hoc analysis of the Pickwick trial, we performed a bivariate analysis of baseline characteristics between patients with and without PH. Variables with a P value ≤ .10 were defined as potential risk factors and were grouped by theoretical pathogenic mechanisms in several adjusted models. Similar analysis was carried out for the 2 OHS phenotypes, with and without severe concomitant obstructive sleep apnea.
Results:
Of 246 patients with OHS, 122 (50%) had echocardiographic evidence of PH defined as systolic pulmonary artery pressure ≥ 40 mm Hg. Lower levels of awake PaO2 and higher body mass index were independent risk factors in the multivariate model, with a negative and positive adjusted linear association, respectively (adjusted odds ratio 0.96; 95% confidence interval 0.93 to 0.98; P = .003 for PaO2, and 1.07; 95% confidence interval 1.03 to 1.12; P = .001 for body mass index). In separate analyses, body mass index and PaO2 were independent risk factors in the severe obstructive sleep apnea phenotype, whereas body mass index and peak in-flow velocity in early/late diastole ratio were independent risk factors in the nonsevere obstructive sleep apnea phenotype.
Conclusions:
This study identifies obesity per se as a major independent risk factor for PH, regardless of OHS phenotype. Therapeutic interventions targeting weight loss may play a critical role in improving PH in this patient population.
Clinical Trial Registration:
Registry: Clinicaltrial.gov; Name: Alternative of Treatment in Obesity Hypoventilation Syndrome; URL: https://clinicaltrials.gov/ct2/show/NCT01405976; Identifier: NCT01405976.
Citation:
Masa JF, Benítez ID, Javaheri S, et al. Risk factors associated with pulmonary hypertension in obesity hypoventilation syndrome. J Clin Sleep Med. 2022;18(4):983–992.
Keywords: sleep apnea, hypoxemia, hypercapnia, noninvasive ventilation, CPAP
BRIEF SUMMARY
Current Knowledge/Study Rationale: What are the risk factors associated with pulmonary hypertension (PH) in untreated obesity hypoventilation syndrome (OHS), and do these risk factors vary based on OHS phenotype? There are no systematic large-scale studies examining mechanistic risk factors associated with PH in untreated OHS.
Study Impact: This is the first cross-sectional study evaluating potential pathophysiological risk factors for PH in a large sample of patients with untreated OHS. Obesity is a shared risk factor for both OHS phenotypes, with or without severe obstructive sleep apnea, suggesting weight loss may play a critical role in improving PH. The mechanisms implicating obesity with PH are discussed.
INTRODUCTION
Obesity hypoventilation syndrome (OHS) is defined by obesity, daytime hypercapnia, and presence of sleep-disordered breathing, after excluding other causes of hypoventilation.1 Concomitant obstructive sleep apnea (OSA) is common (90%),2 and 73% of patients with OHS also have severe OSA.3 Compared with patients with eucapnic OSA4,5 and eucapnia with obesity, patients with OHS have a higher prevalence of cardiovascular and respiratory morbidity, hospitalization, health care resource utilization, and overall mortality.6–17
Pulmonary hypertension (PH) is frequently under recognized in patients with OHS. In the largest randomized clinical trial to date (Pickwick study), approximately 8% of patients had been diagnosed with PH upon study enrollment. However, baseline assessment revealed that nearly 50% of the patients had echocardiographic evidence of PH defined as pulmonary artery systolic pressure ≥ 40 mm Hg.18 Other observational studies have also reported a high prevalence of PH in patients with OHS (from 52% to 88%).2,19–22
From a mechanistic standpoint, there are several pathogenic conditions that could be involved in the increased risk of PH in patients with OHS. These include presence of OSA with associated nocturnal hypoxemia and hypercapnia promoting pulmonary arteriolar vasoconstriction,23 awake arterial blood hypoxemia and hypercapnia, and left ventricular diastolic dysfunction elevating left ventricular end-diastolic pressure.24 Furthermore, obesity per se is an inflammatory condition and its associated comorbidities (ie, hypertension and insulin resistance) may further contribute to PH.25
Despite the overwhelming pathophysiological mechanisms linking OHS to PH, to our knowledge, there are no systematic large-scale studies examining risk factors associated with PH in untreated OHS. We hypothesized that the presence of PH is associated with the severity of hypoxemia and hypercapnia, severity of OSA, degree of obesity, and presence of diastolic dysfunction. To test our hypothesis, we performed a cross-sectional analysis of the baseline data of patients with untreated OHS, with and without concomitant severe OSA phenotypes, enrolled in the Pickwick randomized controlled trials.3,17,18,26–31
METHODS
Trial design
We carried out a cross-sectional study of a multicenter, open-label, randomized clinical trial with 2 parallel groups conducted at 16 clinical sites in Spain.
Participants
From May 2009 to March 2013, we successively screened patients between 15 and 80 years of age who were referred for pulmonary consultations due to suspected OHS or OSA at 16 tertiary hospitals in Spain (see supplemental material). OHS was defined as obesity, with a body mass index (BMI) ≥ 30 kg/m2, stable hypercapnic respiratory failure (Partial pressure of carbon dioxide in the arterial blood (PaCO2) ≥ 45 mm Hg, pH ≥ 7.35, and no clinical exacerbation during the previous 2 months), no noteworthy spirometric evidence of chronic obstructive pulmonary disease (forced expiratory volume in the first second had to be above 70% of predicted in cases where forced expiratory volume in the first second/forced vital capacity was below 70), and no evidence of neuromuscular, chest wall, or metabolic disease to explain hypercapnia. Other inclusion criteria were absence of narcolepsy or restless legs syndrome and a correctly executed 30-minute continuous positive airway pressure/noninvasive ventilation treatment test (see supplemental material). The exclusion criteria were as follows: (1) a psycho-physical inability to complete questionnaires, (2) severe chronic debilitating illness, (3) severe chronic nasal obstruction, and (4) a lack of informed consent.
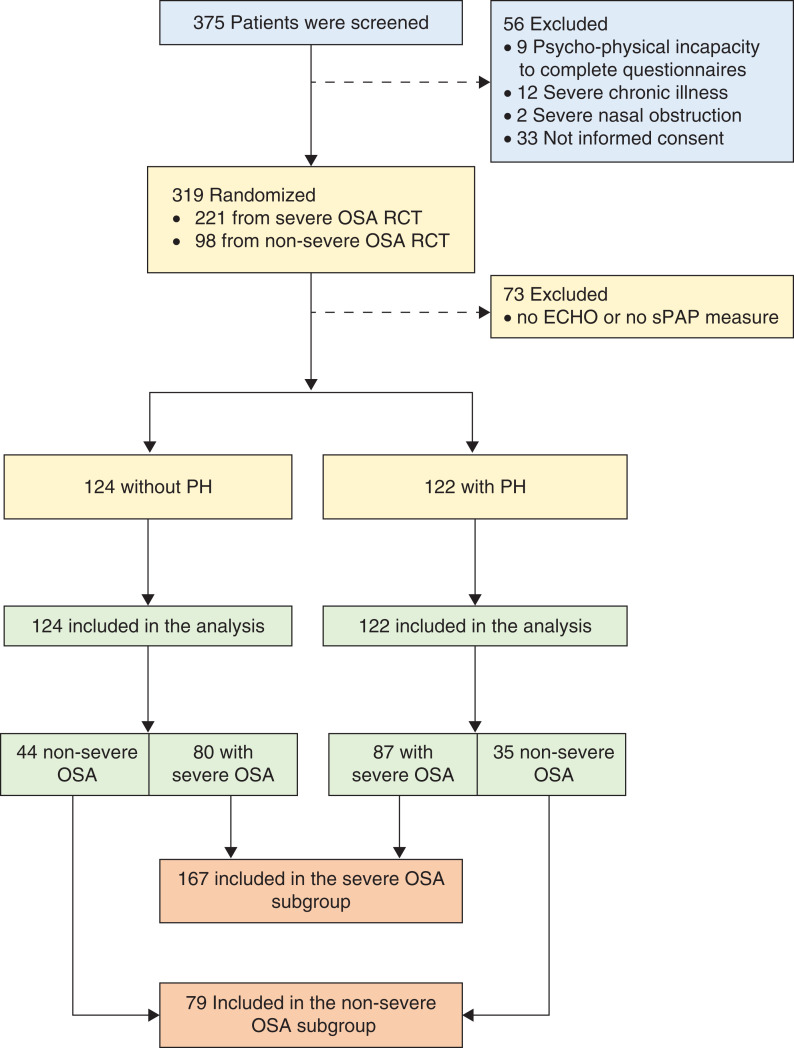
The Pickwick project comprised 2 parallel randomized clinical trials based on the presence of severe OSA [apnea-hypopnea index (AHI) ≥ 30 events/h] or nonsevere OSA (AHI < 30 events/h). Both randomized clinical trials were conducted in 2 phases29 (see figure S1 in supplemental material). In the present cross-sectional analysis, we used data obtained during the baseline evaluation of patients enrolled in both randomized controlled trials who had adequate measure of systolic pulmonary artery pressure obtained from baseline conventional transthoracic echocardiogram (Figure 1). The study was approved by the ethics committees of all 16 centers, and written informed consent was obtained from all patients.
