Abstract
Vaccine anaphylaxis is rare; however, severe allergic reactions after administration of a coronavirus disease 2019 (COVID-19) vaccines have been reported. Excipients in the vaccine may play a role in severe allergic reactions post-vaccination. Various mechanisms, including IgE-mediated pathways, direct mass cell stimulation via the Mas-related G protein-coupled receptor-X2, and complement pathway activation, have been proposed to cause the anaphylaxis. Skin testing, using the basophil activation test, has been used to clarify the mechanism of the anaphylaxis and provide safety information for the next injection. Here, we review the current evidence and suggested approaches for patients who experienced an immediate severe allergic reaction to the first dose of a COVID-19 vaccine.
Keywords: COVID-19 vaccine safety, anaphylaxis, IgE mediated, allergy, hypersensitivity
Introduction
Owing to the outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV2), the US Food and Drug Administration (FDA) approved an emergency use authorization or two highly effective coronavirus disease 2019 (COVID-19) vaccines from Pfizer-BioNTech (1) and Moderna (2) on December 2020. A third vaccine, the Janssen COVID-19 vaccine, was authorized for use by the US FDA on February 27, 2021 (3). Following the implementation of COVID-19 vaccination programs, severe allergic reactions presenting as anaphylaxis have been reported.
Several criteria for anaphylaxis diagnosis have been proposed by the National Institutes of Allergy and Infectious Diseases (NIAID) (4) and the World Allergy Organization (WAO) (5). All organizations have accepted the concept that anaphylaxis is an “acute onset, serious, systemic, allergic, or hypersensitivity reaction that can be life-threatening or fatal” (6). Regarding immunization safety assessment, the Brighton Collaboration case definition criteria for anaphylaxis were developed in 2004 to facilitate the comparability of immunization safety data and distinguish different levels of diagnostic certainty (7).
According to NIAID in 2006 (4), anaphylaxis has rapid onset with more than one organ involved following an exposure to a likely allergen. However, a severe allergic reaction can present with only one organ system involved, such as isolated respiratory or cardiovascular symptoms (8). In addition, skin/mucosal involvement may be absent in 10–20% of all episodes, and decreased blood pressure in infants is often unrecognized (9), resulting in underreporting of anaphylaxis (10). Therefore, WAO in 2020 (5) amended the criteria for the diagnosis of anaphylaxis as acute onset of hypotension or bronchospasm or laryngeal involvement occurring in minutes to several hours after an exposure to a known or highly probable allergen, even in the absence of skin involvement.
Shimabukuro et al. have reported 21 cases of anaphylaxis according to the Brighton Collaboration case definition criteria for anaphylaxis after administration of 1,893,360 first doses of the Pfizer-BioNTech COVID-19 vaccine from December 14 to 23, 2020, corresponding to an estimated initial rate of 11.1 cases per million doses administered (11). Ten cases of anaphylaxis following 4,041,396 doses of the Moderna COVID-19 vaccine were reported in the Vaccine Adverse Event Reporting System (VAERS) from December 21, 2020 to January 10, 2021, representing 2.5 cases per million doses. From December 14, 2021 to January 18, 2021, 66 case reports met the Brighton Collaboration case definition criteria for anaphylaxis received by VAERS: 47 following administration of the Pfizer-BioNTech vaccine and 19 following the Moderna vaccine, corresponding to reporting rates of 4.7 and 2.5 cases per million doses administered, respectively (12). COVID-19 vaccine immunization can reduce the risk and severity of SARS-CoV2 infection (13). Therefore, understanding the mechanism of the allergic reaction and providing safety guidance for the next injection are crucial. This article reviews the causative mechanism, risk stratification, and the guidance for safety in anaphylaxis caused by COVID-19 vaccination.
Allergic Reactions to COVID-19 Vaccines
Vaccine hypersensitivity reactions are infrequent, estimated to be 1.31 cases per million vaccine doses from a population-based study (14). Most reports of vaccine adverse reactions are not severe. However, severe allergic reactions, such as anaphylaxis, can occur (15). Immunological-mediated hypersensitivity can have either an immediate or a delayed onset of action (16).
