Abstract
Patients who receive a kidney transplant commonly experience failure of their allograft. Transplant failure often comes with complex management decisions, such as when and how to wean immunosuppression and start the transition to a second transplant or to dialysis. These decisions are made in the context of important concerns about competing risks, including sensitization and infection. Unfortunately, the management of the failed allograft is, at present, guided by relatively poor-quality data and, as a result, practice patterns are variable and suboptimal given that patients with failed allografts experience excess morbidity and mortality compared with their transplant-naive counterparts. In this review, we summarize the management strategies through the often-precarious transition from transplant to dialysis, highlighting the paucity of data and the critical gaps in our knowledge that are necessary to inform the optimal care of the patient with a failing kidney transplant.
Key words: kidney transplantation, chronic graft deterioration, transplant nephrectomy, transplant outcomes, allografts, Kidney Transplantation Series
Introduction
Much of the focus of kidney transplantation is invested into guiding patients through listing, waitlist management, and transplant, with the goal of preserving allograft function for as long as possible (1,2). Although kidney transplantation outcomes in the short term have shown significant gains over time, improvements in long-term outcomes have been less impressive, thus ensuring that many recipients of a transplant will experience a failed allograft and the attendant physical and emotional consequences (3). Currently, one in five patients will lose their kidney transplant within 5 years, and over half will experience allograft loss by 10 years (4). With graft failure often comes unique and challenging management considerations, including questions related to relisting, continued immunosuppression exposure, graft nephrectomy, and overseeing dialysis initiation. Such decisions often represent competing risks that are subsequently magnified by fragmented transitions of care among providers and health systems.
Allograft loss is associated with a high burden of psychologic and medical morbidity, with patients faring poorly across many traditional measures of quality of care, including anemia management and phosphate control (5,6). These patients experience a higher risk of hospitalization, including an associated 1.5-fold higher risk of infection-related hospitalizations and an association with lower quality-of-life scores, lower physical functioning, and higher burden of depression (6). Patients with failed allografts who return to dialysis are also noted to have notably excess mortality (7). This higher risk of mortality on dialysis with a failed graft is seen across the full age spectrum from <18 years to >70 years of age—in both individuals with and without diabetes—and includes a nearly three-fold higher risk in infection-related mortality (6,8). Despite the relatively large number of patients affected by the deleterious consequences of allograft failure, the subject has received little attention from the transplant community, and practice patterns continue to vary widely across centers. In this review, we summarize prevailing management criteria for the failing allograft, highlighting gaps in knowledge that would ensure optimal care of patients through this challenging transition.
Relisting and Repeat Transplant
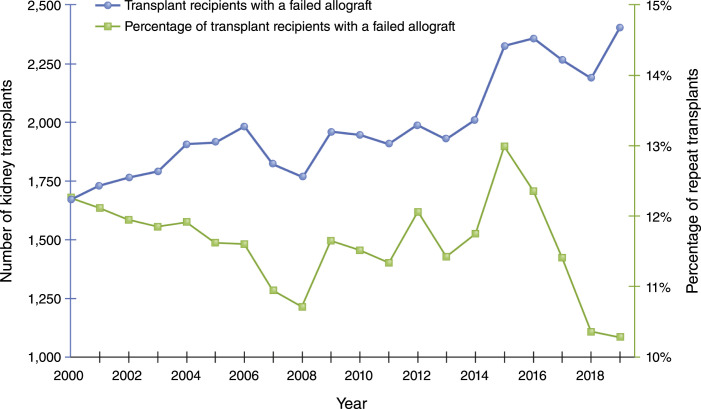
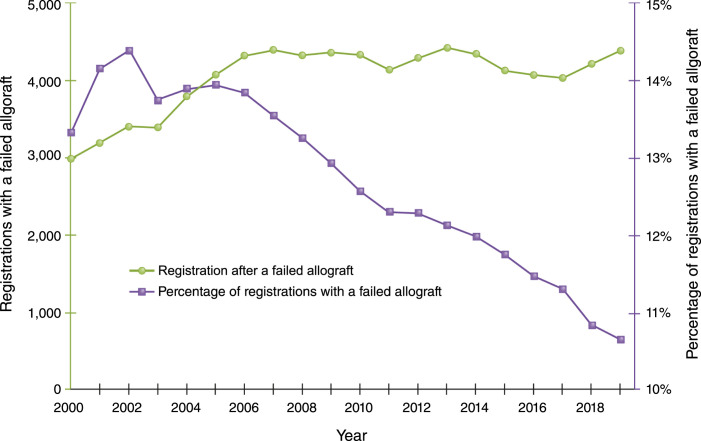
Over the past 2 decades, transplantation after a failed allograft has increased slowly in absolute terms and actually decreased overall in relative terms (Figure 1), which is somewhat surprising given the rising number of total transplants and the modest gains in long-term allograft outcomes. Preemptive waitlisting and transplantation is recognized as the transplantation strategy associated with the best outcomes for patients with kidney failure. Currently, in the United States, individuals are allowed to be waitlisted and start to accrue allocation time once their kidney function is <20 ml/min. This approach is perhaps underused and rife with disparities that are, at least in part, linked to inadequate provider knowledge/awareness (9–11). Individuals with failing allografts, despite having successfully navigated the system for their first transplant and having established relationships with a transplant center, are placed on the waitlist preemptively less frequently than expected—even with provider awareness not being a limiting factor (12). The rates of preemptive relisting and/or transplantation appear to be highly variable across transplant centers, declining over time, and significantly lower among racial minorities and socioeconomically disadvantaged populations (12). The marked variation in clinical practice underscores the absence of a clear focus on optimizing this transition, or consensus on the management of patients with failing allografts with respect to relisting and repeat transplantation (12). Perhaps more concerning is the fact that, although the total number of candidates being added to the waitlist has increased steadily, the number of candidates with a prior transplant has not kept up; instead, the absolute number of such candidates has remained relatively flat since 2007, resulting in a sharp decline as a proportion of the total number of candidates added to the waitlist (Figure 2). Although there are increasingly older patients receiving their first transplant, there is likely a fairly equal number of older patients receiving their second transplant, given the comparable outcomes between the two, and this cannot wholly account for the trend. This reduction in the proportion of patients retransplanted is seen despite the fact that there is increasing evidence demonstrating acceptable allograft and patient outcomes with a second and third transplant, and the exclusion of these individuals from punitive regulatory oversight in the United States in regard to patient survival metrics (3,13).
