Abstract
Platelet-activating factor (PAF) is a potent endogenous proinflammatory mediator implicated in the pathogenesis of septic shock. A double-blind randomized placebo-controlled trial of an intravenous PAF receptor antagonist (lexipafant) was conducted with 131 adult Thai patients with suspected severe sepsis (66 of whom had positive blood cultures). Detailed serial clinical, biochemical, and cytokine measurements were performed. Lexipafant treatment was well tolerated. The 28-day mortality in the lexipafant group (61.4%) was similar to that in the placebo group (62.6%). There was also no evidence that lexipafant affected clinical or biochemical measures of disease severity or the profile of sequentially measured plasma cytokine levels. PAF may not have an important role in the pathogenesis of severe sepsis.
Despite significant advances in antimicrobial treatment and intensive care support, sepsis remains a difficult medical management problem with mortality rates between 30 and 50% (5, 11). Proinflammatory cytokines and platelet-activating factor (PAF) are generated in large amounts during the septic response (1). PAF, an ether-linked phospholipid, is one of the most hypotensive and inflammatory agents yet discovered (1, 2, 3, 7). The effects of PAF are mediated through specific PAF receptors. PAF is produced by a broad range of cell types, including monocytes, macrophages, eosinophils, and platelets as well as vascular, kidney glomerular, and gastrointestinal endothelial cells. A wide variety of mediators stimulate these cells to produce PAF; many of these mediators are secreted during the cytokine cascade associated with septic shock. These include tumor necrosis factor (TNF), thrombin, leukotrienes, and bradykinin. PAF has several biological actions characteristic of a proinflammatory agent. When administered systemically to animals, it produces many of the features of septic shock. In experimental septic shock, blocking either the proinflammatory cytokines TNF and interleukin 1 (IL-1) or lipid mediators such as PAF decreases the severity of the disease (12, 13). In one study, the PAF antagonist BN 52021 was shown to be a safe and promising treatment of patients with severe gram-negative sepsis (6).
Lexipafant (BB-882; British Biotechnology Ltd., Watlington, Oxford, United Kingdom) is another newly developed PAF antagonist. Lexipafant was shown to be a potent antagonist of PAF in in vitro studies involving the inhibition of [3H]PAF receptor binding and in a PAF receptor binding assay conducted on human platelet membranes. In the latter system, lexipafant bound to the receptor seven times more avidly than native PAF (unpublished data). We report here results of a randomized placebo-controlled study to evaluate the clinical safety and efficacy of lexipafant as an adjunct to the treatment of severe sepsis. Lexipafant has been shown to be well tolerated when given intravenously to volunteers, to patients with pancreatitis, and to patients with sepsis (unpublished data).
MATERIALS AND METHODS
Study design and patient recruitment.
This study was a double-blind, placebo-controlled trial conducted at two centers; Sappasitprasong Hospital, Ubon Ratchatani, Thailand, and Siriraj Hospital, Mahidol University, Bangkok, Thailand. The objective of the study was to assess the safety of lexipafant and to determine its effects on the concentrations of proinflammatory cytokines and the clinical course of sepsis. A sample size of 112 patients provided 80% power to detect a reduction in mortality from 50 to 25% with 95% confidence. The study was performed in accordance with the Declaration of Helsinki. The study protocol was approved by the Ethical Review Committee of the Ministry of Public Health for Thailand and the Committee on Human Rights Related to Research Involving Human Subjects, Faculty of Medicine Siriraj Hospital, Mahidol University. Witnessed written informed consent (in Thai) was obtained from patients or from the accompanying relatives following a full explanation of the study.
Patient selection.
