Abstract
Purpose:
Men with metastatic castration-resistant prostate cancer (mCRPC) have limited treatment options after progressing on hormonal therapy and chemotherapy. Here, we evaluate the safety and efficacy of atezolizumab (anti–PD-L1) + radium-223 dichloride (radium-223) in men with mCRPC.
Patients and Methods:
This phase Ib study evaluated atezolizumab + radium-223 in men with mCRPC and bone and lymph node and/or visceral metastases that progressed after androgen pathway inhibitor treatment. Following safety assessment of concurrent dosing, 45 men were randomized 1:1:1 to concurrent or one of two staggered dosing schedules with either agent introduced one cycle before the other. This was followed by a safety–efficacy expansion cohort (randomized 1:1:1). The primary endpoints were safety and objective response rate (ORR) by RECIST 1.1. Secondary endpoints included radiographic progression-free survival (rPFS), PSA responses, and overall survival (OS).
Results:
As of October 4, 2019, 44 of 45 men were evaluable. All 44 had ≥1 all-cause adverse event (AE); 23 (52.3%) had a grade 3/4 AE. Fifteen (34.1%) grade 3/4 and 3 (6.8%) grade 5 AEs were related to atezolizumab; none were related to radium-223. Confirmed ORR was 6.8% [95% confidence interval (CI), 1.4–18.7], median rPFS was 3.0 months (95% CI, 2.8–4.6), median PSA progression was 3.0 months (95% CI, 2.8–3.3), and median OS was 16.3 months (95% CI, 10.9–22.3).
Conclusions:
This phase Ib study demonstrated that atezolizumab + radium-223, regardless of administration schedule, had greater toxicity than either drug alone, with no clear evidence of additional clinical benefit for patients with mCRPC and bone and lymph node and/or visceral metastases.
Translational Relevance.
Patients with metastatic castration-resistant prostate cancer face significant morbidity from bone and lymph node and/or visceral metastases and have limited treatment options after progressing on hormonal therapy and chemotherapy. Immune checkpoint inhibitors may provide benefit in this setting, particularly when combined with therapies with complementary mechanisms of action. We hypothesized that adding immune checkpoint inhibitors to radiotherapy might enhance systemic anti-tumor immune responses. To test this hypothesis, we conducted a phase Ib study evaluating the safety and clinical activity of the anti–PD-L1 immune checkpoint inhibitor atezolizumab combined with radium-223 dichloride (radium-223), a systemically administered radiopharmaceutical indicated for patients with metastatic castration-resistant prostate cancer and bone metastases. The combination of approved doses of atezolizumab and radium-223 used in various dosing schedules did not demonstrate improved clinical efficacy and led to increased treatment-related toxicities. Therefore, further development of this combination in the studied patient population does not seem to be justified.
Introduction
Metastatic castration-resistant prostate cancer (mCRPC) is a leading cause of cancer-related death in men worldwide (1), resulting in a median life expectancy of less than 3 years overall and less than 1 year for patients who have received at least two lines of therapy (2, 3). Patients who develop complications such as progressive bone and visceral metastases have even worse outcomes (4,5,6). Despite treatment advances with immunotherapy, chemotherapy, androgen receptor antagonists, androgen synthesis inhibitors, and radionuclide therapy, the prognosis for men with mCRPC remains poor (2, 3, 7, 8), highlighting the need for new therapeutics and/or combinations.
Cancer immunotherapy is used to treat a wide range of cancer types. Checkpoint inhibitors [anti–programmed death-ligand 1/anti–programmed death-1 (anti–PD-L1/anti–PD-1)] in particular appear promising for prostate cancer. PD-L1 has been shown to be upregulated on tumors and dendritic cells, and to a lesser extent on macrophages, following radiotherapy (9, 10). Single-agent PD-1 inhibition has shown limited activity in men with chemorefractory mCRPC and measurable disease; however, the observed objective response rate (ORR) was a modest 5%, while ≥ 50% decrease in PSA level was seen in only 9% of patients, and radiographic progression-free survival (rPFS) ranged from 2.1 to 3.7 months (11). Cancer immunotherapy monotherapy has not demonstrated dramatic response rates in mCRPC; therefore, searches for combination treatments have been undertaken (12, 13). As there have been reports of partial or complete eradication of tumors distant from the local radiation field (i.e., abscopal effect) in patients receiving both anti–CTLA-4 and local radiotherapy (14,15,16), the concurrent administration of PD-L1 antibodies may enhance the efficacy of ionizing radiation through a mechanism dependent on cytotoxic T cells (17, 18). Thus, we hypothesized that the combination of radiotherapy and cancer immunotherapy would have synergistic potential (18).
Radium-223 dichloride (radium-223) has been approved for the treatment of patients with CRPC and symptomatic bone metastases but without known visceral metastatic disease (19, 20). This systemic treatment is a targeted high-energy α-particle emitter that selectively induces double-stranded DNA breaks at areas of increased bone cell turnover, thereby delivering a targeted cytotoxic effect in bone metastases (17, 21). Atezolizumab (anti–PD-L1) has demonstrated safety and durable long-term clinical benefit in patients with a variety of advanced malignancies and is approved for urothelial carcinoma, triple-negative breast cancer, small cell and non–small cell lung cancer, and hepatocellular carcinoma (22,23,24,25,26,27,28,29,30,31). This mAb blocks the immune checkpoint protein PD-L1 that is expressed on tumor cells and tumor-infiltrating immune cells in many cancer types (22). Here, we report on a phase Ib study conducted to evaluate the safety and therapeutic potential of combining atezolizumab with radium-223 in patients with mCRPC, concomitant bone metastases, and visceral metastases.
Patients and Methods
Study design and treatment
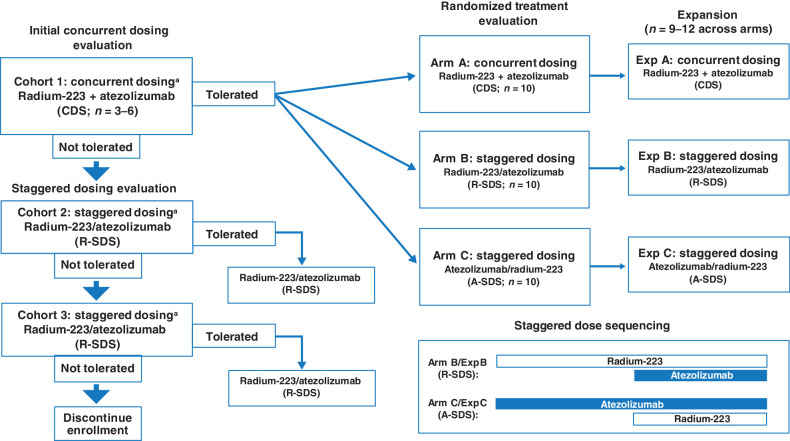
BO30013 (ClinicalTrials.gov ID NCT02814669) was a phase Ib, multicenter, open-label study designed to evaluate the safety and tolerability of concurrent or staggered dosing of atezolizumab and radium-223 in patients with mCRPC and identify a recommended treatment schedule. This study included an initial cohort phase which was followed by a potential randomization phase and an expansion phase. The initial cohort phase evaluated the safety and tolerability of a concurrent dosing schedule (CDS), in which the first cycle of atezolizumab and radium-223 were administered on the same day. If CDS was not tolerated in cohort 1, a minimum of 3 patients were to be enrolled in cohort 2 [staggered dosing schedule (SDS) 28 days of radium-223 run-in] and subsequently into cohort 3 (SDS 56-day run-in of radium-223). If the cohort 2 schedule was not tolerable, cohort 3 would be enrolled. Upon successful completion of the CDS, defined as no dose-limiting toxicities (DLT) observed, patients were then randomized 1:1:1 to a CDS treatment arm or one of two treatment arms with SDS in which atezolizumab or radium-223 was introduced one full cycle (28 days) before the other. Treatment regimens that were found to be safe and tolerable were then expanded. Cohort 1, treatment, and expansion arms are shown in Fig. 1. Atezolizumab was administered by intravenous infusion at a fixed dose of 840 mg on day 1 and day 15 of each 28-day cycle. Radium-223 was administered by slow bolus intravenous injection as a weight-based dose of 55 kBq/kg on day 1 of each 28-day cycle at a maximum of six times until unacceptable toxicity or loss of clinical benefit. Patients who discontinued one agent because of an adverse event (AE) were eligible to continue the other agent.