Figure 1.
Percentage of transplants after failed allograft has decreased over time.
Figure 2.
Percentage of waitlist registrations for candidates with failed (or failing) allografts have decreased over time.
In the current allocation system and with variable rapidity of decline in failing allografts, only a minority of patients with failing allografts will receive preemptive transplants. The overwhelming majority of patients will instead need KRT, and these patients must be adequately prepared for this transition. Despite being under the care of nephrologists for extended periods, two thirds of patients with failed allografts initiate dialysis with a catheter—and >50% have no permanent access already in place—contrary to the goals of the Fistula-First Breakthrough Initiative (14,15). While these proportions are marginally better than the overall incident kidney failure population, these are likely to be underestimates of the failure of nephrologists, given that many patients potentially continue to have functioning accesses that were placed before their initial transplant. Rather, these numbers underscore the failure of transplant centers and nephrologists who are caring for patients with failing allografts to adequately prepare their patients for life without a functioning kidney transplant on multiple fronts, beyond vascular access (14).
Immunosuppression Management
Immunosuppression management is one of the most important and complicated aspects in caring for patients with failing and failed allograft, given the potential for affecting the probability of repeat transplant, protecting residual kidney function, and preventing the development of graft-intolerance syndrome, which can be both painful and precipitate the need for a graft nephrectomy.
Although reduced immunosuppression is commonly recommended in failing allografts, this approach may be counterintuitive in some individuals—such as those with ongoing, chronic, antibody-mediated rejection—and may contribute to more rapid loss of the allograft. One strategy to consider in such patients is the late conversion to belatacept-based regimens, which may allow for an improvement in kidney function and retard the rate of decline, primarily from the elimination of calcineurin inhibitors coupled with improvement in acidosis and other metabolic parameters (16,17). Although there is reason for cautious optimism in this approach for prolonging the life of a failing allograft, supporting data remain limited to small observational cohorts and more robust prospective data are needed (18). This strategy may have the additional benefit of lowering the possible development of de novo donor-specific antibodies, which, if confirmed, would have significant prognostic value for subsequent transplants and is now the subject of a prospective clinical trial (NCT01921218).
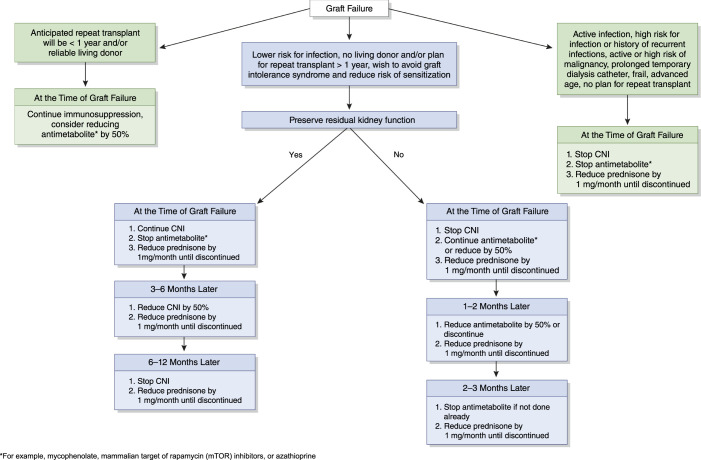
Upon allograft failure, the benefits of continued immunosuppression must be weighed against the risk of complications from their ongoing exposure, such as infection, malignancy, secondary adrenal insufficiency, and cost. The higher risk of morbidity and mortality immediately after dialysis initiation for individuals with failed allografts appears to be driven largely by infection, underscoring the need for a better understanding of the appropriate immunosuppression strategy that would weigh the risks of infections with the benefit of avoiding sensitization, which would result from abrupt cessation of immunosuppression (Figure 3) (19). This risk/benefit calculation is not a one-size-fits-all approach, and it has to take into consideration the likely interval before a subsequent transplant and the current kidney allocation policy, which prioritizes the most sensitized of patients to help mitigate the effect of sensitization on access to a future transplant (20).
Figure 3.
