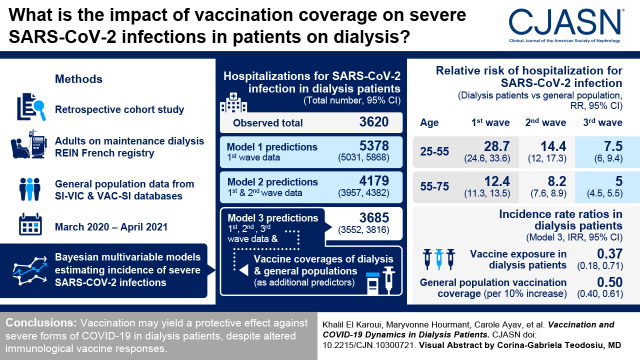
Visual Abstract
Keywords: dialysis, COVID-19, immunology, vaccination, SARS-CoV-2, hospitalization, hemodialysis
Abstract
Background and objectives
Dialysis patients have a high mortality risk after coronavirus disease 2019 (COVID-19) and an altered immunologic response to vaccines, but vaccine clinical effectiveness remains unknown in this population.
Design, setting, participants, & measurements
Using Bayesian multivariable spatiotemporal models, we estimated the association between vaccine exposure and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) severe infections (with hospital admission) in dialysis patients from simultaneous incidence in the general population. For dialysis patients, cases were reported within the French end-stage kidney disease REIN registry from March 11, 2020, to April 29, 2021, and vaccine exposure (first dose) was reported in weekly national surveys since January 2021. Cases in the general population were obtained from the national exhaustive inpatient surveillance system (SI-VIC database), and vaccination coverage (first dose) was obtained from the national surveillance system (VAC-SI database).
Results
During the first wave, incidence in dialysis patients was approximately proportional to the general population. However, we showed a lower relative incidence for dialysis patients during the second wave (compared with that observed in nondialysis patients), suggesting an effect of prevention measures. Moreover, from the beginning of the vaccination rollout, incidence in dialysis patients was lower compared with predictions based on the first and second waves. Adding vaccination coverages in dialysis and nondialysis patients as predictors allowed the reported cases to be fit correctly (3685 predicted cases, 95% confidence interval, 3552 to 3816, versus 3620 reported). Incidence rate ratios were 0.37 (95% confidence interval, 0.18 to 0.71) for vaccine exposure in dialysis patients and 0.50 (95% confidence interval, 0.40 to 0.61) per 10% higher in vaccination coverage in the same-age general population, meaning that vaccine exposure in dialysis patients and the general population was independently associated with lower hospitalization rate of dialysis patients.
Conclusions
Our findings suggest that vaccination may yield a protective effect against severe forms of COVID-19 in dialysis patients, despite altered immunologic vaccine responses.
Introduction
Patients with kidney failure requiring dialysis have a 20%–25% risk of mortality after infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (1–3). Besides, in-center hemodialysis is associated with a higher risk of contact with health care professionals or other high-risk patients, which may explain the elevated incidence of coronavirus disease 2019 (COVID-19) observed in dialysis patients in early 2020 (4,5). To limit the transmission rate, infection prevention steps have been progressively taken in dialysis centers during the successive waves of the pandemic (4,6). Although these measures certainly had a major role in limiting disease transmission, precise quantification of their effect is lacking.
The rapid development of vaccination against SARS-CoV-2 had a major role in controlling COVID-19 spread because vaccines have shown a dramatic efficacy in preventing symptomatic forms of COVID-19, both in clinical trials and in nationwide studies (7). However, dialysis patients have been largely excluded from vaccine trials for safety reasons. Moreover, altered vaccine responses have been previously described in the dialysis population, which justified urgently characterizing SARS-CoV-2 vaccine responses in dialysis patients. To date, this characterization remains mainly based on humoral responses after mRNA vaccines: most studies showed an 80%–95% rate of positive serology in dialysis patients after complete vaccination (8), although this response appeared delayed and antibody levels slightly lower when compared with the nondialysis population (9). These results led to the prioritization of vaccination in dialysis patients in France in early 2021, leading to a high vaccination coverage compared with the nondialysis population. However, the evaluation of vaccine effectiveness against severe forms of COVID-19 in this population remains an unmet need.
In this work, we sought to characterize COVID-19 dynamics in dialysis patients at the population scale by using the general population incidence over time and space as a reflection of SARS-CoV-2 spread.
