Abstract
The objective of this study was to conduct a prospective population pharmacokinetic and pharmacodynamic evaluation of lumefantrine during blinded comparisons of artemether-lumefantrine treatment regimens in uncomplicated multidrug-resistant falciparum malaria. Three combination regimens containing an average adult lumefantrine dose of 1,920 mg over 3 days (four doses) (regimen A) or 2,780 mg over 3 or 5 days (six doses) (regimen B or C, respectively) were given to 266 Thai patients. Detailed observations were obtained for 51 hospitalized adults, and sparse data were collected for 215 patients of all ages in a community setting. The population absorption half-life of lumefantrine was 4.5 h. The model-based median (5th and 95th percentiles) peak plasma lumefantrine concentrations were 6.2 (0.25 and 14.8) μg/ml after regimen A, 9.0 (1.1 and 19.8) μg/ml after regimen B, and 8 (1.4 and 17.4) μg/ml after regimen C. During acute malaria, there was marked variability in the fraction of drug absorbed by patients (coefficient of variation, 150%). The fraction increased considerably and variability fell with clinical recovery, largely because food intake was resumed; taking a normal meal close to drug administration increased oral bioavailability by 108% (90% confidence interval, 64 to 164) (P, 0.0001). The higher-dose regimens (B and C) gave 60 and 100% higher areas under the concentration-time curves (AUC), respectively, and thus longer durations for which plasma lumefantrine concentrations exceeded the putative in vivo MIC of 280 μg/ml (median for regimen B, 252 h; that for regimen C, 298 h; that for regimen A, 204 h [P, 0.0001]) and higher cure rates. Lumefantrine oral bioavailability is very dependent on food and is consequently poor in acute malaria but improves markedly with recovery. The high cure rates with the two six-dose regimens resulted from increased AUC and increased time at which lumefantrine concentrations were above the in vivo MIC.
The combination of the artemisinin derivative artemether and lumefantrine (previously called benflumetol) in a 1:6 ratio is a new, effective, and well-tolerated antimalarial therapy (12). Trials, first in China (where the drugs were discovered) and subsequently in Asia and Africa, have shown that this combination is active even against multidrug-resistant falciparum malaria and is associated with no serious toxicity. Lumefantrine was synthesized originally by the Academy of Military Medical Sciences in Beijing, China. It is a racemic fluorene derivative with the chemical name 2-dibutylamino-1-[2,7-dichloro-9-(4-chlorobenzylidene)-9H-fluoren-4-yl]-ethanol. It conforms structurally, physicochemically, and in mode of action to the aryl amino alcohol group of antimalarial agents including quinine, mefloquine, and halofantrine. Preliminary studies of the pharmacokinetic properties of lumefantrine are reminiscent of those of halofantrine (7), with variable oral bioavailability (augmented considerably by fats), a large apparent volume of distribution, and a terminal elimination half-life for malaria estimated initially at approximately 4 to 5 days (3). A four-dose regimen of artemether-lumefantrine has proved highly effective in studies conducted in Africa, India, and China, but in Thailand, which harbors the most drug-resistant Plasmodium falciparum in the world, cure rates were inferior to those seen with the 3-day artesunate-mefloquine combination (12). Previous studies had suggested that the area under the plasma lumefantrine concentration-time curve was the principal determinant of cure, and it was predicted that an increase in dose should improve efficacy (3). These earlier studies also suggested an increase in oral bioavailability with time but were confined to only four dose schedules.
Blind dose optimization trials to test these predictions were therefore conducted; patients with acute uncomplicated multidrug-resistant falciparum malaria were randomized to receive one of two six-dose regimens of artemether-lumefantrine or the conventional four-dose regimen (14). This study combined a conventional inpatient pharmacokinetic study of adults with a population-based community study of patients of all ages and in whom recently validated capillary blood sampling was used (13). The objective of the pharmacokinetic investigation was to characterize the factors which affect blood lumefantrine concentrations and thus the therapeutic response. The higher-dose regimens were designed to provide more sustained blood lumefantrine levels and thereby improve cure rates in patients receiving six-dose schedules.
MATERIALS AND METHODS
This study took place between September 1996 and February 1997 in two locations: the Hospital for Tropical Diseases in Bangkok, Thailand, and the malaria research facility at Mae La, a camp for displaced persons of the Karen ethnic minority located on the western border of Thailand. Patients were recruited for the study if they had acute symptomatic uncomplicated falciparum malaria, were more than 2 years old (in Bangkok, only adults were recruited), and had received no artemisinin derivatives within the previous 7 days. Pregnant women and patients with signs of severe malaria were excluded. All patients or their attendant guardians or relatives gave fully informed consent. The clinical results of this study will be published elsewhere. This study was approved by the Ethical and Scientific Committees of the Faculty of Tropical Medicine, Mahidol University, and the Karen Refugee Committee.
Procedures.
Patients were enrolled after a thin or thick blood smear showed asexual forms of P. falciparum. On enrollment, a full clinical examination was performed and a blood sample was taken for hematocrit and quantitative parasite count determinations. Artemether-lumefantrine was dispensed as a fixed-dose combination tablet as described previously (12). Each tablet contained 20 mg of artemether and 120 mg of lumefantrine. The minimum dosage for patients weighing less than 15 kg was one tablet per dose; for adults weighing more than 35 kg, four tablets per dose were given.
This study was a double-blind comparison of three dose regimens, A, B, and C. Regimen A was a four-dose regimen given at 0, 8, 24, and 48 h, regimen B was a six-dose regimen given over 3 days at 0, 8, 24, 36, 48, and 60 h, and regimen C was another six-dose regimen given over 5 days at 0, 8, 24, 48, 72, and 96 h. The exact time for each dose was recorded on the case report form. Drug administration was observed in all cases. If the first dose was vomited during the first hour after intake, the whole dose was repeated. All patients received either active drug or placebo such that the tablet numbers were the same and neither the patient nor the investigators were aware of the randomization. Hospitalized patients (Bangkok) received the drugs at times that were documented precisely; therefore, the approximate intervals between drug administration and food intake were known. Community-based patients (Mae La) were asked about meals, so the time interval and the caloric content estimates were less precise. Meal times were not altered prospectively for this study. Patients were seen daily for recording of temperature, subjective adverse effects, and a neurologic examination. All patients were seen daily for 5 days and were then seen on days 7, 14, 21, 28, 35, 42, 49, 56, and 63.
