Abstract
This study evaluated the impact of human immunodeficiency virus (HIV) and combination antiretroviral therapy (cART) on immune activation during pregnancy in a Zambian cohort of HIV-exposed but uninfected children followed up from birth. Activated CD8+ T cells (CD38+ and HLA-DR+) were compared among HIV-uninfected (n = 95), cART experienced HIV-infected (n = 111), and cART-naive HIV-infected (n = 21) pregnant women. Immune activation was highest among HIV-infected/cART-naive women but decreased during pregnancy. Immune activation HIV-infected women who started cART during pregnancy was reduced but not to levels similar to those in HIV-uninfected women. The effects of elevated maternal immune activation in pregnancy on subsequent infant health and immunity remain to be determined.
Keywords: Zambia, Mother-Child health, HIV-positive, HIV-negative, pregnancy, CD38, HLA-DR
Human immunodeficiency virus (HIV)–infected women who started combination antiretroviral therapy (cART) during pregnancy experienced reduced T-cell immune activation but not to levels similar to those in pregnant cART-experienced HIV-infected or HIV-uninfected women. The effect of maternal immune activation during early life on infant immunity remains undefined.
Activation of CD8+ T cells (hereafter referred to as “immune activation”) is a key response required for clearance of viral infection. With chronic infection, levels may remain elevated in peripheral blood [1] and are a hallmark of human immunodeficiency virus (HIV) infection [2]. HIV pathogenesis, including chronic CD8+ T-cell activation, is based on 3 key findings: (1) the persistent detection of HIV-specific effector cytotoxic T cells, (2) the presence of cell surface protein receptors that stimulate naive T cells into differentiated effector phenotypes [3], and (3) an acute/active cytokine profile detected in serum [4]. Ongoing immune activation and resulting inflammation are thought to lead to poor immune reconstitution, increased risk of cardiovascular disease, and metabolic changes, despite effective antiretroviral therapy (ART) [5].
Although combination ART (cART) reduces viral load and immune activation, some individuals retain a degree of immune activation even with undetectable viral load [6]. This is particularly concerning in pregnancy, when immunity is tightly regulated to permit immune activation to fight maternal infections [7] (protection of the mother) and immune quiescence to accommodate the presence of paternal antigens (protection of the fetus) [8]. During pregnancy, perturbations in this process due to acute and chronic infections have severe effects on fetal development [8].
Early studies of pregnancy and HIV disease progression identified several unique immune alterations in HIV-infected pregnant women. Mikyas et al [9] report immune activation in HIV-infected pregnant women (not on cART) above levels observed in HIV-uninfected pregnant and nonpregnant controls, including increases in percentages of CD8+ T cells expressing HLA-DR and CD38 (markers of chronic immune activation). These changes were observed earlier in pregnancy and persisted until delivery in HIV-infected women, compared with transient changes observed in HIV-uninfected pregnant women [9]. Of concern, in utero exposure to maternal HIV infection that does not lead to congenital infection (ie, HIV-exposed, uninfected [HEU] infants) can lead to increased infectious disease morbidity and mortality rates in newborns and alterations in immune cell populations consistent with T-cell immune activation [10].
Between 2000 and 2018, the number of childhood HIV infections decreased by >60%, owing mostly to antenatal ART [11]. This is tempered by increased infectious diseases morbidity and mortality rates among HEU children compared with HIV-unexposed children [12]. The cause of this increased disease burden is unknown, but possible maternal factors include HIV disease progression, exposure to antiretrovirals, and/or coinfections. These maternal factors may have a common immunologic pathway of antigen/microbial exposure leading to sustained immune activation [13]. Given the links of chronic inflammation/immune activation during pregnancy with poor pregnancy outcomes, there is an urgent need to understand the role of maternal immune activation during pregnancy and how it is modified by cART, whether initiated before or after conception. Our hypotheses are that immune activation is highest in ART-naive HIV-infected and lowest in HIV-uninfected women and that it would increase toward delivery.
METHODS
Study Population and Enrollment
These results were collected as part of an ongoing observational cohort study designed to determine morbidity and mortality rates among HEU children compared with children born to HIV-uninfected control women. Initiated in May 2019, the Zambia Infant Cohort Study intends to enroll 750 HIV-infected and 750 HIV-uninfected pregnant women to be followed up for infant clinical outcomes through 6 months postpartum. Women are recruited from a primary health facility in the Chawama township located in Lusaka, Zambia, which has a sociodemographically homogenous population. Prevention of mother-to-child-transmission services are part of routine antenatal care, including opt-out HIV testing and immediate cART provision.
