Abstract
Purpose:
Ceralasertib is a potent and selective oral inhibitor of the serine/threonine protein kinase ataxia telangiectasia and Rad3-related (ATR) protein.
Patients and Methods:
Eligible patients with solid tumors, enriched for melanoma, received ceralasertib in combination with a fixed dose of paclitaxel (80 mg/m2 on D1, D8, D15) in 28-day cycles. The dose of ceralasertib was escalated to reach an MTD in a rolling 6 design. The starting dose of ceralasertib was 40 mg QD. Fifty-seven patients (33 patients with melanoma who failed prior PD1/L1 treatment) were enrolled in 7 dose cohorts ranging from 40 mg QD to 240 mg BD plus weekly paclitaxel.
Results:
The RP2D was established as ceralasertib 240 mg BD days 1–14 plus paclitaxel 80 mg/m2 on D1, D8, D15 every 28 days. The most common toxicities were neutropenia (n = 39, 68%), anemia (n = 25, 44%), and thrombocytopenia (n = 21, 37%). In the full analysis set of 57 patients, the overall response rate (ORR) was 22.6% (95% CI, 12.5–35.3). In 33 patients with melanoma, resistant to prior anti-PD1 therapy, the ORR was 33.3% (95% CI, 18.0–51.8). In the melanoma subset, the mPFS was 3.6 months (95% CI, 2.0–5.8), the median duration of response was 9.9 months (95% CI, 3.7–23.2), and the mOS was 7.4 months (95% CI, 5.7–11.9).
Conclusions:
Ceralasertib in combination with paclitaxel was well tolerated in patients with advanced malignancies and showed evidence of antitumor activity. Durable responses were observed in patients with advanced cutaneous, acral, and mucosal melanoma resistant to anti-PD1/L1 treatment.
See related commentary by Ashworth, p. 4667
Translational Relevance.
Ceralasertib in combination with paclitaxel was well tolerated in patients with advanced malignancies and showed evidence of antitumor activity. Durable responses were observed in patients with advanced cutaneous, acral, and mucosal melanoma resistant to anti-PD1/L1 treatment.
Introduction
Ataxia telangiectasia–mutated (ATM) and ataxia telangiectasia and Rad3-related protein kinase (ATR) are essential components of the cellular DNA damage response (DDR) in human cells (1). ATM is involved in the response to DNA double-stranded breaks (DSB), and ATR is activated by replication stress resulting in single-stranded DNA breaks. In tumor cells, the expression of oncoproteins, such as mutant RAS isoforms, the Myc family of oncoproteins, and overexpression of Cyclin E, disrupt normal cell-cycle regulation and cause replication stress (2). Previous studies have shown that inhibition of ATR is selectively toxic to tumor cells, with high levels of oncogene-induced replication stress (3,4,5,6). Hence, these studies provide support for the development of ATR inhibitors as a therapy in patients with cancer.
Ceralasertib is a potent, selective oral inhibitor of the serine/threonine-specific protein kinase ATR, with good margin of selectivity against PI3Ks and other phosphatidylinositol 3-kinase–related kinase family members, including mTOR, ATM, and DNA-PK (7, 8). Kinase biochemical and cell assay screening data showed excellent selectivity for ATR over 400 other kinases; the nearest hit was mTOR with a GI50 of 5.7 μmol/L based on p70S6K (detection of pSer235/236) in MDAMB-468 cells (8), and thus is not relevant at maximum clinical doses. Ceralasertib inhibits ATR and suppresses the replication stress response induced by DNA damage in the S-phase of the cell cycle in tumor cells. Preclinical studies with ceralasertib have demonstrated antitumor activity in combination with DNA-damaging anticancer therapies, and clinical studies testing these hypotheses are ongoing (9). Paclitaxel, an antimicrotubule agent, is a widely used chemotherapeutic that is a standard of care in several cancer types, including gastric cancer, lung cancer, breast cancer, sarcoma, and ovarian cancer (10,11,12,13,14,15). Paclitaxel disrupts microtubules, which causes chromosome mis-segregation and thus prevents mitosis in M-phase of the cell cycle (16,17,18). Although paclitaxel does not directly damage DNA, a replication-independent role for ATR has recently been described in preventing chromosomal mis-segregation in M-phase (19). An additive effect of weekly paclitaxel and ceralasertib has been shown in an in vitro synergy panel, including in a subset of gastric cancer cell lines (4 of 14 cell lines) as well as in in vivo models of breast cancer cell lines 4T1 and BT-474 (unpublished data). Another rationale for combination treatment is that compromised Aurora B activation by ATR inhibition may weaken the spindle assembly checkpoint and promote micronucleation along with that already induced by taxane (19).
The purpose of this open-label, phase I study (ClinicalTrial.gov identifier: NCT02630199) was to evaluate the safety, tolerability, pharmacodynamics, and preliminary efficacy of ceralasertib in combination with paclitaxel in patients with advanced solid tumors. The original goal of achieving the RP2D was to test the efficacy of the combination in gastric cancer in the umbrella VIKTORY trial (20), but the development plan was amended to include patients with metastatic melanoma who failed prior immunotherapy."
Patients and Methods
Study design and treatments
This trial was a single-center, open-label, non-randomized phase I study at the Samsung Medical Center (Seoul, Korea), recruiting patients between February 2016 and July 2019. The data cutoff for this publication was August 29, 2020. Patients were enrolled in a rolling 6 dose-escalation design, with subsequent cohort escalation or expansion up to an additional 12 patients per cohort based on review of the safety data and dose-limiting toxicities (DLT) during the first cycle of treatment. The trial was conducted in accordance with the Declaration of Helsinki and the Guidelines for Good Clinical Practice. The trial protocol was approved by the Institutional Review Board of Samsung Medical Center (Seoul, Korea), and all patients provided written informed consent before enrollment.
Ceralasertib was administered for between 7 and 21 days beginning on day 1 of cycle 1 in combination with a fixed dose of paclitaxel (80 mg/m2) on days 1, 8, and 15 of every 28-day treatment cycle. In addition, this was preceded by 5 days in cycle 0 in which ceralasertib was dosed once on day 1 with intensive pharmacokinetic (PK) sampling on days 1 to 5 to determine single-dose PK. The starting dose of ceralasertib was 40-mg once daily (QD) days 1 to 21 (D1–21) in cohort 1 based on the experience of ceralasertib in the PATRIOT (NCT02223923) investigator-initiated monotherapy study that was ongoing at the time of protocol development, in which the daily dose of ceralasertib explored was 40 to 480 mg (9, 20). The dose, number of doses per day, and number of days of ceralasertib dosing per cycle in subsequent cohorts was determined on the basis of the emerging safety and tolerability data. The dose levels of ceralasertib tested in each cohort were as follows: Cohort 1, 40 mg QD D1–21; cohort 2, 60 mg QD D1–21; cohort 3, 80 mg QD D1–21; cohort 4, 160 mg QD D1–7; cohort 5a, 240 mg QD D1–7; cohort 5b, 160 mg QD D1–14; cohort 6, 160 mg twice daily (BD) D1–14; and cohort 7, 240 mg BD, D1–14 (Fig. 1). Note that cohort 5 tested two different schedules in parallel, where the daily dose was escalated in cohort 5a (from 160 to 240 mg QD) and the number of days dosing was escalated in cohort 5b (from 7 to 14 days). Paclitaxel was concomitantly administered with ceralasertib for up to 4 cycles, and continuation beyond cycle 4 was dependent on investigator discretion.
