Abstract
Background
Liver kinase B1 (LKB1) has been studied extensively as a tumor suppressor gene (Stk11) in the context of cancer. We hypothesized that myeloid LKB1 plays a role in innate immunity during pneumonia.
Methods
Mice deficient for LKB1 in myeloid cells (LysM-cre × Stk11fl/fl) or neutrophils (Mrp8-cre × Stk11fl/fl) were infected with Klebsiella pneumoniae via the airways. LysM-cre × Stk11fl/fl mice were also intranasally challenged with lipopolysaccharide (LPS).
Results
Mice with myeloid LKB1 deficiency, but not those with neutrophil LKB1 deficiency, had increased bacterial loads in lungs 6–40 hours after infection, compared with control mice, pointing to a role for LKB1 in macrophages. Myeloid LKB1 deficiency was associated with reduced cytokine release into the airways on local LPS instillation. The number of classic (SiglecFhighCD11bneg) alveolar macrophages (AMs) was reduced by approximately 50% in the lungs of myeloid LKB1–deficient mice, which was not caused by increased cell death or reduced proliferation. Instead, these mice had AMs with a “nonclassic” (SiglecFlowCD11bpos) phenotype. AMs did not up-regulate glycolysis in response to LPS, irrespective of LKB1 presence.
Conclusion
Myeloid LKB1 is important for local host defense during Klebsiella pneumonia by maintaining adequate AM numbers in the lung.
Keywords: liver kinase B1, Klebsiella pneumonia, lipopolysaccharide, pneumonia, alveolar macrophage
Myeloid liver kinase B1 (LKB1) depletion reduces alveolar macrophages and impairs host defense against Klebsiella pneumoniae in the lung. LKB1 deficiency in alveolar macrophages does not affect glucose metabolism or immune functions, such as tumor necrosis factor production or phagocytosis.
Liver kinase B1 (LKB1; also known as serine-threonine kinase 11 [STK11]) is known as the tumor suppressor gene mutated in the inherited cancer disorder Peutz-Jeghers syndrome [1]. LKB1 has been studied extensively in the context of cancer, but it may also contribute to an adequate innate immune response during infection. First, LKB1 has been reported as a negative regulator of nuclear factor–κB activation in bone marrow–derived macrophages (BMDMs) exposed to lipopolysaccharide (LPS), a membrane component of gram-negative bacteria, and thereby to reduce the expression of proinflammatory proteins [2].
In accordance, mice with a myeloid cell–specific deletion of Stk11, the gene encoding LKB1, demonstrated increased serum cytokine levels on intraperitoneal LPS administration [2]. On the other hand, LKB1 can inhibit intracellular glycolysis through activation of adenosine monophosphate–activated protein kinase (AMPK) and subsequent inhibition of the mammalian target of rapamycin [3–5]. Of relevance in this context, it has become evident that immune cells reprogram their cellular metabolism to fit different immunological functions [6]. Macrophages [7] and other immune cells [8–10] show an increase in glycolysis on activation, suggesting an important role for glycolysis in inflammation. Indeed, inhibition of glycolysis diminishes proinflammatory cytokine production by macrophages stimulated with bacterial agonists [11, 12]. Hence, LKB1 may influence inflammatory responses by macrophages in seemingly opposite ways, where the direction of the effect may depend on macrophage type and tissue environment.
Alveolar macrophages (AMs) have been implicated as key players in host defense against pathogens that invade the lower airways to induce pneumonia [13]. Pneumonia is responsible for a disproportionate disease burden worldwide [13]. Aerobic gram-negative bacteria are the most common causative microorganisms in nosocomial pneumonia, among which Klebsiella pneumoniae is especially troublesome, considering its increasing antimicrobial resistance [14]. In addition, in certain areas of the world K. pneumoniae has emerged as a rising cause of community-acquired pneumonia [15]. The primary aim of the current study was to determine the role of myeloid cell LKB1 in the host response during pneumonia caused by K. pneumoniae. For this, we made use of mice with a myeloid-specific LKB1 deficiency and a well-established model of Klebsiella-induced pneumonia [16–18].
METHODS
Detailed methods can be found in the online supplement.
Animals
Homozygous Stk11fl/fl mice (014143; Jackson Laboratory) [19] were crossed with LysM-cre [20] or Mrp8-cre mice (021614; Jackson Laboratory) [21] to generate mice that were deficient in myeloid-specific LKB1 (LysM-cre × Stk11fl/fl) and neutrophil-specific LKB1 (Mrp8-cre × Stk11fl/fl) [22]. Stk11fl/fl Cre-negative littermates were used as controls in all experiments. Experiments were approved by the Central Commission for Animal Experiments.
Mouse Models
Pneumonia was induced by intranasal inoculation with approximately 1 × 104 colony-forming units of K. pneumoniae serotype 2 (American Type Culture Collection no. 43816). Infection and processing of organs were done as described elsewhere [16–18]. Lung inflammation was induced by intranasal administration of 1 µg of ultrapure LPS (Escherichia coli O111:B4; InvivoGen) in 50 µL of normal saline.
Assays
LKB1 Western blotting was done as described in the online Supplement. Interleukin 1β (IL-1β), interleukin 10 (IL-10), interleukin 6 (IL-6), tumor necrosis factor (TNF), and CXCL1 were measured by means of enzyme-linked immunosorbent assay according to the manufacturers protocol (R&D Systems). Lactate was quantified using an enzymatic assay, as described elsewhere [23].
Histopathology
Lungs were processed and scored by an independent pathologist, as described elsewhere [16–18].
AM Isolation and Stimulation
AMs were harvested by means of bronchoalveolar lavage (BAL), seeded in 96-well flat-bottom culture plates (Greiner Bio-One) at a density of approximately 3 × 104 cells per well in Roswell Park Memorial Institute 1640 complete medium (containing 10% fetal bovine serum, penicillin-streptomycin, 2 mmol/L L-glutamine, and 25 mmol/L HEPES; Gibco, Thermo Fisher Scientific), and left to adhere overnight. AMs were stimulated for 24 hours with heat-killed K. pneumoniae (multiplicity of infection, 10:1; American Type Culture Collection no. 43816) or 100 ng/mL ultrapure LPS (E. coli O111:B4; InvivoGen). TNF and lactate were measured in supernatants, using assays as described above.
Lung Digestion and Stimulation
Lungs were washed in phosphate-buffered saline (PBS), minced into pieces, and incubated at 37°C for 30 minutes with warm PBS containing 10 mg/mL DNase I (Roche) and 5 mg/mL Liberase TM (Sigma-Aldrich). Cells were filtered, washed several times with PBS, seeded at a density of approximately 1 × 106 cells per well in Roswell Park Memorial Institute 1640 complete medium, and stimulated for 2.5 hours with ultrapure LPS (E. coli O111:B4; InvivoGen) or left untreated.