A suggested approach to immunosuppression weaning upon allograft failure. CNI, calcineurin inhibitor.
Unfortunately, most data on immunosuppression strategies are restricted to single-center, retrospective analyses that provide little guidance on when and how to reduce medications. Consequently, practices vary widely regarding who takes ownership for management decisions (transplant versus dialysis center) and protocols for how immunosuppression withdrawal is carried out, including the duration of taper and the sequence in which each medication is reduced (21,22). Fractured care that occurs across transitions of care, coupled with this profound knowledge gap, likely plays a role in the excess morbidity and mortality among this patient population, and highlights the urgent need for quality, focused clinical research to inform clinical practice (19).
Sensitization is a primary concern for patients who may be eligible for a repeat transplant. Prior studies have demonstrated that complete withdrawal of immunosuppression is associated with a higher risk for the formation of human leukocyte antigen antibodies, although the interval over which the immunosuppression was weaned appears to be influential (20,22,23). The immunosuppression taper may also need to be individualized on the basis of the degree to which the original donor and recipient were mismatched (23). Increasing data support eplet mismatch as more precise than whole-antigen mismatch to risk stratify patients for the development of donor-specific antibodies during transplant and may also prove to be a useful tool to guide immunosuppression management upon graft failure (24).
It has also been argued that continued immunosuppression in the failed allograft may preserve residual kidney function. However, a few early studies evaluated residual function in a small number of patients with failed allografts initiating peritoneal dialysis and demonstrated a much more rapid decline in function compared with patients with kidney failure without transplant, typically occurring over 6–12 months (25). Ultimately, the chance that immunosuppression can preserve some residual kidney function likely depends on whether the underlying etiology of graft failure was immunologic in nature, one of the most common causes of intermediate and late graft loss. Continuing the calcineurin inhibitor may be the best choice for the small subset of patients in which maintaining function is a priority over the ensuing 6–12 months on dialysis, particularly peritoneal dialysis (Figure 3). For the majority of patients, a more rapid taper is likely warranted because there are no published studies that adequately address whether continuing immunosuppression will affect the time course of kidney function decline, especially when taken in the context of the complications associated with these medications.
The benefits of continued immunosuppression exposure on dialysis come with substantial risk, the extent of which has yet to be clearly delineated. The higher mortality in this patient population is related to cardiovascular, malignancy, and infectious etiologies, all of which can be exacerbated by immunosuppressive therapies. Many of these drugs have important adverse effects that likely affect cardiovascular health after return to dialysis, including diabetes, dyslipidemia, hypertension, and accelerated atherosclerotic vascular disease. The higher risk of infection-related malignancy among patients who have received a transplant compared with those on dialysis is well documented, and this risk likely decreases with withdrawal of immunosuppression, although only a few studies on this topic have been performed (26,27). Several small, single-center, retrospective studies have evaluated infection in patients on dialysis who are on immunosuppression. Although the risk of graft-intolerance syndrome is decreased, this often comes at the cost of more infectious complications, with the most common being venous catheter–related bloodstream infections and pyelonephritis, followed by pneumonia, cellulitis, and clostridium difficile colitis (28).
The paucity of data to guide immunosuppressant management in the failed allograft mandates an individualized and thoughtful approach, one that includes clear communication among transplant and dialysis centers. The critical first step is to determine eligibility and timing of repeat transplant. In patients without an imminent plan for repeat transplant, the rate and sequence of immunosuppression wean must be a personalized assessment of risk-benefit for each patient, with a few expert-opinion algorithms available to serve as guidance, and most centers weaning patients off immunosuppression by 1 year after starting dialysis (Figure 3) (29).
Allograft Nephrectomy
The role of graft nephrectomy remains controversial and clinical practice varies widely, largely dependent on regional preferences rather than compelling data. In the absence of urgent indications like infection or hemorrhage, the most salient reason for surgical removal is the treatment of graft-intolerance syndrome. Overt symptoms of rejection can include malaise, pain, fever, and hematuria, which may be refractory to pulse dose steroids. However, the more important question is whether nephrectomy is indicated as prophylaxis against this syndrome, including its more subtle manifestations. An often-cited study by Lopez-Gomez et al. (30), reported in 2004, compared 43 patients who initially kept their transplant in situ to 121 patients who underwent allograft nephrectomy. Patients who did not undergo surgical removal of the allograft had worse anemia, erythropoietin resistance, lower serum albumin, and higher inflammatory markers. Two thirds of patients had persistent symptoms that ultimately required nephrectomy, with subsequent improvement in many biochemical parameters compared with patients who were asymptomatic and retained their grafts. The timing of graft failure was not reported in this study, and the risk of graft-intolerance syndrome may be associated with the timing and nature of graft failure. As a result, some advocate for preemptive nephrectomy in patients with graft failure within 12 months of transplant, although robust data in support of this strategy are lacking.
The potential benefits of nephrectomy must be weighed against the risks of the procedure. There is evidence that removal of the allograft generates donor-specific antibodies, independent of immunosuppression withdrawal (31). In a recent systematic review of 12 studies, levels of panel reactive antibody ranged from 10% to 55% in patients without allograft nephrectomy, compared with 20%–72% in patients who underwent the procedure (32). A few hypotheses have been proposed to explain this phenomenon beyond a notably higher risk of blood transfusions (32). For one, the kidney may behave like a “sponge” that attracts and absorbs formed donor-specific antibodies to their antigenic targets, preventing detection in the serum until the graft is removed (33). Alternatively, surgery may engender an inflammatory response that promotes antibody formation in the setting of mechanical manipulation, remnant allograft tissue, and increased exposure of antigens.