Figure 1.
An overview of the trial design.
DLT was evaluated during cycle 1 (28 days) for each patient. At the end of each dose cohort, the safety and tolerability data were reviewed for DLT evaluation and the decision whether to escalate the dose, de-escalate, and/or expand a cohort was made. A dose was considered non-tolerated and dose escalation ceased if ≥2 of up to 6 evaluable patients experienced a DLT at a dose level. The primary objective of this study was to assess the safety and tolerability of the combination, to determine the MTD and recommend a dose (RP2D) to take into phase 2 studies. Secondary objectives included assessment of the antitumor activity of the combination, characterization of the PK of ceralasertib, and pharmacodynamics of ATR inhibition.
Patients
Eligible patients were ages >19 years with metastatic solid cancer and measurable disease according to RECIST version 1.1 (21). Patients must have received standard-of-care chemotherapy, be Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, and have adequate hematologic parameters (hemoglobin ≥9 g/dL, ANC ≥1,500 per μL, and platelets ≥75,000 per μL), hepatic (ALT and AST ≤2.5 × institutional upper limit of normal unless liver metastases are present in which case it must be ≤5x ULN), and renal functions (estimated creatinine clearance ≥45 mL/min). Although it was not a requirement for study entry, all patients with metastatic melanoma undergoing palliative immune-chemotherapy received annual brain MRI as a screening for asymptomatic brain metastases. Key exclusion criteria were prior receipt of more than four chemotherapy regimens or previous treatment with an ATR inhibitor or paclitaxel. Patients must not have received any palliative chemotherapy or investigational therapies within 14 days of the first administration of study drugs, and were excluded if they had symptomatic brain metastases requiring local therapy. From cohort 5 onwards, based on encouraging preliminary efficacy, the study population was enriched for patients with metastatic melanoma who had received prior anti–PD-L1 therapy. Patients with melanoma with primary resistance to anti–PD-L1 [defined according to SITC recommendations (ref. 22) as having ≥6 weeks drug exposure and best response of progressive disease (PD) or stable disease (SD) <6 months] or had anti-PD1/L1 secondary resistance [defined as having drug exposure ≥6 months and best response of complete response (CR) or partial response (PR) or SD for >6 months, then progressed] were included in the study population. Archival pathology blocks were collected from all enrolled patients for genomic analysis of tumor DNA.
Definition of DLT
DLT was defined as any event of grade 4 neutropenia (ANC <500 cells/mm3) lasting longer than 4 consecutive days, febrile neutropenia or grade 3 neutropenia with documented infection, grade 3 thrombocytopenia with bleeding requiring medical intervention, and other hematologic toxicities ≥ grade 4 (by CTCAE v4.03). Non-hematologic DLTs included any toxicity of ≥ grade 3, QTc prolongation of >500 ms, or any other toxicity that was worse than at baseline, and was clinically significant and/or unacceptable, and did not respond to supportive care.
Study assessments
At baseline, the medical history, physical examination, blood tests, urinalysis, electrocardiography, echocardiogram, and chest, abdomen, and pelvis CT scan results of the patients were reviewed for eligibility. Physical examinations, vital signs, ECOG performance status, and blood tests were repeated before beginning each cycle of therapy. Safety assessments included treatment-emergent adverse events (AE), occurrence of DLTs, blood tests, electrocardiogram, and vital signs. AEs and DLTs were reported using NCI-CTCAE version 4.03, recorded from the first study drug administration to 28 days after study drug administration, whereas DLT events were recorded until the end of treatment cycle 1. Safety information was used to inform dose-expansion/escalation decisions at the Safety Review Committee. In addition, in order for a patient to be evaluable for DLT assessment, they must have received at least 75% of the specified dose of ceralasertib during cycles 0 and 1, or experienced a DLT during the same period. Tumor responses were evaluated every 2 cycles for the first 10 cycles, followed by every 3 cycles until progression using computed tomography according to the RECIST 1.1 criteria.
Blood for PK assessment was collected during cycle 0 day 1 (pre-dose, 30, 60, and 90 minutes, 2, 4, 8, and 12 hours), and days 2 to 5. Limited PK sampling was also obtained during cycle 1 on days 1, 8, and 15 pre-doses of ceralasertib, end of each paclitaxel infusion, and 3 and 6 hours after the end of each paclitaxel infusion. Ceralasertib bioassay was performed using an established assay (Covance). PK samples were collected and banked should bioanalysis be required on the basis of data collected elsewhere in the program. To date, PK samples have been analyzed after a single dose of ceralasertib in cohorts 1 to 4. Optional biopsies were collected from consenting patients with biopsiable disease at baseline, on treatment, and at progression.
NGS using a custom panel
Next-generation sequencing (NGS) was performed on formalin-fixed, paraffin-embedded (FFPE) specimens using the extensively validated OCA v2 platform (Thermo Fisher Scientific; www.thermofisher.com). The methods for DNA/RNA extraction and for sequencing/reporting/validation of the assay were carried out according to previously published reports (23).
IHC for ATM
Representative tumors from each participant were obtained from FFPE archival biopsy samples. The tissue was arranged in a new recipient paraffin block (tissue array block) using a trephine apparatus (Superbiochips Laboratories). Immunohistochemical staining was performed using an automatic immunostainer (DAKO) according to the manufacturer's instructions. Anti-ATM antibody was used as the primary antibody (Y170). Loss of ATM expression was defined as a greater than 80% loss of nuclear staining. Multiplex immunofluorescence was kindly performed by J Tau be at Johns Hopkins University (Baltimore, MD).
cfDNA sequencing
All exons from a custom panel of 601 cancer-related genes were enriched and sequenced with a minimum of ×446 coverage as described previously (24). Raw data were curated to exclude technical and biological artifacts and single-nucleotide variants and short insertions and deletions (no more than 50 bp) called with a limit of detection of 0.2%.