For the study of intracellular TNF, cells were treated with a protein transport inhibitor (containing brefeldin A; BD Biosciences). For the study of glucose uptake and mitochondrial mass and membrane potential in lung suspensions, cells were incubated for 3 hours with addition of 50 µg/mL 2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino]-2-deoxyglucose (2NBDG; Cayman Chemical), 50 nmol/L Mitotracker Green (Invitrogen), or 50 nmol/L Mitotracker Red CMX Ros (Invitrogen) during the last 30 minutes of incubation. Reactive oxygen species (ROS) production was measured by stimulating lung cell suspensions with heat-killed K. pneumoniae or medium control for 3 hours with addition of 10 µmol/L carboxy-H2DCFDA (Invitrogen). Phagocytosis was analyzed by means of incubation with 250 µg/mL pHrodo Red E. coli BioParticles Conjugate (Invitrogen) for 3 hours. Cells were analyzed using flow cytometry, as described below.
Flow Cytometry
Total cell counts in BAL fluid (BALF) and lung digestions were determined using a Coulter cell counter (Beckman Coulter). Cell subsets were identified by staining with fixable viability dye eFluor 780 (Invitrogen) and the following antibodies: rat anti-mouse CD16/CD32 (clone 93), rat anti-mouse CD45 phycoerythrin (PE)–eFluor610 or fluorescein isothiocyanate (FITC) (clone 30-F11), hamster anti-mouse CD11c peridinin-chlorophyll protein (PerCP)–cyanine 5,5 (Cy5,5) (clone HL3), rat anti-mouse CD11b PE–cyanine 7 (clone M1/70), rat anti-mouse Siglec-F Alexa Fluor 647 (clone E50-2440), rat anti-mouse Ly-6G Alexa Fluor 700 or allophycocyanin (clone 1A8), rat anti-mouse major histocompatibility complex class II Alexa Fluor 700 (clone M5/114.15.2), and rat anti-mouse CD24 allophycocyanin (clone M1/69) (all from BD Biosciences); mouse anti-mouse CD64 PerCP-Cy5,5 (clone X54-5/7.1), rat anti-mouse MerTK PE (clone 2B10C42), rat anti-mouse Ly-6G FITC (clone 1A8), and hamster anti-mouse CD103 PerCP-Cy5,5 (clone 2E7) (all from Biolegend); and rat anti-mouse Ly6C FITC (clone 1G7.G10; Miltenyi Biotec). Intracellular staining with rat anti-mouse TNF Alexa Fluor 488 (clone MP6-XT22; Biolegend) and rat anti-mouse Ki-67 FITC (clone SolA15; Thermo Fisher Scientific) was performed using Foxp3/Transcription Factor Staining Buffer Set (eBioscience). Flow cytometry was performed using a FACSCanto II cytometer (BD Biosciences), and data were analyzed using FlowJo software, version 10.7 (BD Biosciences).
Statistical Analysis
Nonparametric variables were analyzed using the Mann-Whitney U test. Parametric variables were analyzed using Student t tests (2-group comparison) or 2-way analysis of variance (comparison between ≥3 groups) with Sidak multiple comparisons test where appropriate. Analyses were done using GraphPad Prism software, version 8 (GraphPad Software).
RESULTS
Importance of Macrophage LKB1 for Host Defense During K. pneumoniae–Induced Pneumonia
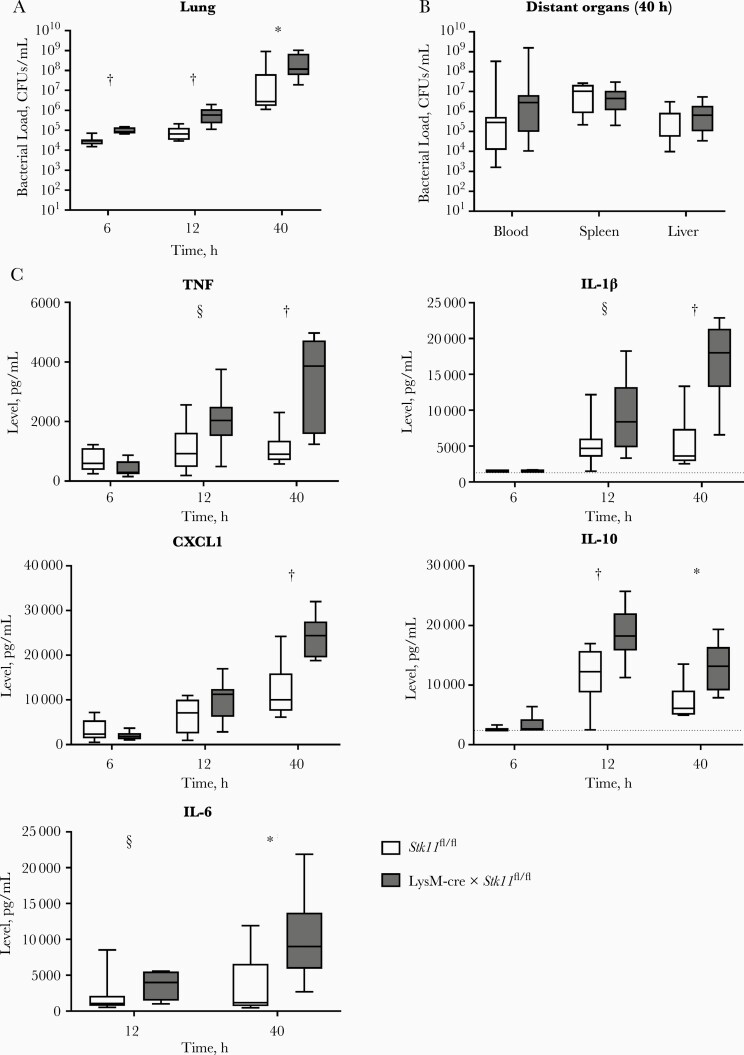
To investigate the role of macrophage LKB1 in host defense during gram-negative pneumonia, we generated myeloid-specific LKB1-deficient mice by crossing LKB1-floxed (Stk11fl/fl) mice with LysM-cre mice. BMDMs and AMs from LysM-cre × Stk11fl/fl mice expressed very low LKB1 protein levels compared with cells from control mice, and BMDM LKB1 levels were not influenced by LPS stimulation (Supplementary Figure 1A,B). LysM-cre × Stk11fl/fl and control mice were infected with K. pneumoniae via the airways, and bacterial loads were determined 6, 12, and 40 hours after inoculation. LysM-cre × Stk11fl/fl mice had increased bacterial loads in the lung 6 hours after inoculation as compared with littermate controls, which was sustained at later time points (Figure 1A). Bacterial loads in blood and distant organs were sporadically detectable 12 hours after infection and did not differ between LysM-cre × Stk11fl/fl and control mice (data not shown).
