Abstract
Study Objectives:
Children with overweight or obesity are more likely to experience sleep disorders, although the role of weight in pediatric insomnia treatment has not been examined. The current study examined the relationships of high body mass with pretreatment insomnia severity and global sleep problems and the potential moderating impact of weight on changes in insomnia severity following insomnia treatment.
Methods:
Participants included 1,133 youth ages 2–18 years clinically referred for insomnia treatment. The Pediatric Insomnia Severity Index was collected at the initial assessment and throughout treatment as part of routine clinical care. Treatment status was coded as no treatment, early termination, and completed treatment. Secondary measures of global sleep problems at the initial assessment included the Adolescent Sleep Wake Scale, Adolescent Sleep Hygiene Scale, and Children’s Sleep Habits Questionnaire. Medical chart review of visits within ± 3 months of baseline was used to obtain age-adjusted and sex-adjusted body mass index Z-score.
Results:
Among adolescents, regression analyses found that higher body mass index Z-score modestly predicted baseline insomnia severity (P = .021) and worse sleep hygiene (P < .001). For children, higher body mass index Z-score was modestly associated with baseline total sleep problems (P = .006) but not insomnia severity (P = .792). Across ages, body mass index Z-score predicted neither treatment status nor insomnia improvement (P > .05). Findings were similar in categorical analyses comparing patients with overweight/obesity to healthy weight.
Conclusions:
Although there is evidence that children of higher body mass present for insomnia treatment with greater sleep concerns, body mass does not predict treatment completion or insomnia improvement. Data suggest insomnia treatment is effective irrespective of weight status.
Citation:
Duraccio KM, Simmons DM, Beebe DW, Byars KC. Relationship of overweight and obesity to insomnia severity, sleep quality, and insomnia improvement in a clinically referred pediatric sample. J Clin Sleep Med. 2022;18(4):1083–1091.
Keywords: pediatric insomnia, weight, sleep quality, obesity
BRIEF SUMMARY
Current Knowledge/Study Rationale: Little is known regarding how weight is related to insomnia severity in youth, nor how weight impacts insomnia symptom improvement following evidence-based insomnia treatment.
Study Impact: This study is among the first to demonstrate that youth of a higher weight are more likely to experience more insomnia symptoms and decreased sleep quality. However, that effect was modest, and youth with overweight/obesity respond similarly to evidence-based insomnia treatment compared to youth of a healthy weight, suggesting that clinicians need not significantly modify insomnia treatment on the basis of weight alone.
INTRODUCTION
Pediatric sleep problems are common1 and have been linked to overweight and obesity (OV/OB) during childhood and adolescence.2–4 Youth reporting poorer sleep quality and more sleep disturbances have higher body mass index (BMI) than those who report better sleep quality.5 Furthermore, longitudinal studies have found that shorter sleep earlier in childhood is associated with greater risk of OV/OB in older youth6–8 and in adulthood,9 even after controlling for baseline weight status.7,8 Insomnia, which also affects over one-third of youth at some point across childhood, is similarly associated with poor health outcomes.10–12
The relationship between sleep and weight status is complex. Youth with OV/OB are more likely to demonstrate symptoms of sleep-disordered breathing, later sleep onset time, and shorter and more disrupted sleep than youth of average weight.13,14 Furthermore, children with OV/OB whose sleep was experimentally shortened across several nights experienced increased daily caloric consumption and weight, compared to when they were well rested.15 Findings suggest a possible vicious cycle, in which individuals with OV/OB have worse sleep, which may in turn contribute to weight gain. However, little is understood about how OV/OB is associated with sleep changes over time.
Insomnia is the most prevalent sleep disorder in the pediatric population.11,12 Insomnia is marked by the individual’s self-reported dissatisfaction with the quality and/or quantity of sleep, particularly regarding their ability to fall and stay asleep and how rested they feel after sleep.16 Pediatric insomnia is related to worse interpersonal, somatic, and psychological functioning.17 Behavioral interventions are considered first-line treatments for pediatric insomnia, as the evidence base is strong in younger children and children with neurodevelopmental disabilities18 and highly promising in adolescents19 and children with chronic medical conditions and psychiatric disorders.11 However, treatment effectiveness can vary, and factors that moderate effectiveness are not well understood. While it has been suggested that adults with OV/OB have greater insomnia symptoms than adults of average weight,20,21 that relationship has not been explored in youth. Furthermore, it is unclear whether youth with OV/OB differentially respond to behavioral intervention for insomnia. If weight relates to insomnia severity in children and adolescents or affects response to treatment, this would suggest that health care providers should consider weight, which is uniformly gathered during medical visits, when screening for insomnia in youth and when designing and implementing behavioral treatments for insomnia.
To begin to address this issue in a manner that is quickly generalizable to a clinical context, we conducted a retrospective analysis of a large dataset collected in the course of routine care at a pediatric behavioral sleep medicine clinic. This study had the following aims: 1) describe the prevalence of OV/OB in youth presenting for insomnia treatment in a behavioral sleep medicine clinic context, 2) examine how weight status is related to insomnia severity and sleep quality at baseline (pretreatment) evaluation, and 3) determine whether OV/OB moderates insomnia severity changes over the course of insomnia treatment. We hypothesized that youth with OV/OB would have worsened insomnia severity and sleep quality at baseline and that those with OV/OB would have less insomnia symptom improvement compared to youth of healthy weight following insomnia treatment.
METHODS
Participants and procedures
Participants included youth aged 2 to 18 years who were clinically referred to a behavioral sleep medicine clinic within a large pediatric hospital for comprehensive sleep evaluation and subsequent insomnia treatment. Participants included in this study were seen from June 2009 through April 2017, met diagnostic criteria for insomnia22,23 following a diagnostic interview completed by a licensed psychologist or a psychology resident/fellow under the direct supervision of a licensed psychologist during their baseline visit, and had height/weight data available within a 3-month window of such baseline evaluation. Presence of organic sleep disorders was determined via chart review. Patients were excluded from analysis if weight status data were not available within their medical record.