Figure 1. Study flowchart.
Flow chart of the study protocol. Of 375 selected patients, 56 were excluded and 319 were randomized: 221 in the severe OSA trial and 98 in the nonsevere OSA trial. Of this 319, 73 were excluded because of inadequate echocardiogram. Of the remaining 246, 122 had and 124 did not have PH. Of the 122 with PH, 87 had severe OSA and 35 had nonsevere OSA who were included in the subgroup analysis, severe OSA and nonsevere OSA, respectively. Of the 124 without PH, 80 had severe OSA and 44 had nonsevere OSA who were included in the subgroup analysis, severe OSA and nonsevere OSA, respectively. Therefore, the severe OSA subgroup had 167 patients and nonsevere OSA had 79 patients. ECHO = transthoracic echocardiogram, OSA = obstructive sleep apnea, PH = pulmonary hypertension, RCT = randomized controlled trial, sPAP = systolic pulmonary artery pressure.
Outcomes
At baseline visit we assessed anthropometric data; smoking habits and alcohol consumption; arterial blood gases during wakefulness while resting and breathing ambient air to assess PaCO2, partial pressure of oxygen in arterial blood (PaO2), pH, and calculated bicarbonate (see page 7 in supplemental material); sphygmomanometric blood pressure32 (see supplemental material); spirometry,33 6-minute walk distance test;34 sleepiness based on the Epworth Sleepiness Scale; comorbidities (hypertension, diabetes, dyslipidemia, ischemic cardiomyopathy, chronic heart failure, stroke, pulmonary hypertension, cardiac arrhythmia, and leg arteriopathy) obtained from the electronic medical records and during face-to-face interview with patients; health-related quality of life tests using the Functional Outcomes of Sleep Questionnaire, the Medical Outcome Survey Short Form 36, the visual analog well-being scale,35,36 oxygen therapy, conventional polysomnography (see pages 8 and 9 in the supplemental material), and conventional transthoracic echocardiogram.
Echocardiography
All 2-dimensional (2D) and Doppler echocardiograms were recorded using available echocardiographic equipment in each center (see supplemental material). Left ventricle size and wall thickness were measured according to international guidelines.37 All these parameters were derived from 2D-guided M-mode imaging or from linear measurements obtained from 2D images. Left ventricle ejection fraction was calculated from end diastolic and end systolic volume estimates, using volumetric measurements. Left ventricle mass was estimated by linear method with Cube’s formula (0.8 × 1.04 × [(IVS + LVDD + LVPW)3 − LVDD3] + 0.6 g), where LVDD is the diastolic diameter of left ventricle (internal diameter), LVPW is the left ventricle posterior wall thickness, and IVS is the interventricular septum thickness, all measured at end-diastole in the long axis parasternal view using either 2D-guided M-mode or linear measurements from 2D echocardiographic images. Left ventricle mass index was obtained dividing the left ventricle mass by the body surface area according to Dubois’ formula. To assess left ventricle diastolic function, we obtained peak early (E), peak atrial (A) mitral valve in-flow velocities, as well as their ratio (E/A), deceleration time and the antero-posterior diameter of the left atrium measured in the parasternal long-axis view. Systolic pulmonary artery pressure was assessed from the maximum velocity of tricuspid regurgitation signal (continuous wave Doppler) by the addition of right atrial pressure, estimated from inferior cava vein and its collapsibility. Right ventricular systolic function was evaluated using the right ventricle index of myocardial performance index.
Statistical analysis
Baseline bivariate analysis between the presence of PH and anthropometric, clinical, polysomnographic, and echocardiographic characteristics was carried out. We considered a systolic pulmonary artery pressure ≥ 40 mm Hg as evidence of PH.30
We performed an adjusted logistic regression model with presence of PH as the dependent variable, and the predictors included in the model were variables that showed a P value < .10 in bivariate analysis between patients with and without PH. Recognizing that certain independent variables of interest can be highly correlated with each other, we used data from the literature, as well as biologic plausibility, to identify the variables best suited for the models. Although age was not statistically different in bivariate analysis, it was included in the models as a confounding risk factor.38 The 6-minute-walk distance test as well as the right ventricular systolic function based in the right ventricle index of myocardial performance index were not included in the model because they are more likely a consequence of PH. The oxygen desaturation index (ODI), AHI, and arousal index were log-transformed to normalize their distribution. A generalized additive model with penalized cubic regression spline was used to evaluate the type of association between each potential risk factor and the presence of PH. The mean oxygen saturation during sleep and PaCO2 were categorized by tertiles after observing a nonlinear relationship with the risk of PH.
We created models including variables according to a pathogenic perspective as follows: (1) obesity, ie, BMI and spirometric parameters; (2) persistent hypoxemia, ie, PaO2 and oxygenation parameters during sleep; (3) OSA, ie, polysomnographic OSA-related parameters such as arousal index, AHI, and ODI; (4) hypoventilation, ie, PaCO2; and (5) postcapillary mechanism, ie, diastolic and systolic dysfunction parameters based on echocardiography. To assess all pathogenic groups together, a process of selection of potential risk factors using a relaxed Least Absolute Shrinkage and Selection Operator (LASSO) model was carried out.39 A 5-fold cross validation was carried out to select the lambda parameter of the LASSO model. Lambda was selected as the largest at which the mean square error was within 1 standard error of the minimal mean square error. A Spearman correlation test between independent risk factors based on the LASSO analysis with the rest of the variables was performed. To perform LASSO analysis, missing values were replaced by the means of the nonmissing values. A similar analysis was repeated for the 2 recognized OHS phenotypes (subgroup analysis), OHS with severe OSA and OHS without severe OSA.
Data management and statistical analyses were performed using R software (R Core Team 2017, Version 3.4.2, Vienna, Austria) and SPSS software (IBM-SPSS Statistics, Version 22.0. IBM Corp., Armonk, NY).
