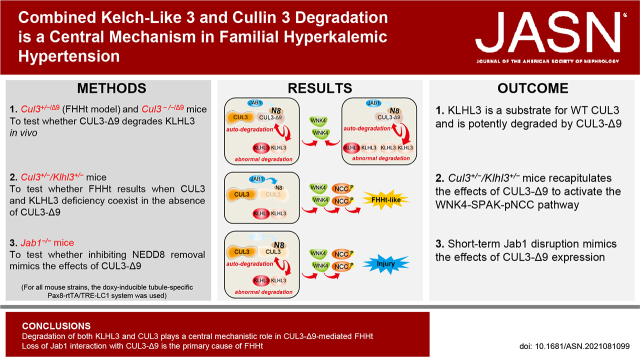
Significance Statement
Familial hyperkalemic hypertension (FHHt) results from inappropriate activation of the Na+Cl– cotransporter (NCC). Causative mutations have been identified in the gene encoding Cullin3 (CUL3). Cullin3 with the substrate binding adaptor Kelch-like 3 (KLHL3) forms an E3 ubiquitin ligase that mediates degradation of NCC regulatory kinases. The mechanism by which mutant CUL3 causes FHHt is unclear, but lower abundance of CUL3 and KLHL3 proteins and loss of binding to the CUL3 regulator JAB1 have been implicated. Using several mouse models, we found that mutant CUL3 potently induced KLHL3 degradation. Mice heterozygous for CUL3 and KLHL3 displayed an FHHt-like phenotype. JAB1 disruption mimicked the effects of mutant CUL3 expression. Our data show that reduced functional KLHL3 and CUL3 play a central role in FHHt.
Keywords: WNK4, NaCl cotransporter, familial hyperkalemic hypertension, Cullin 3, Kelch-like 3, hypertension, ion transport
Visual Abstract
Abstract
Background
Mutations in the ubiquitin ligase scaffold protein Cullin 3 (CUL3) gene cause the disease familial hyperkalemic hypertension (FHHt). In the kidney, mutant CUL3 (CUL3-Δ9) increases abundance of With-No-Lysine (K) Kinase 4 (WNK4), inappropriately activating sterile 20/SPS-1–related proline/alanine-rich kinase (SPAK), which then phosphorylates and hyperactivates the Na+Cl– cotransporter (NCC). The precise mechanism by which CUL3-Δ9 causes FHHt is unclear. We tested the hypothesis that reduced abundance of CUL3 and of Kelch-like 3 (KLHL3), the CUL3 substrate adaptor for WNK4, is mechanistically important. Because JAB1, an enzyme that inhibits CUL3 activity by removing the ubiquitin-like protein NEDD8, cannot interact with CUL3-Δ9, we also determined whether Jab1 disruption mimicked the effects of CUL3-Δ9 expression.
Methods
We used an inducible renal tubule-specific system to generate several mouse models expressing CUL3-Δ9, mice heterozygous for both CUL3 and KLHL3 (Cul3+/−/Klhl3+/−), and mice with short-term Jab1 disruption (to avoid renal injury associated with long-term disruption).
Results
Renal KLHL3 was higher in Cul3−/− mice, but lower in Cul3−/−/Δ9 mice and in the Cul3+/−/Δ9 FHHt model, suggesting KLHL3 is a target for both WT and mutant CUL3. Cul3+/−/Klhl3+/− mice displayed increased WNK4-SPAK activation and phospho-NCC abundance and an FHHt-like phenotype with increased plasma [K+] and salt-sensitive blood pressure. Short-term Jab1 disruption in mice lowered the abundance of CUL3 and KLHL3 and increased the abundance of WNK4 and phospho-NCC.
Conclusions
Jab1−/− mice and Cul3+/−/Klhl3+/− mice recapitulated the effects of CUL3-Δ9 expression on WNK4-SPAK-NCC. Our data suggest degradation of both KLHL3 and CUL3 plays a central mechanistic role in CUL3-Δ9–mediated FHHt.
