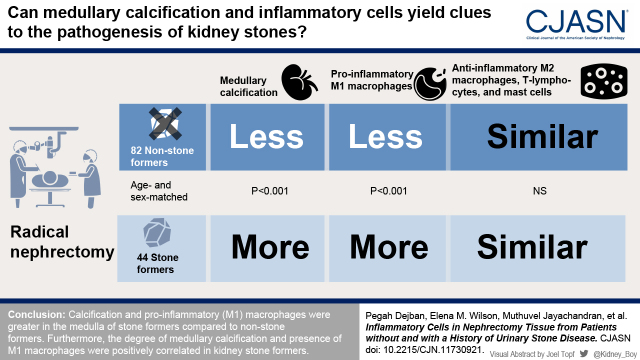
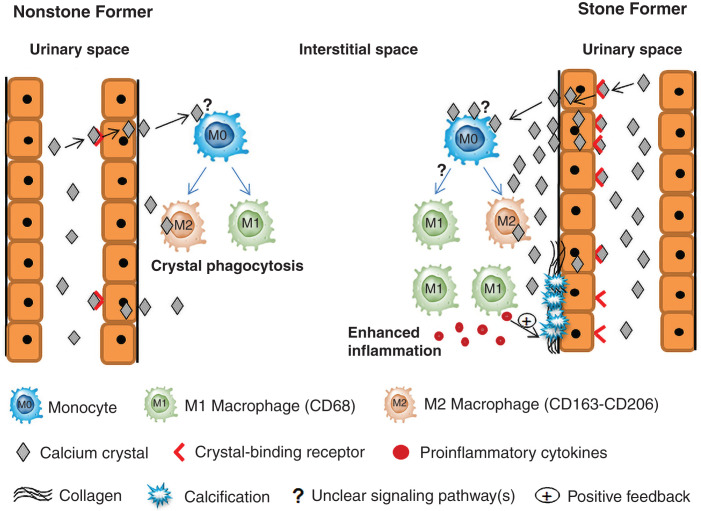
Visual Abstract
Keywords: calcification, inflammation, macrophage, medulla, nephrolithiasis, nephrectomy, urologic diseases
Abstract
Background and objectives
Urinary stone disease has been associated with inflammation, but the specific cell interactions that mediate events remain poorly defined. This study compared calcification and inflammatory cell patterns in kidney tissue from radical nephrectomy specimens of patients without and with a history of urinary stone disease.
Design, setting, participants, & measurements
Nontumor parenchyma of biobanked radical nephrectomy specimens from age- and sex-matched stone formers (n=44) and nonstone formers (n=82) were compared. Calcification was detected by Yasue staining and inflammatory cell populations by immunohistochemistry for CD68 (proinflammatory M1 macrophages), CD163 and CD206 (anti-inflammatory M2 macrophages), CD3 (T lymphocytes), and tryptase (mast cells). Calcifications and inflammatory cells were quantified in cortex and medulla using Image-Pro analysis software.
Results
Calcification in the medulla of stone formers was higher than in nonstone formers (P<0.001). M1 macrophages in the cortex and medulla of stone formers were greater than in nonstone formers (P<0.001), and greater in stone former medulla than stone former cortex (P=0.02). There were no differences in age, sex, body mass index, tumor characteristics (size, stage, or thrombus), vascular disease status, or eGFR between the groups. M2 macrophages, T lymphocytes, and mast cells did not differ by stone former status. There was a correlation between M1 macrophages and calcification in the medulla of stone formers (rho=0.48; P=0.001) and between M2 macrophages and calcification in the medulla of nonstone formers (rho=0.35; P=0.001). T lymphocytes were correlated with calcification in the cortex of both nonstone formers (rho=0.27; P=0.01) and stone formers (rho=0.42; P=0.004), whereas mast cells and calcification were correlated only in the cortex of stone formers (rho=0.35; P=0.02).
Conclusions
Higher medullary calcification stimulated accumulation of proinflammatory rather than anti-inflammatory macrophages in stone formers.
Introduction
Urinary stone disease is a painful and recurrent disease. The prevalence of the disease is approximately one in 11 people in the US population, and similarly high in many parts of the world (1,2). Calcium-containing stones (calcium oxalate and/or calcium phosphate) constitute about 80%–90% of patients with urinary stone disease (3,4). These “idiopathic calcium stones” are thought to arise anchored to precursor lesions, either microscopic papillary calcifications called Randall’s plaques (calcium phosphate calcifications that initially form in the basement membrane of the thin loop of Henle) or ductal plugs (calcium phosphate calcifications that form in the lumen of the terminal collecting ducts) (5,6).
An association between inflammation and urinary stone disease has long been suggested (7). Recent analyses of kidney tissue from humans with urinary stone disease and rodent models of calcium oxalate nephrolithiasis have identified increased expression of genes related to inflammation, immunity, and complement activation pathways (8,9). It has been postulated that immune/inflammatory cells can respond to crystals and calcifications much like an infectious entity, in the process stimulating the innate and adaptive immune systems to recruit a cellular response (10). Among inflammatory cells, macrophages play an important role in normal intrarenal homeostasis, and anti-inflammatory macrophages (M2) appear to contribute to crystal phagocytosis (11). However, cortical and medullary monocyte conversion into proinflammatory (M1) and anti-inflammatory (M2) macrophages, and their distribution and pathophysiological functions within the kidneys of stone formers and nonstone formers, are not clear. This study was designed to quantify the distribution of selected immune/inflammatory cell populations within regions of the kidney where urinary stone precursor lesions typically do (medullary) or do not (cortex) form, by examining large wedge sections of kidney tissues from patients who have undergone unilateral nephrectomy with and without a history of urinary stone disease.