Figure 1.
Macrophage liver kinase B1 (LKB1) is important for host defense against Klebsiella pneumoniae in the lung. A, B, Bacterial loads (colony-forming units [CFUs] per milliliter) in the lungs of LysM-cre × Stk11fl/fl mice and littermate controls 6, 12 and 40 hours after intranasal inoculation with approximately 104 CFUs of K. pneumoniae (A) or in distant organs 40 hours after inoculation (B). C, Cytokine levels (tumor necrosis factor [TNF], IL-1β, CXCL1, and interleukin 1β, 10, and 6 [IL-1β, IL-10, and IL-6]) in the lung at 6, 12, and 40 hours after infection. Data are shown as box-and-whisker diagrams representing 7–8 mice per group at each time point. Bacterial loads and cytokine levels of the LysM-cre × Stk11fl/fl mice were compared with those in littermate control (Stk11fl/fl) mice using the Mann-Whitney test. *P < .05; †P < .01; §P < .1.
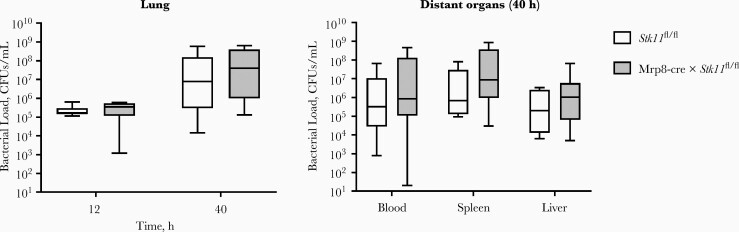
At 40 hours after infection, all mice showed dissemination to distant organs, and bacterial loads did not differ between groups (Figure 1B). At 6 hours after infection, cytokine and chemokine levels in the lungs were low and not significantly different between LysM-cre × Stk11fl/fl and control mice (Figure 1C). Of note, however, at this early time point LysM-cre × Stk11fl/fl mice tended to have lower lung TNF and CXCL1 concentrations in spite of higher bacterial loads. At later time points, especially at 40 hours, LysM-cre × Stk11fl/fl mice had higher lung TNF, IL-1β, CXCL1, IL-10, and IL-6 levels as compared with control mice, likely reflecting the higher bacterial burdens. The extent of lung disease was not different between groups (Supplementary Figure 2). Because the LysM promoter drives Cre expression in all myeloid cells, including neutrophils [22, 24], we determined a possible role for LKB1 in neutrophils in defense against K. pneumoniae. To that end, we generated neutrophil-specific LKB1-deficient mice by crossing Mrp8-cre with Stk11fl/fl mice [22]. These mice did not show differences in bacterial loads in lungs or distant organs (Figure 2). Altogether, these results suggest that LKB1 in macrophages, but not neutrophils, is important for host defense against K. pneumoniae in the lung.
Figure 2.
Neutrophil liver kinase B1 (LKB1) is not important for host defense against Klebsiella pneumoniae. Bacterial loads (colony-forming units [CFUs] per milliliter) in the lungs of Mrp8-cre × Stk11fl/fl mice 12 and 40 hours after intranasal inoculation with approximately 104 CFUs K. pneumoniae or in distant organs 40 hours after inoculation. Data are shown as box-and-whisker diagrams representing 7–8 mice per group at each time point. Bacterial loads of the Mrp8-cre × Stk11fl/fl mice were compared with those in littermate control (Stk11fl/fl) mice, using the Mann-Whitney test.
Reduced Cytokine Release in the Airways upon LPS Challenge in Mice With Macrophage LKB1 Deficiency
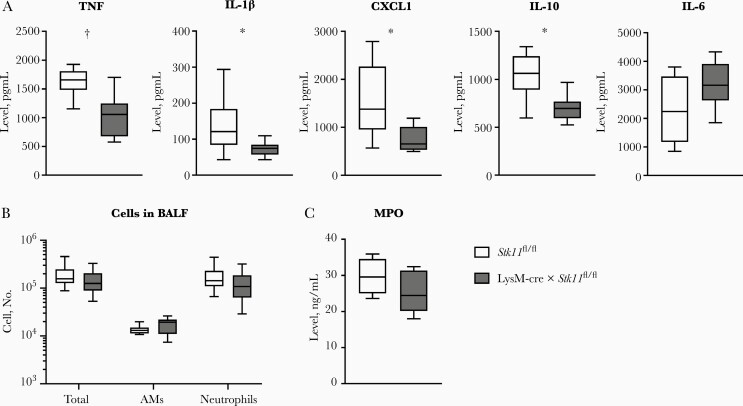
The model of Klebsiella-induced pneumonia used is associated with a gradually growing bacterial load accompanied by steadily increasing proinflammatory cytokine levels (Figure 1) [16–18]; this model is therefore less suitable for studying the impact of host factors on cytokine release, with potential deficiencies being concealed by differences in bacterial numbers. Therefore, we investigated the role of macrophage LKB1 in the induction of cytokines and chemokines in an acute lung inflammation model elicited by local administration of LPS—that is, a more robust and stabile challenge with relevance for gram-negative infection. LysM-cre × Stk11fl/fl mice had significantly lower levels of TNF, IL-1β, CXCL1, and IL-10 in BALF than littermate controls 6 hours after LPS inoculation; IL-6 levels were similar in both mouse strains (Figure 3A). The cell number and proportions of AMs and neutrophils in BALF did not diff between the groups (Figure 3B). BALF levels of myeloperoxidase were also similar between LysM-cre × Stk11fl/fl and control mice (Figure 3C). These data suggest that macrophage LKB1 contributes to cytokine release in the airways during LPS-induced lung inflammation.
Figure 3.