Upon referral to the sleep disorders center, caregivers completed a brief history and referral questionnaire. Patients were subsequently triaged to be seen by a licensed psychologist certified in behavioral sleep medicine in the Behavioral Sleep Medicine Clinic and a board-certified sleep physician in the sleep disorders center if there were concerns for a primary (organic) sleep disorder (eg, obstructive sleep apnea, restless legs syndrome, narcolepsy). Prior to being seen in the Behavioral Sleep Medicine Clinic, as a part of routine clinical care patients and caregivers completed a battery of pre-evaluation screening measures. Screening measures were used during the Behavioral Sleep Medicine Clinic evaluation alongside a sleep diary (when available) and comprehensive clinical interview to make International Classification of Sleep Disorders22,23 diagnosis and provide behavioral treatment recommendations. During this initial appointment, families were invited to have their baseline questionnaire and treatment outcome data archived for research purposes. Caregivers provided written informed consent, and adolescents provided assent for this Institutional Review Board-approved clinical archival database.
Measures
Background questionnaire
Caregivers completed a background history form that included information regarding child age, sex, race or ethnicity, household income, and relevant medical and mental health history.
Anthropometrics
Patient height to the nearest 0.1 cm and weight to the nearest 0.1 kg were obtained from medical chart review. Measurements obtained closest to the baseline visit (± 3 months) were used to compute body mass index Z-scores (BMIz) using age-corrected and sex-corrected norms from the US Centers for Disease Control and Prevention.
Primary sleep outcome: insomnia severity
The 6-item Pediatric Insomnia Severity Index (PISI),24,25 was completed by parents (children aged 2–10 years) or patients (11 years and older) at their initial evaluation as well as each follow-up intervention visit. Respondents answered questions regarding the youth’s prior week’s sleep, including problems initiating sleep, difficulty maintaining sleep, daytime sleepiness, and sleep duration. Items were summed and total scores ranged from 0 to 30 as an indicator of overall insomnia severity, with higher scores reflecting greater sleep problems. The PISI has adequate reliability and validity and is sensitive to treatment change in children 0 to 18 years of age.25 PISI factor scores reflecting sleep onset problems and sleep maintenance problems also have demonstrated reliability (Cronbach’s alpha = .81 and .62, respectively) and adequate convergent and discriminant validity in pediatric patients with insomnia.24 For our primary analyses, we used the PISI total score, 2 factor scores (ie, sleep onset problems, sleep maintenance problems), and the single daytime sleepiness item as our primary sleep outcomes. Within the current sample, the baseline PISI total score, sleep onset factor, and sleep maintenance factor all reflected adequate internal validity (Cronbach’s alpha = .705, .795, and .650, respectively).
Secondary sleep outcomes
The Children’s Sleep Habits Questionnaire (CSHQ)26 was used in secondary analysis as a measure of specific and global sleep concerns in children under the age of 11 years. The CSHQ is a 33-item parent report measure of sleep behavior and sleep disorder symptoms developed for use with children 4–10 years old. The CSHQ has demonstrated validity for use with toddler, preschool, and school-aged children and has been shown to differentiate clinical from control groups.26,27 Published psychometrics demonstrate adequate internal consistency and test–retest reliability for clinical and community comparison groups.26 Higher scores reflect greater sleep problems.26 Internal consistency of the CSHQ total score within this sample was .731.
Patients aged 11–18 years (adolescents) completed 2 secondary sleep outcome measures. The Adolescent Sleep Wake Scale (ASWS) is a 33-item self-report measure that assesses sleep quality in adolescents.28 Scores are yielded along 5 behavioral subscales as well as total sleep quality, each ranging from 1 to 6, with higher scores indicating better sleep quality. Psychometrics for the ASWS demonstrate adequate reliability for the subscales and full scale, as well as concurrent validity.28 Independent peer review of the ASWS supports its use as a measure of sleep quality.29 Internal consistency of the ASWS total score within this sample was .863. The revised version of the Adolescent Sleep Hygiene Scale (ASHS) is a 33-item self-report measure that assesses sleep facilitating and sleep-inhibiting practices.30 The measure yields 6 subscales: physiological, sleep environment, cognitive/emotional, sleep stability, daytime sleep, and behavioral arousal, each ranging from 1 to 6, with higher scores indicating better sleep hygiene. The ASHS has satisfactory psychometric properties with adequate to good internal consistency for subscales and good evidence of concurrent and convergent validity.30 Internal consistency of the ASHS total score within this sample was .806.
Insomnia treatment and treatment status (secondary sleep outcome)
Evidenced-based treatment provided in the context of the Behavioral Sleep Medicine Clinic has been described elsewhere.25 Briefly, during the baseline evaluation visit, subsequent to making insomnia diagnosis, behavioral sleep medicine intervention included: 1) psychoeducation about sleep, sleep health, and sleep hygiene; 2) provision of patient-specific rationale for insomnia treatment, 3) discussing the core components of evidence-based treatment for insomnia (eg, extinction-based interventions; stimulus control); and 4) integration of all treatment components in a written treatment plan with a specific plan for follow-up treatment session(s). Treatment Status was coded 1–3 for this study: 1) no treatment (never returned as recommended after baseline evaluation), 2) early termination (received treatment but did not attend a mutually agreed-upon treatment termination session), 3) treatment completion (treatment terminated after completion of such treatment termination session).