Materials and Methods
Study Patients
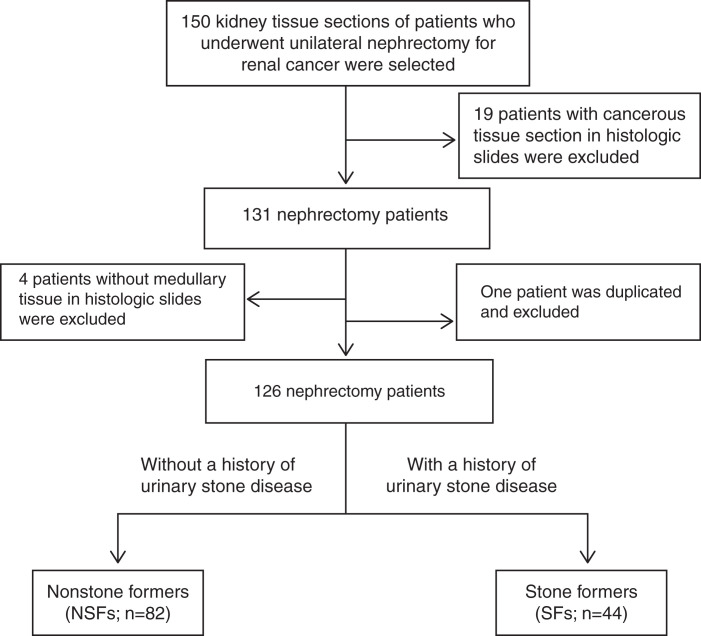
This study was approved by the Institutional Review Board at the Mayo Clinic, Rochester, MN, and all procedures were in accordance with the Declaration of Helsinki. The age-/sex-matched kidney tissue sections were obtained for this study from the Mayo Clinic Nephrectomy Registry (12,13). Patients were studied that underwent complete unilateral nephrectomy for kidney cancer between 2000 and 2012, with no metastatic lesions or positive lymph nodes at the time of surgery. Clinical data at the time of surgery including age, sex, body mass index, tumor size, tumor stage, and the presence of tumor thrombus; comorbidities, including a history of peripheral vascular disease, cardiovascular disease, diabetes mellitus, and hypertension; laboratory data, including serum creatinine and eGFR; and stone former status were collected from a database maintained in the Department of Urology. Stone former status and additional details regarding urinary stone disease history were abstracted from the medical record. The large wedge tissue section of nontumor parenchyma of the biobanked formalin-fixed kidneys from patients with and without a history of urinary stone disease was used to create paraffin-embedded blocks (14). Use of their clinical data and biobanked samples for research was approved by the Mayo Clinic Institutional Review Board. For this study, 50 stone formers were identified on the basis of a history of urinary stone disease documented in their medical record and were age- (±5 years) and sex-matched 2:1 to patients without history of urinary stone disease. Within the two groups, 19 patients were excluded due to cancerous tissue visible in the histologic slide. An additional four slides did not have a medullary region and were excluded from the study, and one nonstone former was found to be duplicated, leaving 82 nonstone formers and 44 stone formers for this analysis (Figure 1).
Figure 1.
Flow diagram for exclusion and inclusion of patients who have undergone unilateral nephrectomy without (NSFs) and with (SFs) a history of urinary stone disease. NSFs, nonstone formers; SFs, stone formers.
Chemicals, Reagents, and Antibodies
Background sniper (catalog BS966), Van Gogh diluent (catalog PD902L), and MACH 3 mouse horseradish peroxidase-polymer detection (catalog M3M530L) were purchased from BioCare Medical, Concord, CA. ADVANCE enzyme link detection kit (catalog K4068), 3,3′ diaminobenzidine (catalog K3468), Dako wash buffer (catalog S3006), mouse antihuman CD68 (catalog M0876), rabbit antihuman CD3 (catalog A0452), and mouse antihuman tryptase (catalog M7052) were purchased from Dako Cytomation, Agilent Technologies, Cedar Creek, TX. Mouse antihuman CD163 (catalog NCL-L-CD163) and mouse antihuman CD206 (catalog LS-B5474) were provided from Leica Biosystem and LifeSpan Biosciences, respectively. EDTA (catalog E4884), silver nitrate (catalog 7761–88–8), rubeanic acid (dithiooxamide, catalog D5141), and ammonium hydroxide solution (catalog 1336–21–6) were obtained from Sigma-Aldrich, St. Louis, MO. All reagents and buffers were of analytical grade.
Yasue Staining for Evaluation of Calcification in Kidney Tissue
Kidney tissue blocks were used to make fresh 4-μm-thick sections. The tissue sections were stained by the Yasue method to detect microscopic calcifications (15). In brief, tissue sections were incubated at 60°C for 1 hour and then cooled at room temperature. Tissue sections were deparaffinized by immersing in xylene solution twice (10 minutes each). Slides were then rehydrated in graded ethanol (100%, 95%, 70%, and 50%; 5 minutes each), followed by distilled water for 10 minutes. Each slide was covered with a few drops of 5% aqueous silver nitrate for 20 minutes and then rinsed with distilled water for 5 minutes. Slides were next covered by a few drops of rubeanic acid solution (50 mg of rubeanic acid in 5–10 ml of 70% ethanol) for 1 minute and then rinsed with distilled water. Slides were finally dehydrated via serial immersion in 50%, 70%, and 95% ethanol (each for 3 minutes) and then absolute ethanol (for 5 minutes), paraffinized by immersion in two xylene solutions for 5 and 10 minutes, and coverslipped with Permount mounting medium for microscopic quantification.
Immunohistochemistry for Quantification of Selected Inflammatory Cell Markers in Kidney Tissue
Histologic slides containing 4-μm-thick kidney tissue sections were warmed to 80°C (10 minutes), deparaffinized via immersion in two xylene solutions, each for 5 minutes, rehydrated in graded ethanol (three absolute ethanol baths followed by a 95% ethanol bath, 2 minutes each), and then incubated in a 1:1 ratio of absolute methanol and 3% hydrogen peroxide to block endogenous peroxidase, followed by a running tap water rinse for 5 minutes. Thereafter, tissue sections underwent heat-induced antigen retrieval via a vegetable steamer (100°C, 30 minutes) containing EDTA (7449 g EDTA in 2 L deionized water at pH 8), allowed to cool to room temperature (5 minutes), and then subjected to a cold tap water rinse before a final Dako wash buffer rinse.
