Adherence rates for initial colorectal cancer screening by FIT or mt-sDNA and for colonoscopy follow-up of a positive initial test influence the comparative effectiveness of these screening strategies. Using adherence rates based on published data for stool-based testing and colonoscopy follow-up yielded superior outcomes with an mt-sDNA versus FIT-screening strategy.
Abstract
Colorectal cancer–screening models commonly assume 100% adherence, which is inconsistent with real-world experience. The influence of adherence to initial stool-based screening [fecal immunochemical test (FIT), multitarget stool DNA (mt-sDNA)] and follow-up colonoscopy (after a positive stool test) on colorectal cancer outcomes was modeled using the Colorectal Cancer and Adenoma Incidence and Mortality Microsimulation Model. Average-risk individuals without diagnosed colorectal cancer at age 40 undergoing annual FIT or triennial mt-sDNA screening from ages 50 to 75 were simulated. Primary analyses incorporated published mt-sDNA (71%) or FIT (43%) screening adherence, with follow-up colonoscopy adherence ranging from 40% to 100%. Secondary analyses simulated 100% adherence for stool-based screening and colonoscopy follow-up (S1), published adherence for stool-based screening with 100% adherence to colonoscopy follow-up (S2), and published adherence for both stool-based screening and colonoscopy follow-up after positive mt-sDNA (73%) or FIT (47%; S3). Outcomes were life-years gained (LYG) and colorectal cancer incidence and mortality reductions (per 1,000 individuals) versus no screening. Adherence to colonoscopy follow-up after FIT had to be 4%–13% higher than mt-sDNA to reach equivalent LYG. The theoretical S1 favored FIT versus mt-sDNA (LYG 316 vs. 297; colorectal cancer incidence reduction 68% vs. 64%; colorectal cancer mortality reduction 76% vs. 72%). The more realistic S2 and S3 favored mt-sDNA versus FIT (S2: LYG 284 vs. 245, colorectal cancer incidence reduction 61% vs. 50%, colorectal cancer mortality reduction 69% vs. 59%; S3: LYG 203 vs. 113, colorectal cancer incidence reduction 43% vs. 23%, colorectal cancer mortality reduction 49% vs. 27%, respectively). Incorporating realistic adherence rates for colorectal cancer screening influences modeled outcomes and should be considered when assessing comparative effectiveness.
Prevention Relevance:
Adherence rates for initial colorectal cancer screening by FIT or mt-sDNA and for colonoscopy follow-up of a positive initial test influence the comparative effectiveness of these screening strategies. Using adherence rates based on published data for stool-based testing and colonoscopy follow-up yielded superior outcomes with an mt-sDNA versus FIT-screening strategy.
Introduction
Screening for colorectal cancer reduces colorectal cancer incidence and mortality by enabling detection and treatment of adenomas and pre-symptomatic cancers (1). Noninvasive stool-screening tests [including multitarget stool DNA (mt-sDNA) and fecal immunochemical test (FIT)] are widely endorsed, but for screening to be complete a positive stool-test needs to be followed by a colonoscopy (1). Not surprisingly, in real-world settings, adherence to mt-sDNA and FIT is reportedly imperfect (2,3,4,5). In addition, the currently available published data suggest that colonoscopy non-adherence following a positive noninvasive colorectal cancer–screening test is common and may differ between FIT and mt-sDNA. Within 6 months after a positive stool-test, colonoscopy follow-up rates range from 43% to 81% for FIT and 73% to 96% for mt-Sdna (6,7,8,9,10,11). Non-adherence to colonoscopy follow-up undermines the achievable benefits of screening. One study found that lack of colonoscopy follow-up after a positive stool-test increased the risk of colorectal cancer by 1.83-fold and increased the risk of colorectal cancer–related death by 1.56-fold over a period of 6 years (12). Delays in colonoscopy follow-up can also significantly increase the risk of incident and fatal colorectal cancer. In a large retrospective study of patients with a positive stool-test, a ≥13 month delay in follow-up colonoscopy increased the odds of colorectal cancer, and a ≥19 month delay increased the odds of colorectal cancer–related mortality when compared with a follow-up colonoscopy completed within 1–3 months (13).