Pregnant women aged >15 years seeking routine antenatal care, who were in in their first 26 weeks of pregnancy by ultrasonographic gestational dating and accepted informed consent, were enrolled. Women requiring delivery at a higher level of care were excluded. Recorded maternal enrollment characteristics included sociodemographic, cART regimen (if HIV infected) and initiation date. HIV serostatus was verified using Alere Determine HIV-1/2 immunoassay with confirmation by SD Bioline HIV-1/2 antibody immunoassay (Abbott). First-line cART was initiated according to Zambian guidelines. “cART experienced” was defined as receipt of cART for any duration before enrollment, versus “cART naive.” Baseline determinations were done for CD4+ T-cell count and viral load, with lower limit of detection of 20 HIV RNA copies/mL plasma (Cobas Ampliprep/Cobas Taqman, version 2.0; Roche Diagnostic). Participants were scheduled to return approximately 3 weeks before the expected delivery date for repeated ultrasonography and end-of-pregnancy immune activation status. HIV-uninfected women were retested at delivery to confirm serostatus.
Flow Cytometry
T-cell immune activation was determined with flow cytometry using freshly collected whole blood directly stained with a cocktail of antibodies in conjunction with absolute counting beads (LeukoSure Fluorospheres; Beckman Coulter Life Sciences). Prepared cells were acquired with a FC500 flow cytometer (Beckman Coulter). CD4+ and CD8+ populations were gated from the CD3+ T-cell population, and evaluated for coexpression of CD38 and HLA-DR. Antibody-fluorochrome combination and clones are as follows: CD3–fluorescein isothiocyanate (UCHT1), CD4-PC7 (RPA-T4), CD8-phycoerythrin (SK1), CD38-PC5 (HIT2) and HLA-DR (G46-6) (Beckman Coulter). The degree of immune activation was calculated as a percentage of CD38+HLA-DR+ expressed on CD3+CD8+ lymphocytes.
Statistical Analyses
Baseline maternal factors, HIV infection characteristics and immune status were compared between HIV-infected and HIV-uninfected women and between cART-experienced and cART-naive HIV-infected women, using χ 2 Fisher exact and 2-sample t tests. Immune activation in HIV-uninfected women was measured at 2 time points during pregnancy. Differences in mean immune activation were compared at baseline and at the third trimester between HIV-uninfected women and cART-naive and cART-experienced HIV-infected women. A mixed effects model was used to compare mean immune activation across groups (HIV infection and cART status), controlling for gestational age. Two sensitivity analyses were also performed: (1) excluding women whose second immune activation measurement was within 1 week of birth, to account for possible increase in immune activation markers at onset of delivery, and (2) excluded cART-experienced women if the duration of cART was ≤6 mo. The conclusions from these sensitivity models did not differ from those from our main analysis (see Supplementary Data).
RESULTS
Of the first 206 deliveries into the Zambia Infant Cohort Study, 111 women (54%) were HIV infected at enrollment and 95 (46%) were HIV uninfected. Baseline characteristics in HIV-infected and HIV-uninfected pregnant women were similar for educational attainment, smoking, and body mass index (Table 1). HIV-infected women were older (30 vs 25 years for HIV-uninfected women; P < .001) and more often employed (37.8% vs 22.1%; P < .01). HIV-uninfected women had fewer prior pregnancies than HIV-infected women (1 vs 2, respectively) and were enrolled earlier in gestation than HIV-infected mothers (19 vs 20 weeks; P = .04).
Table 1.
