ABSTRACT
Background
Life‐space mobility (LSM) captures a broad spectrum of mobility in physical and social environments; however, it has not been extensively studied in Parkinson's disease. Using a multiple‐methods approach, individual, social and environmental factors that impact LSM were explored in PD.
Methods
Two hundred twenty‐seven participants with PD (n = 113) and a comparative group without PD (n = 114) were recruited from the community. Within a cross‐sectional survey, LSM (University of Alabama Birmingham Life‐Space Assessment, LSA) was compared in the two groups. Using multiple linear regression, socio‐demographics, lifestyle behaviors, medical, mobility and social factors were examined to identify factors that explained LSM. A qualitative narrative inquiry was completed to augment the findings from the survey; 10 participants with PD were interviewed regarding facilitators and barriers to mobility.
Results
The mean overall LSA‐composite score for the PD group was 64.2 (SD = 25.8) and 70.3 (SD = 23.1) for the community comparative group (mean difference = 6 points, 95%CI:‐0.4, 12.5) indicating most participants moved independently beyond their neighborhoods. A higher proportion of the PD group required assistance with mobility than the community comparison group. Not driving, receiving caregiving, lower social participation, and lower monthly family finances were associated with restricted LSM in the PD group. Data from qualitative interviews supported quantitative findings and offered insights into the features of the built environment that facilitate and restrict mobility.
Conclusion
Individual, social and environmental factors are associated with the LSM among persons with PD. Clinicians and policy‐makers should include both individual and community‐based factors when developing interventions to encourage the LSM of the PD population.
Keywords: Parkinson’s disease, life‐space mobility, social participation, survey, qualitative research
Parkinson's disease (PD) is a movement disorder impacting a person's mobility, function and well‐being. Mobility is a key component to remaining active and maintaining independence in the community. Unlike measures of activities of daily living (ADL) or instrumental activities of daily living (IADL), life‐space mobility (LSM) is the purposeful movement in one's environment over a specified time, and captures a broad spectrum of mobility in physical and social environments, regardless of assistance required or transportation needed. 1 , 2 Life‐space mobility is an emerging concept influenced by individually‐related factors such as cognition and physical capacity, along with social and environmental factors. 3 , 4 , 5 , 6 Limitations of LSM may be an early marker of functional decline. 1 In older adults, measures of LSM are strongly associated with individual factors (executive functioning and motor performance), social and environmental factors. 7 , 8 , 9 , 10 Life‐space mobility has been evaluated in PD 11 and other chronic conditions including cognitive impairment, 6 , 12 , 13 chronic heart failure, 14 respiratory conditions, 15 , 16 spinal cord injury, 17 musculoskeletal/orthopedic conditions, 18 impaired renal function, 19 and palliative care patients. 20 Limited LSM in older adults predicts healthcare utilization, cognitive decline, mortality and lower health status, 21 , 22 , 23 making it an indicator of overall health and resilience in later life. Early work of LSM in PD reported most people reached the highest life‐space level but needed assistive devices. 11 Rantakokko et al. identified restricted LSM with perceived walking difficulties, pain and depressive symptoms but did not include environmental and social factors.
We explored the LSM of people with PD (PwPD) residing in urban settings using a multiple methods approach. The primary objective of the cross‐sectional survey was to identify individual factors (eg, socio‐demographic, function and medical‐related), along with social and transportation factors that explain LSM in PD. We also examined whether these factors differed from an age‐matched community comparative group without PD. A second objective was to explore PwPD's lived experiences of identified barriers and facilitators to LSM that could be targeted by interventions, and policies to promote community mobility of PwPD.
Methods
Participants
A staged multiple‐methods study was conducted in a cohort of PwPD and an age‐matched community cohort, with a concurrent qualitative narrative inquiry of PwPD. Recruitment of eligible (≥6 months diagnosis of PD) PwPD (n = 113) took place between January and November 2019 in Edmonton and Calgary, Alberta, Canada. The PD cohort was recruited from a community‐based PD activity program, the provincial Parkinson's Association, private neurology clinics, and a PD‐specific research participant registry. Adults without PD (n = 114) were recruited from senior's community associations, matching on age within 5‐year age strata to the participants with PD. Inclusion criteria for all participants included: (1) residing in the community, (2) 50 years of age or older, and (3) ability to speak and understand English. Participants completed hard copies of the survey which were then returned either in‐person at the time of completion or by mail. Participants in both groups were permitted to have assistance, such as a caregiver or research assistant, to help them complete the survey. 24 To maintain anonymity of participants, no surveys were completed at the neurology clinics. Ethics approval was obtained from the Health Research Ethics Board, University of Alberta (Pro00086390).
In addition to measuring LSM, socio‐demographics, medical conditions (comorbidities and self‐rated health status), 25 , 26 lifestyle behaviors (smoking and alcohol use), 27 caregiving, social participation, 28 , 29 physical activity 30 and mobility (walking distance and limitations, and transportation) 27 data were collected using standardized questions from national population surveys. 26 , 27 Participants with PD completed the Parkinson's Disease Questionnaire (PDQ)‐8 31 and were asked about disease duration.
For the qualitative narrative inquiry, PwPD from the Edmonton area (Alberta, Canada) were invited to share experiences navigating within their homes and communities. To capture a variety of experiences, individuals with the highest and lowest LSM, alternating male and female participants, were included. Ten participants agreed to participate and were interviewed before data saturation was achieved. In‐person interviews occurred in a setting of the participant's choice (eg, participant's home, café). All interviews were conducted by one person with qualitative research training (CRB). Participants were asked four open‐ended questions about their understanding of mobility and their lived mobility. Probes were used, when necessary but a natural discussion was encouraged. Key discussion points were summarized and their accuracy was confirmed with participants. The generated field notes commented on body language, emotions and responses of participants, and reflected CRB's thoughts regarding the interview and her position within it. Field notes informed data interpretation and code generation.