Materials and Methods
The French kidney failure REIN registry (2,10) is intended to include all dialysis patients living in France. The national coordination center is based at the Agence de la Biomédecine, a public health agency that oversees the activity of organ and tissue procurement and transplantation. The REIN registry relies on a nationwide network of health professionals, with exhaustive continuous registration of all dialysis patients. The participation rate of centers in all contributing regions is 100%. Thirty-two clinical research assistants regularly visit each dialysis center to check the completeness of patient and event registration and to ensure the quality of data. This study included all patients on dialysis on the French mainland and in French overseas territories.
We analyzed aggregated weekly counts of severe SARS-CoV-2 infections (defined by infections followed by hospital admissions) in dialysis patients and in the French general population.
Cases in dialysis patients were reported within the REIN registry from March 11, 2020, to April 29, 2021 (2,10). Vaccine exposure in dialysis patients (first dose) was reported in weekly surveys sent to all French nephrology centers since January 2021. To account for possible missing data regarding reporting of severe cases (some patients may have been recently diagnosed with COVID-19 or reported as having a mild disease because information on their hospitalization or death was not available until a retrospective reclassification could be made), we set a 6-week delay between the end of the period considered for the analysis (April 29, 2021) and data extraction (June 10, 2021).
Weekly incidences of severe infections in the general population were obtained from the national exhaustive inpatient surveillance system (SI-VIC database), assuming an 11-day time lapse from illness onset to hospitalization. The chosen 11-day lag that was applied to hospitalization dates relies on previous estimates (11) and has been used previously in several modeling studies (12–14). Weekly vaccination coverage (first dose) in the general population was obtained from the national surveillance system (VAC-SI database) (15).
We estimated the expected weekly incidence of severe infections in dialysis patients from simultaneous cases reported for the same age class in the general population using Bayesian hierarchical Poisson regressions accounting for spatial autocorrelation, with the log number of at-risk dialysis patients as an offset. Data were aggregated spatially at the department level (administrative division of France; there are 96 metropolitan departments) and temporally at the week level. Incidence rates and vaccination coverage were considered by 10-year age classes (25–35, 35–45, 45–55, 55–65, 65–75, 75–85, and >85 years). Notably, in order to estimate the association between vaccination in dialysis patients and their risk of severe infections at the population scale, we used data on time- and space-dependent incidence in the general population as a reflection of French incidence of SARS-CoV-2 infections (however, our study was not designed to provide a direct comparison of individual infections in dialysis and nondialysis groups).
We built three statistical models. Model M1 only used incident cases from the first pandemic wave (up to June 30, 2020). Model M2 used similar data up to the end of the second wave and beginning of the vaccination campaign in dialysis patients (January 7, 2021), considering a possible risk change between the two waves. Model M3 used similar data up to April 29, 2021, with vaccine coverage in the general population and in dialysis patients as additional predictors.
Among these models, M1 (fitted from first-wave data) was used to compare second-wave predictions with reported cases and identify changes in factors associated with incidence in dialysis patients from the beginning of the second wave. M2 (fitted from first- and second-wave data) was used to compare third-wave predictions with reported cases and identify changes in factors associated with incidence in dialysis patients from the beginning of the third wave. M3 (fitted from first- to third-wave data) was used to identify factors associated with incidence in dialysis patients globally.
Weekly vaccination coverage data were lagged by 3 and 5 weeks in the general population and dialysis patients, respectively, to account for a possible effect of constituted immunity on infection risk (8,16–18). We used a prolonged lag from vaccination in dialysis patients, given the delayed vaccine response consistently observed in this population (9), and conducted a sensitivity analysis using 3-week lags in both populations. Only patients aged ≥25 years were considered from all data sources. Statistical analyses are detailed in the Supplemental Material. All analyses were performed using R Statistical Software (Foundation for Statistical Computing, Vienna, Austria) and the INLA package.
The institutional review board of the REIN registry gave ethical approval for this study protocol (04/28/2021), in adherence with the Declaration of Helsinki.
