Abstract
Measures implemented to prevent transmission of severe acute respiratory syndrome coronavirus 2 in outpatient dialysis facilities may also help to prevent catheter-associated bloodstream infections in patients receiving hemodialysis. We used United States Renal Data System data to examine rates of antibiotic administration within dialysis facilities and rates of hospital admission for catheter-associated bloodstream infection from March 2018 through November 2020, and rates of hospitalization for sepsis, to address overall changes in hospitalization during the coronavirus disease 2019 (COVID-19) pandemic. Using logistic regression, we estimated year-over-year adjusted odds ratios of these events in 3-month intervals. During the first 6 months of the pandemic, rates of antibiotic administration were between 20% and 21% lower, and rates of hospitalization for catheter-associated bloodstream infection were between 17% and 24% lower than during corresponding periods in 2019, without significant changes in rates of hospitalization for sepsis. However, rates of catheter-associated events also decreased between 2018 and 2019, driven by reductions in facilities operated by a large dialysis provider. These data suggest that significant reductions in catheter-associated infections occurred during the pandemic, superimposed on nonpandemic-related reductions in some facilities before the pandemic. Even after the pandemic, it may be prudent to continue some COVID-19 mitigation measures to prevent catheter-associated bloodstream infections.
Keywords: United States Renal Data System, hemodialysis, vascular access, COVID-19
Introduction
Catheter-associated bloodstream infections are an important source of morbidity and mortality among patients receiving hemodialysis through a central venous catheter. Preventing and monitoring these events were identified as a major priority by the United States Department of Health and Human Services. Multiple federal initiatives have aimed to reduce catheter utilization, improve infection-control practices inside and outside the dialysis facility, and ultimately lower the rate of catheter-associated bloodstream infections. The ESRD Networks’ Fistula First initiative was introduced in the early 2000s to increase the use of fistulae (and decrease the use of catheters) among patients receiving maintenance hemodialysis, and the Centers for Medicare & Medicaid Services has included standardized fistula ratios and long-term catheter rates into the ESRD Quality Incentive Program. At the end of 2019, only 13% of hemodialysis patients used a catheter for ≥3 months (1). However, more than 80% of patients who initiate hemodialysis rely on a catheter for first access (2). Thus, efforts to prevent catheter-associated bloodstream infections remain important. The Centers for Disease Control and Prevention (CDC) has developed guidelines and toolkits for the prevention of infections, and the National Healthcare Safety Network monitors these events. Although the ESRD Quality Incentive Program includes a measure pertaining to the incidence of bloodstream infections reported to the National Healthcare Safety Network, that measure is adjusted for the prevalence of catheter utilization in a facility. As such, there is no measure that specifically tracks the absolute number of catheter-associated infections.
In 2020, the coronavirus disease 2019 (COVID-19) pandemic led to widespread and unprecedented implementation of new infection-control measures to reduce transmission of severe acute respiratory syndrome coronavirus 2 within dialysis facilities (3). These measures included use of personal protective equipment such as masks, gowns, and eye protection by staff, and masks by patients. Frequent hand hygiene, surface cleaning, and disinfection of dialysis machines and stations were also employed. We hypothesized that these measures may have also served to reduce rates of catheter-associated bloodstream infections in 2020. To investigate this possibility, we ascertained rates of intravenous antibiotic administration in dialysis facilities and hospitalization for bloodstream infection among Medicare beneficiaries undergoing hemodialysis with a catheter from March 1, 2018, to November 30, 2020. We also ascertained rates of hospitalization admission for non–catheter-associated sepsis events, so that secular trends in hospitalization for bloodstream infection might be contrasted.
Materials and Methods
We used Medicare Part B claims submitted by outpatient dialysis facilities to identify patients undergoing full-care, in-facility hemodialysis with a central venous catheter during the last 7 days of each calendar month from February 2018 to October 2020. Only those patients alive on the last day of the calendar month were retained for follow-up in the subsequent month. The use of a catheter was ascertained from the last hemodialysis treatment of the calendar month on the basis of modifier code V5; continued use of a catheter was presumed during the subsequent month. For each patient, we ascertained age (at the end of the calendar month), sex, and race and ethnicity.