The surgical procedure may be technically challenging depending on the indication, existing inflammation, and early or late graft failure. Complication rates, in single-center studies and a recent meta-analysis, ranged from 5% to 48% and included bleeding, hematoma formation, and infection (32). Several studies have also evaluated the effect of allograft nephrectomy on complications related to repeat transplant. In another systematic review and metanalysis by Lin et al. (34), patients who underwent allograft nephrectomy had a higher risk of delayed graft function, acute rejection, and allograft and patient survival. However, many of the included studies were affected by methodologic challenges and results were mixed, making it difficult to draw meaningful conclusions. Percutaneous allograft embolization has emerged as an intriguing alterative to nephrectomy and may be associated with lower morbidity, and even mortality, among a growing number of reported case series (35). Although the common postembolization inflammatory syndrome can usually be managed with analgesics, up to 20% of patients may still require surgical removal for persistent graft-intolerance syndrome (36).
CKD Management and Dialysis Modality
Although the initiation of dialysis is often determined by patient symptoms, estimates of glomerular filtration and rates of decline may provide additional information. There are several limitations in estimating GFR as a marker of CKD stage in the kidney allograft. Both the Modification of Diet in Renal Disease study and Chronic Kidney Disease Epidemiology Collaboration creatinine equations likely do not perform as well in patients who have received a transplant compared with those without transplants. Moreover, studies comparing the two have produced heterogeneous results that have made it difficult to advocate strongly for one equation over another. Analyses evaluating cystatin C–based equations were previously hindered by lack of a standardized assay, have not consistently demonstrated superiority, and are not widely used in clinical practice (37–39). For prognostication, all of these equations likely perform reasonably well when placed within the context of eGFR trend; other biochemical parameters, such as proteinuria and presence of donor-specific antibodies; and histologic data evaluating inflammation and fibrosis scores (40,41).
While the time course of kidney function deterioration may be slower or less predictable in failing allografts, the clinical response sometimes focuses too heavily on salvaging what is left (35,36). Regardless of the reason, patients who have received a transplant often receive suboptimal CKD management. Prior studies have noted worse BP control, anemia, lower bicarbonate levels, and higher phosphate levels in patients with failed transplants compared with those with native CKD (6,42). Reports also indicate these patients are less likely to undergo appropriate dialysis planning and more often initiate dialysis with a central venous catheter (14). This is particularly troubling given evidence that early referral for patients with native kidney disease is associated with significantly improved clinical care at the start of dialysis and improved longer-term outcomes—a trend that does not appear to extend to transplant nephrologists and their patients with failing allografts (43). Appropriate planning, including modality counseling and the use of peritoneal dialysis, has also been shown to be associated with improved early outcomes for individuals with a failed allograft (41). While this difference has not been consistent across analyses, it is difficult to know if differences in immunosuppression did not contribute to higher risk of infections and adverse outcomes in some of these analyses (41,44).
Recent studies that suggest the possibility of improved prognostication of outcomes after kidney transplant may help identify failing allografts sooner and encourage better planning of transitions of care (45). Moreover, innovative solutions to provide more comprehensive care for this high-risk cohort show early promise. Multidisciplinary clinics dedicated to patients with low-functioning kidney allografts may improve KRT planning, and even reduce emergency-department visits and hospital admissions, although more research is needed (46,47). In the absence of such clinics and clear guidelines to assist the nontransplant nephrologist, transplant providers should take some responsibility for patients during this time period. There should be clear coordination of care among the transplant center and nontransplant nephrologist, with the former focusing on allograft prognosis, immunosuppression weaning, and relisting, and the latter ensuring optimal CKD management and guiding the patient through dialysis planning. Transplant centers may improve this communication by ensuring that patients have a final transplant clinic visit around the time of graft failure, with a standardized, templated note serving as a checklist to make recommendations regarding these issues and establishing continued lines of communications, should the need for shared decision making arise.
The optimal timing of dialysis initiation in patients who have received a transplant has been evaluated by several observational studies, but conclusions are limited due to inherent biases and the lack of a specific threshold of function, with similar measures used in native kidney disease to guide care; however, some have raised concerns that early initiation of dialysis could have detrimental consequences on patient outcomes (44,48). Moreover, dialysis modality should be individualized to each patient because there are no compelling data to support one form of replacement therapy over another at this time (44).
Palliative Care
Palliative care has a much broader scope beyond end-of-life care and has been associated with an improvement in various outcomes in other noncancer, advanced-organ-failure models—including quality of life, illness understanding, hospitalizations, health care costs, and even lower risk of death (49). Allograft failure is often associated with significant symptom burden, greater utilization of health care resources, transitions between health care systems, and high patient morbidity and mortality. Moreover, patient and provider decisions about repeat transplant and the ensuing perioperative and post-transplant risks are likely to be more complex compared with the first transplant because patients are older, may face extended waitlist time on dialysis, and often have a greater burden of comorbid disease. Finally, a common refrain among patients who have been newly transplanted is that they would rather die than go back to dialysis. Although not always ultimately true, such statements emphasize the significant emotional trauma that can accompany the failing allograft. Palliative care is uniquely equipped to address many of the needs of this patient population, yet routine integration of palliative care services has been slow and currently lacks supporting data. Helping patients understand the role of palliative care and offering referral may be important to include in their management plan, with particular consideration for patients who experience significant change in functional status, worsening disease symptoms, and increasing visits to the emergency department (49).