Results
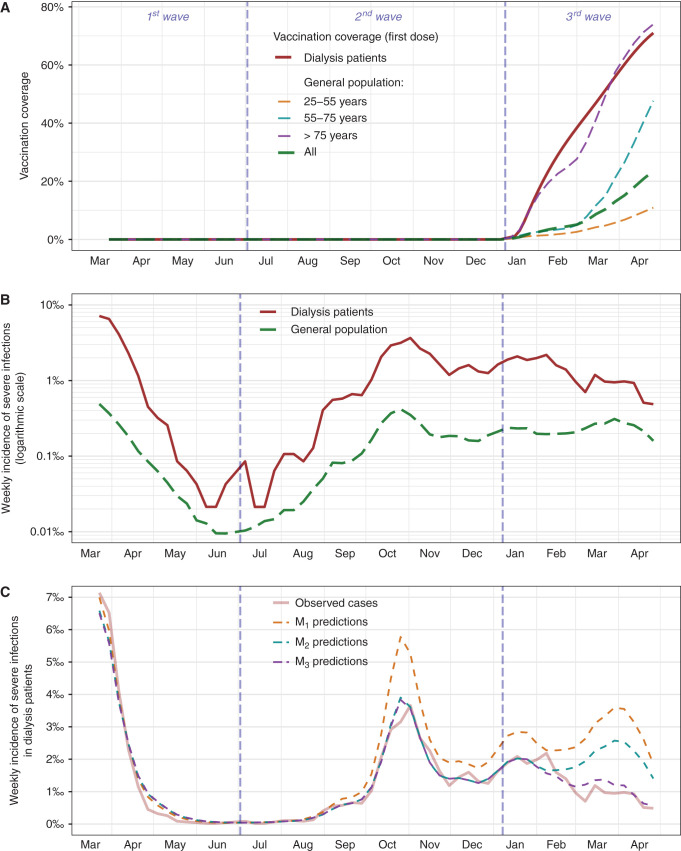
Vaccination coverage in dialysis patients and the general population are shown in Figure 1A. On April 8, 2021 (last date for which vaccinations were considered in this study), the cumulative numbers of vaccine shots in mainland France were 10,255,204 and 3,406,601 for doses 1 and 2, respectively. The global shares were 72% (9.8 million), 20% (2.7 million), and 7% (1 million) for Pfizer/BioNTech, AstraZeneca, and Moderna vaccines, respectively (15). A total of 53,635 at-risk dialysis patients were included in this analysis, with an average follow-up time of 11.7 (SD 2.1) months (627,529 person-months) (Supplemental Table 1). Among them, 3620 SARS-CoV-2 severe infections were reported (Figure 1B, Supplemental Table 2). Notably, throughout the period of the study, we observed no significant change in the size of the dialysis population, which varied from 45,035 to 46,750 (M=46,212). The size of the at-risk dialysis population continuously changed because some patients entered the registry after the beginning of the study, while others were removed because they presented the end point (severe SARS-CoV-2 infection), died, or received a kidney transplant. In the meantime, 446,890 hospital admissions were reported in the general population (Figure 1B, Supplemental Table 2). Cumulative incidences of severe infections in dialysis patients were two- to 28-fold higher in dialysis patients compared with nondialysis patients, according to pandemic wave (first, second, or third) and age strata (Supplemental Table 2). Model M1 (using incident cases from the first pandemic wave) showed that during the first wave, incidence in dialysis patients was approximately proportional to incidence in the general population (Figure 1C, Table 1). Incidence rate ratios (IRRs) describe how a factor modulates the hospitalization risk of dialysis patients, assuming proportionality otherwise with the same-age general population. IRRs were higher in young dialysis patients: compared with patients aged 55–65 years, IRRs were 4.04 (95% confidence interval [CI], 2.38 to 6.51) and 0.87 (95% CI, 0.73 to 1.04) in patients aged 25–35 and 75–85 years, respectively. This counterintuitive result suggests that dialysis patients show a higher hospitalization risk than the general population globally, especially for younger dialysis patients, who have a relatively higher risk of hospitalization than older dialysis patients, when compared with the same-age general population (Table 2).
Figure 1.
Nationwide vaccination coverage and severe acute respiratory syndrome coronavirus 2 severe infections in patients needing maintenance dialysis and in the general population (adults aged ≥25 years). (A) Vaccine policies started in mid-January and first targeted patients with compromising health conditions such as dialysis patients among whom vaccination quickly reached a high coverage. National vaccination policies in the healthy population first targeted older individuals (nursing homes patients from December 27, patients aged >75 years from January 18, patients aged >65 years from early March, and patients aged >50 years from late March). (B) Incidence of severe infections in dialysis patients was approximately proportional to the dynamics in the general population during the first two pandemic waves. The relative incidence in dialysis patients dropped in early 2021 after the beginning of the vaccine policies. (C) Model M1 fitted on first-wave data failed to capture an incidence drop in dialysis patient incidence from the beginning of the second wave. Model M2 fitted on first- and second-wave data failed to capture the early 2021 dynamics of incidence in dialysis patients, contrary to model M3 using vaccine exposures as additional predictors.
Table 1.