Our coprimary events during each follow-up month were intravenous administration of any antibiotic in an outpatient dialysis facility and the incidence of any hospitalization with a discharge diagnosis of infection related to a catheter. Regarding antibiotics, we included only administrations without modifier code AY, which indicates treatment unrelated to kidney failure. Infection related to a catheter was defined by International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) diagnosis codes T80.211A, T80.212A, T80.218A, T80.219A, or T82.7XXA (4). Because hospitalization use decreased nationwide in 2020, we also assessed the incidence of hospitalization with a principal discharge diagnosis of sepsis, so that we could compare changes in hospitalization for infection due to catheters and sepsis.
We used logistic regression to estimate secular trends in the incidence of each event during 3-month intervals beginning in March 2018. We used a generalized estimation equation to account for multiple months per patient, and we adjusted for age, sex, and race and ethnicity. During our analysis, we discovered that the quality of intravenous antibiotic administration data in facilities operated by a large dialysis provider, henceforth referred to as “dialysis provider 2,” deteriorated during the last 4 months of 2019, leading to a clinically implausible scenario of almost no utilization of vancomycin and daptomycin, according to claims in 2020. For this reason, we modeled antibiotic administration only in patients dialyzing in facilities not operated by this provider (and hospitalization in all facilities), with stratification by dialysis provider (dialysis provider 1, dialysis provider 2, all other organizations). Analyses were conducted in SAS v9.4 (SAS Institute, Cary, NC).
Results
There were a mean of 43,755 patients dialyzing via a catheter at the beginning of each month from March 2018 to November 2020. Among all patient months, the mean age was 64.9 years, 51% were women, and 36% were Black; characteristics were unchanged during the study era.
Unadjusted rates of antibiotic administration in the dialysis facility and admissions for catheter-associated bloodstream infection showed seasonal variation with peaks in July–September of each year, whereas admissions for non-catheter-associated sepsis did not display substantial seasonal variation (Figure 1). Between March 2019 and February 2020, adjusted year-over-year declines in the rate of antibiotic administration in dialysis facilities were between 12% and 16%. Between June 2019 and February 2020, adjusted year-over-year declines in the rate of hospitalization for catheter-associated bloodstream infection were between 11% and 14% (Table 1).
Figure 1.
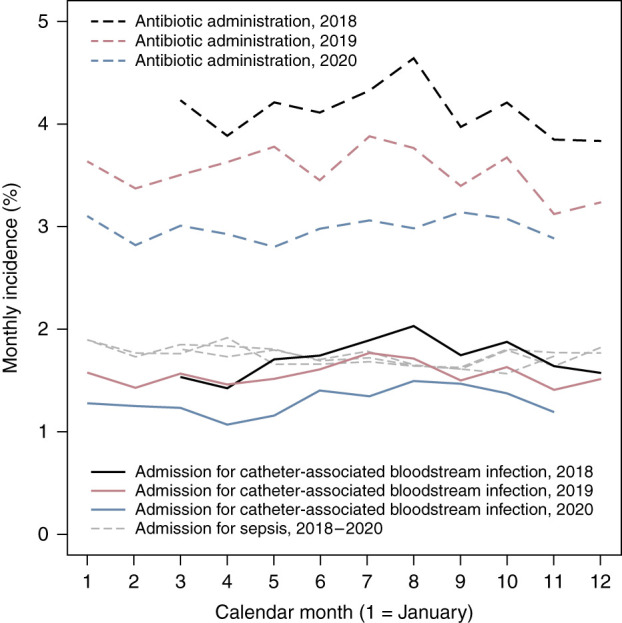
Monthly incidence of intravenous antibiotic administration in an outpatient dialysis facility, hospital admission for catheter-associated bloodstream infection, and hospital admission for sepsis among Medicare beneficiaries undergoing in-facility hemodialysis with a central venous catheter, March 2018–November 2020. Data regarding intravenous antibiotic administration are limited to facilities not operated by dialysis provider 2.
