Abstract
Study Objectives:
The main cause of death in patients with obesity hypoventilation syndrome (OHS) is cardiac rather than respiratory failure. Here, we investigated autonomic-respiratory coupling and serum cardiac biomarkers in patients with OHS and obstructive sleep apnea (OSA) with comparable body mass index and apnea-hypopnea index.
Methods:
Cardiopulmonary coupling (CPC) and cyclic variation of heart rate analysis was performed on the electrocardiogram signal from the overnight polysomnogram. Cardiac serum biomarkers were obtained in patients with OHS and OSA with a body mass index > 40 kg/m2. Samples were obtained at baseline and after 3 months of positive airway pressure (PAP) therapy in both groups.
Results:
Patients with OHS (n = 15) and OSA (n = 36) were recruited. No group differences in CPC, cyclic variation of heart rate, and serum biomarkers were observed at baseline and after 3 months of PAP therapy. An improvement in several CPC metrics, including the sleep apnea index, unstable sleep (low-frequency coupling and elevated low-frequency coupling narrow band), and cyclic variation of heart rate were observed in both groups with PAP use. However, distinct differences in response characteristics were noted. Elevated low-frequency coupling narrow band coupling correlated with highly sensitive troponin-T (P < .05) in the combined cohort. Baseline highly sensitive troponin-T inversely correlated with awake oxygen saturation in the OHS group (P < .05).
Conclusions:
PAP therapy can significantly improve CPC stability in patients with obesity with OSA or OHS, with key differences. Elevated low-frequency coupling narrow band may function as a surrogate biomarker for early subclinical cardiac disease. Low awake oxygen saturation could also increase this biomarker in OHS.
Clinical Trial Registration:
Registry: Australian New Zealand Clinical Trials Registry; Name: Obesity Hypoventilation Syndrome and Neurocognitive Dysfunction; URL: https://anzctr.org.au/Trial/Registration/TrialReview.aspx?id=367492; Identifier: ACTRN12615000122550.
Citation:
Sivam S, Wang D, Wong KKH, et al. Cardiopulmonary coupling and serum cardiac biomarkers in obesity hypoventilation syndrome and obstructive sleep apnea with morbid obesity. J Clin Sleep Med. 2022;18(4):1063–1071.
Keywords: obesity hypoventilation syndrome, obstructive sleep apnea, cardiovascular disease, biomarker, electrocardiogram
BRIEF SUMMARY
Current Knowledge/Study Rationale: The main cause of death in patients with obesity hypoventilation syndrome is cardiac rather than respiratory failure. While positive airway pressure therapy reduces the morbidity and mortality in obesity hypoventilation syndrome, an increase in mortality persists in this population even with treatment compared with patients with obstructive sleep apnea on positive airway pressure therapy.
Study Impact: In patients with obesity hypoventilation syndrome and obstructive sleep apnea with comparable severity, positive airway pressure improves electrocardiogram-derived cardiopulmonary coupling metrics of sleep stability. Elevated low-frequency coupling narrow band (a marker of unstable sleep) also correlates with a key marker of subclinical cardiac disease, highly sensitive troponin-T. Low awake oxygen saturation may also increase highly sensitive troponin in obesity hypoventilation syndrome.
INTRODUCTION
Obesity is associated with cardiometabolic disease, and approximately 70% of patients with obesity have concurrent obstructive sleep apnea (OSA).1 Obesity hypoventilation syndrome (OHS) is a less frequent, although clinically more severe condition, also associated with obesity. OHS is classically defined by the presence of awake hypercapnia (partial pressure of carbon dioxide > 45 mm Hg) in a patient with a body mass index (BMI) > 30 kg/m2 when other causes of awake hypercapnia have been excluded. The main cause of death in OHS is cardiac rather than respiratory failure.2 Cardiac failure in patients with OHS is more common than in patients with obesity with eucapnia.
While positive airway pressure (PAP) therapy reduces the morbidity and mortality in OHS, an increase in mortality persists in this population even with treatment compared with patients with OSA on PAP therapy.2,3 In this context, several studies have attempted to assess whether PAP therapy confers any benefit on markers of cardiac stress in OHS, including highly sensitive C-reactive protein, troponin T or I, and N-terminal brain natriuretic peptide (NT-proBNP). Overall, results have been variable, with some studies showing a benefit,4 while others have not.5–7 Prior studies have also explored PAP effects on the newer highly sensitive troponin (hs-troponin) in OSA8 but not in patients with OHS.9 Cardiopulmonary coupling (CPC) is another potentially useful biomarker of cardiac function that has been explored in OSA10–13 but not in OHS. The evaluation of CPC in OSA has used the polysomnogram electrocardiogram channel to explore sleep-based interactions between autonomic and respiratory oscillations and compare metrics of stable (high-frequency coupling [HFC]) and unstable sleep (low-frequency coupling [LFC]), and sleep fragmentation (elevated LFC broad and narrow bands [e-LFCBB and e-LFCNB]).
In this study, we compare electrophysiologic (CPC) and cardiac serum biomarkers, including hs-troponin, between hypercapnic patients with OHS and eucapnic OSA patients with equal obesity with a comparable apnea-hypopnea index (AHI), on night 1 of PAP therapy. We also assessed the impact of 3 months of PAP therapy on these cardiac parameters.