Standardized Measures
Life‐Space Assessment
The University of Alabama Birmingham Life‐Space Assessment (LSA) is a self‐report measure describing the area in which a person moves purposefully in a specific time period. 1 , 4 It includes 15 items related to mobility in five distinct life‐space areas: bedroom, home, outside the home, neighborhood, outside of town. Participants report their frequency of travel to each life‐space area (1x/week, 2‐3x/week, 4‐6x/week, daily) and mobility devices or help of another person required for a “typical week in the last month.” 1 , 4 A composite score (LSA‐C) is calculated (0–120) where higher values indicate greater mobility. 23 An LSA‐C score of <60 is considered “restricted” 32 , 33 and a five‐point change a minimally important difference. 34 The minimally important difference is the smallest difference in scores that is perceived by patients as beneficial or harmful and is regarded as a change by the clinician. 35 This type of difference is dependent upon the patient population and contextual characteristics. It should be recognized that statistical difference does not imply clinical difference. In addition to an overall score, three LSM indicators are generated from the LSA, representing the frequency of reaching each level of life‐space: (1) independent life‐space (LSA‐I): maximum level achieved without assistance from equipment or another person; (2) life‐space with equipment (LSA‐A): maximum level achieved using equipment but without assistance from another person; and (3) maximum life‐space (LSA‐M): maximum level achieved regardless of assistance required. The LSA is reported to be valid and reliable in populations of community‐dwelling older adults. 1
Social Participation
Respondents were asked about participation frequency in eight social activities over the past 12 months: (1) with family/friends, (2) sports/physical activities, (3) church/religious, (4) educational/cultural, (5) service club, (6) neighborhood/community/professional, (7) other recreational activities, and (8) volunteer/charity work. These social activities have undergone rigorous development and testing, and have been used in nationally representative surveys. 29 , 36 A social participation index (SPI) (range 0–32) indicates the level of social participation based on the frequency of activities, where higher scores indicate more social participation. 28
Parkinson's Disease Questionnaire‐8
Quality of life and experiences living with PD were captured using the Parkinson's Disease Questionnaire‐8 (PDQ‐8). 31 The PDQ‐8 total score ranges from 0 (least impairment) to 100 (worst impairment), based on a scaled response to eight items related to mobility, ADLs, emotional well‐being, stigma, social support, cognition, communication and bodily discomfort.
Statistical Analysis
Quantitative Analysis
Quantitative measures such as the LSA, 34 SPI 28 and PDQ‐8 31 were scored. Students’ t test compared LSA‐C scores between the two groups. Simple linear regressions were performed for the individual contributions of each potential factor on the dependent variable, LSA‐C, with the two groups. Because this was a cross‐sectional survey, an explanatory model rather than a predictive model, was built to identify factors that were associated with LSM. Multivariable linear regression models examined individual (sociodemographic, medical, function, lifestyle behaviors), mobility/transportation (driving status), and social (social participation, caregiving) factors associated with LSM. Variables were collected on clinical relevance and guided by a mobility framework for older adults for inclusion in the regression models. 37 Separate multivariable models identified whether covariates explaining LSM differed by group. In the final multivariable models, all variables with a P‐value ≤0.05 or that were deemed clinically important variables, such as age and sex, were kept in the model regardless of their statistical significance. Analyses were conducted using Stata Version 16 (StataCorp, 2019).
Qualitative Analysis
Interviews were audio‐recorded and transcribed verbatim, using pseudonyms to replace names. Content analysis was conducted on transcript data using the phases of preparation, organizing and reporting to objectively and systematically derive a description of mobility determinants affecting LSM. 38 This analysis was guided by Webber et al.’s comprehensive framework for mobility in older adults, outlining five broad, interrelated categories determining mobility: financial, psychosocial, environmental, physical, and cognitive, each influenced by gender, culture and biography. 37 One author (CRB) selected a subset of transcripts to review, creating an initial codebook, then consulting with other authors (CN, MW, CAJ) to confirm the codebook was representative of transcript substance and emerging themes. The coding scheme was applied to all transcripts and iteratively updated to reflect new topics captured by the remaining transcripts. Data saturation was deemed met when interviews no longer yielded new themes and collecting more data (via additional interviews) would become redundant. This approach has been described by several qualitative researchers, whose perspectives are summarized in Saunders. 39
Results
Participants with PD (n = 113) were younger (mean 71, SD 9.0) than the community comparative group (mean 75, SD 7.6) (mean difference 4, 95% CI 1.7, 6.1) with a greater proportion of males in the PD group (60%) than the comparative group (37%) (P < .001). Both groups were comparable in their monthly family finance and employment status (P > 0.05) (Table 1). Assistive mobility devices were regularly used by 44% of PwPD as compared to 15% of the community comparative group without PD (P < 0.001). Those participants without a driving license included 12% of the PD group and 22% of the community comparative group. Both groups had similar lifestyle behaviors of smoking, alcohol use and physical activity (P > 0.05). The mean number of comorbidities was similar for both groups (PD, mean 2.1, SD 1.6; without PD, mean 2.3, SD 1.5; P = 0.53) with musculoskeletal conditions (49%), cardiovascular conditions (35%) and depression (34%) the most prevalent chronic condition reported by PwPD. Fewer PwPD reported excellent or very good overall health (27.4%) and mental health (31.9%) as compared to the community comparative cohort (overall health 42.0%; mental health 60.2%). The mean duration since diagnosis for PwPD was 8.3 years (SD 6.3, range 0.5–25.4) and the mean PDQ‐8 score was 27.4 (SD 18.5, range 0–78.1).
TABLE 1.