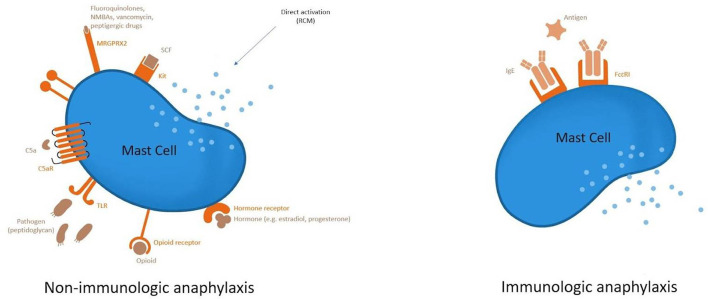
Type I hypersensitivity reactions can manifest as severe life-threatening anaphylaxis and typically occur in minutes or within 4 h of exposure. An IgE-mediated systemic reaction to the excipients in the COVID-19 vaccine may play a role in the potential causative mechanism of the anaphylaxis as in Figure 1. Systemic reactions involving multiple organs have resulted from the release of mediators caused by mast cell degranulation (17).
Figure 1.
Mechanism of severe allergic reaction post-vaccination.
Complement activation-related pseudoallergy (CARPA) is another mechanism considered as a cause of the severe allergic reaction. Pre-existing anti-PEG IgM may trigger the classical complement pathway entailing anaphylatoxins (C3a, C5a) release that causes hypersensitivity reaction (18–21).
Suspected Allergenic Components of the Vaccine
Allergic reactions following COVID-19 vaccination can result from vaccine components, including the active immunizing antigen, adjuvants, preservatives, stabilizers, antibiotics, cell culture materials, and leached packaging components (22). The vaccine antigen rarely, if ever, causes an allergic reaction and any reaction is more likely to be caused by excipients in the vaccine (15). The excipients in the COVID-19 vaccines and their functions are described in Table 1 (14).
Table 1.
Excipients of COVID-19 vaccine and their function.
| Excipient | mRNA BNT162b2 Pfizer | mRNA-1273 Moderna | Janssen |
|---|---|---|---|
| Active | Nucleoside-modified mRNA encoding the viral spike glycoprotein of SAR-CoV-2 | Nucleoside-modified mRNA encoding the viral spike glycoprotein of SAR-CoV-2 | Recombinant, replication-incompetent Ad26 vector, encoding a stabilized variant of the SARS-CoV-2 spike (S) protein |
| Lipid nanoparticles: stability and transport of mRNA PEGylated lipids | 2-[(Polyethylene glycol)-2000]-N,N-ditetradecylacetamide (ALC-0159), 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC), ((4hydroxybutyl)azanediyl)bis (hexane-6,1-diyl)bis(2-hexyldecanoate) (ALC-0315), cholesterol | 1,2-dimyristoyl-rac-glycero-3-methoxypolyethylene glycol-2000 (PEG2000 DMG), 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC), lipid SM-102 (patented ionizable lipid), cholesterol | |
| Buffer: stability of lipid nanoparticles/radical oxidation inhibition | Potassium dihydrogen phosphate/disodium phosphate dihydrate | Tromethamol/tromethamol hydrochloride, acetic acid/sodium acetate trihydrate | |
| Other stabilizers: ionic strength, surfactant, metal-ion chelates | Potassium chloride, sodium chloride | Polysorbate 80, 2-hydroxypropyl-β-cyclodextrin, citric acid monohydrate, trisodium citrate dihydrate, sodium chloride, ethanol | |
| Thermostabilization | Sucrose | Sucrose |
Polyethylene glycol (PEG) has been identified as the cause of severe allergic reactions (23–25). mRNA COVID-19 vaccines contain PEG2000 in the PEGylated lipids, which are used to form lipid nanoparticles that stabilize and transport the mRNA. Although PEG has not previously been used in a vaccine, PEG is used in compounds for polymer-based drug delivery and household products, such as cosmetics (26). Polyethylene glycol-associated anaphylaxis during colonoscopy preparation or laxative use has occurred in an average of four cases (range, 2–8) per year according to US FDA voluntary reporting data from 2005 to 2017 (23). Stone et al. have reported evidence for IgE-mediated hypersensitivity from skin and provocation testing in two patients with a history of anaphylaxis to PEG. One of the patients had skin test positivity and developed anaphylaxis symptoms during intradermal testing (23). However, non-IgE-mediated immune responses to PEG may be responsible for severe allergic reactions (19).