Histologic slides were processed using a Dako autostainer. Antigen-specific antibodies were diluted in Van Gogh diluent. Background Sniper was used as a blocker, and 3,3′ diaminobenzidine was used as the chromagen. Primary antibodies were monoclonal mouse antihuman CD68 (dilution 1:100 for M1 macrophages), mouse antihuman CD163 (1:200 for M2 macrophages), mouse antihuman CD206 (1:1000 for M2 macrophages), and antihuman tryptase (dilution 1:1000 for mast cells), while the MACH 3 mouse horseradish peroxidase-polymer kit was used for detection. For rabbit antihuman CD3 (dilution 1:100 for T lymphocytes), the ADVANCE enzyme link detection kit was used for detection. All tissue samples were processed in accordance with the Dako autostainer program as specified for each antibody. After staining, all slides were rinsed in running tap water, counterstained with hematoxylin (30 seconds), dehydrated, and cover slipped with Permount mounting medium for microscopic quantification.
Quantification of Calcification and Inflammatory Cell Populations in the Cortex and Medulla
To calculate the percentage of calcification and each cell-specific biomarker in the cortex and medulla separately, tissue sections were processed using the whole slide imaging Motic Microscope scanner and quantified using Image-Pro software (version 10.0.1; Media Cybernetics, Silver Spring, MD). All tissue sections were blinded regarding stone former status during this analysis. The percent of calcification and the presence of each cell-specific marker were calculated in the cortex and medulla separately by dividing positively stained tissue area (µm2) by total scanned tissue area (µm2) as previously described (16).
Statistical Analysis
Data were analyzed using GraphPad Prism (version 8.4.3) and JMP (version 14, SAS Institute, Cary, NC). Clinical characteristics are presented as median with interquartile range for continuous variables and as number (percentage) for categorical variables. Given the right-skewedness, the calcification and cell-specific marker percentages are depicted on an antilog scale. There were no missing data in the study except for stone composition in most, because the majority of patients were referred from other institutions. Significant differences between nonstone formers and stone formers were determined using the nonparametric Wilcoxon rank sum or Mann–Whitney test for continuous variables or the chi square (Fisher’s exact) test for categorical variables. Correlations between intrarenal calcification and each inflammatory cell marker were analyzed separately in cortex and medulla of nonstone formers and stone formers using Spearman’s rank correlation coefficient. P<0.05 was considered statistically significant.
Results
The baseline clinical characteristics, comorbidities, and available laboratory data did not differ between groups (Table 1). Kidney stone composition was unknown in most of the patients (91%). As expected in the general population, three out of four cases (75%) with known composition were calcium oxalate or calcium oxalate admixed with calcium phosphate.
Table 1.
Baseline clinical characteristics of study patients
| Characteristics | Nonstone Formers (n=82) | Stone Formers (n=44) |
|---|---|---|
| Age, years | 62 (58, 72) | 61 (57, 71) |
| Male, n (%) | 55 (67) | 31 (71) |
| Body mass index, kg/m2 | 29 (25, 33) | 29 (26, 36) |
| Tumor size (cm3) | 103 (38, 281) | 94 (30, 177) |
| Tumor stage 3 or 4, n (%) | 31 (37) | 21 (47) |
| Presence of tumor thrombus, n (%) | 14 (17) | 12 (27) |
| Peripheral vascular disease, n (%) | 8 (9) | 3 (6) |
| Cardiovascular disease, n (%) | 7 (8) | 6 (13) |
| Diabetes mellitus, n (%) | 19 (23) | 8 (18) |
| Hypertension before surgery, mm Hg, n (%) | 66 (80) | 35 (79) |
| Serum creatinine, mg/dl | 1.1 (0.9, 1.2) | 1.1 (1.0, 1.3) |
| eGFR, ml/min per 1.73 m2 | 74 (61, 85) | 66 (60, 78) |
Data were analyzed by Wilcoxon rank sum and chi-square (Fisher’s exact) tests and are expressed as median (25th, 75th percentiles) or number (percentage). P<0.05 was accepted as statistically significant difference between groups. There was no significant difference between groups. eGFR was determined via serum creatinine on the basis of the Chronic Kidney Disease Epidemiology Collaboration equation (34).