METHODS
This protocol has previously been reported in a study that assessed the effect OHS on neurocognitive function.14 Patients were recruited from consecutive referrals to the Sleep Centre at Royal Prince Alfred Hospital, a tertiary teaching hospital of the University of Sydney, from 2015 to 2017. Inclusion criteria were age between the 18 and 75 years, body mass index (BMI) > 40 kg/m2, and evidence of definite OSA with an AHI > 20 events/h or hypoventilation on their diagnostic sleep study. The diagnosis of sleep-disordered breathing and nocturnal hypoventilation was based on standard scoring criteria.15,16
Exclusion criteria included chronic obstructive lung disease (chronic obstructive pulmonary disease, forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) < 0.7), uncontrolled medical conditions, eg, cardiac failure, acute decompensated hypercapnic respiratory failure, or known renal failure. The study protocol was approved by the Human Research Ethics Committee of the Sydney Local Health District (X14-0385 and HREC/14/RPAH/510) at Royal Prince Alfred Hospital (Sydney, Australia). All participants gave written informed consent prior to study participation. This trial was registered with the Australian and New Zealand Clinical Trials Registry (ACTRN12615000122550).
Study design
Patients who met the inclusion and exclusion criteria based on their diagnostic sleep study were invited to participate in a 3-month treatment study. Patients underwent a CPAP and/or noninvasive ventilation titration study at baseline (visit 1). The latter was performed if CPAP failed to adequately control hypoventilation during the CPAP titration study. Titration resulted in all patients maintaining their oxygen saturation > 90% and AHI < 10 events/h. Transcutaneous carbon dioxide monitoring was performed during all sleep studies. A follow-up PAP review study was performed at 3 months (visit 2), confirming the results of the titration study. Polysomnography was performed as per routine hospital protocol.14 Patients were provided a loan CPAP (Remstar Auto; Philips Respironics, Murrysville, PA) or noninvasive ventilation (VPAP S9; ResMed, Bella Vista, Australia) machine for the study duration. Subjects did not change their medications throughout the duration of the study but were allowed to continue with weight loss efforts.
Fasting early morning blood tests were performed pre-PAP therapy and 3 months post-PAP therapy. A panel of biomarkers associated with inflammation or increased cardiovascular mortality including hs-troponin, NT-proBNP, highly sensitive C reactive protein, gamma-glutamyl transferase, alkaline phosphatase, leptin, and insulin-like growth factor 1 were performed.9,17–24
Cardiopulmonary coupling and cyclic variation of heart rate analyses
CPC analysis of the electrocardiogram (ECG) signal was performed as previously described.10,11 The ECG signal from the initial PAP titration study and the follow-up 3-month PAP review study was used. Briefly, the method uses a single-channel ECG to extract heart rate variability and ECG-derived respiration (amplitude variations in the QRS complex due to shifts in the cardiac electrical axis relative to the electrodes during respiration and changes in thoracic impedance as the lungs fill and empty). Several distinct patterns of CPC are observed: HFC (0.1–0.5 Hz), LFC (0.01 to less than 0.1 Hz), and e-LFC. HFC is associated with stable breathing during non-rapid eye movement sleep; e-LFC detects apneas and hypopnoeas.11,25 The final component, very-low frequency coupling (v-LFC, 0 to less than 0.01Hz) has two subsets, wake and rapid eye movement sleep.11 Sleep-breathing phenotypes can be determined with CPC in the e-LFC band; narrow spectral band e-LFCNB detects central sleep apnea, periodic breathing, or complex sleep apnea. The sleep quality index is an automatically calculated proprietary and trademarked summary index incorporating measures of sleep stability, fragmentation, and periodic breathing, and is displayed as a number between 0 and 100. The sleep apnea indicator based on analysis of cyclic variation of heart rate (CVHR) correlates with AHI.13
Statistical methods
Analysis of patient demographics and characteristics were performed in the OSA and OHS cohorts. Blood test, CPC, and CVHR results were compared on the initial night of PAP therapy (visit 1) and 3 months post (visit 2)-PAP therapy in both cohorts using mixed model analysis. Categorical variables were analyzed using chi-squared or Fisher’s exact test. Correlations were tested by either Pearson’s or Spearman’s tests. Statistical significance was set at P < .05 (2-tailed). Data were analyzed with the statistical software package STATA version 14 (StataCorp, College Station, TX). All serum cardiovascular biomarkers were measured at Royal Prince Alfred Hospital Biochemistry Services, an accredited Australian National Association of Testing Authorities pathology provider.
RESULTS
Subject characteristics
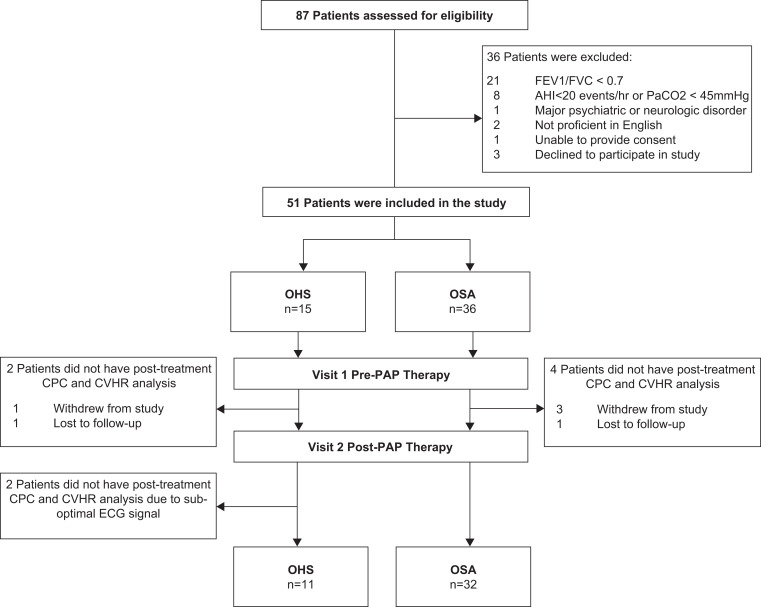
Fifteen patients with OHS and 36 patients with OSA were recruited (Table 1). Diagnostic sleep study results are also provided in Table 1. CPC data were available for 32 and 11 patients with OSA and OHS, respectively (Figure 1). All patients in the OSA group received CPAP and 6 of 15 patients in the OHS group received noninvasive ventilation (5 ST mode; 1 S mode) instead of CPAP. At baseline, there were no differences in patient characteristics except for worse awake and overnight oxygen parameters and daytime partial pressure of CO2 in the OHS group (Table 1). A comparison of AHI and minimum oxygen saturation across all sleep studies are shown in Table 2. Adherence with therapy was good, with the OHS and OSA groups using PAP for more than 4 hours per night on 73% and 80% of days, respectively.
