Direct infection of the kidney by severe acute respiratory syndrome coronavirus 2 has been proposed as a mechanism of AKI in coronavirus disease 2019 (COVID-19) (1). Severe acute respiratory syndrome coronavirus 2 enters the host cell via the angiotensin-converting enzyme II protein, which is expressed in renal tubular epithelial cells (2,3). Medications that inhibit the renin-angiotensin-aldosterone system (RAASi) have been postulated to upregulate expression of angiotensin-converting enzyme II and increase infectivity and organ dysfunction (4). The effect of RAASi use on AKI in COVID-19 is not known.
We previously examined AKI incidence in COVID-19 compared with influenza in a national veteran cohort and found that AKI was more common and severe in COVID-19 (5). In this follow-up evaluation, we used similar methods to compare the risk of AKI by RAASi status among 27,189 veterans hospitalized with either COVID-19 or influenza between October 1, 2019 and February 28, 2021. The primary outcome was AKI, which was defined using peak in-hospital serum creatinine and staged using modified Kidney Disease Improving Global Outcomes creatinine-based criteria: stage 1, ≥0.3 mg/dl creatinine increase from baseline or 1.5–1.9 times baseline; stage 2, creatinine 2.0–2.9 times baseline; and stage 3, creatinine 3.0 times baseline or initiation of dialysis. The primary exposure was prevalent RAASi use, which was defined as having pills on hand within 14 days before admission. Nonuse was defined by no RAASi fills within 365 days before admission. To control for confounding, propensity score weighting (i.e., matching weights) was used to balance baseline demographics (four variables), vitals (six variables), conditions (20 variables), and medications (17 variables) in four exposure groups: RAASi users with COVID-19, nonusers with COVID-19, RAASi users with influenza, and nonusers with influenza. All standardized mean differences for variables used for propensity score weights were <0.1 in the weighted cohort (across all four exposure groups and by pairwise comparison). A logistic regression model using the weighted cohort estimated the main effects of RAASi exposure and COVID-19 and their interaction. Statistical analyses were conducted using R software version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria).
Among RAASi nonusers, we identified 1908 hospitalizations for influenza and 15,028 hospitalizations for COVID-19; among RAASi users, we identified 1276 hospitalizations for influenza and 8977 hospitalizations for COVID-19. Incidence rates of AKI are shown in Figure 1. Among all-comers, AKI was more common among patients with COVID-19 versus influenza (30% versus 26%, P<0.001) and was associated with a 23% higher odds of AKI (odds ratio [OR], 1.23; 95% confidence interval [95% CI], 1.10 to 1.37). The incidence of AKI was greater in RAASi users compared with nonusers in both the influenza and COVID-19 groups, with a 26% higher odds of AKI among all-comers (OR, 1.26; 95% CI, 1.13 to 1.40). The association of RAASi use and AKI was not proportionally different between COVID-19 and influenza (P=0.52; OR, 1.07; 95% CI, 0.87 to 1.33 for RAASi by COVID-19 interaction). Stage 2–3 AKI was more common in COVID-19 versus influenza (11% versus 6%, P<0.001; OR, 1.87; 95% CI, 1.50 to 2.32). The incidence of Stage 2–3 AKI was greater in RAASi users (10% versus 7%, P<0.001; OR, 1.55; 95% CI, 1.29 to 1.85). Similarly, the interaction was not statistically significant (P=0.31; OR, 0.79; 95% CI, 0.51 to 1.24).
Figure 1.
