Abstract
Hematopoietic stem cells (HSCs) are considered to originate from the aorta-gonad-mesonephros, migrate into fetal liver for a rapid expansion, and eventually reside into a unique hypoxic bone marrow niche, where they maintain their homeostasis throughout their life span. HSCs have been widely used for the treatment of many begin or malignant hematopoietic disorders. However, the unavailability of sufficient amount of HSCs still impedes their applications in the clinic. It is urgent to understand how HSC stemness or cell fates are determined at different developmental stages. Although many intrinsic and extrinsic factors (niche components) have been identified in the regulation of HSC origination, expansion, migration, and localization, the underlying mechanisms remain largely unknown. In this article, we summarize current views on the metabolic profiles of HSCs and related regulatory networks, which shows that intrinsic metabolic regulation may be critical for the cell fate determinations of HSCs: HSCs utilize glycolysis as their major energy sources; mitochondrial respiration is also required for the homeostasis of HSCs; amino acids, lipids, or other nutrient metabolisms also have unique roles in sustaining HSC activities. Mechanistically, many important regulatory pathways, such as MEIS1/HIF1A, MYC, PPM1K/CDC20, and ROS signals, are identified to fine-tune the nutrient metabolisms and cell fate commitments in HSCs. Nevertheless, more effort is required for the optimization or establishment of sensitive and specific metabolic techniques/systems for the metabolism studies in HSCs with limited cell numbers and exploring the metabolic profiles and fundamental regulatory mechanisms of different types of nutrients at each developmental stage of HSCs.
Keywords: Bone marrow niche, Glycolysis, Hematopoietic stem cells, Oxidative phosphorylation, Stemness
1. INTRODUCTION
Hematopoietic stem cells (HSCs) can self-renew and give rise to all the downstream hematopoietic progenitors and mature blood cells. HSCs have been reported to be used for the treatment of many types of benign and malignant hematopoietic disorders, including lymphoma, leukemia, aplastic anemia, and immune diseases. However, the difficulties in the limited availability of HSCs and major histocompatibility complex (MHC) matching impede the application of HSCs in the clinic for many hematopoietic disorders. Therefore, unraveling the intrinsic and extrinsic regulators that contribute to HSC stemness can lead to the development of new strategies in obtaining alternative HSC sources or MHC matching to overcome current bottlenecks in clinical applications.
HSCs originate from the aorta-gonad-mesonephros (AGM) region, migrate into the fetal liver for a rapid expansion, and then reside in a unique hypoxic bone marrow microenvironment (niche).1–3 At different developmental stages, many intrinsic and extrinsic factors fine-tune the origination, proliferation, homeostasis, aging, and malignant transformation of HSCs.4,5 Intrinsic metabolic regulators are reported to be key players in the regulation of HSC stemness. For example, several lines of evidence have shown that HSCs mainly utilize glycolysis as the energy source.6,7 Nevertheless, how the metabolism of different nutrients controls HSC activities at different developmental stages remains largely unknown.
2. HSCS RESIDE IN HYPOXIC BONE MARROW NICHES
HSCs have been found to be able to tightly communicate with their unique niches to maintain their activities throughout their life span. The HSC niche was first proposed to be important for normal hematopoiesis in the bone marrow by Schofield in 1978.8 Increasing evidence shows that HSC niches have unique physical and functional structures and contain many different supporting cells, including endothelial cells, stromal cells (mesenchymal stem cells), osteoblasts, adipocytes, sympathetic nerve cells, and several types of hematopoietic cells (megakaryocytes). All of these niches may produce various growth factors, cytokines, or extracellular matrix components to sustain HSC homeostasis. Recently, fetal liver HSC niches have also been identified in the region near portal vessels, which is mainly composed of Nestin+NG2+ pericyte cells.9 Although the endosteal niche, which contains osteoblasts, was initially reported as the niche of quiescent HSCs,10 recent studies show that early lymphoid progenitors mainly reside in the endosteal niche, while HSCs prefer the perivascular niche close to the sinusoid or arteriole, which is constituted by endothelial cells.11 However, several hints also indicate that the endosteal niche and perivascular niche are well connected and orchestrate a functional dynamic niche network. Interestingly, studies have also shown that both quiescent HSCs and proliferating HSCs may localize to the perivascular niche, further indicating that the perivascular niche may be the main niche for HSCs in the bone marrow. Moreover, many other types of niches orchestrated by different niche cells (such as mesenchymal stem cells, megakaryocytes, and adipocytes) have been identified as critical for the maintenance of HSC stemness in recent years.12–15
It has been suggested that the unique anatomic structure and high rate of total oxygen consumption of hematopoietic cells in the bone marrow eventually contribute to the shaping of hypoxic niches with much lower oxygen tension than that of other tissues.16 The average oxygen saturation in the bone marrow is ∼87.5%, and the pO2 is ∼55 mmHg.17 Consistent with this finding, previous findings show that most quiescent HSCs are sustained in regions with a relatively slow blood flow, and a hypoxia indicator, pimonidazole (a hypoxic marker), can mark HSCs in a hypoxic region.18 Low levels of oxygen tension (1%–3%) may enhance HSC expansion in vitro or their engraftment in vivo and promote their differentiation to downstream progenitors and mature hematopoietic cells.19,20 The hypoxic niche may afford a very narrow range of oxygen tension to sustain HSC stemness since maintaining a specific level (not too low or too high) of hypoxia-inducible factor 1 alpha (Hif-1α) is critical for HSC activities.21 Moreover, many niche components derived from niche cells, including endothelial cells, Nestin+ MSCs, and osteoblasts, may exert metabolic effects to support HSC stemness in the bone marrow. For example, studies from Miharada et al report that HSCs highly express surface receptor GPR78 to sustain a relatively high level of glycolysis but low mitochondrial potential. Inhibition of the expression of GPR78 in HSCs leads to a marked migration out of the bone marrow niche.22 Nevertheless, the location of the hypoxic niche in the bone marrow remains controversial since studies also revealed that the perivascular niche may be more hypoxic than the endosteal niche.23 Current studies also indicate that HSCs may be able to tolerate hypoxia with a relatively low oxygen tension by remodeling the regulation of intrinsic metabolic pathways. More efforts are required to unravel the underlying mechanisms related to the metabolic regulations in HSCs in hypoxic niches.