Observed and predicted number of hospital admissions for severe acute respiratory syndrome coronavirus 2 infections in dialysis patients
| First Wave | Second Wave | Third Wave | Total | |
|---|---|---|---|---|
| Observed cases | 1233 | 1447 | 940 | 3620 |
| M1 predictions | 1265 (1156 to 1397) | 2092 (1888 to 2317) | 2021 (1874 to 2194) | 5378 (5031 to 5868) |
| M2 predictions | 1246 (1137 to 1377) | 1475 (1371 to 1577) | 1457 (1356 to 1557) | 4179 (3957 to 4382) |
| M3 predictions | 1258 (1190 to 1333) | 1461 (1386 to 1544) | 957 (938 to 998) | 3685 (3552 to 3816) |
Predicted number of severe infections (infection leading to hospital admissions) in dialysis patients with 95% confidence intervals from models M1 (fitted on first-wave data, March 11–June 30, 2020), M2 (fitted on first- and second-wave data, up to January 7, 2021), and M3 (fitted on all three waves, up to April 29, 2021).
Table 2.
Coefficients from the Poisson regression models for incident hospitalizations for severe acute respiratory syndrome coronavirus 2 infection in dialysis patients
| Variable | IRR (95% CI) |
|---|---|
| Model M1 | |
| Age class (years) | |
| 25–35 | 4.04 (2.38 to 6.51) |
| 35–45 | 2.57 (1.85 to 3.52) |
| 45–55 | 1.37 (1.06 to 1.76) |
| 55–65 | 1 (reference) |
| 65–75 | 0.85 (0.71 to 1.02) |
| 75–85 | 0.87 (0.73 to 1.04) |
| >85 | 0.90 (0.73 to 1.10) |
| Model M2 | |
| Age class (years) | |
| 25–35 | 2.82 (1.90 to 4.06) |
| 35–45 | 2.17 (1.70 to 2.75) |
| 45–55 | 1.26 (1.04 to 1.52) |
| 55–65 | 1 (reference) |
| 65–75 | 0.83 (0.74 to 0.94) |
| 75–85 | 0.74 (0.66 to 0.85) |
| >85 | 0.82 (0.72 to 0.95) |
| Pandemic wave ≥2 | 0.70 (0.64 to 0.76) |
| Model M3 | |
| Age class (years) | |
| 25–35 | 2.92 (2.03 to 4.09) |
| 35–45 | 2.19 (1.74 to 2.73) |
| 45–55 | 1.32 (1.11 to 1.56) |
| 55–65 | 1 (reference) |
| 65–75 | 0.88 (0.78 to 0.98) |
| 75–85 | 0.78 (0.70 to 0.88) |
| >85 | 0.86 (0.76 to 0.97) |
| Pandemic wave ≥2 | 0.70 (0.64 to 0.76) |
| Vaccination coverage in the general population (per 10% higher) | 0.50 (0.40 to 0.61) |
| Vaccine exposure in dialysis patients | 0.37 (0.18 to 0.71) |
| Age–vaccine interaction in dialysis patients (years) | |
| 25–35 | 0.00 (0.00 to 4.66) |
| 35–45 | 0.04 (0.00 to 0.46) |
| 45–55 | 0.55 (0.16 to 1.72) |
| 55–65 | Reference |
| 65–75 | 0.94 (0.43 to 2.11) |
| 75–85 | 1.74 (0.80 to 3.86) |
| >85 | 2.30 (0.95 to 5.56) |
Coefficients from the Poisson regression models are exponentiated and reported as incidence rate ratios (IRRs) with their 95% confidence intervals (95% CI).
We next studied the observed cases during the second wave. Predictions from model M1 overestimated incidence during the second wave, with 2092 predicted cases (95% CI, 1888 to 2317) versus 1447 reported (Figure 1C, Table 1). This suggests that prevention steps toward dialysis patients during the second wave were associated with a reduction in observed cases compared with predicted cases based on first-wave data.
We next developed model M2 based on data up to the end of the second wave. M2 estimated that the risk in dialysis patients decreased between waves 1 and 2, with an IRR of 0.70 (95% CI, 0.64 to 0.76; Table 2). While M2 correctly estimated the reported dialysis cases up to the end of the second wave (Figure 1C), predictions from this model overestimated the expected number of cases from the beginning of the vaccination campaign (third wave): 1457 (95% CI, 1356 to 1557) versus 940 reported (Figure 1C, Table 1). This suggests that other epidemiologic factors that occurred during the third wave have been associated with a reduction of observed cases compared with prediction modeled from the dynamics of the first and second waves. We thus hypothesized that vaccination policy might have been involved in this observation.