Large cohort- and population-level studies demonstrate an association with lower risk of death for all patients who receive a transplant compared with those who remain on dialysis—in part, because of the selection bias that exists for the patients who did get a transplant. However, given that transplant centers also decline patients for relisting, there is clearly a subset of individuals in which the benefits of a second transplant are potentially outweighed by the risks (50–52). Despite the focus on inadequate referrals for waitlisting and increased delisting of patients from the waitlist, there are currently no clear data available to provide a rigorous approach for who should, or should not, be a candidate for a subsequent transplant (50). Recognizing which individuals would not experience a substantial improvement in their quality of life and other patient-centered outcome measures is perhaps as important, so as to be able to facilitate appropriate transitions of care and manage patient expectations/outcomes.
Future Directions
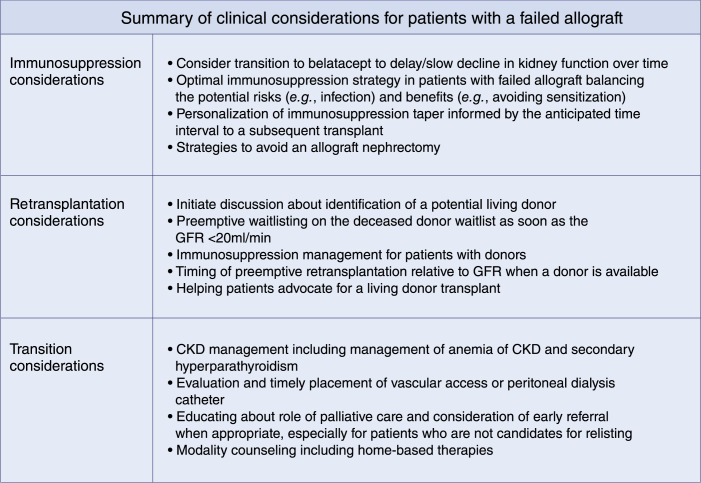
Critical questions about the ideal strategy for managing patients with failing allografts persist on several fronts (Figure 4). We urgently need more data to address the gaps in our clinical understanding of how to manage patients with failing allografts (Table 1).
Figure 4.
Summary of clinical considerations for patients with a failed allograft.
Table 1.
Summary of key knowledge gaps in the optimal clinical management of patients with failing allografts
| Key Knowledge Gaps | |
|---|---|
| Immunosuppression | Potential benefit, timing, and strategy of introducing calcineurin inhibitor–avoidance protocols that would prolong the life of a failing allograft |
| Timing for reduction of immunosuppression in patients with failing allografts and evidence of ongoing chronic rejection | |
| Immunosuppression withdrawal strategy after allograft failure and initiation of KRT to minimize the risk of sensitization | |
| Factors to consider for personalization of the risk/benefit ratio of continued immunosuppression (e.g., infectious risk versus decreased sensitization) in patients on dialysis with different expected intervals before a subsequent transplant | |
| Determining when the potential benefit of a transplant nephrectomy outweighs the potential risks | |
| Transitions of care | Timing of initiation of discussion for the transition to dialysis in the absence of a living donor |
| Timing of modality counseling and appropriate access placement, especially for those patients who are not candidates for a subsequent transplant | |
| Optimal strategy to offer either preemptive listing while encouraging seeking a living donor | |
| Timing of preemptive transplantation when available as an option | |
| Transition of care from transplant clinic to CKD clinic | |
| Consideration for, and timing of, palliative care referral for patients who are not candidates for a subsequent transplant |
Conclusions
Many patients undergoing transplant ultimately experience loss of their allograft, and improving intermediate- and long-term graft survival continues to be a paramount goal for the transplant community. Until this goal is achieved, it is critical that the care of patients with kidney failure acknowledge and optimize the transition to dialysis and, potentially, back to transplant. In the current environment, patients with a failing allograft often receive suboptimal chronic disease management and dialysis planning and poor continuity of care, and important decisions, with high stakes, are guided by low-quality data. The lack of robust data on optimal clinical management of these patients has contributed to significant variations in clinical practice patterns, with suboptimal outcomes being commonplace for patients. Going forward, if we are to avoid failing our patients, it will be important for the transplant community to recognize these challenges and allocate resources to support the focused, higher-quality research that is required to improve long-term graft survival.
Disclosures
S. Mohan reports serving as a member of the American Society of Nephrology Quality Committee, on the Angion Pharma scientific advisory board, as deputy editor of Kidney International Reports (International Society of Nephrology), as member of the Scientific Registry of Transplant Recipients visiting committee, and as vice chair of the United Network for Organ Sharing data advisory committee; having consultancy agreements with Angion Biomedica; and receiving research funding from the National Institutes of Health (National Institute of Biomedical Imaging and Bioengineering, National Institute of Diabetes and Digestive and Kidney Diseases, and National Institute on Minority Health and Health Disparities). The remaining author has nothing to disclose.