Macrophage liver kinase B1 (LKB1)–deficient mice show reduced cytokine release into the airways on lipopolysaccharide (LPS) challenge. Cytokine concentrations (tumor necrosis factor [TNF], CXCL1, and interleukin 1β, 10, and 6 [IL-1β, IL-10, and IL-6]) (A); numbers of total cells, alveolar macrophages (AMs) and neutrophils (B); and myeloperoxidase (MPO) concentration (C) measured in bronchoalveolar lavage fluid (BALF) 6 hours after intranasal inoculation with 1 µg LPS. Data are shown as box-and-whisker diagrams representing 8 mice per group. Cytokine and MPO levels of the LysM-cre × Stk11fl/fl mice were compared with those in littermate control (Stk11fl/fl) mice using the Mann-Whitney test. Cell counts were compared using the Student t test with Holm-Sidak correction for multiple testing. *P < .05; †P < .01.
Impact of LKB1 Deficiency on AM Phenotype, Metabolism, and Function
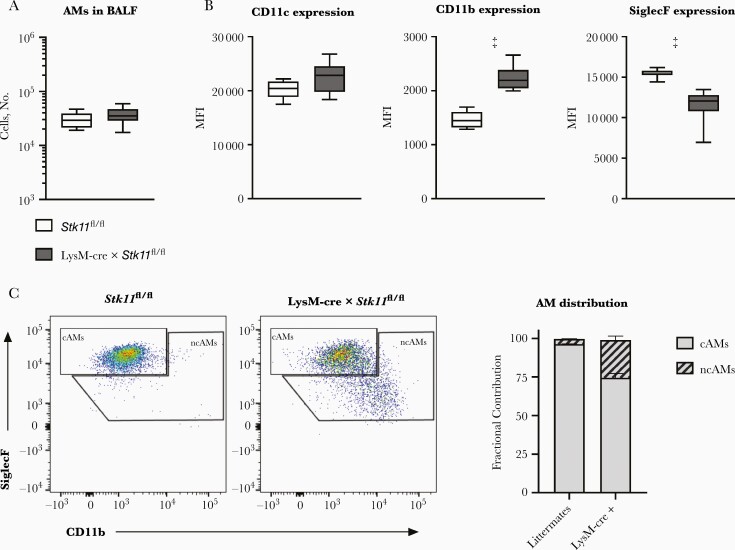
To obtain insight into the impact of LKB1 deficiency on the phenotype of AMs, we performed flow cytometric analysis on macrophages harvested through BAL of uninfected LysM-cre × Stk11fl/fl and control mice. The number of AMs in BALF was equal between groups (Figure 4A). Further analysis showed the (expected) presence of “classic” CD11cposSiglecFhighCD11bneg AMs (cAMs) in BALF of Stk11fl/fl control mice (Figure 4C). Remarkably, LysM-cre × Stk11fl/fl mice had a relatively high proportion of “nonclassic” CD11cposSiglecFlowCD11bpos AMs (ncAMs), which comprised approximately a fourth of the total AM population in these animals (Figure 4B and 4C).
Figure 4.
Macrophage liver kinase B1 (LKB1) deficiency is associated with the presence of “nonclassic” alveolar macrophages (ncAMs). A, Number of all alveolar macrophages (AMs) (CD45+, CD11c+, Ly6G−) in bronchoalveolar lavage fluid (BALF) from naive LysM-cre × Stk11fl/fl mice and littermate controls (Stk11fl/fl). B, CD11c, CD11b, and SiglecF expression of AMs. C, Fractional contribution of “classic” AMs (cAMs) (SiglecFhighCD11bneg) and ncAMs (SiglecFlowCD11bpos) in BALF. Data are shown as box-and-whisker diagrams or as bar graphs showing mean percentage with standard error of the mean for 7–9 mice per group. Cell surface markers and cell numbers of the LysM-cre × Stk11fl/fl mice were compared with those of littermate control (Stk11fl/fl) mice, using the Student t test. ‡P < .001.
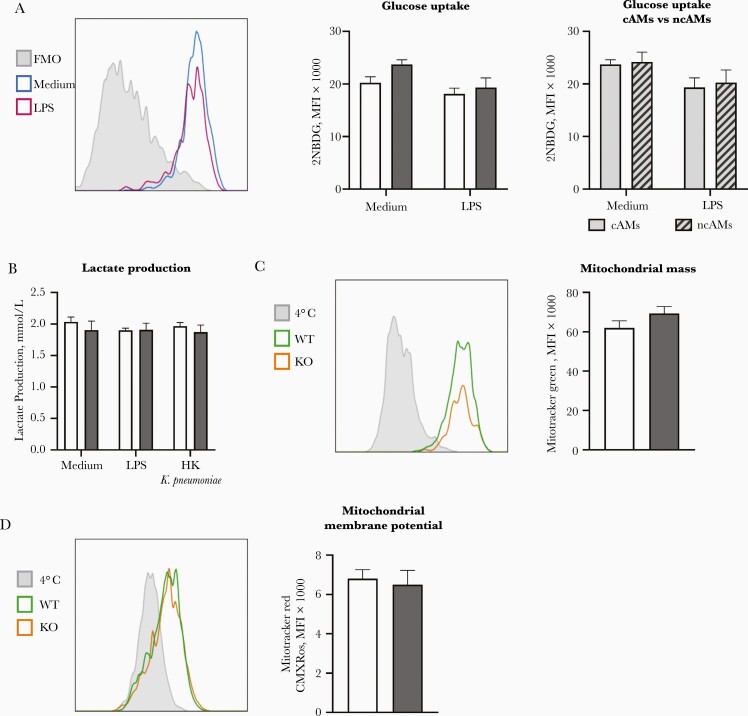
We next determined the effect of LKB1 deficiency on glucose metabolism and mitochondrial mass and membrane potential of AMs. To this end, we analyzed the uptake of 2NBDG, a fluorescent analogue of glucose, by flow cytometry as a measure of glucose uptake. We found no difference in glucose uptake between LKB1-deficient cAMs and control cAMs or between LKB1-deficient cAMs and LKB1-deficient ncAMs (Figure 5A). Lactate production did not differ between in vitro stimulated LKB1-deficient or wild-type AMs, nor was it induced by stimulation with LPS or K. pneumoniae (Figure 5B). Together, these findings suggest that—unlike BMDMs [6, 12, 25]—AMs do not up-regulate glycolysis on stimulation and that this is not altered by LKB1 deficiency. To verify that the mitochondria in AMs are not affected by LKB1 deficiency, we measured mitochondrial mass and mitochondrial membrane potential using flow cytometry. No differences were found between LysM-cre × Stk11fl/fl and control AMs (Figure 5C and 5D).
Figure 5.