3. METABOLIC FEATURES OF HSCS IN HYPOXIC NICHES
Most normal cells prefer to utilize mitochondrial respiration as the major source of energy under aerobic conditions, which produces approximately 18-fold more ATP than that derived from glycolysis in anaerobic conditions. Under hypoxic conditions, the ATP level is mainly maintained by the glycolytic process, where only 2 mol ATP is produced from 1 mol glucose and the intermediate metabolite pyruvate can be converted into lactate to supply the NAD+ level needed for glycolysis. The glycolytic rate is tightly controlled by the expression levels and activities of several rate-limiting enzymes, such as hexokinase and pyruvate kinase M2. Although hypoxic conditions result in an increase in the energy generated from glycolysis, this is less consequential than the fact that most normal cells tend to reduce energy demands in these stress situations. This phenomenon suggests that it is critical to shut down unnecessary processes to decrease energy consumption, which is also defined as “turning off the pilot light.”24,25 Interestingly, HSCs become metabolically quiescent to maintain their activities under “pilot light” conditions and are content with the limited energy supplementation from glycolysis. This observation is also consistent with the fact that HSCs are mainly in a quiescent state in a hypoxic bone marrow niche.25
Oxidative phosphorylation or mitochondrial respiration is reported to generate most reactive oxygen species (ROS), which are mainly derived from electron leakage and can further convert oxygen into superoxide anions. Superoxide anions serve as the precursors of many other oxygen radicals, including nitrogen peroxide and hydroxyl radicals. ROS levels are known to be tightly associated with many degenerative disorders or aging in many tissues or organs, such as in the hematopoietic system (especially in HSCs). HSCs usually have relatively low levels of ROS to sustain their self-renewal capacities and quiescent status.26 Increasing evidence suggests that high levels of glycolysis in HSCs may avoid stress not only from low oxygen tension but also from ROS-mediated oxidative stress from mitochondrial respiration.
Due to the limited availability of HSCs, it is very difficult to analyze the metabolic status of HSCs by using conventional techniques. HSC metabolic characteristics still remain largely unknown, although accumulated studies indicate that HSCs mainly rely on glycolysis for their energy source. For example, HSCs usually have a much lower mitochondrial membrane potential, as displayed by low rhodamine-123 staining, but give rise to more primitive colonies compared to their counterparts27; a mathematics model indicates that HSCs mainly reside in hypoxic niches3; more pimonidazole can be incorporated in HSCs1,6,21; high Hif-1α levels are stably expressed in HSCs6,21; and treatment with tirapazamine (a hypoxic toxic regent) leads to a notable reduction in total HSC numbers.1 Proteomics data have also revealed that the HSC-enriched Lin−sca-1+c-Kit+ (LSK) cell population prefers to adopt glycolysis rather than mitochondrial respiration as their main energy source. In contrast, more differentiated hematopoietic progenitor or mature cells have a much higher level of oxidative phosphorylation than LSK cells. Consistent with these observations, the proteomics analysis28 also demonstrated that more pathways related to glycolysis are involved in the HSC population compared to differentiated cells. Our studies have also revealed that myeloid ecotropic viral integration site 1 (Meis1) can sufficiently transactivate Hif-1α to precisely regulate glycolysis in both murine bone marrow HSCs and human-mobilized peripheral blood HSCs,6,7,29 which indicates that the observed metabolic signatures may not be a consequence resulting of the hypoxic niches but tightly orchestrated by intrinsic regulatory networks.