Recrudescent infections were documented as infections which recurred within the period of observation. In order to distinguish recrudescent from newly acquired infections, the parasite genotypes of the admission and recrudescent isolates were compared using a validated PCR method as described previously (1). Recrudescence was defined as a pair of identical genotypes.
Pharmacokinetics.
In Bangkok, samples of venous blood (7 ml) were obtained. Blood was withdrawn by venipuncture into heparinized tubes and centrifuged without delay at 1,000 × g for 15 min. The plasma was transferred immediately into polypropylene tubes and stored at −70°C until shipment to Basel. In order to characterize the lumefantrine concentration profile accurately for the different regimens while maintaining the blind aspect of the study, each treatment was dispensed with a predefined printed plasma sampling schedule as follows: regimen A—baseline and 4, 8, 24, 28, 32, 44, 48, 60, 72, 80, 120, 168, and 240 h; regimen B—baseline and 8, 24, 36, 44, 48, 60, 64, 72, 96, 108, 120, 168, and 240 h; and regimen C—baseline and 8, 24, 32, 48, 52, 64, 72, 80, 96, 108, 120, 168, and 240 h. Deviations from the above sampling times of ±1 h were allowed. Persons taking the blood samples were not involved in patient management.
At Mae La, only five items of data were collected; up to four capillary blood samples were taken from all patients on days 4, 5, 6, 7, and 8 for the evaluation of lumefantrine concentrations. Additionally, from a subset of 26 patients, 91 pairs of capillary and venous blood samples were collected to test for differences in concentration due to the method of blood sampling (13). Venous blood samples of 4 ml each were withdrawn by venipuncture into heparinized tubes and centrifuged without delay at 1,000 × g for 15 min. The plasma was transferred immediately into polypropylene tubes and stored at −70°C until shipment.
Plasma lumefantrine assay.
Lumefantrine in plasma was measured by a high-pressure liquid chromatography method with UV detection (19). The following minor modifications in the procedure were made: the extracts were centrifuged prior to injection, and the UV wavelength was fixed at 335 nm instead of 215 nm. For plasma, interassay coefficients of variation (CV) for prepared plasma samples at lumefantrine concentrations of 57, 83, 138, 220, 608, 1,223, 2,000, and 2,340 ng/ml were 21.1, 10.3, 3.2, 7.2, 4.8, 4.6, 4, and 3.7%, respectively. The lower limit of quantification was therefore set to 57 ng/ml of blood sample. For capillary measurements, interassay CV at concentrations of 35, 195, 578, and 1,185 ng/ml were 19, 16, 10, and 9%, respectively. The lower limit of quantification was therefore set to 35 ng/ml.
Pharmacokinetic modeling.
As sampling schedules differed and, in the community study, only sparse data were available, a population approach to pharmacokinetic modeling was taken. This approach helped to characterize the pharmacokinetics for patients who were only sparsely sampled and, more importantly, to explore pharmacokinetic relationships with the therapeutic response.
Pharmacokinetic modeling was carried out using NLME, a nonlinear mixed-effect algorithm, part of the statistical package S-plus (Mathsoft, version 3.4 for Unix supplement; Data Analysis Products Division, Mathsoft, Seattle, Wash.). This function uses the same estimation methodology (FOCE) as the widely used NONMEM system (Mathsoft) and also provides individual parameter estimates similar to the so-called post hoc estimates (3, 6; Mathsoft). Characterization of the pharmacokinetics of lumefantrine was carried out as follows. A two-compartment model (4) was fitted to the venous concentration data provided from patients at the Bangkok hospital. The model was refitted and modified by adding the less frequently sampled capillary concentration data provided from patients at the Mae La clinic. Pooling of capillary and venous blood sample data was considered appropriate, as there is no significant difference between capillary lumefantrine and venous lumefantrine concentrations (13).
The effects of the demographic and clinical characteristics and the measures of disease status (e.g., parasite count at baseline) on the pharmacokinetic parameters of the fitted model were then explored. A lag time of 2 h between oral administration and the onset of absorption was assumed in the estimation of the rate of absorption of lumefantrine (3). This estimate of lag time was obtained from previous studies of acute malaria which provided more frequent concentration data during the absorption phase (3). The sampling schedule was biased toward the characterization of area under the curve (AUC) rather than absorption rate. Plasma concentrations were modeled as Cj(t) = Cpred,j(t) + εj(t), where Cj(t) and Cpred,j(t) are the measured and predicted concentrations for the jth patient at time t, respectively. The intrapatient variability in plasma drug concentrations, including measurement and assay error εj(t), was assumed to come from a normal distribution (with a mean of 0 and a variance of ςε2). The interpatient variability in the pharmacokinetic parameters was modeled as a proportionality term. For instance, clearance (CL) for the jth patient was defined as CLj = CL × exp(ηj,CL). CL, as a fixed effect, is the population median estimate, and exp(ηj,CL) is the deviation from the population of CL for the jth patient. Random effects ηj,CL were assumed to come from a normal distribution (with a mean of 0 and a variance of ςCL2). The interpatient CV in CL is thus approximated by the estimate of ςCL. Patient characteristics, e.g., body weight (BWT), as a fixed effect, were incorporated in the model for CL as CLj = [CL + θ (BWT)] × exp(ηj,CL), where θ is the coefficient of BWT. Observed BWT and the other continuous covariates were centered by their median values so that the population estimates would represent those of an average patient. The interpatient variability (η) values for the different parameters were assumed to be independent of each other.