Liver kinase B1 (LKB1) deficiency does not affect alveolar macrophage (AM) glucose metabolism nor mitochondrial parameters. A, Glucose uptake after 3 hours of lipopolysaccharide (LPS) stimulation or medium control, as measured by the median fluorescence intensity (MFI) of 2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino]-2-deoxyglucose (2NBDG), of LysM-cre × Stk11fl/fl “classic” AMs (cAMs) and control cAMs and of “nonclassic” AMs (ncAMs) compared with cAMs of LysM-cre × Stk11fl/fl mice. B, Lactate production of AMs stimulated in vitro with LPS or heat-killed (HK) Klebsiella pneumoniae or left untreated for 24 hours. C, Mitchondrial mass as measured by the MFI of Mitotracker Green probe of LysM-cre × Stk11fl/fl cAMs (knockout [KO]) and control cAMs (wild type [WT]) after 3 hours of incubation in medium. D, Mitochondrial membrane potential as measured by the MFI of Mitotracker Red CMX Ros probe of LysM-cre × Stk11fl/fl AMs (KO) and control AMs (WT) after 3 hours of incubation in medium. Data are shown as bar graphs representing mean with standard error of the mean for 7–9 mice per group. Groups were compared using Student t tests. A, C, and D, The left graph is a representative flowjo picture of the right graph. The Y-axis is a modal scale. The x-axis is the fluorescent intensity.
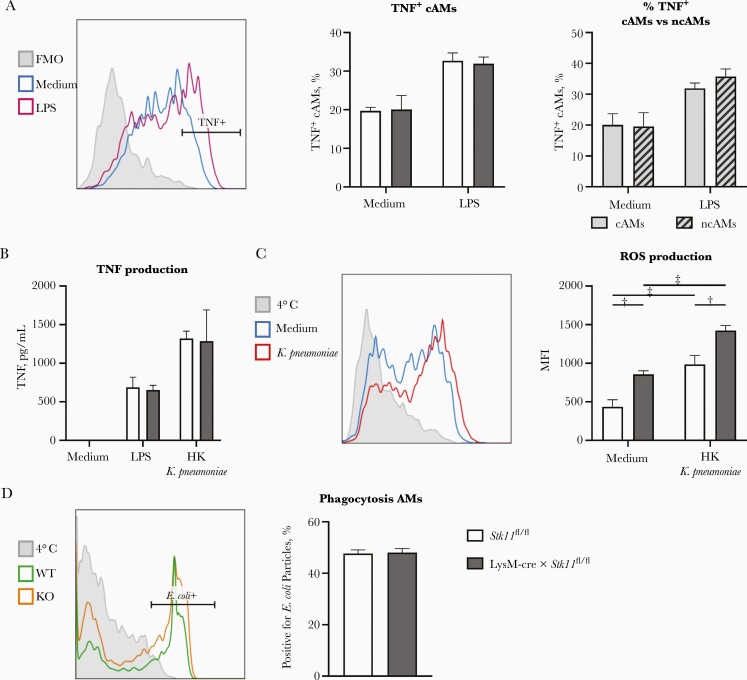
Finally, we assessed the effect of LKB1 deficiency of the functionality of AMs by measuring the capacity to produce TNF and ROS, and to phagocytose. We determined the capacity to produce TNF as the of percentage of TNF-positive cells in the individual AM populations by staining intracellular TNF and analyzing with flow cytometry. We detected no difference between the percentages of TNF-positive cAMs in LysM-cre × Stk11fl/fl and control mice, or between the percentages of TNF-positive cAMs and positive ncAMs (Figure 6A). In addition, AMs obtained via BAL from LysM-cre × Stk11fl/fl mice and littermate controls produced similar amounts of TNF in vitro when stimulated with LPS or heat-killed K. pneumoniae (Figure 6B).
Figure 6.
Liver kinase B1 (LKB1) deficiency does not affect tumor necrosis factor (TNF) production and phagocytosis but enhances reactive oxygen species (ROS) production by alveolar macrophages (AMs). A, Percentage of TNF-positive (TNF+) “classic” AMs (cAMs) from LysM-cre × Stk11fl/fl and control mice and of TNF+ cAMs and “nonclassic” AMs (ncAMs) from LysM-cre × Stk11fl/fl mice after stimulation of lung suspensions for 3 hours with lipopolysaccharide (LPS) or medium control. B, TNF production of AMs stimulated in vitro with LPS or heat-killed (HK) Klebsiella pneumoniae or left untreated for 24 hours. C, ROS production by AMs from LysM-cre × Stk11fl/fl and control mice after stimulation of lung suspensions for 3 hours with HK K. pneumoniae or medium control. D, Percentage of AMs positive for pHrodo Red Escherichia coli particles after a 3-hour incubation. Data are shown as bar graphs representing mean with standard error of the mean for 5–8 mice per group. Median fluorescence intensities (MFIs) and percentage positive cells were compared using Student t tests or 2-way analysis of variance with Sidak multiple comparisons test. A, C, and D, The left graph is a representative flowjo picture of the right graph. The Y-axis is a modal scale. The x-axis is the fluorescent intensity. †P < .01; ‡P < .001. Abbreviation: FMO, fluorescence minus one.
Stimulation with heat-killed K. pneumoniae induced more ROS production relative to medium control; AMs from LysM-cre × Stk11fl/fl mice produced more ROS than AMs of control mice in both stimulated and unstimulated conditions (Figure 6C). The phagocytic capacity of LKB1-deficient and control AMs did not differ (Figure 6D). Together, these data shows that the lungs of LysM-cre × Stk11fl/fl mice contain a significant proportion of SiglecFlow and CD11bpos ncAMs. However, LKB1 deficiency does not affect glucose metabolism or mitochondrial parameters of AMs or negatively affect immune functions.