Polysorbate 80 is widely used as a stabilizing agent in food and pharmacological products, including vaccines. Previous studies have reported that polysorbate 80 can induce local and systemic allergic reactions, including IgE-mediated and non-immune anaphylaxis (27–29). Cross-reactivity to PEG has also been reported (23).
Risk Stratification and Guidance for the Initial Administration of the COVID-19 Vaccine
According to the VAERS data from 55 states and US territories from December 14, 2020 to February 5, 2021, the relative incidence of anaphylaxis following administration of COVID-19 vaccines was two and seven times higher for recipients with a prior history of allergies and recipients who had experienced previous anaphylaxis, respectively (30). However, an allergy to food, drugs, inhalant allergens, or insect venoms is not a contraindication for vaccines. The only contraindications for vaccination with the COVID-19 vaccines are a proven diagnosis of vaccine component hypersensitivity or a previous history of severe allergic reaction to the first dose of the vaccine (31).
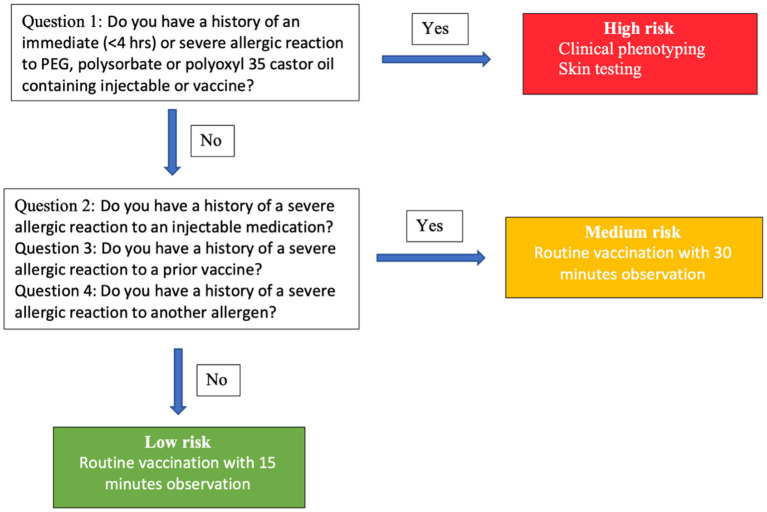
The Centers for Disease Control (CDC) has recommended pre-screening using a questionnaire for risk stratification as in Figure 2 (32). In individuals who have a history of an immediate (<4 h) or severe allergic reaction to a PEG-, polysorbate-, or polyoxyl 35 castor oil-containing injectable or vaccine, clinical phenotyping evaluation and skin testing with a standard concentration should be performed. In individuals who have a history of a severe allergic reaction to an injectable medication, a prior vaccine, or a history of a severe allergic reaction to another allergen, routine vaccination with observation for 30 min is recommended. If the answer to all the questions is “no,” the individual can receive a routine vaccination with 15 min observation.
Figure 2.
Risk stratification and guidance for initial administration of COVID-19 vaccine based on Mass General Brigham and Vanderbilt allergy expert consensus.
Diagnosis and Future Management of Vaccine Hypersensitivity
Skin tests are recommended for risk evaluation in patients with allergic reactions to COVID-19 vaccines by the WAO, American Academy of Allergy and Immunology (AAAAI), and European Academy of Allergy and Clinical Immunology (EAACI) but there is controversy over the recommendations for revaccination by graded administration (32–34). The Canadian Society of Allergy and Clinical immunology (CSACI) has suggested that skin testing is not required for a confirmed severe allergic reaction to a COVID-19 vaccine. However, graded administration can be considered in this case (35).
An individual who has a previous history of hypersensitivity to an excipient or has had a severe allergic reaction to the first dose of a COVID-19 vaccine should be evaluated by an allergist (36). Clinical phenotyping and shared decision making regarding the next injection should be performed. For an unconfirmed diagnosis who suspected allergic reaction to COVID-19 vaccine, a skin test to PEG and polysorbate at standard concentrations should be performed. However, the standardized concentration is not known (23).