Demographic Characteristics of Study Cohort
| Characteristic | Women, No. (%)a | P Value | |
|---|---|---|---|
| HIV Uninfected (n = 95) | HIV Infected (n = 111) | ||
| Baseline maternal | |||
| Age, mean (SD) y | 25 (5) | 30 (6) | <.001 |
| Married | 76 (80.0) | 98 (88.3) | .10 |
| Employed | 21 (22.1) | 42 (37.8) | .01 |
| Educational level | |||
| No formal education | 3 (3.2) | 3 (2.7) | .54 |
| Less than college | 81 (85.3) | 100 (90.1) | |
| College | 11 (11.6) | 8 (7.2) | |
| Nonsmoker during pregnancy | 95 (100) | 111 (100.0) | |
| No alcohol use during pregnancy | 92 (96.8) | 95 (85.5) | .005 |
| BMI, mean (SD)b | 24.77 (4.19) | 24.81 (4.10) | .92 |
| Obstetric | |||
| GA, mean (SD), wk | 19 (3) | 20 (4) | .04 |
| Parity, mean (SD) | 1 (1) | 2 (2) | <.001 |
| Immune status | |||
| Previous tuberculosis diagnosis | 1 (1.1) | 5 (4.5) | .22 |
| CD4+ T-cell count, mean (SD), cells/µL | 807 (1735) | 405 ( 205) | <.001 |
| Baseline immune activation, mean (SD), %c | 26.2 (13.0) | 39.6 (18.8) | <.001 |
Abbreviations: BMI, body mass index; cART, combination antiretroviral therapy; GA, gestational age; HIV, human immunodeficiency virus; SD, standard deviation.
aData represent no. (%) of women unless otherwise specified.
bBMI is calculated as weight in kilograms divided by height in meters squared.
cImmune activation is expressed as the percentage of CD8+CD3+ T cells expressing CD38 and HLA-DR.
At enrollment the majority (81%) of HIV-infected women were cART experienced (n = 90), and 19% were cART naive (n = 21; Table 2). cART-naive women HIV-infected were younger than cART-experienced women (mean age [SD], 29 [3] vs 30 [6] years; P = .18) and had higher body mass index at similar weeks of gestation (26.70 [4.58] vs 24.37 [3.87] [calculated as weight in kilograms divided by height in meters squared].; P = .02). Among cART-experienced women, 72 (80%), had taken cART for >6 months and 89 (99%) were on first-line cART regimen. cART-naive women had lower mean (SD) CD4+ T-cell counts (264/µL [195/µL] vs 438/µL [194l/µL]; P = .02) than the cART-experienced women, and higher mean viral loads (4.5 [0.8] vs 3.0 [1.2] log10 HIV RNA copies/mL plasma; P < .001).
Table 2.
Characteristics of Human Immunodeficiency Virus–Infected Women
| Characteristic | Women Tested, No. | cART-Naive Women | Women Tested, No. | cART-Experienced Women | P Value |
|---|---|---|---|---|---|
| Baseline maternal | |||||
| Age, mean (SD), y | 21 | 29 (3) | 90 | 30 (6) | .18 |
| BMI, mean (SD)a | 21 | 26.70 (4.58) | 90 | 24.37 (3.87) | .02 |
| Obstetric | |||||
| GA, mean (SD), wk | 21 | 19 (4) | 90 | 20 (4) | .47 |
| HIV infection | |||||
| CD4+ T-cell count, mean (SD), cells/µL | 21 | 264 (195) | 90 | 438 (194) | <.001 |
| HIV diagnosis before conception, no. (%) | 21 | 2 (9.5) | 90 | 69 (78.4) | <.001 |
| Detectable viral load, (>20 log10 HIV RNA copies/mL plasma), no. (%) | 19 | 16 (84.2) | 79 | 30 (38.0) | .001 |
| Viral load, mean (SD), log10 HIV RNA copies/mL plasma | 16 | 4.5 (0.8) | 30 | 3.0 (1.2) | <.001 |
| Duration of cART no. (%) | |||||
| None | 21 | NA | .58 | ||
| <1 mo | 0 | 9 (10.0) | |||
| 1–6 mo | 0 | 9 (10.0) | |||
| >6 mo | 0 | 72 (80.0) | |||
| First-line cART regimen, (%) | 21 | 21 (100) | 90 | 89 (99) | <.001 |
| Immune activation, mean (SD), %b | |||||
| Baseline | 21 | 57.0 )16.2) | 90 | 35.5 (17.0) | <.001 |
| Third trimester | 21 | 47.0 (19.8) | 90 | 36.1 (13.7) | .003 |
Abbreviations: BMI, body mass index; cART, combination antiretroviral therapy; GA, gestational age; HIV, human immunodeficiency virus; NA, not applicable; SD, standard deviation.
aBMI is calculated as weight in kilograms divided by height in meters squared.
bImmune activation is expressed as the percentage of CD8+CD3+ T cells expressing CD38 and HLA-DR.