RESULTS
Study participants
Of the 375 patients who met inclusion criteria, 56 were excluded (Figure 1). Of the 319 remaining patients, 73 (23%) were excluded due to lack of appropriate echocardiograms. Of the 246 available for analysis, 122 (49.6%) had evidence of PH. The median of systolic pulmonary artery pressure in the group without PH was 34.0 mm Hg (28.0–36.4) vs 46.0 mmHg (43.0–55.8) in the PH group. Table 1 summarizes the bivariate analysis of baseline characteristics and polysomnographic and echocardiographic parameters based on PH status. Patients with PH were more obese, had higher AHI, lower PaO2, slightly higher PaCO2, and worse exercise tolerance and right ventricular systolic function.
Table 1.
Baseline characteristics of patients with and without PH.
| No PH (n = 124) | PH (n = 122) | Unadjusted Odds Ratio (95% CI) | P | |
|---|---|---|---|---|
| Age, years | 66.0 [55.0;73.0] | 66.0 [56.0;72.0] | 1.00 [0.98;1.02] | .825 |
| Sex, female | 83 (66.9%) | 79 (64.8%) | 0.91 [0.53;1.54] | .721 |
| Smokers | 28 (22.6%) | 21 (17.2%) | 0.71 [0.38;1.34] | .298 |
| Alcohol drinkers‡ | 20 (16.3%) | 21 (17.2%) | 1.07 [0.54;2.11] | .844 |
| BMI, kg/m2 | 39.1 [35.7;45.1] | 43.6 [38.1;49.2] | 1.08 [1.04;1.12] | < .001 |
| ESS | 9.00 [6.00;13.0] | 10.0 [7.00;15.0] | 1.03 [0.98;1.08] | .273 |
| FOSQ | 76.0 [58.8;90.2] | 73.0 [57.2;88.0] | 1.00 [0.98;1.01] | .610 |
| SF 36-Physical | 38.2 [29.9;45.0] | 34.3 [27.5;43.5] | 0.98 [0.96;1.01] | .191 |
| SF 36-Mental | 43.3 [33.6;53.5] | 44.9 [31.9;52.5] | 1.00 [0.98;1.02] | .898 |
| VAWS | 50.0 [37.9;66.0] | 48.5 [39.0;58.3] | 0.99 [0.98;1.01] | .409 |
| Hypertension | 85 (69.1%) | 87 (71.3%) | 1.11 [0.64;1.93] | .709 |
| Systolic BP, mm Hg | 138 [130;142] | 140 [130;148] | 1.00 [0.99;1.02] | .598 |
| Diastolic BP, mm Hg | 80.0 [70.0;85.2] | 80.0 [70.0;90.0] | 1.00 [0.98;1.02] | .826 |
| Diabetes | 44 (35.5%) | 50 (41.0%) | 1.26 [0.75;2.12] | .379 |
| Dyslipidemia | 57 (46.0%) | 53 (43.8%) | 0.92 [0.55;1.52] | .735 |
| Stroke | 7 (5.65%) | 9 (7.38%) | 1.32 [0.47;3.89] | .595 |
| Ischemic heart disease | 10 (8.06%) | 9 (7.44%) | 0.92 [0.35;2.39] | .860 |
| Arrhythmia | 8 (6.45%) | 12 (9.92%) | 1.58 [0.62;4.24] | .335 |
| Chronic heart failure | 20 (16.1%) | 24 (19.8%) | 1.28 [0.67;2.50] | .456 |
| Leg arteriopathy | 12 (9.76%) | 7 (5.79%) | 0.57 [0.20;1.50] | .260 |
| Pulmonary hypertension diagnosis | 7 (5.65%) | 18 (14.9%) | 2.87 [1.19;7.75] | .018 |
| pH | 7.40 [7.38;7.42] | 7.40 [7.38;7.42] | 0.42 [0.00;730] | .822 |
| PaO2, mm Hg | 64.0 [57.7;71.0] | 59.0 [55.0;64.0] | 0.95 [0.93;0.98] | .001 |
| PaCO2, mm Hg | 49.0 [47.0;51.0] | 50.0 [48.0;53.1] | 1.09 [1.02;1.17] | .008 |
| Bicarbonate, mmol/L | 29.6 [27.7;31.4] | 29.1 [28.0;31.0] | 1.03 [0.95;1.11] | .537 |
| FEV1, % of predicted | 80.0 [67.8;91.2] | 75.0 [63.0;84.0] | 0.99 [0.98;1.00] | .079 |
| FVC, % of predicted | 81.2 (19.8) | 77.8 (21.1) | 0.99 [0.98;1.00] | .183 |
| 6-MWD, meters | 377 (104) | 334 (132) | 1.00 [0.99;1.00] | .010 |
| C-reactive protein, mg/L | 0.94 [0.59;1.72] | 1.00 [0.70;2.00] | 1.04 [0.84;1.28] | .725 |
| Polysomnographic parameters† | ||||
| TST, hours | 5.44 (1.41) | 5.24 (1.22) | 0.89 [0.74;1.08] | .248 |
| Sleep efficiency, % | 74.1 [61.8;86.1] | 72.3 [60.7;81.3] | 1.00 [0.98;1.01] | .739 |
| Non-REM 1 and 2, % | 78.0 [65.1;88.4] | 84.1 [70.3;91.6] | 1.02 [1.00;1.03] | .084 |
| On-REM 3, % | 10.2 [3.00;21.3] | 6.15 [0.23;16.9] | 0.98 [0.96;1.00] | .118 |
| REM sleep, % | 9.60 [4.22;14.7] | 8.60 [2.86;14.7] | 0.99 [0.96;1.01] | .317 |
| Arousal index | 36.0 [17.8;62.2] | 49.8 [26.0;75.4] | 1.01 [1.00;1.02] | .014 |
| AHI | 43.2 [17.6;75.5] | 51.8 [23.8;88.8] | 1.01 [1.00;1.01] | .052 |
| Severe OSA, %‡ | 80 (64.5%) | 87 (71.3%) | 1.36 [0.80;2.35] | .258 |
| ODI | 49.8 [21.4;78.2] | 57.8 [28.9;91.8] | 1.01 [1.00;1.02] | .029 |
| Mean SpO2 | 87.0 [82.8;90.0] | 85.0 [82.0;89.0] | 0.96 [0.92;1.01] | .097 |
| TST with SpO2 < 90%, % | 75.0 [44.5;94.8] | 77.0 [49.9;95.6] | 1.00 [1.00;1.01] | .374 |
| Oxygen therapy¶ | 27 (21.8%) | 37 (30.3%) | 1.56 [0.88;2.80] | .130 |
| Echocardiogram parameters | ||||
| TEI | 0.36 [0.26;0.49] | 0.39 [0.28;0.53] | 4.43 [1.03;19.1] | .046 |
| E/A ratio | 0.85 [0.71;1.04] | 0.88 [0.72;1.09] | 1.01 [0.44;2.33] | .975 |
| Deceleration time, ms | 232 [196;261] | 222 [190;265] | 1.00 [1.00;1.00] | .623 |
| Left atrial diameter, mm | 41.0 [36.0;46.0] | 43.0 [39.0;47.0] | 1.02 [0.99;1.04] | .245 |
| LVTDD, mm | 48.9 [43.1;52.0] | 48.6 [44.0;53.0] | 1.02 [0.98;1.06] | .255 |
| LVTSD, mm | 30.4 [27.0;34.0] | 31.0 [27.0;35.0] | 1.02 [0.98;1.05] | .405 |
| LVEF, % | 64.0 [60.0;70.0] | 64.0 [59.0;69.0] | 0.98 [0.96;1.01] | .240 |
| Left ventricular mass index, g/m3 | 109 [85.8;123] | 109 [93.7;127] | 1.00 [1.00;1.01] | .293 |
Data are presented as n (%), median (25;75 IQR), or mean (SD). †Polysomnography was performed in baseline conditions without CPAP/NIV or oxygen therapy in-place. ‡Severe OSA means an AHI ≥ 30 events/h. ¶Oxygen therapy was prescribed during the baseline visit. AHI = apnea-hypopnea index, BMI = body mass index, BP = blood pressure, CI = confidence interval, CPAP = continuous positive airway pressure, E/A= E and A waves, ESS = Epworth Sleepiness Scale, FEV1 = forced expiratory volume in the first second, FVC = forced vital capacity, IQR = interquartile range, LVEF = left ventricular ejection fraction, LVTDD = left ventricular telediastolic diameter, LVTSD = left ventricular telesystolic diameter, NIV = noninvasive ventilation, ODI = 3% oxygen desaturation index, OSA = obstructive sleep apnea, PaCO2 = Partial pressure of carbon dioxide in the arterial blood, PaO2 = pressure of oxygen in arterial blood, PH = pulmonary hypertension (systolic pulmonary artery pressure ≥ 40 mm Hg), REM = rapid eye movement, SD = standard deviation, SpO2 = oxygen saturation by pulse oximetry, TEI = right ventricle index of myocardial performance, TST = total sleep time, VAWS = visual analog well-being scale; 6-MWD = 6-minute walk distance.