Funding
S. Mohan is supported by National Institute of Diabetes and Digestive and Kidney Diseases grants R01 DK114893 and U01 116066 and National Institute on Minority Health and Health Disparities grant R01014161.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Paul S, Melanson T, Mohan S, Ross-Driscoll K, McPherson L, Lynch R, Lo D, Pastan SO, Patzer RE: Kidney transplant program waitlisting rate as a metric to assess transplant access [published online ahead of print August 18, 2020]. Am J Transplant 10.1111/ajt.16277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schold JD, Patzer RE, Pruett TL, Mohan S: Quality metrics in kidney transplantation: Current landscape, trials and tribulations, lessons learned, and a call for reform. Am J Kidney Dis 74: 382–389, 2019 [DOI] [PubMed] [Google Scholar]
- 3.Mohan S, Husain SA: Patient-centered outcomes with second kidney transplant. Clin J Am Soc Nephrol 14: 1131–1132, 2019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saran R, Robinson B, Abbott KC, Agodoa LYC, Bragg-Gresham J, Balkrishnan R, Bhave N, Dietrich X, Ding Z, Eggers PW, Gaipov A, Gillen D, Gipson D, Gu H, Guro P, Haggerty D, Han Y, He K, Herman W, Heung M, Hirth RA, Hsiung JT, Hutton D, Inoue A, Jacobsen SJ, Jin Y, Kalantar-Zadeh K, Kapke A, Kleine CE, Kovesdy CP, Krueter W, Kurtz V, Li Y, Liu S, Marroquin MV, McCullough K, Molnar MZ, Modi Z, Montez-Rath M, Moradi H, Morgenstern H, Mukhopadhyay P, Nallamothu B, Nguyen DV, Norris KC, O’Hare AM, Obi Y, Park C, Pearson J, Pisoni R, Potukuchi PK, Repeck K, Rhee CM, Schaubel DE, Schrager J, Selewski DT, Shamraj R, Shaw SF, Shi JM, Shieu M, Sim JJ, Soohoo M, Steffick D, Streja E, Sumida K, Kurella Tamura M, Tilea A, Turf M, Wang D, Weng W, Woodside KJ, Wyncott A, Xiang J, Xin X, Yin M, You AS, Zhang X, Zhou H, Shahinian V: US Renal Data System 2018 annual data report: Epidemiology of kidney disease in the United States. Am J Kidney Dis 73[Suppl 1]: A7–A8, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huml AM, Sehgal AR: Hemodialysis quality metrics in the first year following a failed kidney transplant. Am J Nephrol 50: 161–167, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perl J, Zhang J, Gillespie B, Wikström B, Fort J, Hasegawa T, Fuller DS, Pisoni RL, Robinson BM, Tentori F: Reduced survival and quality of life following return to dialysis after transplant failure: The dialysis outcomes and practice patterns study. Nephrol Dial Transplant 27: 4464–4472, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kabani R, Quinn RR, Palmer S, Lewin AM, Yilmaz S, Tibbles LA, Lorenzetti DL, Strippoli GF, McLaughlin K, Ravani P; Alberta Kidney Disease Network : Risk of death following kidney allograft failure: A systematic review and meta-analysis of cohort studies. Nephrol Dial Transplant 29: 1778–1786, 2014 [DOI] [PubMed] [Google Scholar]
- 8.Rao PS, Schaubel DE, Jia X, Li S, Port FK, Saran R: Survival on dialysis post-kidney transplant failure: Results from the scientific registry of transplant recipients. Am J Kidney Dis 49: 294–300, 2007 [DOI] [PubMed] [Google Scholar]
- 9.King KL, Husain SA, Jin Z, Brennan C, Mohan S: Trends in disparities in preemptive kidney transplantation in the United States. Clin J Am Soc Nephrol 14: 1500–1511, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patzer RE, Pastan SO: Policies to promote timely referral for kidney transplantation. Semin Dial 33: 58–67, 2020 [DOI] [PubMed] [Google Scholar]
- 11.Patzer RE, Basu M, Smith KD, Plantinga L, Mohan S, Escoffery C, Kim JJ, Melanson T, Pastan SO: Awareness of the new kidney allocation system among United States dialysis providers with low waitlisting. Am J Nephrol 47: 115–119, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schold JD, Augustine JJ, Huml AM, O’Toole J, Sedor JR, Poggio ED: Modest rates and wide variation in timely access to repeat kidney transplantation in the United States. Am J Transplant 20: 769–778, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clark S, Kadatz M, Gill J, Gill JS: Access to kidney transplantation after a failed first kidney transplant and associations with patient and allograft survival: An analysis of national data to inform allocation policy. Clin J Am Soc Nephrol 14: 1228–1237, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan MR, Oza-Gajera B, Chapla K, Djamali AX, Muth BL, Turk J, Wakeen M, Yevzlin AS, Astor BC: Initial vascular access type in patients with a failed renal transplant. Clin J Am Soc Nephrol 9: 1225–1231, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lynch JR, Mohan S, McClellan WM: Achieving the goal: Results from the fistula first breakthrough initiative. Curr Opin Nephrol Hypertens 20: 583–592, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Dürr M, Lachmann N, Zukunft B, Schmidt D, Budde K, Brakemeier S: Late conversion to belatacept after kidney transplantation: Outcome and prognostic factors. Transplant Proc 49: 1747–1756. e1, 2017; [DOI] [PubMed] [Google Scholar]