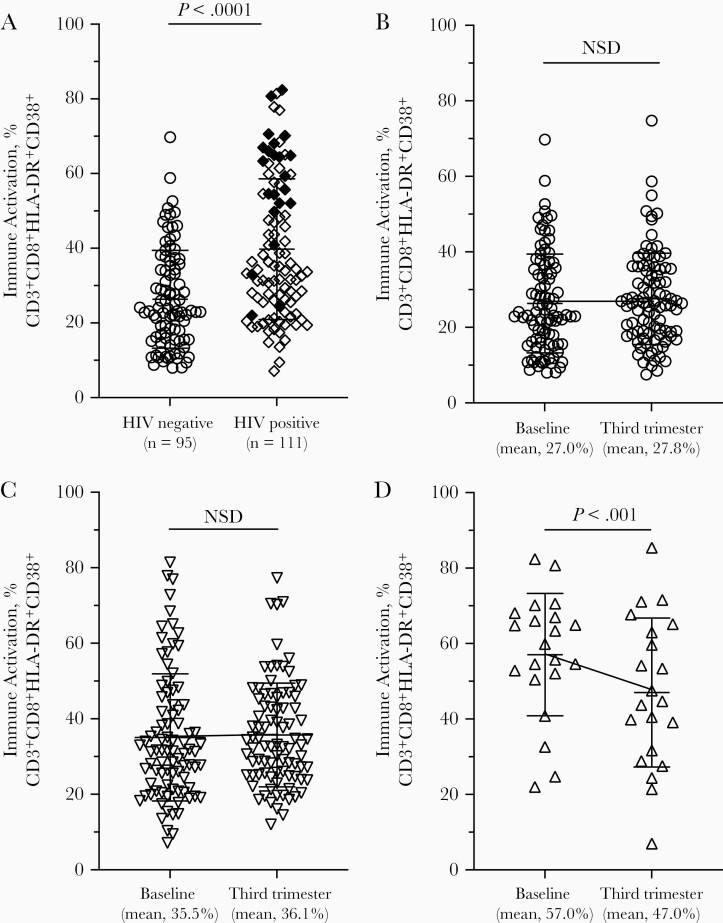
HIV-uninfected women had significantly lower levels of immune activation at baseline compared with HIV-infected women (26% vs 40%; P < .001) (Figure 1A). At baseline, immune activation levels in HIV-uninfected, cART-experienced HIV-infected, and cART-naive HIV-infected women were 26%, 36% and 57%, respectively (P < .001, pairwise comparisons) (Figure 1A). Late third trimester immune activation levels in the HIV-uninfected, cART-experienced HIV-infected, and cART-naive HIV-infected groups were 27%, 36% and 47% (P < .004, pairwise comparisons), respectively. Immune activation remained constant throughout pregnancy for HIV-uninfected and cART-experienced HIV-infected women. cART-naive HIV-infected women had a significant 10% decline in immune activation during the same interval (P = .001) (Figure 1B–1D).
Figure 1.
Immune activation levels in peripheral blood of human immunodeficiency virus (HIV)–uninfected and HIV-infected women. CD8+ T cells coexpressing activation markers HLA-DR and CD38 were identified using flow cytometry and expressed as the mean and standard deviation of the percentage of CD3+CD8+ population double-positive for CD38 and HLA-DR. Statistical assessment was done with Student t test. A, Distribution of immune activation levels from pregnant HIV-uninfected (circles) and HIV-infected (diamonds) women early in the second trimester (baseline). Closed diamonds represent HIV-infected women who were naïve to combination antiretroviral therapy (cART) at baseline. B–D, Trajectory of immune activation during pregnancy; the third trimester time point was approximately 3 weeks before delivery. A reference line connecting the means is included for clarity. B, HIV-uninfected women. C, HIV-infected women on cART before this pregnancy. D, HIV-infected women with cART initiated at the baseline visit. Abbreviation: NSD, no significant difference.
We observed a significant interaction between gestational age and mean immune activation (P < .001). There were no significant changes in immune activation with weeks of gestation in either HIV-uninfected women (slope, 0.01; 95% confidence interval, −01 to .04; P = .29) or cART-experienced HIV-infected women (0.01; −.02 to .03; P = .66); the mean percentage of immune activation increased in both populations by about 1% per week of gestation. There was, however, a significant decrease in immune activation in cART-naive HIV-infected women (slope, −0.11; 95% confidence interval, −.16 to −.06; P <.001), indicative of an 11% reduction in immune activation per week of gestation/time on cART.