Statistical analysis
The number of patients enrolled was based on the desire to obtain adequate tolerability, safety, PK, and pharmacodynamic data while exposing as few patients as possible to the investigational product and procedures. The rolling 6 study design was adopted for the review of DLTs and determination of the MTD. The safety analysis set included all patients who received at least one dose of ceralasertib (n = 57). The full analysis set included all patients who received at least one dose of ceralasertib or paclitaxel (n = 57) and was used for the reporting of efficacy endpoints. Descriptive statistics were applied to summarize patient characteristics. Categorical variables are presented as frequencies, and continuous variables are summarized by medians. All analyses to evaluate the association between genetic alterations and responses to ceralasertib plus paclitaxel were performed using R, and other analyses were carried out using the Statistical Package for the Social Sciences (SPSS), version 19.0 (SPSS Inc.).
Results
Patient demographics
Fifty-seven patients were recruited into 7 dose-cohorts at a single site. The baseline demographics and disease characteristics are shown in Table 1. For cohorts 1 to 4, patients were recruited irrespective of the primary site and histology of their tumor. From cohort 5 onwards, only patients with metastatic melanoma were recruited, all of whom had received prior immunotherapy with an anti-PD1/L1 agent. This followed preliminary evidence of durable responses in patients with melanoma recruited into cohorts 1 to 4. All enrolled patients failed prior standard chemotherapy for metastatic solid cancer (median number of lines of prior treatment 2; range, 1–4). 75% of the patients (43 of 57) received two or more prior lines of palliative systemic therapy before enrollment. Of the total recruited, 33 had melanoma, 15 had gastric cancer, 4 sarcoma, 3 colon cancer, 1 neuroendocrine, and 1 hepatocellular cancer. The melanoma subtypes included cutaneous melanoma (n = 11), mucosal melanoma (n = 11), acral melanoma (n = 10), and unknown (n = 1). Among the 57 patients, 35 patients (61.4%) received prior anti-PD1/L1 therapy; all of those with melanoma received immunotherapy as part of their prior standard of care.
Table 1.
Patient characteristics.
| Variable | N (%) | |
|---|---|---|
| All patients (N = 57) | ||
| Age | ||
| Median (range) | 58.5 (35–75) | |
| Gender | ||
| Male | 27 (47.4) | |
| Female | 30 (52.6) | |
| Tumor types | ||
| Gastric cancer | 15 (26.3) | |
| Colon cancer | 3 (5.3) | |
| Sarcoma | 4 (7.0) | |
| Hepatocellular carcinoma | 1 (1.7) | |
| Neuroendocrine carcinoma | 1 (1.7) | |
| Melanoma | 33 (57.9) | |
| Prior lines of chemotherapy | ||
| 1 regimen | 25 (43.8) | |
| 2 regimens | 18 (31.6) | |
| ≥3 regimens | 14 (24.6) | |
| Metastatic site | ||
| Liver | 24 (42.1) | |
| Prior anti-PD1 therapy | 35 (61.4) | |
| Melanoma patients (N = 33) | ||
| Prior anti–PD-L1 | 33 (100.0) | |
| Prior anti-CTLA4 therapy | 0 | |
| Prior RAF inhibitor | 6 (18.1) | |
| Prior lines of chemotherapy | ||
| 1 regimen | 14 (42.4) | |
| 2 regimens | 19 (57.6) | |
| LDH expression | ||
| ≤ULN | 5 (15.2) | |
| >ULN to ≤2x ULN | 10 (30.3) | |
| >2x ULN | 18 (54.5) | |
| Stage, M category (AJCC 8th) | ||
| M1a | 4 (12.1) | |
| M1b | 5 (15.1) | |
| M1c | 21 (63.8) | |
| M1d | 3 (9.0) | |
| Best response to prior anti–PD-1/L1 | ||
| CR or PR | 4 (12.1) | |
| SD | 16 (48.5) | |
| PD | 13 (39.4) | |
| BRAF mutation | 3 (9.0) | |
| NRAS mutation | 6 (18.2) | |
| Brain metastasis | 3 (9.0) | |
| Melanoma subtypes | ||
| Acral | 10 (30.3) | |
| Cutaneous | 11 (33.3) | |
| Mucosal | 11 (33.3) | |
| Unknown | 1 (3.1) | |
| Stage IV melanoma | 33 (100.0) | |
Dose-escalation and safety analysis
Fifty-seven patients received at least one dose of ceralasertib and were evaluable for safety analysis. No DLTs were observed in cohorts 1 to 3, but an increase in ≥G3 AEs, especially hematological, was observed in cohort 3 (2 events of G3 anemia and 1 of G4 neutropenia in cohort 3 compared with only 1 event of G3 neutropenia in cohort 1). This triggered a change in the dosing schedule of ceralasertib from days 1 to 21 in cohorts 1 to 3, to days 1 to 7 in cohort 4, but at a higher dose of 160 mg QD. This change, with a longer “off period” for ceralasertib, was intended to allow for an increased period of recovery for the bone marrow before beginning the next cycle while increasing the daily dose of ceralasertib. There were no DLTs in cohort 4 and ceralasertib was escalated to 240 mg QD days 1 to 7 for cohort 5a (n = 3) and 160 mg QD days 1 to 14 for cohort 5b (n = 3). No DLTs were observed in cohort 5a or 5b, and ceralasertib was escalated to 160 mg BD for 14 days in cohort 6. Clinical data generated elsewhere in the ceralasertib program suggested that a 14-day drug holiday was necessary for bone marrow recovery; therefore, a “14 day on–14 day off” schedule was adopted. A single DLT of grade 3 neutropenic fever was observed in cohort 6 (160 mg BD ceralasertib days 1–14 plus weekly paclitaxel) that required IV antibiotics and G-CSF support. The patient recovered from neutropenia 6 days after the occurrence but elected to withdraw from further participation in the trial. As per protocol, the dose of ceralasertib was escalated to 240 mg BD days 1 to 14 in Cohort 7. A DLT of grade 3 neutropenic fever was observed in Cohort 7, and the patient subsequently died due to septic shock, attributed to a pre-existing disseminated melanoma-related wound infection present at study entry. Unfortunately, this patient refused all further medical treatment, including IV antibiotics and fluid resuscitation; thus, the death was not considered a grade 5 drug-related event in this study. Although cohort 7 was declared tolerated, it was decided not to increase the dose of ceralasertib further because at 240 mg BD and above we do not see proportionate increases in exposure, presumably due to the limited solubility of the current formulation of ceralasertib. Of 57 patients receiving study drugs, the median number of cycles of paclitaxel was 3 (range, 1–18), and 24 patients continued to receive paclitaxel beyond cycle 4.