Figure 1.
Schema and enrollment of BO30013. A-SDS, atezolizumab run-in staggered dosing schedule; CDS, concurrent dosing schedule; exp, expansion; R-SDS, radium-223 run-in staggered dosing schedule. Agent + agent represents concurrent dosing. Agent/agent represents staggered dosing, with the first agent listed as the run-in agent. During staggered dosing, the first agent was administered for one cycle (28 days) prior to the initiation of the second agent. aPatients were closely monitored for DLTs during one 28-day cycle during cycle 1 for cohort 1, cycle 2 for cohort 2, and cycle 3 for cohort 3. Arms A, B, and/or C may have been selected for expanded enrollment on the basis of an evaluation of safety and tolerability, preliminary efficacy, and immune correlates.
Patients
Eligible patients were ages 18 years or older with known progressive mCRPC defined as castrate serum testosterone level ≤ 50 ng/dL (1.7 nmol/L), bilateral orchiectomy or maintenance on androgen ablation therapy with luteinizing hormone-releasing agonist or antagonist or polyestradiol phosphate throughout study and follow-up period, serum PSA progression, and serum PSA ≥ 2 ng/mL. Disease progression was defined according to Prostate Cancer Working Group 2 (PCWG2) criteria during or following treatment with at least 28 days of treatment with a second-generation androgen pathway inhibitor. Other key eligibility criteria included history of treatment with a taxane-containing regimen or ineligibility/refusal of a taxane-containing regimen. Patients were required to have two or more bone metastases and visceral metastases or malignant lymphadenopathy. Measurable disease per RECIST 1.1 was also required. Additional eligibility criteria included participants having disease that was not amenable to curative or locoregional therapies or had progressed thereafter, an Eastern Cooperative Oncology Group performance status score of 0 or 1, and adequate hematologic and organ function. Key exclusion criteria included a history of autoimmune disease, significant liver disease, prior radionuclide therapy, coinfection with hepatitis B and hepatitis C virus, and malignancies other than CRPC within 5 years prior to initiation of study treatment. Full eligibility criteria are provided in the trial protocol.
The study protocol was approved by local Institutional Review Boards prior to patient recruitment and was conducted in accordance with the Declaration of Helsinki International Conference on Harmonization E6 Guidelines for Good Clinical Practice. Written informed consent was obtained for all patients prior to performing study-related procedures in accordance with federal and institutional guidelines. This clinical study is being sponsored globally by F. Hoffmann-La Roche Ltd of Basel, Switzerland, and was registered on ClinicalTrials.gov (NCT02814669).
Outcomes
The primary objective of the study was to evaluate the safety and occurrence of DLTs of atezolizumab when given in combination with radium-223 and to identify a recommended treatment schedule for the treatment combination. DLTs were defined as grade ≥ 4 AEs (neutropenia, anemia, and thrombocytopenia) lasting ≥ 7 days, grade ≥ 3 (febrile neutropenia) lasting > 48 hours, grade ≥ 3 symptomatic hepatic toxicities lasting > 48 hours or asymptomatic hepatic toxicities lasting > 7 days, and grade ≥ 3 non-hematologic or non-hepatic organ toxicity. The primary efficacy objective was to evaluate combination treatment with atezolizumab and radium-223 as measured by ORR (per RECIST 1.1). Exploratory efficacy endpoints included ORR [investigator-assessed modified RECIST (mRECIST) based on RECIST 1.1 conventions and immune-related response criteria; refs. 32,33,34], duration of response (PCWG2 and mRECIST until the time of first observation of disease progression after the first objective response), rPFS (PCWG2), overall survival (OS), PSA response rate (>50% decrease in PSA from baseline after ≥ 4 weeks with a confirmatory PSA measurement), time to PSA progression (25% increase and ≥ 2 ng/mL absolute increase above baseline ≥ 12 weeks after baseline), confirmed total-alkaline phosphatase (ALP) response rate (proportion of enrolled patients who had a ≥ 30% reduction in blood levels compared with the baseline value, confirmed by a second total-ALP value obtained approximately ≥ 4 weeks later), time to total ALP progression (≥25% increase from baseline value or ≥ 25% increase above nadir value), and time to first symptomatic skeletal-related event (a skeletal-related event is considered to be external beam radiotherapy to relieve skeletal symptoms, new symptomatic pathologic bone fracture, occurrence of spinal cord compression, or tumor-related orthopedic surgical intervention) as measured from treatment start date in symptomatic patients.
Biomarker assessments included rate of circulating tumor cell conversion (patients with decrease to < 5 circulating tumor cells per 7.5 mL during treatment in those with ≥ 5 circulating tumor cells per 7.5 mL at baseline) and identification of biomarkers associated with treatment response and disease progression. Tumor assessments and bone scans were conducted at baseline and at weeks 12, 20, 28, and 36 and every 12 weeks thereafter until confirmed disease progression per PCWG2 criteria.
This study enrolled patients regardless of PD-L1 status, and both investigators and patients were blinded to PD-L1 expression status. IHC was conducted for PD-L1 (centrally evaluated per SP142 assay; Ventana). IC0, 1, 2 or 3 refers to < 1%, ≥ 1% and < 5%, ≥ 5% and < 10% or ≥ 10% PD-L1–expressing tumor-infiltrating immune cells within the tumor area, respectively. TC0, 1, 2, or 3 refers to < 1%, ≥ 1% and < 5%, ≥ 5% and < 50%, or ≥ 50% of tumor cells expressing PD-L1, respectively. IHC was also performed to detect CD8+ T cells in the tumor bed [Clone C8/144B (Dako)]. Patients with enough available tumor sample were also surveyed in an exploratory manner for RNA expression by Illumina TruSeq RNAaccess (MedGenome), including immune signature expression (35). Tumor whole-exome sequencing was performed on a limited number of patient tumors with germline subtraction by Agilent SureSelect (MedGenome). Biomarkers were collected on treatment just before the secondary schedule dose was administered, with only one drug on-board for samples. The gene signatures used to evaluate RNA sequencing (RNA-seq) data were described previously (35, 36).