Data analysis
We utilized hierarchical linear regressions for our primary analyses examining the predictive association of BMIz with the baseline PISI scores for total insomnia severity, sleep onset problems factor, sleep maintenance problems factor, and daytime sleepiness, and the role of BMIz on change from preintervention to postintervention for total insomnia severity. Secondary analyses examined the associations of BMIz with baseline sleep problems in children (CSHQ), sleep quality (ASWS), and sleep hygiene in adolescents (ASHS) and treatment status in each age range. To determine whether the assumptions for linear regression were met, we confirmed that the relationship between BMIz and our primary outcomes were linear (examination of scatterplots), the standardized residuals of each regression were normally distributed (via P-P Plots and Shapiro-Wilk tests of normality), and the residuals were homoscedastic (scatterplots of the standardized residual against the standardized predicted value). To determine which covariates would be included on the first step of the models, we ran a series of bivariate regressions to examine whether sex, income, race, age, or the presence of a primary (organic) sleep disorder (eg, sleep apnea) significantly predicted baseline insomnia severity. Only income emerged as a significant predictor and, as such, was included in the first steps of all the regression models. BMIz was entered in the second step of each model. Because Treatment Status was an ordinal variable, associations with BMIz were assessed via Kruskal-Wallace tests. Models were run independently for the 2 age groups that corresponded to PISI respondent (2–10.9 years; 11–18 years), as these groups differed both in respondent (parent report vs self-report) and several secondary outcome measures.
As a complement to running analyses on BMIz as a continuous variable, we also categorically classified body mass in terms of the commonly used Centers for Disease Control-defined clinical groups of healthy weight (< 85th percentile), overweight (85–94th percentile), and obese (≥ 95th percentile). Because such a small percentage of the sample met criteria for underweight (< 5th percentile), those with underweight were placed in the “healthy weight” category for analyses. Preliminary tests of analytic assumptions showed that baseline insomnia severity was not normally distributed in each weight group (Shapiro-Wilk test P < .001) and sample cell sizes across weight groups were nonequivalent. As such, the nonparametric Kruskal-Wallis was utilized to compare baseline insomnia severity across weight groups to determine whether weight group predicted change in insomnia severity from preintervention to postintervention and to determine whether weight group predicted treatment status. In instances where the Kruskal-Wallis test emerged as significant, we ran Mann-Whitney U-tests to contrast weight groups (ie, healthy weight compared to overweight, healthy weight compared to obese). The P value was set to .05 for all primary analyses and .01 for all secondary analyses to adjust for multiple comparisons.
RESULTS
Participant characteristics and treatment outcomes
Of the 1,550 children and adolescents seen within the behavioral sleep medicine clinic with a diagnosis of insomnia, we were able to gather objective height and weight estimates for 1,133 individuals (n = 744 children ages 2–10.9 years; n = 389 adolescents ages 11–18 years). Those excluded from the study due to a lack of height/weight estimates had lower income (t(1,995) = 2.37, P = .018), were older (t(2,129) = −4.663, P < .0001), and had higher baseline and follow-up PISI scores (t(2,128) = −2.61, P = .009; t(2,128) = 4.673, P < .001) than those included. As depicted in Table 1, 12.6% of included children were classified as overweight and 19.9% as obese and 18.5% of adolescents were classified as overweight and 31.1% as obese. A total of 4.4% of children and 3.6% of adolescents fell within the underweight category. Our final sample was 43.7% female and 79.1% Caucasian, with the majority of children being male (62.1%), Caucasian (77.6%), and lower income (54.4%), and the majority of adolescents being female (54.8%), Caucasian (82%), and higher income (59%). Following initial evaluation, 7.9% of the sample was not recommended for a follow-up appointment, 35.0% were recommended for follow-up but only attended the initial evaluation appointment, 32.7% of the sample terminated treatment early, and 24.4% of the sample successfully completed treatment. Participants attended an average of 2.12 (standard deviation = 1.51) appointments. Similar to what was previously reported from this population base,25 for those in our sample with at least 1 follow-up appointment, insomnia severity significantly improved for both children (t(743) = 17.42, P < .0001) and adolescents (t(388) = 11.74, P < .0001) over time. See Table 1 for additional demographic information for each age group.
Table 1.
Sample characteristics at initial evaluation.
| Entire Sample (n = 1,133) | Children, Ages 2–10.9 years (n = 744) | Adolescents, Ages 11–18 years (n = 389) | |
|---|---|---|---|
| Female (%) | 43.7 | 37.9 | 54.8 |
| Race/ethnicity (%) | |||
| White | 79.1 | 77.6 | 82.0 |
| Black | 10.2 | 11.3 | 8.0 |
| Hispanic | 1.6 | 1.6 | 1.5 |
| Asian | 1.1 | 0.7 | 1.8 |
| Native American | 0.2 | 0.1 | 0.3 |
| Multiracial | 7.7 | 8.4 | 6.2 |
| Other | 0.1 | 0.3 | 0.2 |
| Age (years) | 9.05 (4.86) | 6.02 (2.59) | 14.84 (2.18) |
| Family income (%) | |||
| 50K or less | 46.8 | 54.4 | 41.0 |
| > 50K | 47.0 | 45.6 | 59.0 |
| BMIz | 0.64 (1.28) | 0.52 (1.28) | 0.86 (1.24) |
| Weight group (%) | |||
| Underweight | 4.1 | 4.4 | 3.6 |
| Healthy weight | 57.5 | 63.1 | 46.8 |
| Overweight | 14.7 | 12.6 | 18.5 |
| Obese | 23.7 | 19.9 | 31.1 |
| Initial insomnia severity | 17.72 (6.1) | 17.43 (5.97) | 18.28 (6.30) |
| Presence of organic sleep disorder | |||
| Obstructive sleep apnea | 6.2 | 6.3 | 5.9 |
| Central sleep apnea | 0.4 | 0.5 | 0.0 |
| Snoring | 20.3 | 23.0 | 15.2 |
| Hypoventilation | 0.4 | 0.5 | 0.3 |
| Restless legs syndrome | 0.7 | 0.5 | 1.0 |
| Periodic limb movement | 4.5 | 4.4 | 4.6 |
| Narcolepsy | 1.0 | 0.4 | 2.1 |
| Nocturnal seizures | 0.4 | 0.3 | 0.5 |
Sample features at time of baseline appointment. Categorical variables are listed in percentages, while continuous variables are listed as mean ± standard deviation. BMIz = body mass index Z-score.