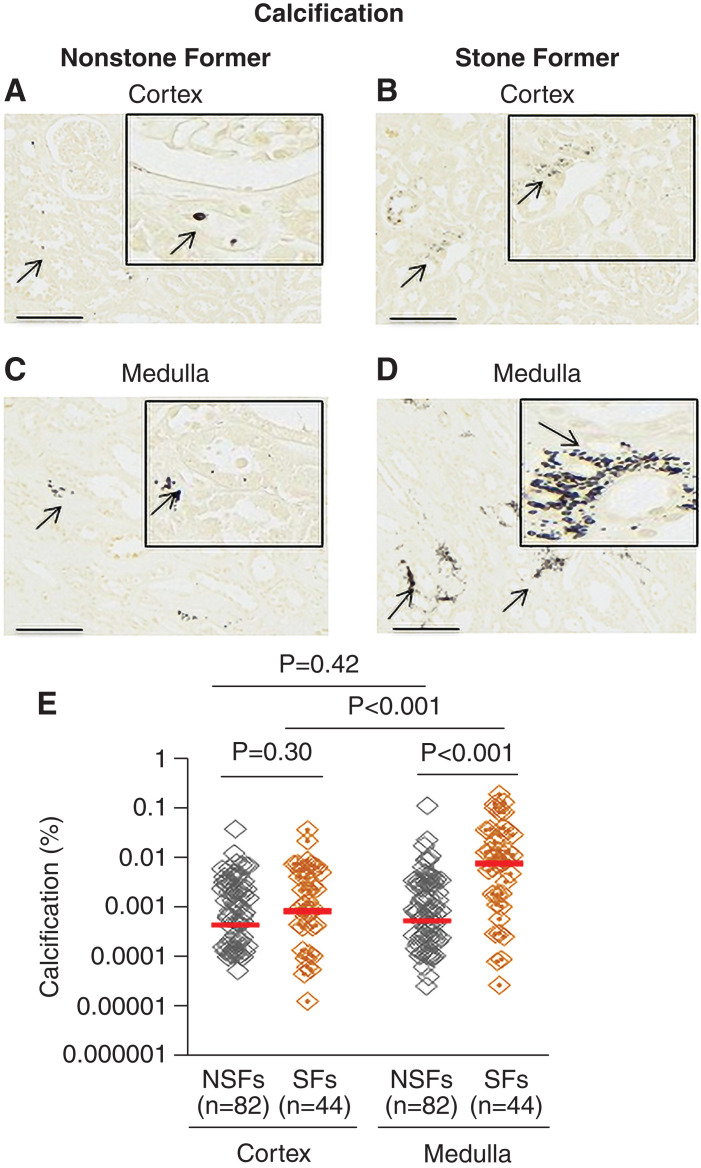
Medullary calcifications were significantly greater in the medulla of stone formers versus nonstone formers (P<0.001; Figure 2). Moreover, among stone formers, the amount of medullary calcification was higher than that in the cortex (P<0.001; Figure 2). Intraluminal calcifications that did not polarize, and were thus consistent with calcium phosphate, were also noted in the majority of nonstone formers (73%) and stone formers (84%) and did not differ between the groups.
Figure 2.
Presence of calcification in the cortex and medulla of kidney tissues from patients who have undergone a unilateral nephrectomy without (NSFs) and with (SFs) a history of urinary stone disease. Representative Yasue staining for calcification in the cortex of nonstone formers (A) and stone formers (B) and medulla of nonstone formers (C) and stone formers (D). The percentage of calcification present in cortex and medulla of nonstone formers and stone formers are presented in (E). Images were taken using 20× visual field. Scale bars=60 µm. Arrows indicate examples of positive calcification staining (A–D). Red lines represent the median. Each gray or orange diamond represents calcification percent in an individual patient. P<0.05 indicates significant differences.
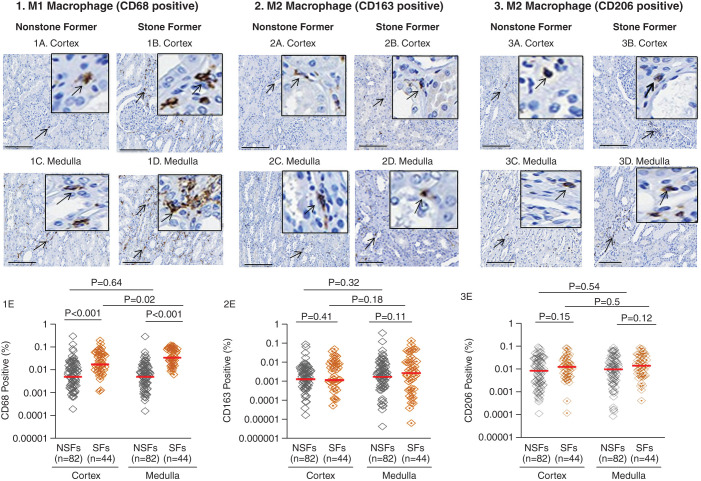
M1 macrophages (CD68 positivity) were significantly greater in the cortex and medulla of stone formers compared with the cortex and medulla of nonstone formers (P<0.001). M1 macrophages were also higher in the medulla of stone formers compared with the cortex of stone formers (P=0.02) (Figure 3, 1E). There was no significant difference in the populations of M2 macrophages (CD163 [Figure 3, 2E] and CD206 [Figure 3, 3E] positivity), T lymphocytes (CD3 positivity; Supplemental Figure 1, 1E) and mast cells (tryptase positivity; Supplemental Figure 1, 2E) did not differ between stone former and nonstone formers as a group or between the cortex and medulla within the nonstone former or stone former groups.
Figure 3.
Presence of M1 macrophages (CD68 positive) and M2 macrophages (CD163 and CD206) in the cortex and medulla of kidney tissues from patients who have undergone a unilateral nephrectomy without (NSFs) and with (SFs) a history of urinary stone disease. Example of CD68-positive M1 macrophage in the cortex of nonstone formers (1A) and stone formers (1B) and medulla of nonstone formers (1C) and stone formers (1D). The percentage of CD68-positive M1 macrophage in kidney tissue from individual nonstone formers and stone formers are presented in (1E). An example of CD163-positive M2 macrophage in the cortex of nonstone formers (2A) and stone formers (2B) and medulla of nonstone formers (2C) and stone formers (2D). The percentage of CD163-positive M2 macrophages in kidney tissue of nonstone formers and stone formers are presented in (2E). An example of CD206-positive M2 macrophages in the cortex of nonstone formers (3A) and stone formers (3B) and the medulla of nonstone formers (3C) and stone formers (3D). The percentage of CD206-positive M2 macrophages in the kidney tissue of nonstone formers and stone formers are presented in (3E). Images were taken using 20× visual field. Scale bars=60 µm. Arrows show positive staining for each cell-specific marker. Red lines represent the median. Each gray or orange diamond represents the percentage of each cell-specific marker positivity from an individual patient. P<0.05 indicates significant differences.