Table 2 shows the potentially relevant risk factors from pathogenic age-adjusted and multivariate model.
Table 2.
Association between pathogenic mechanisms and pulmonary hypertension.
| Age-Adjusted Model | Multivariate Model* | |||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Obesity | ||||
| BMI, kg/m2 | 1.08 (1.04;1.13) | <.001 | 1.07 (1.03;1.12) | .001 |
| FEV1, % of predicted | 0.98 (0.97;0.99) | .013 | ||
| Sustained hypoxemia | ||||
| PaO2, mm Hg | 0.95 (0.92;0.98) | .001 | 0.96 (0.93;0.98) | .003 |
| Mean SpO2 during sleep | ||||
| First tertile | Reference | |||
| Second tertile | 0.67 (0.35;1.26) | .214 | ||
| Third tertile | 0.7 (0.38;1.31) | .269 | ||
| OSA | ||||
| Arousal index† | 1.18 (1.03;1.36)‡ | .017 | ||
| Oxygen desaturation index† | 1.13 (1.01;1.27)‡ | .049 | ||
| Apnea-hypopnea index† | 1.09 (0.98;1.21)‡ | .1 | ||
| Non-REM 1 and 2, % | 1.02 (1.00;1.03) | .079 | ||
| Hypoventilation | ||||
| PaCO2, mm Hg | ||||
| First tertile | Reference | |||
| Second tertile | 1.23 (0.66;2.30) | .512 | ||
| Third tertile | 2.2 (1.18;4.12) | .013 | ||
*Selection variables using Least Absolute Shrinkage and Selection Operator (LASSO). †Log-transformed variable was used. ‡OR for a 50% increase in variable. Mean SpO2 during sleep and PaCO2 were categorized by tertiles due to their nonlinear relationship with the presence of pulmonary hypertension (see Figure S3 (1.1MB, pdf) ). BMI = body mass index, CI = confidence interval, FEV1 = forced expiratory volume in the first second, OR = odd ratio, OSA = obstructive sleep apnea, PaCO2 = Partial pressure of carbon dioxide in the arterial blood, PaO2 = pressure of oxygen in arterial blood, REM = rapid eye movement, SpO2 = oxygen saturation by pulse oximetry.
Obesity model
BMI showed a significant positive linear association and forced expiratory volume in the first second showed a significant negative linear association with the presence of PH after adjusting for age (Table 2 and Figure S2 (1.1MB, pdf) in the supplemental material).
Arterial blood hypoxemia model
PaO2 showed a significant negative linear association with the presence of PH after adjusting for age (Table 2 and Figure S2 (1.1MB, pdf) ).
OSA model
Arousal index and ODI showed a significant positive linear association with the presence of PH after adjusting for age (Table 2 and Figure S2 (1.1MB, pdf) ).
Hypoventilation model
PaCO2 showed a negative nonlinear relationship with the presence of PH (Figure S2 (1.1MB, pdf) ). After categorizing PaCO2 by tertiles, the third tertile showed a significantly higher relationship with PH than the first tertile (Table 2).
Multivariate model
Variable selection process, based on LASSO regression, selected BMI with an adjusted odds ratio of 1.07 (95% confidence interval [CI] 1.03–1.12; P = .001) and PaO2 with an adjusted odds ratio of 0.96 (95% CI 0.93–0.98; P = .003) as independent risk factors for PH (Table 2 and Figure S3 (1.1MB, pdf) in the supplemental material).
Subgroups analysis for OHS with and without severe OSA phenotypes
Of the 246 patients available for the analysis, 167 (67.9%) had severe OSA (Figure 1). Of the 167 with severe OSA, 87 (52.1%) had PH. Of the 79 with nonsevere OSA, 35 (44.3%) had PH. In the severe OSA subgroup, the median pulmonary artery systolic pressure was 32.0 mm Hg (interquartile range = 26.0–35.1) in the group without PH and 47.0 mm Hg (43.6–57.0) in the PH group. In the nonsevere OSA subgroup, the pulmonary artery systolic pressure was 35.5 mm Hg (31.8–37.0) in the group without PH and 43.4 mm Hg (41.1–51.5) in the PH group.
Table S1 (1.1MB, pdf) and Table S2 (1.1MB, pdf) in the supplemental material summarize the baseline characteristics, polysomnography, and echocardiographic parameters for each OSA phenotype with bivariate analysis between those with and without PH. In the severe OSA subgroup, patients with PH were more obese, with worse daytime oxygenation, hypoventilation, exercise tolerance, OSA severity, and better left ventricular diastolic function. In the nonsevere OSA subgroup, patients with PH were more obese, with a higher prevalence of diabetes mellitus, with worse quality of life, exercise tolerance, and worse left ventricular diastolic function.
Table S3 (1.1MB, pdf) and Table S4 (1.1MB, pdf) in the supplemental material summarize the potential risk factors and the adjusted models in the severe and nonsevere OSA subgroups. BMI, PaCO2, and PaO2 were potential independent risk factors for PH in the severe OSA subgroup in the age-adjusted model. However, in the multivariate model, only BMI and PaO2 were independent risk factors with an adjusted odds ratio of 1.07 (95% CI 1.02–1.12; P = .008) for BMI and 0.95 (95% CI 0.92–0.99; P = .024) for PaO2 (Table S3 (1.1MB, pdf) and Figure S4 (1.1MB, pdf) in the supplemental material). E/A ratio, presence of diabetes mellitus, and BMI were potential independent risk factors for PH in the nonsevere OSA subgroup in the age-adjusted model. However, in the multivariate model, only BMI and E/A ratio were independent risk factors with an adjusted odds ratio of 1.09 (95% CI 1.01–1.19; P = .008) for BMI and 0.05 (95% CI 0.00–0.59; P = .017) for E/A ratio (Table S4 (1.1MB, pdf) and Figure S5 (1.1MB, pdf) in the supplemental material).
