Abstract
Kidney cortex apoptosis was studied with female Wistar rats treated for 10 days with gentamicin and netilmicin at daily doses of 10 or 20 mg/kg of body weight and amikacin or isepamicin at daily doses of 40 mg/kg. Apoptosis was detected and quantitated using cytological (methyl green-pyronine) and immunohistochemical (terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling) staining, in parallel with a measurement of drug-induced phospholipidosis (cortical phospholipids and phospholipiduria), cortical proliferative response (3H incorporation in DNA and histoautoradiography after in vivo pulse-labeling with [3H]thymidine), and kidney dysfunction (blood urea nitrogen and creatinine). Gentamicin induced in proximal tubules a marked apoptotic reaction which (i) was detectable after 4 days of treatment but was most conspicuous after 10 days, (ii) was dose dependent, (iii) occurred in the absence of necrosis, and (iv) was nonlinearly correlated with the proliferative response (tubular and peritubular cells). Comparative studies revealed a parallelism among the extents of phospholipidosis, apoptosis, and proliferative response for three aminoglycosides (gentamicin >> amikacin ≅ isepamicin). By contrast, netilmicin induced a marked phospholipidosis but a moderate apoptosis and proliferative response. We conclude that rats treated with gentamicin develop an apoptotic process as part of the various cortical alterations induced by this antibiotic at low doses. Netilmicin, and still more amikacin and isepamicin, appears safer in this respect. Whereas a relation between aminoglycoside-induced tubular apoptosis and cortical proliferative response seems to be established, no simple correlation with phospholipidosis can be drawn.
Aminoglycosides have long been and still are an essential component of our armamentarium against severe, life-threatening infections caused by gram-negative bacilli (20, 49). Their nephrotoxicity and ototoxicity, however, remain of concern to clinicians, even though the optimization of their mode of administration (21) and the use of intrinsically less toxic derivatives (48) already offer some increased safety. An intriguing aspect of aminoglycoside nephrotoxicity remains the fact that very large amounts of drug (usually 10 times the therapeutic doses) must be administered to animals in order to cause clear-cut acute tubular necrosis and concomitant alteration of the renal function (20, 52). In contrast, rats treated with low, more therapeutically relevant dosages show neither extended tubular necrosis nor gross kidney dysfunction but merely an array of alterations involving the apical and basolateral membrane, the lysosomes, and various other subcellular components of proximal tubule cells, none of which, however, has unambiguously been associated with cell death and organ dysfunction (49). Yet, the development of these alterations is associated with an increased tissue DNA synthesis, which develops in parallel with the appearance of dedifferentiated cells in the proximal tubules and a proliferation of interstitial cells (38, 67). It has long been thought that this process was a reaction to focal tubular necroses (39), which are difficult to quantitatively assess because dead cells are quickly swept away in the lumen and the urine. Based on nonsystematic morphological observations performed during our early studies, however, we suggested that gentamicin could also induce the apoptotic death of epithelial tubular cells (38), but the importance of this phenomenon was not examined. Apoptosis, or programmed cell death, was originally described in the study of insect tissue degeneration (43) and was later found to be a prominent process in most instances of tissue remodeling (33). It designates a form of cell demise characterized by specific cytological features which make it quite distinct from necrosis, such as cell shrinkage, increased cytoplasmic density, and compaction of chromatin which segregates into well-defined clumps abutting the nuclear membrane (10). While apoptosis is a physiological process during embryogenesis and the establishment of immune self-tolerance (1, 44, 73), it can also be triggered by various pharmacological agents, including anticancer drugs (74) and antibiotics (2). The ototoxicity of aminoglycosides has been attributed to apoptosis of cochlear and vestibular hair cells in the inner ear sensory epithelia (36, 50). The recognition that apoptosis entails the cleavage of internucleosomal DNA (DNA laddering) by endogenous endonucleases such as caspase-activated endonuclease (CAD) (16), has led to the development of immunohistochemical techniques allowing one to easily observe and quantitate the occurrence of apoptotic cells in tissue sections (19). This offered a valuable opportunity to reexamine our original suggestion that gentamicin causes apoptosis in kidney tissues. We therefore present here a quantitative evaluation of apoptosis induced in renal cortex by gentamicin at low, therapeutically relevant doses in correlation with the development of other early, subclinical tissue alterations. Netilmicin, amikacin, and isepamicin have been included to better assess the significance of our findings in relation to the lower nephrotoxic potential of these derivatives (48).
(Preliminary accounts of this work have been given at the 35th and the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy [M. El Mouedden, H. Taper, G. Laurent, and P. M. Tulkens, Abstr. 35th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-123, 1995, and M. El Mouedden, G. Laurent, M. P. Mingeot-Leclercq, and P. M. Tulkens, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-88, 1998, respectively].)
MATERIALS AND METHODS
Overall design of the experiments.
The administration of low doses of gentamicin (typically 4 to 10 mg/kg of body weight) is known to cause within 10 days in renal proximal tubular cells (i) the development of a conspicuous lysosomal phospholipidosis which can be assessed directly by the measurement of total cortical phospholipids (37), and indirectly by the determination of phospholipiduria (27, 28, 32) and (ii) a significant cellular proliferation which can be visualized and quantitated by histoautoradiography and measurement of DNA radioactivity in [3H]thymidine-pulsed animals (38). Tubular necroses become clearly detectable only at larger doses (20 mg/kg or more [52]). The severity of the lysosomal alterations and the extent of cellular proliferation at low doses and of the cortical necrosis and the kidney dysfunction at large doses are increased if the daily dose of the aminoglycosides is divided into two or three administrations over 24 h (P. M. Tulkens, M. E. De Broe, P. Maldague, G. Verpooten, and S. Scharpe, Abstr. Annu. Meet. Am. Soc. Microbiol. 1982, abstr. A 30, 1982; 6, 38). Based on these considerations, on previous studies with aminoglycosides (53, 69, 72), and on pilot studies, we selected the experimental conditions defined in Table 1. These were aimed at providing an appropriate model of the clinical use of aminoglycosides and at mimicking as much as possible the actual drug exposure experienced by patients. Moreover, the doses, treatment duration, and schedules used were known by us to elicit a frank phospholipidosis in the absence of visible tubular necrosis at the lower dose and detectable tubular necrosis at the higher dose. Netilmicin was systematically compared to gentamicin throughout these experiments since it causes a marked phospholipidosis (69) but less necrosis than does gentamicin (53). The comparison was thereafter extended to amikacin and isepamicin, both of which cause a mild phospholipidosis and little necrosis (69, 72).
TABLE 1.
Experimental groups, conditions of treatments, and relevance to the clinical use of aminoglycosides
| Drug | Dose (mg/kg)a | Duration (days) | Fold increase over:
|
|
|---|---|---|---|---|
| Clinical doseb | Clinical daily drug exposurec | |||
| Gentamicin | 10 | 4–10 | ∼2 | ∼0.5 |
| 20 | 4–10 | ∼4 | ∼1 | |
| Netilmicin | 10 | 4–10 | ∼1.7 | ∼0.4 |
| 20 | 4–10 | ∼3.3 | ∼0.8 | |
| Amikacin | 40 | 10 | ∼2.7 | ∼0.7 |
| Isepamicin | 40 | 10 | ∼2.7 | ∼0.7 |
Twice-a-day schedule (daily dose split into two administrations at 12-h intervals). This schedule (or even a three-times-a-day schedule) was long considered mandatory for aminoglycosides but is known to increase toxicities at both low and high doses in animals (38, 52). Data for patients are less definite, even though a trend toward less toxicity is commonly observed with a once-a-day schedule (21, 48).
Suggested maintenance doses for an adult patient with an estimated creatinine clearance of 90 ml/min (20) (gentamicin, 5.1; netilmicin, 6; and amikacin, 15 mg/kg, respectively) or based on the registered dosage in Belgium and many other countries for isepamicin.
Based on estimated ratio of areas under the serum concentration-time curve, AUC ratio, using the dose ratio defined in footnote b and assuming apparent half-lives of ∼30 min in rats and ∼120 min in humans (β-elimination phases).
Animals and treatments.
All studies were performed with female Wistar rats (180 to 200 g in body weight) purchased from a commercial breeding farm (Iffa-Credo, l'Arbresle, France). Before and during treatment, animals were housed in a central facility submitted to a 12-h light-dark cycle, provided with regular rat chow and tap water ad libitum, and handled and treated according to the guidelines of the Belgian Ministry of Agriculture (agreement no. LA 12303116). Twenty-four hours before termination of the treatment, the rats were transferred to metabolic cages designed for urine collection. All daily doses (see data) were split into halves given as two separate administrations at around 9 a.m. and 5 p.m., respectively. Drugs were injected by the intraperitoneal route after appropriate dilution in 0.9% NaCl in order to deliver a volume of 0.5 ml per 200 g of animal and per injection (the actual volume injected into each rat was adjusted according to the body weight which was recorded immediately before each drug administration). For control animals, only 0.9% NaCl (0.5 ml per 200 g of animal) was administered. Each experimental group, including controls, contained six animals. Treated and control rats were killed approximately 16 h after the last drug injection and exactly 1 h after intraperitoneal injection of 200 μCi of [methyl-3H]thymidine (40 Ci/mmol; Amersham International plc, Little Chalfont, United Kingdom).