Table 1.
Patient characteristics, comorbidities, sleep study parameters, and PAP adherence.
| OSA (n = 36) | OHS (n = 15) | P | |
|---|---|---|---|
| Demographics | |||
| Age | 48 ± 2.3 | 53 ± 3.2 | ns |
| Male, n (%) | 15 (42) | 4 (27) | ns |
| BMI (kg/m2) | 50 (13) | 58 (21) | ns |
| Ethnicity, n (%) | |||
| Caucasian | 23 (64) | 8 (53) | ns |
| Pacific Islander | 7 (19) | 4 (27) | ns |
| Aboriginal | 3 (8) | 1 (6) | ns |
| Epworth sleepiness score | 13 ± 6.0 | 14 ± 6.9 | ns |
| Never smoker, n (%) | 20 (55) | 4 (27) | ns |
| Pack-years | 0 (13) | 10 (20) | ns |
| Comorbidities, n (%) | |||
| Diabetes | 15 (42) | 9 (60) | ns |
| Hypertension | 21 (58) | 8 (53) | ns |
| Hypercholesterolemia | 5 (14) | 4 (27) | ns |
| Depression | 4 (11) | 4 (27) | ns |
| Stroke | 1 (0.03) | 0 (0) | ns |
| Coronary artery disease | 5 (0.14) | 1 (0.06) | ns |
| Medications, n (%) | |||
| ACE-inhibitor/ARB | 18 (50) | 6 (40) | ns |
| Beta-blocker | 6 (17) | 4 (27) | ns |
| Diuretic | 7 (19) | 1 (6) | ns |
| Aspirin or anticoagulant | 6 (17) | 6 (40) | ns |
| Cholesterol-lowering agent | 12 (33) | 6 (40) | ns |
| Oral hypoglycemic agent | 16 (44) | 6 (40) | ns |
| Diagnostic sleep study parameters | |||
| AHI (events/h) | 71 ± 31.2 | 78 ± 47.4 | ns |
| Minimum SpO2 (%) | 63 ± 18.8 | 48 ± 16.1 | < .01 |
| TST with SpO2 < 90% (min) | 140 ± 149.2 | 308 ± 139.6 | < .01 |
| TST with SpO2 < 80% (min) | 55 ± 19.9 | 112 ± 107.7 | ns |
| PAP compliance (minutes) | 317.9 ± 92.9 | 375.1 ± 102.1 | ns |
| % Days with > 4 hours use | 73 ± 26 | 80 ± 15.6 | ns |
| Daytime PaCO2 (mm Hg) | |||
| Visit 1 | 40 (7) | 49 (6) | < .01 |
| Visit 2 | 42 (4) | 43 (6) | ns |
| Daytime SpO2 (mm Hg) | |||
| Visit 1 | 96 (2) | 93 (5) | < .01 |
| Visit 2 | 96 (2) | 95 (1) | < .01 |
Data are presented as mean ± standard deviation or median (interquartile range) unless otherwise stated. ACE = angiotensin-converting enzyme, AHI = apnea-hypopnea index, ARB = angiotensin receptor blocker, BMI = body mass index, ns = not significant, OHS = obesity hypoventilation syndrome, OSA = obstructive sleep apnea, PaCO2 = partial pressure of carbon dioxide, PAP = positive airway pressure, SpO2 = oxygen saturation by pulse oximetry, TST = total sleep time.
Figure 1. Patient recruitment flow diagram.
AHI = apnea-hypopnea index, CPC = cardiopulmonary coupling, CVHR = cyclic variation of heart rate, ECG = electrocardiogram; FEV1/FVC = spirometric ratio, OHS = obesity hypoventilation syndrome, OSA = obstructive sleep apnea, PAP = positive airway pressure.
Table 2.
Diagnostic, PAP titration, and PAP review study AHI and minimum oxygen saturation.
| AHI (Events/h) | Min O2 (%) | |
|---|---|---|
| OSA | ||
| Diagnostic study | 71 ± 31.2 | 63 ± 18.8 |
| Titration study | 10 ± 13.6 | 81 ± 9.6* |
| PAP review study | 7 ± 14.6 | 88 ± 4.9* |
| OHS | ||
| Diagnostic study | 78 ± 47.4 | 48 ± 16.1 |
| Titration study | 19 ± 27.5 | 68 ± 13* |
| PAP review study | 4 ± 3.6 | 79 ± 10.5* |
Data are presented as mean ± standard deviation. *P < .05 comparing the PAP titration and PAP review studies. AHI = apnea-hypopnea index, Min O2 = minimum oxygen saturation during an overnight sleep study, OHS = obesity hypoventilation syndrome, OSA = obstructive sleep apnea, PAP = positive airway pressure.