Since volume of distribution of the central compartment (V) and the bioavailability fraction (F) cannot be estimated simultaneously, F1 (fraction of the first dose absorbed) was fixed at one to allow estimation of the relative volume (V/f). The F values of the subsequent doses (F2, … , Fn), where n = 8, could then be estimated relative to F1 in order to estimate changes in bioavailability over time, assuming a constant V. F1, F2, F3, and F5 are common for all dose regimens. F4 and F6 are specific for regimen B, and F7 and F8 are specific for regimen C. Under this parameter specification, the estimated interpatient variability in F1 is the combined variabilities in F1 and in V. The terminal slope, β, and the rate constant for transfer from the peripheral compartment to the central compartment, K21, did not vary significantly between patients; therefore, their variance terms, ςβ2 and ςK212, respectively, were set at zero.
The statistical significance of subject demographics and disease status (i.e., parasite density) were first examined by plotting the η values of each pharmacokinetic parameter against each covariate. If a pattern was suspected, then the covariate was included in the pharmacokinetic model and tested formally using the likelihood ratio test. A new model was preferred over another if the change in −2 log likelihood was greater than χα;υ2, where α (=0.05) is the significance level and υ is the number of additional parameters in the new model. Models with different covariance structures, e.g., having different numbers of random effects, were compared using Akaike's information criterion. A model having a smaller value for Akaike's information criterion was considered better. Distribution assumptions were examined for validity using normality tests and graphic methods. Model goodness of fit was assessed using analysis of residuals.
Data on food intake from 51 patients at the Bangkok hospital were analyzed to assess the influence of food on the pharmacokinetics of lumefantrine. The type of food intake was recorded prospectively as either none, liquids only (zero fat), light meal (500 calories, 10 g of fat), or normal meal (estimate of 1,000 calories, 20 g of fat). The exact time of food intake was not recorded, but information was available on the size and content and whether the meal or drink was taken before dosing. Using the estimated F1 to F8, a linear mixed-effect model was fitted using the procedure MIXED of the SAS statistical package (SAS Institute Inc., Cary, N.C.). The vector for F1 to F8 was used as the response variable, the type of food was used as the main fixed effect, and patient identification number was used as a random effect.
EKG correlates.
For the 51 adult patients in Bangkok, EKG recordings were taken prior to treatment and at 3, 4, 5, 8, and 29 days after treatment. Standard EKG intervals were recorded automatically at a paper speed of 50 mm/s, with manual checking of machine estimates (14a). The Q-T interval was corrected (QTc) using Bazett's formula: QTc = QT/RR0.5. Repeated QTc measures as the response variables were evaluated using a linear mixed-effect model. The effects of age, gender, BWT, initial parasite count, baseline QTc, heart rate, day of EKG recording, and log lumefantrine concentration were considered fixed, while patient effect was considered random. Three models (I, II, and III) were compared. They all included the variables QTc at baseline, heart rate, age, and center. However, in addition, model I included the variable day of EKG recording, model II included the variable plasma lumefantrine concentration, and model III included both variables. Variables on the continuous scale were centered by their median values before analysis. A total of 238 observations were available for evaluation. QTc data on day 4 were also evaluated using a fixed-effect model (without patient effect) with model-based estimated lumefantrine concentration or log AUC (from baseline until the zero time of the EKG recording) as the main independent variable. This procedure was done to take into account any possible cumulative effect of changes in plasma drug concentrations and pharmacodynamic effect or any asynchrony between them. These data are included in a larger series examining possible cardiac effects of lumefantrine to be reported elsewhere (van Vugt et al., Am. J. Trop. Med. Hyg., in press).
RESULTS
Clinical response.
In total, 266 patients were recruited for the pharmacokinetic studies (51 adults in Bangkok and 215 patients of all ages in Mae La). There were 15 children ranging in age from 6 to 12 years. Full clinical and laboratory details and the therapeutic response are presented in detail elsewhere (14). All patients made an uncomplicated recovery from their malaria infection. Treatment with artemether-lumefantrine was very well tolerated, and there were no serious drug-attributable adverse effects. The median (25th to 75th percentile) parasite clearance times were the same in each of the three treatment groups: 44 h (26 to 53 h). The median fever clearance times were 37 (27 to 55) h in Bangkok and 21 (20 to 43) h in Mae La (P, <0.001) but were not significantly different among the three treatment groups. Thus, the acute responses to treatment were very similar in the three treatment groups. Overall cure rates, adjusted for reinfection by PCR parasite genotyping and assessed at 28 days, were 83% in the four-dose regimen and 97 and 99% in the two high-dose regimens (P, <0.01).
Dosing.
Dosing in Bangkok was done according to a fixed schedule, i.e., ideally at 0, 8, 24, 36, 48, 60, 72, and 96 h from the start of treatment, but this schedule was adapted to avoid disrupting nursing schedules. Median clock times were 14, 19, 14, 04, 14, 04, 14, and 14 h, respectively. Median dosing times from the start of treatment in Mae La were 0, 8, 20, 27, 44, 51, 68, and 92 h. Median clock times were 13, 21, 09, 16, 09, 16, 09, and 09 h, respectively. Exact actual dosing times were noted.
Pharmacokinetics.