Primary analyses
Insomnia severity and BMIz
As shown in Table 2, BMIz did not significantly predict baseline insomnia severity overall nor on any PISI subscales for children (P > .15). However, for adolescents, higher BMIz emerged as a small but significant predictor for greater baseline insomnia severity (P = .021), particularly sleep onset problems (P = .048) and daytime sleepiness (P = .021) rather than sleep maintenance problems (P = .16; see Table 2). BMIz did not significantly predict change in insomnia severity from preintervention to postintervention in either children (β = −0.01, t(703) = −0.27, P = .790) or adolescents (β = 0.02, t(753) = 0.29, P = .771).
Table 2.
Association between baseline BMIz and insomnia severity, stratified by age.
| Children (Ages 2–10.9 years) | Adolescents (Ages 11–18 years) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Step | Predictor | R2 | β (SE) | T | Step | Predictor | R2 | β | T |
| PISI Total Score | |||||||||
| 1 | Income | .040*** | −0.20 (0.07) | −5.44*** | 1 | Income | .012* | −0.11 (0.11) | −2.03* |
| 2 | .040*** | 2 | .026** | ||||||
| Income | −0.20 (0.07) | −5.39*** | Income | −0.10 (0.11) | −1.91 | ||||
| BMIz | 0.00 (0.17) | 0.01 | BMIz | 0.12 (0.27) | 2.32* | ||||
| PISI Factor 1 (Difficulty Falling Asleep) | |||||||||
| 1 | Income | .054*** | −0.23 (0.05) | −6.35*** | 1 | Income | .017* | −0.13 (0.07) | −2.47* |
| 2 | .054*** | 2 | .028** | ||||||
| Income | −0.23 (0.05) | −6.32*** | Income | −0.12 (0.07) | −2.36* | ||||
| BMIz | −0.01 (0.11) | −0.13 | BMIz | 0.10 (0.17) | 1.98* | ||||
| PISI Factor 2 (Difficulty Staying Asleep) | |||||||||
| 1 | Income | .028*** | −0.17 (0.04) | −4.51*** | 1 | Income | .016* | −0.13 (0.06) | −2.40* |
| 2 | 2 | .021* | |||||||
| Income | .028*** | −0.17 (0.04) | −4.50*** | Income | −0.12 (0.06) | −2.31* | |||
| BMIz | −0.01 (0.10) | −0.26 | BMIz | 0.08 (0.16) | 1.41 | ||||
| PISI Daytime Sleepiness | |||||||||
| 1 | Income | .002 | −0.05 (0.02) | −1.28 | 1 | Income | .002 | 0.04 (0.03) | 0.83 |
| 2 | .005 | 2 | .017* | ||||||
| Income | −0.04 (0.02) | −1.10 | Income | 0.05 (0.03) | 0.97 | ||||
| BMIz | 0.05 (0.05) | 1.41 | BMIz | 0.12 (0.07) | 2.31* | ||||
*P < .05; **P < .01; ***P < .001. BMIz = body mass index Z-score, PISI = Pediatric Insomnia Severity Index, SE = standard error.
Insomnia severity and weight group
Parallel nonparametric analyses across categorical weight groups yielded similar results (Table 3). In children, there were no differences across weight groups in overall baseline insomnia severity or any PISI subscales (P > .15). However, weight group was modestly associated with baseline insomnia severity overall (P = .015) and sleep onset problems (P = .017) for adolescents (ages 11–18 years), but there were no significant differences for sleep maintenance problems (P = .105) or daytime sleepiness (P = .498). Follow-up comparisons found that adolescents of healthy weight did not differ on baseline insomnia severity from those with overweight (Z = −1.21, P = .226), but did differ from those with obesity (Z = −2.87, P = .004), with those with obesity having somewhat higher baseline insomnia severity than those of a healthy weight. Similar patterns emerged when examining sleep onset problems, adolescents of healthy weight did not differ from those with overweight (Z = −.91, P = .362), but did differ from those with obesity (Z = −2.80, P = .005), with those with obesity having somewhat higher baseline sleep onset problems than those of a healthy weight.
Table 3.
Baseline insomnia severity and its associated factor scores by weight group, stratified by age.
| Healthy Weight | Overweight | Obese | Kruskal-Wallis H | P | |
|---|---|---|---|---|---|
| Children, Ages 2–10.9 years | |||||
| PISI total score | 17.38 (6.08) | 17.09 (5.49) | 18.05 (6.02) | 1.96 | .376 |
| PISI factor 1 | 9.30 (4.00) | 8.98 (4.13) | 9.59 (3.76) | 1.13 | .569 |
| PISI factor 2 | 8.46 (3.57) | 8.39 (3.17) | 8.72 (3.52) | 0.64 | .725 |
| PISI DS | 2.43 (1.59) | 2.43 (1.58) | 2.72 (1.62) | 3.32 | .191 |
| Adolescents, Ages 11–18 years | |||||
| PISI total score | 17.47 (6.28) | 18.48 (6.14) | 19.69 (6.31)* | 8.41 | .015 |
| PISI factor 1 | 9.23 (4.15) | 9.79 (3.68) | 10.68 (3.56)* | 8.21 | .017 |
| PISI factor 2 | 7.32 (3.67) | 8.09 (3.69) | 8.85 (3.72) | 4.51 | .105 |
| PISI DS | 3.25 (1.61) | 3.43 (1.65) | 3.42 (1.57) | 1.40 | .498 |
*Mann-Whitney U comparisons showing significant differences (P < .01) between adolescents of healthy weight vs those with obesity. DS = daytime sleepiness, PISI = Pediatric Insomnia Severity Inventory.