CPC and CVHR: Baseline vs treatment
No differences in CPC and CVHR variables were observed at baseline (visit 1) and after 3 months of PAP therapy between the OSA and OHS groups. A reduction in the sleep apnea indicator, metrics of unstable sleep (LFC, e-LFCNB), and CVHR were observed in both groups with PAP use (Table 3). In the OSA group, an improvement in the sleep quality index and measures of stable sleep (HFC) were also seen after 3 months of PAP use.
Table 3.
Cardiopulmonary coupling and cyclic variation of heart rate of participants by group before and after 3 months of PAP therapy.
| OSA (n = 32) | OHS (n = 11) | Baseline OSA vs OHS Comparison (P) | Between Visit OSA vs OHS Comparison (P) | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Post-Treatment | P | Baseline | Post-Treatment | P | |||
| SQI | 37.1 (20.2) | 56.5 (16.9) | .004 | 30.5 (17.2) | 47.0 (17.7) | .06 | .37 | .77 |
| SAI (%) | 38.7 (26.5) | 15.2 (15.3) | .002 | 51.7 (32.4) | 25.3 (15.4) | .02 | .25 | .96 |
| HFC (%) | 28.3 (23.7) | 52.3 (22.0) | .001 | 20.6 (12.1) | 38.8 (23.0) | .06 | .41 | .56 |
| LFC (%) | 53.0 (27.4) | 26.7 (12.2) | .001 | 60.4 (19.8) | 40.2 (16.1) | .03 | .48 | .47 |
| vLFC (%) | 17.0 (11.6) | 17.6 (10.7) | .50 | 17.4 (10.4) | 19.1 (8.1) | .58 | .83 | .87 |
| e-LFCBB (%) | 24.7 (21.1) | 9.2 (6.4) | .001 | 20.2 (10.0) | 15.6 (11.4) | .50 | .56 | .18 |
| e-LFCNB (%) | 11.2 (14.4) | 1.6 (2.5) | .001 | 18.1 (23.9) | 1.8 (3.8) | .04 | .54 | .67 |
| Total eLFC (%) | 35.9 (31.4) | 10.8 (7.5) | .001 | 38.3 (24.0) | 17.4 (11.2) | .05 | .98 | .59 |
| LFC/HFC ratio | 7.1 (17.4) | 1.6 (4.3) | .15 | 5.6 (6.1) | 2.4 (3.0) | 1.00 | .05 | .15 |
| CVHR (%) | 45.1 (22.2) | 26.0 (17.2) | .02 | 57.6 (33.1) | 31.4 (13.5) | .01 | .24 | .67 |
Data are presented as mean ± standard deviation or median (interquartile range) unless otherwise stated. Bold values: P < .05. CVHR = cyclic variation of heart rate, eLFC = elevated low-frequency coupling, e-LFCBB = elevated low-frequency coupling broad band, e-LFCNB = elevated low-frequency coupling narrow band, OHS = obesity hypoventilation syndrome, OSA = obstructive sleep apnea, PAP = positive airway pressure, SAI = sleep apnea indicator, SQI = sleep quality index, HFC = high-frequency coupling, LFC = low-frequency coupling, vLFC = very-low-frequency coupling.
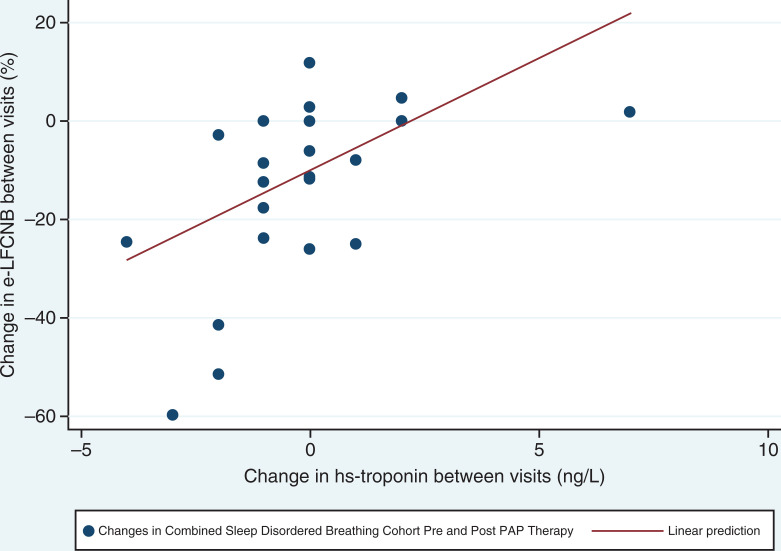
As there were no differences between patients with OSA and OHS at baseline and after 3 months of PAP therapy, correlations were performed for both CPC variables and CVHR as a combined cohort. The e-LFCNB cardiopulmonary coupling variable which is increased with unstable sleep correlated with hs-troponin-T (P < .05) in this combined sleep-disordered breathing cohort (Table 4, Figure 2). No other metrics of hypoxemia, carbon dioxide, or serum cardiovascular biomarkers demonstrated an association with CPC or CVHR.
Table 4.