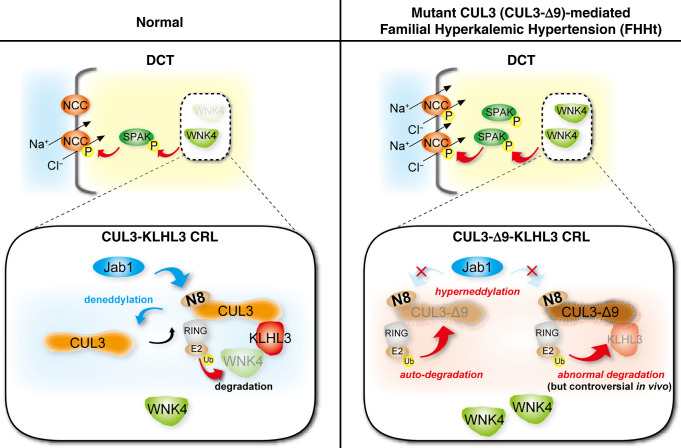
The renal thiazide-sensitive Na+Cl– cotransporter (NCC) plays a key role in sodium and potassium homeostasis. NCC is activated via aminoterminal phosphorylation by STE20-Proline Alanine-rich Kinase (SPAK), which in turn is phosphorylated and activated by With-No-Lysine (K) (WNK) kinases (Figure 1).1–4 WNK kinase abundance is regulated by an E3 ubiquitin ligase complex (Cullin-RING ligase, CRL) consisting of a scaffold (Cullin 3, CUL3), a substrate adaptor (Kelch-Like 3, KLHL3), and a ubiquitin-conjugating enzyme (Really Interesting New Gene, RING). Polyubiquitination of WNK kinases tags them for proteasomal degradation (Figure 1). NCC is activated by dietary potassium deficiency and by hypokalemia, via WNK and SPAK, leading to sodium chloride and potassium retention.5 Both hypokalemia and angiotensin II modulate KLHL3 activity, by altering its phosphorylation and reducing its abundance,6,7 marking the CRL as a key regulatory pathway in distal convoluted tubule cells. Mutations in the WNKs,8 CUL3,9,10 or in KLHL39,10 cause familial hyperkalemic hypertension (FHHt, also known as Gordon syndrome or Pseudohypoaldosteronism Type II).
Figure 1.
NCC activity regulation by the CUL3-WNK-SPAK pathway. (Left) In normal conditions the NCC is activated by SPAK, which is in turn activated by WNK4. WNK4 abundance is determined by activity of a CRL consisting of the scaffold Cullin 3 (CUL3), the substrate adaptor Kelch-like 3 (KLHL3), and a RING ubiquitin ligase. The ubiquitin-like protein NEDD8 (N8) is added by to CUL3 by NEDD8 activating enzyme (NAE, not shown), and removal is catalyzed by c-Jun activation domain-binding protein-1 (JAB1). Cycling of NEDD8 addition (neddylation) and removal (deneddylation is essential to maintain CRL stability and activity. (Right) In FHHt, WNK4 accumulates, resulting in hyperactivation of NCC. The precise mechanism by which CUL3 mutations cause WNK4 accumulation has remained unclear. Most CUL3 mutations that cause FHHt cause mis-splicing that results in deletion of exon 9 and generation of a form of CUL3 with a 57 amino acid deletion (CUL3-Δ9). CUL3-Δ9 is unable to interact with JAB1, leading to hyperneddylation. In vitro, this leads to CUL3-Δ9 autoubiquitination. Consistent with this, CUL3-Δ9 expression is extremely low in several CUL3-Δ9–expressing mouse models. In vitro, CUL3-Δ9–mediated degradation of KLHL3 has also been reported, but this has not been consistently found. The aim of this study was to resolve this issue and determine the mechanisms underlying CUL3-Δ9–mediated FHHt.
CUL3 normally undergoes covalent modification by addition (catalyzed by NEDD8-activating enzyme) and then removal (catalyzed by c-Jun activation domain-binding protein-1, JAB1), of the 9KDa ubiquitin-like protein NEDD8. This cycling of NEDD8 addition/removal (“neddylation”/“deneddylation”) is essential for the stability and activity of the CRL, and has been shown to be physiologically important in multiple organs including the heart,11 the brain,12 and the immune system.13 In the kidney, recent evidence from studies in mice suggests that cullin neddylation status changes in response to dietary potassium,6 or in models of diabetes.14,15 Two types of FHHt-causing CUL3 mutation have been identified. The first cause mis-splicing of exon 9 from the mRNA,10 generating a mutant form of CUL3 with a 57 amino-acid deletion (Δ403–459) (CUL3-Δ9). More recently, a novel mutation within the coding region of exon 10 (Δ474–477, CUL3Δ474–477) was identified.16 Both types of mutation prevent interactions between the mutant CUL3 and JAB1.16–18 In FHHt, normal function of the CRL is disrupted due to “hyperneddylation” of the mutant CUL3.17,19,20
The precise mechanism by which CUL3-Δ9 hyperneddylation leads to FHHt has been unclear. We reported that CUL3-Δ9 inappropriately degrades KLHL3 in vitro and suggested this plays a central role in CUL3-Δ9–mediated FHHt17 (Figure 1). Although CUL3-Δ9 knock-in mice were reported to have lower KLHL3 than controls,21 this was not observed in other CUL3-Δ9–expressing mouse models.19,22,23 Furthermore, mice heterozygous for Klhl3 do not phenocopy FHHt.24 CUL3-Δ9 also promotes its own ubiquitination and degradation both in vitro and in vivo19,21–23 (Figure 1). This led to an alternative hypothesis that functional CUL3 haploinsufficiency causes FHHt, but mice heterozygous for Cul3 also lack the FHHt phenotype.22 Thus, partial deficiency of either KLHL3 or CUL3 alone is not sufficient to cause FHHt.