DISCUSSION
This is the first cross-sectional study evaluating potential pathophysiological risk factors for PH in a large sample of patients with untreated OHS. The patients with OHS enrolled in the study were ambulatory and had stable chronic hypercapnic respiratory failure. The results demonstrate that obesity per se is the most important pathogenic mechanism implicated in the pathogenesis of PH in patients with OHS being present in the entire cohort, as well as in both phenotypes of OHS, those with and without severe OSA. Interestingly, however, in the severe OSA phenotype, arterial hypoxemia and in the nonsevere OSA phenotype, left ventricular diastolic dysfunction were also additional independent risk factors associated with PH.
Pathophysiologically, obesity can lead to PH by a variety of mechanisms including its association with OSA, altered blood gas chemistry, and left ventricular diastolic dysfunction, particularly in heart failure with preserved ejection fraction causing postcapillary PH.40 An important mechanistic question is how obesity per se could be linked to PH. We found that elevated BMI is associated with PH in both phenotypes of OHS, those with or without severe OSA. In this context, a previous study of a large cohort of otherwise normal participants showed a linear correlation between increasing BMI and pulmonary artery pressure.41 The authors proposed that the link is related to increased cardiac output in obesity. However, there are other obesity-related pathophysiological factors potentially associated with PH, independent of the aforementioned mechanisms. First, there is a U-shaped relationship between lung volume and pulmonary vascular resistance, which is minimal at functional residual capacity, but pulmonary vascular resistance increases as lung volume deviates from functional residual capacity. Notably, with extreme obesity, as expiratory reserve volume decreases and resting lung volume moves toward residual volume, pulmonary vascular resistance increases, as lower lung volumes progressively decrease the caliber of the pulmonary vessels (specifically, the so called extra alveolar vessels).42 Notably, vascular resistance is inversely proportional to the radius of a vessel to the power of 4, indicating that small reduction in vascular radius increases the vascular resistance considerably. The reduction in vascular lung volume by obesity is similar to its effect on reducing the caliber of the airways increasing lower and upper airways resistance, which leads to reduction in alveolar ventilation and at the same time increases the work of breathing.43
In addition to reduction in lung volume leading to a decrease in pulmonary vascular volume and increasing airway resistance, evidence from animal and human studies indicate that with obesity there is accumulation of fat in the perivascular tissue, mechanically decreasing the size of the vascular bed on the one hand, and also creating an inflammatory cascade (endothelial dysfunction) further contributing to reduction in vascular bed, increasing pulmonary vascular resistance, and eventually leading to the remodeling of pulmonary circulation and sustained PH. The mechanisms implicated in obesity-induced microvascular disease and perivascular adipose tissue accumulation are multiple and complex.44,45 Specifically, adipocytes secrete inflammatory molecules and impair healthy endothelial function with diminished generation and release of nitric oxide, which acts as a vasodilator and an anti-inflammatory molecule. Furthermore, release of tumor necrosis factor-α by perivascular adipose tissue in small arteries increases vascular endothelin-1 and endothelin-1 A receptor expression further contributing to PH.46
Importantly, bariatric surgery reverses the perivascular adipose tissue-mediated vascular procontractile effect in patients with obesity to the vascular anticontractile effect in lean patients by reducing local adipose tissue inflammation and oxidative stress.44 Finally, as noted earlier, obesity is associated with an increased cardiac output,47 which may further contribute to PH in the face of noncompliant vascular bed associated with extreme obesity. The implication of these findings is that treatment of obesity and weight loss, by itself, should play a significant role in improving PH. It goes without saying, however, that other benefits of weight loss, such as improving OSA, lowering blood pressure, and reduction in CO2 production, which lowers PaCO2 and reciprocally increases PaO2,48 could also play additional role in improving PH.
We also found that low PaO2 is another risk factor potentially involved in the pathogenesis of PH. Patients with OHS and severe OSA experience pronounced intermittent hypoxemia while asleep and sustained hypoxemia while awake. It is well known that alveolar hypoxia causes pulmonary vasoconstriction (hypoxic pulmonary vasoconstriction), presumably via smooth muscle cell oxygen-sensitive voltage-dependent potassium channels.49 In this context, we found that daytime arterial hypoxemia was significantly more severe in the OHS phenotype with severe OSA than in nonsevere OSA phenotype, whereas time below saturation of 90% during sleep was not (Table S1 (1.1MB, pdf) and Table S2 (1.1MB, pdf) ). This suggests that daytime hypoxemia plays a more significant role in the pathogenesis of PH. On the other hand, it is notable that parameters related to OSA severity, such as AHI, ODI, and arousal index did not remain significant in the severe OSA model (Table S3 (1.1MB, pdf) ). One potential explanation of the lack of association between OSA severity and PH in the severe OSA subgroup may be related to a “ceiling effect”, since the AHI is above ≥ 30 events/h in all patients in this subgroup.
Interestingly, we found that in OHS phenotype without severe OSA, left ventricular diastolic dysfunction causing postcapillary PH became a risk factor. This suggests that in the absence of arterial hypoxemia, the contribution of left ventricular diastolic dysfunction and presumably elevated left ventricular diastolic pressure becomes unmasked; this likely is caused by obesity itself,50 as there were no significant differences in prevalence of ischemic heart disease and heart failure between the 2 groups (Table S2 (1.1MB, pdf) ).
The results of this study are consistent with those of Thayer et al.51 Their study included a cohort of 5,038 patients referred for echocardiography and 1,043 who underwent right heart catheterization. Similar to our study, they defined pulmonary hypertension as echocardiographic evidence of pulmonary artery systolic pressure of ≥ 40 mm Hg. BMI was positively associated with pulmonary arterial pressures measured by echocardiography and by right heart catheterization. However, BMI was not associated with hemodynamic evidence of pulmonary vascular remodeling (increased transpulmonary pressure). However, the lack of pulmonary vascular remodeling may not be applicable to our patients with OHS who have a much higher BMI. Only 36.7% of their patients had obesity (BMI ≥ 30 kg/m2) and the median BMI was below 30 kg/m2. Moreover, sleep studies were not performed systematically to assess for sleep-disordered breathing. In contrast, the average BMI of our cohort without PH was 39 kg/m2 and those with PH was 43.6 kg/m2. Our findings are in line with a meta-analysis that assessed the effect of weight loss on pulmonary artery hemodynamics, with an average decrease in mean pulmonary artery pressure of 5 mm Hg after a mean weight loss of 43 kg.52 Of note, the largest effect of weight loss was observed in patients with OHS undergoing bariatric surgery, with an average reduction in the mean pulmonary artery pressure of 13 mm Hg.20
In the severe OSA phenotype, there was a statistical trend between better E/A ratio (ie, less diastolic dysfunction) and PH in bivariate analysis (Table S2 (1.1MB, pdf) ). Although intriguing and in the opposite direction of what would have been expected, this association became even less significant in the multivariate analysis, in part because the result of the bivariate analysis may have been influenced by having multiple comparisons. We speculate that in severe OSA, other mechanisms such as repeated negative intrathoracic pressure swings, hypercapnia, and mainly hypoxemia have a larger impact on pulmonary hemodynamics than diastolic dysfunction.