Characteristics of participants
| Parkinson's disease (n = 113) | Without Parkinson's disease (n = 114) | |
|---|---|---|
| Socio‐demographics | ||
| Age, mean (SD) | 71.2 (9.0) | 75.2 (7.6) |
| Gender (male), n (%) | 68 (60.2) | 42 (36.8) |
| Education, n (%) | ||
| Less than secondary | 18 (15.9) | 5 (4.4) |
| Completed postsecondary | 25 (22.1) | 28 (24.6) |
| At least some postsecondary | 70 (62.0) | 80 (70.8) |
| Employment status, n (%) | ||
| Working or volunteer | 7 (6.2) | 6 (5.3) |
| Retired | 97 (85.8) | 104 (91.2) |
| On disability or unemployed | 9 (8.0) | 4 (3.5) |
| Marital status, n (%) | ||
| Married/common law | 94 (83.2) | 58 (50.9) |
| Widowed | 8 (7.1) | 30 (26.3) |
| Single | 4 (3.5) | 10 (8.8) |
| Divorced/separated | 7 (6.2) | 16 (14.0) |
| Monthly family finances, n (%) | ||
| Some money left over | 64 (56.6) | 68 (60.7) |
| Just enough/unable to make ends meet | 28 (30.4) | 29 (23.7) |
| Ever smoked, n (%) | 49 (43.4) | 56 (50.0) |
| Use of alcohol, n (%) | ||
| ≤1x/mo | 65 (58.0) | 56 (53.3) |
| 2‐4x/mo | 23 (20.5) | 27 (25.7) |
| 2‐7x/wk | 24 (21.4) | 22 (21.0) |
| Function | ||
| Assistive walking devices | ||
| None | 63 (55.8) | 94 (85.5) |
| Cane/walking poles | 32 (28.3) | 12 (10.9) |
| Walker | 16 (14.2) | 3 (2.7) |
| Wheelchair | 2 (1.8) | 1 (0.9) |
| Walking distance, n (%) | ||
| Unlimited | 40 (35.4) | 60 (54.1) |
| 6–10 blocks | 23 (20.4) | 10 (9.0) |
| 1–5 blocks | 32 (28.3) | 26 (23.4) |
| <1 block/indoor only | 18 (15.9) | 15 (13.5) |
| Walking limitations, n (%) | ||
| No limitations | 18 (16.2) | 46 (42.2) |
| Pain and/or discomfort | 44 (39.6) | 34 (31.2) |
| Fatigue | 39 (35.1) | 12 (11.0) |
| Balance | 10 (9.0) | 17 (15.6) |
| Self‐reported physical activity, ≥150 min per week | 42 (37.8) | 42 (38.2) |
| Social participation index, mean (SD) | 12.8 (5.7) | 16.8 (6.1) |
| No driver's license, n (%) | 14 (12.4) | 24 (21.8) |
| Receiving formal caregiving, n (%) | 12 (10.7) | 8 (7.1) |
| Receiving informal caregiving, n (%) | 43 (38.4) | 12 (10.7) |
| Health status | ||
| Chronic conditions, n (%) | ||
| Cardiovascular | 35 (32.1) | 39 (34.8) |
| Respiratory | 7 (6.4) | 14 (12.7) |
| Depression | 34 (32.1) | 16 (14.5) |
| Diabetes | 13 (12.0) | 23 (21.1) |
| Musculoskeletal | 49 (45.4) | 54 (48.2) |
| Neurological (PD excluded) | 11 (10.2) | 5 (4.5) |
| Hearing loss | 33 (30.3) | 45 (40.5) |
| Vision impairment | 32 (29.6) | 43 (38.7) |
| Total number of conditions, mean (SD) | 2.1 (1.6) | 2.3 (1.5) |
| Self‐rated overall health, n (%) | ||
| Excellent or very good | 31 (27.4) | 47 (42.0) |
| Good | 45 (39.8) | 50 (44.6) |
| Fair or poor | 37 (32.7) | 15 (13.4) |
| Self‐rated overall mental health, n (%) | ||
| Excellent or very good | 36 (31.9) | 68 (60.2) |
| Good | 47 (41.6) | 36 (31.9) |
| Fair or poor | 30 (26.5) | 9 (8.0) |
Participants with PD were less socially active (mean SPI score 12.8, SD 5.7) than participants with the community comparative group (mean SPI score 16.8, SD 6.1) (mean difference 4.1, 95% CI 2.2, 6.0). Activities with family and/or friends were the most reported activities for both groups. Engagement in volunteer/charity, club/organizational, neighborhood‐related or other recreational activities outside of the home contributed to lower overall participation relative to the community comparative group (Fig. 1).
FIG. 1.
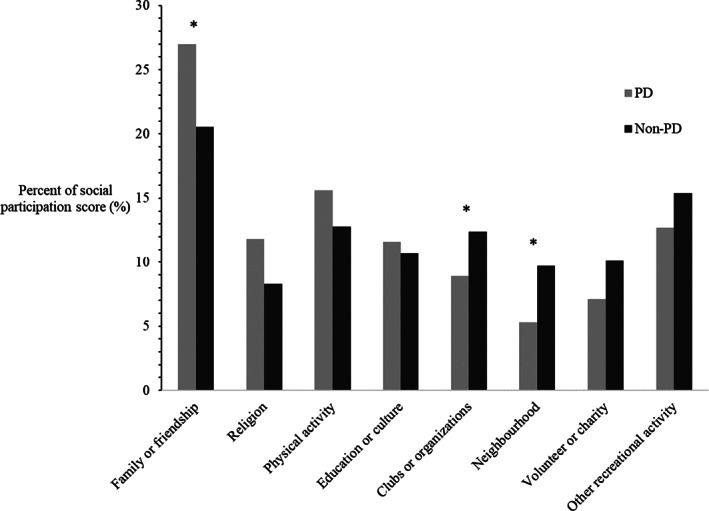
Percentage of each domain of social activity (activities done with other people) contributing to overall social participation among participants with and without Parkinson's disease. *P < 0.05.
The mean overall LSA‐C score was not statistically different between groups (PD mean 64.2, SD 25.8; without PD mean 70.3, SD 23.1) (mean difference 6, 95% CI: −0.36, 12.45), although a clinically meaningful difference (>5 points) was seen between the two groups (Table 2). While all participants reported reaching the lowest level of LSM (bedroom), a smaller proportion of both groups reported being able to travel beyond their “towns” without assistance (PD, n = 56, 50%; community comparative, n = 62, 54%) (Table 2). A higher proportion of the PD group reported attaining the highest level of LSM regardless of assistive devices or personal assistance (65%) as compared to the community comparative group (55%). Interestingly, 83% of the PD group were married and 38% received informal caregiving compared to 51% of the community comparative cohort who were married and 11% received informal caregiving (P < 0.05) (Table 1).
TABLE 2.
Life‐space mobility composite score and life‐space levels reached among participants with and without Parkinson's disease
| Life‐space composite score, mean (SD) | Parkinson's disease (n = 113) | Without‐Parkinson's disease (n = 114) | ||||
|---|---|---|---|---|---|---|
| 64.2 (25.8) | 70.3 (23.1) | |||||
| Life‐space level reached | Independent* | Assisted** | Maximal*** | Independent* | Assisted** | Maximal*** |
| Bedroom, n (%) | 113 (100.0) | 113 (100.0) | 113 (100.0) | 114 (100.0) | 114 (100.0) | 114 (100.0) |
| Home, n (%) | 87 (77.0) | 101 (89.4) | 113 (100.0) | 108 (94.7) | 112 (98.2) | 114 (100.0) |
| Outside home, n (%) | 82 (72.6) | 98 (86.7) | 110 (97.3) | 101 (88.6) | 109 (95.6) | 111 (97.4) |
| Neighborhood, n (%) | 70 (61.9) | 90 (79.6) | 103 (91.2) | 92 (80.7) | 104 (91.2) | 107 (93.9) |
| Within town, n (%) | 73 (64.6) | 95 (84.1) | 109 (96.5) | 97 (85.1) | 108 (94.7) | 110 (96.5) |
| Beyond town, n (%) | 47 (41.6) | 56 (49.6) | 73 (64.6) | 58 (50.9) | 62 (54.4) | 63 (55.3) |
Independent life‐space (LSA‐I) is mobility achieved without the help of an assistive mobility device or another person.