We therefore sought unifying mechanistic insights into CUL3-mediated FHHt. We first used several mouse models to address the controversy regarding CUL3-Δ9–mediated KLHL3 degradation in vivo. Next, we tested the hypothesis that the FHHt phenotype results when deficiency of CUL3 and KLHL3 coexist, by generating mice heterozygous for both Klhl3 and Cul3 in the absence of CUL3-Δ9 expression. Finally, we tested whether interference with deneddylation in mice, by disrupting JAB1, would also affect CUL3, KLHL3, WNK4, and NCC in a manner resembling that observed in the human disease. Our findings further emphasize the physiologic importance of CUL3 regulation by neddylation and deneddylation.
Methods
Animals
Animal studies were approved by the Oregon Health and Science University Institutional Animal Care and Use Committee (protocol IP00286). For all mouse strains, the doxycycline-inducible tubule-specific Pax8-rtTA/TRE-LC1 system was used.17,22,25 CUL3-Δ9 was expressed from the Loxp-STOP-Loxp-Cul3-Δ9-IRES-tdTomato transgene.26 Cul3wt/flox/CUL3-Δ9 (Cul3+/−/Δ9) mice were generated as previously described.22 CUL3 knockout mice (Cul3−/−) and littermates expressing CUL3-Δ9 on a Cul3−/− background (Cul3−/−/Δ9) were generated by breeding Cul3+/−/Δ9 with Cul3flox/flox mice. To generate Klhl3flox/flox mice, mice carrying a trapping cassette “SA-geo-pA” flanked by the flippase recombinase target sites upstream of exon 3 of the Klhl3 allele (C57BL/6N-Atm1BrdKlhl3tm1a(KOMP)Wtsi/HMmucd, RRID:MMRRC_048345-UCD) were obtained from the Mutant Mouse Resource and Research Center at University of California, Davis. To excise the trapping cassette, these were crossed with B6.CgTg(Pgk1-flpo)10Sykr/J mice (011065, The Jackson Laboratory, Bar Harbor, ME) expressing Flp recombinase under the control of the ubiquitously active Pgk1 promoter. Klhl3flox/+ were bred with mice carrying Pax8-rtTA/TRE-LC1 (Pax8/LC1) to obtain Klhl3flox/+-Pax8-rtTA/LC1 mice, which were then interbred to obtain Klhl3flox/flox-Pax8-rtTA/LC1 mice (Klhl3−/−). Cul3wt/flox -Klhl3wt/flox mice (Cul3+/−/Klhl3+/−) were generated by crossing Cul3−/− mice17 with Klhl3−/− mice. Jab1−/− mice were generated as previously described.20 To induce recombination at floxed sites, 2 mg/ml doxycycline (ThermoFisher Scientific, Waltham, MA) in 5% sucrose in drinking water was administered for 2 or 3 weeks. After doxycycline treatment, mice were returned to regular drinking water for ≥2 weeks before experiments were performed. Control mice given 5% sucrose drinking water were phenotypically equivalent to wild-type (WT) mice and were genetically identical littermates of those receiving doxycycline. To assess salt sensitivity in Cul3+/−/Klhl3+/− mice, mice were fed a high-sodium/normal-K+ (HS/NK) diet (4% NaCl, 0.8% K+) (Teklad Custom Diet, TD.92034, Envigo) for 7 days, followed by an HS/low-K+ (LK) diet (3.5% Na, 0.0015%–0.003% K+) (Teklad Custom Diet, TD.170969, Envigo) for 7 days. Both male and female mice were used in approximately equal numbers for all experiments except for blood pressure measurement, in which only male mice were used, and all mice were on a mixed C57Bl/6J;sv129 background.