Sacrifice and sampling of renal tissue.
Animals were killed by decapitation, and blood samples were collected from the stump for the measurement of serum creatinine and blood urea nitrogen (BUN). Both kidneys were exposed by laparotomy and excised. After longitudinal bisection, the renal cortex was removed by sharp dissection. For light microscopy studies, two necropsy specimens (each equivalent to approximately a quarter of each kidney) were fixed separately in Carnoy's mixture and in 10% neutral buffered formalin (4.2% formaldehyde) at room temperature for 24 and 48 h, respectively. The remaining renal cortex tissue was snap-frozen in dry ice and stored at −80°C until biochemical analysis.
Morphological studies. (i) Histological demonstration of apoptotic cells.
Kidney specimens, fixed in formalin or Carnoy's mixture, were dehydrated in graded ethanol solutions and embedded in paraffin. Sections (approximately 7 μm thick) of formalin-fixed tissue were used for hematoxylin-eosin staining or terminal deoxynucleotidyltransferase (TdT)-mediated dUTP-biotin nick end labeling (TUNEL), whereas other sections (also approximately 7 μm thick) from tissue fixed in Carnoy's mixture were stained with methyl green-pyronine according to the method of Brachet (8).
(ii) Immunohistochemical demonstration of apoptosis.
Apoptotic cells were detected by TdT-mediated extension of 3′ OH ends of fragmented DNA using either digoxigenin- or fluorescein-labeled dUTP as a precursor (TUNEL) (this method is an adaptation from the original work of Gavrieli et al. [19], who used biotin-labeled dUTP). DNA-bound digoxigenin or fluorescein was revealed by reaction with antidigoxigenin or antifluorescein antibodies labeled with peroxidase. Reagents were purchased as commercial kits (Apoptag; Oncor, Gaithersburg, Md.; in situ cell death detection kit POD; Boehringer Mannheim, Mannheim, Germany [presently Roche Diagnostics]). The immunohistochemical staining procedure was carried out in accordance with the suppliers' recommendations. Briefly, sections of formalin-fixed and paraffin-embedded specimens were dewaxed, rehydrated, and pretreated with 20 μg of proteinase K per ml for 15 min at room temperature. After rinsing, the sections were incubated for 2 h in a reaction buffer containing TdT, dATP, and digoxigenin-11-dUTP or fluorescein-11-dUTP. At the end of the incubation, the sections were rinsed with a stop-wash buffer for 30 min at 37°C, and the mixture was then replaced by peroxidase-conjugated antidigoxigenin or antifluorescein antibody. Immunocomplexes were visualized by exposure to H2O2 and diaminobenzidine. Finally, sections were counterstained with methyl green prior to mounting for light microscopy examination. Two positive controls were made to check that the immunostaining performed under our experimental conditions specifically revealed nuclei containing fragmented DNA. For the first one, DNA nicking was produced by exposing sections of control kidneys to micrococcal nuclease (10 μg/ml, 10 min, 37°C). In the second one, apoptosis was induced in cultured thymocytes by exposure to dexamethasone (3, 19). In both cases, we clearly observed the appearance of a large number of immunostained nuclei. Negative controls were also run by omitting the addition of TdT in the reaction mixture. No labeling was seen in this case in both kidney sections and smears of dexamethasone-treated thymocytes.
(iii) Morphological demonstration of cell proliferation.
S-phase cells were detected by histoautoradiography as described in the work of Laurent et al. (38). Paraffin sections were dewaxed and coated in the dark by dipping them in K-5 Ilford nuclear emulsion (Ilford Ltd., Mobberley, Cheshire, United Kingdom). Histoautoradiographs, stored at 4°C in sealed boxes, were allowed 5 weeks for proper exposure. After processing, tissue sections were counterstained with hemalun and eosin.
(iv) Quantitative analyses.
Enumeration of labeled nuclei (TUNEL histological or histoautoradiography) was performed for slides picked at random for each experimental animal (three slides for Brachet staining; two slides for apoptosis immunolabeling and histoautoradiography). These slides were assigned an arbitrary code which was disclosed to the observer (M.E.) only after final pooling of the results. Examination was made on a Zeiss light microscopy at ×40 magnification for the enumeration of S-phase cells and at ×40 or ×63 magnification for apoptotic nuclei. For calibration purposes, a square grid was mounted in one eyepiece, determining a square field of 0.04 or 0.016 mm2, depending on the magnification. The number of fields examined per slide was 20 for the methyl green-pyronine-stained sections and the immunostained sections and 40 for the histoautoradiographs (yielding total surfaces of 0.966, 0.644, and 3.22 mm2 per slide, respectively). For the histological assessment of apoptosis, we counted all cells with a pyknotic and karyorrhectic nucleus (typically, the total numbers of nuclei actually counted per animal were ≈3 for controls and ≈60 and ≈100 for rats treated for 10 days with 10 and 20 mg of gentamicin per kg, respectively). For the assessment of apoptosis by TUNEL, we counted all nuclei exhibiting a frank brown labeling. These nuclei most often displayed typical alterations such as pyknosis, crescent-like condensation of the chromatin, or formation of apoptotic bodies. Clusters of apoptotic bodies were given a single count (typical numbers of TUNEL-positive nuclei counted per animal, including clusters of apoptotic bodies, were ≈2 for controls and ≈65 and ≈130 for rats treated with 10 and 20 mg of gentamicin per kg for 10 days, respectively). For the assessment of DNA synthesis by histoautoradiography, we counted all cells with clearly recognizable silver grains over or closely associated with the nucleus (typical numbers of positive cells counted per animal were ≈6 for controls and ≈176 and ≈460 for animals receiving 10 and 20 mg of gentamicin per kg for 10 days, respectively).
Biochemical and microbiological studies.
Cortical tissue samples were thawed and homogenized at 0°C in distilled water for measurement of their contents in total lipid phosphorus and protein, using well-established procedures (5, 7, 45) which were fully validated in the present study (coefficients of variation of 1.5 and 1.8% for the assays of tissue phospholipids and tissue proteins, respectively [n = 20 in each case]). The same samples were then used for the determination of DNA specific radioactivity (38), with a minimum of 1,000 counts recorded over background per vial. The urine collected during the 24 h preceding the sacrifice was carefully mixed and stored at −20°C until analysis. Samples were thawed, mixed thoroughly, and centrifuged at 25,000 rpm (Rotor 30; Beckman Instruments, Palo Alto, Calif.) for 1 h. The resulting pellets were resuspended in 1 ml of distilled water and used for the determination of total lipid phosphorus, as detailed previously (27, 28, 34). The coefficient of variation of this determination was 4.7% (n = 20). Serum creatinine and BUN were determined by the routine procedures (4, 15, 31) used for human clinical samples in our University Hospital (Cliniques Universitaires Saint-Luc, Brussels, Belgium), with intrarun and interrun coefficients of variations of 0.6 and 1.2, and 3 and 2.5%, respectively (Hitachi 717 autoanalyzer; Hitachi Ltd., Chiyoda-ku, Tokyo, Japan). The aminoglycoside content of the renal cortex tissue was assayed by a plate diffusion bioassay, using Bacillus subtilis (ATCC 6633) as the test organism, using the technique developed earlier for cultured cell extracts (68). All samples were assayed in triplicate against a series of known standards (extracts from control animals spiked with the corresponding antibiotic after homogenization; no interference of homogenate protein was noted) over a 2.5- to 20-μg/ml range (typical r ≈ 0.987) and covering the range of drug concentrations found in the samples from treated animals.
Statistical analyses.
The statistical analysis was performed according to a two-step procedure, the first step being the determination of the type of data distribution for each parameter studied, and the second being the analysis of the effects of the independent variables. The first step used the Kolmogorov-Smirnov test, and distributions of data were found to be log normal in all cases. Using then the logarithmic transformation of data, the effects of independent variables were first assessed by analysis of variance (ANOVA). When the latter disclosed nonrandom variations, significant differences between groups were identified by the Scheffe post hoc test. Unless stated otherwise, the level of significance was set at P values of <0.05. These analyses were made using STATISTICA (Statsoft, Tulsa, Okla.). Correlations were made and plotted using GraphPad InStat (GraphPad Software, San Diego, Calif.).
Materials.
Gentamicin, netilmicin, and amikacin were the injectable products distributed in Belgium for human clinical use as Géomycine and Nétromycine (both from Schering-Plough Labo, Heist-op-den-Berg, Belgium) and Amukin (Bristol Italiana Sud, S.P.A., Latina, Italy), respectively. Isepamicin was an investigational material for clinical trials (isepamicin sulfate; 250 mg/ml; solution for intravenous-intramuscular injection; batch 35684-038; Schering-Plough S.A., Brussels, Belgium). Unless specified otherwise, all other products and reagents were purchased from established commercial suppliers and were either of analytical grade or certified as fit for the projected purpose by the provider.