Assisted life‐space (LSA‐A) is mobility achieved with or without the help of an assistive mobility device.
Maximal life‐space (LSA‐M) is mobility achieved by any means, whether that be with or without help from an assistive mobility device or another person.
SD, standard deviation.
Statistically significant variables associated with the LSA‐C with the simple linear regression were considered in the multivariable linear models (Table 3). After statistically adjusting for age and sex for both final multivariable models, receiving caregiving and lower monthly finances were significant factors in explaining lower LSM as measured by the LSA‐C (Table 3). Other variables in the final PD model included having no driver's license (β = −0.40) and less frequent social participation (β = 0.36). For example, those without a driver's license had a 25‐point decrease with LSA‐C score. The variables in this model explained 56.6% of the variation seen with LSA‐C. Not having a driver's license and social participation were not significant factors in the final model for the community comparative group, yet having a respiratory condition (β = −0.23) was reflective of a 15‐point decrease in LSM. The variables in this parsimonious model explained 17% of the variations seen with LSA‐C.
TABLE 3.
Univariate and multivariable analysis examining the association between life‐space mobility composite score
| Covariate (reference) | Parkinson's disease | Without Parkinson's disease | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariable | Univariate | Multivariable | |||||||
| Coeff | 95% CI | Coeff | Standardized Coeff (β) | 95% CI | Coeff | 95% CI | Coeff | Standardized Coeff (β) | 95% CI | |
| Age, yr | −0.8 | −1.3, −0.3 | −0.5 | −0.10 | −0.9, −0.1 | −0.2 | −0.8, 0.4 | −0.3 | −0.14 | −0.9, 0.2 |
| Sex, male | 1.4 | −8.5, 11.2 | −3.8 | −0.17 | −10.7, 3.0 | −1.7 | −10.6, 7.3 | −6.4 | −0.11 | −15.0, 2.3 |
| No driver's license | −34.4 | −44.2, −24.7 | −25.3 | −0.40 | −34.6, −16.4 | −9.5 | −22.6, 3.6 | NS | ||
| Social participation index | 2.2 | 1.4, 2.9 | 1.6 | 0.36 | 1.0, 2.2 | 1.2 | 0.5, 1.9 | NS | ||
| Receiving caregiving | −21.0 | −30.3, −11.9 | −12.7 | −0.24 | −19.7, −5.4 | −19.0 | −29.0, −1.6 | −16.8 | −0.27 | −28.0, −5.6 |
| No extra money in the house | −12.5 | −24.2, −0.8 | −13.4 | −0.22 | −21.8, −5.1 | −18.3 | −28.8, −7.8 | −13.2 | −0.23 | −24.2, −2.2 |
| Respiratory condition | −0.5 | −20.7, 19.7 | NS | −15.3 | −27.8, −2.8 | −15.1 | −0.23 | −27.4, −2.8 | ||
| R square | 56.6% | 17.0% | ||||||||
NS, not significant.
Semi‐Structured Interviews
Interviewees for the qualitative component had a mean age of 68.9 years (SD = 6.0), five were male, six reported using assistive mobility devices, three did not having a driver's license, and two received informal caregiving. Interviewees had a mean LSA‐C score of 64.6 (SD = 21.6) and a mean SPI score of 12.9 (SD = 6.4). The average disease duration was 10.9 years (SD = 8.0) and two had undergone deep brain stimulation surgery.
Mobility Determinant: Physical Health
Four themes related to physical health emerged: “Non‐motor and motor symptoms,” “Experiences with medication,” “Managing symptoms,” and “Ability to participate.” Mobility for most participants was impacted by difficulties with balance, gait, freezing of gait (FOG), incontinence and/or anxiety, which are often unpredictable. (Supplementary Information) PD‐specific medications, assistive devices and physical activity were prominent approaches to managing symptoms. These strategies, however, may further reduce mobility, that is, ON–OFF effects of dopaminergic medications, environmental challenges using assistive devices, or risk of injury during exercise.
Physical symptoms directly the limited activities in which individuals could comfortably partake, and indirectly impacted participation by making getting to the activity a challenge. For example, in response to PD symptoms, many PwPD ceased driving entirely, requiring alternate transportation arrangements. If family and/or friends were unavailable to drive, or if PwPD felt they were being a burden by asking for a ride, an activity may be entirely avoided.
Mobility Determinant: Cognition
“Navigation and dual‐tasking” was a theme relating to cognition as a mobility determinant. Participants noticed cognitive symptoms (memory loss and slower processing speed), affected the ability to navigate to new or familiar destinations. They described changes to concentration affecting ability to dual‐task, particularly when focus was needed for ambulation. Situations requiring split attention, such as simultaneous walking and talking, were challenging.
Mobility Determinant: Environment
Barriers to mobility in the built environment were represented by themes of “Challenging spaces,” specific to PD, and “Accessibility of public spaces,” relevant to anyone experiencing mobility limitations. For PwPD, moving through crowded or confined spaces resulted in instability and FOG episodes.
“If there's too many people around—like the hockey game—sometimes the concourse is really quite crowded… I can't take big steps so I take micro‐steps all over the place and then it's hard to keep your balance when you're doing that. It's just difficult to move around when, when the crowd is that bad.” (P71, male)
Descriptions of difficult experiences with the accessibility of public washrooms were reported. Avoiding locations or events without easily accessible facilities was frequently reported. Poor lighting, stairs, or spaces inappropriate for the use of assistive devices hampered mobility. Washrooms with ample space and a suitable solution for temporarily storing assistive devices were the most desirable. For participants with incontinence, proximity was essential.