Using vaccination coverages as additional predictors in M3 allowed the overall incidence during the third wave to be estimated accurately (3685 predicted cases; 95% CI, 3552 to 3816 versus 3620 reported) and the weekly reported number of cases to be approximated correctly (Figure 1C, Table 1). Interestingly, using M3, the IRR was 0.37 (95% CI, 0.18 to 0.71) for vaccine exposure in dialysis patients, showing around a three-fold reduction of hospitalization risk associated with vaccination. Moreover, for dialysis patients, the IRR was 0.50 (95% CI, 0.40 to 0.61) per 10% higher vaccination coverage in the same-age general population, showing an inverse association between incidence of severe infections in dialysis patients and vaccination of the general population. This suggests that vaccine exposure in both dialysis patients and the general population was independently associated with a reduction of hospitalization rates in dialysis patients when compared with the same-age general population (Table 2). As for estimates from previous models, IRRs calculated from model M3 in dialysis patients were higher in younger patients: compared with dialysis patients aged 55–65 years, IRRs were 2.92 (95% CI, 2.03 to 4.09) and 0.78 (95% CI, 0.70 to 0.88) in dialysis patients aged 25–35 and 75–85 years, respectively. This observation using model M3 confirms that young dialysis patients had a higher risk than older ones throughout the follow-up when compared with the same-age general population. We then hypothesized that IRRs associated with vaccine exposure may change according to age strata; therefore, we estimated the interaction between age and vaccine exposure in dialysis patients. We thus identified a lower IRR for vaccine exposure in younger patients: compared with patients aged 55–65 years, IRRs were 0.04 (95% CI, 0.00 to 0.46) and 1.74 (95% CI, 0.80 to 3.86) in dialysis patients aged 25–35 and 75–85 years, respectively. These results suggest a higher impact of vaccination in reducing hospital admissions for younger dialysis patients compared with the same-age general population (Supplemental Table 3). Coefficients from these models are reported in Table 2. Sensitivity analysis using 3-week lags in both populations disclosed similar results (Supplemental Table 4).
Discussion
By modeling the dynamics of SARS-CoV-2 infections leading to hospital admissions among dialysis patients in France as a proportion of cases reported in the general population, we identified a relative reduction in cases in the second wave compared with a model based on the first wave only. This result suggests that, accounting for the epidemiologic trends in the general population, the hospitalization rate of dialysis patients in the second wave was lower than expected from data from the first wave. This observation may be explained by the progressive implementation of prevention steps, earlier testing, and better management of COVID-19 in dialysis patients during the second wave. Indeed, since the beginning of the pandemic, protective strategies have been broadcasted by the French Society of Nephrology with weekly COVID-19 webinars. However, this effect could also be confounded by a trend to limit hospitalization of dialysis patients with milder cases. Notably, although a similar downward trend in COVID-19–related hospitalization and mortality since the first wave has been observed in the general population in the United States (19), our results suggest an added reduction in adverse outcomes in dialysis patients.
Moreover, our models also suggest a reduction in hospitalized cases starting in early 2021 compared with a model based on the first and second waves only. Therefore, we hypothesized that vaccination exposure could have had a role in limiting hospital admissions in dialysis patients, who were prioritized by French vaccine policies (Figure 1A). Taking into account vaccine exposure in both the general population and dialysis patients using model M3, (1) we could adequately predict the observed incidence during the third wave, and (2) we found that the reduction of cases was independently associated with vaccine exposure in both dialysis patients and the same-age general population. These findings suggest that vaccination may have a protective effect from the first dose in dialysis patients. Moreover, these patients may indirectly benefit from vaccine policies at the population scale, probably thanks to lessened exposure to the virus from their encounters.
Interestingly, we quantified an IRR of 0.37 (95% CI, 0.18 to 0.71) for vaccine exposure in dialysis patients, meaning that vaccination was associated with around a three-fold reduction in hospitalization rate for dialysis patients when accounting for the epidemiologic trends in the same-age general population (Table 2). In the context of a more contagious new variant and waning antibody levels in vaccinated patients, the statistical method presented in this study could allow the future persistence/decline of the protective effect of vaccination in dialysis patients to be quantified at the population scale.
Importantly, in our study, vaccine exposure data were dependent on weekly reports from dialysis centers. Vaccine exposure could therefore be overestimated because dialysis centers with high vaccination rates might tend to report their data better. However, despite this potential overestimation that would tend to undervalue the preventive effect of vaccination, we were able to show a reduction of hospitalization rates associated with vaccine exposure in dialysis patients. Consequently, vaccine exposure appears as a major factor associated with lower hospitalization in dialysis patients in early 2021.