RESULTS
As shown in Table 2, a clear-cut phospholipidosis (assessed by the measurement of cortical phospholipid content and of urinary phospholipid excretion) was observed in all animals treated for 10 days with 10 mg of gentamicin per kg. Yet, these animals did not suffer from kidney dysfunction as revealed by the measurement of their serum creatinine and BUN. When the gentamicin dose was raised to 20 mg/kg, no further increase but rather a trend toward a decrease of the cortical phospholipid content was seen with, however, a large variation among animals. By contrast, phospholipiduria was clearly dose dependent in the range investigated. The mean values of serum creatinine and BUN were elevated in animals treated with 20 mg/kg, but the overall statistical analysis failed to disclose a significant effect of the treatment because of the large variations seen. Nevertheless, it was clear that some animals had developed definite renal dysfunction and that the dose interval chosen for gentamicin in this experiment clearly spanned the threshold zone separating the subtoxic alterations from those causing the onset of acute tubular necrosis and acute renal failure. Table 2 also shows that netilmicin, as expected, induced a more severe phospholipidosis (assessed by the assay of cortical phospholipids and phospholipiduria) than did gentamicin but did not cause significant kidney dysfunction since all values of BUN or creatinine remained within the range of controls.
TABLE 2.
Cortical (drug content and phospholipids), urinary phospholipiduria, and serum (creatinine and BUN) parameters in control rats and in rats treated with gentamicin and netilmicin
| Sample type and parameter | Value (mean ± SD) at daily dosage (mg/kg)a
|
||||
|---|---|---|---|---|---|
| 0 (controls) | Gentamicin
|
Netilmicin
|
|||
| 10 | 20 | 10 | 20 | ||
| Cortex | |||||
| Drug contentb | NA | 9.3 ± 2.7 | ND | 9.3 ± 1.9 | 12.2 ± 1.9 |
| Phospholipidsc | 100.0 ± 5.0 | 118.9 ± 6.1f | 111.4 ± 15.1 | 132.7 ± 5.1fh | 139.2 ± 8fh |
| Urine | |||||
| Phospholipiduriad | 100.0 ± 18.7 | 373.2 ± 30.8f | 488.8 ± 88.8fg | 564.3 ± 48.1fh | 714.7 ± 99.5fgh |
| Serume | |||||
| Creatinine | 0.6 ± 0.1 | 0.6 ± 0.1 | 1.2 ± 0.6 | 0.7 ± 0.1 | 0.7 ± 0.1 |
| BUN | 37.2 ± 10.5 | 32.7 ± 6.3 | 75.7 ± 52.3 | 27.3 ± 5.0 | 33.3 ± 7.8 |
Daily dosage administered for 10 days in two separate administrations at 9 a.m. and 5 p.m. NA, not applicable; ND, not determined.
Micrograms per milligram of protein.
Percentage of control value (controls, 323 ± 16 nmol/mg of protein).
Percentage of control value (controls, 122.12 ± 22.87 nmol/24 h).
Milligrams per deciliter.
Significantly different from controls.
Significantly different from the group treated at the lower dose.
Significantly different from the other aminoglycoside at the same dosage.
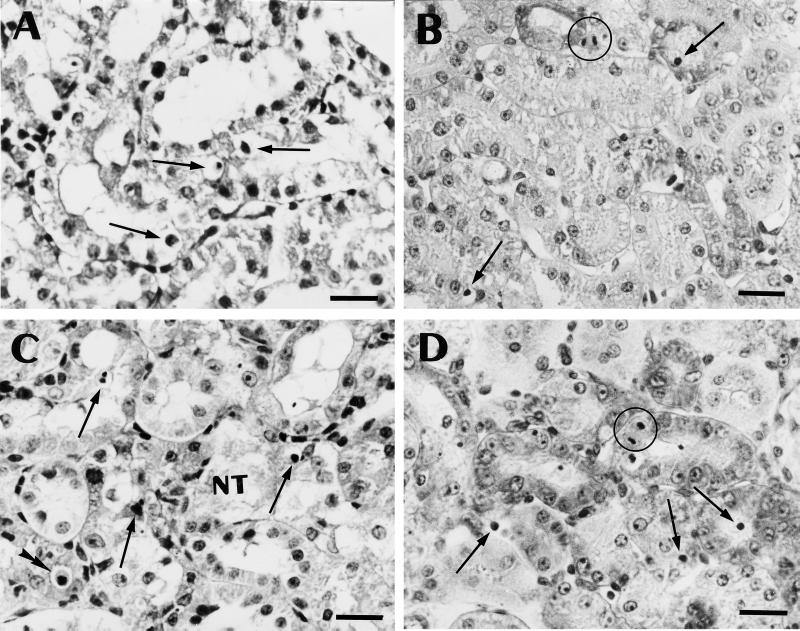
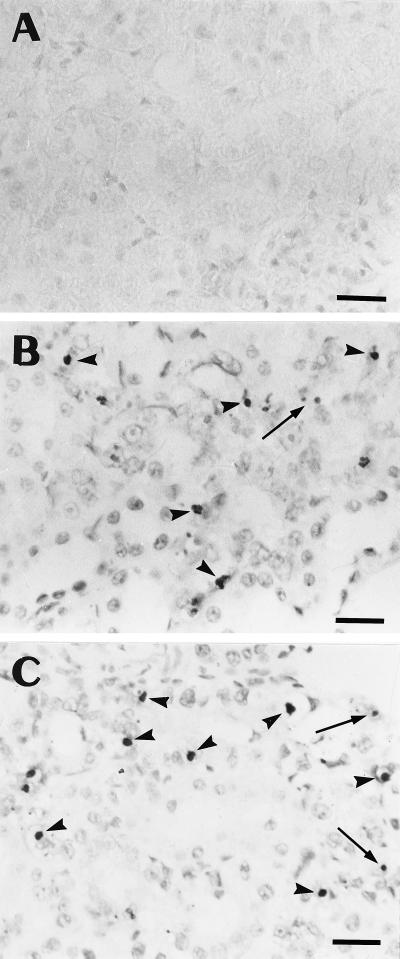
The morphological appearance of the cortex of the animals treated at the two dosages of gentamicin is shown in Fig. 1. At 10 mg/kg, the tubules looked essentially normal when sections stained with hematoxylin and eosin were examined at low to medium magnification. Close examination of these sections at high magnification, however, revealed the appearance of cells with alterations typical of apoptosis (cell shrinkage and cytoplasm eosinophilia and presence of a small and shrunken nucleus with chromatin condensation [Fig. 1A]). The use of the periodic acid-Schiff reaction confirmed that these cells were almost exclusively found in proximal tubules (data not shown). The nuclear alterations characteristic of apoptosis were more clearly visible after using the Brachet technique (methyl green-pyronine staining [Fig. 1B]). When the daily dose of gentamicin was increased to 20 mg/kg, apoptotic figures became more frequent (Fig. 1C and D) and, at the same time, necrotic tubules were clearly and unambiguously seen in several parts of the cortex (Fig. 1C). Apoptotic cells were most often found in tubule sections that otherwise looked unremarkable but were also observed in necrotic tubules. In parallel with these conventional staining techniques, we examined kidney sections after in situ immunohistochemical demonstration of DNA fragmentation (TUNEL technique). As shown in Fig. 2, scattered, peroxidase-labeled nuclei could easily be detected over the entire cortex from all treated animals, due to their brownish appearance contrasting with the light background (Fig. 2B and C). Most of these labeled nuclei were seen in proximal tubule epithelium, but some were also observed in the lumen. Sections from control animals showed only a very few labeled nuclei (Fig. 2A).
FIG. 1.
Light microscopy appearance of paraffin sections of kidney cortex. (A and C) Sections stained with hematoxylin and eosin; (B and D) sections stained with methyl green-pyronine (Brachet staining [8]). Animals were treated with gentamicin for 10 days (10 [A and B] or 20 [C and D] mg/kg). Single arrows point to cells showing clear evidence of nuclear alterations related to apoptosis; the double arrowhead (in panel C) shows a cell with clear shrinkage and eosinophilia of the cytoplasm; NT (in panel C) denotes a necrotic tubule. Two mitotic figures are circled in panels B and D. Bars, 20 μm.
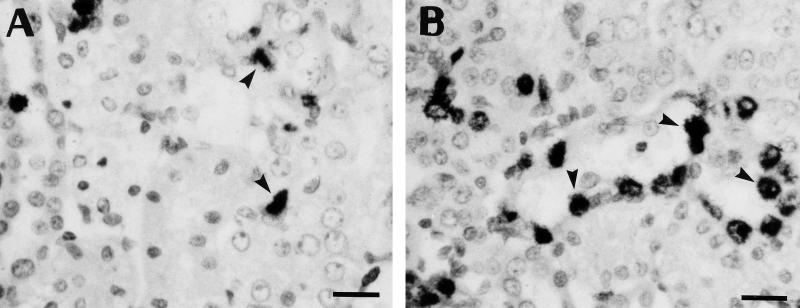
FIG. 2.