Discussion
These results show 3 major findings. First, immune activation in HIV-uninfected women is stable throughout pregnancy, in contrast to the findings of Mikyas et al [9], in which a distinct increase in immune activation was seen in the third trimester in both HIV-uninfected and HIV-infected women minimally suppressed on azidothymidine (AZT) monotherapy. Second, provision of cART significantly reduced immune activation but not to levels observed in HIV-uninfected women, indicating that cART regimens to prevent mother-to-child-transmission do not normalize maternal immune activation in all women. Third, our data highlight the broad range of immune activation that exists in both HIV-infected and HIV-uninfected women, possibly owing to non-HIV comorbid conditions. The effects of sustained elevated immune activation during pregnancy on in utero immune development are not fully understood.
cART in pregnant women initiated as early in pregnancy as possible is now the global standard of care. A decrease in viral load is thought to be the mechanism by which the marked decrease in vertical transmission in the presence of cART occurs. However, less is known about the increased infant morbidity and mortality rates seen among HEU children. Persistent in utero exposure to maternal inflammation, either through or accompanied by immune activation, could play a role in alterations in infant immune dysregulation [14]. Compared with HIV-infected women who had been receiving cART for at least 6 months before enrollment, cART-naive women had significantly increased levels of immune activation.
An earlier study of HIV and immune activation during pregnancy found that HIV-infected women (receiving AZT alone) had consistently higher levels of immune activation than HIV-uninfected women [15]. Our data differ from that study in cART exposure, participant ethnicity, time points of sample collection during gestation and in methodology. Despite prolonged cART, baseline immune activation levels in cART-experienced women remained slightly higher than those in HIV-uninfected pregnant women (35% vs 27%; P < .001). Regardless of a significant reduction in immune activation levels during pregnancy in the cART naive women, the reduction did not normalize to the levels found in either cART-experienced women or HIV-uninfected pregnant women. This residual high level of immune activation throughout pregnancy in cART-naive women results in fetal exposure to a proinflammatory milieu plausibly associated with untoward immune effects in the developing fetus and early infancy [10].
Our study had some limitations and strengths. We are unable to determine whether maternal immune activation is a marker for poor maternal health overall or a step in the pathogenesis of infant morbidity and/or mortality. We captured standard changes in the CD8+ T-cell population, however, our panel may not be directly reflective of the level of other important immune cell populations, such as regulatory T cells, that are believed to play a role in the immune tolerance [16]. Our study strengths included a sample of HIV-uninfected pregnant women large enough to generate normative values, allowing for direct comparisons with HIV-infected women. and the longitudinal nature of the cohort. allowing observation of both populations of HIV-infected women: those already receiving cART and those newly starting it.
Importantly, our data highlight individual variation in immune activation across all pregnant women. A small proportion of HIV-uninfected women have immune activation levels similar to those in women with newly diagnosed HIV. Similarly, a subset of cART-experienced women have levels of immune activation similar to those in cART-naive women, indicative of the many triggers of immune activation. Finally, our study design that leveraged a homogeneous community helped to reduce confounding variables and also reflects the proportions of HIV-infected pregnant women on pre- and postconception cART. Given the associations between maternal infections during pregnancy and effects on fetal development, in utero exposure to elevated maternal immune activation—regardless of the causative agent—may place the infant at increased risk of illness or death. To date there is no predictive marker observed in HEU infants that indicates susceptibility to untoward outcomes. Given the wealth of data from HEU infants indicative of infant immune disruptions present at birth as well as increased infectious morbidity and mortality rates, it is plausible that maternal immune activation has a role in infant immune dysfunction.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank the women of Chawama for their participation in the Zambia Infant Cohort Study. We also heartily thank all the team members in Lusaka, Boston and Providence. REDCap electronic data capture tools are hosted at Boston University (CTSI 1UL1TR001430).
This article is dedicated to the memory of Roy Chavuma—“Dr Laugh”—who died of coronavirus disease 2019 during the preparation of the manuscript.