Table 1.
Year-over-year adjusted odds ratios of intravenous antibiotic administration in an outpatient dialysis facility, hospital admission for catheter-associated bloodstream infection, and hospital admission for sepsis, by 3-month interval, March 2019–November 2020
| Interval | Referent | IV Antibiotic Administrationa | Hospital Admissionb | ||||
|---|---|---|---|---|---|---|---|
| Catheter-Associated BSI | Sepsis | ||||||
| AOR | 95% CI | AOR | 95% CI | AOR | 95% CI | ||
| Prepandemic | |||||||
| 2019:3–2019:5 | 2018:3–2018:5 | 0.88 | 0.84 to 0.93 | 0.97 | 0.92 to 1.04 | 1.03 | 0.97 to 1.09 |
| 2019:6–2019:8 | 2018:6–2018:8 | 0.84 | 0.80 to 0.89 | 0.89 | 0.84 to 0.95 | 0.99 | 0.93 to 1.05 |
| 2019:9–2019:11 | 2018:9–2018:11 | 0.84 | 0.80 to 0.88 | 0.86 | 0.81 to 0.91 | 1.03 | 0.97 to 1.10 |
| 2019:12–2020:2 | 2018:12–2019:2 | 0.84 | 0.79 to 0.88 | 0.88 | 0.83 to 0.94 | 1.00 | 0.94 to 1.06 |
| Pandemic | |||||||
| 2020:3–2020:5 | 2019:3–2019:5 | 0.79 | 0.75 to 0.84 | 0.76 | 0.71 to 0.81 | 0.98 | 0.92 to 1.04 |
| 2020:6–2020:8 | 2019:6–2019:8 | 0.80 | 0.76 to 0.85 | 0.83 | 0.78 to 0.89 | 0.99 | 0.93 to 1.05 |
| 2020:9–2020:11 | 2019:9–2019:11 | 0.89 | 0.84 to 0.94 | 0.89 | 0.83 to 0.95 | 0.95 | 0.89 to 1.00 |
IV, intravenous; BSI, bloodstream infection; AOR, adjusted odds ratio; 95% CI, 95% confidence interval.
In facilities not operated by dialysis provider 2.
In all facilities.
During the first 6 months of the COVID-19 pandemic, adjusted rates of antibiotic administration were between 20% and 21% lower, while corresponding rates of hospitalization for catheter-associated bloodstream infection were between 17% and 24% lower, compared with already-lower rates in 2019 (Table 1). Adjusted year-over-year declines in these rates were nearer 10% between September and November 2020. There were no significant changes in hospital admissions for non–catheter-associated sepsis during 2019 or 2020. Unadjusted rates of antibiotic administration in the dialysis facility and hospitalization for catheter-associated bloodstream infections, stratified by dialysis provider, are displayed in Supplemental Figures 1–3. Year-over-year odds ratios of hospitalization for catheter-associated bloodstream infection, stratified by dialysis provider organization, are displayed in Table 2. In facilities operated by dialysis provider 1, year-over-year declines were apparent beginning in June 2019; during the first 3 months of the pandemic, the year-over-year decline was even larger (35%). In all other facilities, year-over-year declines during the prepandemic era were modest and generally nonsignificant. During the first 3 months of the pandemic, year-over-year declines were 17% and 21% in facilities operated by dialysis provider 2 and facilities not operated by either dialysis provider 1 or dialysis provider 2, respectively.
Table 2.