Statistical analysis
This signal-seeking phase I study was designed with a planned enrollment of 45 participants and different dose run-ins. Determination of sample sizes was based primarily on the evaluation of safety and preliminary assessments of antitumor activity and biological activity (biomarkers). Demographics, clinical characteristics, exploratory biomarkers, and IHC outcomes were summarized using descriptive statistics. Safety assessments included all patients who received at least one dose of any study treatment and were also summarized using descriptive statistics. ORR and exploratory efficacy assessments included all treated patients and were summarized by treatment schedules and by subgroups according to patient characteristics. Objective response was defined per RECIST 1.1 as a complete response or partial response (PR), as determined by investigator assessment and confirmed by repeat assessment ≥ 4 weeks apart. Patients not meeting this criterion, including patients without a post-baseline tumor assessment, were classified as nonresponders. Ninety-five percent confidence intervals (95% CI) for ORR were calculated using the Clopper–Pearson method. The OS, radiographic PFS, and PSA PFS endpoints were calculated from time since randomization. For OS, patients who were alive or lost to follow-up as of the clinical cutoff date were censored at the last known date they were alive. Milestone rates for OS, rPFS, PSA, and PFS were estimated using the Kaplan–Meier method, with 95% CIs calculated using the Brookmeyer and Crowley method.
Data sharing
Qualified researchers may request access to individual patient-level data through the clinical study data request platform (https://vivli.org/). Further details on Roche criteria for eligible studies are available here (https://vivli.org/members/ourmembers/). For further details on Roche Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here (https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm).
Results
Patients
As of the data cutoff of October 4, 2019, 45 patients with mCRPC were enrolled in the study. Three patients were enrolled in the initial CDS cohort and showed acceptable tolerability. Thirty patients were randomized into a CDS arm or one of two SDS arms. Twelve patients were also randomized into one of three expansion arms (one CDS or one of two SDS). One patient enrolled into expansion arm B did not receive treatment. Baseline characteristics were generally similar among treatment arms (Table 1). The median age was 69.0 years (range, 41–85 years), median PSA was 51.6 μg/L (range, 3–3,051 ug/L), and median lactate dehydrogenase was 234.0 U/L (range, 109–2,363 U/L). For prior treatment, 14 patients (31.8%) had prior use of a taxane, 37 (84.1%) had prior radiotherapy, and 13 (29.5%) had received ≥ 3 lines of therapy. All 44 patients (100%) had confirmed metastatic bone disease. Of these patients, 8 patients (18.2%) had one to three bone lesions, 4 (9.1%) had four to six, 8 (18.2%) had seven to nine, and 24 (54.5%) had ≥ 10 bone lesions. Twenty-one (47.6%) patients had confirmed metastases to visceral organs, 36 (81.8%) had confirmed metastases to lymph nodes, and 17 (38.6%) had confirmed metastases to other sites at study entry.
Table 1.
Patient demographics.
| Cohort 1 | Arm A | Exp A | Arm B | Exp B | Arm C | Exp C | All | |
|---|---|---|---|---|---|---|---|---|
| CDS | CDS | CDS | R-SDS | R-SDS | A-SDS | A-SDS | Patients | |
| n (%) | (n = 3) | (n = 10) | (n = 4) | (n = 10) | (n = 3) | (n = 10) | (n = 4) | (N = 44) |
| Median age (range), y | 70.0 (44–70) | 67.5 (41–82) | 67.5 (58–82) | 74.0 (62–78) | 68.0 (68–69) | 67.5 (49–85) | 71.5 (66–72) | 69.0 (41–85) |
| Age ≥ 65 y | 2 (66.7) | 7 (70.0) | 3 (75.0) | 9 (90.0) | 3 (100.0) | 7 (70.0) | 4 (100.0) | 35 (79.5) |
| White race | 2 (66.7) | 9 (90.0) | 1 (25.0) | 8 (80.0) | 1 (33.3) | 7 (70.0) | 3 (75.0) | 31 (70.5) |
| Baseline ECOG | ||||||||
| 0 | 2 (66.7) | 5 (50.0) | 4 (100.0) | 6 (60.0) | 2 (66.7) | 5 (50.0) | 2 (50.0) | 26 (59.1) |
| 1 | 1 (33.3) | 5 (50.0) | 0 | 4 (40.0) | 1 (33.3) | 5 (50.0) | 2 (50.0) | 18 (40.9) |
| Baseline PSA, median (range), μg/L | 21.7 (19–3,051) | 77.7 (4–608) | 154.8 (3–2,027) | 45.1 (6–186) | 55.2 (43–161) | 21.2 (7–365) | 280.7 (40–1,029) | 51.6 (3–3,051) |
| Baseline ALP, median (range), U/L | 75.0 (43–104) | 116.0 (42–284) | 134.5 (56–448) | 98.5 (51–506) | 167.0 (130–488) | 220.0 (36–1,064) | 119.5 (80–313) | 125.0 (36–1,064) |
| Stage at initial diagnosis | ||||||||
| I | 0 | 0 | 0 | 0 | 0 | 0 | 1 (25.0) | 1 (2.3) |
| II | 1 (33.3) | 1 (10.0) | 1 (25.0) | 3 (30.0) | 0 | 1 (10.0) | 0 | 7 (15.9) |
| III | 1 (33.3) | 0 | 0 | 2 (20.0) | 0 | 4 (40.0) | 0 | 7 (15.9) |
| IV | 1 (33.3) | 5 (50.0) | 2 (50.0) | 4 (40.0) | 0 | 2 (20.0) | 2 (50.0) | 16 (36.4) |
| Unknown | 0 | 4 (40.0) | 1 (25.0) | 1 (10.0) | 3 (100.0) | 3 (30.0) | 1 (25.0) | 13 (29.5) |
| Number of bone lesions | ||||||||
| 1–3 | 3 (100.0) | 0 | 1 (25.0) | 4 (40.0) | 0 | 0 | 0 | 8 (18.2) |
| 4–6 | 0 | 2 (20.0) | 0 | 1 (10.0) | 0 | 1 (10.0) | 0 | 4 (9.1) |
| 7–9 | 0 | 1 (10.0) | 0 | 2 (20.0) | 0 | 3 (30.0) | 2 (50.0) | 8 (18.2) |
| ≥ 10 | 0 | 7 (70.0) | 3 (75.0) | 3 (30.0) | 3 (100.0) | 6 (60.0) | 2 (50.0) | 24 (54.5) |
| Prior surgery, n (%) | 1 (33.3) | 6 (60.0) | 4 (100.0) | 8 (80.0) | 3 (100.0) | 9 (90.0) | 3 (75.0) | 34 (77.3) |
| Prior use of taxane, n (%) | 1 (33.3) | 5 (50.0) | 2 (50.0) | 1 (10.0) | 0 | 2 (20.0) | 3 (75.0) | 14 (31.8) |
| Prior radiotherapy, n (%) | 3 (100.0) | 8 (80.0) | 2 (50.0) | 8 (80.0) | 3 (100.0) | 9 (90.0) | 4 (100.0) | 37 (84.1) |
| Max line of therapy, n (%) | ||||||||
| 1 | 3 (100.0) | 5 (50.0) | 1 (25.0) | 2 (20.0) | 3 (100.0) | 2 (20.0) | 1 (25.0) | 17 (38.6) |
| 2 | 0 | 2 (20.0) | 0 | 4 (40.0) | 0 | 2 (20.0) | 2 (50.0) | 10 (22.7) |
| 3+ | 0 | 3 (30.0) | 3 (75.0) | 3 (30.0) | 0 | 4 (40.0) | 0 | 13 (29.5) |
| Missing | 0 | 0 | 0 | 1 (10.0) | 0 | 2 (20.0) | 1 (25.0) | 4 (9.1) |
| Metastatic sites, n (%) | ||||||||
| Adrenal | 1 (33.3) | 1 (10.0) | 0 | 0 | 0 | 4 (40.0) | 0 | 6 (13.6) |
| Bone | 3 (100.0) | 10 (100.0) | 4 (100.0) | 10 (100.0) | 3 (100.0) | 10 (100.0) | 4 (100.0) | 44 (100.0) |
| Liver | 0 | 0 | 1 (25.0) | 0 | 0 | 1 (10.0) | 1 (25.0) | 3 (6.8) |
| Lung | 2 (66.7) | 5 (50.0) | 1 (25.0) | 0 | 0 | 2 (20.0) | 0 | 10 (22.7) |
| Lymph | 3 (100.0) | 7 (70.0) | 3 (75.0) | 10 (100.0) | 2 (66.7) | 7 (70.0) | 4 (100.0) | 36 (81.8) |
| Mediastinum | 0 | 1 (10.0) | 1 (25.0) | 0 | 0 | 0 | 0 | 2 (4.5) |
| Othera | 0 | 5 (50.0) | 1 (25.0) | 6 (60.0) | 1 (33.3) | 3 (30.0) | 1 (25.0) | 17 (38.6) |
Abbreviations: A-SDS, atezolizumab run-in staggered dosing schedule; CDS, concurrent dosing schedule; ECOG, Eastern Cooperative Oncology Group; R-SDS, radium-223 run-in staggered dosing schedule; y, years.