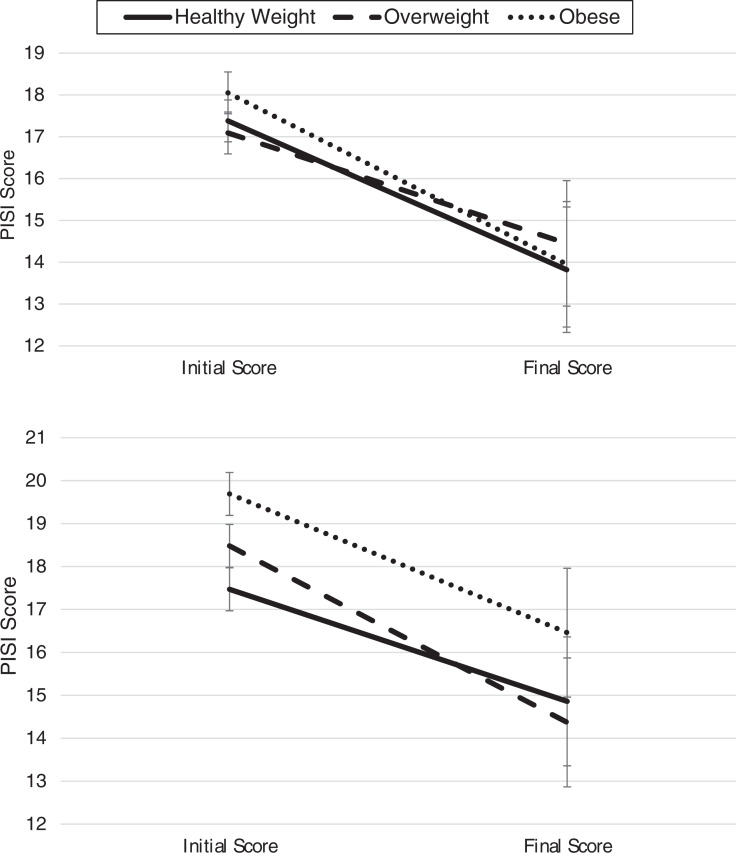
Weight group was not associated with change in insomnia severity from preintervention to postintervention in either children (H = 3.27, P = .195) or adolescents (H = 2.66, P = .265; Figure 1).
Figure 1. Interaction of weight group and time on insomnia severity.
(Top) Changes in PISI score in children (ages 2–10.9 years). (Bottom) Changes in PISI score in adolescents (ages 11–18 years). PISI = Pediatric Insomnia Severity Index.
Secondary analyses
Body mass and treatment status
Weight group was not associated with treatment status (no treatment, early termination, successful treatment completion) for children (H = 3.16, P = .210) or adolescents (H = 4.18, P = .123). BMIz also was not associated with treatment status for children (H = 3.20, P = .783) or adolescents (H = 11.67, P = .07).
Baseline sleep problems, sleep quality, and sleep hygiene
Higher child BMIz was significantly related to higher CSHQ total sleep problems (P = .006). Closer examination of CSHQ subscales found that higher BMIz was primarily related to higher daytime sleepiness (β = 0.12, t(701) = 3.32, P = .001), rather than sleep-disordered breathing, bedtime resistance, sleep anxiety, sleep onset delay, sleep duration, night wakings, or parasomnias (P ≥ .011).
For adolescents, the relationship between BMIz and overall sleep quality on the ASWS approached significance (β = −0.12, t(332) = −2.18, P = .03), but no clear effects were evident on the ASWS subscales (P ≥ .046). Higher adolescent BMIz was associated with a lower overall sleep hygiene on the ASHS (β = −0.23, t(332) = −4.28, P = < .001). For the ASHS subscales, there were significant associations between higher BMIz and higher cognitive/emotional activation (β = −0.19, t(332) = −3.57, P < .001) and higher levels of daytime sleep (β = −0.15, t(332) = −2.82, P = .005), but not behavioral arousal, physiological arousal, sleep stability, or sleep environment (P ≥ .023).
DISCUSSION
As prevalence of pediatric obesity and sleep problems in youth continue to rise,1,31 this study aimed to understand better the association between weight status and insomnia. We examined the role of weight in evidence-based insomnia treatment in a large, clinically referred sample of over 1,000 children and adolescents with International Classification of Sleep Disorders-confirmed insomnia. The study aimed to characterize prevalence of OV/OB within a clinically referred sample, examine the relation between weight group and pretreatment insomnia severity and sleep quality, and determine the moderating impact of weight status on insomnia treatment outcomes following evidence-based behavioral insomnia treatment.