Patients were selected for inclusion in the study if the admitting clinicians considered a fatal outcome likely (i.e., they estimated the probability of death as being >50%). The minimum inclusion criteria included a clinical suspicion of sepsis with two or more of the following: (i) fever (>38.3°C), hypothermia (<36°C), or proven site of infection; (ii) tachycardia (>90 beats/min); (iii) tachypnea (respiratory rate of >30 breaths/min, requirement for mechanical ventilation, or partial pressure of CO2 in arterial blood <4.3 kPa); and (iv) hypotension (supine systolic blood pressure of ≤90 mm Hg or sustained drop in systolic blood pressure of ≥40 mm Hg despite adequate fluid challenge). Exclusion criteria were age of <15 years, pregnancy or lactation, or receipt of concomitant treatment with coumarin-like anticoagulants.
Study procedures.
On admission, the inclusion criteria were checked and informed consent was obtained. Vital signs were recorded, and blood samples (minimum, 15 ml), urine samples, and throat swabs (and pus and sputum, if available) were obtained and cultured. A detailed clinical examination, including assessment of Glasgow Coma Scale score, was recorded on a standard form. Blood samples were used for culturing and hematology, biochemistry, cytokine level, and coagulation tests. Urine output and vital signs were monitored a minimum of once every 4 h by a dedicated team of research nurses.
Study drug.
At the start of the study in 1993, 500-ml infusion bottles containing lexipafant (0.2 mg/ml) or normal saline (placebo) were provided by British Biotechnology Ltd. with certificates of analysis. Lexipafant and placebo were supplied in identical 10-ml clear-glass ampoules containing lexipafant (5 mg/ml) or an equal volume of saline (0.9%) (placebo). The two could not be distinguished. The treatment for each patient was supplied as a treatment pack allocated in order of trial entry. Patients receiving lexipafant were given a loading dose of 4 mg in 10 min followed by an infusion of 4 mg/h. This dosage was given for 72 h to 30 patients who were studied in 1993 and for 7 days to all patients who were included in the study thereafter.
This trial did not interfere in any way with antibiotic treatment, resuscitation, or any other aspects of patient management. Intravenous antibiotics were started as soon as appropriate cultures were taken. All patients we treated initially with either a combination of parenteral benzylpenicillin or cloxacillin and gentamicin if they were previously healthy or a broad-spectrum cephalosporin and gentamicin if they had an underlying disease. Patients with suspected melioidosis were empirically treated with ceftazidime. The antibiotic regimen was modified 72 to 96 h after admission, according to the microbiological diagnosis and clinical response to the initial drugs. Concomitant treatment with oral anticoagulant therapy, aspirin, nonsteroidal anti-inflammatory drugs, or other investigational new drugs was not given. Other drugs were given as indicated clinically.
Cytokine measurements.
For the first 30 patients studied, levels of serum TNF alpha (TNF-α), IL-6, IL-8, and soluble TNF receptors (TNF-RI and TNF-RII), and plasma lactate were measured on admission, and then at 4, 8, 12, 24 h, and then daily until day 7 after initiation of treatment. In subsequent patients, these cytokines were measured only on admission and at 24, 48, 120, and 168 h after initiation of treatment. Cytokine levels were determined using a quantitative immunoenzymatic sandwich technique (Quantikine kit; Research and Diagnostic Systems, Minneapolis, Minn.). A monoclonal antibody specific for the cytokine to be measured was used to coat the microtiter plate provided. Standards with known amounts of cytokines and samples were pipetted into the wells. Any cytokine present was bound by the immobilized antibody. Unbound sample proteins were then washed away, and a horseradish peroxidase-linked polyclonal antibody specific for the cytokine was added to the wells and allowed to bind to the antibody-cytokine complex. A substrate solution was then added to the wells. The intensity of color developed was in proportion to the amount of bound cytokine. The reaction was stopped, and the amount of cytokine in a sample was determined from its optical density and the standard curve of optical density plotted against known concentrations of cytokines.
The limits of detection were 4.4 pg/ml for TNF-α, 0.35 pg/ml for IL-6, 18 pg/ml for IL-8, 25 pg/ml for TNF-RI, and 12 pg/ml for TNF-RII. There was no significant cross-reactivity with or interference from other cytokines when each cytokine measurement was carried out. For each cytokine assay, the intra-assay coefficient of variation varied from 2.2 to 5.1% and the interassay coefficient of variation varied from 4.1 to 12.2%.