Using our different models, we detected that IRRs were higher in younger dialysis patients compared with older dialysis patients. An explanation might be that dialysis is an additional risk factor for all patients, but this higher risk is not as important in older patients (in whom age is already a major risk factor) as it is in younger ones in whom severe infections are very rare in the general population (1). We thus hypothesized that vaccine impact on hospital admissions may also vary according to age strata. Indeed, we identified an interaction between age and vaccine exposure in dialysis patients, leading to a lower IRR for vaccine exposure in younger dialysis patients (Table 2). This result suggests a higher impact of vaccination in lowering hospital admissions for younger dialysis patients compared with older dialysis patients, accounting for the epidemiologic trends in the same-age general population (Supplemental Table 3). These data could be related to a better vaccine response in young dialysis patients, as suggested in the general population (20). However, vaccine policies in the general population may also explain this interaction. Indeed, in early 2021, vaccine policies for the nondialysis population targeted older individuals (nursing home patients from December 27, patients aged >75 years from January 18, patients aged >65 years from early March, and patients aged >50 years from late March). Consequently, it is likely that a higher benefit of vaccine exposure in young dialysis patients could be linked to the low vaccination coverage in the same-age general population (Figure 1A).
The strength of our analysis is the large number of patients followed in the dialysis population since the beginning of the SARS-CoV-2 spread in France, thanks to the nationwide REIN registry (2,10). However, in the absence of individual data on vaccination, we were not able to evaluate the individual risk of severe COVID-19 after vaccination (21). Moreover, our models did not take into account the impact of previous SARS-CoV-2 asymptomatic or pauci-symptomatic infection, given the difficulty in reliably detecting such infections in dialysis patients (5,22). Besides, whether this analysis could be extended to other populations with different prevention measures (including types of vaccines and vaccination schemes) remains to be studied. Of note, our analyses ended before the strategy of a third vaccine injection started in France (in late April 2021). Lastly, our data did not include important virologic factors such as the spread of variant of concern types in both populations (23). However, no evidence to date suggests that SARS-CoV-2 variants may differently impact the relative susceptibility of dialysis patients compared with nondialysis ones.
In conclusion, this study identifies vaccination coverage in both dialysis patients and the general population as independently associated with protection against severe infection in dialysis patients. Although causal relationships cannot be demonstrated from such an observational study, our findings suggest that both individual and herd vaccine-induced immunity may yield a protective effect against severe forms of COVID-19 in dialysis patients.
Disclosures
C. Couchoud reports serving on the Editorial Boards of BMC Nephrology, CJASN, and Nephrologie et Thérapeutique and other interests or relationships as a member of a not-for-profit association that aims to promote collaborations and research in Renal Epidemiology “L’Association des Néphrologues pour la Recherche en Epidémiologie (EPINEPHRO).” K. El Karoui reports employment with INSERM U955; research funding from Amgen, Otsuka, and Sanofi; and honoraria from Alexion, AstraZeneca, and Otsuka. F. Glowacki reports research funding from Sanofi and honoraria from AstraZeneca. M. Hourmant reports ownership interest in Sanofi. All remaining authors have nothing to disclose.
Funding
None.
Supplementary Material
Acknowledgments
We gratefully acknowledge all participants of the REIN registry—nephrologists and research assistants alike. The centers participating in the registry are listed in the REIN annual report: http://www.agence-biomedecine.fr/Le-programme-REIN.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Growing Understanding of the Clinical and Serologic Effects of COVID-19 Vaccines in Patients Undergoing Long-Term Dialysis,” on pages 335–337.
Author Contributions
C. Ayav, C. Couchoud, and N. Lapidus curated the data; C. Couchoud, K. El Karoui, and N. Lapidus conducted the formal analysis, were responsible for validation and visualization, and reviewed and edited the manuscript; C. Couchoud, F. Glowacki, M. Hourmant, and N. Lapidus carried out the investigation; C. Couchoud and N. Lapidus were responsible for the methodology; C. Couchoud was responsible for resources; C. Couchoud, K. El Karoui, M. Hourmant, and N. Lapidus provided supervision and wrote the original draft of the manuscript; K. El Karoui and N. Lapidus conceptualized the study; and N. Lapidus was responsible for software.
Data Sharing Statement
All data used in this study are available in this article.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.10300721/-/DCSupplemental.
Supplemental Summary 1. Collaborator information.
Supplemental Table 1. Description of the study populations.
Supplemental Table 2. Crude number of hospitalizations, total at-risk population, cumulative incidence in dialysis and the general population, and relative risk of hospitalization according to age classes and pandemic waves.