aSoft tissue, retroperitoneal, pleura, soft tissue, peritoneum, bladder, pelvis, chest, prostate, kidney, right ureter, retroperitoneal soft tissue, right periureteral mass, retrocrural node, peritoneum, right perirectal mass, rectum pre-sacral.
Safety
All 44 patients received atezolizumab and radium-223 and were evaluable for safety. A total of 17 patients (38.6%) received treatment on the CDS, 13 (29.5%) received treatment on the SDS with radium run-in, and 14 (31.8%) received treatment on the SDS with atezolizumab run-in. The median duration of atezolizumab therapy was 97.9 days (range, 0–562 days), and the median duration for radium-223 was 83.9 days (range, 26–179 days). Patients receiving atezolizumab had a median of 7.5 doses (range, 1–39 doses), and those receiving radium-223 had a median of 4.0 doses (range, 2–6 doses). All-cause and treatment-related AE data are summarized in Table 2. AEs that occurred in ≥ 20% of patients are listed in Supplementary Table S1. The most common AEs observed in patients receiving atezolizumab + radium-223 were fatigue (56.8%), decreased appetite (50.0%), diarrhea (45.5%), and nausea (43.2%). Of the grade 3/4 AEs, 15 (34.1%) were related to atezolizumab, and 12 (27.3%) were related to radium-223. In patients receiving atezolizumab, anemia (n = 5, 11.4%) and decreased lymphocyte count (n = 4, 9.1%) were the most common grade 3/4 AEs. In patients receiving radium-223, anemia (n = 5, 11.4%) and decreased lymphocyte count (n = 3, 6.8%) were also the most common grade 3/4 AEs.
Table 2.
Summary of AEs in the safety-evaluable population.
| Patients with adverse events, n (%) | Patients (N = 44) |
|---|---|
| ≥ 1 all-cause AE | 44 (100.0) |
| Grade 1/2 | 13 (29.5) |
| Grade 3/4 | 23 (52.3) |
| Grade 5 | 8 (18.2) |
| Serious AEs | 20 (45.5) |
| Grade 5 AEs | 8 (18.2) |
| Prostate cancer progression | 4 (9.1) |
| Othera | 4 (9.1) |
| Treatment-related AEs | |
| Atezolizumab related | 38 (86.4) |
| Grade 1/2 | 21 (47.7) |
| Grade 3/4 | 15 (34.1) |
| Grade 5b | 3 (6.8) |
| Radium-223 related | 35 (79.5) |
| Grade 1/2 | 23 (52.3) |
| Grade 3/4 | 12 (27.3) |
| Grade 5 | 0 |
| Serious treatment-related AEs | |
| Atezolizumabc | 8 (18.2) |
| Radium-223d | 2 (4.5) |
| AEs of special interest | 35 (79.5) |
| AEs leading to withdrawal of treatment | |
| Atezolizumab | 3 (6.8) |
| Radium-223 | 1 (2.3) |
Note: Clinical cutoff date: October 4, 2019.
aEnteritis, autoimmune myositis, respiratory failure, cerebral vascular accident.
bEnteritis, autoimmune myositis, respiratory failure.
cHypoxia, limbic encephalitis, neuropathy peripheral, dyspnea, respiratory failure, anemia, thrombocytopenia, sinus tachycardia, alanine aminotransferase increased, aspartate aminotransferase increased, autoimmune myositis, confusional state, hydronephrosis.
dAnemia, febrile neutropenia, thrombocytopenia, hydronephrosis.
A summary of causes of death is provided in Supplementary Table S2. Of the 44 patients in the safety-evaluable population, 27 (61.4%) died. Per protocol, all deaths occurring during the protocol-specified AE reporting period, regardless of relationship to study treatment and including death attributed to progression of prostate cancer, were reported on the AE electronic case report form. Nineteen deaths (43.2%) were attributed to disease progression, four (9.1%) of which occurred during the AE reporting period. Another four deaths (9.1%) occurred because of AEs [1 case (2.3%) each of enteritis, autoimmune myositis, cerebrovascular accident, and respiratory failure]. Three of these deaths (due to enteritis, autoimmune myositis, and respiratory failure) were considered to be related to atezolizumab; none were associated with radium-223. One case of cerebrovascular accident was assessed by the investigator to be related to concurrent illness and not related to either atezolizumab or radium-223. The remaining four deaths (9.1%) were due to unknown cause.
The 3 patients who died from a related AE are as follows:
(i) Grade 5 enteritis: An 82-year-old man with a past treatment history of hormonal therapy, sipuleucel-T, CPI-444, and radiotherapy to the lumbar spine was randomized to the concurrent dosing regimen (expansion arm A). Biopsies showed that the patient had metastases in the bone, lymph node, and peritoneum. The patient had completed five doses of atezolizumab and radium-223 each before discontinuing both study agents due to disease progression. The patient was then started on pelvic radiotherapy for over 3 weeks before developing grade 3 enteritis. Steroids were given, but the patient did not respond. The laboratory test for bacterial infections also had negative findings. The patient was then transferred to hospice care and died approximately 1 week later. The investigator considered this grade 5 enteritis event to be related to both atezolizumab and the recent radiotherapy.
(ii) Grade 5 autoimmune myositis: An 85-year-old man with a past treatment history of bilateral testicular surgery, abiraterone, and prednisone was randomized to atezolizumab run-in staggered dosing schedule (arm C). Metastases in the bone and liver were confirmed via biopsies. After three cycles of atezolizumab and radium-223, the patient was initially diagnosed with grade 3 myositis, initially asymptomatic, which led to the interruption of atezolizumab and treatment with steroids. The patient was later diagnosed with grade 3 autoimmune myositis, which required more aggressive therapy. The patient had been previously treated with a statin for hyperlipidemia, and his refractory myositis was felt to be related to atezolizumab or statin-induced autoimmune necrotizing myopathy. The patient died approximately 3 weeks later, and the grade 5 autoimmune myositis was considered by the investigator to be related to atezolizumab.