CPC and CVHR variables correlation at baseline and after 3 months of PAP use for patients with sleep-disordered breathing.
| PaCO2 | AHI | Min O2 | ODI | T90 | AI | Awake SpO2 | hs-Troponin | |
|---|---|---|---|---|---|---|---|---|
| Baseline | ||||||||
| SQI | −0.09 | −0.68 | 0.195 | −0.09 | −0.19 | −0.01 | −0.11 | 0.15 |
| SAI | 0.15 | 0.64 | −0.12 | 0.01 | 0.23 | −0.17 | 0.001 | −0.04 |
| HFC | −0.19 | −0.73 | 0.26 | −0.18 | −0.26 | 0.26 | −0.001 | −0.04 |
| LFC | 0.08 | 0.69 | −0.23 | 0.15 | 0.24 | −0.01 | 0.11 | −0.16 |
| e-LFCNB | 0.06 | 0.53 | −0.20 | 0.16 | 0.02 | 0.05 | 0.09 | −0.18 |
| Total eLFC | 0.12 | 0.62 | −0.09 | −0.02 | 0.23 | −0.01 | 0.07 | −0.18 |
| CVHR | 0.23 | 0.62 | −0.09 | -0.02 | 0.2 | −0.17 | −0.11 | −0.01 |
| Change between visits | ||||||||
| SQI | −0.35 | 0.17 | −0.12 | 0.08 | 0.22 | −0.33 | −0.17 | |
| SAI | 0.56 | −0.33 | −0.06 | 0.14 | 0.01 | 0.21 | 0.28 | |
| HFC | −0.33 | 0.20 | 0.06 | 0.19 | 0.06 | −0.15 | 0.04 | |
| LFC | 0.21 | −0.04 | −0.25 | −0.12 | 0.02 | 0.07 | 0.29 | |
| e-LFCNB | 0.43 | −0.15 | −0.03 | −0.03 | 0.01 | 0.01 | 0.55 | |
| Total eLFC | 0.35 | −0.16 | −0.19 | −0.05 | 0.03 | 0.01 | 0.21 | |
| CVHR | 0.35 | −0.18 | −0.18 | 0.05 | 0.16 | −0.08 | 0.21 |
Bold values: P < .05. AHI = apnea-hypopnea index, AI = arousal index, CPC = cardiopulmonary coupling, CVHR = cyclic variation of heart rate, eLFC = elevated low-frequency coupling, e-LFCNB = elevated low-frequency coupling narrow band, HFC = high-frequency coupling, hs-troponin = highly sensitive troponin, LFC = low-frequency coupling, Min O2 = minimum oxygen saturation during an overnight sleep study, ODI = oxygen desaturation index, PaCO2 = partial pressure of carbon dioxide, PAP = positive airway pressure, SpO2 = oxygen saturation by pulse oximetry, SAI = sleep apnea indicator, SQI = sleep quality index, T90 = total time with oxygen saturation < 90% during an overnight sleep study.
Figure 2. Correlation between change in NB percent and change in hs-troponin between visits.
r = .56, P = .001 by Spearman’s rank correlation coefficient. E-LFCNB = elevated low-frequency coupling narrow band, hs-troponin = highly sensitive troponin, PAP = positive airway pressure.
Serum biomarkers: Baseline vs treatment
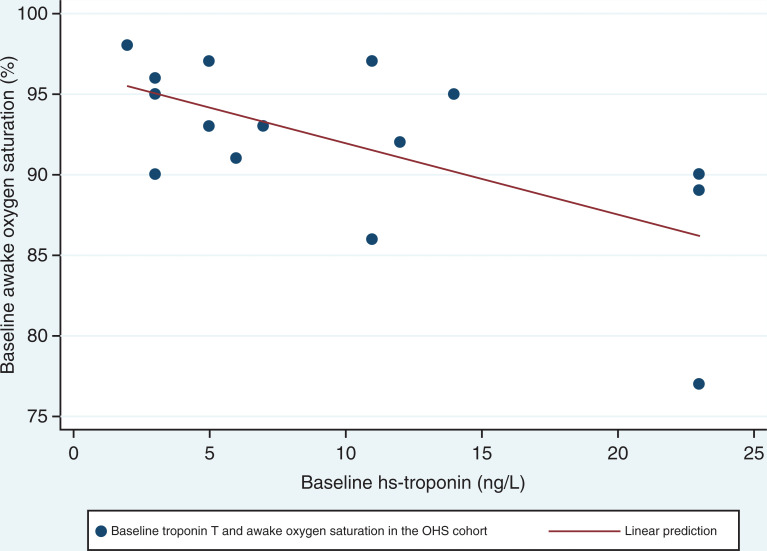
There was a reduction in hs-troponin-T in the OSA group with the use of PAP; however, the mean value remained in the normal range pre-PAP therapy and post-PAP therapy (P = .03). Only 15% of the sleep-disordered breathing cohort demonstrated hs-troponin-T levels above the normal range (> 14 ng/L). No differences in serum cardiovascular biomarkers (NT-proBNP, highly sensitive C reactive protein, gamma-glutamyl transferase, alkaline phosphatase, leptin, and insulin-like growth factor 1) were observed at baseline and post 3 months of PAP in either group. Gamma-glutamyl transferase baseline hs-troponin-T inversely correlated with awake oxygen saturation in the OHS group (r = −.69, P < .05; Figure 3).
Figure 3. Correlation between pre-PAP awake oxygen saturation and hs-troponin in OHS.
r = −.69, P = .004 by Spearman’s rank correlation coefficient. hs-troponin = highly sensitive troponin; OHS = obesity hypoventilation syndrome, PAP = positive airway pressure.
DISCUSSION
Our analysis had the following key results: 1) 3 months of PAP therapy improves ECG-derived cardiopulmonary coupling and heart rate variability metrics of sleep stability in both patients with OHS and those with OSA; 2) there were no between group differences in sleep fragmentation or quality indices at baseline and after 3 months post-PAP therapy between these groups; 3) serum biomarker hs-troponin-T correlated with e-LFCNB, suggesting that in this population with obesity with sleep-disordered breathing, this biomarker may be related to early subclinical cardiac disease; 4) low awake oxygen saturation demonstrated an inverse relationship with hs-troponin-T in the OHS group, suggesting that poor daytime oxygen saturation may be associated with early subclinical cardiac disease in patients with OHS, who had worse awake oxygen saturation compared to patients with OSA.