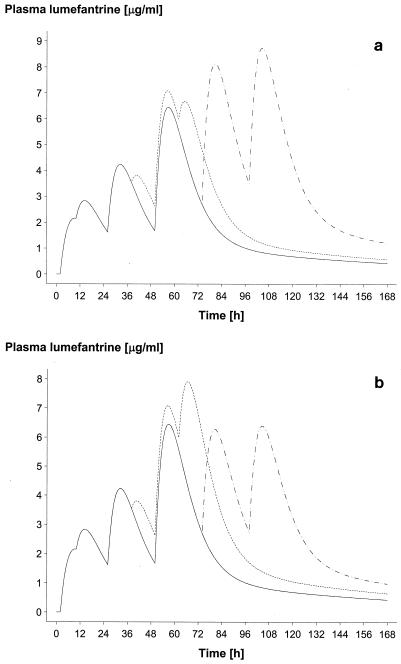
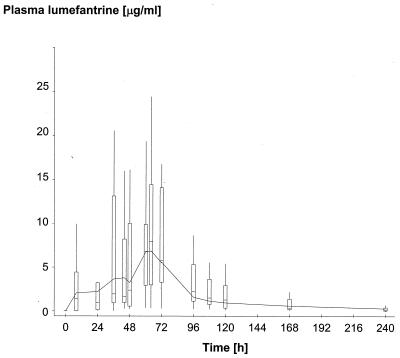
Approximately 14 venous samples per patient were obtained from 51 patients in Bangkok (16, 18, and 17 in regimens A, B, and C, respectively), giving a total of 655 samples (excluding baseline samples). At Mae La, approximately four capillary samples per patient were obtained from 215 patients (74, 72, and 69 in regimens A, B, and C, respectively), giving a total of 793 samples. The capillary samples were collected at approximately 69 h (n = 212), 93 h (n = 209), 116 h (n = 104), 140 h (n = 91), and 165 h (n = 170) and at other unscheduled times (n = 7). The final pharmacokinetic model selected is shown in Table 1; this is a two-compartment open model with first-order absorption and with elimination from the central compartment. Figure 1 shows two plots of the model-based median population profile for the three dose regimens at Bangkok and Mae La. To demonstrate goodness of fit, Fig. 2 shows a plot of the model (median) profile for regimen B at Bangkok, superimposed on a box plot (minimum, lower quartile, median, upper quartile, and maximum) of the observed lumefantrine concentrations. Residual plots (not provided) indicated that there was no bias in estimation and that patient-specific profiles were characterized adequately (data not shown).
TABLE 1.
Lumefantrine parameter estimates of the pharmacokinetic model derived from 266 Thai patients with acute falciparum malaria
| Parameter | Estimatea | SE | CV (%) |
|---|---|---|---|
| Ka (h−1) | 0.17 | 0.024 | 72 |
| α (h−1) | 0.114 | 0.012 | 40 |
| β (h−1) | 0.009 | 0.0013 | Not significantb |
| K21 (h−1) | 0.015 | 0.002 | Not significantb |
| V (liters) | 103 | 13 | Confounded with F1 |
| F1 | 1.0 | Fixed parameter | 149 |
| F2–F1 | −0.49 | 0.11 | 149 |
| F3–F1 | 0.49 | 0.11 | 94 |
| F4–F1 | −0.49 | 0.11 | 102 |
| F5–F1 | 1.54 | 0.31 | 53 |
| F6–F1 | 56 | ||
| Mae La | 0.68 | 0.34 | |
| Bangkok | 0.1 | 0.24 | |
| F7–F1 | 51 | ||
| Mae La | 1.1 | 0.28 | |
| Bangkok | 2.0 | 0.43 | |
| F8–F1 | 56 | ||
| Mae La | 1.1 | 0.28 | |
| Bangkok | 2.0 | 0.43 | |
| Lumefantrine assay error (μg/ml) | 0.69 |
Median estimates of fixed effects.
Random effects were not significantly different from zero.
FIG. 1.
Model-based median population profile of plasma lumefantrine concentrations for regimens A (four doses of four tablets) (solid line), B (six doses of four tablets, 60 h) (dotted line), and C (six doses of four tablets, 96 h) (dashed line). Four tablets equalled 80 mg of artemether and 480 mg of lumefantrine. (a) Bangkok (16, 18, and 17 patients for regimens A, B, and C, respectively). (b) Mae La (74, 72, and 69 patients for regimens A, B, and C, respectively).
FIG. 2.
Model-based median population profile of plasma lumefantrine concentrations for regimen B (six doses of four tablets, 60 h), superimposed on a box plot (minimum, lower quartile, median, upper quartile, and maximum) of the observed plasma drug concentrations derived from frequent sampling (dense data) in 18 patients to illustrate goodness of fit.
The population absorption half-life of lumefantrine was 4.5 h (90% confidence interval [CI], 3.5 to 4.9). Peak plasma lumefantrine concentrations varied considerably. The model-based median (lower and upper 5th percentiles) maximum plasma drug concentrations for the three dose regimens were 6.2 μg/ml (0.25 and 14.8) for regimen A, 9.0 μg/ml (1.1 and 19.8) for regimen B, and 8.0 μg/ml (1.4 and 17.4) for regimen C. The corresponding median (lower and upper 5th percentiles) times to maximum plasma drug concentrations were estimated to be 50 h (29 and 66), 54 h (41 and 66), and 53 h (26 and 114) following the first dose. The estimated distribution (α) half-life was 6.1 h (90% CI, 5.2 to 7.4), and the terminal (β) elimination half-life was 3.2 days (90% CI, 2.6 to 4.1). The interpatient variability in first-order absorption rate constant (Ka,), CL, and F values in the acute phase of the disease was high. The variability in F declined with subsequent doses, such that the CV for F1 was 150% while those for F6, F7, and F8 were about 50%. Compared to F1, F2 (8 h) and F4, (36 h) fell by 50%. Apparent bioavailability increased at other doses, rising by 50% for F3 (24 h) and by 154% for F5 (48 h). F6 (60 h), F7 (72 h), and F8 (96 h) were found to differ between the two centers. F6 was similar to F1 in Bangkok, but it was 68% higher in Mae La. Compared to F1, F7 and F8 were 110% higher in Mae La and 200% higher in Bangkok. Because the F values for each patient changed considerably over time, accurate individual and thus population estimates of oral CL and V could not be obtained.