Most patients who have had a previous severe allergic reaction to a COVID-19 vaccine can tolerate revaccination. Wolfson et al. have reported that 74% of patients who had an allergic reaction after the first dose of a mRNA vaccine and underwent allergic assessment by a skin test to PEG and polysorbate could be revaccinated safely. The negative predictive value of a negative skin test to PEG has been found to predict a 75% likelihood of tolerating the second dose of the vaccine (37). This study also found that patients who had a positive intradermal test to PEG (methylprednisolone acetate 4 mg/ml) could tolerate a second dose of mRNA vaccine without any reaction. Four patients who tested positive in a polysorbate skin test using refresh tears which contains polysorbate, received the second dose of mRNA vaccine safely, suggesting an irritant concentration of refresh tears (37).
Non-IgE-mediated allergic reactions may be responsible for COVID-19 allergies. Warren et al. performed allergy assessments by skin testing to PEG and polysorbate 80, basophil activation tests and measurement of PEG IgG levels in 11 patients who were suspected of having an allergic reaction to a mRNA COVID-19 vaccine (19). All the patients had positive basophil activation tests to PEG and mRNA vaccine; however, the skin tests to PEG and polysorbate 80 were negative.
The CDC have suggested an interchangeable vaccine platform using a vaccine that contains different excipients for patients who had reactions to excipients in the first dose of the vaccine. The adenoviral vector-based Janssen COVID-19 vaccine can be considered for individuals who had a severe allergic reaction to an mRNA-based COVID-19 vaccine. An mRNA COVID-19 vaccine can be considered for individuals who are contraindicated for the adenoviral vector-based Janssen COVID-19 vaccine. However, vaccination with caution is recommended because of the possibility of cross-reactivity between PEG and polysorbate 80 (38). Although graded challenge dosing has been used for other vaccines (39), there is no supporting data regarding the safety and efficacy of graded challenge dosing for mRNA vaccines.
Premedication with an antihistamine is controversial. The WAO (33), AAAAI (32), and BASCI (40) have recommended that premedication may mask the initial symptoms of a systemic reaction resulting in delayed diagnosis. However, pretreatment with a second-generation antihistamine can be considered in individuals with mild symptoms (pruritus or urticaria only) (32).
Alternative Vaccine Options
Mixing and matching of two different COVID-19 vaccines could be an option if an individual is suspected to have had an allergic reaction to the first dose, or if there is a shortage of certain vaccine. Previous studies found priming with the ChadOx1 nCoV-19 vaccine followed by boosting with BNT162b2 resulted in significantly greater humoral and cellular immune responses (41–43). In solid organ transplant recipients who have limited humoral immune response to mRNA-based COVID-19 vaccines, heterologous vaccination seems promising because it induced specific antibodies and SARS-CoV2-specific CD4 and CD8 T cells (44).
Nordstrom et al. reported that individuals who used a heterologous ChadOx1 nCoV-19 and mRNA prime-boost vaccination strategy were 68% less likely to develop a symptomatic COVID-19 infection compared with those who were unvaccinated whereas those who used a homologous ChadOx1 nCoV-19 were 50% less likely to develop a symptomatic COVID-19 infection compared with those who were unvaccinated (45). Additionally, 88% effectiveness for preventing of SAR-CoV-2 infection was reported, which is similar to an individual receiving two doses of BNT162b2 (46).
Heterologous priming with the ChadOx1 nCoV-19 vaccine, followed by boosting with BNT162b2, is an effective alternative to induce strong humoral and cellular immune responses and acceptable reactogenicity (42, 46). This should be an alternative vaccination strategy for an individual who cannot complete the course using the same type of COVID-19 vaccine. However, there is a lack of evidence and knowledge about the necessity and timing of a booster dose following such a strategy.
Conclusion
Allergic reactions to the COVID-19 vaccines are rare, and various components of the vaccines could be responsible for causing the reaction. To date, PEG and polysorbate 80 have been identified as possible factors for the etiology of reactions to the mRNA vaccine. However, the precise mechanism of action is not yet known. Therefore, the CDC has recommended that individuals with a history of anaphylaxis to PEG should avoid both COVID-19 mRNA vaccines. Furthermore, clinical phenotyping, allergic assessment, and shared decision making before the next injection should be performed in conjunction with an allergist. The future recommendations may change as additional data are obtained.
Author Contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank Victoria Muir, Ph.D. and Dr. Anthony Tan edited a draft of this manuscript.