Microsimulation modeling of colorectal cancer–screening strategies allows for estimating outcomes in an average risk population under a variety of changeable assumptions (14). Colorectal cancer models have been used to guide colorectal cancer–screening recommendations (15). However, many published modeling studies incorporate a theoretical assumption of 100% patient adherence for all screening and follow-up tests. The Colorectal Cancer and Adenoma Incidence and Mortality Microsimulation Model (CRC-AIM) was previously used to comprehensively analyze the impact of adherence at reported real-world adherence rates for stool-based testing, as well as outcomes over a full spectrum of adherence ranging from 10% to 100% for each screening test, or assuming varying numbers of completed stool-tests (14). The results demonstrated that altering adherence rate assumptions had a substantial impact on the predicted outcomes of colorectal cancer–screening strategies and shifted the order of model-recommend strategies to favor mt-sDNA over FIT. Although the previous analyses demonstrated the impact of initial screening adherence on predicted outcomes, the influence of more realistic adherence to follow-up colonoscopy (when indicated) on achievable screening outcomes remains incompletely defined. To build upon the impact of adherence to colorectal cancer outcomes previously demonstrated using CRC-AIM, microsimulation analyses were conducted to model the effects of (i) the traditional modeling approach of perfect adherence and (ii) reported adherence rates for FIT and mt-sDNA and colonoscopy follow-up for a positive initial test on estimated life-years gained (LYG), colorectal cancer incidence, and colorectal cancer mortality.
Materials and Methods
The CRC-AIM model has been validated and full details are available elsewhere (14, 16). CRC-AIM incorporates assumptions related to the natural history of colorectal cancer and colorectal cancer screening and uses these assumptions to calculate predicted outcomes in a simulated population. The natural history component of CRC-AIM models the sequence of adenoma to carcinoma progression and includes assumptions regarding the adenoma growth rate, adenoma location, adenoma to colorectal cancer transition probabilities, and growth of a colorectal cancer. The screening component incorporates assumptions about each colorectal cancer–screening test's sensitivity and specificity, as well as assumptions regarding complications and screening adherence.
For the present study, an average-risk US population birth cohort free of diagnosed colorectal cancer at age 40 that underwent triennial mt-sDNA or annual FIT screening from ages 50 to 75 was simulated using the CRC-AIM model. Primary analyses were performed using adherence published rates of 71% for initial mt-sDNA (2) and 43% for initial FIT (3, 4) screening, with colonoscopy follow-up adherence modeled across a wide range (40%–100%). Patients without a colonoscopy follow-up were assumed to be non-adherent until colorectal cancer symptom onset.
Three adherence scenarios were further modeled in secondary analyses: Scenario 1 included the traditional assumption of 100% adherence for all stool-based screening and colonoscopy follow-up tests (best case); Scenario 2 included published adherence rates for initial mt-sDNA (71%; ref. 2) or FIT (43%; refs. 3, 4) screening, with assumed 100% adherence to colonoscopy follow-up; and Scenario 3 included published adherence rates for initial mt-sDNA (2) or FIT (3, 4) screening and published adherence rates for colonoscopy follow-up after positive mt-sDNA (73%) or FIT (47%; ref. 6).
The predicted outcomes of LYG and percentage of reductions in colorectal cancer–related incidence and mortality in the simulated population were calculated for those undergoing a screening strategy (e.g., triennial mt-sDNA or annual FIT) and compared with those who were not screened. The outcome of LYG is defined as the number of LYG from screening that delayed or prevented death due to colorectal cancer. Outcomes for each scenario are reported per 1,000 individuals.
Sensitivity analyses were further performed using a different published adherence rate for initial FIT screening of 48.2% observed in a retrospective cohort study and was within the context of an organized screening program (5).