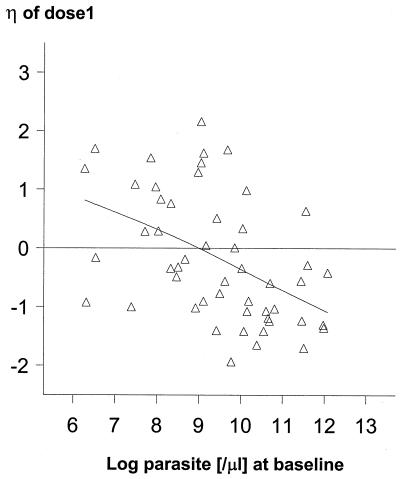
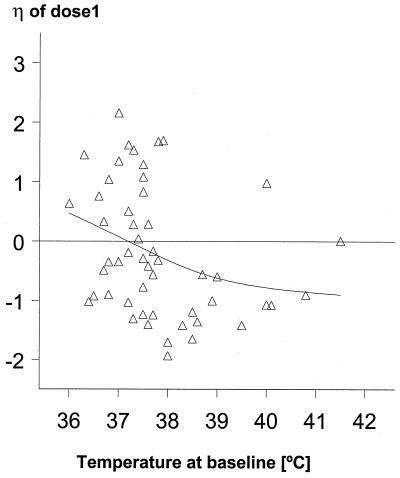
Using patient estimates derived from the study in Bangkok, where more detailed sampling was undertaken, we found that increasing baseline parasite counts were associated with progressive decreases in F1, F2, F3, and F4 but not in F5 to F8 (Fig. 3). For example, a parasite count of 26,000/μl was associated with an F1 20% lower than a count of 13,000/μl, the median count in this group. The decreases in F2, F3, and F4 were 15, 10, and 10%, respectively. A similar effect on F1 was observed for baseline body temperature (Fig. 4). Based on estimates from all patients, age, BWT, and gender had no effects on any of the main pharmacokinetic parameters (Ka, CL, or F1 to F8); for example, Fig. 5 plots the interpatient deviation in estimated CL against age.
FIG. 3.
Relationship between the interpatient deviation (η) in F1 and the log parasite count on admission.
FIG. 4.
Relationship between the interpatient deviation (η) in F1 and body temperature on admission.
FIG. 5.
Relationship between the interpatient deviation (η) in estimated CL and age on admission.
AUC.
Based on the population estimated pharmacokinetic model and patient-specific deviations from it, AUC0–∞ was calculated for each individual dose and for all doses combined. The correlation estimates indicated that AUC varied substantially within each patient for the initial doses (1, 2, and 3) but that this variation decreased with subsequent doses. The correlations (r) were 0.13 between doses 2 and 3 and 0.56 between doses 3 and 5 and rose to 0.87 for doses 7 and 8. Thus, the substantial intrapatient variability in the fraction of the drug absorbed in the acute phase of the disease declined with recovery. Median AUC (lower and upper 5th percentiles) for regimens A, B, and C were estimated to be 356 (215 and 973), 561 (231 and 1,668), and 712 (393 and 1,560) ng/ml/h, respectively. These values correspond to approximately 60 and 100% higher bioavailabilities with regimens B and C, respectively, than with regimen A.
Effect of food intake on the pharmacokinetics of lumefantrine.
The estimated mean increase in oral bioavailability as a result of taking a light meal close to the time of drug intake (i.e., within 1 h before or after) was 48% (P, 0.007) (90% CI, 16 to 84); for a normal meal, this value was 108% (P, 0.0001) (90% CI, 64 to 164) compared with taking liquids alone. A normal meal was associated with a 42% (90% CI, 17 to 72) increase in bioavailability compared with a light meal (P, 0.003). After 24 to 48 h of treatment, the majority of patients were eating normally; two-thirds of the meals were of the normal type. Thus, most patients contributed data to all four food effect possibilities. The estimated increase in oral bioavailability of the first dose in patients who took a normal meal (n = 13) compared to patients who took liquids only (n = 24) was 336% (P, 0.0001) (90% CI, 156 to 640).
Time until plasma lumefantrine concentrations fell below 280 ng/ml.
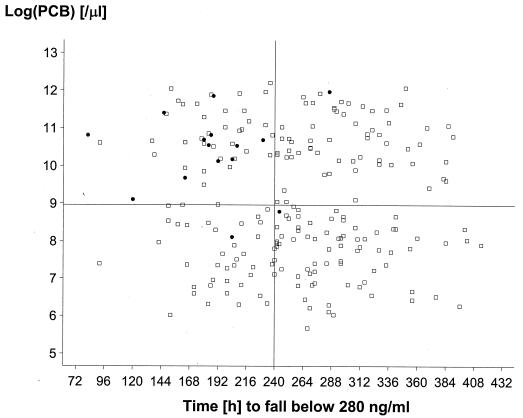
In an earlier dose range study, in which lower doses of artemether-lumefantrine were used, a plasma lumefantrine concentration of 280 ng/ml 7 days from the start of treatment was found to provide a useful cutoff value for determining the risk of therapeutic failure (i.e. recrudescence) (3). In that study, 75% of patients with day 7 lumefantrine levels higher than this threshold were cured, compared to only 51% of patients with lower lumefantrine levels. Further modeling of predicted parasite CL profiles (F. Ezzet and N. J. White, unpublished observations) also suggests that this total plasma lumefantrine concentration may be close to the in vivo MIC. Thus, the longer the time before plasma lumefantrine concentrations fall below this value, the less likely the chance of recrudescence. In this study, the median times required for plasma lumefantrine concentrations to fall below 280 ng/ml were estimated to be 204, 252, and 298 h for regimens A, B, and C, respectively (P, 0.0001). Figure 6 plots baseline (log) parasite count against time (hours) for which the plasma lumefantrine concentration exceeded 280 ng/ml. Twelve out of the 15 cases of recrudescence occurred in patients with the highest baseline parasite counts and shortest times to fall below the cutoff point.
FIG. 6.