The most common treatment-emergent AEs (all causality, all grades) were anemia (n = 27, 47%) and neutropenia, including neutrophil count decreased (n = 24, 42%; Table 2). Most patients were neutropenic only and there were only two cases of febrile neutropenia, both of which were G3. Other toxicities (with frequency ≥ 10%) included anorexia (n = 17, 30%), nausea (n = 17, 30%), alopecia (n = 15, 26%), fatigue/general weakness (n = 10, 17%), thrombocytopenia, including platelet count decreased (n = 7, 12%), pruritis (n = 7, 12%), vomiting (n = 7, 12%), and rash (n = 7, 12%). The main toxicities of grade 3 or higher were neutropenia (n = 17, 30%), anemia (n = 13, 23%), and thrombocytopenia (n = 5, 9%). Two patients in cohort 6 had a dose reduction of ceralasertib due to toxicity, one due to G4 neutropenia and required 2 successive dose reductions (from 160 mg BD to 160 mg QD during cycle 1, then to 80 mg QD at the beginning of cycle 2) and another patient due to G2 fatigue at the end of cycle 1. Two patients in cohort 7 required dose reductions, one in cycle 6 and another in cycle 5 (both from 240 mg BD to 160 mg BD) due to infection of the ear (otitis media) and neutropenia, respectively. One patient discontinued treatment in cycle 1 due to variceal bleeding that was unrelated to either ceralasertib or paclitaxel and there were no treatment-related deaths. There were no clinically meaningful TEAEs related to ceralasertib identified from vital signs or ECG parameters. An MTD was not established as dose escalation was stopped following Cohort 7, which was tolerated. Therefore, per protocol, the RP2D was declared as 240 mg BD ceralasertib, days 1 to 14 plus paclitaxel 80 mg/m2 on days 1, 8, and 15 every 28 days.
Table 2.
Treatment-emergent AEs occurring in >10% of patients in the safety analysis set by CTCAE grade.
| All patients and doses (N = 57) | 160 mg BD melanoma (N = 6) | 240 mg BD melanoma (N = 9) | ||||
|---|---|---|---|---|---|---|
| Any grade | Grade ≥3 | Any grade | Grade ≥3 | Any grade | Grader ≥3 | |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Patients with any AE | 57 (100%) | 28 (49.1%) | 6 (100%) | 4 (67%) | 5 (56%) | 5 (56%) |
| Anemia | 27 (47%) | 13 (23%) | 5 (83.3%) | 3 (50%) | 2 (22.2%) | 2 (22.2%) |
| Neutropenia and neutrophil count decreased | 24 (42%) | 17 (30%) | 4 (83.3%) | 4 (67%) | 5 (56%) | 5 (56%) |
| Anorexia | 17 (30%) | 0 | 2 (33.3%) | 0 | 2 (22.2%) | 0 |
| Nausea | 17 (30%) | 1 (2%) | 2 (33.3%) | 0 | 3 (33.3%) | 0 |
| Alopecia | 15 (26%) | 0 | 2 (33.3%) | 0 | 0 | 0 |
| Fatigue | 10 (17%) | 0 | 1 (17%) | 0 | 3 (33.3%) | 0 |
| Platelet count decreased and thrombocytopenia | 7 (12%) | 5 (9%) | 1 (17%) | 1 (17%) | 4 (44.4%) | 3 (33.3%) |
| Pruritus | 7 (12%) | 0 | 2 (33.3%) | 0 | 4 (44.4) | 0 |
| Vomiting | 7 (12%) | 1 (2%) | 1 (17%) | 0 | 2 (22.2%) | 0 |
| Rash | 7 (12%) | 0 | 2 (33.3%) | 0 | 1 (11.1%) | 0 |
PK analysis
PK data after a single dose of ceralasertib are available for the first 3 cohorts and 6 patients in cohort 4 (Supplementary Fig. S1). The PK data showed dose proportionality and were consistent with the PK concentrations from other company-sponsored studies, indicating that there is no meaningful difference in PK between patients of European and Asian ancestry. Ceralasertib PK shows a rapid Tmax (1.5–2 hours) and a terminal half-life of 12–16 hours.
Efficacy analysis
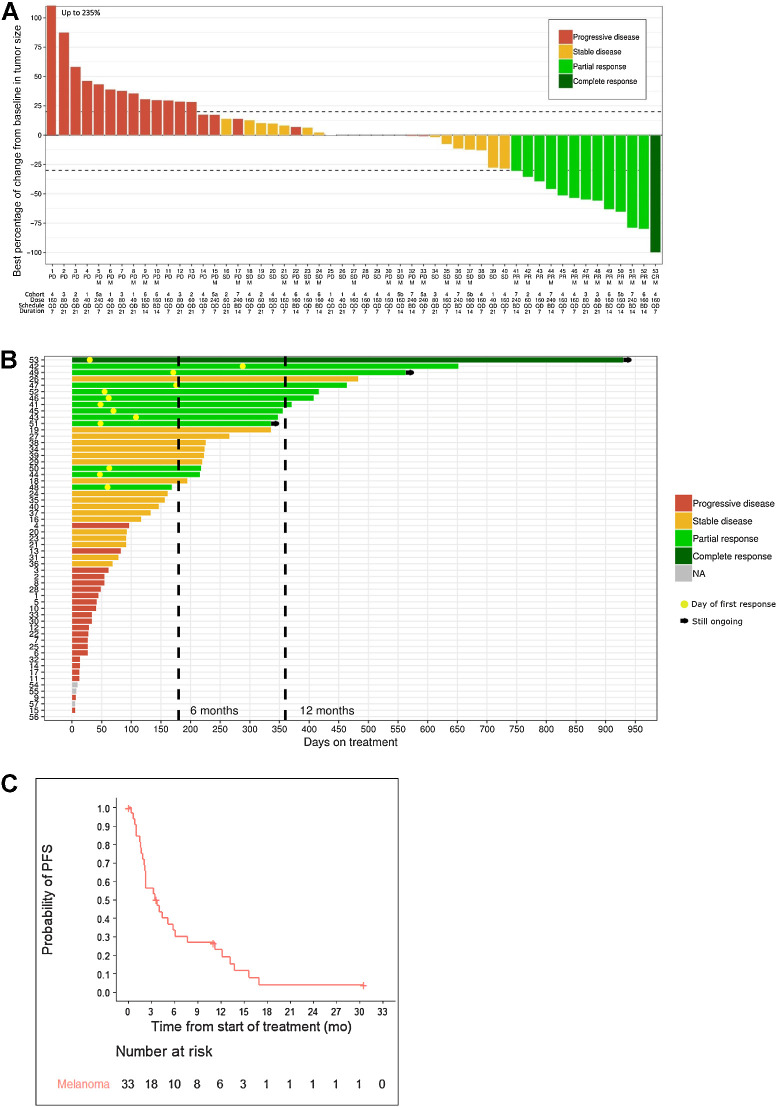
In the efficacy set (of 57 patients who received at least one dose of ceralasertib or paclitaxel), there was 1 CR (1.8%, melanoma), 12 confirmed PRs [12/57 (21.1%); 2 gastric, 10 melanoma, all of the melanoma cases having received prior immunotherapy], 18 patients with a best response of SD (31.6%), 22 patients with PD, and 4 were non-evaluable (Fig. 2A; Supplementary Table S1). The four non-evaluable patients (not included in Fig. 2A) discontinued treatment in cycle 1 for reasons other than disease progression and toxicity (1 due to an unrelated AE and 3 withdrew consent). At the data cutoff of August 29, 2020, three patients were still receiving study drugs (Fig. 2B). In the subgroup of patients with melanoma (n = 33), the overall response rate (ORR) was 33.3% (95% CI, 18.0–51.8) and the DCR was 60.6% (95% CI, 42.1%–77.1%). The median PFS for the patients with melanoma was 3.6 months (95% CI, 2.0–5.8), the median duration of response was 9.9 months (95% CI, 3.7–23.2), and mOS was 7.4 months (95% CI, 5.7–11.9). Twenty-three of the 33 patients with melanoma had died. Responses did not appear related to the baseline level of LDH (there were 8 responders from 28 with LDH >ULN) or PD-L1 expression (there were 6 responders from 12 with PD-L1 expression ≤5%). Furthermore, responses were observed across all histological subtypes of melanoma, including in 3 of 11 cutaneous, 3 of 10 acral, and 5 of 11 mucosal. Two of the patients with melanoma who responded to study treatment had responses to prior PD-1 immunotherapy; 6 had SD as the best response and 3 had PD as the best response.