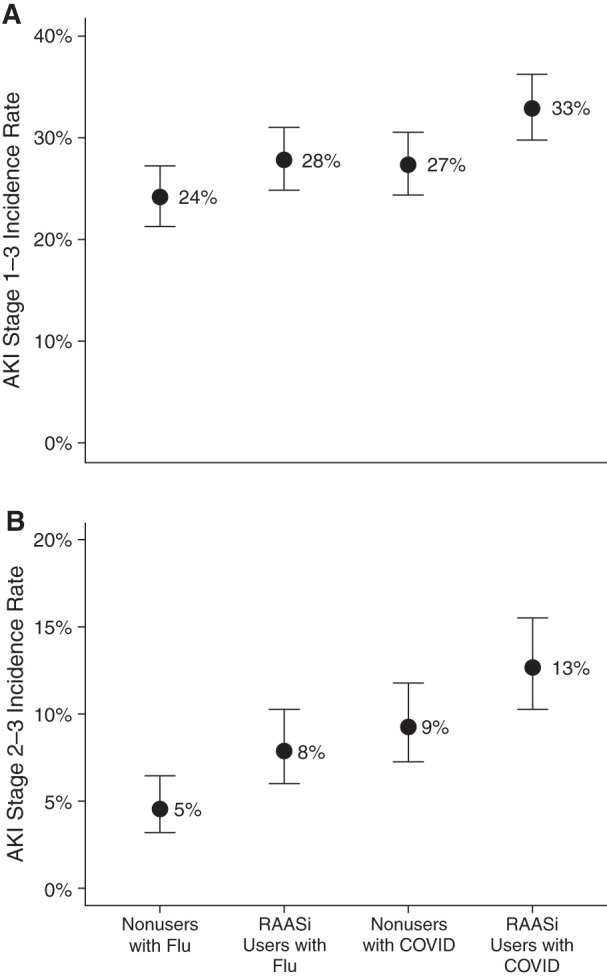
Incidence rates of (A) AKI Stage 1–3 and (B) AKI Stage 2–3 in medications that inhibit the renin-angiotensin-aldosterone (RAASi) nonusers with influenza, RAASi users with influenza, RAASi nonusers with coronavirus disease 2019 (COVID-19), and RAASi users with COVID-19. Proportion of patients in each exposure group with AKI (weighted cohort). Error bars represent 95% confidence intervals. Note the similar proportional increase in AKI incidence with RAASi exposure in both the COVID-19 and influenza groups.
In this study, we observed a higher risk of AKI associated with RAASi use in both influenza and COVID-19, a finding that has been observed in other clinical settings and consistent here when examining stage 2–3 AKI. To our knowledge, this study is the first to examine the effect of RAASi use on AKI in COVID-19. Although our findings do not rule out the possibility of a unique pathophysiology of AKI in COVID-19, the proportionally similar risk associated with RAASi use in both illnesses does not support the hypothesis of direct infectivity amplified by RAASi as a major mechanism of AKI in COVID-19. Although RAASi use does not appear to carry a unique AKI risk in patients with COVID-19, our findings underscore the importance of careful assessment of an individual’s AKI risk factors when considering RAASi use in patients who are hospitalized.
The strengths of our study include the use of national data and rigorous attempts to adjust for baseline conditions that could affect AKI risk. Limitations include the possibility of confounding by illness severity or other in-hospital factors; however, we anticipate that the greater illness severity in the COVID-19 group would result in a greater risk of AKI with RAASi use compared with influenza and bias our results away from the null. Our definition of RAASi use (pills on hand within 14 days of admission) aimed to enrich for patients actively taking RAASi; however, we acknowledge as a limitation that this does not guarantee medication adherence. Other limitations include a lack of histologic data and reduced generalizability to females and non-White patients.