Clinical definitions.
The following prospective criteria (6) were used, in the absence of known chronic organ failure, to define organ dysfunction associated with sepsis: (i) septic shock was defined as either refractory systemic hypotension (systolic blood pressure, of <90 mm Hg), despite an apparently adequate fluid challenge or the use of vasopressor drugs to maintain blood pressure; (ii) respiratory failure included hypoxemia (alveolar O2 pressure/fractional concentration of inspired O2 of 200) or the need for mechanical ventilation; (iii) renal dysfunction included a serum creatinine level of >3.4 mg/dl (>300 μmol/liter) or the need for dialysis; (iv) hepatic dysfunction included either a total bilirubin level of >3.5 mg/dl (>60 μmol/liter) or an alkaline phosphatase, aspartate aminotransferase, or alanine aminotransferase level three times the upper limit of normal; and (v) neurologic dysfunction included either a Glasgow Coma Scale score of <7 without sedative drugs or coma not related to meningitis, neurologic disease, or trauma.
Statistical analysis.
Demographic data, clinical characteristics and outcome, severity of the diseases (assessed with the Apache II score), and hematology, biochemistry, and coagulation parameters were compared for lexipafant- and placebo-treated groups. For continuous variables, the Mann-Whitney U test was used; for categorical variables, either the chi-square test with Yates' correction or Fisher's exact test was used.
Mortality was analyzed by plotting the Kaplan-Meier survival curve for both treatment groups. The Mantel-Haenszel log-rank test was performed to compare the survival distributions for lexipafant and placebo treatments. Patients who had not died by day 28 were right censored. Stepwise Cox regression analysis was used to determine the independent predictors of mortality. All analyses were done on an intention-to-treat basis.
Cytokine profiles.
The cytokine data were presented graphically with median values over time. When levels were not recorded, smoothing techniques were used to replace the missing values. When the first value was missing, it was replaced by the next value; missing last values were replaced by the last observation recorded. Otherwise, the averages of the former and latter values were calculated. For individuals with more than 50% of observations missing, missing values were not replaced by the smoothing techniques mentioned above.
Admission cytokine levels were compared for lexipafant and placebo treatments and for patients that died or lived. To summarize the change of cytokine levels over time, the maximum percent decrease from admission was calculated for each individual, i.e., [(admission value − minimum value)/admission value] × 100.
RESULTS
The study was conducted between August 1993 and August 1995. In total, 131 patients were entered into the study, and all received study treatment; 64 patients were treated with placebo (26 males and 38 females) and 67 patients were treated with lexipafant (32 males and 35 females). The mean (standard deviation) ages in the two groups were similar: 50 (15) years and 54 (15) years, respectively. Clinical characteristics of both groups on admission were also similar (Table 1). Overall, three patients were withdrawn from the study because of adverse events.
TABLE 1.