(iii) Grade 5 respiratory failure: A 65-year-old man with a past treatment history of hormonal therapy and radiotherapy to the sacrum was randomized to the radium run-in staggered dosing schedule (arm B). Biopsies showed that the patient had metastasis in bone, lymph node, and peritoneum (right base and left mid) and a right perirectal mass. This patient received two doses of radium-223 and one dose of atezolizumab prior to discontinuing both study treatments due to disease progression. Approximately 8 weeks after study drug discontinuation and during treatment with subsequent docetaxel chemotherapy, the patient was hospitalized with fever, weakness, and fatigue. He was noted to have grade 3 hypoxia (worsened to grade 4) and respiratory failure. A CT scan showed increased patchy areas of consolidation in bilateral lung bases and progression in peritoneal and retroperitoneal tumor implants. Because of the patient's worsening condition, he received antibiotics, oxygen support, and steroids but died approximately 3 weeks after the initial hospital admission despite the proactive treatment for possible Pneumocystis jirovecii. The grade 5 respiratory failure was reported as related to atezolizumab-induced pneumonitis, concurrent illness, or docetaxel-related pneumonitis, and the patient's underlying disease.
Efficacy
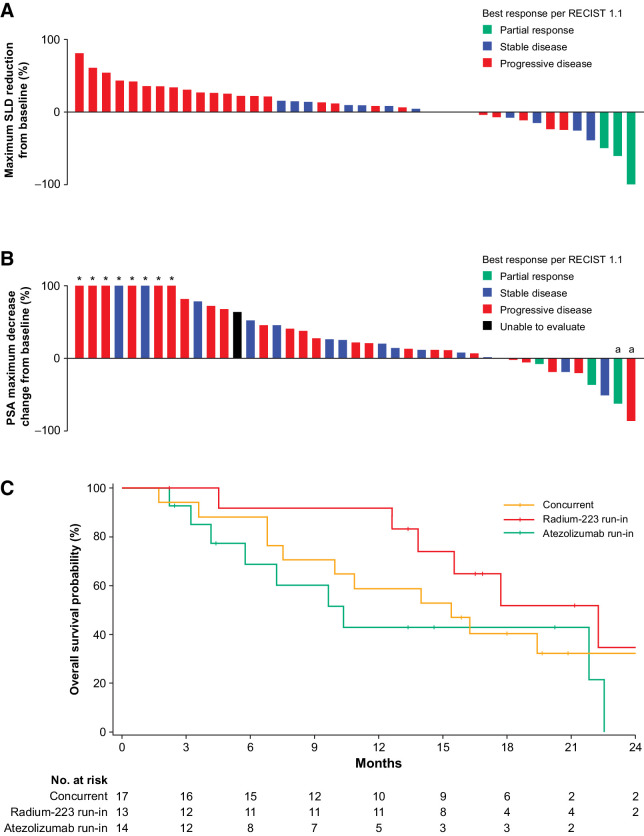
The median duration of follow-up for the efficacy-evaluable population was 13.9 months (range, 1.7–34.2 months), and all 44 patients had measurable disease at baseline. Responses are presented in Table 3 and Supplementary Fig. S1. Three patients (6.8%) had confirmed PR per RECIST 1.1; 1 patient each from cohort 1, arm B, and expansion arm B. Sites of response were lymph node for the patients from cohort 1 and arm B and soft-tissue mass from left fourth rib for the patient from expansion arm B. Durations of confirmed response for each patient in cohort 1, arm B, and expansion arm B were 6.3, 9.0, and 11.1 months, respectively. These 3 patients (6.8%) also had confirmed PRs per mRECIST. Stable disease as best response was seen in 14 patients: 3 patients (30.0%) in arm A, 4 (40.0%) in arm B, 5 (50.0%) in arm C, and 2 (50.0%) in expansion arm C (Table 3; Supplementary Fig. S1). There were no complete responses. The sum of longest diameters (SLD) decreased from 41 to 16 mm (61.0% reduction) in the patient from cohort 1 and 22 to 12 mm (45.0%) in the patient from expansion arm B. SLD decreased from 23 to 15 mm (34.8% reduction) in the patient from arm B (Fig. 3A).
Table 3.
Responses for patients with measurable disease by arms.
| Cohort 1 | Arm A | Exp A | Arm B | Exp B | Arm C | Exp C | ||
|---|---|---|---|---|---|---|---|---|
| CDS | CDS | CDS | R-SDS | R-SDS | A-SDS | A-SDS | ||
| Response criteria, n (%) | (n = 3) | (n = 10) | (n = 4) | (n = 10) | (n = 3) | (n = 10) | (n = 4) | All patients (N = 44) |
| Confirmed ORR per RECIST 1.1 (95% CI) | 1 (33.3) (0.8–90.6) | 0 (0.0–30.9) | 0 (0.0–60.2) | 1 (10.0) (0.3–44.5) | 1 (33.3) (0.8–90.6) | 0 (0.0–30.9) | 0 (0.0–60.2) | 3 (6.8) (1.4–18.7) |
| PR (95% CI) | 1 (33.3) (0.8–90.6) | 0 (0.0–30.9) | 0 (0.0–60.2) | 1 (10.0) (0.3–44.5) | 1 (33.3) (0.8–90.6) | 0 (0.0–30.9) | 0 (0.0–60.2) | 3 (6.8) (1.4–18.7) |
| SD (95% CI) | 0 (0.0–70.8) | 3 (30.0) (6.7–65.3) | 0 (0.0–60.2) | 4 (40.0) (12.2–73.8) | 0 (0.0–70.8) | 5 (50.0) (18.7–81.3) | 2 (50.0) (6.8–93.2) | 14 (31.8) (18.6–47.6) |
| PD (95% CI) | 2 (66.7) (9.4–99.2) | 7 (70.0) (34.8–93.3) | 4 (100.0) (39.8–100.0) | 4 (40.0) (12.2–73.8) | 2 (66.7) (9.4–99.2) | 5 (50.0) (18.7–81.3) | 1 (25.0) (0.6–80.6) | 25 (56.8) (41.0–71.7) |
| Missing or unevaluable | 0 | 0 | 0 | 1 (10.0) | 0 | 0 | 1 (25.0) | 2 (4.5) |
| ORR per mRECIST (95% CI) | 1 (33.3) (0.8–90.6) | 0 (0.0–30.9) | 0 (0.0–60.2) | 1 (10.0) (0.3–44.5) | 1 (33.3) (0.8–90.6) | 0 (0.0–30.9) | 0 (0.0–60.2) | 3 (6.8) (1.4–18.7) |
| PSA response rate (95% CI) | 1 (33.3) (0.8–90.6) | 0 (0.0–30.9) | 0 (0.0–60.2) | 1 (10.0) (0.3–44.5) | 0 (0.0–70.8) | 0 (0.0–30.9) | 0 (0.0–60.2) | 2 (4.5) (0.6–15.5) |
| ALP response rate (95% CI) | 1 (33.3) (0.8–90.6) | 4 (40.0) (12.2–73.8) | 2 (50.0) (6.8–93.2) | 5 (50.0) (18.7–81.3) | 3 (100.0) (29.2–100.0) | 5 (50.0) (18.7–81.3) | 1 (25.0) (0.6–80.6) | 21 (47.7) (32.5–63.3) |
Abbreviations: A-SDS, atezolizumab run-in staggered dosing schedule; CDS, concurrent dosing schedule; R-SDS, radium-223 run-in staggered dosing schedule.
Figure 3.
Clinical activity of efficacy-evaluable population. A, Waterfall plot of the maximum reduction in the SLD in the efficacy-evaluable population. B, Waterfall plot of maximum change in PSA decrease in efficacy-evaluable population. C, Kaplan–Meier plot for OS for all treatment arms in efficacy-evaluable population. aPatients with confirmed PSA decreased.