4. OXIDATIVE PHOSPHORYLATION AND HSC STEMNESS
Early studies show that glycolysis may serve as the major metabolic mechanism of HSCs. Recent evidence reveals that a certain amount of mitochondria still exists in HSCs, indicating that HSC activities may also rely on oxidative phosphorylation at different developmental stages. For example, studies from Manesia et al demonstrate that fetal liver HSCs have increased levels of oxidative phosphorylation, which may be different from that of adult HSCs.30 It is conceivable that the metabolic states may be very dynamic when quiescent HSCs exit from the G0 stage and initiate self-renewal and expansion since more energy and macromolecular materials are required for cell division. Many of the macromolecules are the intermediate metabolites derived from the TCA cycle, suggesting that oxidative phosphorylation may contribute to the normal cell fate commitments of HSCs. Previous studies indicate that many mitochondria in HSCs may be inactive and only act as an energy reserve under some stress situations; nevertheless, a recent report also shows that adult HSCs contain very high levels of mitochondrial mass and enhanced dye-efflux abilities yet possess limited respiratory and turnover capacities.31 Further evidence from other groups also suggests that oxidative phosphorylation is important for HSC stemness.32 Although glycolysis may quickly produce ATP to fuel many biomacromolecule synthesis processes, an enhanced cell proliferation also heavily depends on the different types of intermediate metabolites from oxidative phosphorylation.
Oxidative phosphorylation is regulated by many signaling pathways to sustain HSC activities, such as the LKB1- and PGC-1-mediated pathways.33 LKB1 deletion results in the loss of quiescence and a decrease in mitochondrial mass in HSCs, which is similar to the phenotypes exhibited in Hif-1α-null HSCs. PGC-1α is able to markedly enhance the expression of several antioxidants for ROS detoxication. Guanine nucleotide exchange factor binding protein 5 sustains the mitochondrial potential and quiescent status of HSCs.34 Mitochondrial malfunction is usually accompanied by an increased level of ROS, which further impairs HSC activities. Moreover, mutations in key enzymes in oxidative phosphorylation can cause malignant transformation of HSCs. For example, mutations in IDH1 and IDH2 result in the generation of the onco-metabolite 2-hydroxyglutarate, which further contributes to the development of acute myeloid leukemia (AML).35 Wild-type IDH1 and IDH2 localize to the cytoplasm and mitochondria, respectively, and convert isocitric acid into α-ketoglutaric acid. However, mutants of IDH1 and IDH2 catalyze isocitric acid into 2-hydroxyglutarate. α-Ketoglutaric acid is a key cofactor of many oxygenases, for example, TET2. TET2 converts 5mC into 5hmC to initiate DNA demethylation in an α-ketoglutaric acid-dependent manner.36 IDH1/2 mutants cause the loss of function of TET2 and enhance the abnormal self-renewal of HSCs, which gradually results in the development of myelodysplastic syndrome (MDS) or AML. Nevertheless, it remains unclear whether HSCs with mitochondrial malfunction also have abnormal glycolytic levels and how mitochondrial respiration and glycolysis are connected under either physiological or pathological conditions. More efforts are required to delineate the roles of mitochondrial respiration in the cell fate commitments of HSCs.
ROS are mainly generated from oxidative phosphorylation, and a certain level of ROS is required to determine the cell fate of various cell types under physiological conditions. Abnormally increased ROS levels may cause an imbalance in redox and oxidative stress, which further causes the oxidation of lipids, nucleic acids, proteins and carbohydrates, cell apoptosis, aging, and malignant transformation of many cell types, such as HSCs. ROS mainly contain superoxide, hydrogen peroxide, and oxygen radicals. Superoxide is generated via the interaction between free oxygen and uncoupled electrons released from the electron transfer chain (also termed electron leakage), which can be subsequently catalyzed into hydrogen peroxide by superoxide dismutase, followed by conversion into hydroxyl radicals or hydrogen oxides by glutathione, catalase, and peroxidase.
Exposure to high levels of ROS may cause loss of stemness or cell death in HSCs. For example, when HSCs are separated into DCFDAhigh and DCFDAlow cell populations on the basis of DCFDA (a ROS indictor) staining and transplanted into recipient mice, DCFDAlow cells contributed a higher donor repopulation with a distinct myeloid differentiation26 compared to the DCFDAhigh cells. Because aged HSCs are usually biased toward lineage differentiation to myeloid cells, high ROS levels may be considered a potent driver of HSC aging. Currently, several pathways have been found to be involved in the regulation of ROS levels in HSCs: (1) Polycomb complexes, such as Bmi1, have been found to be highly upregulated in HSCs and sustain HSC self-renewal by downregulating ROS levels, as well as p16Ink4a/p19Arf expression37; (2) the DNA damage response is another pathway used to maintain low ROS levels and genome stability, and loss of function of ATM leads to enhanced ROS levels and deficiencies in the DNA damage response and impairs normal hematopoiesis38; (3) FOXO signaling is also important for the maintenance of the redox state of HSCs,39 and FOXO3a collaborates with ATM to inhibit ROS production and activate DDR to sustain the self-renewal capacities of HSCs; and (4) HO-1 has a stress-dependent antioxidase role to sustain the homeostasis of the redox state in HSCs. Nrf2 and Keap1 transactivate HO-1 expression to inhibit ROS generation to maintain HSC stemness.40 These studies indicate that both exposure duration and ROS levels may significantly affect the self-renewal and differentiation capacities of HSCs. However, it is urgent to develop novel techniques to sensitively and precisely monitor the subtle and dynamic changes of ROS levels and their specific components in HSCs to unravel their additional functions in hematopoiesis.