Comparison of clinical and biochemical characteristics and distribution of organ dysfunction on admission
| Parameter | Results for the following group:
|
|
|---|---|---|
| Placebo (n = 64) | Lexipafant (n = 67) | |
| Primary disease (blood culture positive), n (%) | 34 (53) | 32 (48) |
| B. pseudomallei | 19 (30) | 17 (25) |
| Escherichia coli | 7 (11) | 4 (6) |
| Other gram-negative bacteria | 4 (6) | 4 (6) |
| Gram-positive cocci | 4 (6) | 7 (10) |
| Apache II score | ||
| ≤20, n (%) | 42 (76) | 46 (78) |
| ≤20, n (%) | 13 (24) | 13 (22) |
| Overall median (range) | 16 (4–29) | 16 (5–42) |
| Organ dysfunction (n) | ||
| Septic shock | 27 | 22 |
| Renal dysfunction | 40 | 33 |
| Respiratory failure | 36 | 37 |
| Neurologic dysfunction | 14 | 14 |
| Liver dysfunction | 29 | 27 |
| Median (range) laboratory values | ||
| Hematocrit, % | 34.0 (10–56) | 33.0 (19–52) |
| Creatinine, mg/dl | 2.1 (0.7–11.3) | 2.1 (0.6–15.0) |
| Total bilirubin, mg/dl | 1.2 (0.3–16.8) | 1.3 (0.2–16.9) |
| Albumin, g/dl | 2.8 (1.2–4.9) | 2.8 (1.8–4.5) |
| Lactate, mmol/liter | 3.2 (0.8–13.3) | 2.9 (1.1–16.8) |
The proportions of patients who were blood culture positive were similar in the two treatment groups: 53% in the placebo group and 48% in the lexipafant group. Burkholderia pseudomallei was the most common organism isolated in both groups. Among blood culture-negative patients, the sites of infection and the causative organisms were also similar in the two treatment groups. For 19 patients (30%) in the placebo group and 18 patients (27%) in the lexipafant group, all cultures from suspected sources of infection were negative. The clinical severity of disease for both treatment groups, as assessed by Apache II scores, and clinical and biochemical evidence of vital organ dysfunction were also similar (Table 1). No patient in either treatment group received steroid treatment in this study.
Mortality.
The 28-day cumulative mortality was 62.9% in this study (61.4% in the placebo group and 62.6% in the lexipafant group; P, 0.4). For patients who died, 67.5% died within the first 48 h in the placebo group and 52.8% did so in the lexipafant group (P, 0.28). The 28-day cumulative mortality was higher in patients who were blood culture positive (69.6%) than in blood culture-negative patients (55%) (P, 0.044). Patients with B. pseudomallei septicemia had a significantly higher mortality than patients with septicemia caused by other pathogens: 76.8 versus 57.2%, respectively (P, 0.03). Among the blood culture-positive patients, the 28-day cumulative mortalities were 61.3% in the lexipafant group and 73.4% in the placebo group (P, 0.09). There were also no significant differences in the duration of hospital stay between the two groups.
Cytokine profiles.
All admission cytokine levels, except for that of TNF-α, were not significantly different between the two treatment groups. The TNF-α levels were significantly higher in the placebo group than in the lexipafant group (Table 2). Patients who died had significantly higher admission cytokine levels than those who survived. No differences were found for the maximum proportional decrease from the baseline values for all cytokine levels when lexipafant and placebo treatments were compared (Table 3). However, the maximum percent decrease from the baseline for levels of lactate, IL-6, TNF-α, TNF-RI, and TNF-RII was significantly smaller in patients with B. pseudomallei septicemia than in those with septicemia caused by other organisms (8.7 versus 36.4%, 22.8 versus 84.7%, 0 versus 56.9%, 3.8 versus 42.2%, and 2.8 versus 31.6%, respectively; P, <0.05). There was a significantly larger maximum percent decrease from the baseline for patients who survived than for patients who died. Cytokine levels remained elevated in fatal cases, with a median maximum percent decrease from the baseline ranging from 0 to 19.5% (Table 4).
TABLE 2.
Cytokine profiles on admission
| Cytokine | Median (range) cytokine level, pg/ml, in the following group:
|
|
|---|---|---|
| Placebo (n = 62) | Lexipafant (n = 65) | |
| TNF-α | 51 (4–1,349) | 30 (14–1,753)a |
| IL-6 | 890 (13–2,136,000) | 408.5 (11–772,000) |
| IL-8 | 290.4 (18–189,936) | 221 (18–124,656) |
| TNF-RI | 13,170 (2,072–69,720) | 14,315 (2,912–83,380) |
| TNF-RII | 32,710 (4,177–175,160) | 25,737 (5,396–434,585) |
Significantly different from the corresponding value for the placebo group (P = 0.03; Mann-Whitney U test).
TABLE 3.