Two responders (4.5%; 1 patient from cohort 1 and 1 patient from arm B) had a confirmed PSA response (Table 3). The maximum PSA decrease from baseline for each patient, along with best response per RECIST 1.1, is shown in Fig. 3B. Relative to baseline, there were 4 patients (9.1%) with ≥ 30% PSA decrease, 3 patients (6.8%) with ≥ 50% PSA decrease, and no patients with ≥ 90% PSA decrease. Confirmed ALP responses were seen across all seven treatment arms (Table 3). There were no significant differences between treatment arms for OS in Fig. 3C and Supplementary Table S4; median OS for all patients was 16.3 months (95% CI, 10.9–22.3; Supplementary Table S3). Median rPFS for all patients was 3.0 months (95% CI, 2.8–4.6; Supplementary Table S3).
Biomarker analyses
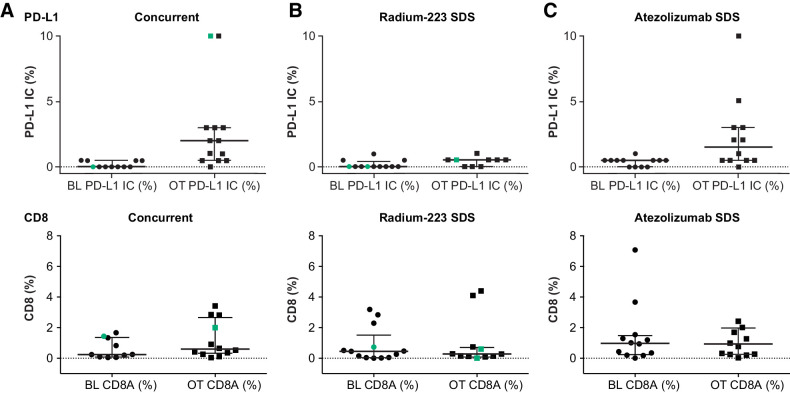
Changes in PD-L1 and CD8 IHC were consistent with the known mechanism of action of atezolizumab, and changes in alkaline phosphatase were consistent with radium-223 activity (Fig. 2A and B; refs. 22, 37, 38). Increases in PD-L1 expression on tumors occurred only in patients who received scheduled atezolizumab first (cohort 1, arm C, and expansion arm C; Fig. 2A). Furthermore, no significant CD8 changes were observed across all arms (Fig. 2B), and significant differences in immune signatures were not broadly seen (Supplementary Fig. S2). Although some patients achieving PR or stable disease (SD) exhibited hallmarks of inflammation, some patients with progressive disease (PD) also exhibited inflamed signatures. Looking specifically at the 3 patients who achieved a PR: tumor samples collected at baseline and during cycle 1 from the cohort 1 patient showed an increase in PD-L1 expression levels from a baseline of IC0/TC0 to IC3/TC1 (Supplementary Fig. S3), increased infiltration of CD8 from 1.4% at baseline to 2.0% following cycle 1 from the tumor stroma into tumor nests (Supplementary Fig. S3), and an increase in favorable immune gene signature expression with treatment (Supplementary Fig. S2). One PR patient from expansion arm B had tumor with a PD-L1 expression level of IC1 at baseline. Tumor samples showed an increase in the presence of CD8 T cells from 0.45% at baseline to 0.6% at cycle 1. This patient also had high expression of IFNγ, IFNα/β, and antigen presentation machinery gene signatures following cycle 1 (Supplementary Fig. S2). The tumor sample from the other arm B PR patient was PD-L1 IC0/TC0 at baseline, and only 0.01% of CD8 cells stained in the tumor area at cycle 1. There was insufficient sample for RNA-seq to be performed at both time points for this patient.
Figure 2.
PD-L1 and CD8 biomarker analyses across patients with concurrent treatment (A), radium-223 run-in (B), or atezolizumab run-in (C). Green indicates a partial responder. BL, baseline; IC, tumor-infiltrating immune cells; OT, on treatment.
Discussion
This is the first report on the safety, efficacy, and biomarker analyses associated with the combination of atezolizumab and radium-223 in patients with mCRPC, concomitant bone metastases, and lymph node and/or visceral metastases. Greater toxicities were observed with the combination than with each individual agent alone, and we found no evidence for additive or synergistic efficacy based on objective PSA responses. Most responses were not durable across a range of treatment dosing schedules.
One goal of BO30013 was to identify a recommended dosing schedule for atezolizumab in combination with radium-223, with both concurrent and staggered dosing schedules being examined. Staggered dosing schedules have the potential to alter treatment outcomes (39, 40) and have been employed successfully in patients with melanoma (12, 41). Although the seven cohorts examined here included various arms and expansions of CDS and SDS with atezolizumab or radium-223 run-ins, a recommended dosing schedule for the combination treatment was not identified.
In addition, no dose de-escalation or escalation plans were included as part of the study design. Given the available clinical safety data, atezolizumab 840 mg every 2 weeks was considered an appropriate starting dose to evaluate in combination with radium-223 dichloride, with both agents administered according to their standard dose and schedule. Both atezolizumab and radium-223 have been well tolerated as monotherapy and demonstrated little overlapping toxicity (19, 22, 29, 30). The likelihood of significant drug–drug interactions between atezolizumab + radium-223 was considered low so that the benefit–risk ratio of this combination was considered acceptable. Furthermore, the potential for immune-related toxicities is not mitigated by lower doses of immune checkpoint inhibitors.
Instead of dose de-escalation or escalation plans, BO30013 included a cohort phase before randomization. No DLTs, as defined per protocol, were reported during the initial phase. The toxicity to benefit ratio of the combination in the studied patient population was unfavorable compared with those of individual agents alone. Fatigue was the most common AE. Other common AEs included decreased appetite, diarrhea, nausea, vomiting, and anemia. Although the grade 5 AE rate was higher than expected, this was the result of recording four deaths due to PD (9.1%) as AEs because they occurred during the AE reporting period. Of the treatment-related grade 5 AEs, three (7%; enteritis, autoimmune myositis, and respiratory failure) were reported as related to atezolizumab, with no grade 5 AEs reported to be related to radium-223.
Of the 44 patients in the efficacy-evaluable population, 3 patients (6.8%, 95% CI, 1.4–18.7) had PR to CDS or radium-223 run-in SDS. Although no clinical responses were seen with the atezolizumab run-in SDS, the efficacy of the combination was not definitively influenced by the run-in or changing the order of drug delivery. The radium-223 SDS arms had trends of numerically longer PFS versus both CDS and atezolizumab run-in SDS; however, these results were not statistically significant.
Changes in PD-L1 and CD8 IHC were consistent with the known mechanism of action of atezolizumab (22), and changes in alkaline phosphatase levels were consistent with the mechanism of action of radium-223 (17, 38, 42). As in other trials and indications, PD-L1 increases were observed in arms with atezolizumab run-ins (cohort 1, arm C, and expansion arm C; refs. 22, 37). However, changes in PD-L1 and CD8 levels were not associated with the limited clinical efficacy seen with this combination. CD8 levels did not change across all three arms. Although samples from tumors achieving PR or SD were more inflamed, PD samples also exhibited inflamed signatures. However, biomarker analyses did not reveal any significant differences in these immune signatures.
This study had several limitations, including its small size and lack of power due to being a phase I pilot study. This small size was also potentially limiting for the exploratory biomarker analyses as the ability to associate changes with activity was likely reduced. In addition, a dose de-escalation or escalation phase was not performed to find the MTD for this combination due to the lack of overlapping toxicities. This potentially limited the effectiveness of this combination versus if lower doses had been evaluated as part of the study design.