Correlations between inflammatory cell markers and calcification are depicted in Table 2. The presence of M1 macrophages significantly correlated with greater calcification within medulla of stone formers (rho=0.48 and P=0.001; Supplemental Figure 2D), whereas the presence of M2 macrophages correlated with medullary calcifications in nonstone formers (rho=0.26, P=0.01 for CD163 [Supplemental Figure 3C]; and rho=0.35, P=0.001 for CD206 [Supplemental Figure 4C]). The presence of mast cells correlated with stone former cortical calcification (rho=0.35, P=0.02). There was also a correlation between the presence of T lymphocytes and greater cortical calcification among both nonstone formers (rho=0.27, P=0.01) and stone formers (rho=0.42, P=0.004).
Table 2.
Correlation between inflammatory cell markers and calcification in kidney tissue from patients who underwent unilateral nephrectomy without (nonstone formers) and with (stone formers) a history of urinary stone disease
| Cell Type | Nonstone Formers (n=82) | Stone Formers (n=44) | ||
|---|---|---|---|---|
| Spearman (rho) Value | P Value | Spearman (rho) Value | P Value | |
| Cell markers | Calcification in cortex | Calcification in cortex | ||
| CD68 (M1 macrophage) | 0.17 | 0.11 | 0.003 | 0.99 |
| CD163 (M2 macrophage) | 0.16 | 0.14 | 0.23 | 0.13 |
| CD206 (M2 macrophage) | 0.17 | 0.12 | 0.28 | 0.07 |
| CD3 (T lymphocyte) | 0.27 | 0.01 | 0.42 | 0.004 |
| Tryptase (mast cell) | −0.05 | 0.63 | 0.35 | 0.02 |
| Cell markers | Calcification in medulla | Calcification in medulla | ||
| CD68 (M1 macrophage) | 0.05 | 0.62 | 0.48 | 0.001 |
| CD163 (M2 macrophage) | 0.26 | 0.01 | 0.29 | 0.06 |
| CD206 (M2 macrophage) | 0.35 | 0.001 | 0.25 | 0.10 |
| CD3 (T lymphocyte) | 0.19 | 0.08 | 0.22 | 0.14 |
| Tryptase (mast cell) | 0.20 | 0.07 | 0.16 | 0.29 |
Association of kidney tissue inflammatory cells and calcification were analyzed by Spearman correlation test. P<0.05 was considered statistically significant.
Multivariable analyses indicated that M1 macrophages (CD68 positivity) associated with stone former status independent of tumor stage and tumor thrombus.
Discussion
This study examined the relationship between intrarenal calcification and distribution of specific inflammatory cell populations within the cortex and medulla of nephrectomy tissue from patients without and with a history of urinary stone disease. Calcifications were remarkably higher in the medullary region of stone formers compared with nonstone formers. Furthermore, there was a higher percentage of calcification in the medulla of stone formers compared with the cortex of stone formers. M1 (but not M2) macrophages were more common in stone former cortex and medulla. In the stone former group, medullary M1 macrophages correlated with medullary calcification, whereas medullary M2 macrophages correlated with medullary calcification in the nonstone former group.
Calcium crystals (micro or macro) can form within tubular lumens. Many crystals simply pass through the kidney and are excreted in the urine, ultimately detected as crystalluria. However, observations in cell culture (17), animal models (18), and human pathologic samples (19) suggest some of these crystals may adhere to kidney tubular epithelial cells, become internalized, and transcytose to the interstitium. A crystal diffusion study also demonstrated that kidney tissues from both nonstone formers and stone formers provided evidence for transmigration of crystals from tubular lumen to the interstitium. However, the papilla and medulla of stone formers had a markedly greater number of crystal deposits compared with the papilla and medulla of nonstone formers (19).
In this study, M1 macrophage populations were higher in the medulla of stone formers compared with nonstone formers. Moreover, the medullary M1 macrophages were greater than cortical M1 macrophages among the stone formers. However, the population of M2 macrophages within the cortex and medullary regions of kidneys did not differ by stone former status. Thus, interstitial crystals could potentially attract and activate monocytes and macrophages (20,21). Under normal physiologic conditions, a small population of intrarenal resident immune cells are present, including macrophages, lymphocytes, mast cells, and dendritic cells (22). Among these, macrophages play a crucial role in normal homeostasis and the regulation of immunity through phagocytic processes (23). In general, activated tissue monocytes can convert into either M1 or M2 macrophages, depending on the stimuli (24). It has been suggested that M1 and M2 macrophages within the kidney play an opposing role in nephrolithiasis.
Significant findings and possible implications of this study are depicted in Figure 4. In previous studies, the expression of M1-related genes was associated with stone formation, whereas the expression of M2-related genes was associated with decreased stone formation, presumably due to increased crystal phagocytosis and clearance (24,25). Indeed, M2 macrophage differentiation and crystal phagocytosis were observed after exposure of human monocytes to calcium oxalate crystals in vitro. However, when the exposure time exceeded 6 days, monocyte differentiation into M1 macrophages was observed (21,24). It was hypothesized that prolonged exposure of monocytes to calcium oxalate crystals impaired mitochondrial function, thus favoring M1 differentiation (21,24). In addition, oxalate and/or calcium oxalate crystals can also activate the inflammasome, triggering an inflammatory cascade and cytokine release (IL-1β and IL-18) (26). This in turn favors tubulointerstitial damage by stimulating inflammatory cell recruitment. The resulting tubular epithelial cell injury could favor crystal retention and stone formation via several unknown and known mechanisms, including increased interstitial and basement membrane collagen synthesis and expression of injury-related crystal-binding molecules on the apical surface of tubular epithelial cells (Figure 4) (27,28). More recently, it has been observed that culture of renal epithelial cells with exosomes derived from macrophages pre-exposed to calcium oxalate monohydrate crystals also increased production of IL-1β (29).
Figure 4.