Nearly one-third of children (2–10 years; 32.5%) and half of adolescents (49.6%; 11–18 years) in this sample had OV/OB. The overall prevalence of OV/OB within this sample is higher than what is observed in national statistics,31 consistent with studies that have observed higher levels of sleep problems (later sleep onset, shorter sleep duration, poor sleep quality, greater sleep disturbances, and symptoms of sleep-disordered breathing) among youth with higher BMIz and/or categorically defined overweight or obesity.5–8,13 However, the nature of this study did not allow for the detection of causality between sleep and weight status; multidirectional effects are certainly possible.32,33
Our hypothesis that individuals with OV/OB would have worse pretreatment insomnia severity was only partially supported, primarily through significant findings observed in adolescent patients. Higher adolescent BMIz was associated with a modest increase in baseline overall insomnia severity, sleep onset problems, and daytime sleepiness after controlling for family income. More severe baseline insomnia severity and sleep onset problems were particularly evident in adolescents meeting Centers for Disease Control cutoffs for obesity. However, we did not observe that either BMIz or weight group was associated with insomnia severity in children. Given that previous research has established that adults with OV/OB have greater insomnia symptoms than adults of healthy weight,20,21 it is possible that the positive relationship between insomnia symptoms and weight emerges later in development. However, it is also possible that effects may differ based on reporter; sleep outcomes were based on parent report for children in this study, whereas sleep outcomes were self-reported both in our adolescent sample and that of prior studies on adults.
Secondary analyses focused on the relationship between weight status and multifactored sleep symptoms yielded findings fairly consistent with prior literature, showing youth with higher body mass are more likely to have sleep problems.13 Higher BMIz in children was associated with greater total sleep problems and daytime sleepiness. Higher adolescent BMIz was associated with worse overall total sleep hygiene, greater cognitive/emotional activation, and greater daytime sleep. There were no statistically significant findings for sleep-disordered breathing symptoms, bedtime resistance, sleep onset delay, sleep duration, night wakings, or parasomnias in children, nor for overall sleep quality, sleep stability, sleep environment factors, returning to wakefulness, and behavioral/physiologic arousal in adolescents.
Our study findings did not support our hypothesis that youth with overweight or obesity would have less insomnia symptom improvement compared to youth of healthy weight. Despite a very large clinical sample that affords considerable statistical power, neither BMIz nor categorical weight group (healthy, overweight, obese) seemed to alter the trajectory of improvements in insomnia severity from preintervention to postintervention for children or adolescents. There was also no significant difference across weight groups in whether families did not pursue treatment, partially completed treatment, or formally completed insomnia treatment.
Although findings were inconsistent with several expectations, the observation that patients of varying weight groups experienced similar reduction in insomnia severity posttreatment (see Figure 1) is encouraging. Combined with the previously summarized findings, this observation suggests that while youth (particularly adolescents) with OV/OB may present with greater insomnia, increased multifactored sleep disorder symptoms, poorer sleep hygiene, and worse sleep quality, their sleep improves during evidence-based insomnia treatment. In the adult literature, meta-analyses34 have described variability between studies regarding the obesity-insomnia relation and suggested that perhaps the relationship is more complex in terms of possible moderators or mediators (eg, appetite regulating hormones, executive functioning, emotional distress) or other relevant sleep variables (eg, sleep timing or variability). Mechanisms underlying the relationship between poor sleep and obesity have also been proposed for adolescents,32 although research and models in pediatrics have thus focused more heavily on the consequences of disrupted sleep on obesity risk. There continues to be a clear need for research on potential bidirectional relationships between sleep and obesity in clinical trials and longitudinal studies that consider a variety of relevant sleep domains (eg, insomnia symptoms, sleep duration, sleep timing, variability of sleep) and possible family/cultural and contextual factors.32
The current study had a number of strengths, including a sample that was both large (increasing statistical power) and clinically derived (maximizing applicability to clinical care), and a study design that examined insomnia severity over the course of treatment that expands a literature that has largely been confined to cross-sectional studies. However, there are also several limitations worth noting. There are no established PISI cut-off scores for insomnia diagnosis nor prior research including a healthy population of youth to provide normative insomnia data, complicating the clinical interpretation of our findings. However, it is worth noting that cross-group differences in PISI scores was roughly a one-third to one-half the size of the effect of treatment, which is not trivial. The availability of anthropometrics proximal to the initial visit impacted our ability to examine the bidirectional weight-insomnia relation and prevented examination of weight gain or loss across the intervention period. The focus on a treatment-seeking sample of youth also may limit generalizability of findings to those with clinically significant insomnia in community-based settings. Furthermore, those excluded from the study because of lacking height/weight estimates had worse insomnia severity scores at baseline and following treatment, suggesting that the included data are not fully representative of a treatment-seeking sample. Our study sample was also predominantly Caucasian, and although there were no significant differences in insomnia outcomes across race in this sample, future research may benefit from further examination of sleep and health parameters in more diverse samples while also considering interactions with socioeconomic (eg, income, family size) and environmental factors. Lastly, our study included a greater proportion of those lost to follow-up compared to randomized trials in adolescents.35,36 This may be attributable to the fact that randomized control trials tend to have more homogeneous samples of highly motivated volunteers, often incentivize attendance, and generally have predefined and often short courses of treatment. There is evidence of attrition rates ranging from 22% in insomnia treatment for adolescents with comorbid psychiatric conditions37 and as high as 30–38% in adult patients presenting to clinical settings, including sleep clinics.38–40 Further research is needed to clarify the potential for attrition to reflect symptom improvement, who is most at risk for drop-out, and implement interventions to reduce sleep health disparities in terms of both presentation or referral to and retention in treatment.
CONCLUSIONS
Youth presenting for insomnia treatment very often have overweight or obesity. At the time they present for insomnia treatment, adolescents with higher body mass tend to have greater overall insomnia severity, more sleep onset concerns, worse sleep hygiene, greater arousal prior to bed, and daytime sleepiness, after accounting for income. Among younger children seeking insomnia treatment, higher body mass is also associated with more baseline sleep problems and daytime sleepiness. However, while youth with overweight or obesity may present with more insomnia symptoms and sleep problems, they respond similarly to behavioral insomnia treatment based on reduction in insomnia severity. Future research should assess change in both weight status and insomnia over the course of treatment, long-term outcomes related to insomnia remission or symptom relapse and the role of family, cultural, and societal factors that may impact weight and sleep health.