5. MECHANISMS OF THE REGULATION OF GLYCOLYSIS IN HSCS
Hif-1α is known as the key mediator for sustaining glycolytic levels in HSCs in hypoxic bone marrow niches that controls the expression of many downstream targets involved in glycolysis. Although Hif-1α can interact with Hif-1β to form a heterodimer and is relatively stable under hypoxic conditions, Hif-1α appears to be expressed in HSCs even under normoxic conditions.29 Several signaling pathways have been reported to be critical in sustaining the stability of Hif-1α. For example, the VHL-mediated deubiquitination pathway is essential to maintain Hif-1α levels and stability41; AMPK- or SIRT1-mediated signaling is also involved in the maintenance of certain Hif-1α levels42; arene receptor AhR can form an AhR/aryl complex to suppress the binding of ARNT with Hif-1α to downregulate its protein level, which also indicates that AhR antagonist may serve as an idea expander to in ex vivo expansion of HSCs43; and we previously also showed that cytoskeleton protein PFN1 maintains Hif-1α expression through EGR1/Gα13 pathways to fine-tune the glycolysis and homeostasis of HSCs.44 All these regulatory molecules or pathways are important for maintaining HSC activities under hypoxic conditions or some normoxic conditions and avoiding the ROS stress resulting from oxidative phosphorylation.
Meis1 is a HOX family member and an evolutionarily conserved DNA-binding transcriptional factor that is highly expressed in HSCs at many developmental stages.45 Although Meis1 deletion is embryonically lethal in mice due to multiple defects in hematopoiesis and vasculogenesis, the detailed roles of Meis1 in HSC stemness await further investigations. Interestingly, our studies show that Meis1 can bind to the promoter region of Hif-1α and directly activate its expression,6 and Meis1 also controls the glycolytic level and self-renewal ability of HSCs via the Hif-2α/ROS/p16 pathway.7 CDC20 serves as a key E3 ligase to maintain Meis1 protein levels through ubiquitination pathways.46 Meis1 deletion leads to a notable decrease in Hif-1α and Hif-2α expression levels, increased ROS levels, and loss of quiescence of HSCs. The ROS scavenger NAC can almost fully rescue the loss of function of Meis1-null HSCs.7 Intriguingly, human mobilized peripheral blood HSCs also prefer to utilize glycolysis for their energy source via Meis1/Hif-1α/Pbx1/HoxA9 pathways,29 indicating that intrinsic metabolic networks regulate the cell fates of HSCs.
Hif-1α is well known to regulate HSC glycolysis and function by the transactivation of many downstream target genes, including LDHA, PKM2, GLUT1, PFKL, PDK2, and Cripto. Hif-1α enhances PDK activities to efficiently inhibit pyruvate entry into oxidative phosphorylation or the tricarboxylic acid cycle (TCA).47 Deletion of the Hif-1α downstream targets LDHA or PKM2 results in a notable impairment in the HSC repopulation ability.48 Quiescent HSCs express high levels of Hif-1α, which is also quite stable when the cells are migrating from the hypoxic bone marrow niche into peripheral blood.29,49 Hif-1α deletion in adult HSCs leads to significantly reduced reconstitution capacities and loss of quiescence, suggesting that a certain level of Hif-1α is important for sustaining the HSC pool.21 Mutations in the Hif-1α binding sites in the promoter motif of vessel endothelial growth factor impair HSC stemness.50 Conditional deletion of Hif-1α in HSCs results in a swift switch from glycolysis to mitochondrial respiration and notably increased Hif-2α levels that may result from a compensation effect.7 Nevertheless, a recent report showed some controversial results showing that loss of Hif-1α has no impact on HSC activities,51 although these studies cannot totally exclude the possibility that Hif-1α may also play a key role in bone marrow niche cells to support hematopoiesis.
Moreover, Hif-1α also interacts with Notch and Wnt signaling to determine the cell fates of HSCs. Hif-1α can directly interact with the intracellular domain of Notch (NICD) to maintain its stability to ultimately transactivate downstream target molecules.52 The interaction between Hif-1α and β-catenin results in a significant downregulation of certain Wnt-mediated target genes while enhancing Hif-1α target gene expression.53 In addition, Hif-1α has also been found to be important for normal hematopoiesis at different developmental stages. As mentioned previously, Hif-1α deletion is embryonically lethal in mice because of several defects in vasculogenesis, hematopoiesis, or neurogenesis. The transcriptional profile of Hif-1α in HSCs shares many similarities with human mesenchymal stem cells and embryonic stem cells.54 Hif-1α deletion in mice leads to a notable decrease in the size of the yolk sac and a much lower number of hematopoietic cells compared to WT cells.55 Overall, although several lines of evidence show how glycolysis is fine-tuned in HSCs, the detailed underlying regulatory networks require further exploration.