Disclosures
A.M. Hung reports employment with Veterans Affairs; reports receiving research funding from Department of Veterans Affairs Clinical Service Research and Development Merit “Genetics of Kidney Disease & Hypertension, Risk Prediction and Drug Response” and a Vertex Grant to Vanderbilt University Medical Center; and reports serving as Co-Chair of Million Veteran Program Publications & Presentation committee of Veterans Affairs, Co-chair of Pharmacogenomics for COVID-19 Million Veteran Program, Section Editor of Clinical Nephrology for Journal of Renal Nutrition, standing member of Scientific Review Committee Health Services Research and Development (HSR&D) bioinformatics, ad hoc Scientific Review Committee National Heart Lung and Blood Institute, ad hoc Scientific Review Committee CSR&D, and ad hoc Scientific Review Committee Kidney, Nutrition, Obesity and Diabetes. E.D. Siew reports employment with Nashville Veterans Affairs; reports having consultancy agreements with Akebia Therapeutics on 4/19; reports receiving honoraria for an invited educational talk on AKI epidemiology at the DaVita Annual Physician Leadership Conference 2/19; reports serving as an Associate Editor of CJASN; and reports receiving royalties as an author for UpToDate. M.E. Matheny reports employment with the Department of Veterans Affairs; reports having consultancy agreements with National Institutes of Health-Department of Veterans Affairs-Department of Defense Pain Management Grant Consortium; and reports serving as a scientific advisor or member of Steering Committee for the Indianapolis Department of Veterans Affairs (VA) HSR&D Center of Innovation Center, Steering Committee of the Scientific Merit Review Board Study Section, VA HSR&D, Informatics & Methods Section, and VA HSR&D VA Information Resource Center. S.C. Shah reports having consultancy agreements with Phathom Pharmaceuticals. All remaining authors have nothing to disclose.
Funding
This work was supported by the Veterans Affairs HSR&D grants COVID-19 Rapid Response Project C19 20-214 and T32DK007569-32 (to B.C. Birkelo); by the Million Veteran Program grant SDR 18-194; and the Vanderbilt O’Brien Kidney Center P30-DK114809 Clinical and Translational Research Core (to E.D. Siew).
Acknowledgments
A preliminary version was presented in abstract form at the American Society of Nephrology Kidney Week in November 2021. Because Dr. Edward D. Siew is an Associate Editor of CJASN, he was not involved in the peer review process for this manuscript. Another editor oversaw the peer review and decision-making process for this manuscript.
Author Contributions
J.P. Arroyo Ornelas, B.C. Birkelo, R.A. Greevy, A.M. Hung, M.E. Matheny, S.K. Parr, A.M. Perkins, S.C. Shah, and E.D. Siew conceptualized the study; M.E. Matheny, S.K. Parr, and A.J. Vincz were responsible for data curation; R.A. Greevy, M.E. Matheny, A.M. Perkins, and E.D. Siew were responsible for the formal analysis; M.E. Matheny and E.D. Siew were responsible for the funding acquisition; J.P. Arroyo Ornelas, B.C. Birkelo, R.A. Greevy, A.M. Hung, T. Kapoor, M.E. Matheny, S.K. Parr, S.C. Shah, and E.D. Siew were responsible for the investigation; J.P. Arroyo Ornelas, B.C. Birkelo, R.A. Greevy, A.M. Hung, M.E. Matheny, S.K. Parr, A.M. Perkins, S.C. Shah, and E.D. Siew were responsible for the methodology; M.E. Matheny and E.D. Siew were responsible for the resources; R.A. Greevy, M.E. Matheny, and E.D. Siew provided supervision; B.C. Birkelo, R.A. Greevy, S.K. Parr, A.M. Perkins, and E.D. Siew wrote the original draft; and J.P. Arroyo Ornelas, B.C. Birkelo, R.A. Greevy, A.M. Hung, T. Kapoor, M.E. Matheny, S.K. Parr, A.M. Perkins, S.C. Shah, E.D. Siew, and A.J. Vincz reviewed and edited the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Ng JH, Bijol V, Sparks MA, Sise ME, Izzedine H, Jhaveri KD: Pathophysiology and pathology of acute kidney injury in patients with COVID-19. Adv Chronic Kidney Dis 27: 365–376, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL: A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579: 270–273, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang YM, Zhang H: Genetic roadmap for kidney involvement of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Clin J Am Soc Nephrol 15: 1044–1046, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soler MJ, Barrios C, Oliva R, Batlle D: Pharmacologic modulation of ACE2 expression. Curr Hypertens Rep 10: 410–414, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birkelo BC, Parr SK, Perkins AM, Greevy RA Jr, Hung AM, Shah SC, Arroyo JP, Denton J, Vincz AJ, Matheny ME, Siew ED: Comparison of COVID-19 versus influenza on the incidence, features, and recovery from acute kidney injury in hospitalized United States Veterans. Kidney Int 100: 894–905, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]