CPC-based analysis seems to be able to capture beneficial therapy effects in both OSA and OHS. Although the mean minimum oxygen saturation is lower in the PAP titration compared to the 3-month follow-up study, overall, it is improved when compared to the diagnostic sleep study. The mean AHI is comparable between the PAP titration and 3-month follow-up PAP review study. This suggests that the improvements observed over the 3-month period are likely secondary to use of PAP over a 3-month period rather than secondary to the immediate impact of PAP on alleviation of sleep-disordered breathing. Previous studies have shown similar CPC and CVHR improvements in OSA with CPAP and non-CPAP modalities of treatment.26,27 When retrospectively assessing CPC variables in 177 diagnostic and PAP titration polysomnography, HFC increased and LFC and e-LFC decreased only in the PAP titration group, reflecting reduced unstable sleep parameters associated with improving AHI with PAP therapy.26 Both HFC and LFC also improved with surgery for OSA or mandibular advancement splint usage in 98 patients, where a 50% reduction in AHI was achieved.27 More recent studies have also demonstrated a relationship between improvements in sleep quality index and blood pressure in patients with OSA at a high-cardiovascular risk.28 In a secondary analysis of 241 participants in the Heart Biomarker Evaluation in Apnea Treatment study, PAP therapy, rather than oxygen supplementation and/or sleep hygiene education, improved 24-hour (−3 mm Hg) and sleep (−5 mm Hg) mean arterial blood pressure in patients with a baseline sleep quality index < 55. Patients with OSA and those with OHS in our study had a baseline sleep quality index < 55. In addition, CPC variables correlate with AHI and OSA severity and worsen with aging and obesity.29–31 In a study comparing 29 patients with obesity referred for a sleep clinic assessment prior to bariatric surgery with 10 healthy weight control participants, alterations in CPC were most pronounced in the morbidly obese (BMI 40–49.9 kg/m2) and super obese (BMI > 50 kg/m2) groups compared with the severely obese group (BMI 35–39.9 kg/m2).30 In our study, both the OHS and OSA groups had comparable BMI and thus no differences in CPC variables between groups.
hs-Troponin is detectable in increased concentrations in over 70% of patients with severe OSA and is associated with an increased risk of heart failure and subclinical myocardial injury in this group of patients.32,33 In 1,645 male and female patients free of coronary heart disease and heart failure partaking in the Atherosclerosis Risk in the Communities and the Sleep Heart Health Studies, OSA severity was associated with increased hs-troponin-T in middle-aged and older individuals.33 Over a median of 12.4 years follow-up, hs-troponin-T was related to increased risk of death and incident heart failure in all OSA categories. No association was found with NT-proBNP in that study, even after adjustments for multiple confounders. In another cohort free of overt cardiovascular disease followed over a 10-year period, baseline hs-troponin-T even in the normal range (mean ≥ 7.42 ng/L) demonstrated a graded association with gadolinium enhanced cardiac magnetic resonance imaging related structural changes, which may precede symptoms of heart disease.34
In our study, e-LFCNB was associated with increased hs-troponin-T levels in the population with OSA and those with OHS, a marker of early subclinical cardiac disease. In a retrospective study involving automated ECG analysis of 5,247 polysomnograms from the Sleep Heart Health Study, this parameter was associated with greater sleep apnea severity, hypertension, and stroke risk.35 There was a 2% higher hypertension prevalence and 3% increased odds of prevalent stroke for every 1% increase of e-LFCNB across the total sleep time. CPC parameters, including e-LFCNB, have also previously been shown to be significantly worse in patients with severe OSA compared to those with nonsevere OSA.29 In their cohort of 221 male and female patients with a mean BMI of 26 kg/m2, a mean AHI of 55 events/h and minimum oxygen saturation of 71% compared with a mean AHI 14 events/h and minimum oxygen saturation of 82% was observed in the severe and nonsevere groups, respectively. While hs-troponin-T inversely correlated with awake oxygen saturation in the OHS group, this did not translate to a difference between the OSA and OHS groups in baseline or post-PAP therapy hs-troponin-T levels, despite patients with OHS having lower awake oxygen saturation compared to the intermittent sleep-related hypoxia in patients with OSA.
There are several limitations to our study. First, this was a post hoc analysis of a small clinical trial, and a lack of power may have diminished our ability to detect a difference in serum cardiac biomarkers, including hs-troponin-T. Second, we lacked a control group with normal respiratory parameters and a sham-treated PAP arm. Third, the severity of hypercapnia in the OHS cohort may not be adequate to detect a difference in ECG-derived and serum cardiac biomarkers between groups with otherwise similar BMIs and apnea severity. Our OHS group also had a severe degree of obstructive events. It is unclear if patients with pure hypoventilation without a severe AHI would demonstrate different biomarkers.
In summary, 3 months of PAP therapy can significantly improve autonomic-respiratory coupling parameters in patients with obesity with severe sleep-disordered breathing, regardless of hypercapnia. Sleep quality improvements are more prominent in nonhypercapnic patients. A CPC signal biomarker of high loop gain, e-LFCNB, which correlates with periodic breathing, may function as a surrogate biomarker for early subclinical cardiac disease, correlating with hs-troponin-T. Low awake oxygen saturation could also increase this serum biomarker in OHS. Longitudinal studies with larger sample sizes, however, are needed to confirm these findings.