Results
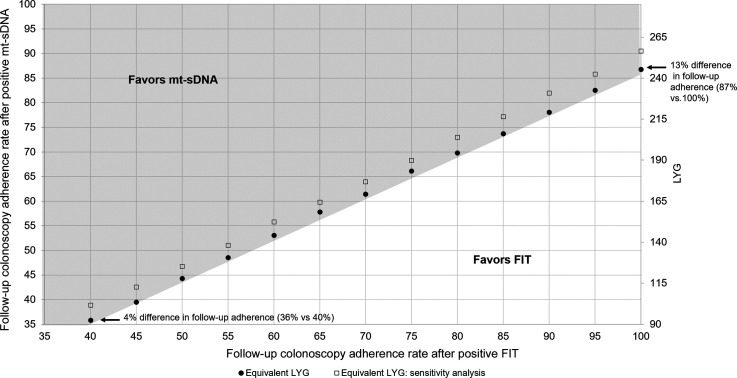
With published adherence for stool-screening tests, over the range of adherence rates for colonoscopy follow-up, mt-sDNA achieved more LYG than FIT (Fig. 1). Adherence to colonoscopy follow-up after FIT had to be 4% to 13% higher than mt-sDNA to reach equivalent LYG (Fig. 1), 6% to 17% higher to reach equivalent reductions in colorectal cancer incidence, and 4% to 14% higher to reach equivalent reductions in colorectal cancer mortality.
Figure 1.
Equal predicted life years-gained (LYG) for triennial multitarget stool DNA (mt-sDNA) and annual fecal immunochemical test (FIT) by the follow-up colonoscopy adherence rate. Circles indicate equivalent LYG when adherence to initial mt-sDNA and FIT screening is assumed to be 71% and 43%, respectively. Squares indicate equivalent LYG when adherence to initial mt-sDNA and FIT screening is assumed to be 71% and 48.2%, respectively (sensitivity analysis).
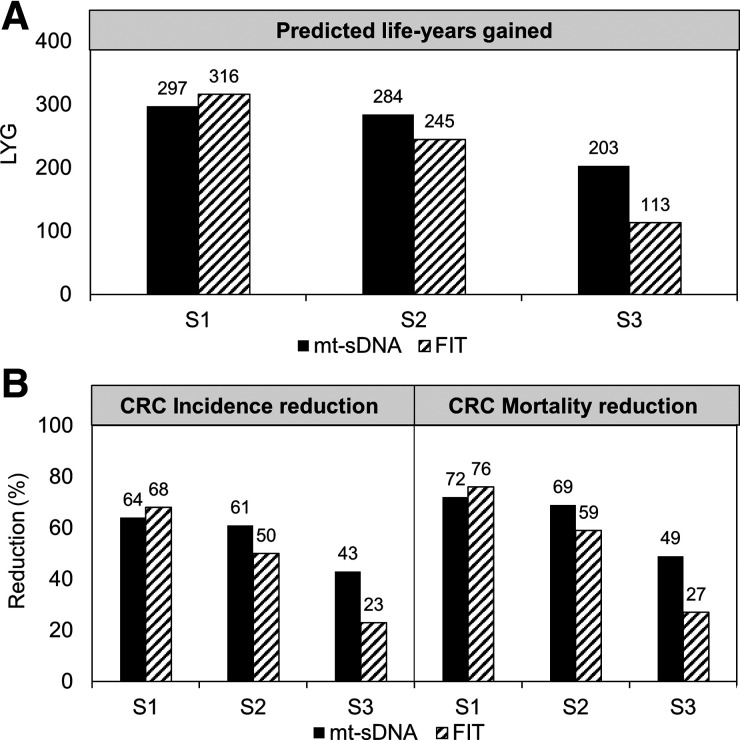
In secondary analyses, the theoretical scenario 1 yielded better outcomes with FIT versus mt-sDNA (LYG 316 vs. 297; colorectal cancer incidence reduction 68% vs. 64%; colorectal cancer mortality reduction 76% vs. 72%), whereas the more realistic scenario 2 and scenario 3 favored mt-sDNA vs. FIT (Scenario 2: LYG 284 vs. 245, colorectal cancer incidence reduction 61% vs. 50%, colorectal cancer mortality reduction 69% vs. 59%; Scenario 3: LYG 203 vs. 113, colorectal cancer incidence reduction 43% vs. 23%, colorectal cancer mortality reduction 49% vs. 27%, respectively; Fig. 2). Each scenario yielded LYG and reductions in colorectal cancer incidence and colorectal cancer mortality with either mt-sDNA or FIT screening compared with no colorectal cancer screening (Fig. 2).