Maximum decrease in plasma cytokine and lactate concentrations in treatment groups after admission
| Parameter | Placebo
|
Lexipafant
|
||
|---|---|---|---|---|
| No. of patients | Median (range) % decrease in concn | No. of patients | Median (range) % decrease in concn | |
| Lactate | 58 | 27.6 (0–86.2) | 59 | 36.4 (0–85.5) |
| IL-6 | 59 | 69.1 (0–100) | 62 | 67.8 (0–100) |
| IL-8 | 59 | 45.9 (0–99.8) | 62 | 45.3 (0–98.5) |
| TNF-α | 59 | 33.1 (0–96.4) | 62 | 13.3 (0–98.5) |
| TNF-RI | 58 | 21.7 (0–79.9) | 62 | 17.8 (0–90.1) |
| TNF-RII | 58 | 15.8 (0–92.3) | 62 | 8.6 (0–83.6) |
TABLE 4.
Maximum decrease in plasma cytokine and lactate concentrations in patient groups after admissiona
| Parameter | Survivors
|
Fatalities
|
||
|---|---|---|---|---|
| No. of patients | Median (range) % decrease in concn | No. of patients | Median (range) % decrease in concn | |
| Lactate | 53 | 40.7 (0–85.5) | 64 | 19.5 (0–86.2) |
| IL-6 | 53 | 88.7 (0–100) | 68 | 19.5 (0–99.7) |
| IL-8 | 53 | 66.1 (0–99.6) | 68 | 6.7 (0–99.8) |
| TNF-α | 53 | 50.0 (0–98.7) | 68 | 0.0 (0–93.9) |
| TNF-RI | 53 | 44.4 (0–89.6) | 67 | 0.0 (0–90.1) |
| TNF-RII | 53 | 35.8 (0–83.6) | 67 | 0.0 (0–92.3) |
For the comparison of survivors versus fatalities, P values were 0.01 for all parameters, as determined by the Mann-Whitney U test.
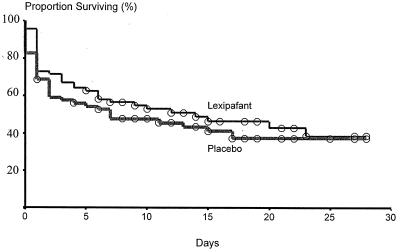
Stepwise Cox regression analysis revealed that higher admission plasma lactate and IL-8 levels were significant factors associated with the outcome of treatment in this study (P for both variables, 0.001). Age (P, 0.88), sex (P, 0.25), cytokines (IL-6 [P, 0.52], TNF-α [P, 0.76], TNF-RI [P, 0.80], and TNF-RII [P, 0.15]), and type of organism (B. pseudomallei versus other organisms; P, 0.14) were not significantly associated with mortality. Also, lexipafant treatment was not associated with a significant reduction in mortality compared to placebo (P, 0.80). A Kaplan-Meier plot comparing the survival of patients in both treatment groups is shown in Fig. 1.
FIG. 1.
Kaplan-Meier plot comparing the survival of patients treated with lexipafant and placebo. Subjects still in the hospital at the end of the study were censored at 28 days.
Adverse effects.
The adverse events reported in this study were similar in both groups. Fatal multiorgan failure was the most common serious adverse event in this study, and in every case this event was considered to result from the primary disease rather than the study drugs. Three patients who survived did not complete the study because of adverse events—two in the drug treatment group and one in the placebo group. Two patients (one in the placebo group and one in the lexipafant group) were taken out of the study because they developed generalized seizures, considered possibly related to both the study treatment and the primary disease. The third patient who was treated with lexipafant had a transient rise in creatinine which subsequently resolved. This event was considered in retrospect to have resulted from the primary disease.