Our study has several limitations. Although 73 patients were excluded from the original cohort due to missing echocardiographic data, our cohort remains the largest thus far examining factors associated with PH in this patient population. We recognize the inherent limitations of echocardiography for accurate diagnosis of PH, but as noted our results are similar to those of Thayer et al51 who used both echocardiography and invasive hemodynamic monitoring by right heart catheterization. Of course, echocardiography is a practical and readily accessible noninvasive tool used regularly in clinical practice. In the Pickwick study, echocardiograms were performed at baseline and annually over 3 years.18 As such, performing multiple right heart catheterizations in such a large cohort of patients would have not been pragmatic. We did not use transcutaneous CO2 monitoring as a direct measure of hypoventilation during baseline polysomnography3; however, participants had established diagnosis of OHS and had chronic stable hypercapnia, as evidenced by arterial blood gas measurements and elevated bicarbonate concentration. Some patients included in the study had been on home oxygen therapy at baseline. Although there was no significant difference in the prevalence of home oxygen therapy in patients with and without PH, we do not know if these patients were using their supplemental oxygen therapy at home or for how long they were being treated with oxygen therapy. As such, we cannot accurately assess the effect of home oxygen supplementation on pulmonary hemodynamics. Importantly, oxygen treatment was not associated with PH in the bivariate analysis (Table 1). Lastly, the cross-sectional nature of the study does not address the direction of causality.
In conclusion, increasing levels of obesity and sustained awake hypoxemia measured by PaO2 are independent risk factors for PH in patients with untreated OHS. Obesity is a shared risk factor for both OHS phenotypes, with or without severe OSA, suggesting weight loss may play a critical role in improving PH, based on a number of unique pathophysiological mechanisms. Other risk factors vary between OHS phenotypes. In patients with concomitant severe OSA, high degree of sustained hypoxemia remains a significant risk factor, while in patients without severe OSA, left ventricular diastolic dysfunction (postcapillary mechanism) emerges as an important risk factor.
DISCLOSURE STATEMENT
All authors have seen and approved this manuscript. This study was funded by Instituto de Salud Carlos III (Fondo de Investigaciones Sanitarias, Ministerio de Sanidad y Consumo) PI050402, Spanish Respiratory Foundation 2005 (FEPAR), and Air Liquide Spain. The sponsors and funders of the study had no involvement or any influence in study design; in the collection, analysis, and interpretation of data; in the writing of the manuscript; and in the decision to submit the manuscript for publication. The corresponding author (J.F.M.) confirms that he had full access to all the data in the study and he had the final responsibility for the decision to submit for publication. The authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors are indebted to Vanessa Iglesias for her technical assistance. J.F.M. had full access to all data from the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Spanish Sleep Network: Isabel Utrabo, MD; Nicolás González-Mangado, MD, PhD; Maria A. Martinez-Martinez, MD; Elena Ojeda-Castillejo, MD; Daniel López-Padilla, MD; Santiago J. Carrizo, MD, PhD, Prof; Begoña Gallego, MD, PhD; Mercedes Pallero, MD; Odile Romero MD; Maria A. Ramón, PT, MSc; Eva Arias, MD; Jesús Muñoz-Méndez, MD, PhD; Cristina Senent, MD, PhD; Jose N. Sancho-Chust, MD, PhD; Nieves B. Navarro-Soriano, MD; Emilia Barrot, MD, PhD; José M. Benítez, MD; Jesús Sanchez-Gómez, MD; Rafael Golpe, MD, PhD; María A. Gómez-Mendieta, MD, PhD; Silvia Gomez, MD; Mónica Bengoa, MD.
Author contributions: Substantial contributions to study conception and design, acquisition of data, or analysis and interpretation of data: Juan F. Masa, MD, PhD; Babak Mokhlesi, MD, M.Sc; Shahrokh Javaheri, MD; M-Ángeles Sánchez-Quiroga, MD; Francisco Javier Gomez de Terreros, MD, PhD; Rocio Gallego, MD; Iván Benítez, BSc(Stat); Francisco-José Vázquez-Polo PhD; Miguel A. Negrín PhD; María Martel-Escobar PhD; Ferran Barbe, MD, PhD, Auxiliadora Romero, MD, Candela Caballero-Eraso MD, PhD, Mónica González, MD, PhD, Soledad López-Martín, MD; José M. Marin, MD, PhD; Odile Romero, MD; Trinidad Díaz-Cambriles, MD; Eusebi Chiner, MD, PhD; Carlos Egea, MD, PhD; Babak Mokhlesi, MD, M.Sc; Jaime Corral, MD; Galo Fernandez, MD; Isabel Utrabo, MD; Javier Barca, MD; Estrella Ordax, MD; Nicolás González-Mangado, MD, PhD; Maria F. Troncoso, MD, Maria-Ángeles Martinez-Martinez, MD; Elena Ojeda-Castillejo, MD; Daniel López Padilla, MD; Santiago J. Carrizo, MD, PhD; Begoña Gallego, MD, PhD; Mercedes Pallero, MD; Odile Romero, MD; Sergi Martí, MD, PhD; Maria Antonia Ramón, PT, M.Sc; Eva Arias, MD; Jesús Muñoz-Méndez, MD, PhD; Cristina Senent, MD; Jose N. Sancho-Chust, MD; Nieves Belén Navarro Soriano, MD; Emilia Barrot, MD, PhD; José M. Benítez, MD; Jesús Sanchez-Gómez, MD; Rafael Golpe, MD, PhD; Ana Santiago-Recuerda, MD, PhD; Silvia Gomez, MD; and Mónica Bengoa, MD. Drafting the article or revising the article critically for important intellectual content: Juan F. Masa, MD, PhD; Babak Mokhlesi, MD, M.Sc; Iván Benítez, BSc(Stat); Shahrokh Javaheri, MD; Francisco Javier Gomez de Terreros, MD, PhD; Maria Ángeles Sánchez-Quiroga, MD; Rocio Gallego, MD; Auxiliadora Romero, MD; Candela Caballero-Eraso, MD, PhD; Estrella Ordax-Carbajo, MD, PhD; Maria F. Troncoso, MD; Mónica González, MD, PhD; Soledad López-Martín, MD; José M. Marin, MD, PhD; Sergi Martí, MD, PhD; Trinidad Díaz-Cambriles, MD; Eusebi Chiner, MD, PhD; Carlos Egea, MD, PhD; Francisco-José Vázquez-Polo. PhD; Miguel A. Negrín, PhD; María Martel-Escobar, PhD; Ferran Barbe, MD, PhD; and Jaime Corral, MD. Approval of the version to be published: Juan F. Masa, MD, PhD; Babak Mokhlesi, MD, M.Sc.; Ivan Benitez, BSc (Stat); Shahrokh Javaheri, MD; Francisco-José Vázquez-Polo, PhD; Miguel A. Negrín PhD; and Jaime Corral, MD. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: Juan F. Masa, MD, PhD.
Data sharing statement: Additional related documents such as study protocol, statistical analysis plan, and informed consent form will be available upon request from the Pickwick Project principal investigator (Dr. Juan Fernando Masa). Deidentified patient data can be requested by researchers for use in independent scientific research and will be provided following review and approval of the research proposal (including statistical analysis plan) and completion of a data sharing agreement with the Pickwick Project Publications Committee. Investigator Data requests can be made anytime from 1 to 2 years after the publication of this trial. Requests should be sent to the corresponding author (Dr. Juan Fernando Masa, fmasa@separ.es).