LKB1 Deficiency Leading to Reduced Lung AMs
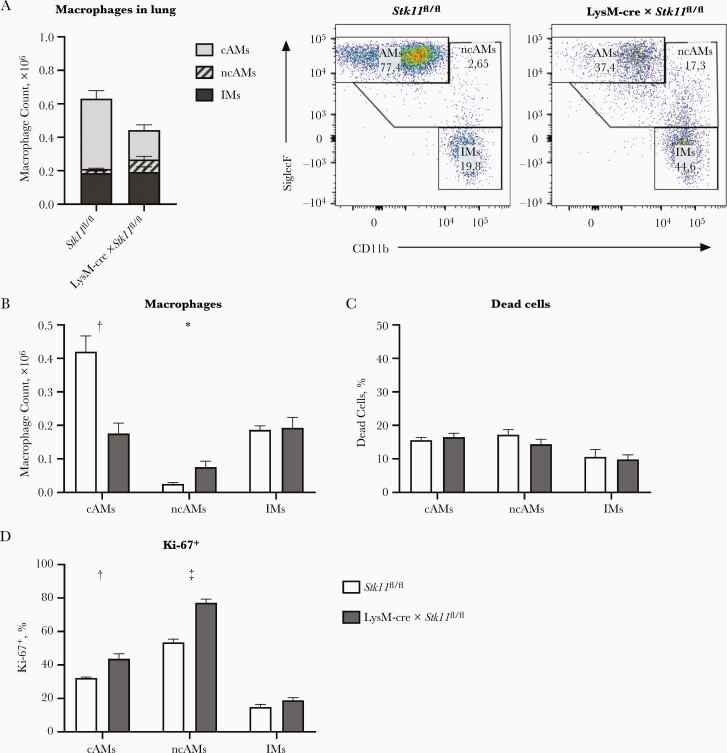
To explain the discrepant results regarding LPS-induced TNF production in the respiratory tract in vivo (reduced in LysM-cre × Stk11fl/fl mice; Figure 3A) and by AMs in vitro (not affected; Figure 6A and 6B), we analyzed immune cell populations in whole lungs by means of flow cytometry, allowing analysis of other immune cells, that is, interstitial macrophages (IMs) and dendritic cells (DCs). First, we determined the number of AMs and IMs in the lungs of LysM-cre × Stk11fl/fl mice and littermate controls. Surprisingly, the total number of macrophages was reduced in the lungs of LysM-cre × Stk11fl/fl mice compared with control mice (Figure 7A), which was caused by a >50% reduction in the number of cAMs (Figure 7B). The number of ncAMs was higher in the lungs of LysM-cre × Stk11fl/fl mice, while IM numbers were similar. The strong reduction in cAMs is surprising, because the numbers of AMs obtained with BAL were similar in LysM-cre × Stk11fl/fl and control mice.
Figure 7.
Liver kinase B1 (LKB1) deficiency leads to a reduced number of alveolar macrophages (AMs) in the lung. A, Macrophage (CD45+, MerTK+, and CD64+) populations in lung suspensions of LysM-cre × Stk11fl/fl and littermate control (Stk11fl/fl) mice: “classic” AMs (cAMs) (SiglecFhigh and CD11bneg), “nonclassic” AMs (ncAMs) (SiglecFlow and CD11bpos), and interstitial macrophages (IMs) (SiglecFneg and CD11bhigh). B–D, Numbers of cAMs, ncAMs, and IMs (B), percentage of dead cells (C), and percentage Ki-67–positive cells (D) in lung suspensions of LysM-cre × Stk11fl/fl mice and littermate controls (Stk11fl/fl). Data are shown as bar graphs representing mean with standard error of the mean for 5 mice per group. LysM-cre × Stk11fl/fl mice were compared with littermate control (Stk11fl/fl) mice using Student t tests. *P < .05; †P < .01; ‡P < .001.
Quantification of the number of cAMs and ncAMs in whole lungs compared with lungs after BAL showed that almost all AMs of LysM-cre × Stk11fl/fl mice were removed after BAL, whereas the lungs of control mice still contained a substantial amount of cAMs after BAL (Supplementary Figure 3A). The number of cAMs removed from the lungs, calculated as the difference in number of cAMs with or without BAL, was similar between LysM-cre × Stk11fl/fl mice and littermate controls (Supplementary Figure 3B). The reduced number of cAMs in the lungs of LysM-cre × Stk11fl/fl mice was not associated with an increased proportion of dead cells; likewise, the proportions of dead ncAMs and IMs were similar in lungs of LysM-cre × Stk11fl/fl and control mice (Figure 7C).
The percentages of proliferating cAMs and ncAMs as measured by Ki-67 positivity were higher in the lungs of LysM-cre × Stk11fl/fl mice compared with control mice; the proportion of Ki-67–positive IMs was low and did not differ between groups (Figure 7D). The high CD11b expression of the ncAMs suggests a hematopoietic origin [26], possibly monocyte derived. We therefore analyzed the number of monocytes in the lung and found that the numbers of monocytes, both Ly6C+ and Ly6C−, were indeed higher in the lungs of LysM-cre × Stk11fl/fl mice (Supplementary Figure 3C). Finally, the number of DCs in the lung, both CD11b+ and CD103+ DCs, was not affected by LKB1 deficiency (Supplementary Figure 3D). Together these data show that the number of cAMs was strongly reduced in the lungs of LysM-cre × Stk11fl/fl mice, which is not explained by an increase in cell death (similar to control values) or a decrease in proliferation (higher than control values).
DISCUSSION
Earlier studies on the role of LKB1 in immune cell function established important contributions of LKB1 to normal hematopoietic stem cell [19, 27] and T-lymphocyte functions [28]. To the best of our knowledge, the potential role of LKB1 in the host response to infection has not been studied thus far. We here used a murine model of gram-negative bacterial pneumonia to show that myeloid-specific deficiency of LKB1 results in enhanced growth of the common human pathogen K. pneumoniae in the lungs. The impaired host defense in mice with myeloid LKB1 deficiency was accompanied by a reduced cytokine response in the airways to locally administered LPS and a diminished number of cAMs in the lungs. AM immune functions, like TNF production and phagocytosis, were not negatively affected by LKB1 deficiency, suggesting that the impaired host defense was mainly attributable to the reduced number of AMs. We also showed that neutrophil LKB1 is not important for the host defense against K. pneumoniae.
The impaired host defense against K. pneumoniae in LysM-cre × Stk11fl/fl mice could be a direct result of the reduced AM numbers in these mice. Previous research documented that depleting AMs [29] or reducing the fitness and number of AMs [30] in mice results in increased bacterial outgrowth and reduced survival after infection with K. pneumoniae. In addition—and these possibilities are not mutually exclusive—decreased TNF production associated with reduced AM numbers could also impair host defense against K. pneumoniae [31, 32]. An earlier investigation reported that LKB1-deficient BMDMs produced more TNF and IL-6 on stimulation with LPS when compared with control cells [2]. However, we found no differences in the AM TNF production capacity between LysM-cre × Stk11fl/fl and control mice, suggesting that the role of LKB1 in TNF production might be macrophage type specific. We consider it likely that the lower TNF, IL-1β, CXCL1, and IL-10 levels in BALF obtained from LysM-cre × Stk11fl/fl mice 6 hours after LPS challenge are related to lower numbers of AMs (the main source of these mediators). BALF IL-6 levels were similar in both mouse strains, possibly because of distinct cellular sources of this cytokine [33, 34].