Mobility Determinant: Psychosocial
Five psychosocial themes emerged: “Activity avoidance,” “Receiving help from others,” “Planning excursions,” “Setting expectations,” and “Navigating the social environment.” Participants avoided activities where they doubted their self‐confidence (eg, driving), feared falling, or risked embarrassment. Conversely, social networks (family, friends, healthcare professionals and other PwPD) helped meet participation and mobility goals. Notably, participation in PD support groups was crucial to many PwPD for knowledge sharing, encouragement and emotional comfort.
Taking time to plan excursions facilitated successful community trips. Spouses and PwPD coordinated schedules, chose accessible destinations for socializing, and planned safe routes for driving. Psychosocial factors influenced self‐expectation, shaping participants' understanding of what activities could and could not be done safely.
“My anxiety level was so high that I couldn't drive. I did recognize that and it's been that way a couple of times.” (P71, male)
Lastly, some participants found self‐advocacy to be a valuable tool when navigating social environments. Those who openly talked about their health succeeded in having their financial, physical and psychosocial needs met, thus facilitating mobility and participation in the community. Not all participants were able to advocate for themselves in personal relationships successfully and, consequently, had fewer opportunities for participation outside the home.
“I used to be able to run and jog, and kick a football and soccer ball with my grandsons. I don't see them very much. They live ten minutes from here, but they want to play, and I can't—so I don't. And so we're drifting apart.” (P41, male)
Mobility Determinant: Finance
One participant talked about the impact of financial constraints on mobility, specifically when PD symptoms prevented driving and reliance on taxis was needed. This finding was not sufficient to constitute a theme but signals the importance of considering finances as a determinant of mobility.
“I don't want to take the taxi to the [Parkinson's Association]. It depends on [which driver] you get. You either get someone who goes really slow, and it costs you $40 each way, or you can get somebody who's pretty fast and it takes, it's $20 each way and that can be expensive for something you want to do every day.” (P97, female)
Influence of Gender, Culture, and Biography
The theme of “Identity” was present in many interviews. Mobility was discussed as an essential aspect of independence, for which participants closely associated with their identity: the person that they are and wish to continue to be.
Interviewer: “And why is mobility important to you?”
Participant: “Independence. I don't want somebody having to walk with me. I like to walk by myself.” (P78, female)
Discussion
Life‐space mobility of PwPD did not statistically differ from an age‐matched community cohort without PD, but factors explaining LSM differed between the groups. Individual and social factors (driver's license, level of social participation, receiving caregiving and monthly family finances) were associated with LSM for PwPD. Respiratory conditions explained LSM for the community comparative cohort, whereas the status of driving license and social participation were not significant factors. Qualitative interviews offered insights into individual, social and environmental factors that impacted LSM, supporting and broadening the quantitative findings. In particular, physical themes related to driving; psychosocial themes related to participation in the community, and environmental themes related to the accessibility of public spaces, emerged as important mobility factors for PwPD. Collectively, findings from our multiple‐methods research suggest the multidimensional aspects of LSM seen in PwPD support the application of the mobility framework developed by Webber et al. 37 in PD.
In our study, nearly half (44%) of PwPD used assistive mobility devices to reach similar levels of life‐space as their counterparts without PD. The LSM of PwPD was lower in relation to another cross‐sectional study examining LSM in 164 community‐dwelling PwPD living in Sweden. 11 Participants in these two studies were comparable in independent (LSA‐I) and assisted (LSA‐A) life‐space, but our study found 25% fewer participants reached the highest life‐space level with maximal assistance (LSA‐M). Although age and gender/sex were similar in both studies, our cohort was based in urban areas compared to the Swedish cohort. 11 Participants living rurally are more likely to leave their community to access shops, services, healthcare or social gathering places, resulting in additional trips into higher levels of life‐space.
Possessing a valid driver's license emerged as a meaningful facilitator of LSM for PwPD but not those without PD. Driving is intimately linked with autonomy and mobility in North American society and is the preferred means of transportation by Canadians. 40 , 41 It is a complex task balancing individual, social and environmental factors. Older adults who do not drive, or who have reduced driving capacity, have reduced LSM, and this association appears to be exacerbated in people with walking difficulties. 42 , 43 Other studies of older adults have shown driving cessation leads to progressive life‐space restriction, a pattern that may be expected because an individual who is a car passenger requires help from another person (the driver) to reach their destination. 44 , 45 Requiring assistance is a criterion for scoring LSM; therefore, that individual would reflect a lower score, even if their level and frequency of travel stayed the same. 45 Participants who drove often adapted their driving behaviors in response to PD, learning to carefully plan excursions around medication schedules, symptoms and driving conditions. Modifying driving behaviors (ie, avoiding driving in the dark or traffic) was a theme of a previous qualitative study exploring experiences of driving in PwPD. 46 Participants who did not drive acknowledged worrying about burdening loved ones by asking for rides and thus, were not as participatory as they may like to be. 46
Social participation was significantly associated with LSM with the PD cohort; however, the directional relationship between social participation and LSM was undetermined because of the cross‐sectional survey design. In this, and previous studies, PwPD discussed activity avoidance, including social participation, due to concerns about self‐efficacy, feeling embarrassed, and falling. 47 , 48 In addition to intentional activity avoidance, interviewees in our study described missing social activities for reasons beyond their control. The unpredictable nature of symptoms (FOG, tremors) and medications (sudden, unpredictable ON–OFF) affected their ability to engage with family and/or friends. An earlier qualitative study conducted among PwPD at all PD stages found unpredictable symptoms influenced social participation, but restrictions were most profound in participants with severe PD. 49
Interviewees discussed aspects of the environment facilitating or restricting mobility in their community. Crowded and confined spaces were problematic because frequent stopping, starting and changing directions exacerbated motor symptoms and anxiety. Interviewees discussed avoiding or requiring help with activities involving large crowds, such as outdoor festivals. Lamont et al. 50 found that crowded environments were overwhelmingly disliked by PwPD, noting that these circumstances lead to more frequent FOG episodes. The inability to manage walking difficulties can negatively impact an individual's self‐concept and ability to socially participate. 51 Walking outdoors is an activity used for leisure, socializing, and transportation, but not all outdoor spaces provide a safe, enjoyable physical walking environment. In recent quantitative studies, features of the built environment (slope, sidewalk conditions) were associated with changes in gait speed, while perceived neighborhood usability was found to be a determinant of mobility. 52 , 53 This relationship highlights the interplay between individual‐level factors (socioeconomic status, health), and the physical condition of communities, which are shaped by local politics and economics.