6. METABOLIC PROFILES OF HSCS AT DEVELOPMENTAL STAGES OR INVOLVING OTHER NUTRIENTS
Increasing evidence indicates that HSCs may have different metabolic characteristics and molecular signatures at different developmental stages: initiation, expansion, differentiation, homeostasis, and aging.56 Many intrinsic factors and extrinsic components may be involved in the regulation of the metabolic dynamics during these processes.45 For example, the metabolic properties may be different between adult HSCs and fetal liver HSCs30; Wip1 coordinates with p53 and mTORC1 pathways to slowdown HSC aging57; glycolysis is switched to mitochondrial respiration while quiescent HSCs exit the G0 stage and differentiate to the downstream progenies; and HSC differentiation heavily depends on oxidative phosphorylation, as shown by the fact that PTPMT1 deletion inhibits HSC differentiation, resulting in a rapid failure of hematopoiesis.58
Currently, most studies focus on glycolysis and mitochondrial respiration with respect to glucose metabolism in adult HSCs. Very limited findings on other nutrient or metabolic pathways (pentose phosphate pathway, amino acid, or lipid metabolism) have been reported, and the metabolic profiles of HSCs at individual developmental stages (AGM, fetal liver, or aged HSCs) have not been systemically or precisely evaluated. An increasing number of studies suggest that different nutrient metabolisms are also important for the maintenance of HSC stemness. For example, PML-PPARδ-FAO-mediated lipid acid oxidation efficiently preserves the capabilities of symmetric divisions or self-renewal of HSCs59; protein synthesis rate controls HSC activities60; branched-chain amino acids are essential for the homeostasis and ex vivo expansion of HSCs46,61; vitamin A-retinoic acid signaling regulates HSC dormancy62; and many other regulators, such as LKB1/AMPK, FOXOs, Wnt, c-Myc, OCT1, PI3K/AKT, and PTEN, may also be involved in the metabolism of various nutrients.
7. NOVEL TECHNIQUES FOR THE ANALYSES OF HSC METABOLISMS
To precisely delineate these complicated metabolic networks in a limited number of HSCs, it is critical and urgent to develop novel tools or techniques for the analyses of different nutrient metabolisms. Due to the limited numbers of HSCs, many routine metabolic assays or tools are not suitable for the characterization of the metabolic properties of HSCs. Recently, several new/optimized techniques have been developed to satisfy the requirements of the analyses of different nutrient metabolisms in HSCs. For example, by using a Seahorse XF analyzer, glycolysis and mitochondrial respiration in HSCs can be measured as the extracellular acidification rate (ECAR) and oxygen consumption rate (OCAR), respectively.59 A Seahorse XF analyzer can also be used for the measurement of lipid metabolism. LC-MS has been developed and optimized to measure various intermediate metabolites with a small number of HSCs, which makes it possible to perform metabolomic analyses. Recent studies provide intriguing results showing that it is possible to conduct metabolomic analysis with approximately 104 HSCs to unravel the metabolisms of different types of nutrients.63 In addition, catabolism or anabolism is a redox bioreaction that involves electron transfer between different substrates mediated by specific coenzymes, such as nicotinamide adenine dinucleotide (NAD+) and nicotinamide adenine dinucleotide phosphate (NADP+), and results in energy synthesis and release. Recent studies show that the ratio of NAD+/NADH or NADP+/NADPH changes in live cells can be monitored by genetically encoded sensors both in vitro and in vivo.64,65 We also demonstrate that these genetically encoded sensors can be used for the evaluation of glucose or amino acid changes in HSCs or leukemia cells.46,66 These new metabolic techniques may provide potent tools to sensitively and precisely evaluate the subtle dynamic metabolic changes in HSCs or other types of stem cells.
8. CONCLUDING REMARKS
Although several important properties of HSC metabolisms, as well as their downstream metabolic pathways, have been illustrated (Fig. 1), it still remains largely unknown about the precise metabolic features and their underlying regulatory networks of different HSCs at different developmental stages, due to the relatively rare frequencies and the complicated interconversion or crosstalk among different types of nutrients including glucose, amino acid, and lipid. Comprehensive and novel powerful tools/techniques are critical for depicturing all the detailed metabolic properties of HSCs, including single cell sequencing, metabolomics, genetically encoded metabolic sensors, animal models/definitive functional assays, and so on. Currently, delineation of metabolisms in stem cells has become one of the leading scientific fields attributed to many newly developed techniques and novel insights into how intrinsic metabolisms affect the cell fate determinations. The studies in metabolisms of HSCs or other types of stem cells will definitely shed a new light on understanding stem cell biology and its related pathogenesis, which may provide a unique angle for the development of novel strategies for the treatments of hematopoietic disorders or other diseases.
Figure 1.