Immunohistochemical demonstration of apoptotic nuclei in sections of kidney cortical specimens of controls (A) and animals treated for 10 days with gentamicin at 10 (B) and 20 (C) mg/kg. Sections were treated for TdT-mediated labeling of fragmented DNA (TUNEL [19]). Positive nuclei were identified as shrunken, darkly stained bodies with either a condensed or a fragmented appearance (arrowheads). Labeling is also detected in small granules which probably originate from the disruption of an apoptotic nucleus (arrows). When these granules occurred in clusters, only one count was recorded in the quantitative analysis (Fig. 3). Bars, 20 μm.
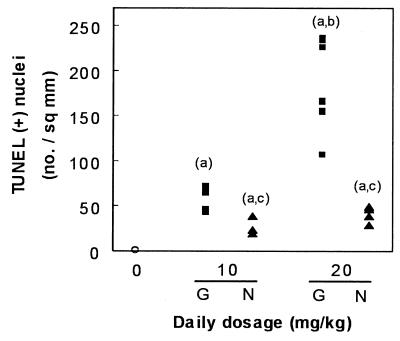
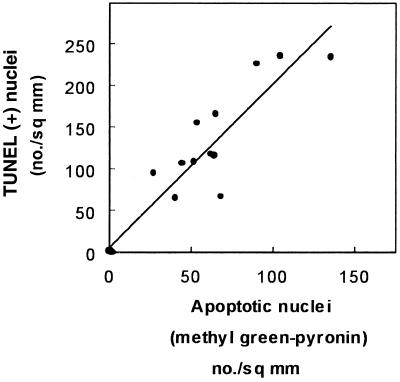
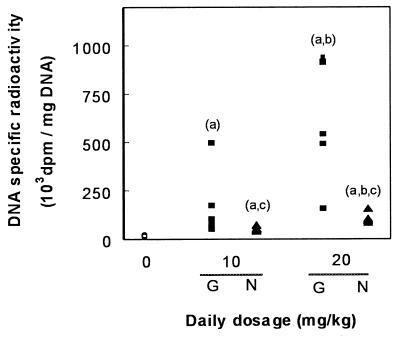
Having thus demonstrated qualitatively by both conventional histology and TUNEL the occurrence of apoptosis in the kidney cortex of animals treated with gentamicin, we undertook a quantification of this process in comparison with netilmicin. Figure 3 shows the results obtained with TUNEL. Although there was a fair degree of variability among individual animals at the highest dose of gentamicin (20 mg/kg), the results clearly showed a dose dependency of the apoptotic process, with values reaching up to 240 times those of control animals. A clear-cut response was also noted for netilmicin but to a markedly lesser extent than for gentamicin. The occurrence of the apoptotic process was also quantitated using methyl green-pyronine staining. Figure 4 shows that a highly significant correlation was obtained between the values given by these two techniques, indicating that the variation recorded for the apoptotic response was linked to a variability among animals and not to the technique used. Yet, about twice as many apoptotic figures were scored using the TUNEL technique as were scored by the methyl green-pyronine staining method, suggesting a major difference in the sensitivities of the two methods (see Discussion).
FIG. 3.
Enumeration of apoptotic nuclei observed in cortex sections after application of TUNEL (Fig. 2). Open circles, control animals; filled squares, animals treated for 10 days with gentamicin (G); filled triangles, animals treated with netilmicin (N). Each point refers to the pooled counts of one individual animal (n = 6 in each group). Statistical analysis (ANOVA followed by Scheffe post hoc test with P < 0.05): a, significantly higher than control; b, significantly higher than the lower dose; c, significantly lower than the other aminoglycoside at the same dosage.
FIG. 4.
Correlation between the frequency of apoptotic nuclei detected by TdT-mediated labeling of fragmented DNA (TUNEL) and revealed by methyl green-pyronine (Brachet staining) in control animals and in animals treated for 10 days with gentamicin at 10 or 20 mg/kg (r = 0.89; n = 18; P < 0.001).
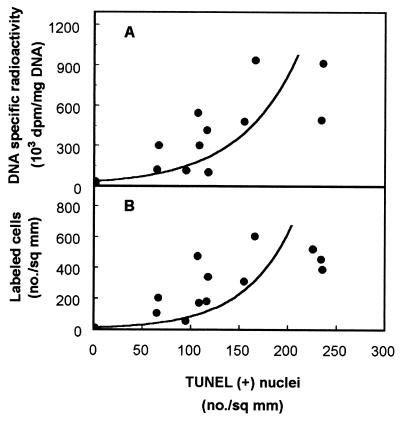
We knew from a previous study (38) that gentamicin, at the dose of 10 mg/kg, induces a marked proliferative response in kidney cortex. This reaction was therefore quantitated in the present study using the [3H]thymidine incorporation approach to draw quantitative correlations with the apoptotic process. Figure 5 shows that a large number of labeled nuclei could easily be observed in both the proximal tubules and the interstitium of gentamicin-treated rats. Using the criterium of 3H incorporation in cortical DNA (Fig. 6), we noted that the response was highly variable but nevertheless clearly dose dependent (this variability was already noted in earlier studies [38] and was shown to result from true variation among treated animals in their ability to incorporate [3H]thymidine in cortical tissue [54]). A marked difference between gentamicin-treated and netilmicin-treated animals was also observed. A reasonable correlation was obtained when comparing the biochemical data (DNA radioactivity) with the enumeration of cells labeled by histoautoradiography in the kidney cortex of the same animals. Finally, Fig. 7 shows that there was a statistically significant correlation between the proliferative process (quantitated by either the measurement of the cortical DNA radioactivity or the enumeration of labeled cells after histoautoradiography) and the apoptotic response (quantitated by TUNEL). The statistical analysis suggested, however, that the correlation was not linear.
FIG. 5.
Light microscopy appearance of cortex sections processed for histoautoradiography. Animals were treated for 10 days with gentamicin (10 [A] or 20 [B] mg/kg). Bars, 20 μm. Each animal received 200 μCi of [3H]thymidine 1 h before sacrifice for the demonstration of DNA synthesis in S-phase cells (arrowheads).
FIG. 6.
Determination of the DNA specific radioactivity in renal tissue cortex. Open circles, controls; filled squares, animals treated for 10 days with gentamicin (G); filled triangles, animals treated with netilmicin (N). Each animal received an intraperitoneal injection of 200 μCi of [3H]thymidine 1 h before sacrifice. Each symbol refers to an individual animal (n = 6 in each group). Statistical analysis (ANOVA followed by Scheffe post hoc test with P < 0.05): a, significantly higher than control; b, significantly higher than the lower dose; c, significantly lower than the other aminoglycoside at the same dosage.
FIG. 7.
Correlation between cortical DNA radioactivity (A) or frequency of labeled cells in cortex (B) and apoptosis (detected by TUNEL) in controls and in animals treated for 10 days with gentamicin at 10 or 20 mg/kg. Curvilinear, polynomial functions were fitted by GraphPad software (for panel A, r = 0.90, n = 36, and P < 0.01; for panel B, r = 0.88, n = 18, and P < 0.01) after a first analysis disclosed a statistically significant nonlinear relationship.