This novel study used a multiple‐methods design of a qualitative narrative inquiry to add depth and context to the quantitative findings from surveying a community‐based PD cohort and comparative group without PD. A healthy, age‐matched comparison group allowed observation of differences in the LSM patterns between PwPD and community older adults living in a similar geographical area. Perhaps more importantly, LSM is a multi‐dimensional construct that reflects the type, frequency and independence of movement within and outside of a persons’ community. To this end, LSM provides unique information from traditional measures of mobility used in clinical settings, particularly for those whose ability may be gradually deteriorating, such as with PD. In light of these strengths, several limitations should be noted. Although LSM has been validated in several older adult populations, and adults with chronic health conditions, psychometric properties of the LSA in a PD population have not been well‐established. 54 , 55 , 56 , 57 Recall of mobility over a 4‐week period may be another limitation. A recent comparison of methods for community mobility reported poor convergent validity between LSA and a wireless inertial measurement unit with GPS, suggesting LSA has poor discrimination. 58 Additionally, disease severity of PwPD and cognition of all participants could not be measured in a survey format. Lastly, the cross‐sectional nature of this survey design did not permit us to identify predictive factors of LSM. Further research could examine LSM over time to identify determinants of LSM in PD.
Relative to a community comparative group, this cohort of PwPD did not experience lower LSM; however, they relied more heavily on assistive devices and supportive environments to maintain these comparable levels of mobility. These findings have important implications for clinicians caring for PwPD, as well as their care‐partners. A measure of LSM, as part of the general assessment of mobility in PD, will alert the care team to issues impacting the navigation and planning of trips outside the home as well as potential care‐partner fatigue or burnout. Awareness of these challenges will allow the care team to implement specific strategies to target these challenges. Clinicians and policy‐makers must consider factors beyond the capacity of the individual, such as social and environmental factors, when designing and implementing interventions to support community mobility, with all the attendant benefits for people with PD.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript Preparation: A. Writing of the first draft, B. Review and Critique.
C.R.B.: 1B, 1C, 2A, 2B, 2C, 3A
M.W.: 1A, 1B, 2C, 3B
C.I.J.N.: 1A, 1B, 2A, 2C, 3B
C.A.J.: 1A, 1B, 1C, 2A, 2C, 3B
Disclosures
Ethical Compliance Statement: This study was approved by the Health Ethics Research Board (HREB) at the University of Alberta, Edmonton, Alberta, Canada. (Pro00086390). Written informed consent was obtained by participants for the interviews. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm this work is consistent with those guidelines.
Funding Sources and Conflicts of Interest: C. Ryder‐Burbidge was funded, in part, by a studentship from the Parkinson Association of Alberta. The authors declare no conflicts of interest relevant to this work.
Financial Disclosures for the Previous 12 Months: The authors declare no additional disclosures to report.
Supporting information
Appendix S1. Code tree representing themes and sub‐themes emerging from interviews with 10 individuals with Parkinson's disease and relating to Webber et al. (2010) key determinants of mobility for older adults and including the overarching influence of biography.
Appendix S2. Additional quotations from semi‐structured interviews with participants with PD representing barriers and facilitators to mobility organized by Webber et al. (2010) key determinants of mobility.
Acknowledgments
We thank the participants for their time and effort in participating in this study.
References
- 1. Baker PS, Bodner EV, Allman RM. Measuring life‐space mobility in community‐dwelling older adults. J Am Geriatr Soc 2003;51(11):1610–1614. 10.1046/j.1532-5415.2003.51512.x. [DOI] [PubMed] [Google Scholar]
- 2. Taylor JK, Buchan IE, van der Veer SN. Assessing life‐space mobility for a more holistic view on wellbeing in geriatric research and clinical practice. Aging Clin Exp Res 2019;31(4):439–445. 10.1007/s40520-018-0999-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shumway‐Cook A, Patla A, Stewart A, Ferrucci L, Ciol MA, Guralnik JM. Environmental components of mobility disability in community‐living older persons. J Am Geriatr Soc 2003;51(3):393–398. 10.1046/j.1532-5415.2003.51114.x. [DOI] [PubMed] [Google Scholar]
- 4. Peel C, Baker PS, Roth DL, Brown CJ, Bodner EV, Allman RM. Assessing mobility in older adults: the UAB study of aging life‐space assessment. Phys Ther 2005;85(10):1008–1019. 10.1093/ptj/85.10.1008. [DOI] [PubMed] [Google Scholar]
- 5. Levasseur M, Filiatrault J, Lariviere N, et al. Influence of lifestyle redesign R on health, social participation, leisure, and mobility of older French‐Canadians. Am J Occup Ther 2019;73(5):7305205030p1–7305205030p18. 10.5014/ajot.2019.031732. [DOI] [PubMed] [Google Scholar]
- 6. De Silva NA, Gregory MA, Venkateshan SS, et al. Examining the association between life‐space mobility and cognitive function in older adults: a systematic review. J Aging Res 2019;2019:3923574. 10.1155/2019/3923574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Poranen‐Clark T, von Bonsdorff MB, Rantakokko M, et al. The temporal association between executive function and life‐space mobility in old age. J Gerontol A Biol Sci Med Sci 2018;73(6):835–839. 10.1093/gerona/glx217. [DOI] [PubMed] [Google Scholar]
- 8. Portegijs E, Iwarsson S, Rantakokko M, Viljanen A, Rantanen T. Life‐space mobility assessment in older people in Finland; measurement properties in winter and spring. BMC Res Notes 2014;7(1):323. 10.1186/1756-0500-7-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rantakokko M, Iwarsson S, Portegijs E, Viljanen A, Rantanen T. Associations between environmental characteristics and life‐space mobility in community‐dwelling older people. J Aging Health 2015;27(4):606–621. 10.1177/0898264314555328. [DOI] [PubMed] [Google Scholar]