Figure 2.
A, Predicted life years-gained (LYG). B, Colorectal cancer incidence and mortality reduction for triennial multitarget stool DNA (mt-sDNA) and annual fecal immunochemical test (FIT) in 3 different adherence scenarios. Results are per 1,000 individuals free of diagnosed colorectal cancer at age 40 and screened between 50 and 75 years. S1, 100% screening adherence and 100% colonoscopy follow-up adherence; S2, reported screening adherence and 100% colonoscopy follow-up adherence; S3, reported screening adherence and reported colonoscopy follow-up adherence.
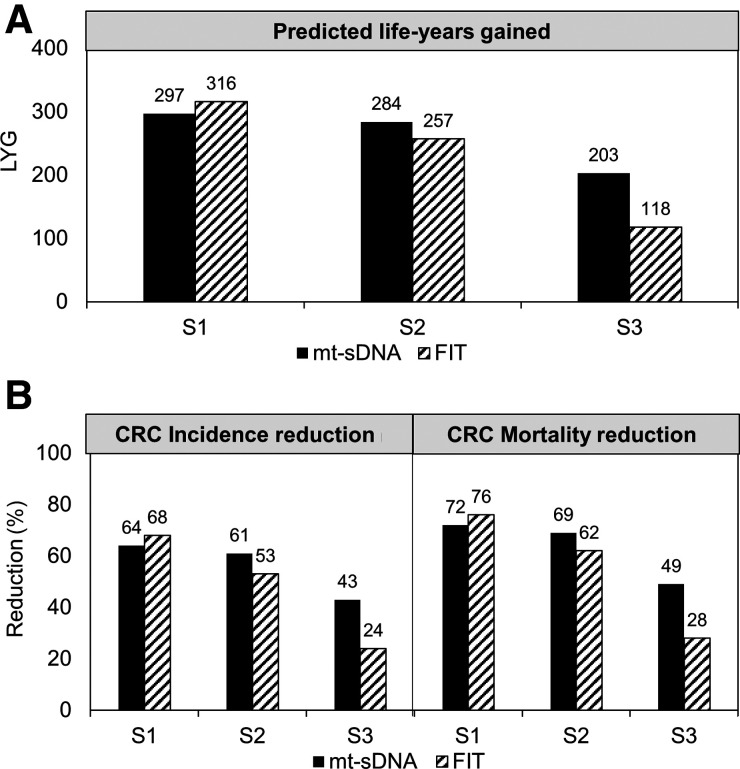
The results in the sensitivity analysis were similar to the primary results (Figs. 1 and 3).
Figure 3.
Sensitivity analysis. A, Predicted life years-gained (LYG), B, Colorectal cancer incidence and mortality reduction for triennial multitarget stool DNA (mt-sDNA) and annual fecal immunochemical test (FIT) in 3 different adherence scenarios. Results are per 1,000 individuals free of diagnosed colorectal cancer at age 40 and screened between 50 and 75 years. S1, 100% screening adherence and 100% colonoscopy follow-up adherence; S2, sensitivity analysis rates for reported screening adherence and 100% colonoscopy follow-up adherence; S3, sensitivity analysis rates for reported screening adherence and reported colonoscopy follow-up adherence.
Discussion
When published initial screening and colonoscopy follow-up adherence rates are used instead of assuming 100% adherence, the predicted benefits of the colorectal cancer–screening process are substantially decreased. When adherence rates are modeled under a wide range of likely real-world scenarios, mt-sDNA yields greater colorectal cancer–screening benefits than FIT. Furthermore, the overall results from the analysis support the concept that even under less than ideal adherence conditions, any colorectal cancer screening is beneficial and “the best test is the one that gets done” (17, 18).