Proposed intrarenal cellular response to urinary crystals. Interstitial calcium crystals bind to monocytes via an unknown receptor (?) to trigger differentiation of monocytes into M1 and M2 macrophages. In nonstone formers, M2 macrophages effectively metabolize interstitial crystals by phagocytosis to minimize intrarenal crystal accumulation and prevent stone formation. In stone formers, increased stimulation of monocytes by a greater number of interstitial crystals promotes differentiation of monocytes into inflammatory M1 macrophages rather than M2 macrophages by a mechanism yet to be determined (?). M1 macrophages secrete proinflammatory cytokines to enhance a fibrotic response, including basement membrane collagen accumulation. These local changes favor interstitial calcification (Randall’s plaque) and tubular epithelial cell expression of crystal-binding molecules. These processes function as positive feedback to increase further crystal adhesion and retention and M1 macrophage accumulation.
A previous study demonstrated that human monocytes exposed to calcium oxalate crystals differentiated into M1 macrophages and increased production of the proinflammatory cytokines TNFα, IL-1β, IL-8, and IL-10 (24). Microarray and immunohistological study demonstrated greater expression of M1 macrophages within the papillary tissue of 23 stone formers compared with seven age- and sex-matched nonstone formers (9). M1 macrophages appeared mainly in the interstitial region of the papilla and the tubular lumen. However, CD68 gene expression did not differ between nonstone formers and stone formers (19). A recent study published by our group demonstrated that urinary excretion of CD68-positive extracellular vesicles (derived from activated M1 macrophages) was significantly lower in stone formers compared with nonstone formers, suggesting greater intrarenal expression of proinflammatory macrophages and their vesicles within stone formers (30). In addition, experiments using a hyperoxaluric experimental mouse model demonstrated that daily intra-abdominal administration of IL-4 and IL-13 and transfusion of CD206-positive macrophages increased intrarenal M2 macrophage accumulation and suppressed calcium oxalate crystal formation compared with a control group (25).
This study did not reveal a significant difference in the cortical or medullary T lymphocyte distribution of nonstone formers compared with stone formers. Upregulation of the T helper signaling pathway within the Randall’s plaque-containing papilla of calcium phosphate stone formers has been reported (31), and other studies suggest the possibility that T cell stimulation is followed by macrophage activation (32). It has also been reported that T cell migration was stimulated by treatment with exosomes derived from calcium oxalate–treated macrophages (29).
The medullary and cortical mast cell distributions did not differ between nonstone formers and stone formers. Normally, kidney tissue contains small numbers of mast cells that can increase dramatically in response to inflammation. A role for mast cells in the pathogenesis of urinary stone disease has not been well described to date. However, a recent report of patients with systemic mastocytosis suggested that enhanced stone formation may be observed in this patient population (33). Thus, future studies may yet reveal an association between inflammation, mast cells, and stone risk.
In this study, calcification within the medulla of stone formers was positively associated with M1 macrophages, which further suggests a role for M1 macrophages in stone pathogenesis. Moreover, there was a positive association between medullary calcification and M2 macrophages in the nonstone formers, which may suggest M2 macrophages play a protective role against stone pathogenesis. Further molecular studies are required to identify the exact role of M2 macrophages in the prevention of urinary stone disease. There was also a positive correlation between cortical calcification and the presence of T lymphocytes in both nonstone formers and stone formers, which may reflect enhanced cortical crystal dissolution among both groups. Calcifications and mast cells were positively correlated in the cortex, but not medulla, of stone formers in this study. Thus, the role of mast cells in kidney stone pathogenesis, whether protective or exacerbating risk, could be complicated and requires further study.
This study has certain limitations. Kidney tissues were obtained from nephrectomy samples after resection for tumors (typically renal cell cancer), and there is a possibility the presence of cancer may have influenced the inflammatory cell populations. However, both stone formers and nonstone formers underwent nephrectomy as a cancer treatment, and the available baseline clinical characteristics, including tumor size, tumor stage 3 or 4, and presence of tumor thrombus, did not differ between groups. Multivariable models also suggested that tumor status did not influence the results. The temporal relationship between kidney stone episodes, onset of parenchymal crystals, and onset of parenchymal inflammation could not be assessed from these cross-sectional data. Furthermore, because most patients were only referred for the cancer treatment, detailed longitudinal data regarding their stone history such as single or recurrent episode(s); naïve or on treatment stone; history of hydronephrosis, urinary tract infection, and antibiotic therapy; and information about the composition of stone were not available. Regarding stone composition, it was calcium oxalate and/or calcium phosphate in the majority, when known. This suggests our cohort is representative of stone formers in the community, which are >80% calcium oxalate and/or calcium phosphate. Of note, our experience mirrors most studies in the literature, because stone composition is usually not available for over half of cases. Limiting our analysis to those with known composition would greatly reduce the value of the study (3).
In conclusion, both calcification and M1 macrophages were greater in the medulla of stone formers compared with nonstone formers. Furthermore, the degree of medullary calcification and presence of M1 macrophages were positively correlated in stone formers. This study and other published studies suggest that excess interstitial crystals could potentially stimulate differentiation of monocytes into M1 macrophages, triggering an inflammatory cascade and higher risk of kidney stone formation. Continued elucidation of the role of M1 and M2 macrophage–mediated cascades in urinary stone disease risks in various and larger population-based studies could lead to novel targets for the development of future preventative strategies and treatment options for this highly prevalent, chronic condition worldwide.
Disclosures
A.D. Rule reports employment with the Mayo Clinic; reports serving as a scientific advisor or member of the National Institute of Diabetes and Digestive and Kidney Diseases Urological Diseases of America Contract Management Board; reports serving as an Associate Editor of JASN and a Section Editor of the Mayo Clinic; and reports other interests/relationships with UpToDate. J.C. Lieske reports employment with the Mayo Clinic; reports having consultancy agreements with Allena, Alnylam, the American Board of Internal Medicine, Dicerna, Federation Bio, Novobiome, Orfan, Oxidien, OxThera, Siemens, and Synlogic; reports receiving research funding from Allena, Alnylam, Dicerna, OxThera, Retrophin, Siemens, and Synlogic; reports receiving honoraria from the American Board of Internal Medicine and UpToDate; and reports serving as a scientific advisor or member of American Board of Internal Medicine, Hyperoxaluria Foundation, Kidney International, and Oxalosis. K. Koo reports employment with the Mayo Clinic and other interests/relationships with UpToDate. M. Jayachandran reports employment with the Mayo Clinic and serving in an advisory or leadership role for Journal of Extracellular Vesicles. A. Denic, L.E. Wellik, L.P. Herrera Hernandez, P. Dejban, S. Sinha, and Z. Haskic report being employed by the Mayo Clinic. All remaining authors have nothing to disclose.