Other agents with complementary mechanisms of action are also being examined in combination with cancer immunotherapy for patients with prostate cancer. The IMbassador250 study demonstrated that the addition of atezolizumab to enzalutamide did not improve OS in patients with mCRPC (43). No new safety signals were identified in IMbassador250 (43). However, targeting a different part of the cancer immunity cycle might still result in an efficacious combination. To this end, the combination of radium-223 with sipuleucel-T (NCT02463799) has produced encouraging results, as improved clinical outcomes were seen compared with sipuleucel-T alone (44). The combinations of atezolizumab and cabozantinib (NCT03170960) and pembrolizumab and docetaxel (NCT02861573) are also being explored.
In summary, this phase Ib study showed no new safety signals, but more AEs, including atezolizumab-related grade 5 AEs, were observed with the combination of atezolizumab + radium-223. The combination also did not appear to demonstrate improved clinical benefit for patients with mCRPC, regardless of the sequence of study treatments. No further studies are ongoing for this combination.
Authors' Disclosures
L. Fong reports grants from AbbVie, Bavarian Nordic, Dendreon, Merck, and Roche/Genentech, as well as grants and personal fees from Bristol Myers Squibb and Janssen during the conduct of the study; L. Fong has ownership interests in Actym, Alector, Atreca, BioAlta, Bolt, Keyhole, Immunogenesis, Nutcracker, RAPT, Scribe, Senti, Soteria, and TeneoBio. M.J. Morris reports non-financial support from Roche during the conduct of the study, as well as personal fees from ORIC Pharmaceuticals and Curium outside the submitted work; M.J. Morris also reports being an uncompensated advisor to Bayer, Endocyte, Advanced Accelerator Applications, Johnson & Johnson, and Athenex. MSK received funding for clinical trials in which M.J. Morris received salary support from Bayer, Endocyte, Progenics, Corcept, Roche/Genentech, and Janssen. O. Sartor reports grants and personal fees from Advanced Accelerator Applications, AstraZeneca, Bayer, Constellation, Dendreon, Janssen, Progenics, and Sanofi; personal fees from Astellas, Blue Earth Diagnostics, Inc., Bavarian Nordic, Bristol Myers Squibb, Clarity Pharmaceuticals, Clovis, EMD Serono, Fusion, Isotopen Technologien Muenchen, Myovant, Myriad, Noria Therapeutics, Inc., Novartis, Noxopharm, POINT Biopharma, Pfizer, TeneBio, Telix, and Theragnostics; and grants from Invitae, Merck, and SOTIO during the conduct of the study. C.S. Higano reports other from F. Hoffmann-La Roche Ltd, AstraZeneca, Bayer, Clovis, Dendreon, eFFECTOR Therapeutics, Emergent, Ferring, Genentech, Medivation, Pfizer, Astellas, Aptevo, and Aragon, as well as personal fees from Astellas, Bayer, Blue Earth Diagnostics, Clovis, Dendreon, Ferring, Genentech, Carrick Therapeutics, Hinova, Janssen, Merck, Orion, Pfizer, Novartis, Tolmar, Menarini, Myovant, and Vaccitech during the conduct of the study. L. Pagliaro reports non-financial and other support from Roche/Genentech during the conduct of the study. L. Pagliaro also reports non-financial and other support from Exelixis, Pfizer, and Merck, as well as personal fees from Merck outside the submitted work. A. Alva reports grants from Genentech during the conduct of the study. A. Alva also reports grants and personal fees from Bristol Myers Squibb, Merck, and AstraZeneca, as well as grants from Progenics, Ionis, Esanik, and Arcus outside the submitted work; A. Alva is a NCCN panel member and member of ASCO and SWOG. L.J. Appleman reports grants from Roche during the conduct of the study, as well as non-financial support from Bayer outside the submitted work. W. Tan reports other from Medscape WebMD outside the submitted work. U. Vaishampayan reports grants from Genentech during the conduct of the study, as well as personal fees from Bayer, Sanofi, and Pfizer outside the submitted work. D. Tayama reports other from Genentech/Roche outside the submitted work. E.E. Kadel III reports other support from Clinuvel, Epizyme, MannKind, Merck, Roche/Genentech, and Teladoc outside the submitted work. K.C. Yuen reports personal fees from Genentech, Inc during the conduct of the study. A. Datye reports employment by F. Hoffmann-La Roche and has stock ownership in F. Hoffmann-La Roche. A.J. Armstrong reports grants from Genentech/Roche during the conduct of the study. A.J. Armstrong also reports grants and personal fees from Pfizer/Astellas, Janssen, Celgene/Bristol Myers Squibb, Merck, AstraZeneca, and Forma; grants from Constellation; and personal fees from Clovis outside the submitted work. D.P. Petrylak reports other support from Bellicum Pharmaceuticals and TYME; grants and personal fees from Advanced Accelerator Applications, AstraZeneca, Bayer, Bristol Myers Squibb, Clovis Oncology, Lilly, Pfizer, Roche, and Seattle Genetics; personal fees from Amgen, Astellas Pharma, Boehringer Ingelheim, Exelixis, Incyte, Janssen, Pharmacyclics, UroGen Pharma, Sanofi, and Celgene; and grants from Astellas Medivation, Endocyte, Genentech, Innocrin Pharma, MedImmune, Merck, Novartis, and Progenics outside the submitted work. No disclosures were reported by the other author.
Supplementary Material
Acknowledgments
This study was sponsored by F. Hoffmann-La Roche Ltd/Genentech, Inc, a member of the Roche Group. We thank the patients participating in this trial and their families, the nurses, research coordinators, data managers, and clinical study site investigators. Medical writing assistance for this article was provided by Priscilla Hong, PharmD, of Health Interactions, Inc, and funded by F. Hoffmann-La Roche, Ltd.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
#L. Fong, M.J. Morris, A.J. Armstrong, and D.P. Petrylak contributed equally to this article.
ClinicalTrials.gov identifier: NCT02814669.