ABBREVIATIONS
- ASHS
Adolescent Sleep Hygiene Scale
- ASWS
Adolescent Sleep Wake Scale
- BMI
body mass index
- BMIz
body mass index Z-score
- CSHQ
Children’s Sleep Habits Questionnaire
- OV/OB
overweight/obesity
- PISI
Pediatric Insomnia Severity Index
DISCLOSURE STATEMENT
All authors have seen and approved this manuscript. This study was funded by Cincinnati Children’s Hospital Medical Center Division of Behavioral Medicine and Clinical Psychology for maintenance of the data repository. The primary author was supported by the Health Resources and Services Administration (HRSA) of the US Department of Health and Human Services (HHS) as part of the General Pediatrics Research Fellowship (T32HP10027-23-00). The authors report no conflicts of interest.
REFERENCES
- 1. Wheaton AG , Jones SE , Cooper AC , Croft JB . Short sleep duration among middle school and high school students - United States, 2015 . MMWR Morb Mortal Wkly Rep. 2018. ; 67 ( 3 ): 85 – 90 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cappuccio FP , Taggart FM , Kandala N-B , et al . Meta-analysis of short sleep duration and obesity in children and adults . Sleep. 2008. ; 31 ( 5 ): 619 – 626 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Miller MA , Kruisbrink M , Wallace J , Ji C , Cappuccio FP . Sleep duration and incidence of obesity in infants, children, and adolescents: a systematic review and meta-analysis of prospective studies . Sleep. 2018. ; 41 ( 4 ): 1 – 19 . [DOI] [PubMed] [Google Scholar]
- 4. Chen X , Beydoun MA , Wang Y . Is sleep duration associated with childhood obesity? A systematic review and meta-analysis . Obesity (Silver Spring). 2008. ; 16 ( 2 ): 265 – 274 . [DOI] [PubMed] [Google Scholar]
- 5. Jarrin DC , McGrath JJ , Drake CL . Beyond sleep duration: distinct sleep dimensions are associated with obesity in children and adolescents . Int J Obes Lond. 2013. ; 37 ( 4 ): 552 – 558 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Agras WS , Hammer LD , McNicholas F , Kraemer HC . Risk factors for childhood overweight: a prospective study from birth to 9.5 years . J Pediatr. 2004. ; 145 ( 1 ): 20 – 25 . [DOI] [PubMed] [Google Scholar]
- 7. Bell JF , Zimmerman FJ . Shortened nighttime sleep duration in early life and subsequent childhood obesity . Arch Pediatr Adolesc Med. 2010. ; 164 ( 9 ): 840 – 845 . [DOI] [PubMed] [Google Scholar]
- 8. Krueger PM , Reither EN , Peppard PE , Burger AE , Hale L . Cumulative exposure to short sleep and body mass outcomes: a prospective study . J Sleep Res. 2015. ; 24 ( 6 ): 629 – 638 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Landhuis CE , Poulton R , Welch D , Hancox RJ . Childhood sleep time and long-term risk for obesity: a 32-year prospective birth cohort study . Pediatrics. 2008. ; 122 ( 5 ): 955 – 960 . [DOI] [PubMed] [Google Scholar]
- 10. Cook F , Conway LJ , Giallo R , Gartland D , Sciberras E , Brown S . Infant sleep and child mental health: a longitudinal investigation . Arch Dis Child. 2020. ; 105 ( 7 ): 655 – 660 . [DOI] [PubMed] [Google Scholar]
- 11. Meltzer LJ , Wainer A , Engstrom E , Pepa L , Mindell JA . Seeing the Whole Elephant: a scoping review of behavioral treatments for pediatric insomnia . Sleep Med Rev. 2021. ; 56 : 101410 . [DOI] [PubMed] [Google Scholar]
- 12. Johnson EO , Roth T , Schultz L , Breslau N . Epidemiology of DSM-IV insomnia in adolescence: lifetime prevalence, chronicity, and an emergent gender difference . Pediatrics. 2006. ; 117 ( 2 ): e247 – e256 . [DOI] [PubMed] [Google Scholar]
- 13. Beebe DW , Lewin D , Zeller M , et al . Sleep in overweight adolescents: shorter sleep, poorer sleep quality, sleepiness, and sleep-disordered breathing . J Pediatr Psychol. 2007. ; 32 ( 1 ): 69 – 79 . [DOI] [PubMed] [Google Scholar]
- 14. Graef DM , Janicke DM , McCrae CS . Sleep patterns of a primarily obese sample of treatment-seeking children . J Clin Sleep Med. 2014. ; 10 ( 10 ): 1111 – 1117 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hart CN , Carskadon MA , Considine RV , et al . Changes in children’s sleep duration on food intake, weight, and leptin . Pediatrics. 2013. ; 132 ( 6 ): e1473 – e1480 . [DOI] [PubMed] [Google Scholar]
- 16. Morin CM , Benca R . Chronic insomnia . Lancet. 2012. ; 379 ( 9821 ): 1129 – 1141 . [DOI] [PubMed] [Google Scholar]
- 17. Roberts RE , Roberts CR , Duong HT . Chronic insomnia and its negative consequences for health and functioning of adolescents: a 12-month prospective study . J Adolesc Health. 2008. ; 42 ( 3 ): 294 – 302 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Meltzer LJ , Mindell JA . Systematic review and meta-analysis of behavioral interventions for pediatric insomnia . J Pediatr Psychol. 2014. ; 39 ( 8 ): 932 – 948 . [DOI] [PubMed] [Google Scholar]