Figure 2.
The drug-efficacy data. A, Waterfall plot of best percentage of change in sum of target lesions in the full analysis set. The y-axis represents the percentage of maximum tumor reduction assessed according to RECIST v1.1 criteria. M, melanoma. The bars are coded by best overall response; B, Swimmer plot showing duration of treatment. The bars are coded by best response as in A. The yellow circle in responders indicates the day of first response. C, PFS (N = 33).
Biomarker analysis
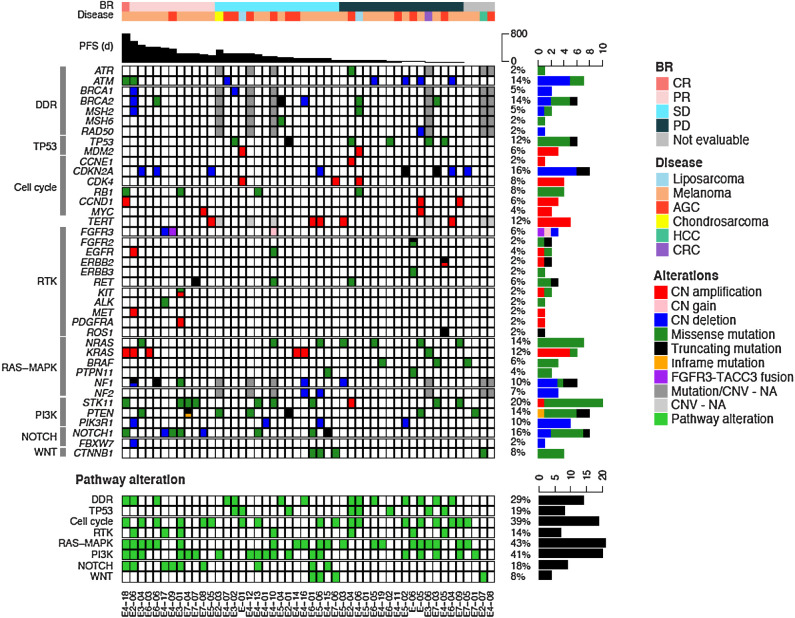
NGS was performed on archival tumor tissue biopsies before treatment from 48 patients. Although patients' tumors showed diverse mutation profiles, there was no significant correlation between the mutational profiles and tumor response (Fig. 3). Two patients with responses, including the CR, had missense mutations in ATM (ATM T2035P; ATM I2888T), but neither of these mutations were thought to be pathogenic. IHC for ATM was performed on tumor samples from 33 patients with melanoma (Supplementary Fig. S2). Loss of ATM, defined by ≤20% tumor nuclei staining, was observed in 3 of the 33 patients with melanoma, and occurred in patients with a best response of PR, SD, and non-evaluable for efficacy.
Figure 3.
Pathologic–genomic landscape of 48 patients from the full analysis set. The top shows the clinical characteristics of the patients, including best response (BR), disease type, and progression-free survival. The middle bottom shows alterations in select genes, derived from sequencing archival tumor DNA. Genes are grouped by pathway or function, and frequency across sequenced patients. The bottom summarizes the pathway alterations in each patient sample. CN, copy number; Amplification, 4< CN; Gain, 3<CN≤4; Deletion, CN<1; NA, not available.