DISCLOSURE STATEMENT
All authors have seen and approved this manuscript. Work for this study was performed at Royal Prince Alfred Hospital, Sydney, Australia. Cardiopulmonary coupling and cyclic variation of heart rate analysis was performed in the United States. This study was funded by a National Health and Medical Research Council Senior Principal Research Fellowship (1106974) to R.R.G., a National Health and Medical Research Council Leadership Fellowship (1139625) to C.L.P., a National Health and Medical Research Council Postgraduate Scholarship to S.S., and a top-up postgraduate scholarship from the Royal Australasian College of Physicians to S.S. The authors report no conflicts of interests.
ACKNOWLEDGMENTS
The authors thank the patients who participated in this study for their patience and enthusiasm.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- BMI
body mass index
- CPAP
continuous positive airway pressure
- CPC
cardiopulmonary coupling
- CVHR
cyclic variation of heart rate
- ECG
electrocardiogram
- HFC
high-frequency coupling
- hs-troponin
highly sensitive troponin
- LFC
low-frequency coupling
- NT-proBNP
N-terminal brain natriuretic peptide
- OHS
obesity hypoventilation syndrome
- OSA
obstructive sleep apnea
- PAP
positive airway pressure
REFERENCES
- 1. Lopez PP , Stefan B , Schulman CI , Byers PM . Prevalence of sleep apnea in morbidly obese patients who presented for weight loss surgery evaluation: more evidence for routine screening for obstructive sleep apnea before weight loss surgery . Am Surg. 2008. ; 74 ( 9 ): 834 – 838 . [PubMed] [Google Scholar]
- 2. Castro-Añón O , Pérez de Llano LA , De la Fuente Sánchez S , et al . Obesity-hypoventilation syndrome: increased risk of death over sleep apnea syndrome . PLoS One. 2015. ; 10 ( 2 ): e0117808 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kreivi HR , Itäluoma T , Bachour A . Effect of ventilation therapy on mortality rate among obesity hypoventilation syndrome and obstructive sleep apnoea patients . ERJ Open Res. 2020. ; 6 ( 2 ): 00101 – 2019 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Held M , Walthelm J , Baron S , Roth C , Jany B . Functional impact of pulmonary hypertension due to hypoventilation and changes under noninvasive ventilation . Eur Respir J. 2014. ; 43 ( 1 ): 156 – 165 . [DOI] [PubMed] [Google Scholar]
- 5. Borel JC , Tamisier R , Gonzalez-Bermejo J , et al . Noninvasive ventilation in mild obesity hypoventilation syndrome: a randomized controlled trial . Chest. 2012. ; 141 ( 3 ): 692 – 702 . [DOI] [PubMed] [Google Scholar]
- 6. Kauppert CA , Dvorak I , Kollert F , et al . Pulmonary hypertension in obesity-hypoventilation syndrome . Respir Med. 2013. ; 107 ( 12 ): 2061 – 2070 . [DOI] [PubMed] [Google Scholar]
- 7. Salord N , Mayos M , Miralda RM , et al . Continuous positive airway pressure in clinically stable patients with mild-to-moderate obesity hypoventilation syndrome and obstructive sleep apnoea . Respirology. 2013. ; 18 ( 7 ): 1135 – 1142 . [DOI] [PubMed] [Google Scholar]
- 8. Chang YS , Yee BJ , Hoyos CM , et al . The effects of continuous positive airway pressure therapy on Troponin-T and N-terminal pro B-type natriuretic peptide in patients with obstructive sleep apnoea: a randomised controlled trial . Sleep Med. 2017. ; 39 : 8 – 13 . [DOI] [PubMed] [Google Scholar]
- 9. Monneret D , Giral P , Bonnefont-Rousselot D , Roche F . Introducing high-sensitivity cardiac troponin t as a biomarker of OSA-related cardiovascular morbidity in obesity hypoventilation syndrome . Chest. 2016. ; 150 ( 6 ): 1408 – 1409 . [DOI] [PubMed] [Google Scholar]
- 10. Thomas RJ , Mietus JE , Peng CK , Goldberger AL . An electrocardiogram-based technique to assess cardiopulmonary coupling during sleep . Sleep. 2005. ; 28 ( 9 ): 1151 – 1161 . [DOI] [PubMed] [Google Scholar]
- 11. Thomas RJ , Wood C , Bianchi MT . Cardiopulmonary coupling spectrogram as an ambulatory clinical biomarker of sleep stability and quality in health, sleep apnea, and insomnia . Sleep. 2018. ; 41 ( 2 ): zsx196 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Magnusdottir S , Hilmisson H . Ambulatory screening tool for sleep apnea: analyzing a single-lead electrocardiogram signal (ECG) . Sleep Breath. 2018. ; 22 ( 2 ): 421 – 429 . [DOI] [PubMed] [Google Scholar]
- 13. Wu JG , Wang D , Rowsell L , et al . The effect of acute exposure to morphine on breathing variability and cardiopulmonary coupling in men with obstructive sleep apnea: A randomized controlled trial . J Sleep Res. 2020. ; 29 ( 2 ): e12930 . [DOI] [PubMed] [Google Scholar]
- 14. Sivam S , Poon J , Wong KKH , et al . Slow-frequency EEG activity during wake and sleep in obesity hypoventilation syndrome . Sleep. 2020. ; 43 ( 2 ): zsz214 . PMID: 31552426. [DOI] [PubMed] [Google Scholar]
- 15. Berry RB , Chediak A , Brown LK , et al. ; NPPV Titration Task Force of the American Academy of Sleep Medicine . Best clinical practices for the sleep center adjustment of noninvasive positive pressure ventilation (NPPV) in stable chronic alveolar hypoventilation syndromes . J Clin Sleep Med. 2010. ; 6 ( 5 ): 491 – 509 . [PMC free article] [PubMed] [Google Scholar]