- 19. de Bruin EJ , Bögels SM , Oort FJ , Meijer AM . Efficacy of cognitive behavioral therapy for insomnia in adolescents: a randomized controlled trial with internet therapy, group therapy and a waiting list condition . Sleep. 2015. ; 38 ( 12 ): 1913 – 1926 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pearson NJ , Johnson LL , Nahin RL . Insomnia, trouble sleeping, and complementary and alternative medicine: Analysis of the 2002 national health interview survey data . Arch Intern Med. 2006. ; 166 ( 16 ): 1775 – 1782 . [DOI] [PubMed] [Google Scholar]
- 21. Singareddy R , Vgontzas AN , Fernandez-Mendoza J , et al . Risk factors for incident chronic insomnia: a general population prospective study . Sleep Med. 2012. ; 13 ( 4 ): 346 – 353 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. American Academy of Sleep Medicine . International Classification of Sleep Disorders: Diagnostic and Coding Manual. 2nd ed . Westchester, IL: : American Academy of Sleep Medicine; ; 2005. . [Google Scholar]
- 23. American Academy of Sleep Medicine . International Classification of Sleep Disorders. 3rd ed . Darien, IL: : American Academy of Sleep Medicine; ; 2014. . [Google Scholar]
- 24. Byars KC , Simon SL , Peugh J , Beebe DW . Validation of a brief insomnia severity measure in youth clinically referred for sleep evaluation . J Pediatr Psychol. 2017. ; 42 ( 4 ): 466 – 475 . [DOI] [PubMed] [Google Scholar]
- 25. Byars KC , Simon SL . Practice patterns and insomnia treatment outcomes from an evidence-based pediatric behavioral sleep medicine clinic . Clin Pract Pediatr Psychol. 2014. ; 2 ( 3 ): 337 – 349 . [Google Scholar]
- 26. Owens JA , Spirito A , McGuinn M . The Children’s Sleep Habits Questionnaire (CSHQ): Psychometric properties of a survey instrument for school-aged children . Sleep. 2000. ; 23 ( 8 ): 1043 – 1051 . [PubMed] [Google Scholar]
- 27. Goodlin-Jones BL , Sitnick SL , Tang K , Liu J , Anders TF . The Children’s Sleep Habits Questionnaire in toddlers and preschool children . J Dev Behav Pediatr. 2008. ; 29 ( 2 ): 82 – 88 . [DOI] [PubMed] [Google Scholar]
- 28. LeBourgeois MK , Giannotti F , Cortesi F , Wolfson AR , Harsh J . The relationship between reported sleep quality and sleep hygiene in Italian and American adolescents . Pediatrics. 2005. ; 115 , 1, Suppl : 257 – 265 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lewandowski AS , Toliver-Sokol M , Palermo TM . Evidence-based review of subjective pediatric sleep measures . J Pediatr Psychol. 2011. ; 36 ( 7 ): 780 – 793 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Storfer-Isser A , Lebourgeois MK , Harsh J , Tompsett CJ , Redline S . Psychometric properties of the Adolescent Sleep Hygiene Scale . J Sleep Res. 2013. ; 22 ( 6 ): 707 – 716 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Skinner AC , Ravanbakht SN , Skelton JA , Perrin EM , Armstrong SC . Prevalence of obesity and severe obesity in US children, 1999–2016 . Pediatrics. 2018. ; 141 ( 3 ): e20173459 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Duraccio KM , Krietsch KN , Chardon ML , Van Dyk TR , Beebe DW . Poor sleep and adolescent obesity risk: a narrative review of potential mechanisms . Adolesc Health Med Ther. 2019. ; 10 : 117 – 130 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Krietsch KN , Chardon ML , Beebe DW , Janicke DM . Sleep and weight-related factors in youth: A systematic review of recent studies . Sleep Med Rev. 2019. ; 46 : 87 – 96 . [DOI] [PubMed] [Google Scholar]
- 34. Chan WS , Levsen MP , McCrae CS . A meta-analysis of associations between obesity and insomnia diagnosis and symptoms . Sleep Med Rev. 2018. ; 40 : 170 – 182 . [DOI] [PubMed] [Google Scholar]
- 35. de Bruin EJ , Bögels SM , Oort FJ , Meijer AM . Improvements of adolescent psychopathology after insomnia treatment: results from a randomized controlled trial over 1 year . J Child Psychol Psychiatry. 2018. ; 59 ( 5 ): 509 – 522 . [DOI] [PubMed] [Google Scholar]
- 36. Clarke G , McGlinchey EL , Hein K , et al . Cognitive-behavioral treatment of insomnia and depression in adolescents: A pilot randomized trial . Behav Res Ther. 2015. ; 69 : 111 – 118 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Åslund L , Lekander M , Wicksell RK , Henje E , Jernelöv S . Cognitive-behavioral therapy for insomnia in adolescents with comorbid psychiatric disorders: A clinical pilot study . Clin Child Psychol Psychiatry. 2020. ; 25 ( 4 ): 958 – 971 . [DOI] [PubMed] [Google Scholar]
- 38. Perlis ML , Sharpe M , Smith MT , Greenblatt D , Giles D . Behavioral treatment of insomnia: treatment outcome and the relevance of medical and psychiatric morbidity . J Behav Med. 2001. ; 24 ( 3 ): 281 – 296 . [DOI] [PubMed] [Google Scholar]
- 39. Perlis M , Aloia M , Millikan A , et al . Behavioral treatment of insomnia: a clinical case series study . J Behav Med. 2000. ; 23 ( 2 ): 149 – 161 . [DOI] [PubMed] [Google Scholar]
- 40. Verbeek I , Schreuder K , Declerck G . Evaluation of short-term nonpharmacological treatment of insomnia in a clinical setting . J Psychosom Res. 1999. ; 47 ( 4 ): 369 – 383 . [DOI] [PubMed] [Google Scholar]