Relationship between the log parasite count on admission and the time to fall below a plasma lumefantrine concentration of 280 ng/ml. Horizontal line, median log parasite count; vertical line, median time to reach 280 ng/ml. The closed circles represent patients whose infections subsequently recrudesced, and the open squares represent patients who were treated successfully.
EKG findings (effects on QTc).
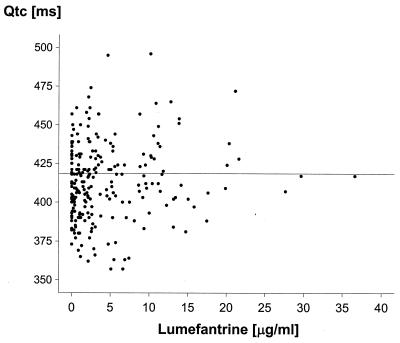
QTc was estimated to have median (standard deviation) values of 418 (30) ms at baseline, 410 (31) ms at 52 h, 417 (26) ms at 76 h, 413 (23) ms at 100 h, 408 (22) ms at 163 h, and 412 (20) ms at 667 h after the start of treatment. A series of mixed-effect models were fitted to the data as described above. Baseline QTc, heart rate, BWT, and day of electrocardiogram were all found to have a statistically significant effect on QTc, but plasma lumefantrine concentrations did not, indicating that plasma lumefantrine concentrations do not correlate with QTc. The results of the fit of model 1 are shown in Table 2 (model 2 proved inferior [P, 0.0003]), and model 3 was not significantly different from model 1 [P, 0.065]). The variables age, gender, and dose regimen were also found not to be significant contributors. QTc at baseline and heart rate correlated positively with QTc, indicating inadequate rate correction with Bazett's formula, while BWT correlated negatively. The estimates derived from this model gave the multiplication factor for every unit increase, e.g., 0.63-ms increase in QTc for an increase in heart rate of one beat per minute. The model gave an estimated increase in QTc of about 10 ms on day 4. The QTc data on day 4 (49 patient observations) were examined to test for differences in QTc as a result of differences in plasma lumefantrine concentrations. Plasma lumefantrine concentrations and the lumefantrine AUC were found to have no effect on QTc (P, 0.9), while the effects of QTc at baseline, heart rate, and BWT were found to be similar to those in the best-fit model. Since measured plasma drug concentrations were not available for every QTc value recorded, predicted lumefantrine concentrations were obtained. Figure 7 provides a scatter plot of QTc against the predictions. Taken together, these data all indicate that lumefantrine does not prolong QTc. Further data on the relationship between QTc and plasma lumefantrine concentrations in a larger series are presented elsewhere (van Vugt et al., Am. J. Trop. Med. Hyg., in press).
TABLE 2.
Parameter estimates of the linear mixed-effect model for QTc
| Effect | Estimatea | SE | P |
|---|---|---|---|
| Intercept | 418.8 | 3.23 | 0.0001 |
| Baseline QTc (s) | 0.36 | 0.07 | 0.0001 |
| Heart rate (per min) | 0.63 | 0.11 | 0.0001 |
| BWT (kg) | −0.71 | 0.26 | 0.008 |
| Day | |||
| 3 | 5.5 | 3.3 | 0.09 |
| 4 | 9.8 | 3.3 | 0.004 |
| 5 | 8.8 | 3.3 | 0.009 |
| 8 | 3.3 | 3.3 | 0.3 |
Median estimates of fixed effects.
FIG. 7.
Relationship between the EKG QTc and the estimated plasma lumefantrine concentrations on days 3 to 29. Horizontal line, median QTc.
Adverse experiences.
Potential adverse drug effects for 51 patients (Bangkok) were examined. Since regimens A, B, and C were of different durations, namely, 48, 60, and 96 h, respectively, potential adverse drug effects that started between days 2 and 4, 2 and 5, and 2 and 6, respectively, were considered for analysis. Artemether-lumefantrine was remarkably well tolerated. Only 13 potential adverse drug effects in total were reported by these patients; all were mild, and only 6 involved the nervous (headache, dizziness) or gastrointestinal (nausea, abdominal discomfort) system. These complaints are common in malaria irrespective of treatment and could not be related to plasma drug concentrations.
DISCUSSION
The fixed combination of artemisinin and lumefantrine achieves its antimalarial effect through the sequential large initial reduction in parasite biomass by artemether and the subsequent removal of all of the remaining viable parasites by the intrinsically less active but more slowly eliminated lumefantrine (17, 18). Artemether probably achieves fractional parasite biomass reduction of up to 104 per cycle, so treatment regimens involving 5 days of artemether cover three asexual cycles and leave relatively few viable parasites for lumefantrine to remove (17). The combination also provides mutual protection of the two antimalarial drugs from the development of resistance, as parasites are never exposed to artemether alone and relatively few are exposed to lumefantrine unprotected by the artemisinin derivative (18). In clinical practice, the combination is well tolerated and rapidly and reliably effective. However, overall cure depends on the presence of sufficient lumefantrine to remove the residual parasite biomass left by artemether, and this in turn depends on adequate oral bioavailability.