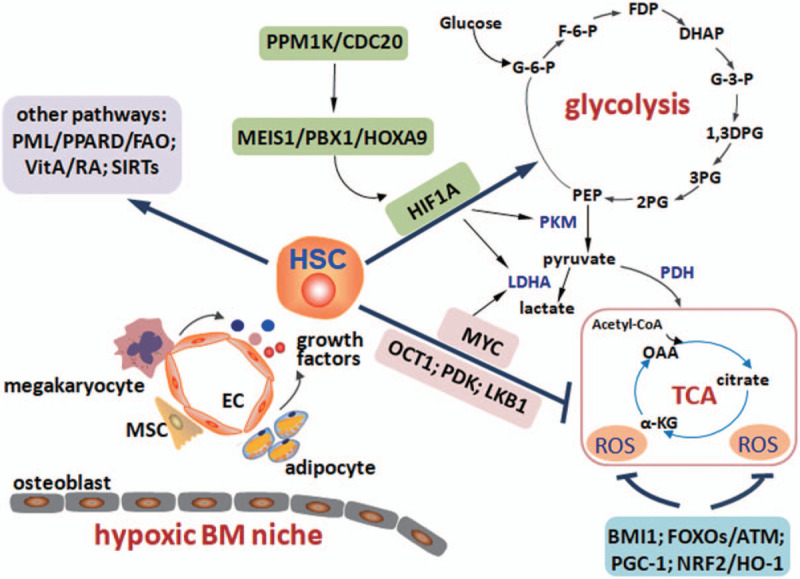
The diagram for metabolic regulations in HSCs. Adult HSCs usually reside in a hypoxic unique bone marrow (BM) niche, which contains many types of niche cells, including osteoblasts (endosteal niche), endothelial cells (vascular niche), megakaryocytes, adipocytes, mesenchymal stem cells, and so on. Different niche cells secrete many growth factors to regulate the HSC metabolisms and stemness. HSCs may mainly locate in the vascular niche and have high level of glycolysis but relatively low level of oxidative phosphorylation. MEIS1/PBX1/HOXA9 complex transactivates Hif-1α and its downstream targets of LDHA and PKM to maintain the glycolytic level. PPM1K/CDC20-mediated branched-chain amino acid metabolism is critical for normal MEIS1 level. OCT1, PDK, LKB1, and MYC signals inhibit oxidative phosphorylation level and/or enhance glycolysis. Reactive oxygen species (ROS) levels are fine-tuned by BMI1, FOXOs/ATM, PGC-1, and NRF2/HO-1 signals. Other metabolic pathways, such as PML/PPARD/FAO, vitamin A/retinoid acid (VitA/RA), and SIRTs, are also required for the maintenance of HSC activities and metabolisms of different nutrients.
ACKNOWLEDGMENTS
This work was supported by grants from National Natural Science Foundation of China (81825001) and the Shanghai Science and Technology Commission (19XD1422100).
REFERENCES
- [1].Parmar K, Mauch P, Vergilio JA, Sackstein R, Down JD. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc Natl Acad Sci U S A 2007;104(13):5431–5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Suda T, Takubo K, Semenza GL. Metabolic regulation of hematopoietic stem cells in the hypoxic niche. Cell Stem Cell 2011;9(4):298–310. [DOI] [PubMed] [Google Scholar]
- [3].Chow DC, Wenning LA, Miller WM, Papoutsakis ET. Modeling pO(2) distributions in the bone marrow hematopoietic compartment. II. Modified Kroghian models. Biophys J 2001;81(2):685–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bryder D, Rossi DJ, Weissman IL. Hematopoietic stem cells: the paradigmatic tissue-specific stem cell. Am J Pathol 2006;169(2):338–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Blank U, Karlsson G, Karlsson S. Signaling pathways governing stem-cell fate. Blood 2008;111(2):492–503. [DOI] [PubMed] [Google Scholar]
- [6].Simsek T, Kocabas F, Zheng J, et al. The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell Stem Cell 2010;7(3):380–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kocabas F, Zheng J, Thet S, et al. Meis1 regulates the metabolic phenotype and oxidant defense of hematopoietic stem cells. Blood 2012;120(25):4963–4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells 1978;4(1–2):7–25. [PubMed] [Google Scholar]
- [9].Khan JA, Mendelson A, Kunisaki Y, et al. Fetal liver hematopoietic stem cell niches associate with portal vessels. Science 2016;351(6269):176–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Calvi LM, Adams GB, Weibrecht KW, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 2003;425(6960):841–846. [DOI] [PubMed] [Google Scholar]
- [11].Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 2012;481(7382):457–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zhou BO, Yue R, Murphy MM, Peyer JG, Morrison SJ. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell 2014;15(2):154–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mendez-Ferrer S, Michurina TV, Ferraro F, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 2010;466(7308):829–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zhao M, Perry JM, Marshall H, et al. Megakaryocytes maintain homeostatic quiescence and promote post-injury regeneration of hematopoietic stem cells. Nat Med 2014;20(11):1321–1326. [DOI] [PubMed] [Google Scholar]
- [15].Naveiras O, Nardi V, Wenzel PL, Hauschka PV, Fahey F, Daley GQ. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature 2009;460(7252):259–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Eliasson P, Jonsson JI. The hematopoietic stem cell niche: low in oxygen but a nice place to be. J Cell Physiol 2010;222(1):17–22. [DOI] [PubMed] [Google Scholar]
- [17].Harrison JS, Rameshwar P, Chang V, Bandari P. Oxygen saturation in the bone marrow of healthy volunteers. Blood 2002;99(1):394. [DOI] [PubMed] [Google Scholar]
- [18].Kubota Y, Takubo K, Suda T. Bone marrow long label-retaining cells reside in the sinusoidal hypoxic niche. Biochem Biophys Res Commun 2008;366(2):335–339. [DOI] [PubMed] [Google Scholar]
- [19].Koller MR, Bender JG, Miller WM, Papoutsakis ET. Reduced oxygen tension increases hematopoiesis in long-term culture of human stem and progenitor cells from cord blood and bone marrow. Exp Hematol 1992;20(2):264–270. [PubMed] [Google Scholar]