Year-over-year adjusted odds ratios of hospital admission for catheter-associated bloodstream infection, by 3-month interval and stratified by dialysis provider organization, March 2019–November 2020
| Interval | Referent | Dialysis Provider 1 | Dialysis Provider 2 | All Other Organizations | |||
|---|---|---|---|---|---|---|---|
| AOR | 95% CI | AOR | 95% CI | AOR | 95% CI | ||
| Prepandemic | |||||||
| 2019:3–2019:5 | 2018:3–2018:5 | 0.96 | 0.87 to 1.07 | 0.94 | 0.85 to 1.04 | 1.04 | 0.92 to 1.17 |
| 2019:6–2019:8 | 2018:6–2018:8 | 0.76 | 0.68 to 0.84 | 0.98 | 0.89 to 1.08 | 0.96 | 0.87 to 1.06 |
| 2019:9–2019:11 | 2018:9–2018:11 | 0.74 | 0.66 to 0.83 | 0.90 | 0.81 to 0.99 | 0.93 | 0.84 to 1.03 |
| 2019:12–2020:2 | 2018:12–2019:2 | 0.78 | 0.69 to 0.88 | 0.96 | 0.86 to 1.06 | 0.90 | 0.80 to 1.02 |
| Pandemic | |||||||
| 2020:3–2020:5 | 2019:3–2019:5 | 0.65 | 0.57 to 0.73 | 0.83 | 0.75 to 0.93 | 0.79 | 0.70 to 0.90 |
| 2020:6–2020:8 | 2019:6–2019:8 | 0.76 | 0.68 to 0.85 | 0.93 | 0.84 to 1.02 | 0.79 | 0.71 to 0.89 |
| 2020:9–2020:11 | 2019:9–2019:11 | 0.89 | 0.79 to 1.00 | 0.97 | 0.87 to 1.08 | 0.79 | 0.70 to 0.89 |
AOR, adjusted odds ratio; 95% CI, 95% confidence interval.
Discussion
There were significant reductions in the administration of intravenous antibiotics in dialysis facilities and hospital admissions for catheter-associated bloodstream infection among Medicare beneficiaries with central venous catheters between March 2018 and November 2020, and those reductions were not accompanied by changes in rates of admission for sepsis. Decreases during March to November 2020 were in keeping with our hypothesis that rates declined during the COVID-19 pandemic. However, we also found that year-over-year reductions in administration of intravenous antibiotics and hospital admissions for catheter-associated bloodstream infection were apparent beginning in June 2019, nearly 1 year before the COVID-19 pandemic began. This suggests that factors other than changes in infection-control practices during the pandemic are partially responsible for the multiyear trend in outcomes. Nevertheless, the parallel reductions in in-facility antibiotic administration and hospitalizations for catheter-associated bloodstream infection suggest that there was a true reduction in these events rather than a shift from inpatient to outpatient management. Furthermore, the lack of reduction in non–catheter-associated admissions for sepsis during the same period suggests that overall changes in hospitalization rates during the pandemic were not the reason for these findings.
We speculate that gowning, masking, and increased disinfection in dialysis facilities to reduce transmission of severe acute respiratory syndrome coronavirus 2 may have reduced the incidence of catheter-associated bloodstream infections, even beyond recent measures designed to reduce catheter-associated bloodstream infections in the hemodialysis population. In 2009, the CDC announced plans for a collaborative project to prevent bloodstream infections and invited outpatient dialysis facilities to participate. The resulting collaborative group developed and implemented a bundled intervention that included surveillance of bloodstream infections and feedback through the National Healthcare Safety Network, staff education and competency, chlorhexidine for skin antisepsis, catheter care observations, and patient education and engagement efforts (5). Implementation of these interventions resulted in a 54% reduction in access-related bloodstream infections at the end of a 15-month intervention (5) that was sustained over 4 years (6). Nevertheless, it appears that further reductions in infection rates occurred in 2020, possibly related to better hygiene and disinfection during the COVID-19 pandemic. This finding is important because it suggests that some measures implemented to reduce intrafacility transmission of COVID-19 should be continued to prevent catheter-associated bloodstream infections. Interestingly, year-over-year declines in infection rates were relatively modest between September and November 2020. Given that this period coincided with a decline in the incidence of COVID-19 infection throughout the United States, it is possible that infection-prevention practices may have partially lapsed during this period. Alternatively, infection rates may have begun to stabilize at a new, lower level.