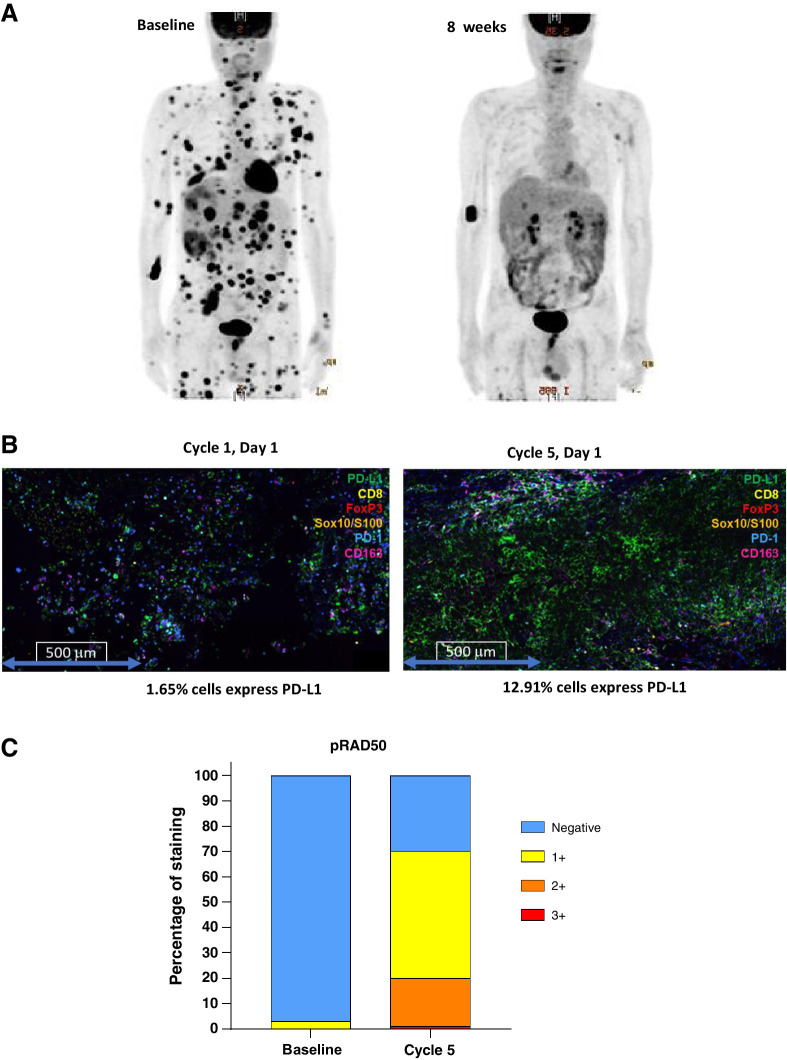
We obtained baseline and on-treatment tumor biopsies from one patient who had a substantial metabolic response (assessed by FDG-PET) to the combination regimen in cohort 7 after 2 cycles of treatment. This patient, who did not have mutations in BRAF or RAS, was refractory to prior pembrolizumab with progression of multiple liver lesions and disseminated skin lesions. After 2 cycles of 240 mg BD ceralasertib days 1–14 plus weekly paclitaxel at 80 mg/m2, the patient achieved a PR (46% tumor reduction) with a significant decrease in metabolic activity as demonstrated in serial PET-CT images (Fig. 4A). The patient maintained PR until cycle 8 as the target lesion continued to shrink (maximum target lesion reduction, 63%). Paired biopsies from this patient were used to examine PD-L1 expression, and an increase from 1.65% at baseline to 12.9% at cycle 5 day 1 was observed (Fig. 4B). Phosphorylated RAD50 (pRAD50) has been reported as a novel biomarker of ceralasertib inhibition in tumors expressing ATM (25). We examined pRAD50 by IHC and observed an increase in expression from 5% at baseline to 70% (of cells positive) at cycle 5 (Fig. 4C) consistent with target engagement within the tumor.
Figure 4.
Pharmacodynamic study from a dramatic responder to AZD6738/paclitaxel. This patient was refractory to prior pembrolizumab with rapid progression to liver and skin lesions (A, left). After 2 cycles of 240-mg BD ceralasertib days 1–14 plus weekly paclitaxel at 80 mg/m2, the patient achieved a PR with a significant decrease in metabolic activity as demonstrated in follow-up PET-CT images (right). Paired biopsies from this patient were used to examine PD-L1 expression, and an increase from 1.65% at baseline to 12.9% at cycle 5 day 1 was observed (B). pRAD50 was increased in expression from 5% at baseline to 70% (of cells positive) at cycle 5, consistent with target engagement within the tumor (C).
Circulating free DNA (cfDNA) was isolated from plasma for 53 patients, and baseline and on treatment samples were sequenced with up to 2,700x coverage for a custom panel of 601 genes as previously described (24). Longitudinal analysis was prioritized for patients that responded. In two patients who were responders in cohorts 6 and 7, a number of mutant alleles were identified in baseline cfDNA that were cleared from plasma within 2 cycles of treatment, consistent with reduction of target lesions (Supplementary Figs. S3 and S4).
Discussion
This study explored the safety, tolerability, PK, pharmacodynamics, and efficacy of ceralasertib in combination with paclitaxel in patients with advanced solid tumors. On the basis of the observed safety profile, the combination of ceralasertib and paclitaxel is considered well tolerated, but an MTD could not be defined according to the protocol. The RP2D to be taken forward in a future study is ceralasertib 240 mg BD days 1–14 in combination with paclitaxel at 80 mg/m2 on days 1, 8, and 15, every 28 days. In this combination, ceralasertib could be administered at the maximum-tolerated monotherapy dose without compromising the approved weekly dose of paclitaxel. This is in contrast with other chemotherapy combinations with ceralasertib, such as with carboplatin, where toxicity prohibited escalation of ceralasertib (26).
Evidence of antitumor activity was observed in patients who had failed standard chemotherapy, especially in patients with advanced melanoma who were resistant immunotherapy. Responses were observed in patients with high baseline LDH, which is a negative prognostic indicator in melanoma, suggesting a benefit even in this group with poor therapeutic outcomes. In addition, as this study was conducted at a single center in South Korea, we were able to recruit patients with acral and mucosal melanoma, which occur at a much lower prevalence than cutaneous melanoma in the Caucasian population, and are associated with poorer treatment outcomes (27). We show that regardless of melanoma subtype, durable responses were observed. These efficacy data are promising for patients with melanoma who have progressed on immunotherapy because existing treatment options offer poor response rates (typically ∼15%) with limited response durability. This study suggests that ceralasertib plus paclitaxel is worthy of further investigation as a novel treatment for recurrent melanoma after failure of anti-PD1–based therapies.
ATM and ATR play essential roles in the DDR by facilitating connections between DNA-damage sensing and DDR effectors (28, 29). ATR participates in functional interactions between repair proteins, especially ATM, during DDR (30). Published preclinical experiments revealed that ceralasertib inhibited the growth of gastric cancer cell lines with ATM deficiency (31). The ATM gene comprises a 4 kilobase-pair coding sequence with many mutations, some of which have been annotated as pathogenic. In our analysis with NGS and ATM IHC, there was no clear correlation between specific genomic alterations or loss of ATM expression and response to treatment, which suggests that multiple factors may underlie response to treatment.
DDR inhibitors such as ATR, ATM, and PARP inhibitors could promote the priming of the anticancer immune response and enhance a Th1-based response as well as modulate the immune microenvironment (32,33,34). DDR inhibitor-mediated catastrophic DNA damage is a favorable factor for immunotherapy (35). In one scenario, exposure to DDR inhibitors triggers induction of the STING pathway to promote antitumor immunological response (36). Consistent with this hypothesis, in an exploratory analysis of one set of paired biopsies in this study from a patient with a significant radiographic response, we found evidence for the induction of PD-L1 expression relative to baseline. Currently, PD-L1 expression by IHC is a widely used biomarker to predict the response to immunotherapy with PD1/L1 checkpoint inhibitors; however, its use in melanoma is controversial (37, 38). The upregulation of PD-L1 is mainly driven by inflammation, and thus the degree of PD-L1 expression could reflect the status of the tumor immune microenvironment (39). In preclinical models, it was observed that PARP inhibition induced PD-L1 upregulation (40), which is consistent with the findings of this study. These findings are being further explored in a phase II trial of durvalumab and ceralasertib in gastric cancer and immunotherapy-resistant melanoma (NCT03780608), with the collection of paired biopsies for analysis of PD-L1 expression and other immune biomarkers. Future work should evaluate potential biomarkers that are predictive of response to ceralasertib, including immune fitness to identify patients most likely to benefit from ceralasertib treatment.