- 17.Schulte K, Vollmer C, Klasen V, Bräsen JH, Püchel J, Borzikowsky C, Kunzendorf U, Feldkamp T: Late conversion from tacrolimus to a belatacept-based immuno-suppression regime in kidney transplant recipients improves renal function, acid-base derangement and mineral-bone metabolism. J Nephrol 30: 607–615, 2017 [DOI] [PubMed] [Google Scholar]
- 18.Gill JS: Cause for cautious optimism: Belatacept for patients with impaired kidney allograft function. Transplantation 102: 347–348, 2018 [DOI] [PubMed] [Google Scholar]
- 19.Ojo A, Wolfe RA, Agodoa LY, Held PJ, Port FK, Leavey SF, Callard SE, Dickinson DM, Schmouder RL, Leichtman AB: Prognosis after primary renal transplant failure and the beneficial effects of repeat transplantation: Multivariate analyses from the United States Renal Data System. Transplantation 66: 1651–1659, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Nimmo AMSA, McIntyre S, Turner DM, Henderson LK, Battle RK: The impact of withdrawal of maintenance immunosuppression and graft nephrectomy on HLA sensitization and calculated chance of future transplant. Transplant Direct 4: e409, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bayliss GP, Gohh RY, Morrissey PE, Rodrigue JR, Mandelbrot DA: Immunosuppression after renal allograft failure: A survey of US practices. Clin Transplant 27: 895–900, 2013 [DOI] [PubMed] [Google Scholar]
- 22.Casey MJ, Wen X, Kayler LK, Aiyer R, Scornik JC, Meier-Kriesche HU: Prolonged immunosuppression preserves nonsensitization status after kidney transplant failure. Transplantation 98: 306–311, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Augustine JJ, Woodside KJ, Padiyar A, Sanchez EQ, Hricik DE, Schulak JA: Independent of nephrectomy, weaning immunosuppression leads to late sensitization after kidney transplant failure. Transplantation 94: 738–743, 2012 [DOI] [PubMed] [Google Scholar]
- 24.Davis S, Wiebe C, Campbell K, Anobile C, Aubrey M, Stites E, Grafals M, Pomfret E, Nickerson P, Cooper JE: Adequate tacrolimus exposure modulates the impact of HLA class II molecular mismatch: A validation study in an American cohort [published online ahead of print September 4, 2020]. Am J Transplant 10.1111/ajt.16290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davies SJ: Peritoneal dialysis in the patient with a failing renal allograft. Perit Dial Int 21[Suppl 3]: S280–S284, 2001 [PubMed] [Google Scholar]
- 26.Vajdic CM, McDonald SP, McCredie MR, van Leeuwen MT, Stewart JH, Law M, Chapman JR, Webster AC, Kaldor JM, Grulich AE: Cancer incidence before and after kidney transplantation. JAMA 296: 2823–2831, 2006 [DOI] [PubMed] [Google Scholar]
- 27.van Leeuwen MT, Webster AC, McCredie MR, Stewart JH, McDonald SP, Amin J, Kaldor JM, Chapman JR, Vajdic CM, Grulich AE: Effect of reduced immunosuppression after kidney transplant failure on risk of cancer: Population based retrospective cohort study. BMJ 340: c570, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woodside KJ, Schirm ZW, Noon KA, Huml AM, Padiyar A, Sanchez EQ, Sarabu N, Hricik DE, Schulak JA, Augustine JJ: Fever, infection, and rejection after kidney transplant failure. Transplantation 97: 648–653, 2014 [DOI] [PubMed] [Google Scholar]
- 29.British Transplant Society : Management of the failing kidney transplant. https://bts.org.uk/wp-content/uploads/2016/09/13_BTS_Failing_Graft-1.pdf. Accessed August 24, 2020
- 30.López-Gómez JM, Pérez-Flores I, Jofré R, Carretero D, Rodríguez-Benitez P, Villaverde M, Pérez-García R, Nassar GM, Niembro E, Ayus JC: Presence of a failed kidney transplant in patients who are on hemodialysis is associated with chronic inflammatory state and erythropoietin resistance. J Am Soc Nephrol 15: 2494–2501, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Del Bello A, Congy-Jolivet N, Sallusto F, Guilbeau-Frugier C, Cardeau-Desangles I, Fort M, Esposito L, Guitard J, Cointault O, Lavayssière L, Nogier MB, Blancher A, Rostaing L, Kamar N: Donor-specific antibodies after ceasing immunosuppressive therapy, with or without an allograft nephrectomy. Clin J Am Soc Nephrol 7: 1310–1319, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghyselen L, Naesens M: Indications, risks and impact of failed allograft nephrectomy. Transplant Rev (Orlando) 33: 48–54, 2019 [DOI] [PubMed] [Google Scholar]