- 10. Ullrich P, Eckert T, Bongartz M, Werner C, Kiss R, Bauer JM, Hauer K. Life‐space mobility in older persons with cognitive impairment after discharge from geriatric rehabilitation. Arch Gerontol Geriatr 2019;81:192–200. 10.1016/j.archger.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 11. Rantakokko M, Iwarsson S, Slaug B, Nilsson MH. Life‐space mobility in Parkinson's disease: associations with motor and non‐motor symptoms. J Gerontol A Biol Sci Med Sci 2019;74(4):507–512. 10.1093/gerona/gly074. [DOI] [PubMed] [Google Scholar]
- 12. O'Connor ML, Edwards JD, Wadley VG, Crowe M. Changes in mobility among older adults with psychometrically defined mild cognitive impairment. J Gerontol B Psychol Sci Soc Sci 2010;65B(3):306–316. 10.1093/geronb/gbq003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Beland F, Julien D, Bier N, et al. Association between cognitive function and life‐space mobility in older adults: results from the FReLE longitudinal study. BMC Geriatr 2018;18(1):227. 10.1186/s12877-018-0908-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lo AX, Flood KL, Kennedy RE, Bittner V, Sawyer P, Allman RM, Brown CJ. The association between life‐space and health care utilization in older adults with heart failure. J Gerontol A Biol Sci Med Sci 2015;70(11):1442–1447. 10.1093/gerona/glv076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Iyer AS, Wells JM, Bhatt SP, et al. Life‐space mobility and clinical outcomes in COPD. Int J Chron Obstruct Pulmon Dis 2018;13:2731–2738. 10.2147/COPD.S170887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gottlieb ER, Smith EC, Wolfenden LL, Allman RM, Tangpricha V. Life‐space mobility is associated with frequency of hospitalization in adults with cystic fibrosis. Clin Respir J 2011;5(4):245–251. 10.1111/j.1752-699X.2010.00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lanzino D, Sander E, Mansch B, Jones A, Gill M, Hollman J. Life space assessment in spinal cord injury. Top Spinal Cord Inj Rehabil 2016;22(3):173–182. 10.1310/sci2203-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McCrone A, Smith A, Hooper J, Parker RA, Peters A. The life‐space assessment measure of functional mobility has utility in community‐based physical therapist practice in the United Kingdom. Phys Ther 2019;99(12):1719–1731. 10.1093/ptj/pzz131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bowling CB, Muntner P, Sawyer P, Sanders PW, Kutner N, Kennedy R, Allman RM. Community mobility among older adults with reduced kidney function: a study of life‐space. Am J Kidney Dis 2014;63(3):429–436. 10.1053/j.ajkd.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Phillips JL, Lam L, Luckett T, Agar M, Currow D. Is the life space assessment applicable to a palliative care population? Its relationship to measures of performance and quality of life. J Pain Symptom Manage 2014;47(6):1121–1127. [DOI] [PubMed] [Google Scholar]
- 21. Crowe M, Andel R, Wadley VG, Okonkwo OC, Sawyer P, Allman RM. Life‐space and cognitive decline in a community‐based sample of African American and Caucasian older adults. J Gerontol A Biol Sci Med Sci 2008;63(11):1241–1245. 10.1093/gerona/63.11.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rantakokko M, Portegijs E, Viljanen A, Iwarsson S, Kauppinen M, Rantanen T. Changes in life‐space mobility and quality of life among community‐dwelling older people: a 2‐year follow‐up study. Qual Life Res 2016;25(5):1189–1197. 10.1007/s11136-015-1137-x. [DOI] [PubMed] [Google Scholar]
- 23. Kennedy RE, Williams CP, Sawyer P, Lo AX, Connelly K, Nassel A, Brown CJ. Life‐space predicts health care utilization in community‐dwelling older adults. J Aging Health 2019;31(2):280–292. [DOI] [PubMed] [Google Scholar]
- 24. Cavanaugh JT, Crawford K. Life‐space assessment and physical activity scale for the elderly: validity of proxy informant responses. Arch Phys Med Rehabil 2014;95(8):1527–1532. 10.1016/j.apmr.2014.03.027. [DOI] [PubMed] [Google Scholar]
- 25. Bailis DS, Segall A, Chipperfield JG. Two views of self‐rated general health status. Soc Sci Med 2003;56(2):203–217. 10.1016/S0277-9536(02)00020-5. [DOI] [PubMed] [Google Scholar]
- 26. Statistics Canada . Canadian Community Health Survey—Annual Component (CCHS). Ottawa: Statistics Canada; 2021. https://www23.statcan.gc.ca/imdb/p2SV.pl?Function=getSurvey&SDDS=32262021. Accessed June, 2021.
- 27. Canadian Longitudinal Study in Aging . 2018. https://www.clsa-elcv.ca/. Accessed January 15, 2018.
- 28. Harasemiw O, Newall N, Shooshtari S, Mackenzie C, Menec V. From social integration to social isolation: the relationship between social network types and perceived availability of social support in a National Sample of older Canadians. Res Aging 2018;40(8):715–739. [DOI] [PubMed] [Google Scholar]
- 29. Taylor R, Conway L, Calderwood L, et al. Health, Wealth and Lifestyles of the Older Population in England: The 2002 English Longitudinal Study of Ageing Technical Report. London: Institute of Fiscal Studies; 2007. [Google Scholar]
- 30. Milton K, Bull FC, Bauman A. Reliability and validity testing of a single‐item physical activity measure. Br J Sports Med 2011;45(3):203–208. 10.1136/bjsm.2009.068395. [DOI] [PubMed] [Google Scholar]
- 31. Jenkinson C, Fitzpatrick R, Peto V, Greenhall R, Hyman N. The PDQ‐8: development and validation of a short‐form Parkinson's disease questionnaire. Psychol Health 1997;12(6):805–814. 10.1080/08870449708406741. [DOI] [Google Scholar]
- 32. Allman RM, Sawyer P, Roseman JM. The UAB study of aging: background and insights into life‐space mobility among older Americans in rural and urban settings. Aging Health 2006;2(3):417–429. 10.2217/1745509X.2.3.417. [DOI] [Google Scholar]
- 33. Portegijs E, Rantakokko M, Mikkola TM, Viljanen A, Rantanen T. Association between physical performance and sense of autonomy in outdoor activities and life‐space mobility in community‐dwelling older people. J Am Geriatr Soc 2014;62(4):615–621. 10.1111/jgs.12763. [DOI] [PubMed] [Google Scholar]
- 34. Kennedy RE, Almutairi M, Williams CP, Sawyer P, Allman RM, Brown CJ. Determination of the minimal important change in the life‐space assessment. J Am Geriatr Soc 2019;67(3):565–569. 10.1111/jgs.15707. [DOI] [PubMed] [Google Scholar]