In conclusion, this phase I study demonstrated that ceralasertib in combination with paclitaxel was well tolerated with evidence for antitumor activity in patients with advanced solid tumors, especially those with recurrent melanoma resistant to prior immunotherapy treatment, and established an RP2D for future studies. Further clinical evaluation, mechanistic studies, and identification of biomarkers predictive of response to ceralasertib are required to understand and refine the patient population most likely to benefit from this combination treatment.
Authors' Disclosures
S.A. Smith reports other support from AstraZeneca during the conduct of the study and reports a patent for Ceralasertib pending to AstraZeneca. P.G. Mortimer reports personal fees from AstraZeneca Pharmaceuticals Ltd. during the conduct of the study and personal fees from AstraZeneca Pharmaceuticals Ltd. outside the submitted work. C. Smith reports other support from AstraZeneca outside the submitted work. S.E. Willis reports personal fees from AstraZeneca during the conduct of the study and personal fees from AstraZeneca outside the submitted work. I. Irurzun-Arana reports other support from AstraZeneca outside the submitted work; and reports employment with AstraZeneca and holds company stock. A.C. Berges reports other support from AstraZeneca outside the submitted work. J. Park reports grants and personal fees from BMS, Servier, and MedPacto outside the submitted work. I. Kozarewa reports other support from AstraZeneca during the conduct of the study and other support from AstraZeneca outside the submitted work. E. Dean reports other support from AstraZeneca during the conduct of the study; other support from AstraZeneca outside the submitted work; and reports employment and is a stockholder at AstraZeneca. J. Lee reports grants from AstraZeneca during the conduct of the study. No disclosures were reported by the other authors.
Supplementary Material
Acknowledgments
This investigator-initiated trial was funded by a study-drug donation and partial funding from AstraZeneca.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
This article is featured in Highlights of This Issue, p. 4665
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
#S.T. Kim and S.A. Smith contributed equally as co-authors of this article.
Clin Cancer Res 2021;27:4700–9
Authors' Contributions
S.T. Kim: Investigation, visualization, methodology, writing–original draft, writing–review and editing. S.A. Smith: Conceptualization, data curation, methodology, writing–original draft, project administration, writing–review and editing. P. Mortimer: Formal analysis, investigation, visualization. A.B. Loembé: Project administration, writing–review and editing. H. Cho: Data curation, formal analysis, writing–review and editing. K.M. Kim: Formal analysis, visualization, writing–review and editing. C. Smith: Writing–review and editing. S. Willis: Visualization, methodology. I. Irurzun-Arana: Formal analysis, validation, investigation, visualization. A. Berges: Supervision, validation. J.Y. Hong: Methodology, writing–original draft. S.H. Park: Data curation, writing–review and editing. J.O. Park: Methodology, writing–original draft. Y.S. Park: Writing–original draft, project administration, writing–review and editing. H.Y. Lim: Writing–original draft, project administration. W.K. Kang: Methodology, writing–original draft. I. Kozarewa: Project administration, writing-review and editing. A.J. Pierce: Data curation, formal analysis. E. Dean: Data curation, formal analysis. J. Lee: Resources, data curation, software, formal analysis, supervision, funding acquisition, validation, investigation, visualization, methodology.
References
- 1. Maréchal A, Zou L. DNA damage sensing by the ATM and ATR kinases. Cold Spring Harb Perspect Biol 2013;5:a012716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Toledo LI, Murga M, Fernandez-Capetillo O. Targeting ATR and Chk1 kinases for cancer treatment: a new model for new (and old) drugs. Mol Oncol 2011;5:368–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schoppy DW, Ragland RL, Gilad O, Shastri N, Peters AA, Murga M, et al. Oncogenic stress sensitizes murine cancers to hypomorphic suppression of ATR. J Clin Invest 2012;122:241–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Höglund A, Nilsson LM, Muralidharan SV, Hasvold LA, Merta P, Rudelius M, et al. Therapeutic implications for the induced levels of Chk1 in Myc-expressing cancer cells. Clin Cancer Res 2011;17:7067–79. [DOI] [PubMed] [Google Scholar]
- 5. Murga M, Campaner S, Lopez-Contreras AJ, Toledo LI, Soria R, Montaña MF, et al. Exploiting oncogene-induced replicative stress for the selective killing of Myc-driven tumors. Nat Struct Mol Biol 2011;18:1331–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gilad O, Nabet BY, Ragland RL, Schoppy DW, Smith KD, Durham AC, et al. Combining ATR suppression with oncogenic ras synergistically increases genomic instability, causing synthetic lethality or tumorigenesis in a dosage-dependent manner. Cancer Res 2010;70:9693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Foote KM, Lau A, Nissink JW. Drugging ATR: progress in the development of specific inhibitors for the treatment of cancer. Future Med Chem 2015;7:873–91. [DOI] [PubMed] [Google Scholar]
- 8. Foote KM, Nissink JWM, McGuire T, Turner P, Guichard S, Yates JWT, et al. Discovery and characterization of AZD6738, a potent inhibitor of ataxia telangiectasia mutated and Rad3 related (ATR) kinase with application as an anticancer agent. J Med Chem 2018;61:9889–907. [DOI] [PubMed] [Google Scholar]
- 9. Dillon MT, Boylan Z, Smith D, Guevara J, Mohammed K, Peckitt C, et al. PATRIOT: a phase I study to assess the tolerability, safety and biological effects of a specific ataxia telangiectasia and Rad3-related (ATR) inhibitor (AZD6738) as a single agent and in combination with palliative radiation therapy in patients with solid tumours. Clin Transl Radiat Oncol 2018;12:16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 2014;15:1224–35. [DOI] [PubMed] [Google Scholar]
- 11. Kelly K, Crowley J, Bunn PA, Jr, Presant CA, Grevstad PK, Moinpour CM, et al. Randomized phase III trial of paclitaxel plus carboplatin versus vinorelbine plus cisplatin in the treatment of patients with advanced non–small-cell lung cancer: a Southwest Oncology Group trial. J Clin Oncol 2001;19:3210–8. [DOI] [PubMed] [Google Scholar]