The oral bioavailability of lumefantrine varies considerably between individual doses and between patients and is reduced significantly in the acute phase of malaria. This large intrapatient variability in the fraction of drug absorbed between doses confounds estimation of conventional pharmacokinetic parameters such as CL and apparent V. The reduction in oral bioavailability may result either from disease-induced changes in the intestinal absorption of this poorly water-soluble drug (8) or from the lack of concomitant food intake and therefore the absence of fats, which have been shown to augment oral bioavailability significantly. Lumefantrine is a highly lipophilic substance; oral bioavailability increases substantially if the drug is administered after a meal rich in fat. In an earlier volunteer trial, this increase was estimated to be approximately 16-fold (M. Bindschedler, P. Degen, Z. L. Lu, et al., Abstr. XIV Int. Congress Trop. Med. Malaria, Nagasaki, Japan, 1996). Therefore, the differences in overall availability between patients and between doses could be explained largely by differences in food intake and the type of food taken. Patients with acute malaria are reluctant to eat and often vomit. The more severe the infection, the less likely it is that the patient will eat, and the longer will be the period of fasting. This situation compromises the efficacy of poorly absorbed drugs, although the administration of other poorly bioavailable compounds, such as halofantrine, together with fats has been feasible in clinical practice (11). Indeed, in phase III studies of atovaquone-proguanil (atovaquone is another poorly bioavailable, highly lipophilic compound), the drug was always taken with food or milk (5). Whether this would be an effective approach for the administration of lumefantrine is not known, although these data suggest that it would. As patients recover from malaria, splanchnic blood flow and gut motility return to normal and they begin to eat normally (8). This both improves lumefantrine absorption and reduces the variability in oral bioavailability between doses. Differences in the bioavailability of the final two doses observed between Bangkok and Mae La samples can be attributed to the timing of meals; in Bangkok, the drugs were administered shortly after the midday meal, whereas in Mae La, the drugs were given several hours after the small morning meal.
The consistent therapeutic response to the artemether component of the antimalarial combination (16, 17) ensures speedy clinical recovery and thus rapid improvement in lumefantrine absorption. As a consequence of the poor initial oral bioavailability in acute malaria, the absorption profiles following the later treatment administrations become disproportionately important in determining the terminal elimination profile and thus antiparasitic effect. Previous studies have shown that the AUC is the principal determinant of antimalarial activity (3). The importance of the improved absorption in the recovery period is illustrated by the difference in lumefantrine AUCs between the 3-day and 5-day regimens. Whereas a 50% increase in dose given over 3 days gave a corresponding 50% increase in AUC, when this same higher dose was given over 5 days, the increase in AUC was nearly 100%. Thus, 5-day treatment regimens with this combination provide more reliable lumefantrine bioavailability, as at least 3 days of the treatment course are given with the patient in a recovered clinical state.
In a previous study (3), a plasma lumefantrine concentration of 280 ng/ml 7 days from the start of treatment was found to provide a useful cutoff value for determining the risk of therapeutic failure. Modeling of parasite clearance kinetics also suggests that this value lies close to the average in vivo MIC (Ezzet and White, unpublished observations). The higher-dose regimens in this study increased the duration for which plasma drug levels remained above this value by approximately 25% for the 3-day regimen and 50% for the 5-day regimen. In this trial, the risk of therapeutic failure was related to baseline parasite count and a rapid fall in lumefantrine concentrations to below 280 ng/ml. The excellent clinical results obtained with the higher-dose regimens in clinical trials compared with the lower-dose 3-day regimen probably do result from the increased lumefantrine AUCs or time for which the MIC is exceeded. However, the increased dose of artemether given may also contribute. There are few data on oral artemether dose-response relationships (10, 17), and it remains possible that 1.3 mg/kg per dose does not give a maximum parasiticidal effect. However, there is extensive evidence that 5-day regimens with artemisinin or its derivatives alone give considerably higher overall cure rates than 3-day regimens. The reasons are that three asexual life cycles rather than two are affected and parasite biomass is reduced by an additional 10,000-fold. These data argue in favor of the administration of 5-day regimens to patients with little or no background immunity both to maximize the benefit from the artemether component and to provide as wide a margin of safety in ensuring that plasma lumefantrine levels exceed the MIC for as long as possible.
Artemether-lumefantrine was very well tolerated in this and previous studies. Indeed, there were no adverse effects that could be ascribed unequivocally to the drug. The cardiotoxic potential for the aryl amino alcohol class of antimalarial agents is well known. Marked ventricular repolarization delay manifested by prolongation of the EKG QTc is associated with quinidine and halofantrine, a drug which shares a highly variable and fat-dependent oral bioavailability with lumefantrine (9). EKG studies in malaria commonly reveal temporal changes in QTc which are variously ascribed to drug or disease effects (15). These and other data suggest that the rate correction provided by Bazett's formula does not eliminate the effect of heart rate completely (R. N. Price and N. J. White, unpublished observations). Empirically, a power constant of between 0.4 and 0.45 instead of 0.5 provides a better correction. However, the inclusion of heart rate as a covariate in the analysis of the Bazett-based QTc in a mixed-effects model, as was done here, would compensate for the inadequacy of the correction. The data in this study and those of a larger series clearly show that lumefantrine has little or no effect on ventricular repolarization (14a). Therefore, there is no a priori reason to withhold this drug from patients with a long QTc or any other cardiac abnormalities.
In areas of malaria endemicity, background immunity acts in synergy with antimalarial chemotherapy, and treatment responses (in terms of cure rates) are always better than those achieved in nonimmune subjects or in young children (17). The excellent clinical results obtained with the higher-dose 3-day regimen in this study suggest that this would be a satisfactory regimen for most areas of endemicity, as the population studied on the western border of Thailand has relatively little background immunity compared to the majority of the populations of the malarious tropics. This is an area of relatively low and unstable transmission (previously termed an area of hypoendemicity), and patients have on average one falciparum malaria infection every 2 years. Elsewhere in the tropics, infection rates may range up to two infectious bites every day, and at high levels of transmission, 3-day regimens should offer a considerable margin of safety. The overall plasma lumefantrine concentration profile might be improved further by coadministration of the drug with fats, although this procedure is unlikely to prove practical in most rural areas of the tropics.
ACKNOWLEDGMENTS
We are very grateful to all members of the staff of the Bangkok Hospital for Tropical Diseases and the Shoklo Malaria Research Unit, in particular, Lucy Phaipun. All antimalarial drugs were provided by Novartis Pharma AG Limited.
This study was supported by Novartis Pharma AG and was part of the Wellcome-Mahidol University Oxford Tropical Medicine Research Programme funded by the Wellcome Trust of Great Britain.