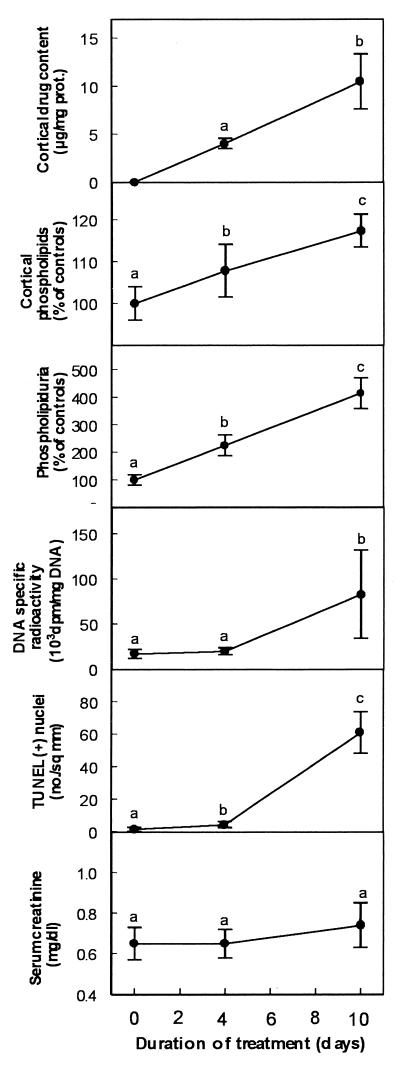
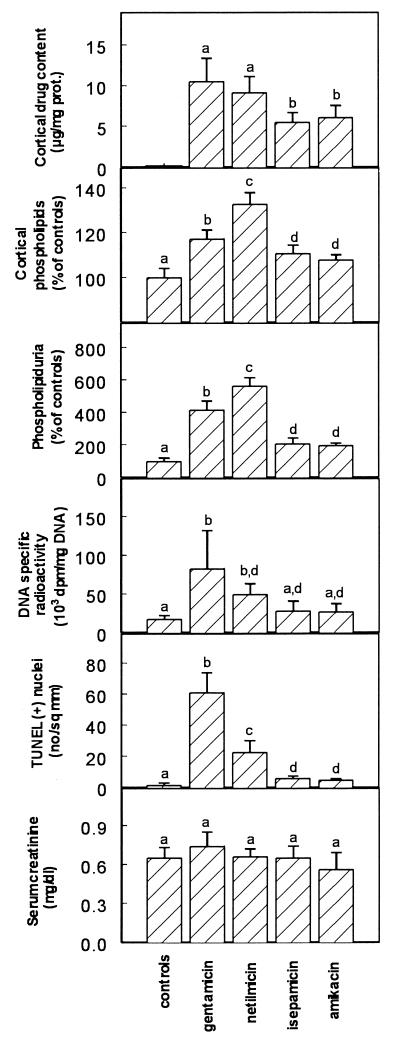
To further delineate a potential relationship between all changes demonstrated so far, we examined the time dependence of their occurrence by treating animals for both 4 and 10 days. We used only gentamicin (to obtain large responses for all criteria) but selected the low dose (10 mg/kg) to fully exclude the possibility of causing extended necrosis. Results presented in Fig. 8 clearly show that, while drug accumulation and development of cortical phospholipidosis and phospholipiduria proceeded in parallel and almost linearly with time, both DNA synthesis and apoptosis were delayed events with only minimal changes detected at day 4 compared to what was seen at day 10. Serum creatinine was only minimally increased at the end of the treatment. In a final series of experiments, we compared gentamicin and netilmicin to amikacin and isepamicin at an equitherapeutic dose. We selected again the low dose of gentamicin (10 mg/kg) and set, accordingly, the doses of amikacin and isepamicin at 40 mg/kg, based on their corresponding dosage ratios to gentamicin in human clinical use. Netilmicin was also included to complete the comparison. The treatment period was set at 10 days to allow for the potential development of all changes while remaining within acceptable clinical criteria of treatment duration. As shown in Fig. 9, amikacin and isepamicin (i) accumulated to lower levels than gentamicin and netilmicin in the kidney cortex in spite of their higher dosage, (ii) caused a milder phospholipidosis as assessed by the two criteria of cortical phospholipid content and phospholipiduria, (iii) caused only a modest increase in cortical DNA radioactivity, and (iv) caused only minimal apoptosis (all values for both amikacin- and isepamicin-treated animals were statistically different from those of gentamicin- and netilmicin-treated ones). No change in renal function was seen for all drugs.
FIG. 8.
Cortical drug content, cortical total phospholipids, urinary excretion of phospholipids, DNA specific radioactivity, apoptotic nuclei in renal cortex (TUNEL), and serum creatinine in rats treated receiving saline (controls) or gentamicin at 10 mg/kg for 4 or 10 days. Values are means ± standard deviations (n = 6 in each group). Symbols with different letters in each graph indicate values significantly different from each other based on statistical analysis by ANOVA followed by Scheffe post hoc test (P < 0.05). prot., protein.
FIG. 9.
Cortical drug content, cortical total phospholipids, urinary excretion of phospholipids, DNA specific radioactivity in renal cortex, apoptotic cells in cortex sections (TUNEL), and serum creatinine in rats treated with saline (controls) or with gentamicin (10 mg/kg), netilmicin (10 mg/kg), amikacin (40 mg/kg), or isepamicin (40 mg/kg) for 10 days. Values are means ± standard deviations (n = 6 in each group). Groups with different letters are significantly different from each other based on statistical analysis by ANOVA followed by Scheffe post hoc test (P < 0.05). prot., protein.
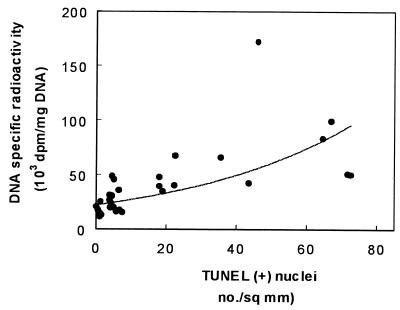
Finally, we tested whether apoptosis and DNA synthesis were correlated at the level of all individual animals used to generate the data of Fig. 9. Figure 10 shows that the DNA specific radioactivity and TUNEL were indeed correlated in a nonlinear fashion in an imperfect but nevertheless significant manner. Similarly, significant, nonlinear correlations were obtained when using the data from the enumeration of S-phase cells to evaluate the proliferative process or when assessing apoptosis by methyl green-pyronine staining (data not shown).
FIG. 10.
Correlation between apoptotic cells (TUNEL) and tubular proliferation (DNA specific radioactivity) in control animals and all animals treated with aminoglycosides (gentamicin, netilmicin, amikacin, and isepamicin) for 10 days. Data and treatments are those used for Fig. 9. A curvilinear, polynomial function was fitted by GraphPad software (r = 0.69; n = 18; P < 0.01) after a first analysis disclosed a statistically significant nonlinear relationship.
DISCUSSION
Low multiples of the human therapeutic doses of aminoglycosides have long been known to cause an array of tubular alterations without signs of extended necrosis and overt tubular dysfunction (48). These alterations were therefore considered by many observers to be unrelated to toxicity defined by histopathological damage (mainly cell death) and alteration of kidney function (decrease of glomerular filtration) (20). Yet, our observation that low-dose treatment is associated with a highly significant increase in cellular proliferation of cortical cells (proximal tubules and interstitium [38]), a process typically considered as denoting tissue repair and inflammation, has cast doubt on the benign character of these alterations (39). The present data now provide unambiguous, quantitative evidence that proximal tubular epithelium actually undergoes a marked process of nonnecrotic cell death, namely, apoptosis, upon exposure to gentamicin at these low multiples of the clinical doses. The morphological techniques that we have used clearly identify the characteristic changes associated with the apoptotic process, namely, a nuclear condensation and fragmentation, together with a shrinkage of the cell body, an eosinophilia of the cytoplasm, and the characteristic cleavage of DNA (demonstrated in situ by the TUNEL technique). The lower incidence of apoptotic cells or clusters of apoptotic bodies detected by Brachet staining than by the TUNEL approach can probably be accounted for by the fact that the former detects cells where chromatin has undergone marked condensation and the cytoplasm has been profoundly modified, corresponding to a late stage in apoptosis, whereas the latter allows one to label all nuclei in which DNA has been fragmented, i.e., at an earlier stage of the apoptotic process. Although transcription and RNA splicing can to some extent interfere with the TUNEL method and lead to an overestimation of apoptotic cells (35), we believe that the apoptotic process present here is probably more extensive than can be evidenced by our approaches. Apoptotic cells, indeed, display changes in surface-exposed sugars and modifications of phospholipid transversal distribution in plasma membrane, together with an increase in vitronectin content (17, 57, 58), that ensure their recognition and swift engulfment by adjacent cells (59). Apoptotic cells, and damaged tubular cells in general, quickly detach from the basement membrane and are shed in the urine (55, 56, 66). All these cells will escape detection in tissue sections. Despite these uncertainties concerning the actual frequency of apoptotic cells, it is probably safe to say that apoptosis induced by gentamicin in proximal tubular epithelium of kidney is widespread and develops in a dose-dependent fashion. The data therefore indicate that apoptosis is clearly part of the array of early changes occurring in renal tissue after treatment with aminoglycosides at low doses, i.e., in the absence of detectable necrosis and functional alterations. We also show that apoptosis and necrosis can simultaneously occur in proximal tubules when the dosage of gentamicin is moderately increased (20 mg/kg). The observations reported here are therefore of a different nature than those of Nouwen et al. (51), who reported the occurrence of apoptosis in distal segments of the nephron after induction of acute necrosis in proximal tubules by massive gentamicin dosage (400 mg/kg for 2 days).
Apoptosis plays a major role in kidney embryogenesis, resulting in large-scale cell death during development (12). By contrast, in the adult and under normal circumstances, evidence of apoptosis is seldom found in the kidney, where the rate of cell turnover is very low. However, there are a number of documented cases related to kidney insult in both pathology and toxicology where the renal tissue, in particular the tubular epithelium, exhibits a substantial increase of apoptotic cells (13, 14). Thus, apoptosis is clearly involved in ischemic renal atrophy (22) and in the recovery of tubular epithelium after temporary ischemia (62). The intercalating anticancer agents doxorubicin and mitoxantrone have also been found to induce apoptosis in renal tubule epithelium (25). At a low concentration, cisplatin, another antitumor drug well known for its renal toxicity, causes apoptosis in cultured proximal tubular cells (41). Subchronic cadmium intoxication of kidney is also associated with cell apoptosis occurring in the absence of necrosis in the S3 segment of proximal tubules (65). Interestingly enough, the frequency of apoptotic cells in that model evolves in parallel with that of S-phase cells, exactly as demonstrated in the present study.