DISCUSSION
Current strategies for the management of bacterial sepsis include early use of antimicrobial agents to kill bacteria, surgical procedures to eradicate the nidus of infection, and early use of intensive life support procedures, such as mechanical ventilation, dialysis, and use of vasoactive drugs. Despite these aggressive approaches, sepsis continues to have a high mortality. In patients with septic shock, death is usually the end result of the development of multiorgan failure. These clinical manifestations can be produced in experimental animals by the administration of purified bacterial endotoxin or TNF. It has been suggested that cytokine-related systemic intravascular inflammation is a common pathogenic link among initial endotoxemia, multiorgan failure, and death in sepsis. The significantly higher levels of all proinflammatory cytokines measured in this study in patients who died than in patients who survived confirm the results from several other studies (8, 10).
This study was conducted on the premise that neutralizing one potentially harmful host mediator, PAF, could stop or attenuate these cytokine-mediated processes linked with sepsis. Earlier results from animal studies of endotoxin-induced sepsis showed that lexipafant, the potent PAF receptor antagonist used in this study, reduced TNF production by 40% compared to that in placebo-treated animals (12). B. pseudomallei is the most common cause of community-acquired septicemia in northeast Thailand, especially in the rainy season (4). Thus, half of the organisms isolated from blood cultures in this study were B. pseudomallei. Although patients with bacteremic melioidosis and patients with bacteremia caused by other organisms had significantly different cytokine profiles and mortality, the proportions of patient with bacteremic melioidosis were similar in both the lexipafant and the placebo groups.
Lexipafant was well tolerated, but it was not associated with a significant reduction in mortality. This trial had only sufficient statistical power to detect large differences in mortality, but there was also no evidence of benefit on clinical or laboratory markers of vital organ dysfunction. These results argue against a central role for PAF in the pathogenesis of lethal septicemia. Levels in blood of the proinflammatory cytokines TNF-α, IL-6, IL-8, and soluble TNF-RI and TNF-RII were very high on admission and remained elevated in patients who developed multiorgan failure and died, whereas these levels fell after the initial 72 h of treatment in patients who survived. The goal of lexipafant treatment was to inhibit the production of these potentially toxic mediators produced by the exaggerated host immune response to bacterial endotoxin, but lexipafant did not lower the levels of any of these cytokines significantly compared to the placebo treatment.
The failure to reduce the mortality of patients with presumed sepsis with single-mediator antagonist treatment, including PAF receptor antagonist (this study), anti-TNF antibody (4a), or antiendotoxin therapy (such as HA-1A [14]), suggests that single therapeutic agents aimed at inhibiting the complex cytokine production cascade may not be the correct treatment strategy (9). The host response process in severe infections is complex and has not been characterized completely. Furthermore, in the majority of patients, irreversible lethal events may already have taken place before admission to the hospital.
ACKNOWLEDGMENTS
We are grateful to the director and staff of both Sappasitprasong Hospital and Siriraj Hospital for their support and, in particular, Vanaporn Wuthiekanun and Amanda Walsh for technical assistance.
This study was part of the Wellcome-Mahidol University Oxford Tropical Medicine Research Programme funded by the Wellcome Trust of Great Britain.