References
- 1.FDA . Fact Sheet for Healthcare Providers Administering Vaccine (Vaccination Providers): Emergency Use Authorization (EUA) of the Pfizer-BioNTech COVID-19 Vaccine to Prevent Coronavirus Disease 2019 (COVID-19). (2021). Available online at: https://www.fda.gov/media/144413/download (accessed January 17, 2021).
- 2.FDA . Fact Sheet for Healthcare Providers Administering Vaccine (Vaccination Providers): Emergency Use Authorization (EUA) of the Moderna COVID-19 Vaccine to Prevent Coronavirus Disease 2019 (COVID-19). (2021). (accessed October 20, 2021). [Google Scholar]
- 3.Emergency Use Authorization (EUA) of the Janssen COVID-19 Vaccine to Prevent Coronavirus Disease 2019 (COVID-19). Available online at: https://www.fda.gov/media/146304/download (accessed October 20, 2021).
- 4.Sampson HA, Munoz-Furlong A, Campbell RL, Adkinson NF, Jr, Bock SA, Branum A, et al. Second Symposium on the definition and management of anaphylaxis: summary report–Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network Symposium. J Allergy Clin Immunol. (2006) 117:391–7. 10.1016/j.jaci.2005.12.1303 [DOI] [PubMed] [Google Scholar]
- 5.Cardona V, Ansotegui IJ, Ebisawa M, El-Gamal Y, Fernandez Rivas M, Fineman S, et al. World allergy organization anaphylaxis guidance 2020. World Allergy Organ J. (2020) 13:100472. 10.1016/j.waojou.2020.100472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simons FE, Ardusso LR, Bilo MB, Cardona V, Ebisawa M, El-Gamal YM, et al. International consensus on (ICON) anaphylaxis. World Allergy Organ J. (2014) 7:9. 10.1186/1939-4551-7-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruggeberg JU, Gold MS, Bayas JM, Blum MD, Bonhoeffer J, Friedlander S, et al. Anaphylaxis: case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. (2007) 25:5675–84. 10.1016/j.vaccine.2007.02.064 [DOI] [PubMed] [Google Scholar]
- 8.Brown SG, Stone SF, Fatovich DM, Burrows SA, Holdgate A, Celenza A, et al. Anaphylaxis: clinical patterns, mediator release, and severity. J Allergy Clin Immunol. (2013). 132:1141.e5–9.e5. 10.1016/j.jaci.2013.06.015 [DOI] [PubMed] [Google Scholar]
- 9.Simons FE, Sampson HA. Anaphylaxis: unique aspects of clinical diagnosis and management in infants (birth to age 2 years). J Allergy Clin Immunol. (2015) 135:1125–31. 10.1016/j.jaci.2014.09.014 [DOI] [PubMed] [Google Scholar]
- 10.Simons FE, Sampson HA. Anaphylaxis epidemic: fact or fiction? J Allergy Clin Immunol. (2008) 122:1166–8. 10.1016/j.jaci.2008.10.019 [DOI] [PubMed] [Google Scholar]
- 11.Shimabukuro T, Nair N. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine. JAMA. (2021) 325:780–1. 10.1001/jama.2021.0600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shimabukuro TT, Cole M, Su JR. Reports of anaphylaxis after receipt of mRNA COVID-19 vaccines in the US-December 14, 2020–January 18, 2021. JAMA. (2021) 325:1101–2. 10.1001/jama.2021.1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abbasi J. COVID-19 mRNA vaccines blunt breakthrough infection severity. JAMA. (2021) 326:473. 10.1001/jama.2021.12619 [DOI] [PubMed] [Google Scholar]
- 14.McNeil MM, Weintraub ES, Duffy J, Sukumaran L, Jacobsen SJ, Klein NP, et al. Risk of anaphylaxis after vaccination in children and adults. J Allergy Clin Immunol. (2016) 137:868–78. 10.1016/j.jaci.2015.07.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McNeil MM, DeStefano F. Vaccine-associated hypersensitivity. J Allergy Clin Immunol. (2018) 141:463–72. 10.1016/j.jaci.2017.12.971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caubet JC, Ponvert C. Vaccine allergy. Immunol Allergy Clin North Am. (2014). 34:597–613, ix. 10.1016/j.iac.2014.04.004 [DOI] [PubMed] [Google Scholar]