Stool-based colorectal cancer screening is not complete until a follow-up colonoscopy is performed after a positive stool-test. However, there are many reasons patients do not undergo a colonoscopy follow-up. Other health issues may be of higher priority or may not permit a colonoscopy to be safely performed (6, 19). Often the patients simply refuse the colonoscopy follow-up (8, 9). Fear or anxiety about the procedure and lack of belief or awareness in the importance are key reasons for refusal (19). Non-adherence to colonoscopy follow-up has also been shown to be driven by provider and system barriers. Some of these barriers include failure to inform the patient of a positive stool-test, failure to order the colonoscopy or a preprocedural evaluation, communication challenges, and lack of action by the gastroenterology clinic staff after the colonoscopy was ordered (6, 8, 19). Colorectal cancer system-level navigation programs that track stool-test positive patients and contact patients by telephone to schedule appointments may increase the adherence to colonoscopy follow-up (20).
The currently reported analyses are limited by availability of existing data referent to real-world initial colorectal cancer screening and follow-up colonoscopy adherence rates. More research is needed to understand why the initial adherence is higher for mt-sDNA than FIT screening. However, a likely reason is the embedded patient reminders and patient navigation programs associated with all mt-sDNA test orders (2). Patient navigation has been shown to increase screening adherence (21,22,23,24), and adherence rates of approximately 50% for FIT in the United States have been observed in the context of a healthcare setting that included patient navigation (5). The published adherence rates may not be generalizable to all populations eligible for screening. For example, reported rates from a Medicare population, or from an integrated healthcare organization, or another socioeconomic or ethnic population may not extrapolate accurately to other age ranges, payers, and clinical settings. A broad range (43%–81%) of colonoscopy follow-up adherence 6 months after a positive FIT has been reported in the literature, depending on the evaluated population (6,7,8,9,10). Fewer studies have evaluated colonoscopy follow-up adherence after a positive mt-sDNA, with reported rates of 73% and 96% (we chose to apply the more conservative rate in our modeling; refs. 6, 7, 11). The higher adherence following positive mt-sDNA may be due to patient and/or provider perceptions regarding the utility of molecularly driven screening represented by this assay approach. To account for potential variability in the colonoscopy follow-up adherence, the analysis used a range of 40% to 100% adherence. Given the uncertainty around the adherence rates, a sensitivity analysis using an initial FIT adherence of 48.2% was conducted and had little impact on the results.
Although adherence is a large driver of the difference in comparative effectiveness between mt-sDNA and FIT in the current analysis, and increasing FIT adherence may mitigate much of the benefit, differences in the tests themselves also contribute to differences in the predicted outcomes. For example, mt-sDNA has a higher sensitivity to detect colorectal cancer than FIT (92% vs. 74%, respectively; ref. 25). A previous CRC-AIM analysis demonstrated that when assuming patients were randomly adherent to the same number of mt-sDNA and FIT tests, an individual would have to take up to 21 FIT tests to match the equivalent LYG as an individual who took up to 9 mt-sDNA tests (14).
These findings demonstrate that incorporating realistic, rather than theoretical, adherence rates for initial stool screening and colonoscopy follow-up provides more clinically applicable outcomes data to inform further discussions referent to the comparative effectiveness of mt-sDNA- versus FIT-based strategies in real-world settings. Choice of test and comprehensive navigation support can favorably impact patient initiation and follow-through, affording great potential to enhance the overall effectiveness of colorectal cancer screening when regularly considered in program planning and evaluation.
Authors' Disclosures
A.M. Fendrick reports other support and has been a consultant for AbbVie, Amgen, Bayer, Centivo, Community Oncology Association, Covered California, EmblemHealth, Exact Sciences, Freedman, Health, GRAIL, Harvard University, Health and Wellness, Innovations*, Health at Scale Technologies*, HealthCorum, Hygieia, MedZed, Merck, Montana, Health Cooperative, Pair Team*, Penguin Pay, Phathom Pharmaceuticals, Risalto, Risk International, Sempre Health*, State of Minnesota, U.S., Department of Defense, Virginia Center for Health, Innovation, Wellth*, Wildflower Health, Yale-New Haven Health System, Zansors*, as well as equity Interest Research: AHRQ, Boehringer-Ingelheim, Gary and Mary West Health Policy Center, Arnold Ventures, National Pharmaceutical Council, PCORI, PhRMA, RWJ Foundation, State of Michigan/CMS Outside Position: AJMC (Co-editor), ME. D.A. Fisher reports grants and other support from Exact Sciences during the conduct of the study; as well as other support from Guardant Health outside the submitted work. L. Saoud reports personal fees from Exact Sciences during the conduct of the study. A. Ozbay reports other support from Exact Sciences during the conduct of the study; as well as other support from Exact Sciences outside the submitted work. J.J. Karlitz reports personal fees from Exact Sciences and other support from Gastro Girl/GI OnDemand outside the submitted work. P.J. Limburg reports other support from Exact Sciences during the conduct of the study; as well as other support from Exact Sciences outside the submitted work.