- [20].LaIuppa JA, Papoutsakis ET, Miller WM. Oxygen tension alters the effects of cytokines on the megakaryocyte, erythrocyte, and granulocyte lineages. Exp Hematol 1998;26(9):835–843. [PubMed] [Google Scholar]
- [21].Takubo K, Goda N, Yamada W, et al. Regulation of the Hif-1alpha level is essential for hematopoietic stem cells. Cell Stem Cell 2010;7(3):391–402. [DOI] [PubMed] [Google Scholar]
- [22].Miharada K, Karlsson G, Rehn M, et al. Cripto regulates hematopoietic stem cells as a hypoxic-niche-related factor through cell surface receptor grp78. Cell Stem Cell 2011;9(4):330–344. [DOI] [PubMed] [Google Scholar]
- [23].Spencer JA, Ferraro F, Roussakis E, et al. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature 2014;508(7495):269–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hochachka PW, Buck LT, Doll CJ, Land SC. Unifying theory of hypoxia tolerance: molecular/metabolic defense and rescue mechanisms for surviving oxygen lack. Proc Natl Acad Sci U S A 1996;93(18):9493–9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Arai F, Hirao A, Ohmura M, et al. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell 2004;118(2):149–161. [DOI] [PubMed] [Google Scholar]
- [26].Jang YY, Sharkis SJ. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood 2007;110(8):3056–3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Spangrude GJ, Johnson GR. Resting and activated subsets of mouse multipotent hematopoietic stem cells. Proc Natl Acad Sci U S A 1990;87(19):7433–7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Unwin RD, Smith DL, Blinco D, et al. Quantitative proteomics reveals posttranslational control as a regulatory factor in primary hematopoietic stem cells. Blood 2006;107(12):4687–4694. [DOI] [PubMed] [Google Scholar]
- [29].Kocabas F, Xie L, Xie J, et al. Hypoxic metabolism in human hematopoietic stem cells. Cell Biosci 2015;5:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Manesia JK, Xu Z, Broekaert D, et al. Highly proliferative primitive fetal liver hematopoietic stem cells are fueled by oxidative metabolic pathways. Stem Cell Res 2015;15(3):715–721. [DOI] [PubMed] [Google Scholar]
- [31].de Almeida MJ, Luchsinger LL, Corrigan DJ, Williams LJ, Snoeck HW. Dye-independent methods reveal elevated mitochondrial mass in hematopoietic stem cells. Cell Stem Cell 2017;21(6):725–729.e724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Anso E, Weinberg SE, Diebold LP, et al. The mitochondrial respiratory chain is essential for haematopoietic stem cell function. Nat Cell Biol 2017;19(6):614–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Gan B, Hu J, Jiang S, et al. Lkb1 regulates quiescence and metabolic homeostasis of haematopoietic stem cells. Nature 2010;468(7324):701–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Chen Y, Yu M, Dai X, et al. Critical role for gimap5 in the survival of mouse hematopoietic stem and progenitor cells. J Exp Med 2011;208(5):923–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Mardis ER, Ding L, Dooling DJ, et al. Recurring mutations found by sequencing an acute myeloid leukemia genome. N Engl J Med 2009;361(11):1058–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ko M, Huang Y, Jankowska AM, et al. Impaired hydroxylation of 5-methylcytosine in myeloid cancers with mutant tet2. Nature 2010;468(7325):839–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Park IK, Qian D, Kiel M, et al. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature 2003;423(6937):302–305. [DOI] [PubMed] [Google Scholar]
- [38].Guo Z, Kozlov S, Lavin MF, Person MD, Paull TT. ATM activation by oxidative stress. Science 2010;330(6003):517–521. [DOI] [PubMed] [Google Scholar]
- [39].Storz P. Forkhead homeobox type O transcription factors in the responses to oxidative stress. Antioxid Redox Signal 2011;14(4):593–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Cao YA, Wagers AJ, Karsunky H, et al. Heme oxygenase-1 deficiency leads to disrupted response to acute stress in stem cells and progenitors. Blood 2008;112(12):4494–4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Li Z, Wang D, Messing EM, Wu G. Vhl protein-interacting deubiquitinating enzyme 2 deubiquitinates and stabilizes Hif-1alpha. EMBO Rep 2005;6(4):373–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Jeong JH, Kang JH, Hwang SL, et al. 4-O-Methylascochlorin, methylated derivative of ascochlorin, stabilizes Hif-1alpha via AMPK activation. Biochem Biophys Res Commun 2011;406(3):353–358. [DOI] [PubMed] [Google Scholar]