ABBREVIATIONS
- AHI
apnea-hypopnea index
- BMI
body mass index
- CI
confidence interval
- E/A
ratio of early to peak atrial mitral valve in-flow velocities
- ODI
oxygen desaturation index
- OHS
obesity hypoventilation syndrome
- OSA
obstructive sleep apnea
- PaCO2
partial pressure of carbon dioxide in the arterial blood
- PaO2
partial pressure of oxygen in arterial blood
- PH
pulmonary hypertension
- 2D
2-dimensional
Contributor Information
on behalf of the Spanish Sleep Network:
Isabel Utrabo, Nicolás González-Mangado, Maria A. Martinez-Martinez, Elena Ojeda-Castillejo, Daniel López-Padilla, Santiago J. Carrizo, Begoña Gallego, Mercedes Pallero, Odile Romero, Maria A. Ramón, Eva Arias, Jesús Muñoz-Méndez, Cristina Senent, Jose N. Sancho-Chust, Nieves B. Navarro-Soriano, Emilia Barrot, José M. Benítez, Jesús Sanchez-Gómez, Rafael Golpe, María A. Gómez-Mendieta, Silvia Gomez, and Mónica Bengoa
REFERENCES
- 1. Mokhlesi B , Kryger MH , Grunstein RR . Assessment and management of patients with obesity hypoventilation syndrome . Proc Am Thorac Soc. 2008. ; 5 ( 2 ): 218 – 225 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kessler R , Chaouat A , Schinkewitch P , et al . The obesity-hypoventilation syndrome revisited: a prospective study of 34 consecutive cases . Chest. 2001. ; 120 ( 2 ): 369 – 376 . [DOI] [PubMed] [Google Scholar]
- 3. Masa JF , Corral J , Alonso ML , et al. ; Spanish Sleep Network . Efficacy of different treatment alternatives for obesity hypoventilation syndrome. Pickwick Study . Am J Respir Crit Care Med. 2015. ; 192 ( 1 ): 86 – 95 . [DOI] [PubMed] [Google Scholar]
- 4. Castro-Añón O , Pérez de Llano LA , De la Fuente Sánchez S , et al . Obesity-hypoventilation syndrome: increased risk of death over sleep apnea syndrome . PLoS One. 2015. ; 10 ( 2 ): e0117808 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Basoglu OK , Tasbakan MS . Comparison of clinical characteristics in patients with obesity hypoventilation syndrome and obese obstructive sleep apnea syndrome: a case-control study . Clin Respir J. 2014. ; 8 ( 2 ): 167 – 174 . [DOI] [PubMed] [Google Scholar]
- 6. Priou P , Hamel JF , Person C , et al . Long-term outcome of noninvasive positive pressure ventilation for obesity hypoventilation syndrome . Chest. 2010. ; 138 ( 1 ): 84 – 90 . [DOI] [PubMed] [Google Scholar]
- 7. Berg G , Delaive K , Manfreda J , Walld R , Kryger MH . The use of health-care resources in obesity-hypoventilation syndrome . Chest. 2001. ; 120 ( 2 ): 377 – 383 . [DOI] [PubMed] [Google Scholar]
- 8. Weitzenblum E , Kessler R , Chaouat A . [Alveolar hypoventilation in the obese: the obesity-hypoventilation syndrome] . Rev Pneumol Clin. 2002. ; 58 ( 2 ): 83 – 90 . [PubMed] [Google Scholar]
- 9. Weitzenblum E , Chaouat A , Kessler R , Oswald M , Apprill M. Krieger J . Daytime hypoventilation in obstructive sleep apnea syndrome . Sleep Med Rev. 1999. ; 3 ( 1 ): 79 – 93 . [DOI] [PubMed] [Google Scholar]
- 10. Rochester D . Obesity and Pulmonary Function . In: Alpert MA , Alexander JK , eds. The Heart and Lung in Obesity. Armonk, NY: : Futura Publishing Company, Inc; ; 1998. : 109 – 131 [Google Scholar]
- 11. Biring MS , Lewis MI , Liu JT , Mohsenifar Z . Pulmonary physiologic changes of morbid obesity . Am J Med Sci. 1999. ; 318 ( 5 ): 293 – 297 . [DOI] [PubMed] [Google Scholar]
- 12. Leech J , Onal E , Aronson R , Lopata M . Voluntary hyperventilation in obesity hypoventilation . Chest. 1991. ; 100 ( 5 ): 1334 – 1338 . [DOI] [PubMed] [Google Scholar]
- 13. Zwillich CW , Sutton FD , Pierson DJ , Greagh EM , Weil JV . Decreased hypoxic ventilatory drive in the obesity-hypoventilation syndrome . Am J Med. 1975. ; 59 ( 3 ): 343 – 348 . [DOI] [PubMed] [Google Scholar]
- 14. MacGregor MI , Block AJ , Ball WC Jr . Topics in clinical medicine: serious complications and sudden death in the Pickwickian syndrome . Johns Hopkins Med J. 1970. ; 126 ( 5 ): 279 – 295 . [PubMed] [Google Scholar]
- 15. Miller A , Granada M . In-hospital mortality in the Pickwickian syndrome . Am J Med. 1974. ; 56 ( 2 ): 144 – 150 . [DOI] [PubMed] [Google Scholar]
- 16. Jennum P , Kjellberg J . Health, social and economical consequences of sleep-disordered breathing: a controlled national study . Thorax. 2011. ; 66 ( 7 ): 560 – 566 . [DOI] [PubMed] [Google Scholar]
- 17. Masa JF , Corral J , Romero A , et al. ; Spanish Sleep Network . Protective cardiovascular effect of sleep apnea severity in obesity hypoventilation syndrome . Chest. 2016. ; 150 ( 1 ): 68 – 79 . [DOI] [PubMed] [Google Scholar]
- 18. Masa JF , Mokhlesi B , Benítez I , et al. ; Long-Term Pickwick Randomized Controlled Clinical Trial . Echocardiographic changes with positive airway pressure therapy in obesity hypoventilation syndrome . Am J Respir Crit Care Med. 2020. ; 201 ( 5 ): 586 – 597 . [DOI] [PubMed] [Google Scholar]
- 19. Castro-Añón O , Golpe R , Pérez-de-Llano LA , et al . Haemodynamic effects of non-invasive ventilation in patients with obesity-hypoventilation syndrome . Respirology. 2012. ; 17 ( 8 ): 1269 – 1274 . [DOI] [PubMed] [Google Scholar]
- 20. Sugerman HJ , Baron PL , Fairman RP , Evans CR , Vetrovec GW . Hemodynamic dysfunction in obesity hypoventilation syndrome and the effects of treatment with surgically induced weight loss . Ann Surg. 1988. ; 207 ( 5 ): 604 – 613 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kauppert CA , Dvorak I , Kollert F , et al . Pulmonary hypertension in obesity-hypoventilation syndrome . Respir Med. 2013. ; 107 ( 12 ): 2061 – 2070 . [DOI] [PubMed] [Google Scholar]
- 22. Alawami M , Mustafa A , Whyte K , Alkhater M , Bhikoo Z , Pemberton J . Echocardiographic and electrocardiographic findings in patients with obesity hypoventilation syndrome . Intern Med J. 2015. ; 45 ( 1 ): 68 – 73 . [DOI] [PubMed] [Google Scholar]
- 23. Minai OA , Pandya CM , Golish JA , et al . Predictors of nocturnal oxygen desaturation in pulmonary arterial hypertension . Chest. 2007. ; 131 ( 1 ): 109 – 117 . [DOI] [PubMed] [Google Scholar]
- 24. Lévy P , Pépin JL , Arnaud C , et al . Intermittent hypoxia and sleep-disordered breathing: current concepts and perspectives . Eur Respir J. 2008. ; 32 ( 4 ): 1082 – 1095 . [DOI] [PubMed] [Google Scholar]
- 25. Zamanian RT , Hansmann G , Snook S , et al . Insulin resistance in pulmonary arterial hypertension . Eur Respir J. 2009. ; 33 ( 2 ): 318 – 324 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Masa JF , Corral J , Caballero C , et al . Non-invasive ventilation in obesity hypoventilation syndrome without severe obstructive sleep apnea . Thorax. 2016. ; 71 : 899 – 906 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Masa JF , Mokhlesi B , Benítez I , et al. ; Spanish Sleep Network . Long-term clinical effectiveness of continuous positive airway pressure therapy versus non-invasive ventilation therapy in patients with obesity hypoventilation syndrome: a multicentre, open-label, randomised controlled trial . Lancet. 2019. ; 393 ( 10182 ): 1721 – 1732 . [DOI] [PubMed] [Google Scholar]