Clin Cancer Res 2021;27:4746–56
Authors' Contributions
L. Fong: Conceptualization, data curation, supervision, investigation, methodology, writing–original draft, writing–review and editing. M.J. Morris: Resources, investigation, writing–review and editing. O. Sartor: Writing–review and editing. C.S. Higano: Resources, investigation, methodology, writing–original draft, writing–review and editing. L. Pagliaro: Formal analysis, investigation, writing–review and editing. A. Alva: Writing–review and editing. L.J. Appleman: Data curation, investigation, writing–review and editing. W. Tan: Writing–review and editing. U. Vaishampayan: Investigation, writing–original draft, writing–review and editing. R. Porcu: Data curation, formal analysis, methodology, writing–original draft, writing–review and editing. D. Tayama: Writing–review and editing. E.E. Kadel III: Data curation, formal analysis, methodology, writing–original draft, writing–review and editing. K.C. Yuen: Formal analysis, visualization, writing–review and editing. A. Datye: Data curation, formal analysis, visualization, methodology, writing–original draft, writing–review and editing. A.J. Armstrong: Conceptualization, resources, supervision, investigation, writing–original draft, writing–review and editing. D.P. Petrylak: Writing–review and editing.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7–30. [DOI] [PubMed] [Google Scholar]
- 2. Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med 2014;371:424–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ryan CJ, Smith MR, Fizazi K, Saad F, Mulders PF, Sternberg CN, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 2015;16:152–60. [DOI] [PubMed] [Google Scholar]
- 4. Armstrong AJ, Lin P, Higano CS, Sternberg CN, Sonpavde G, Tombal B, et al. Development and validation of a prognostic model for overall survival in chemotherapy-naïve men with metastatic castration-resistant prostate cancer. Ann Oncol 2018;29:2200–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pezaro CJ, Omlin AG, Altavilla A, Lorente D, Ferraldeschi R, Bianchini D, et al. Activity of cabazitaxel in castration-resistant prostate cancer progressing after docetaxel and next-generation endocrine agents. Eur Urol 2014;66:459–65. [DOI] [PubMed] [Google Scholar]
- 6. Halabi S, Kelly WK, Ma H, Zhou H, Solomon NC, Fizazi K, et al. Meta-analysis evaluating the impact of site of metastasis on overall survival in men with castration-resistant prostate cancer. J Clin Oncol 2016;34:1652–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Roviello G, Petrioli R, Laera L, Francini E. The third line of treatment for metastatic prostate cancer patients: option or strategy? Crit Rev Oncol Hematol 2015;95:265–71. [DOI] [PubMed] [Google Scholar]
- 8. Sartor O, de Bono JS. Metastatic prostate cancer. N Engl J Med 2018;378:645–57. [DOI] [PubMed] [Google Scholar]
- 9. Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest 2014;124:687–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sharabi AB, Lim M, DeWeese TL, Drake CG. Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. Lancet Oncol 2015;16:e498–509. [DOI] [PubMed] [Google Scholar]
- 11. Antonarakis ES, Piulats JM, Gross-Goupil M, Goh J, Ojamaa K, Hoimes CJ, et al. Pembrolizumab for treatment-refractory metastatic castration-resistant prostate cancer: multicohort, open-label phase II KEYNOTE-199 study. J Clin Oncol 2020;38:395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McArthur GA, Stroyakovskiy D, Gogas H, Robert C, Lewis K, Protsenko S, et al. Evaluation of atezolizumab (A), cobimetinib (C), and vemurafenib (V) in previously untreated patients with BRAFV600 mutation-positive advanced melanoma: primary results from the phase 3 IMspire150 trial. In: Proceedings of the Annual Meeting of the American Association for Cancer Research 2020; 2020. Apr 27–28 and Jun 22-24. Philadelphia (PA): AACR; Cancer Res 2020;80(16 Suppl):Abstract CT012. [Google Scholar]
- 13. Picardo SL, Hansen AR. The PD-1/PD-L1 pathway in advanced prostate cancer-have we milked this cow? Ann Transl Med 2019;7:346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 2012;366:925–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stamell EF, Wolchok JD, Gnjatic S, Lee NY, Brownell I. The abscopal effect associated with a systemic anti-melanoma immune response. Int J Radiat Oncol Biol Phys 2013;85:293–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Formenti SC, Rudqvist NP, Golden E, Cooper B, Wennerberg E, Lhuillier C, et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nat Med 2018;24:1845–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Morris MJ, Corey E, Guise TA, Gulley JL, Kevin Kelly W, Quinn DI, et al. Radium-223 mechanism of action: implications for use in treatment combinations. Nat Rev Urol 2019;16:745–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Slovin SF, Higano CS, Hamid O, Tejwani S, Harzstark A, Alumkal JJ, et al. Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: results from an open-label, multicenter phase I/II study. Ann Oncol 2013;24:1813–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.XOFIGO (radium Ra 223 dichloride). Prescribing information. Bayer HealthCare Pharmaceuticals Inc; 2019.
- 20. Parker C, Nilsson S, Heinrich D, Helle SI, O'Sullivan JM, Fosså SD, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 2013;369:213–23. [DOI] [PubMed] [Google Scholar]
- 21. Goyal J, Antonarakis ES. Bone-targeting radiopharmaceuticals for the treatment of prostate cancer with bone metastases. Cancer Lett 2012;323:135–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014;515:563–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 2016;387:1909–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Balar AV, Galsky MD, Rosenberg JE, Powles T, Petrylak DP, Bellmunt J, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet 2017;389:67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med 2018;379:2108–21. [DOI] [PubMed] [Google Scholar]
- 26. Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med 2018;379:2220–9. [DOI] [PubMed] [Google Scholar]
- 27. Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016;387:1837–46. [DOI] [PubMed] [Google Scholar]
- 28. Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.TECENTRIQ (atezolizumab) Prescribing information. Genentech, Inc.; 2021.
- 30.TECENTRIQ (atezolizumab) Summary of product characteristics. Roche Registration Limited; 2021.
- 31. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med 2020;382:1894–905. [DOI] [PubMed] [Google Scholar]
- 32. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- 33. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wolchok JD, Hoos A, O'Day S, Weber JS, Hamid O, Lebbe C, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 2009;15:7412–20. [DOI] [PubMed] [Google Scholar]
- 35. Mariathasan S, Turley SJ, Nickles D, Castiglioni A, Yuen K, Wang Y, et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 2018;554:544–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lapuk AV, Wu C, Wyatt AW, McPherson A, McConeghy BJ, Brahmbhatt S, et al. From sequence to molecular pathology, and a mechanism driving the neuroendocrine phenotype in prostate cancer. J Pathol 2012;227:286–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kim JW, Shaffer DR, Massard C, Powles T, Harshman LC, Braiteh FS, et al. A phase Ia study of safety and clinical activity of atezolizumab in patients with metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol 36:6s, 2018. (suppl; abstr 187). [DOI] [PubMed] [Google Scholar]
- 38. Prelaj A, Rebuzzi SE, Buzzacchino F, Pozzi C, Ferrara C, Frantellizzi V, et al. Radium-223 in patients with metastatic castration-resistant prostate cancer: efficacy and safety in clinical practice. Oncol Lett 2019;17:1467–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rothschilds A, Tzeng A, Mehta NK, Moynihan KD, Irvine DJ, Wittrup KD. Order of administration of combination cytokine therapies can decouple toxicity from efficacy in syngeneic mouse tumor models. Oncoimmunology 2019;8:e1558678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tzeng A, Kauke MJ, Zhu EF, Moynihan KD, Opel CF, Yang NJ, et al. Temporally programmed CD8α. Cell Rep 2016;17:2503–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Weber JS, Gibney G, Sullivan RJ, Sosman JA, Slingluff CL, Lawrence DP, et al. Sequential administration of nivolumab and ipilimumab with a planned switch in patients with advanced melanoma (CheckMate 064): an open-label, randomised, phase 2 trial. Lancet Oncol 2016;17:943–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Heinrich D, Bruland Ø, Guise TA, Suzuki H, Sartor O. Alkaline phosphatase in metastatic castration-resistant prostate cancer: reassessment of an older biomarker. Future Oncol 2018;14:2543–56. [DOI] [PubMed] [Google Scholar]
- 43. Sweeney C, Gillessen S, Rathkopf D, Matsubara N, Drake C, Fizazi K, et al. IMbassador250: a phase III trial comparing atezolizumab with enzalutamide vs enzalutamide alone in patients with metastatic castration-resistant prostate cancer (mCRPC) [abstract]. In: Proceedings of the Annual Meeting of the American Association for Cancer Research 2020; 2020. Apr 27–28 and Jun 22-24. Philadelphia (PA): AACR; Cancer Res 2020;80(16 Suppl):Abstract nr CT014. [Google Scholar]
- 44. Marshall CH, Fu W, Wang H, Park JC, DeWeese TL, Tran PT, et al. Randomized phase II trial of sipuleucel-T with or without radium-223 in men with bone-metastatic castration-resistant prostate cancer. Clin Cancer Res 2021;27:1623–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.