- 16. Iber C , Ancoli-Israel S , Chesson A , Quan S ; for the American Academy of Sleep Medicine . The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1st ed . Westchester, IL: : American Academy of Sleep Medicine; ; 2007. . [Google Scholar]
- 17. Budweiser S , Luchner A , Jörres RA , et al . NT-proBNP in chronic hypercapnic respiratory failure: a marker of disease severity, treatment effect and prognosis . Respir Med. 2007. ; 101 ( 9 ): 2003 – 2010 . [DOI] [PubMed] [Google Scholar]
- 18. Castellano G , Affuso F , Conza PD , Fazio S . The GH/IGF-1 axis and heart failure . Curr Cardiol Rev. 2009. ; 5 ( 3 ): 203 – 215 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Borel JC , Roux-Lombard P , Tamisier R , et al . Endothelial dysfunction and specific inflammation in obesity hypoventilation syndrome . PLoS One. 2009. ; 4 ( 8 ): e6733 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Möhlenkamp S , Lehmann N , Moebus S , et al. ; Heinz Nixdorf Recall Study Investigators . Quantification of coronary atherosclerosis and inflammation to predict coronary events and all-cause mortality . J Am Coll Cardiol. 2011. ; 57 ( 13 ): 1455 – 1464 . [DOI] [PubMed] [Google Scholar]
- 21. Yousuf O , Mohanty BD , Martin SS , et al . High-sensitivity C-reactive protein and cardiovascular disease: a resolute belief or an elusive link? J Am Coll Cardiol. 2013. ; 62 ( 5 ): 397 – 408 . [DOI] [PubMed] [Google Scholar]
- 22. Aguirre GA , De Ita JR , de la Garza RG , Castilla-Cortazar I . Insulin-like growth factor-1 deficiency and metabolic syndrome . J Transl Med. 2016. ; 14 ( 1 ): 3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tonelli M , Curhan G , Pfeffer M , et al . Relation between alkaline phosphatase, serum phosphate, and all-cause or cardiovascular mortality . Circulation. 2009. ; 120 ( 18 ): 1784 – 1792 . [DOI] [PubMed] [Google Scholar]
- 24. Wannamethee G , Ebrahim S , Shaper AG . Gamma-glutamyltransferase: determinants and association with mortality from ischemic heart disease and all causes . Am J Epidemiol. 1995. ; 142 ( 7 ): 699 – 708 . [DOI] [PubMed] [Google Scholar]
- 25. Wang D , Piper AJ , Wong KK , et al . Slow wave sleep in patients with respiratory failure . Sleep Med. 2011. ; 12 ( 4 ): 378 – 383 . [DOI] [PubMed] [Google Scholar]
- 26. Cho JH , Kim HJ . The effect of continuous positive airway pressure on cardiopulmonary coupling . Sleep Breath. 2017. ; 21 ( 2 ): 341 – 345 . [DOI] [PubMed] [Google Scholar]
- 27. Lee WH , Hong SN , Kim HJ , et al . A comparison of different success definitions in non-continuous positive airway pressure treatment for obstructive sleep apnea using cardiopulmonary coupling . J Clin Sleep Med. 2016. ; 12 ( 1 ): 35 – 41 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Magnusdottir S , Hilmisson H , Thomas RJ . Cardiopulmonary coupling-derived sleep quality is associated with improvements in blood pressure in patients with obstructive sleep apnea at high-cardiovascular risk . J Hypertens. 2020. ; 38 ( 11 ): 2287 – 2294 . [DOI] [PubMed] [Google Scholar]
- 29. Seo MY , Hwang SJ , Nam KJ , Lee SH . Significance of sleep stability using cardiopulmonary coupling in sleep disordered breathing . Laryngoscope. 2020. ; 130 ( 8 ): 2069 – 2075 . [DOI] [PubMed] [Google Scholar]
- 30. Trimer R , Cabiddu R , Mendes RG , et al . Heart rate variability and cardio-respiratory coupling during sleep in patients prior to bariatric surgery . Obes Surg. 2014. ; 24 ( 3 ): 471 – 477 . [DOI] [PubMed] [Google Scholar]
- 31. Trimer R , Cabidu R , Sampaio LL , et al . Heart rate variability and cardiorespiratory coupling in obstructive sleep apnea: elderly compared with young . Sleep Med. 2014. ; 15 ( 11 ): 1324 – 1331 . [DOI] [PubMed] [Google Scholar]
- 32. Randby A , Namtvedt SK , Einvik G , et al . Obstructive sleep apnea is associated with increased high-sensitivity cardiac troponin T levels . Chest. 2012. ; 142 ( 3 ): 639 – 646 . [DOI] [PubMed] [Google Scholar]
- 33. Querejeta Roca G , Redline S , Punjabi N , et al . Sleep apnea is associated with subclinical myocardial injury in the community. The ARIC-SHHS study . Am J Respir Crit Care Med. 2013. ; 188 ( 12 ): 1460 – 1465 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Seliger SL , Hong SN , Christenson RH , et al . High-sensitive cardiac troponin T as an early biochemical signature for clinical and subclinical heart failure: MESA (Multi-Ethnic Study of Atherosclerosis) . Circulation. 2017. ; 135 ( 16 ): 1494 – 1505 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Thomas RJ , Weiss MD , Mietus JE , Peng CK , Goldberger AL , Gottlieb DJ . Prevalent hypertension and stroke in the Sleep Heart Health Study: association with an ECG-derived spectrographic marker of cardiopulmonary coupling . Sleep. 2009. ; 32 ( 7 ): 897 – 904 . [PMC free article] [PubMed] [Google Scholar]