Although the number of AMs obtained via BAL was equal in naive and LPS-challenged LysM-cre × Stk11fl/fl and control mice, whole lungs of LysM-cre × Stk11fl/fl mice contained much lower numbers of cAMs than lungs of control mice. This discrepancy suggests that LysM-cre × Stk11fl/fl AMs are more easily retrieved by BAL than control AMs, which is supported by the finding that the lungs of control mice still contained a substantial amount of AMs after BAL. Westphalen et al [35] described a subtype of AMs, sessile AMs, which are attached to alveolar epithelial cells via connexin 43 containing gap junction channels. They showed that sessile AMs remain attached to the alveoli, where they perform an immunomodulatory role to reduce LPS-induced lung inflammation. It has become evident that LKB1 plays an important role in cell polarity and junction formation in other tissues [36, 37], possibly via regulation of connexins 40 and 43 [37]. This supports the possibility that LKB1 may contribute to the formation of gap junctions between sessile AMs and the alveolar epithelium and that LKB1 deficiency could result in easily retrievable “loose” AMs.
The influx of ncAMs likely presents a compensatory mechanism for the reduced number of cAMs in LysM-cre × Stk11fl/fl lungs. The high CD11b expression by ncAMs suggests that these cells have migrated to the alveolar space and have a hematopoietic stem cell origin [26], unlike cAMs, which have a prenatal origin [38]. This suggests that ncAMs could be monocyte derived. Indeed, the number of monocytes was higher in the lungs of LysM-cre × Stk11fl/fl mice than in littermate controls.
Although LKB1 deficiency neither affected the viability of macrophages nor hampered their proliferation, the mechanism by which LKB1 deficiency resulted in reduced cAMs still needs to be elucidated. Similar to AMs, regulatory T-cell (Treg)–specific deletion of LKB1 in mice causes loss of Treg numbers and function, leading to a fatal, early-onset autoimmune disorder [39]. This loss of Tregs is largely independent of the AMPK-driven metabolic changes. Furthermore, DC-specific LKB1 depletion has been shown to impair the ability of DCs to induce antigen-specific T cell immunity [40, 41] through a mechanism independent of AMPK-induced metabolic adaptations [41], confirming a role for LKB1 beyond metabolism. Taken together, these data suggest a role for LKB1 in the context of cell development and/or differentiation.
Proinflammatory responses are typically associated with enhanced cellular glycolysis, providing a rapid source of energy. A rise in glycolysis on stimulation with LPS has been reported in different macrophage subtypes, including BMDMs and peritoneal macrophages [25]. In the current study, we demonstrated that AMs do not up-regulate glycolysis on exposure to LPS. This finding is in agreement with those of a study showing that murine AMs do not enhance glycolysis in response to LPS and that inhibition of glycolysis does not modify LPS-induced cytokine production in these cells [42]. These data exemplify the notion that the tissue environment of immune cells may influence the particularities of the metabolic changes within the cell driving inflammatory responses [43, 44].
Our study has limitations. Owing to regulatory restrictions, we could not determine the impact of myeloid LKB1 deficiency on survival during Klebsiella pneumonia; previous studies from our group suggest that the elevated bacterial loads detected in LysM-cre × Stk11fl/fl mice may result in accelerated death [45, 46]. We performed experiments with a single dose of K. pneumoniae, and it thus remains to be established whether the observed phenotype of LysM-cre × Stk11fl/fl mice is also present during pneumonia caused by other infectious doses.
In conclusion, we here report that loss of LKB1 function in macrophages is associated with a reduced number of AMs in the lungs, diminished cytokine release in the airways on local LPS instillation, and an impaired ability to restrict the local growth of K. pneumoniae during pneumonia. While the current investigation is the first to indicate that macrophage LKB1 contributes to antibacterial defense in the lungs, further studies are warranted to assess the role of LKB1 in AM development.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Presented in part: New Frontiers in Innate Immunity and Inflammation, Cluj-Napoca, Romania, 4-7 September 2018.
Acknowledgments. We thank Marieke ten Brink and Joost Daalhuisen for their technical assistance with the mouse models. We thank Regina de Beer for her assistance with the lung histopathology.
Financial support. This work was supported by ZonMw (grant 40-00812-98-14016).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Hemminki A, Markie D, Tomlinson I, et al. A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature 1998; 391:184–7. [DOI] [PubMed] [Google Scholar]
- 2. Liu Z, Zhang W, Zhang M, Zhu H, Moriasi C, Zou MH. Liver kinase B1 suppresses lipopolysaccharide-induced nuclear factor κB (NF-κB) activation in macrophages. J Biol Chem 2015; 290:2312–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gwinn DM, Shackelford DB, Egan DF, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell 2008; 30:214–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shaw RJ, Bardeesy N, Manning BD, et al. The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer Cell 2004; 6:91–9. [DOI] [PubMed] [Google Scholar]
- 5. Bolster DR, Crozier SJ, Kimball SR, Jefferson LS. AMP-activated protein kinase suppresses protein synthesis in rat skeletal muscle through down-regulated mammalian target of rapamycin (mTOR) signaling. J Biol Chem 2002; 277:23977–80. [DOI] [PubMed] [Google Scholar]
- 6. O’Neill LA, Kishton RJ, Rathmell J. A guide to immunometabolism for immunologists. Nat Rev Immunol 2016; 16:553–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rodríguez-Prados JC, Través PG, Cuenca J, et al. Substrate fate in activated macrophages: a comparison between innate, classic, and alternative activation. J Immunol 2010; 185:605–14. [DOI] [PubMed] [Google Scholar]
- 8. Krawczyk CM, Holowka T, Sun J, et al. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood 2010; 115:4742–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Donnelly RP, Loftus RM, Keating SE, et al. mTORC1-dependent metabolic reprogramming is a prerequisite for NK cell effector function. J Immunol 2014; 193:4477–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Michalek RD, Gerriets VA, Jacobs SR, et al. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol 2011; 186:3299–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bettencourt IA, Powell JD. Targeting metabolism as a novel therapeutic approach to autoimmunity, inflammation, and transplantation. J Immunol 2017; 198:999–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mills ELL, Kelly B, Logan A, et al. Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell 2016; 167:457–70.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Quinton LJ, Walkey AJ, Mizgerd JP. Integrative physiology of pneumonia. Physiol Rev 2018; 98:1417–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mehrad B, Clark NM, Zhanel GG, Lynch JP 3rd. Antimicrobial resistance in hospital-acquired gram-negative bacterial infections. Chest 2015; 147:1413–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Song JH, Huh K, Chung DR. Community-acquired pneumonia in the Asia-Pacific region. Semin Respir Crit Care Med 2016; 37:839–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Achouiti A, Vogl T, Urban CF, et al. Myeloid-related protein-14 contributes to protective immunity in gram-negative pneumonia derived sepsis. PLoS Pathog 2012; 8:e1002987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Claushuis TAM, de Vos AF, Nieswandt B, et al. Platelet glycoprotein VI aids in local immunity during pneumonia-derived sepsis caused by gram-negative bacteria. Blood 2018; 131:864–76. [DOI] [PubMed] [Google Scholar]