REFERENCES
- 1.Brockman A, Paul R E L, Anderson T J C, Hackford I, Phaiphun L, Nosten F, Day K P. Application of genetic markers to the identification of recrudescent Plasmodium falciparum infections on the northwestern border of Thailand. Am J Trop Med Hyg. 1999;60:14–21. doi: 10.4269/ajtmh.1999.60.14. [DOI] [PubMed] [Google Scholar]
- 2.Davidian M, Giltinan A R. Nonlinear models for repeated measurements data. London, England: Chapman & Hall, Ltd.; 1995. [Google Scholar]
- 3.Ezzet F, Mull R, Karbwang J. The population pharmacokinetics of CGP 56697 and its effects on the therapeutic response in malaria patients. Br J Clin Pharmacol. 1998;46:553–561. doi: 10.1046/j.1365-2125.1998.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gibaldi M, Perrier D. Pharmacokinetics. 2nd ed. New York, N.Y: Marcel Dekker, Inc.; 1982. [Google Scholar]
- 5.Hussein Z, Eaves J, Hutchinson D B, Canfield C J. Population pharmacokinetics of atovaquone in patients with acute malaria caused by Plasmodium falciparum. Clin Pharmacol Ther. 1997;61:518–530. doi: 10.1016/S0009-9236(97)90132-6. [DOI] [PubMed] [Google Scholar]
- 6.Lindstrom M J, Bates D M. Nonlinear mixed effects models for repeated measures data. Biometrics. 1990;46:673–687. [PubMed] [Google Scholar]
- 7.Milton K A, Edwards G, Ward S A, Orme M L, Breckenridge A M. Pharmacokinetics of halofantrine in man: effects of food and dose size. Br J Clin Pharmacol. 1989;28:71–77. doi: 10.1111/j.1365-2125.1989.tb03507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Molyneux M E, Looareesuwan S, Menzies I S, Grainger S L, Philips R E, Wattanagoon Y, Thompson R P H, Warrell D A. Reduced hepatic blood flow and intestinal malabsorption in severe falciparum malaria. Am J Trop Med Hyg. 1989;40:470–476. doi: 10.4269/ajtmh.1989.40.470. [DOI] [PubMed] [Google Scholar]
- 9.Nosten F, ter Kuile F O, Luxemburger C, Woodrow C, Kyle D E, Chongsuphajaisiddhi T, White N J. Cardiac effects of antimalarial treatment with halofantrine. Lancet. 1993;341:1054–1056. doi: 10.1016/0140-6736(93)92412-m. [DOI] [PubMed] [Google Scholar]
- 10.Price R N, Nosten F, Luxemburger C, Kahm A, Brockman A, Chongsuphajaisiddhi T, White N J. Artesunate versus artemether in combination with mefloquine for the treatment of multidrug resistant falciparum malaria. Trans R Soc Trop Med Hyg. 1995;89:523–527. doi: 10.1016/0035-9203(95)90094-2. [DOI] [PubMed] [Google Scholar]
- 11.ter Kuile F O, Dolan G, Nosten F, Edstein M D, Luxemburger C, Phaipun L, Chongsuphajaisiddhi T, Webster H K, White N J. Halofantrine versus mefloquine in the treatment of multi-drug resistant falciparum malaria. Lancet. 1993;341:1044–1049. doi: 10.1016/0140-6736(93)92409-m. [DOI] [PubMed] [Google Scholar]
- 12.van Vugt M, Brockman A, Gemperli B, Luxemburger C, Gathmann I, Royce C, Slight T, Looareesuwan S, White N J, Nosten F. A randomised comparison of artemether-benflumetol and artesunate-mefloquine in the treatment of multidrug-resistant falciparum malaria. Antimicrob Agents Chemother. 1998;42:135–139. doi: 10.1128/aac.42.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Vugt M, Ezzet F, Phaipun L, Nosten F, White N J. The relationship between capillary and venous concentrations of benflumetol. Trans R Soc Trop Med Hyg. 1998;92:564–565. doi: 10.1016/s0035-9203(98)90917-8. [DOI] [PubMed] [Google Scholar]
- 14.van Vugt M, Wilairatana P, Gemperli B, Gathmann I, Phaipun L, Brockman A, Luxemburger C, White N J, Nosten F, Looareesuwan S. Efficacy of six doses of artemether-benflumetol in the treatment of multi-drug resistant falciparum malaria. Am J Trop Med Hyg. 1999;60:936–942. doi: 10.4269/ajtmh.1999.60.936. [DOI] [PubMed] [Google Scholar]
- 14a.van Vugt M, Ezzet F, Nosten F, White N J. No cardiotoxicity with high dose lumefantrine. Am. J; 1999. . Trop. Med. Hyg., in press. [Google Scholar]
- 15.von Seidlein L, Jaffar S, Greenwood B. Prolongation of the QTc interval in African children treated for falciparum malaria. Am J Trop Med Hyg. 1997;56:4494–4497. doi: 10.4269/ajtmh.1997.56.494. [DOI] [PubMed] [Google Scholar]
- 16.White N J. Clinical pharmacokinetics and pharmacodynamics of artemisinin and derivatives. Trans R Soc Trop Med Hyg. 1994;88:41–43. doi: 10.1016/0035-9203(94)90471-5. [DOI] [PubMed] [Google Scholar]
- 17.White N J. Assessment of the pharmacodynamic properties of antimalarial drugs in vivo. Antimicrob Agents Chemother. 1997;41:1413–1422. doi: 10.1128/aac.41.7.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.White N J. Preventing antimalarial drug resistance through combinations. Drug Resistance Updates. 1998;1:3–6. doi: 10.1016/s1368-7646(98)80208-2. [DOI] [PubMed] [Google Scholar]
- 19.Zeng M Y, Lu Z L, Yang S-C, Zhang M, Liao J, Liu S-L, Teng X H. Determination of benflumetol in human plasma by reversed-phase high-performance liquid chromatography with ultraviolet detection. J Chromatogr. 1996;681:299–306. doi: 10.1016/0378-4347(95)00542-0. [DOI] [PubMed] [Google Scholar]