Funding
This work was partially funded by Mayo Clinic O’Brien Urology Research Center (U54-DK101227), the Nephrology and Urology Summer Undergraduate Research Fellowship program (R25-DK101405), National Institute of Diabetes and Digestive and Kidney Diseases grant (R01-DK090358), and the Mayo Foundation.
Supplementary Material
Acknowledgments
We thank all study participants.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Exploring the Role of Inflammation toward the Pathogenesis of Calcium Nephrolithiasis,” on pages 338–339.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.11730921/-/DCSupplemental.
Supplemental Figure 1. Presence of T lymphocytes (CD3 positive) and mast cells (tryptase positive) in the cortex and medulla of kidney tissues from patients who have undergone unilateral nephrectomy without (NSFs, nonstone formers) and with (SFs, stone formers) a history of urinary stone disease.
Supplemental Figure 2. Correlation between calcification and M1 macrophage (CD68 positive) in kidney tissue from patients who have undergone unilateral nephrectomy without (nonstone formers) and with (stone formers) a history of urinary stone disease.
Supplemental Figure 3. Correlation between calcification and M2 macrophage (CD163 positive) in kidney tissue from patients who have undergone unilateral nephrectomy without (nonstone formers) and with (stone formers) a history of urinary stone disease.
Supplemental Figure 4. Correlation between calcification and M2 macrophage (CD206 positive) in kidney tissue from patients who have undergone unilateral nephrectomy without (nonstone formers) and with (stone formers) a history of urinary stone disease.
References
- 1.Scales CD Jr, Smith AC, Hanley JM, Saigal CS; Urologic Diseases in America Project : Prevalence of kidney stones in the United States. Eur Urol 62: 160–165, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Romero V, Akpinar H, Assimos DG: Kidney stones: A global picture of prevalence, incidence, and associated risk factors. Rev Urol 12: e86–e96, 2010 [PMC free article] [PubMed] [Google Scholar]
- 3.Singh P, Enders FT, Vaughan LE, Bergstralh EJ, Knoedler JJ, Krambeck AE, Lieske JC, Rule AD: Stone composition among first-time symptomatic kidney stone formers in the community. Mayo Clin Proc 90: 1356–1365, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lieske JC, Rule AD, Krambeck AE, Williams JC, Bergstralh EJ, Mehta RA, Moyer TP: Stone composition as a function of age and sex. Clin J Am Soc Nephrol 9: 2141–2146, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evan AP, Lingeman JE, Coe FL, Parks JH, Bledsoe SB, Shao Y, Sommer AJ, Paterson RF, Kuo RL, Grynpas M: Randall’s plaque of patients with nephrolithiasis begins in basement membranes of thin loops of Henle. J Clin Invest 111: 607–616, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rivera M, Cockerill PA, Enders F, Mehta RA, Vaughan L, Vrtiska TJ, Herrera Hernandez LP, Holmes DR 3rd, Rule AD, Lieske JC, Krambeck AE: Characterization of inner medullary collecting duct plug formation among idiopathic calcium oxalate stone formers. Urology 94: 47–52, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khan SR, Kok DJ: Modulators of urinary stone formation. Front Biosci 9: 1450–1482, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Okada A, Yasui T, Hamamoto S, Hirose M, Kubota Y, Itoh Y, Tozawa K, Hayashi Y, Kohri K: Genome-wide analysis of genes related to kidney stone formation and elimination in the calcium oxalate nephrolithiasis model mouse: Detection of stone-preventive factors and involvement of macrophage activity. J Bone Miner Res 24: 908–924, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Taguchi K, Hamamoto S, Okada A, Unno R, Kamisawa H, Naiki T, Ando R, Mizuno K, Kawai N, Tozawa K, Kohri K, Yasui T: Genome-wide gene expression profiling of Randall’s plaques in calcium oxalate stone formers. J Am Soc Nephrol 28: 333–347, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams GR, Fierens K, Preston SG, Lunn D, Rysnik O, De Prijck S, Kool M, Buckley HC, Lambrecht BN, O’Hare D, Austyn JM: Immunity induced by a broad class of inorganic crystalline materials is directly controlled by their chemistry. J Exp Med 211: 1019–1025, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kusmartsev S, Dominguez-Gutierrez PR, Canales BK, Bird VG, Vieweg J, Khan SR: Calcium oxalate stone fragment and crystal phagocytosis by human macrophages. J Urol 195: 1143–1151, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denic A, Mathew J, Nagineni VV, Thompson RH, Leibovich BC, Lerman LO, Lieske JC, Alexander MP, Augustine JJ, Kremers WK, Rule AD: Clinical and pathology findings associate consistently with larger glomerular volume. J Am Soc Nephrol 29: 1960–1969, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Denic A, Ricaurte L, Lopez CL, Narasimhan R, Lerman LO, Lieske JC, Thompson RH, Kremers WK, Rule AD: Glomerular volume and glomerulosclerosis at different depths within the human kidney. J Am Soc Nephrol 30: 1471–1480, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Denic A, Elsherbiny H, Mullan AF, Leibovich BC, Thompson RH, Ricaurte Archila L, Narasimhan R, Kremers WK, Alexander MP, Lieske JC, Lerman LO, Rule AD: Larger nephron size and nephrosclerosis predict progressive CKD and mortality after radical nephrectomy for tumor and independent of kidney function. J Am Soc Nephrol 31: 2642–2652, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yasue T: Histochemical identification of calcium oxalate. Acta Histochem Cytochem 2: 83–95, 1969 [Google Scholar]