- 12. Jones SE, Erban J, Overmoyer B, Budd GT, Hutchins L, Lower E, et al. Randomized phase III study of docetaxel compared with paclitaxel in metastatic breast cancer. J Clin Oncol 2005;23:5542–51. [DOI] [PubMed] [Google Scholar]
- 13. Flaherty KT, Lee SJ, Zhao F, Schuchter LM, Flaherty L, Kefford R, et al. Phase III trial of carboplatin and paclitaxel with or without sorafenib in metastatic melanoma. J Clin Oncol 2013;31:373–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Penel N, Bui BN, Bay JO, Cupissol D, Ray-Coquard I, Piperno-Neumann S, et al. Phase II trial of weekly paclitaxel for unresectable angiosarcoma: the ANGIOTAX Study. J Clin Oncol 2008;26:5269–74. [DOI] [PubMed] [Google Scholar]
- 15. Perren TJ, Swart AM, Pfisterer J, Ledermann JA, Pujade-Lauraine E, Kristensen G, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med 2011;365:2484–96. [DOI] [PubMed] [Google Scholar]
- 16. Horwitz SB. Mechanism of action of taxol. Trends Pharmacol Sci 1992;13:134–6. [DOI] [PubMed] [Google Scholar]
- 17. Arnal I, Wade RH. How does taxol stabilize microtubules? Curr Biol 1995;5:900–8. [DOI] [PubMed] [Google Scholar]
- 18. Nogales E, Wolf SG, Khan IA, Luduena RF, Downing KH. Structure of tubulin at 6.5 A and location of the taxol-binding site. Nature 1995;375:424–7. [DOI] [PubMed] [Google Scholar]
- 19. Kabeche L, Nguyen HD, Buisson R, Zou L. A mitosis-specific and R loop-driven ATR pathway promotes faithful chromosome segregation. Science 2018;359:108–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dillon M, Guevara J, Mohammed K, Smith SA, Dean E, McLellan L, et al. A phase I study of ATR inhibitor, AZD6738, as monotherapy in advanced solid tumours (PATRIOT part A, B). Ann Oncol 2019;30:v165–6. [Google Scholar]
- 21. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- 22. Kluger HM, Tawbi HA, Ascierto ML, Bowden M, Callahan MK, Cha E, et al. Defining tumor resistance to PD-1 pathway blockade: recommendations from the first meeting of the SITC immunotherapy resistance taskforce. J Immunother Cancer 2020;8:e000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Frampton GM, Fichtenholtz A, Otto GA, Wang K, Downing SR, He J, et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat Biotechnol 2013;31:1023–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee J, Kim ST, Kim K, Lee H, Kozarewa I, Mortimer PGS, et al. Tumor genomic profiling guides patients with metastatic gastric cancer to targeted treatment: the VIKTORY Umbrella Trial. Cancer Discov 2019;9:1388–405. [DOI] [PubMed] [Google Scholar]
- 25. Jones GN, Rooney C, Griffin N, Roudier M, Young LA, Garcia-Trinidad A, et al. pRAD50: a novel and clinically applicable pharmacodynamic biomarker of both ATM and ATR inhibition identified using mass spectrometry and immunohistochemistry. Br J Cancer 2018;119:1233–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yap TA, Krebs MG, Postel-Vinay S, Bang YJ, El-Khoueiry A, Abida W, et al. Phase I modular study of AZD6738, a novel oral, potent and selective ataxia telangiectasia Rad3-related (ATR) inhibitor in combination (combo) with carboplatin, olaparib or durvalumab in patients (pts) with advanced cancers. Eur J Cancer 2016;69:32607–7. [Google Scholar]
- 27. Kuk D, Shoushtari AN, Barker CA, Panageas KS, Munhoz RR, Momtaz P, et al. Prognosis of mucosal, uveal, acral, nonacral cutaneous, and unknown primary melanoma from the time of first metastasis. Oncologist 2016;21:848–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zeman MK, Cimprich KA. Causes and consequences of replication stress. Nat Cell Biol 2014;16:92–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Saha J, Wang M, Cucinotta FA. Investigation of switch from ATM to ATR signaling at the sites of DNA damage induced by low and high LET radiation. DNA Repair 2013;12:1143–51. [DOI] [PubMed] [Google Scholar]
- 30. Adams BR, Golding SE, Rao RR, Valerie K. Dynamic dependence on ATR and ATM for double-strand break repair in human embryonic stem cells and neural descendants. PLoS ONE 2010;5:e10001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Min A, Im SA, Jang H, Kim S, Lee M, Kim DK, et al. AZD6738, A novel oral inhibitor of ATR, induces synthetic lethality with ATM deficiency in gastric cancer cells. Mol Cancer Ther 2017;16:566–77. [DOI] [PubMed] [Google Scholar]
- 32. Stewart RA, Pilie PG, Yap TA. Development of PARP and immune-checkpoint inhibitor combinations. Cancer Res 2018;78:6717–25. [DOI] [PubMed] [Google Scholar]
- 33. Teo MY, Seier K, Ostrovnaya I, Regazzi AM, Kania BE, Moran MM, et al. Alterations in DNA damage response and repair genes as potential marker of clinical benefit from PD-1/PD-L1 blockade in advanced urothelial cancers. J Clin Oncol 2018;36:1685–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mouw KW, Goldberg MS, Konstantinopoulos PA, D'Andrea AD. DNA damage and repair biomarkers of immunotherapy response. Cancer Discov 2017;7:675–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pilie PG, Gay CM, Byers LA, O'Connor MJ, Yap TA. PARP Inhibitors.: Extending benefit beyond BRCA-mutant cancers. Clin Cancer Res 2019;25:3759–71. [DOI] [PubMed] [Google Scholar]
- 36. Sen T, Rodriguez BL, Chen L, Corte CMD, Morikawa N, Fujimoto J, et al. Targeting DNA damage response promotes antitumor immunity through STING-mediated T-cell activation in small-cell lung cancer. Cancer Discov 2019;9:646–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, et al. Pembrolizumab versus chemotherapy for PD-L1–positive non–small cell lung cancer. N Engl J Med 2016;375:1823–33. [DOI] [PubMed] [Google Scholar]
- 38. Ding W, LaPlant BR, Call TG, Parikh SA, Leis JF, He R, et al. Pembrolizumab in patients with CLL and Richter transformation or with relapsed CLL. Blood 2017;129:3419–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gao Y, Yang J, Cai Y, Fu S, Zhang N, Fu X, et al. IFN-gamma-mediated inhibition of lung cancer correlates with PD-L1 expression and is regulated by PI3K–AKT signaling. Int J Cancer 2018;143:931–43. [DOI] [PubMed] [Google Scholar]
- 40. Jiao S, Xia W, Yamaguchi H, Wei Y, Chen MK, Hsu JM, et al. PARP inhibitor upregulates PD-L1 expression and enhances cancer-associated immunosuppression. Clin Cancer Res 2017;23:3711–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.