Supplemental Table 3. Relative risks (95% confidence intervals) of severe infection in dialysis patients, predicted from model M3, between April 2 and April 8, 2021, in Paris.
Supplemental Table 4. Coefficients from the Poisson regression (exponentiated and reported as incidence rate ratios with their 95% confidence intervals): model M3 (3- and 5-week lags between first dose and expected protection in the general population and in dialysis patients, respectively) and additional model with 3-week lags for all.
References
- 1.Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, Curtis HJ, Mehrkar A, Evans D, Inglesby P, Cockburn J, McDonald HI, MacKenna B, Tomlinson L, Douglas IJ, Rentsch CT, Mathur R, Wong AYS, Grieve R, Harrison D, Forbes H, Schultze A, Croker R, Parry J, Hester F, Harper S, Perera R, Evans SJW, Smeeth L, Goldacre B: Factors associated with COVID-19-related death using OpenSAFELY. Nature 584: 430–436, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Couchoud C, Bayer F, Ayav C, Béchade C, Brunet P, Chantrel F, Frimat L, Galland R, Hourmant M, Laurain E, Lobbedez T, Mercadal L, Moranne O; French REIN registry : Low incidence of SARS-CoV-2, risk factors of mortality and the course of illness in the French national cohort of dialysis patients. Kidney Int 98: 1519–1529, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jager KJ, Kramer A, Chesnaye NC, Couchoud C, Sánchez-Álvarez JE, Garneata L, Collart F, Hemmelder MH, Ambühl P, Kerschbaum J, Legeai C, Del Pino Y Pino MD, Mircescu G, Mazzoleni L, Hoekstra T, Winzeler R, Mayer G, Stel VS, Wanner C, Zoccali C, Massy ZA: Results from the ERA-EDTA Registry indicate a high mortality due to COVID-19 in dialysis patients and kidney transplant recipients across Europe. Kidney Int 98: 1540–1548, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Meester J, De Bacquer D, Naesens M, Meijers B, Couttenye MM, De Vriese AS; NBVN Kidney Registry Group : Incidence, characteristics, and outcome of COVID-19 in adults on kidney replacement therapy: A regionwide registry study. J Am Soc Nephrol 32: 385–396, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clarke C, Prendecki M, Dhutia A, Ali MA, Sajjad H, Shivakumar O, Lightstone L, Kelleher P, Pickering MC, Thomas D, Charif R, Griffith M, McAdoo SP, Willicombe M: High prevalence of asymptomatic COVID-19 infection in hemodialysis patients detected using serologic screening. J Am Soc Nephrol 31: 1969–1975, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cherif A, Willetts JL, Usvyat L, Wang Y, Kotanko P: Comparative analysis of SARS-CoV-2 reproduction rates in the dialysis and general populations. J Am Soc Nephrol 32: 791–794, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dagan N, Barda N, Kepten E, Miron O, Perchik S, Katz MA, Hernán MA, Lipsitch M, Reis B, Balicer RD: BNT162b2 mRNA COVID-19 vaccine in a nationwide mass vaccination setting. N Engl J Med 384: 1412–1423, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ikizler TA, Coates PT, Rovin BH, Ronco P: Immune response to SARS-CoV-2 infection and vaccination in patients receiving kidney replacement therapy. Kidney Int 99: 1275–1279, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Praet J, Reynders M, De Bacquer D, Viaene L, Schoutteten MK, Caluwé R, Doubel P, Heylen L, De Bel AV, Van Vlem B, Steensels D, De Vriese AS: Predictors and dynamics of the humoral and cellular immune response to SARS-CoV-2 mRNA vaccines in hemodialysis patients: A multicenter observational study. J Am Soc Nephrol 32: 3208–3220, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Couchoud C, Stengel B, Landais P, Aldigier J-C, de Cornelissen F, Dabot C, Maheut H, Joyeux V, Kessler M, Labeeuw M, Isnard H, Jacquelinet C: The renal epidemiology and information network (REIN): A new registry for end-stage renal disease in France. Nephrol Dial Transplant 21: 411–418, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B: Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 395: 1054–1062, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hozé N, Paireau J, Lapidus N, Tran Kiem C, Salje H, Severi G, Touvier M, Zins M, de Lamballerie X, Lévy-Bruhl D, Carrat F, Cauchemez S: Monitoring the proportion of the population infected by SARS-CoV-2 using age-stratified hospitalisation and serological data: A modelling study. Lancet Public Health 6: e408–e415, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salje H, Tran Kiem C, Lefrancq N, Courtejoie N, Bosetti P, Paireau J, Andronico A, Hozé N, Richet J, Dubost CL, Le Strat Y, Lessler J, Levy-Bruhl D, Fontanet A, Opatowski L, Boelle PY, Cauchemez S: Estimating the burden of SARS-CoV-2 in France. Science 369: 208–211, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lapidus N, Paireau J, Levy-Bruhl D, de Lamballerie X, Severi G, Touvier M, Zins M, Cauchemez S, Carrat F; SAPRIS-SERO study group : Do not neglect SARS-CoV-2 hospitalization and fatality risks in the middle-aged adult population. Infect Dis Now 51: 380–382, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.data.gouv.fr : Données relatives aux personnes vaccinées contre la Covid-19 (VAC-SI). Available at: https://www.data.gouv.fr/en/datasets/donnees-relatives-aux-personnes-vaccinees-contre-la-covid-19-1/. Accessed July 1, 2021
- 16.Haas EJ, Angulo FJ, McLaughlin JM, Anis E, Singer SR, Khan F, Brooks N, Smaja M, Mircus G, Pan K, Southern J, Swerdlow DL, Jodar L, Levy Y, Alroy-Preis S: Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: An observational study using national surveillance data. Lancet 397: 1819–1829, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walsh EE, Frenck RW Jr, Falsey AR, Kitchin N, Absalon J, Gurtman A, Lockhart S, Neuzil K, Mulligan MJ, Bailey R, Swanson KA, Li P, Koury K, Kalina W, Cooper D, Fontes-Garfias C, Shi PY, Türeci Ö, Tompkins KR, Lyke KE, Raabe V, Dormitzer PR, Jansen KU, Şahin U, Gruber WC: Safety and immunogenicity of two RNA-based COVID-19 vaccine candidates. N Engl J Med 383: 2439–2450, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramasamy MN, Minassian AM, Ewer KJ, Flaxman AL, Folegatti PM, Owens DR, Voysey M, Aley PK, Angus B, Babbage G, Belij-Rammerstorfer S, Berry L, Bibi S, Bittaye M, Cathie K, Chappell H, Charlton S, Cicconi P, Clutterbuck EA, Colin-Jones R, Dold C, Emary KRW, Fedosyuk S, Fuskova M, Gbesemete D, Green C, Hallis B, Hou MM, Jenkin D, Joe CCD, Kelly EJ, Kerridge S, Lawrie AM, Lelliott A, Lwin MN, Makinson R, Marchevsky NG, Mujadidi Y, Munro APS, Pacurar M, Plested E, Rand J, Rawlinson T, Rhead S, Robinson H, Ritchie AJ, Ross-Russell AL, Saich S, Singh N, Smith CC, Snape MD, Song R, Tarrant R, Themistocleous Y, Thomas KM, Villafana TL, Warren SC, Watson MEE, Douglas AD, Hill AVS, Lambe T, Gilbert SC, Faust SN, Pollard AJ; Oxford COVID Vaccine Trial Group : Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): A single-blind, randomised, controlled, phase 2/3 trial. Lancet 396: 1979–1993, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ioannou GN, O’Hare AM, Berry K, Fan VS, Crothers K, Eastment MC, Locke E, Green P, Shah JA, Dominitz JA: Trends over time in the risk of adverse outcomes among patients with SARS-CoV-2 infection [published online ahead of print May 11, 2021]. Clin Infect Dis 10.1093/cid/ciab419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Müller L, Andrée M, Moskorz W, Drexler I, Walotka L, Grothmann R, Ptok J, Hillebrandt J, Ritchie A, Rabl D, Ostermann PN, Robitzsch R, Hauka S, Walker A, Menne C, Grutza R, Timm J, Adams O, Schaal H: Age-dependent immune response to the Biontech/Pfizer BNT162b2 COVID-19 vaccination. Clin Infect Dis 73: 2065–2072, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agence de la biomédecine : Les chiffres du R.E.I.N., 2021. Available at: https://www.agence-biomedecine.fr/Les-chiffres-du-R-E-I-N. Accessed July 2, 2021
- 22.Creput C, Fumeron C, Toledano D, Diaconita M, Izzedine H: COVID-19 in patients undergoing hemodialysis: Prevalence and asymptomatic screening during a period of high community prevalence in a large Paris center. Kidney Med 2: 716–723.e1, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tao K, Tzou PL, Nouhin J, Gupta RK, de Oliveira T, Kosakovsky Pond SL, Fera D, Shafer RW: The biological and clinical significance of emerging SARS-CoV-2 variants. Nat Rev Genet 22: 757–773, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.