- 35. Revicki D, Hays RD, Cella D, Sloan J. Recommended methods for determining responsiveness and minimally important differences for patient‐reported outcomes. J Clin Epidemiol 2008;61(2):102–109. 10.1016/j.jclinepi.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 36. Raina P, Wolfson C, Kirkland S, et al. Cohort profile: the Canadian longitudinal study on aging (CLSA). Int J Epidemiol 2019;48(6):1752–1753. 10.1093/ije/dyz173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Webber SC, Porter MM, Menec VH. Mobility in older adults: a comprehensive framework. Gerontologist 2010;50(4):443–450. 10.1093/geront/gnq013. [DOI] [PubMed] [Google Scholar]
- 38. Elo S, Kyngäs H. The qualitative content analysis process. J Adv Nurs 2008;62(1):107–115. 10.1111/j.1365-2648.2007.04569.x. [DOI] [PubMed] [Google Scholar]
- 39. Saunders B, Sim J, Kingstone T, et al. Saturation in qualitative research: exploring its conceptualization and operationalization. Qual Quant 2018;52(4):1893–1907. 10.1007/s11135-017-0574-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dickerson AE, Molnar LJ, Eby DW, et al. Transportation and aging: a research agenda for advancing safe mobility. Gerontologist 2007;47(5):578–590. 10.1093/geront/47.5.578. [DOI] [PubMed] [Google Scholar]
- 41. Turcotte M. Profile of seniors’ transportation habits. Can Soc Trends 2012;93(2012001):1–16. [Google Scholar]
- 42. Kuspinar A, Verschoor CP, Beauchamp MK, et al. Modifiable factors related to life‐space mobility in community‐dwelling older adults: results from the Canadian longitudinal study on aging. BMC Geriatr 2020;20(1):35. 10.1186/s12877-020-1431-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Viljanen A, Mikkola TM, Rantakokko M, Portegijs E, Rantanen T. The association between transportation and life‐space mobility in community‐dwelling older people with or without walking difficulties. J Aging Health 2016;28(6):1038–1054. 10.1177/0898264315618919. [DOI] [PubMed] [Google Scholar]
- 44. Shah RC, Maitra K, Barnes LL, James BD, Leurgans S, Bennett DA. Relation of driving status to incident life space constriction in community‐dwelling older persons: a prospective cohort study. J Gerontol A Biol Sci Med Sci 2012;67(9):984–989. 10.1093/gerona/gls133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Huisingh C, Levitan EB, Sawyer P, Kennedy R, Brown CJ, McGwin G. Impact of driving cessation on trajectories of life‐space scores among community‐dwelling older adults. J Appl Gerontol 2017;36(12):1433–1452. 10.1177/0733464816630637. [DOI] [PubMed] [Google Scholar]
- 46. Holmes JD, Alvarez L, Johnson AM, et al. Driving with Parkinson's disease: exploring lived experience. Parkinsons Dis 2019;2019:3169679. 10.1155/2019/3169679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jonasson SB, Nilsson MH, Lexell J, Carlsson G. Experiences of fear of falling in persons with Parkinson's disease—a qualitative study. BMC Geriatr 2018;18(1):44. 10.1186/s12877-018-0735-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sjödahl Hammarlund C, Westergren A, Åström I, Edberg AK, Hagell P. The impact of living with Parkinson's disease: balancing within a web of needs and demands. Parkinsons Dis 2018;2018:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Thordardottir B, Nilsson MH, Iwarsson S, Haak M. “You plan, but you never know” – participation among people with different levels of severity of Parkinson's disease. Disabil Rehabil 2014;36(26):2216–2224. 10.3109/09638288.2014.898807. [DOI] [PubMed] [Google Scholar]
- 50. Lamont RM, Morris ME, Woollacott MH, Brauer SG. Community walking in people with Parkinson's disease. Parkinsons Dis 2012;2012:1–8. 10.1155/2012/856237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hammarlund CS, Andersson K, Andersson M, Nilsson MH, Hagell P. The significance of walking from the perspective of people with Parkinson's disease. J Parkinsons Dis 2014;4(4):657–663. 10.3233/JPD-140399. [DOI] [PubMed] [Google Scholar]
- 52. Twardzik E, Duchowny K, Gallagher A, Alexander N, Strasburg D, Colabianchi N, Clarke P. What features of the built environment matter most for mobility? Using wearable sensors to capture real‐time outdoor environment demand on gait performance. Gait Posture 2019;68:437–442. 10.1016/j.gaitpost.2018.12.028. [DOI] [PubMed] [Google Scholar]
- 53. Raggi A, Corso B, De Torres L, et al. Determinants of mobility in populations of older adults: results from a cross‐sectional study in Finland, Poland and Spain. Maturitas 2018;115:84–91. 10.1016/j.maturitas.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 54. Auger C, Demers L, Gélinas I, Routhier F, Jutai J, Guérette C, Deruyter F. Development of a French‐Canadian version of the life‐space assessment (LSA‐F): content validity, reliability and applicability for power mobility device users. Disabil Rehabil 2009;4(1):31–41. 10.1080/17483100802543064. [DOI] [PubMed] [Google Scholar]
- 55. Curcio C‐L, Alvarado BE, Gomez F, Guerra R, Guralnik J, Zunzunegui MV. Life‐space assessment scale to assess mobility: validation in Latin American older women and men. Aging Clin Exp Res 2013;25(5):553–560. 10.1007/s40520-013-0121-y. [DOI] [PubMed] [Google Scholar]
- 56. Ji M, Zhou Y, Liao J, Feng F. Pilot study on the Chinese version of the life space assessment among community‐dwelling elderly. Arch Gerontol Geriatr 2015;61(2):301–306. 10.1016/j.archger.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 57. Kammerlind A‐SC, Fristedt S, Ernsth Bravell M, Fransson EI. Test–retest reliability of the Swedish version of the life‐space assessment questionnaire among community‐dwelling older adults. Clin Rehabil 2014;28(8):817–823. 10.1177/0269215514522134. [DOI] [PubMed] [Google Scholar]
- 58. Zhu L, Duval C, Boissy P, Montero‐Odasso M, Zou G, Jog M, Speechley M. Comparing GPS‐based community mobility measures with self‐report assessments in older adults with Parkinson's disease. J Gerontol A Biol Sci Med Sci 2020;75(12):2361–2370. 10.1093/gerona/glaa012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Code tree representing themes and sub‐themes emerging from interviews with 10 individuals with Parkinson's disease and relating to Webber et al. (2010) key determinants of mobility for older adults and including the overarching influence of biography.
Appendix S2. Additional quotations from semi‐structured interviews with participants with PD representing barriers and facilitators to mobility organized by Webber et al. (2010) key determinants of mobility.