REFERENCES
- 1.Anderson B O, Bensard D D, Harken A H. The role of platelet activating factor and its antagonists in shock, sepsis and multiple organ failure. Surg Gynecol Obstet. 1991;172:415–424. [PubMed] [Google Scholar]
- 2.Braquet P, Paubert-Braquet M, Bourgain R, Bussolino F, Hosford D. PAF/cytokine autogenerated feedback networks in microvascular immune injury: consequences in shock, ischemia and graft rejection. J Lipid Mediators. 1989;1:75–112. [PubMed] [Google Scholar]
- 3.Bussolino F, Porcellini M G, Varese L, Besin A. Intravascular release of platelet activating factor in children with sepsis. Thromb Res. 1987;48:619–620. doi: 10.1016/0049-3848(87)90396-3. [DOI] [PubMed] [Google Scholar]
- 4.Chaowagul W, White N J, Dance D A B, Wattanagoon Y, Naigowit P, Davis T M, Looareesuwan S, Pitakwatchara N. Melioidosis: a major cause of community-acquired septicemia in northeastern Thailand. J Infect Dis. 1989;159:890–899. doi: 10.1093/infdis/159.5.890. [DOI] [PubMed] [Google Scholar]
- 4a.Cohen J, Carlet J. INTERSEPT: an international, multicenter, placebo-controlled trial of monoclonal antibody to human tumor necrosis factor alpha in patients with sepsis. International Sepsis Trial Group. Crit Care Med. 1996;24:1431–1440. doi: 10.1097/00003246-199609000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Cross A S. Antiendotoxin antibodies: a dead end? Ann Intern Med. 1994;121:58–59. doi: 10.7326/0003-4819-121-1-199407010-00011. [DOI] [PubMed] [Google Scholar]
- 6.Dhainaut J-F A, Tenaillon A, Le Tulzo Y, Schlemmer B, Solet J-P, Wolff M, Holzapfel L, Zeni F, Dreyfuss D, Mira J-P, De Vathaire F, Guinot P the BN52021 Sepsis Study Group. Platelet activating factor receptor antagonist BN52021 in the treatment of severe sepsis: a randomized, double-blind, placebo-controlled, multicenter clinical trial. Crit Care Med. 1994;22:1720–1728. [PubMed] [Google Scholar]
- 7.Fink A, Geva D, Zung A, Konichezky S, Eliraz A, Bentwich Z. Adult respiratory distress syndrome (ARDS): roles of leukotriene, C4 and platelet activating factor. Crit Care Med. 1990;18:905–910. [PubMed] [Google Scholar]
- 8.Gårdlund B, Sjolin J, Nilsson A, Roll M, Wickerts C-J, Wretlind B. Plasma levels of cytokines in primary septic shock in humans: correlation with disease severity. J Infect Dis. 1995;172:296–301. doi: 10.1093/infdis/172.1.296. [DOI] [PubMed] [Google Scholar]
- 9.Natanson C, Hoffman W D, Suffredini A F, Eichacker P Q, Danner R L. Selected treatment strategies for septic shock based on proposed mechanisms of pathogenesis. Ann Intern Med. 1994;120:771–783. doi: 10.7326/0003-4819-120-9-199405010-00009. [DOI] [PubMed] [Google Scholar]
- 10.Pinsky M R, Vincent J-L, Deviere J, Alegre M, Dupont E. Serum cytokine levels in human septic shock. Chest. 1993;103:565–575. doi: 10.1378/chest.103.2.565. [DOI] [PubMed] [Google Scholar]
- 11.Pittet D, Thievent B, Wenzel R P, Li N, Gurman G, Suter P M. Importance of pre-existing co-morbidities for prognosis of septicemia in critically ill patients. Intensive Care Med. 1993;19:265–272. doi: 10.1007/BF01690546. [DOI] [PubMed] [Google Scholar]
- 12.Tang H M, Teshima D Y, Lum B K B. Effects of the PAF antagonist Befafant and L-659,989 in endotoxic and septic shock. Drug Dev Res. 1993;29:216–221. [Google Scholar]
- 13.Torley L W, Pickett W C, Carroll M L, Kohler C A, Schaub R E, Wissner A, DeJoy S Q, Oronsky A L, Kerwar S S. Studies of the effect of a platelet-activating factor antagonist, CL 184,005, in animal models of gram-negative bacterial sepsis. Antimicrob Agents Chemother. 1992;36:1971–1977. doi: 10.1128/aac.36.9.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ziegler E J, Fisher C J, Sprung C L, Straube R C, Sadoff J C, Foulke G E, Wortel C H, Fink M P, Dellinger P, Teng N N H, Allen E, Berger H J, Knatterud G L, LoBuglio A F, Smith C R the HA-1A Sepsis Study Group. Treatment of gram-negative bacteremia and septic shock with HA-1A human monoclonal antibody against endotoxin. N Engl J Med. 1991;324:429–436. doi: 10.1056/NEJM199102143240701. [DOI] [PubMed] [Google Scholar]