- 28. Corral J , Mogollon MV , Sánchez-Quiroga MÁ , et al. ; Spanish Sleep Network . Echocardiographic changes with non-invasive ventilation and CPAP in obesity hypoventilation syndrome . Thorax. 2018. ; 73 ( 4 ): 361 – 368 . [DOI] [PubMed] [Google Scholar]
- 29. López-Jiménez MJ , Masa JF , Corral J , et al. ; Grupo Cooperativo . Mid- and long-term efficacy of non-invasive ventilation in obesity hypoventilation syndrome: the Pickwick’s Study . Arch Bronconeumol. 2016. ; 52 ( 3 ): 158 – 165 . [DOI] [PubMed] [Google Scholar]
- 30. Masa JF , Corral J , Romero A , et al. ; Spanish Sleep Network . The effect of supplemental oxygen in obesity hypoventilation syndrome . J Clin Sleep Med. 2016. ; 12 ( 10 ): 1379 – 1388 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Masa JF , Mokhlesi B , Benítez I , et al. ; Spanish Sleep Network . Cost-effectiveness of positive airway pressure modalities in obesity hypoventilation syndrome with severe obstructive sleep apnoea . Thorax. 2020. ; 75 ( 6 ): 459 – 467 . [DOI] [PubMed] [Google Scholar]
- 32. Chobanian AV , Bakris GL , Black HR , et al. ; National High Blood Pressure Education Program Coordinating Committee . Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure . Hypertension. 2003. ; 42 ( 6 ): 1206 – 1252 . [DOI] [PubMed] [Google Scholar]
- 33. García-Río F , Calle M , Burgos F , et al. ; Spanish Society of Pulmonology and Thoracic Surgery (SEPAR) . Spirometry . Arch Bronconeumol. 2013. ; 49 ( 9 ): 388 – 401 . [DOI] [PubMed] [Google Scholar]
- 34. ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories . ATS statement: guidelines for the six-minute walk test . Am J Respir Crit Care Med. 2002. ; 166 ( 1 ): 111 – 117 . [DOI] [PubMed] [Google Scholar]
- 35. Masa JF , Jiménez A , Durán J , et al . Alternative methods of titrating continuous positive airway pressure: a large multicenter study . Am J Respir Crit Care Med. 2004. ; 170 ( 11 ): 1218 – 1224 . [DOI] [PubMed] [Google Scholar]
- 36. Masa JF , Jiménez A , Durán J , et al. ; Spanish Group of Breathing Sleep Disorders . Visual analogical well-being scale for sleep apnea patients: validity and responsiveness : a test for clinical practice . Sleep Breath. 2011. ; 15 ( 3 ): 549 – 559 . [DOI] [PubMed] [Google Scholar]
- 37. Lang RM , Badano LP , Mor-Avi V , et al . Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging . J Am Soc Echocardiogr. 2015. ; 28 ( 1 ): 1 – 39.e14 . [DOI] [PubMed] [Google Scholar]
- 38. Almeneessier AS , Nashwan SZ , Al-Shamiri MQ , Pandi-Perumal SR , BaHammam AS . The prevalence of pulmonary hypertension in patients with obesity hypoventilation syndrome: a prospective observational study . J Thorac Dis. 2017. ; 9 ( 3 ): 779 – 788 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hastie T , Tibshirani R , Tibshirani RJ . Extended comparisons of best subset selection, forward stepwise selection, and the lasso. arXiv. Preprint posted online July 27, 2017. doi: 1707.08692.
- 40. Campos-Rodriguez F , Martínez-García MA , Mohsenin V , Javaheri S . Systemic and Pulmonary Hypertension in Obstructive Sleep Apnea . In: Kryger MH , Roth T , Dement WC , eds. Principles and Practices of Sleep Medicine, 7/e. Philadelphia: : Elsevier; ; 2021. (In Press). [Google Scholar]
- 41. McQuillan BM , Picard MH , Leavitt M , Weyman AE . Clinical correlates and reference intervals for pulmonary artery systolic pressure among echocardiographically normal subjects . Circulation. 2001. ; 104 : 2797 – 2802 . [DOI] [PubMed] [Google Scholar]
- 42. Fischer LG , Aken HV , Bu Combining Diaeresis Rkle H . Management of pulmonary hypertension: physiological and pharmacological considerations for anesthesiologists . Anesth Analg. 2003. ; 96 ( 6 ): 1603 – 1616 . [DOI] [PubMed] [Google Scholar]
- 43. De Backer LA , Vos WG , Salgado R , et al . Functional imaging using computer methods to compare the effect of salbutamol and ipratropium bromide in patient-specific airway models of COPD . Int J Chron Obstruct Pulmon Dis. 2011. ; 6 : 637 – 646 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Aghamohammadzadeh R , Greenstein AS , Yadav R , et al . Effects of bariatric surgery on human small artery function: evidence for reduction in perivascular adipocyte inflammation, and the restoration of normal anticontractile activity despite persistent obesity . J Am Coll Cardiol. 2013. ; 62 ( 2 ): 128 – 135 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ayinapudi K , Singh T , Motwani A , Le Jemtel TH , Oparil S . Obesity and pulmonary hypertension . Curr Hypertens Rep. 2018. ; 20 ( 12 ): 99 . [DOI] [PubMed] [Google Scholar]
- 46. Virdis A , Duranti E , Rossi C , et al . Tumour necrosis factor-alpha participates on the endothelin-1/nitric oxide imbalance in small arteries from obese patients: role of perivascular adipose tissue . Eur Heart J. 2015. ; 36 ( 13 ): 784 – 794 . [DOI] [PubMed] [Google Scholar]
- 47. de Divitiis O , Fazio S , Petitto M , Maddalena G , Contaldo F , Mancini M . Obesity and cardiac function . Circulation. 1981. ; 64 ( 3 ): 477 – 482 . [DOI] [PubMed] [Google Scholar]
- 48. Javaheri S , Simbartl LA . Respiratory determinants of diurnal hypercapnia in obesity hypoventilation syndrome. What does weight have to do with it? Ann Am Thorac Soc. 2014. ; 11 ( 6 ): 945 – 950 . [DOI] [PubMed] [Google Scholar]
- 49. Naeije R , Brimioulle S . Physiology in medicine: importance of hypoxic pulmonary vasoconstriction in maintaining arterial oxygenation during acute respiratory failure . Crit Care. 2001. ; 5 ( 2 ): 67 – 71 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Alpert MA . Obesity cardiomyopathy: pathophysiology and evolution of the clinical syndrome . Am J Med Sci. 2001. ; 321 ( 4 ): 225 – 236 . [DOI] [PubMed] [Google Scholar]
- 51. Thayer TE , Levinson RT , Huang S , et al . BMI is causally associated with pulmonary artery pressure but not hemodynamic evidence of pulmonary vascular remodeling . Chest. 2021. ; 159 ( 1 ): 302 – 310 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Reddy YNV , Anantha-Narayanan M , Obokata M , et al . Hemodynamic effects of weight loss in obesity: A systematic review and meta-analysis . JACC Heart Fail. 2019. ; 7 ( 8 ): 678 – 687 . [DOI] [PMC free article] [PubMed] [Google Scholar]