Our study also identified a substantial reduction in rates of bloodstream infections in late 2019 and early 2020 that occurred before the onset of widespread COVID-19 and COVID-19 mitigation practices in dialysis facilities in the United States. The national reduction was primarily driven by one large dialysis organization. The most likely explanation for this reduction is the introduction of antimicrobial barrier catheter caps into routine use in dialysis facilities across the country. Randomized controlled trials of the ClearGuard HD antimicrobial barrier cap (ICU Medical, San Clemente, CA) showed a 43% lower rate of bloodstream infections compared with standard caps over 12 months (7) and a 63% lower rate compared with a combination of a Tego needlefree connector (ICU Medical) and Curos disinfecting cap for Tego (3M Health Care, St. Paul, MN). Interestingly, dialysis provider 1 introduced ClearGuard HD caps as standard of care in May 2019 (8), and beginning in the next month, we observed a series of year-over-year declines in the rate of hospitalization for catheter-associated bloodstream infection in those facilities. These declines may represent a real-world confirmation of clinical trial results. On the other hand, the CDC has emphasized prevention of vascular access infections during the past decade; some of the ongoing declines in the rate of hospitalization for catheter-related bloodstream infections may be attributable to these efforts.
The strength of this study is its inclusion of recent data on a large percentage of the hemodialysis population dialyzing through catheters. However, there are also limitations. We were able to observe only events that are captured through claims, and therefore, we could not examine rates of positive blood cultures. Practice changes in 2019 resulted in complex changes in rates of outcomes across dialysis provider organizations before the pandemic, complicating interpretation of events in 2020. Stratification by dialysis provider revealed that baseline rates were more stable in some organizations than in others and allowed for better resolution of the changes in 2020 relative to 2019. However, claims do not provide information about utilization of hemodialysis catheter devices, including caps, that may have varied by provider. We lack an explanation for the disappearance of line-item billing regarding vancomycin and daptomycin administration in patients receiving care in facilities operated by dialysis provider 2, which led to our strategy of limiting analysis of this outcome to patients dialyzing in facilities not operated by dialysis provider 2. Finally, we cannot discount the possibility that the threshold for hospitalization changed during the pandemic, although we found that incidence of admissions for sepsis did not significantly decline, even during the first wave of the pandemic.
Rates of hospitalization for catheter-associated bloodstream infection declined between 2018 and 2019 and again between 2019 and 2020. Antibiotic administration rates showed a similar pattern, but admissions for sepsis appeared stable over this period. COVID-19–related infection-control practices appeared to reduce bloodstream infections beyond what was accomplished through directed infection-control practices and introduction of new catheter caps in 2018–2019.
Disclosures
D.T. Gilbertson reports employment with Chronic Disease Research Group; consultancy agreements with Amgen; and research funding from Amgen, AstraZeneca, DaVita, HRSA, and NIH. K.L. Johansen reports employment with Hennepin Healthcare, serving as a member of Steering Committee for GlaxoSmithKline prolyl hydroxylase inhibitor clinical trials program and the Akebia Advisory Board, and serving as an Associate Editor of JASN. J. Liu reports employment with Hennepin Healthcare Research Institute. Y. Peng reports employment with Chronic Disease Research Group. E.D. Weinhandl was an employee of Fresenius Medical Care North America between March 2019 and April 2020 and a consultant to the company between May 2020 and November 2021, providing input in epidemiologic studies of home dialysis. E.D. Weinhandl also reports employment with Chronic Disease Research Group, Hennepin Healthcare Research Institute; consultancy agreements with Fresenius Medical Care North America, Outset Medical; and serving as a scientific advisor or member of Advisory Board of Home Dialyzors United and Board of Directors of Medical Education Institute. J.B. Wetmore reports employment with Hennepin County Medical Center; ad hoc consulting for BMS-Pfizer Alliance; research funding from Amgen, AstraZeneca, BMS/Pfizer, Genentech, Merck, NIH (NIDDK), and OPKO Health; honoraria from Aurinia (for advisory board activities, as noted below), BMS-Pfizer Alliance, and Reata; participating on occasion on ad hoc advisory boards for BMS-Pfizer Alliance; and honoraria for educational activities (CME-eligible) for NephSAP (American Society of Nephrology) and Healio.