Financial support. This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant HD094650 to principal investigator D. M. T.).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. McMichael AJ, Rowland-Jones SL. Cellular immune responses to HIV. Nature 2001; 410:980–7. [DOI] [PubMed] [Google Scholar]
- 2. Rizzardini G, Trabattoni D, Saresella M, et al. . Immune activation in HIV-infected African individuals. Italian-Ugandan AIDS cooperation program. AIDS 1998; 12:2387–96. [DOI] [PubMed] [Google Scholar]
- 3. Liu Z, Cumberland WG, Hultin LE, Prince HE, Detels R, Giorgi J. Elevated CD38 antigen expression on CD8+ T cells Is a stronger marker for the risk of chronic HIV disease progression to AIDS and death in the multicenter AIDS cohort study than CD4+ cell count, soluble immune activation markers, or combinations of HLA-DR. J Acqu Immune Defic Syndr Human Retrovirol 1997; 16:83-92. [DOI] [PubMed] [Google Scholar]
- 4. Freeman ML, Shive CL, Nguyen TP, Younes SA, Panigrahi S, Lederman MM. Cytokines and T-cell homeostasis in HIV infection. J Infect Dis 2016; 214:S51–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Corbeau P, Reynes J. Immune reconstitution under antiretroviral therapy: the new challenge in HIV-1 infection. Blood 2011; 117:5582–90. [DOI] [PubMed] [Google Scholar]
- 6. Tuaillon E, Al Tabaa Y, Baillat V, Segondy M, Picot MC, Reynes J, Vendrell JP. Close association of CD8+/CD38bright with HIV-1 replication and complex relationship with CD4+ T-cell count. Cytometry Part B Clin Cytom 2009; 76:249-260. [DOI] [PubMed] [Google Scholar]
- 7. Yang X, Su B, Zhang X, Liu Y, Wu H, Zhang T. Incomplete immune reconstitution in HIV/AIDS patients on antiretroviral therapy: challenges of immunological non-responders. J Leukoc Biol 2020; 107:597–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Racicot K, Kwon JY, Aldo P, Silasi M, Mor G. Understanding the complexity of the immune system during pregnancy. Am J Reprod Immunol 2014; 72:107–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mikyas Y, Aziz N, Harawa N, et al. . Immunologic activation during pregnancy: serial measurement of lymphocyte phenotype and serum activation molecules in HIV-infected and uninfected women. J Reprod Immunol 1997; 33:157–70. [DOI] [PubMed] [Google Scholar]
- 10. Dauby N, Goetghebuer T, Kollmann TR, Levy J, Marchant A. Uninfected but not unaffected: chronic maternal infections during pregnancy, fetal immunity, and susceptibility to postnatal infections. Lancet Infect Dis 2012; 12:330–40. [DOI] [PubMed] [Google Scholar]
- 11. UNAIDS. Start free stay free. 2019 Report.2019. https://www.unaids.org/sites/default/files/media_asset/20190722_UNAIDS_SFSFAF_2019_en.pdf. Accessed 09 Sept 2020.
- 12. Brennan AT, Bonawitz R, Gill CJ, et al. . A meta-analysis assessing all-cause mortality in HIV-exposed uninfected compared with HIV-unexposed uninfected infants and children. AIDS 2016; 30:2351–60. [DOI] [PubMed] [Google Scholar]
- 13. Afran L, Garcia Knight M, Nduati E, Urban BC, Heyderman RS, Rowland-Jones SL. HIV-exposed uninfected children: a growing population with a vulnerable immune system? Clin Exp Immunol 2014; 176:11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bunders M, Thorne C, Newell ML; European Collaborative Study . Maternal and infant factors and lymphocyte, CD4 and CD8 cell counts in uninfected children of HIV-1-infected mothers. AIDS 2005; 19:1071–9. [DOI] [PubMed] [Google Scholar]
- 15. Rich KC, Siegel JN, Jennings C, Rydman RJ, Landay AL. CD8+ lymphocytes in pregnancy and HIV infection: characterization of CD8+ subpopulations and CD8+ noncytotoxic antiviral activity. AIDS Res Hum Retroviruses 1999; 15:665–70. [DOI] [PubMed] [Google Scholar]
- 16. Yu Y, Ma X, Gong R, Zhu J, Wei L, Yao J. Recent advances in CD8+ regulatory T cell research. Oncol Lett 2018; 15:8187–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.