Acknowledgments
Financial support for this study was provided by a contract with Exact Sciences Corporation. The funding agreement ensured the authors' independence in designing the study, interpreting the data, writing, and publishing the report. Medical writing and editorial assistance were provided by Erin P. Scott, PhD, of Maple Health Group, LLC, funded by Exact Sciences Corporation.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
Cancer Prev Res 2021;14:845–50
Authors' Contributions
A.M. Fendrick: Conceptualization, writing–review and editing. D.A. Fisher: Conceptualization, writing–review and editing. L. Saoud: Data curation, software, formal analysis, investigation, visualization, methodology, writing–review and editing. A.B. Ozbay: Conceptualization, supervision, visualization, methodology, writing–review and editing. J.J. Karlitz: Conceptualization, writing–review and editing. P.J. Limburg: Conceptualization, methodology, writing–review and editing.
References
- 1. Wolf AMD, Fontham ETH, Church TR, Flowers CR, Guerra CE, LaMonte SJ, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin 2018;68:250–81. [DOI] [PubMed] [Google Scholar]
- 2. Weiser E, Parks P, Swartz RK, van Thomme J, Lavin PT, Limburg PJ, et al. Cross-sectional adherence with the multi-target stool DNA test for colorectal cancer screening: real-world data from a large cohort of older adults. J Med Screen 2021;28:18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Akram A, Juang D, Bustamante R, Liu L, Earles A, Ho SB, et al. Replacing the guaiac fecal occult blood test with the fecal immunochemical test increases proportion of individuals screened in a large healthcare setting. Clin Gastroenterol Hepatol 2017;15:1265–70.e1. [DOI] [PubMed] [Google Scholar]
- 4. Hassan C, Giorgi Rossi P, Camilloni L, Rex DK, Jimenez-Cendales B, Ferroni E, et al. Meta-analysis: adherence to colorectal cancer screening and the detection rate for advanced neoplasia, according to the type of screening test. Aliment Pharmacol Ther 2012;36:929–40. [DOI] [PubMed] [Google Scholar]
- 5. Jensen CD, Corley DA, Quinn VP, Doubeni CA, Zauber AG, Lee JK, et al. Fecal immunochemical test program performance over 4 rounds of annual screening: a retrospective cohort study. Ann Intern Med 2016;164:456–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cooper GS, Grimes A, Werner J, Cao S, Fu P, Stange K. Barriers to follow-up colonoscopy after positive FIT or multitarget stool DNA testing. J Am Board Fam Med 2021;34:61–9. [DOI] [PubMed] [Google Scholar]
- 7. Finney Rutten LJ, Jacobson DJ, Jenkins GD, Fan C, Weiser E, Parks PD, et al. Colorectal cancer screening completion: an examination of differences by screening modality. Prev Med Rep 2020;20:101202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Martin J, Halm EA, Tiro JA, Merchant Z, Balasubramanian BA, McCallister K, et al. Reasons for lack of diagnostic colonoscopy after positive result on fecal immunochemical test in a safety-net health system. Am J Med 2017;130:93 e1–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. May FP, Yano EM, Provenzale D, Brunner J, Yu C, Phan J, et al. Barriers to follow-up colonoscopies for patients with positive results from fecal immunochemical tests during colorectal cancer screening. Clin Gastroenterol Hepatol 2019;17:469–76. [DOI] [PubMed] [Google Scholar]