- 17.Sampath V, Rabinowitz G, Shah M, Jain S, Diamant Z, Jesenak M, et al. Vaccines and allergic reactions: the past, the current COVID-19 pandemic, and future perspectives. Allergy. (2021) 76:1640–60. 10.1111/all.14840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klimek L, Novak N, Cabanillas B, Jutel M, Bousquet J, Akdis CA. Allergenic components of the mRNA-1273 vaccine for COVID-19: possible involvement of polyethylene glycol and IgG-mediated complement activation. Allergy. (2021) 76:3307–13. 10.1111/all.14794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Warren CM, Snow TT, Lee AS, Shah MM, Heider A, Blomkalns A, et al. Assessment of allergic and anaphylactic reactions to mRNA COVID-19 vaccines with confirmatory testing in a US regional health system. JAMA Netw Open. (2021) 4:e2125524. 10.1001/jamanetworkopen.2021.25524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szebeni J. Complement activation-related pseudoallergy: a stress reaction in blood triggered by nanomedicines and biologicals. Mol Immunol. (2014) 61:163–73. 10.1016/j.molimm.2014.06.038 [DOI] [PubMed] [Google Scholar]
- 21.Kozma GT, Shimizu T, Ishida T, Szebeni J. Anti-PEG antibodies: properties, formation, testing and role in adverse immune reactions to PEGylated nano-biopharmaceuticals. Adv Drug Deliv Rev. (2020). 154–155:163–75. 10.1016/j.addr.2020.07.024 [DOI] [PubMed] [Google Scholar]
- 22.CDC . What's in Vaccines? (2019). Available online at: https://cdc.gov/vaccines/vacgen/additives.htm (accessed January 14, 2021). [Google Scholar]
- 23.Stone CA, Jr, Liu Y, Relling MV, Krantz MS, Pratt AL, Abreo A, et al. Immediate hypersensitivity to polyethylene glycols and polysorbates: more common than we have recognized. J Allergy Clin Immunol Pract. (2019) 7:1533.e8–40.e8. 10.1016/j.jaip.2018.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sellaturay P, Nasser S, Ewan P. Polyethylene glycol-induced systemic allergic reactions (anaphylaxis). J Allergy Clin Immunol Pract. (2021) 9:670–5. 10.1016/j.jaip.2020.09.029 [DOI] [PubMed] [Google Scholar]
- 25.Cabanillas B, Akdis CA, Novak N. Allergic reactions to the first COVID-19 vaccine: a potential role of polyethylene glycol? Allergy. (2021) 76:1617–8. 10.1111/all.14711 [DOI] [PubMed] [Google Scholar]
- 26.NIH Daily Med. National Library of Medicine. (2018). Available online at: https://dailymed.nlm.nih.gov/dailymed/ (accessed October 20, 2021).
- 27.Price KS, Hamilton RG. Anaphylactoid reactions in two patients after omalizumab administration after successful long-term therapy. Allergy Asthma Proc. (2007) 28:313–9. 10.2500/aap.2007.28.3003 [DOI] [PubMed] [Google Scholar]
- 28.Badiu I, Geuna M, Heffler E, Rolla G. Hypersensitivity reaction to human papillomavirus vaccine due to polysorbate 80. BMJ Case Rep. (2012). 2012:bcr0220125797. 10.1136/bcr.02.2012.5797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palacios Castano MI, Venturini Diaz M, Lobera Labairu T, Gonzalez Mahave I, Del Pozo Gil MD, Blasco Sarramian A. Anaphylaxis due to the excipient polysorbate 80. J Investig Allergol Clin Immunol. (2016) 26:394–6. 10.18176/jiaci.0109 [DOI] [PubMed] [Google Scholar]
- 30.Desai AP, Desai AP, Loomis GJ. Relationship between pre-existing allergies and anaphylactic reactions post mRNA COVID-19 vaccine administration. Vaccine. (2021) 39:4407–9. 10.1016/j.vaccine.2021.06.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sokolowska M, Eiwegger T, Ollert M, Torres MJ, Barber D, Del Giacco S, et al. EAACI statement on the diagnosis, management and prevention of severe allergic reactions to COVID-19 vaccines. Allergy. (2021) 76:1629–39. 10.1111/all.14739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Banerji A, Wickner PG, Saff R, Stone CA, Jr, Robinson LB, Long AA, et al. mRNA vaccines to prevent COVID-19 disease and reported allergic reactions: current evidence and suggested approach. J Allergy Clin Immunol Pract. (2021) 9:1423–37. 10.1016/j.jaip.2020.12.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turner PJ, Ansotegui IJ, Campbell DE, Cardona V, Ebisawa M, El-Gamal Y, et al. COVID-19 vaccine-associated anaphylaxis: a statement of the World Allergy Organization Anaphylaxis Committee. World Allergy Organ J. (2021) 14:100517. 10.1016/j.waojou.2021.100517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klimek L, Jutel M, Akdis CA, Bousquet J, Akdis M, Torres MJ, et al. ARIA-EAACI statement on severe allergic reactions to COVID-19 vaccines - an EAACI-ARIA position paper. Allergy. (2021) 76:1624–8. 10.1111/all.14726 [DOI] [PubMed] [Google Scholar]