- [43].Ichihara S, Yamada Y, Gonzalez FJ, Nakajima T, Murohara T, Ichihara G. Inhibition of ischemia-induced angiogenesis by benzo[a]pyrene in a manner dependent on the aryl hydrocarbon receptor. Biochem Biophys Res Commun 2009;381(1):44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Zheng J, Lu Z, Kocabas F, et al. Profilin 1 is essential for retention and metabolism of mouse hematopoietic stem cells in bone marrow. Blood 2014;123(7):992–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Zhou F, Li X, Wang W, et al. Tracing haematopoietic stem cell formation at single-cell resolution. Nature 2016;533(7604):487–492. [DOI] [PubMed] [Google Scholar]
- [46].Liu X, Zhang F, Zhang Y, et al. PPM1K regulates hematopoiesis and leukemogenesis through CDC20-mediated ubiquitination of meis1 and p21. Cell Rep 2018;23(5):1461–1475. [DOI] [PubMed] [Google Scholar]
- [47].Takubo K, Nagamatsu G, Kobayashi CI, et al. Regulation of glycolysis by Pdk functions as a metabolic checkpoint for cell cycle quiescence in hematopoietic stem cells. Cell Stem Cell 2013;12(1):49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Wang YH, Israelsen WJ, Lee D, et al. Cell-state-specific metabolic dependency in hematopoiesis and leukemogenesis. Cell 2014;158(6):1309–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Piccoli C, D’Aprile A, Ripoli M, et al. The hypoxia-inducible factor is stabilized in circulating hematopoietic stem cells under normoxic conditions. FEBS Lett 2007;581(16):3111–3119. [DOI] [PubMed] [Google Scholar]
- [50].Rehn M, Olsson A, Reckzeh K, et al. Hypoxic induction of vascular endothelial growth factor regulates murine hematopoietic stem cell function in the low-oxygenic niche. Blood 2011;118(6):1534–1543. [DOI] [PubMed] [Google Scholar]
- [51].Vukovic M, Sepulveda C, Subramani C, et al. Adult hematopoietic stem cells lacking Hif-1alpha self-renew normally. Blood 2016;127(23):2841–2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Dunwoodie SL. The role of hypoxia in development of the mammalian embryo. Dev Cell 2009;17(6):755–773. [DOI] [PubMed] [Google Scholar]
- [53].Kaidi A, Williams AC, Paraskeva C. Interaction between beta-catenin and Hif-1 promotes cellular adaptation to hypoxia. Nat Cell Biol 2007;9(2):210–217. [DOI] [PubMed] [Google Scholar]
- [54].Kim CG, Lee JJ, Jung DY, et al. Profiling of differentially expressed genes in human stem cells by cDNA microarray. Mol Cells 2006;21(3):343–355. [PubMed] [Google Scholar]
- [55].Iyer NV, Kotch LE, Agani F, et al. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev 1998;12(2):149–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Tothova Z, Gilliland DG. Foxo transcription factors and stem cell homeostasis: insights from the hematopoietic system. Cell Stem Cell 2007;1(2):140–152. [DOI] [PubMed] [Google Scholar]
- [57].Chen Z, Yi W, Morita Y, et al. Wip1 deficiency impairs haematopoietic stem cell function via p53 and mtorc1 pathways. Nat Commun 2015;6:6808. [DOI] [PubMed] [Google Scholar]
- [58].Yu WM, Liu X, Shen J, et al. Metabolic regulation by the mitochondrial phosphatase PTPMT1 is required for hematopoietic stem cell differentiation. Cell Stem Cell 2013;12(1):62–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Ito K, Carracedo A, Weiss D, et al. A PML-PPAR-delta pathway for fatty acid oxidation regulates hematopoietic stem cell maintenance. Nat Med 2012;18(9):1350–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Signer RA, Magee JA, Salic A, Morrison SJ. Haematopoietic stem cells require a highly regulated protein synthesis rate. Nature 2014;509(7498):49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Taya Y, Ota Y, Wilkinson AC, et al. Depleting dietary valine permits nonmyeloablative mouse hematopoietic stem cell transplantation. Science 2016;354(6316):1152–1155. [DOI] [PubMed] [Google Scholar]
- [62].Cabezas-Wallscheid N, Buettner F, Sommerkamp P, et al. Vitamin A-retinoic acid signaling regulates hematopoietic stem cell dormancy. Cell 2017;169(5):807–823.e819. [DOI] [PubMed] [Google Scholar]
- [63].Agathocleous M, Meacham CE, Burgess RJ, et al. Ascorbate regulates haematopoietic stem cell function and leukaemogenesis. Nature 2017;549(7673):476–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Zhao Y, Jin J, Hu Q, et al. Genetically encoded fluorescent sensors for intracellular NADH detection. Cell Metab 2011;14(4):555–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Tao R, Zhao Y, Chu H, et al. Genetically encoded fluorescent sensors reveal dynamic regulation of NADPH metabolism. Nat Methods 2017;14(7):720–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Hao X, Gu H, Chen C, et al. Metabolic imaging reveals a unique preference of symmetric cell division and homing of leukemia-initiating cells in an endosteal niche. Cell Metab 2019;29(4):950–965 e956. [DOI] [PubMed] [Google Scholar]