- 16.Ding W, LaPlant BR, Call TG, Parikh SA, Leis JF, He R, Shanafelt TD, Sinha S, Le-Rademacher J, Feldman AL, Habermann TM, Witzig TE, Wiseman GA, Lin Y, Asmus E, Nowakowski GS, Conte MJ, Bowen DA, Aitken CN, Van Dyke DL, Greipp PT, Liu X, Wu X, Zhang H, Secreto CR, Tian S, Braggio E, Wellik LE, Micallef I, Viswanatha DS, Yan H, Chanan-Khan AA, Kay NE, Dong H, Ansell SM: Pembrolizumab in patients with CLL and Richter transformation or with relapsed CLL. Blood 129: 3419–3427, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lieske JC, Norris R, Swift H, Toback FG: Adhesion, internalization and metabolism of calcium oxalate monohydrate crystals by renal epithelial cells. Kidney Int 52: 1291–1301, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Mulay SR, Eberhard JN, Desai J, Marschner JA, Kumar SV, Weidenbusch M, Grigorescu M, Lech M, Eltrich N, Müller L, Hans W, Hrabě de Angelis M, Vielhauer V, Hoppe B, Asplin J, Burzlaff N, Herrmann M, Evan A, Anders HJ: Hyperoxaluria requires TNF receptors to initiate crystal adhesion and kidney stone disease. J Am Soc Nephrol 28: 761–768, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okada A, Hamamoto S, Taguchi K, Unno R, Sugino T, Ando R, Mizuno K, Tozawa K, Kohri K, Yasui T: Kidney stone formers have more renal parenchymal crystals than non-stone formers, particularly in the papilla region. BMC Urol 18: 19, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khan SR, Finlayson B, Hackett R: Renal papillary changes in patient with calcium oxalate lithiasis. Urology 23: 194–199, 1984 [DOI] [PubMed] [Google Scholar]
- 21.Dominguez-Gutierrez PR, Kwenda EP, Khan SR, Canales BK: Immunotherapy for stone disease. Curr Opin Urol 30: 183–189, 2020 [DOI] [PubMed] [Google Scholar]
- 22.Kaissling B, Le Hir M: Characterization and distribution of interstitial cell types in the renal cortex of rats. Kidney Int 45: 709–720, 1994 [DOI] [PubMed] [Google Scholar]
- 23.Ta W, Chawla A, Pollard J: Origins and hallmarks of macrophages: Development, homeostasis, and disease. Nature 496: 445–455, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dominguez-Gutierrez PR, Kusmartsev S, Canales BK, Khan SR: Calcium oxalate differentiates human monocytes into inflammatory M1 macrophages. Front Immunol 9: 1863, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taguchi K, Okada A, Hamamoto S, Unno R, Moritoki Y, Ando R, Mizuno K, Tozawa K, Kohri K, Yasui T: M1/M2-macrophage phenotypes regulate renal calcium oxalate crystal development. Sci Rep 6: 35167, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mulay SR, Kulkarni OP, Rupanagudi KV, Migliorini A, Darisipudi MN, Vilaysane A, Muruve D, Shi Y, Munro F, Liapis H: Calcium oxalate crystals induce renal inflammation by NLRP3-mediated IL-1β secretion. J Clin Invest 123: 236–246, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anders H-J, Suarez-Alvarez B, Grigorescu M, Foresto-Neto O, Steiger S, Desai J, Marschner JA, Honarpisheh M, Shi C, Jordan J, Müller L, Burzlaff N, Bäuerle T, Mulay SR: The macrophage phenotype and inflammasome component NLRP3 contributes to nephrocalcinosis-related chronic kidney disease independent from IL-1-mediated tissue injury. Kidney Int 93: 656–669, 2018 [DOI] [PubMed] [Google Scholar]
- 28.Hirose M, Yasui T, Okada A, Hamamoto S, Shimizu H, Itoh Y, Tozawa K, Kohri K: Renal tubular epithelial cell injury and oxidative stress induce calcium oxalate crystal formation in mouse kidney. Int J Urol 17: 83–92, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Singhto N, Kanlaya R, Nilnumkhum A, Thongboonkerd V: Roles of macrophage exosomes in immune response to calcium oxalate monohydrate crystals. Front Immunol 9: 316, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang J, Kumar S, Jayachandran M, Herrera Hernandez LP, Wang S, Wilson EM, Lieske JC: Excretion of urine extracellular vesicles bearing markers of activated immune cells and calcium/phosphorus physiology differ between calcium kidney stone formers and non-stone formers. BMC Nephrol 22: 204, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taguchi K, Hamamoto S, Okada A, Sugino T, Unno R, Ando R, Gao B, Tozawa K, Kohri K, Yasui T: Helper T-cell signaling and inflammatory pathway lead to formation of calcium phosphate but not calcium oxalate stones on Randall’s plaques. Int J Urol 26: 670–677, 2019 [DOI] [PubMed] [Google Scholar]
- 32.Okada A, Yasui T, Fujii Y, Niimi K, Hamamoto S, Hirose M, Kojima Y, Itoh Y, Tozawa K, Hayashi Y, Kohri K: Renal macrophage migration and crystal phagocytosis via inflammatory-related gene expression during kidney stone formation and elimination in mice: Detection by association analysis of stone-related gene expression and microstructural observation. J Bone Miner Res 25: 2701–2711, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Molderings GJ, Solleder G, Kolck UW, Homann J, Schröder D, von Kügelgen I, Vorreuther R: Ureteral stones due to systemic mastocytosis: Diagnostic and therapeutic characteristics. Urol Res 37: 227–229, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.