- 35.Vander Leek TK, Chan ES, Connors L, Derfalvi B, Ellis AK, Upton JEM, et al. COVID-19 vaccine testing and administration guidance for allergists/immunologists from the Canadian Society of Allergy and Clinical Immunology (CSACI). Allergy Asthma Clin Immunol. (2021) 17:29. 10.1186/s13223-021-00529-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Greenhawt M, Abrams EM, Shaker M, Chu DK, Khan D, Akin C, et al. The risk of allergic reaction to SARS-CoV-2 vaccines and recommended evaluation and management: a systematic review, meta-analysis, GRADE assessment, and international consensus approach. J Allergy Clin Immunol Pract. (2021) 9:3546–67. 10.1016/j.jaip.2021.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wolfson AR, Robinson LB, Li L, McMahon AE, Cogan AS, Fu X, et al. First-dose mRNA COVID-19 vaccine allergic reactions: limited role for excipient skin testing. J Allergy Clin Immunol Pract. (2021). 9:3308.e3–20.e3. 10.1016/j.jaip.2021.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Centers for Disease Control and Prevention . Interim Clinical Considerations for Use of COVID-19 Vaccines Currently Authorized in the United States. Atlanta, GA: Centers for Disease Control and Prevention; (2021). Available online at: https://www.cdc.gov/vaccines/covid-19/info-by-product/clinical-considerations.html (accessed March 20, 2021). [Google Scholar]
- 39.Kelso JM, Greenhawt MJ, Li JT, Nicklas RA, Bernstein DI, Blessing-Moore J, et al. Adverse reactions to vaccines practice parameter 2012 update. J Allergy Clin Immunol. (2012) 130:25–43. 10.1016/j.jaci.2012.04.003 [DOI] [PubMed] [Google Scholar]
- 40.Public Health England . COVID-19: the green book (chapter 14). Public Health England. Available online at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/978508/Green_book_chapter_16April2021.pdf (accessed March 20, 2021). [Google Scholar]
- 41.Schmidt T, Klemis V, Schub D, Mihm J, Hielscher F, Marx S, et al. Immunogenicity and reactogenicity of heterologous ChAdOx1 nCoV-19/mRNA vaccination. Nat Med. (2021) 27:1530–5. 10.1038/s41591-021-01464-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Callaway E. Mix-and-match COVID vaccines trigger potent immune response. Nature. (2021) 593:491. 10.1038/d41586-021-01359-3 [DOI] [PubMed] [Google Scholar]
- 43.Borobia AM, Carcas AJ, Perez-Olmeda M, Castano L, Bertran MJ, Garcia-Perez J, et al. Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacS): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet. (2021) 398:121–30. 10.1016/S0140-6736(21)01420-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmidt T, Klemis V, Schub D, Schneitler S, Reichert MC, Wilkens H, et al. Cellular immunity predominates over humoral immunity after homologous and heterologous mRNA and vector-based COVID-19 vaccine regimens in solid organ transplant recipients. Am J Transplant. (2021) 21:3990–4002. 10.1111/ajt.16818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nordstrom P, Ballin M, Nordstrom A. Effectiveness of heterologous ChAdOx1 nCoV-19 and mRNA prime-boost vaccination against symptomatic Covid-19 infection in Sweden: a nationwide cohort study. Lancet Reg Health Eur. (2021) 11:100249. 10.1016/j.lanepe.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Callaway E. Mix-and-match COVID vaccines ace the effectiveness test. Nature. (2021). 10.1038/d41586-021-02853-4 [DOI] [PubMed] [Google Scholar]