We may at this stage only speculate on the mechanisms by which aminoglycosides, and gentamicin in particular, cause apoptosis in proximal tubules. It is currently acknowledged that the process leading to apoptosis can be divided into two major phases (40), namely, the commitment phase and the execution phase. The commitment phase is reversible and determines whether or not a cell will undergo apoptosis. This phase can be triggered by a variety of external or internal stimuli, the influence of which is now viewed as creating inappropriate signals in a given context. This phase is under the control of an intricate network of regulating systems, most of them also involved in cellular physiological processes distinct from apoptosis. It is during the execution phase, which is irreversible, that the cell exhibits the various morphological and biochemical changes typical of apoptosis, including chromatin compaction and internucleosomal DNA cleavage. Despite the fact that apoptosis can result from exposure to a wide variety of stimuli, the execution phase displays a remarkable similarity irrespective of the cell type or apoptosis inducer and is probably mediated by a common pathway. Our current knowledge of the renal disposition of aminoglycosides suggests that these drugs could act upon either phase of the apoptotic process.
Likely mechanisms related to the commitment phase are (i) a direct effect of aminoglycosides on membranes, especially the brush border membrane causing inhibition of phosphatidylinositol phospholipase C and/or impairment of protein kinase C, both processes likely to lead to apoptosis (47, 61); and (ii) a perturbation of mitochondrial integrity leading to the release of caspase-activating proteins (23). The former series of mechanisms, which essentially rely on direct contact between drug and membrane, are demonstrable upon exposure to aminoglycosides (24, 42, 46, 60). In our opinion, however, an effect of gentamicin at this level is difficult to reconcile with the fact that apoptosis, although already detectable at day 4, becomes particularly prominent only at a later stage (Fig. 8). This suggests that it is not the mere contact but the accumulation of the gentamicin within kidney cortex which actually causes, directly or indirectly, apoptosis. Gentamicin accumulated within cells has been shown to interact with mitochondria, causing disruption of electron transport and a drop in ATP (63, 75, 76), and these changes could trigger one or several of the recently described mitochondrial signals involved in the commitment phase of apoptosis (23). However, most if not all metabolic effects of aminoglycosides on mitochondria have been shown to occur after cell death (77).
It is tempting to speculate that aminoglycosides act at the execution phase by disrupting lysosomes (following their swelling through phospholipidosis) and provoking the release of cysteine proteases capable of activating caspase-3 (30), a key effector in the execution phase of apoptosis (16). This process, which was already proposed to account for gentamicin-induced cellular necrosis (37, 71) and appears to operate in other cases of apoptosis (9), would nicely explain (i) the delay in the onset of apoptosis (since a threshold in lysosomal dilation is probably necessary to cause rupture [70]), (ii) the self-limiting character of the cortical phospholipidosis (through sloughing of phospholipid-laden cells), and (iii) the more favorable behavior of amikacin and isepamicin than of gentamicin (mediated by both their lower accumulation [this study] and their lesser inhibitory potential toward lysosomal phospholipases [11, 72]). Yet, a key role of lysosomal alterations in eliciting apoptosis is difficult to reconcile with the divergent effects of gentamicin and netilmicin on apoptosis and their common behavior with respect to phospholipidosis. In the absence of further characterization of the precise process ongoing with netilmicin, the role of lysosomal alterations must therefore remain unsettled with respect to the onset of apoptosis. Generally speaking, a more extensive analysis of the toxicological behavior of netilmicin is warranted, since this aminoglycoside shows a remarkably low overall toxicity in animals upon acute exposure at large doses (29, 53, 64) but not with chronic treatments at low doses (26).
The toxicological implications of our observations need to be critically examined. First, the doses of 10 to 20 mg/kg for gentamicin and netilmicin and of 40 mg/kg for amikacin and isepamicin which were used in this study can probably be considered low multiples of the corresponding human doses. They actually can be considered to provide a total drug exposure (in terms of area under the serum concentration-time curve) lower than or similar to what is observed in humans treated with the recommended clinical doses of these antibiotics (20) (Table 1), taking into consideration the difference in drug elimination half-lives between rats (≈30 min) and humans (≈120 min). The present data are, therefore, likely to be of immediate clinical relevance and may provide the basis for future investigations in humans. Second, the loss of tubular cells by apoptosis can probably be viewed as a mechanism of tissue damage, and more extensive studies, comparing a large array of aminoglycosides with different established toxicities or using various nephroprotectants (48), would certainly be useful in this context. Yet, one may also argue that apoptosis is a favorable event insofar as it results in an early and swift elimination of those cells the viability of which is compromised, thereby allowing an efficient nephrogenic repair that prevents the denudation of the tubular basement membrane. The fact that netilmicin, amikacin, and isepamicin, all of which have a better safety profile than gentamicin (48), also cause less apoptosis pleads strongly, however, for a pejorative view of aminoglycoside-induced apoptosis. Third, the fact that a moderately large dose of gentamicin (20 mg/kg) induces both apoptosis and necrosis actually suggests the existence of two distinct mechanisms of cytotoxicity which could be entirely different but could also be related to a limited versus a massive alteration of a common target. Finally, it now becomes almost certain that the sizeable increase of DNA synthesis detected in the absence of necrosis in tubular epithelium after treatment of animals with low doses of aminoglycosides results from cellular proliferation in response to apoptosis and compensates for the loss of epithelial cells. As shown earlier, labeled cells seen in tubular epithelium after [3H]thymidine pulse appear indeed in tubules displaying signs of regeneration and the extent of DNA labeling in cortex matches the abundance of dedifferentiated cells (38, 67). Yet, we cannot exclude the partial involvement of a DNA repair process associated with apoptosis itself. The statistical analysis revealed that the correlation between the increase in DNA synthesis and apoptosis is not linear, with a more intense cellular proliferation than anticipated at large doses. As discussed above, this observation may merely indicate that the extent of apoptosis is underestimated at large doses because of the swift loss of apoptotic cells from the epithelium. It may, however, also suggest that apoptosis is associated with the release of mitogenic factors which are expected to cause a more than directly proportionate effects (18). In this context, the peritubular proliferation which is also observed and has been fully documented and quantitated in previous studies conducted with the same low doses of aminoglycosides (38, 67) is probably highly indicative of such a mitogenic effect caused by tubular injury, since there is no evidence that aminoglycosides cause direct interstitial damage.
ACKNOWLEDGMENTS
This work was supported by a fellowship (Aides aux Etudes) to M. El Mouedden and a grant-in-aid to the Unité de Pharmacologie Cellulaire et Moléculaire from the French nonprofit organization Vaincre les Maladies Lysosomales, Ozoir-la-Ferrière, France, and grants from the Belgian Fund for Medical Scientific Research (grant no. 3.4516.94), and the Actions de Recherches Concertées (contract no. 94/99-172) of the Direction Générale de la Recherche Scientifique—Communauté Française de Belgique. G. Laurent and M.-P. Mingeot-Leclercq are Senior Research Associate and Research Associate, respectively, of the National Fund for Scientific Research (Belgium).
M. R. Dujardin provided technical assistance for the histological studies, and M. Philippe provided access to clinical laboratory facilities for the measurement of serum creatinine and BUN.