- 33.Bocrie O, Hussein Aly AA, Guignier F, Funes de la Vega M, Rifle G, Mousson C, Martin L: Distribution of donor-specific antibodies in the cortex and the medulla of renal transplants with chronic allograft nephropathy. Transpl Immunol 17: 227–229, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Lin J, Wang R, Xu Y, Chen J: Impact of renal allograft nephrectomy on graft and patient survival following retransplantation: A systematic review and meta-analysis. Nephrol Dial Transplant 33: 700–708, 2018 [DOI] [PubMed] [Google Scholar]
- 35.Al Badaai G, Pernin V, Garrigue V, Monnin V, Murez T, Fadli SED, Molinari N, Thuret R, Iborra F, Mourad G: Renal graft intolerance syndrome in late graft failure patients: Efficacy and safety of embolization as first-line treatment compared to surgical removal. Transpl Int 30: 484–493, 2017 [DOI] [PubMed] [Google Scholar]
- 36.Takase HM, Contti MM, Nga HS, Bravin AM, Valiatti MF, El-Dib RP, Modelli de Andrade LG: Nephrectomy versus embolization of non-functioning renal graft: A systematic review with a proportional meta-analysis. Ann Transplant 23: 207–217, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Delanaye P, Mariat C: The applicability of eGFR equations to different populations. Nat Rev Nephrol 9: 513–522, 2013 [DOI] [PubMed] [Google Scholar]
- 38.Molnar MZ, Ichii H, Lineen J, Foster CE 3rd, Mathe Z, Schiff J, Kim SJ, Pahl MV, Amin AN, Kalantar-Zadeh K, Kovesdy CP: Timing of return to dialysis in patients with failing kidney transplants. Semin Dial 26: 667–674, 2013 [DOI] [PubMed] [Google Scholar]
- 39.Molnar MZ, Ojo AO, Bunnapradist S, Kovesdy CP, Kalantar-Zadeh K: Timing of dialysis initiation in transplant-naive and failed transplant patients. Nat Rev Nephrol 8: 284–292, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beckman BS, Brookins JW, Shadduck RK, Mangan KF, Deftos LJ, Fisher JW: Effect of different modes of dialysis on serum erythropoietin levels in pediatric patients. A report of the Southwest Pediatric Nephrology Study Group. Pediatr Nephrol 2: 436–441, 1988 [DOI] [PubMed] [Google Scholar]
- 41.Perl J, Dong J, Rose C, Jassal SV, Gill JS: Is dialysis modality a factor in the survival of patients initiating dialysis after kidney transplant failure?. Perit Dial Int 33: 618–628, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ansell D, Udayaraj UP, Steenkamp R, Dudley CR: Chronic renal failure in kidney transplant recipients. Do they receive optimum care?: Data from the UK Renal Registry. Am J Transplant 7: 1167–1176, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Aniort J, Kaysi S, Garrouste C, Abdelkader MH, Isnard M, Aguilera D, Ali Y, Bouiller M, Mulliez A, Heng AE: CKD complications in kidney-transplanted patients going back to dialysis: Impact on patients outcomes. J Nephrol 31: 147–155, 2018 [DOI] [PubMed] [Google Scholar]
- 44.Perl J, Hasan O, Bargman JM, Jiang D, Na Y, Gill JS, Jassal SV: Impact of dialysis modality on survival after kidney transplant failure. Clin J Am Soc Nephrol 6: 582–590, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Akbari S, Knoll G, White CA, Kumar T, Fairhead T, Akbari A: Accuracy of kidney failure risk equation in transplant recipients. Kidney Int Rep 4: 1334–1337, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Evans RDR, Bekele S, Campbell SM, Clark SG, Harris L, Thomas A, Jones GL, Thuraisingham R: Assessment of a dedicated transplant low clearance clinic and patient outcomes on dialysis after renal allograft loss at 2 UK transplant centers. Transplant Direct 4: e352, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bissonnette J, Woodend K, Davies B, Stacey D, Knoll GA: Evaluation of a collaborative chronic care approach to improve outcomes in kidney transplant recipients. Clin Transplant 27: 232–238, 2013 [DOI] [PubMed] [Google Scholar]
- 48.Perl J, Bargman JM, Davies SJ, Jassal SV: Clinical outcomes after failed renal transplantation-Does dialysis modality matter?. Semin Dial 21: 239–244, 2008 [DOI] [PubMed] [Google Scholar]
- 49.Wentlandt K, Weiss A, O’Connor E, Kaya E: Palliative and end of life care in solid organ transplantation. Am J Transplant 17: 3008–3019, 2017 [DOI] [PubMed] [Google Scholar]
- 50.Schold JD, Arrigain S, Flechner SM, Augustine JJ, Sedor JR, Wee A, Goldfarb DA, Poggio ED: Dramatic secular changes in prognosis for kidney transplant candidates in the United States. Am J Transplant 19: 414–424, 2019 [DOI] [PubMed] [Google Scholar]
- 51.King KL, Husain SA, Schold JD, Patzer RE, Reese PP, Jin Z, Ratner LE, Cohen DJ, Pastan SO, Mohan S: Major variation across local transplant centers in probability of kidney transplant for wait-listed patients. J Am Soc Nephrol 31: 2900–2911, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mohan S, Chiles MC: Achieving equity through reducing variability in accepting deceased donor kidney offers. Clin J Am Soc Nephrol 12: 1212–1214, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]