- 18. Ding C, Scicluna BP, Stroo I, et al. Prekallikrein inhibits innate immune signaling in the lung and impairs host defense during pneumosepsis in mice. J Pathol 2020; 250:95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nakada D, Saunders TL, Morrison SJ. Lkb1 regulates cell cycle and energy metabolism in haematopoietic stem cells. Nature 2010; 468:653–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Clausen BE, Burkhardt C, Reith W, Renkawitz R, Förster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res 1999; 8:265–77. [DOI] [PubMed] [Google Scholar]
- 21. Passegué E, Wagner EF, Weissman IL. JunB deficiency leads to a myeloproliferative disorder arising from hematopoietic stem cells. Cell 2004; 119:431–43. [DOI] [PubMed] [Google Scholar]
- 22. Abram CL, Roberge GL, Hu Y, Lowell CA. Comparative analysis of the efficiency and specificity of myeloid-Cre deleting strains using ROSA-EYFP reporter mice. J Immunol Methods 2014; 408:89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lachmandas E, Boutens L, Ratter JMM, et al. Microbial stimulation of different Toll-like receptor signalling pathways induces diverse metabolic programmes in human monocytes. Nat Microbiol 2016; 2:1–10. [DOI] [PubMed] [Google Scholar]
- 24. Cramer T, Yamanishi Y, Clausen BE, et al. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell 2003; 112:645–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Diskin C, Pålsson-McDermott EM. Metabolic modulation in macrophage effector function. Front Immunol 2018; 9:270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kierdorf K, Prinz M, Geissmann F, Gomez Perdiguero E. Development and function of tissue resident macrophages in mice. Semin Immunol 2015; 27:369–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gurumurthy S, Xie SZ, Alagesan B, et al. The Lkb1 metabolic sensor maintains haematopoietic stem cell survival. Nature 2010; 468:659–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Blagih J, Krawczyk CM, Jones RG. LKB1 and AMPK: central regulators of lymphocyte metabolism and function. Immunol Rev 2012; 249:59–71. [DOI] [PubMed] [Google Scholar]
- 29. Broug-Holub E, Toews GB, van Iwaarden JF, et al. Alveolar macrophages are required for protective pulmonary defenses in murine Klebsiella pneumonia: elimination of alveolar macrophages increases neutrophil recruitment but decreases bacterial clearance and survival. Infect Immun 1997; 65:1139–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moser EK, Field NS, Oliver PM. Aberrant Th2 inflammation drives dysfunction of alveolar macrophages and susceptibility to bacterial pneumonia. Cell Mol Immunol 2018; 15:480–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Laichalk LL, Kunkel SL, Strieter RM, Danforth JM, Bailie MB, Standiford TJ. Tumor necrosis factor mediates lung antibacterial host defense in murine Klebsiella pneumonia. Infect Immun 1996; 64:5211–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Moore TA, Lau HY, Cogen AL, Standiford TJ. Defective innate antibacterial host responses during murine Klebsiella pneumoniae bacteremia: tumor necrosis factor (TNF) receptor 1 deficiency versus therapy with anti-TNF-alpha. Clin Infect Dis 2005; 41(suppl 3):S213–7. [DOI] [PubMed] [Google Scholar]
- 33. Crestani B, Cornillet P, Dehoux M, Rolland C, Guenounou M, Aubier M. Alveolar type II epithelial cells produce interleukin-6 in vitro and in vivo: regulation by alveolar macrophage secretory products. J Clin Invest 1994; 94:731–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Angeli V, Faveeuw C, Delerive P, et al. Schistosoma mansoni induces the synthesis of IL-6 in pulmonary microvascular endothelial cells: role of IL-6 in the control of lung eosinophilia during infection. Eur J Immunol 2001; 31:2751–61. [DOI] [PubMed] [Google Scholar]
- 35. Westphalen K, Gusarova GA, Islam MN, et al. Sessile alveolar macrophages communicate with alveolar epithelium to modulate immunity. Nature 2014; 506:503–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Porat-Shliom N, Tietgens AJ, Van Itallie CM, et al. Liver kinase B1 regulates hepatocellular tight junction distribution and function in vivo. Hepatology 2016; 64:1317–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ozcan C, Battaglia E, Young R, Suzuki G. LKB1 knockout mouse develops spontaneous atrial fibrillation and provides mechanistic insights into human disease process. J Am Heart Assoc 2015; 4:e001733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gomez Perdiguero E, Klapproth K, Schulz C, et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature 2015; 518:547–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. He N, Fan W, Henriquez B, et al. Metabolic control of regulatory T cell (Treg) survival and function by Lkb1. Proc Natl Acad Sci U S A 2017; 114:12542–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chen S, Fang L, Guo W, et al. Control of Treg cell homeostasis and immune equilibrium by Lkb1 in dendritic cells. Nat Commun 2018; 9:5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang Y, Du X, Wei J, et al. LKB1 orchestrates dendritic cell metabolic quiescence and anti-tumor immunity. Cell Res 2019; 29:391–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Woods PS, Kimmig LM, Meliton AY, et al. Tissue resident alveolar macrophages do not rely on glycolysis for LPS-induced inflammation. Am J Respir Cell Mol Biol 2019; 126990:rcmb.2019-0244OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Stienstra R, Netea-Maier RT, Riksen NP, Joosten LAB, Netea MG. Specific and complex reprogramming of cellular metabolism in myeloid cells during innate immune responses. Cell Metab 2017; 26:142–56. [DOI] [PubMed] [Google Scholar]
- 44. Artyomov MN, Sergushichev A, Schilling JD. Integrating immunometabolism and macrophage diversity. Semin Immunol 2016; 28:417–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hommes TJ, Dessing MC, Veer Cv, et al. Role of triggering receptor expressed on myeloid cells-1/3 in Klebsiella-derived pneumosepsis. Am J Respir Cell Mol Biol 2015; 53:647–55. [DOI] [PubMed] [Google Scholar]
- 46. Anas AA, de Vos AF, Hoogendijk AJ, et al. Endoplasmic reticulum chaperone gp96 in macrophages is essential for protective immunity during gram-negative pneumonia. J Pathol 2016; 238:74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.