Funding
This work was funded by the National Institute of Diabetes and Digestive and Kidney Diseases (contract #75N94019C00006).
Supplementary Material
Acknowledgments
The data reported here have been supplied by the United States Renal Data System (USRDS). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US Government.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental Material
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.11360821/-/DCSupplemental.
Supplemental Figure 1. Monthly incidence of intravenous antibiotic administration in an outpatient dialysis facility and hospital admission for catheter-associated bloodstream infection among Medicare beneficiaries undergoing in-facility hemodialysis with a central venous catheter, March 2018–November 2020 in facilities operated by dialysis provider 1.
Supplemental Figure 2. Monthly incidence of intravenous antibiotic administration in an outpatient dialysis facility and hospital admission for catheter-associated bloodstream infection among Medicare beneficiaries undergoing in-facility hemodialysis with a central venous catheter, March 2018–November 2020 in facilities operated by dialysis provider 2.
Supplemental Figure 3. Monthly incidence of intravenous antibiotic administration in an outpatient dialysis facility and hospital admission for catheter-associated bloodstream infection among Medicare beneficiaries undergoing in-facility hemodialysis with a central venous catheter, March 2018–November 2020 in facilities not operated by dialysis provider 1 or dialysis provider 2.
References
- 1.Centers for Medicare & Medicaid Services : Medicare Dialysis Facilities, 2021. Available at: https://data.cms.gov/quality-of-care/medicare-dialysis-facilities. Accessed August 25, 2021
- 2.United States Renal Data System : 2020 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2020 [Google Scholar]
- 3.Kliger AS, Garrick R: Evidence-based practices to reduce COVID-19 transmission in dialysis facilities. Clin J Am Soc Nephrol 16: 1146–1148, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DialysisData.org : Dialysis Facility Report Methodology. Available at: https://dialysisdata.org/content/dialysis-facility-report-methodology. Accessed August 25, 2021
- 5.Patel PR, Yi SH, Booth S, Bren V, Downham G, Hess S, Kelley K, Lincoln M, Morrissette K, Lindberg C, Jernigan JA, Kallen AJ: Bloodstream infection rates in outpatient hemodialysis facilities participating in a collaborative prevention effort: A quality improvement report. Am J Kidney Dis 62: 322–330, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Yi SH, Kallen AJ, Hess S, Bren VR, Lincoln ME, Downham G, Kelley K, Booth SL, Weirich H, Shugart A, Lines C, Melville A, Jernigan JA, Kleinbaum DG, Patel PR: Sustained infection reduction in outpatient hemodialysis centers participating in a collaborative bloodstream infection prevention effort. Infect Control Hosp Epidemiol 37: 863–866, 2016 [DOI] [PubMed] [Google Scholar]
- 7.Hymes JL, Mooney A, Van Zandt C, Lynch L, Ziebol R, Killion D: Dialysis catheter-related bloodstream infections: A cluster-randomized trial of the ClearGuard HD antimicrobial barrier cap. Am J Kidney Dis 69: 220–227, 2017 [DOI] [PubMed] [Google Scholar]
- 8.Sibble S, Hunt A, Van Wyck DB, Jordan L, Tentori F, Nissenson AR, Brunelli SM: Association between antimicrobial barrier cap use and outcomes among hemodialysis patients using a central venous catheter. Available at: https://www.asn-online.org/education/kidneyweek/2020/program-abstract.aspx?controlId=3442083. Accessed August 25, 2021
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