REFERENCES
- 1.Alison M R, Sarraf C E. Apoptosis: regulation and relevance to toxicology. Hum Exp Toxicol. 1995;14:234–247. doi: 10.1177/096032719501400302. [DOI] [PubMed] [Google Scholar]
- 2.Aoshiba K, Nagai A, Konno K. Erythromycin shortens neutrophil survival by accelerating apoptosis. Antimicrob Agents Chemother. 1995;39:872–877. doi: 10.1128/aac.39.4.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arends M J, Wyllie A H. Apoptosis: mechanisms and roles in pathology. Int Rev Exp Pathol. 1991;32:2523–2541. doi: 10.1016/b978-0-12-364932-4.50010-1. [DOI] [PubMed] [Google Scholar]
- 4.Bartels H, Böhmer M, Heierli C. Serum Kreatininbestimmung ohne Enteiweissen. Clin Chim Acta. 1972;37:193–197. doi: 10.1016/0009-8981(72)90432-9. [DOI] [PubMed] [Google Scholar]
- 5.Bartlett G R. Phosphorus assay in column chromatography. J Biol Chem. 1959;234:466–468. [PubMed] [Google Scholar]
- 6.Bennett W M, Plamp C E, Gilbert D N, Parker R A, Porter G A. The influence of dosage regimen on experimental gentamicin nephrotoxicity: dissociation of peak serum levels from renal failure. J Infect Dis. 1979;140:576–580. doi: 10.1093/infdis/140.4.576. [DOI] [PubMed] [Google Scholar]
- 7.Bligh E G, Dyer W J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 8.Brachet J. The use of basic dyes and ribonuclease for the cytochemical detection of ribonucleic acid. Q J Microsc Sci. 1953;94:1–10. [Google Scholar]
- 9.Brunk U T, Dalen H, Roberg K, Hellquist H B. Photooxidative disruption of lysosomal membranes causes apoptosis of cultured human fibroblasts. Free Radic Biol Med. 1997;23:616–626. doi: 10.1016/s0891-5849(97)00007-5. [DOI] [PubMed] [Google Scholar]
- 10.Buja L M, Eigenbordt M L, Eigenbordt E H. Apoptosis and necrosis: basic types and mechanisms of cell death. Arch Pathol Lab Med. 1993;117:1208–1215. [PubMed] [Google Scholar]
- 11.Carlier M B, Laurent G, Claes P J, Vanderhaeghe H J, Tulkens P M. Inhibition of lysosomal phospholipases by aminoglycoside antibiotics: in vitro comparative studies. Antimicrob Agents Chemother. 1983;23:440–449. doi: 10.1128/aac.23.3.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coles H S R, Burne J F, Raff M C. Large scale normal cell death in the developing rat kidney and its reduction by epidermal growth factor. Development. 1993;118:777–784. doi: 10.1242/dev.118.3.777. [DOI] [PubMed] [Google Scholar]
- 13.Conaldi P G, Biancone L, Bottelli A, Wade-Evans A, Racusen L C, Boccellino M, Toniolo A. HIV-1 kills renal tubular epithelial cells in vitro by triggering an apoptotic pathway involving caspase activation and Fas upregulation. J Clin Investig. 1998;102:2041–2049. doi: 10.1172/JCI3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis M A, Ryan D H. Apoptosis in the kidney. Toxicol Pathol. 1998;26:810–825. doi: 10.1177/019262339802600615. [DOI] [PubMed] [Google Scholar]
- 15.DiGiorgio J. Nonprotein nitrogenous constituents. In: Henry R J, Cannon D C, Winkelman J W, editors. Clinical chemistry: principles and techniques. 2nd ed. New York, N.Y: Harper and Row Publishers, Inc.; 1974. pp. 503–564. [Google Scholar]
- 16.Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A, Nagata S. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature. 1998;391:43–50. doi: 10.1038/34112. [DOI] [PubMed] [Google Scholar]
- 17.Fadok V A, Voelker D R, Campbell P A, Cohen J J, Bratton D L, Henson P M. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148:2207–2216. [PubMed] [Google Scholar]
- 18.Fine L G, Hammerman M R, Abboud H E. Evolving role of growth factors in the renal response to acute and chronic disease. J Am Soc Nephrol. 1992;2:1163–1170. doi: 10.1681/ASN.V271163. [DOI] [PubMed] [Google Scholar]
- 19.Gavrieli Y, Sherman Y, Ben-Sasson S A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gilbert D N. Aminoglycosides. In: Mandell G L, Bennett J E, Dolin R, editors. Principles and practice of infectious diseases. 4th ed. New York, N.Y: Churchill Livingstone; 1995. pp. 279–306. [Google Scholar]
- 21.Gilbert D N. Editorial response: meta-analyses are no longer required for determining the efficacy of single daily dosing of aminoglycosides. Clin Infect Dis. 1997;24:816–819. doi: 10.1093/clinids/24.5.816. [DOI] [PubMed] [Google Scholar]
- 22.Gobe G C, Axelsen R A. Genesis of renal tubular atrophy in experimental hydronephrosis in the rat. Role of apoptosis. Lab Investig. 1987;56:273–282. [PubMed] [Google Scholar]
- 23.Green D R, Reed J C. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 24.Hagiwara M, Inagaki M, Kanamura K, Ohta H, Hidaka H. Inhibitory effects of aminoglycosides on renal protein phosphorylation by protein kinase C. J Pharmacol Exp Ther. 1988;244:355–360. [PubMed] [Google Scholar]
- 25.Herman E H, Zhang J, Hasinoff B B, Clarck J R, Ferrans V J. Comparison of the structural changes induced by doxorubicin and mitoxantrone in the heart, kidney and intestine and characterization of the Fe(III)-mitoxantrone complex. J Mol Cell Cardiol. 1997;29:2415–2430. doi: 10.1006/jmcc.1997.0477. [DOI] [PubMed] [Google Scholar]
- 26.Hottendorf G H, Barnett D, Gordon L L, Christensen E F, Madissoo H. Nonparallel nephrotoxicity dose-response curves of aminoglycosides. Antimicrob Agents Chemother. 1981;19:1024–1028. doi: 10.1128/aac.19.6.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ibrahim S, Clerckx-Braun F, Jacqmin P, Donnez J, Brulein V, Tulkens P M. Effect of the schedule of administration of two aminoglycosides, netilmicin and amikacin on urinary phospholipid excretion in humans. In: Bach P H, Gregg N J, Wilks M F, Delacruz L, editors. Nephrotoxicity: mechanisms, early diagnosis and therapeutic management. New York, N.Y: Marcel Dekker; 1991. pp. 93–97. [Google Scholar]
- 28.Ibrahim S, Van der Auwera P, Meunier F, Tulkens P M. Effect of netilmicin and amikacin on urinary phospholipid excretion in humans. Arch Toxicol. 1989;13(Suppl.):413–416. doi: 10.1007/978-3-642-74117-3_81. [DOI] [PubMed] [Google Scholar]
- 29.Igarashi M, Levy J K, Jerger J. Comparative toxicity of netilmicin and gentamicin in squirrel monkeys (Saimiri sciureus) J Infect Dis. 1978;137:476–480. doi: 10.1093/infdis/137.4.476. [DOI] [PubMed] [Google Scholar]
- 30.Ishisaka R, Utsumi T, Yabuki M, Kanno T, Furuno T, Inoue M, Utsumi K. Activation of caspase-3-like protease by digitonin-treated lysosomes. FEBS Lett. 1998;435:233–236. doi: 10.1016/s0014-5793(98)01080-1. [DOI] [PubMed] [Google Scholar]
- 31.Jaffé M. Über den Niederschlag welchen Pikrinsäure in normalen Harn erzeugt und über eine neue Reaktion des Kreatinins. Z Physiol Chem. 1886;10:391–400. [Google Scholar]
- 32.Josepovitz C, Levine R, Lane B, Kaloyanides G J. Contrasting effects of gentamicin and mercuric chloride on urinary excretion of enzymes and phospholipids in the rat. Lab Investig. 1985;52:375–386. [PubMed] [Google Scholar]
- 33.Kerr J F R, Wyllie A H, Currie A R. Apoptosis: a basic biological phenomenon with wide ranging implications in tissue kinetics. J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kishore B K, Ibrahim S, Lambricht P, Laurent G, Maldague P, Tulkens P M. Comparative assessment of poly-L-aspartic and poly-L-glutamic acids against gentamicin-induced renal lysosomal phospholipidosis, phospholipiduria and cell proliferation in rats. J Pharmacol Exp Ther. 1992;262:424–432. [PubMed] [Google Scholar]
- 35.Kockx M M, Muhring J, Knaapen M W M, de Meyer G R Y. RNA synthesis and splicing interferes with DNA in situ end labeling techniques used to detect apoptosis. Am J Pathol. 1998;152:885–888. [PMC free article] [PubMed] [Google Scholar]
- 36.Lang H, Liu C. Apoptosis and hair cell degeneration in the vestibular sensory epithelia of the guinea pig following a gentamicin insult. Hear Res. 1997;111:177–184. doi: 10.1016/s0378-5955(97)00098-1. [DOI] [PubMed] [Google Scholar]
- 37.Laurent G, Kishore B K, Tulkens P M. Aminoglycoside-induced phospholipidosis and nephrotoxicity. Biochem Pharmacol. 1990;40:2383–2392. doi: 10.1016/0006-2952(90)90078-y. [DOI] [PubMed] [Google Scholar]
- 38.Laurent G, Maldague P, Carlier M B, Tulkens P. Increased renal DNA synthesis in vivo after administration of low doses of gentamicin to rats. Antimicrob Agents Chemother. 1983;24:586–593. doi: 10.1128/aac.24.4.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laurent G, Toubeau G, Heuson-Stiennon J A, Tulkens P, Maldague P. Kidney tissue repair after nephrotoxic injury: biochemical and morphological characterization. Crit Rev Toxicol. 1988;19:147–183. doi: 10.3109/10408448809014903. [DOI] [PubMed] [Google Scholar]