- 10. Corley DA, Jensen CD, Quinn VP, Doubeni CA, Zauber AG, Lee JK, et al. Association between time to colonoscopy after a positive fecal test result and risk of colorectal cancer and cancer stage at diagnosis. JAMA 2017;317:1631–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Prince M, Lester L, Chiniwala R, Berger B. Multitarget stool DNA tests increases colorectal cancer screening among previously noncompliant Medicare patients. World J Gastroenterol 2017;23:464–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee YC, Li-Sheng Chen S, Ming-Fang Yen A, Yueh-Hsia Chiu S, Ching-Yuan Fann J, Chuang SL, et al. Association between colorectal cancer mortality and gradient fecal hemoglobin concentration in colonoscopy noncompliers. J Natl Cancer Inst 2017;109:djw269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. San Miguel Y, Demb J, Martinez ME, Gupta S, May FP. Time to colonoscopy after abnormal stool-based screening and risk for colorectal cancer incidence and mortality. Gastroenterology 2021;160:1997–2005.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Piscitello A, Saoud L, Fendrick AM, Borah BJ, Hassmiller Lich K, Matney M, et al. Estimating the impact of differential adherence on the comparative effectiveness of stool-based colorectal cancer screening using the CRC-AIM microsimulation model. PLoS One 2020;15:e0244431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Knudsen AB, Zauber AG, Rutter CM, Naber SK, D-R VP, Pabiniak C, et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: modeling study for the US preventive services task force. JAMA 2016;315:2595–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Piscitello A, Saoud L, Matney M, Borah BJ, Fendrick AM, Lich KH, et al. Description and validation of the colorectal cancer and adenoma incidence and mortality (CRC-AIM) microsimulation model 2020. Available from: https://www.biorxiv.org/content/10.1101/2020.03.02.966838v1.
- 17. Bibbins-Domingo K, Grossman DC, Curry SJ, Davidson KW, Epling JW, Jr, García FAR, et al. Screening for colorectal cancer: us preventive services task force recommendation statement. JAMA 2016;315:2564–75. [DOI] [PubMed] [Google Scholar]
- 18. Rex DK, Boland CR, Dominitz JA, Giardiello FM, Johnson DA, Kaltenbach T, et al. Colorectal cancer screening: recommendations for physicians and patients from the U.S. multi-society task force on colorectal cancer. Gastroenterology 2017;153:307–23. [DOI] [PubMed] [Google Scholar]
- 19. Cusumano VT, Corona E, Partida D, Yang L, Yu C, May FP. Patients without colonoscopic follow-up after abnormal fecal immunochemical tests are often unaware of the abnormal result and report several barriers to colonoscopy. BMC Gastroenterol 2020;20:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Selby K, Jensen CD, Zhao WK, Lee JK, Slam A, Schottinger JE, et al. Strategies to improve follow-up after positive fecal immunochemical tests in a community-based setting: a mixed-methods study. Clin Transl Gastroenterol 2019;10:e00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Horne HN, Phelan-Emrick DF, Pollack CE, Markakis D, Wenzel J, Ahmed S, et al. Effect of patient navigation on colorectal cancer screening in a community-based randomized controlled trial of urban African American adults. Cancer Causes Control 2015;26:239–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dougherty MK, Brenner AT, Crockett SD, Gupta S, Wheeler SB, Coker-Schwimmer M, et al. Evaluation of interventions intended to increase colorectal cancer screening rates in the United States: a systematic review and meta-analysis. JAMA Intern Med 2018;178:1645–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ritvo PG, Myers RE, Paszat LF, Tinmouth JM, McColeman J, Mitchell B, et al. Personal navigation increases colorectal cancer screening uptake. Cancer Epidemiol Biomarkers Prev 2015;24:506–11. [DOI] [PubMed] [Google Scholar]
- 24. Honeycutt S, Green R, Ballard D, Hermstad A, Brueder A, Haardörfer R, et al. Evaluation of a patient navigation program to promote colorectal cancer screening in rural Georgia, USA. Cancer 2013;119:3059–66. [DOI] [PubMed] [Google Scholar]
- 25. Imperiale TF, Ransohoff DF, Itzkowitz SH, Levin TR, Lavin P, Lidgard GP, et al. Multitarget stool DNA testing for colorectal-cancer screening. N Engl J Med 2014;370:1287–97. [DOI] [PubMed] [Google Scholar]