- 40.Lieberthal W, Levine J S. Mechanisms of apoptosis and its potential role in renal tubular epithelial cell injury. Am J Physiol. 1996;271:F471–F488. doi: 10.1152/ajprenal.1996.271.3.F477. [DOI] [PubMed] [Google Scholar]
- 41.Lieberthal W, Triaca V, Levine J. Mechanisms of death induced by cisplatin in proximal tubular epithelial cells: apoptosis vs necrosis. Am J Physiol. 1996;270:F700–F708. doi: 10.1152/ajprenal.1996.270.4.F700. [DOI] [PubMed] [Google Scholar]
- 42.Lipsky J J, Lietman P S. Aminoglycoside inhibition of a renal phosphatidylinositol phospholipase C. J Pharmacol Exp Ther. 1982;220:287–292. [PubMed] [Google Scholar]
- 43.Lockshin R A, Williams C M. Programmed cell death. I. Cytology of the degeneration in the intersegmental muscles of peryi silkmoth. J Insect Physiol. 1965;11:123–133. doi: 10.1016/0022-1910(65)90099-5. [DOI] [PubMed] [Google Scholar]
- 44.Lockshin R A, Zakeri Z. Programmed cell death and apoptosis. In: Tomei L D, Coppe F O, editors. Apoptosis: the molecular basis of cell death. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1991. pp. 47–60. [Google Scholar]
- 45.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 46.Marche P, Olier B, Girard A, Fillastre J P, Morin J P. Aminoglycoside-induced alterations of phosphoinositide metabolism. Kidney Int. 1987;31:59–64. doi: 10.1038/ki.1987.9. [DOI] [PubMed] [Google Scholar]
- 47.Miao J Y, Kaji K, Hayashi H, Araki S. Inhibitors of phospholipase promote apoptosis of human endothelial cells. J Biochem. 1997;121:612–618. doi: 10.1093/oxfordjournals.jbchem.a021629. [DOI] [PubMed] [Google Scholar]
- 48.Mingeot-Leclercq M P, Tulkens P M. Aminoglycosides: nephrotoxicity. Antimicrob Agents Chemother. 1999;43:1003–1012. doi: 10.1128/aac.43.5.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mingeot-Leclercq M P, Glupczynski Y, Tulkens P M. Aminoglycosides: activity and resistance. Antimicrob Agents Chemother. 1999;43:727–737. doi: 10.1128/aac.43.4.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nagakawa T, Yamane H, Takayama M, Sunami K, Nakai Y. Apoptosis of guinea pig cochlear hair cells following chronic aminoglycoside treatment. Eur Arch Otorhinolaryngol. 1998;255:127–131. doi: 10.1007/s004050050027. [DOI] [PubMed] [Google Scholar]
- 51.Nouwen E J, Verstrepen W A, Buyssens N, Zhu M-Q, De Broe M E. Hyperplasia, hypertrophy, and phenotypic alterations in the distal nephron after acute proximal tubular injury in the rat. Lab Investig. 1994;70:479–493. [PubMed] [Google Scholar]
- 52.Parker R A, Bennett W H, Porter G A. Animal models in the study of aminoglycoside nephrotoxicity. In: Whelton A, Neu H C, editors. The aminoglycosides: microbiology, clinical use and toxicology. New York, N.Y: Marcel Dekker, Inc.; 1982. pp. 235–267. [Google Scholar]
- 53.Parker R A, Gilbert D N, Houghton D C, Porter G A, Bennett W M. Comparative nephrotoxicities of high-dose netilmicin and tobramycin in rats. Antimicrob Agents Chemother. 1980;18:346–348. doi: 10.1128/aac.18.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Porter G A, Laurent G, Maldague P, Tulkens P M. Gentamicin-induced stimulation of DNA synthesis in rat kidney cortex. Comparison between in vivo and in vitro models. Toxicol Lett. 1984;23:205–213. doi: 10.1016/0378-4274(84)90128-0. [DOI] [PubMed] [Google Scholar]
- 55.Racusen L. Epithelial cell shedding in acute renal injury. Clin Exp Pharmacol Physiol. 1998;25:273–275. doi: 10.1111/j.1440-1681.1998.t01-3-.x. [DOI] [PubMed] [Google Scholar]
- 56.Racusen L C, Fivush B A, Li Y L, Slatnik I, Solez K. Dissociation of tubular cell detachment and tubular cell death in clinical and experimental acute tubular necrosis. Lab Investig. 1991;64:546–556. [PubMed] [Google Scholar]
- 57.Savill J, Dransfield I, Hogg N, Haslett C. Vitronectin receptor mediated phagocytosis of cells undergoing apoptosis. Nature. 1990;343:170–173. doi: 10.1038/343170a0. [DOI] [PubMed] [Google Scholar]
- 58.Savill J S, Fadok V, Henson P, Haslett C. Phagocyte recognition of cells undergoing apoptosis. Immunol Today. 1993;14:131–136. doi: 10.1016/0167-5699(93)90215-7. [DOI] [PubMed] [Google Scholar]
- 59.Savill J S, Henson P M, Haslett C. Phagocytosis of aged human neutrophils by macrophages is mediated by novel “charge sensitive” recognition mechanism. J Clin Investig. 1989;84:1518–1527. doi: 10.1172/JCI114328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schwertz D W, Kreisberg J I, Venkatachalam M A. Effects of aminoglycosides on proximal tubule brush border membrane phosphatidylinositol-specific phospholipase C. J Pharmacol Exp Ther. 1984;231:48–55. [PubMed] [Google Scholar]
- 61.Serlachius E, Svennilson J, Schalling M, Asperia A. Protein kinase C in the developing kidney: isoform expression and effects of ceramide and PKC inhibitors. Kidney Int. 1997;52:901–910. doi: 10.1038/ki.1997.411. [DOI] [PubMed] [Google Scholar]
- 62.Shimizu A, Yamada N. Apoptosis and cell desquamation in repair process of ischemic tubular necrosis. Mol Pathol. 1993;64:171–180. doi: 10.1007/BF02915110. [DOI] [PubMed] [Google Scholar]
- 63.Simmons C F, Ronald J, Bogusky T, Humes H D. Inhibitory effects of gentamicin on renal mitochondrial oxidative phosphorylation. J Pharmacol Exp Ther. 1980;214:709–715. [PubMed] [Google Scholar]
- 64.Szot R J, McCormick G, Chung M, Christie B, Weinberg E, Schwartz E. Comparative toxicity of netilmicin and tobramycin in dogs. Toxicol Appl Pharmacol. 1980;55:169–178. doi: 10.1016/0041-008x(80)90233-1. [DOI] [PubMed] [Google Scholar]
- 65.Tanimoto A, Hamada T, Koide O. Cell death and regeneration of renal proximal tubular cells in rats with subchronic cadmium intoxication. Toxicol Pathol. 1993;21:341–352. doi: 10.1177/019262339302100401. [DOI] [PubMed] [Google Scholar]
- 66.Thompson C B. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 67.Toubeau G, Laurent G, Carlier M B, Abid S, Maldague P, Heuson-Stiennon J A, Tulkens P M. Tissue repair in rat kidney cortex after short treatment with aminoglycosides at low doses: a comparative biochemical and morphometric study. Lab Investig. 1986;54:385–393. [PubMed] [Google Scholar]
- 68.Tulkens P, Trouet A. The uptake and intracellular accumulation of aminoglycoside antibiotics in lysosomes of cultured rat fibroblasts. Biochem Pharmacol. 1978;27:415–424. doi: 10.1016/0006-2952(78)90370-2. [DOI] [PubMed] [Google Scholar]
- 69.Tulkens P, Laurent G, Carlier M B, Toubeau G, Heuson-Stiennon J, Maldague P. Comparative study of the alterations induced in rat kidney by gentamicin, dibekacin, netilmicin, tobramycin and amikacin at low doses. In: Spitzy K H, Karrer K, editors. Proceedings of the 13th International Congress of Chemotherapy. 1983. pp. 30–35. Vienna, Austria. [Google Scholar]
- 70.Tulkens P M. Experimental studies on nephrotoxicity of aminoglycosides at low doses: mechanisms and perspectives. Am J Med. 1986;80:105–114. doi: 10.1016/0002-9343(86)90487-0. [DOI] [PubMed] [Google Scholar]
- 71.Tulkens P M, De Broe M E, Maldague P, Heuson-Stiennon J A. Lysosomal alterations in aminoglycoside-induced acute renal failure. In: Solez K, Whelton A, editors. Acute renal failure: correlations between morphology and function. New York, N.Y: Marcel Dekker; 1983. pp. 299–327. [Google Scholar]
- 72.Tulkens P M, Mingeot-Leclercq M P, Laurent G, Brasseur R. Conformational and biochemical analysis of the interactions between phospholipids and aminoglycoside antibiotics in relation with their toxicity. In: Brasseur R, editor. Molecular description of biological membrane components by computer-aided conformational analysis. Boca Raton, Fla: CRC Press, Inc.; 1990. pp. 63–93. [Google Scholar]
- 73.Ueda N, Shah S V. Apoptosis. J Lab Clin Med. 1994;124:169–177. [PubMed] [Google Scholar]
- 74.Vermes I, Haanen C. Apoptosis and programmed cell death in health and disease. Adv Clin Chem. 1994;31:177–246. doi: 10.1016/s0065-2423(08)60336-4. [DOI] [PubMed] [Google Scholar]
- 75.Weinberg J M. The role of cell calcium overload in nephrotoxic renal tubular cell injury. Am J Kidney Dis. 1986;8:284–291. doi: 10.1016/s0272-6386(86)80099-3. [DOI] [PubMed] [Google Scholar]
- 76.Weinberg J M, Humes H D. Mechanisms of gentamicin-induced dysfunction of renal cortical mitochondria. I. Effects on mitochondrial respiration. Arch Biochem Biophys. 1980;205:222–231. doi: 10.1016/0003-9861(80)90102-2. [DOI] [PubMed] [Google Scholar]
- 77.Weinberg J M, Harding P G, Humes H D. Mechanisms of gentamicin-induced dysfunction of renal cortical mitochondria. II. Effects on mitochondrial monovalent cation transport. Arch Biochem Biophys. 1980;205:232–239. doi: 10.1016/0003-9861(80)90103-4. [DOI] [PubMed] [Google Scholar]