PCR Genotyping
Genomic DNA extracts were prepared from tail snips by heating overnight at 55°C in 300 μl of digestion solution containing 5 mM EDTA, 200 mM NaCl, 100 mM Tris (pH 8.0), 0.2% SDS, and 0.4 mg/ml proteinase K, followed by ethanol precipitation. The following primers were used: Pax8 forward, 5′-CCATGTCTAGACTGGACAAGA-3′; Pax8 reverse, 5′-CAGAAAGTCTTGCCATGACT-3′; CRE forward, 5′-TTTCCCGCAGAACCTGAACCTGAAGAT-3′; CRE reverse, 5′-TCACCGGCATCAACGTTTTCTT-3′; Cul3flox forward, 5′-CAGGTTGTATTTTAACTGCTTAAATGTCAAAACCT-3′; Cul3flox reverse, 5′-TTTGTCTGGACCAAATATGGCAGCCAAAACC-3′; Cul3-Δ9 forward, 5′-GGCGCGATTCTTACCAAGTCC-3′; Cul3-Δ9 reverse, 5′-GCGCATGAACTCTTTGATGACTT-3′; Jab1 forward, 5′-GGTCAGAAAGCTAGGCCTAAGAAGG-3′; Jab1 reverse, 5′-GGCATGCATCACCATTTTCAGTAG-3′. PCR genotyping for Klhl3flox and confirmation of Flp recombinase was performed by quantitative PCR by Transnetyx (Cordova, TN).
Antibodies
Antibody sources, species, dilutions, and validation references are provided in Supplemental Table 1.
Western Blot
Harvested kidneys were cut in half and snap frozen in liquid nitrogen, then stored at −80°C. Half kidney was homogenized using a Potter homogenizer in 1 ml cold buffer containing 300 mM sucrose, 50 mM Tris·HCl (pH 7.4), 1 mM EDTA, 1 mM EGTA, 1 mM NaVO4, 50 mM NaF, 1 mM ditiothreitol, 1 mM phenylmethane sulfonyl fluoride, 1 µg/ml aprotinin, 4 µg/ml leupeptin, and phosphatase inhibitor (PhosStop; Roche, Mannheim, Germany). Homogenates were centrifuged at 6000 rpm for 15 minutes at 4°C, and supernatants transferred to a new tube then stored at −80°C. Protein loading was adjusted by densitometric quantitation of total protein after Coomassie staining (see Supplemental Figure 1).22,27,28 Protein samples were separated by electrophoresis on 4%–12% Criterion XT Bis-Tris gels and 4%–15% Criterion TGX stain-free gels (BioRad Laboratories, Hercules, CA) and transferred to low fluorescence polyvinylidene fluoride membranes using the Trans-Blot Turbo transfer system (BioRad Laboratories). Membranes were blocked with 5% nonfat milk in PBS-Tween (ThermoFisher Scientific), followed by incubation with primary antibody overnight at 4°C. Anti-KLHL3 antibody was diluted in CanGetSignal (TOYOBO, Osaka, Japan). Appropriate horseradish peroxidase–conjugated secondary antibody in blocking buffer was added to membranes for 1 hour at room temperature. Membranes were developed using enhanced chemiluminescence, Western Lightning Plus–ECL (Perkin Elmer, Waltham, MA), and visualized using Pxi digital imaging system (Syngene, Frederick, MA), and densitometry was performed with ImageJ (http://rsbweb.nih.gov/ij/).
Immunofluorescence
For immunolocalization, three mice per group were analyzed to confirm findings; representative images are shown. Mice were anesthetized with a ketamine/xylazine/acepromazine cocktail, and kidneys were perfusion-fixed with retrograde abdominal aortic perfusion of 3% paraformaldehyde in PBS (pH 7.4). Kidneys were removed, dissected, and cryopreserved in 800 mOsm sucrose in PBS overnight, before embedding in Tissue-Tek OCT compound (Sakura Finetek, Torrance, CA). Slides were prepared by cutting 5 μm sections and were stored at −80°C until use. Immunofluorescence staining was prepared as follows. Slides were incubated with 0.5% Triton-X 100 in PBS for 30 minutes. Sections were then blocked with 5% BSA in PBS for 30 minutes, followed by incubation with primary antibody, diluted in blocking buffer, overnight at 4°C. Sections were incubated with fluorescent dye–conjugated secondary antibody, diluted in blocking buffer, for 1 hour at room temperature before being mounted with Prolong Diamond Antifade Mountant (Invitrogen, Carlsbad, CA). Fluorescence images were acquired using a Keyence BZ-X810 florescence microscope (Osaka, Japan).
Quantitative PCR
Kidneys were harvested and RNA was preserved by treating with RNAlater RNA Stabilization Reagent (Qiagen, Hilden, Germany) overnight at 4°C. Kidney tissue was then homogenized in PBS and immediately transferred to QIAzol lysis reagent, where it was further homogenized by running the sample through a 20-gauge blunt cannula. Total RNA was isolated using RNeasy Plus Universal Mini kit (Qiagen). The total RNA (1000 ng) was reverse transcribed using a High-Capacity RNA-to-cDNA kit (Applied Biosystems, Foster City, CA). The resulting complementary DNA was diluted 1:10 and real-time quantitative PCR was performed on a QuantStudio 7 Flex Real-Time PCR System using TaqMan Universal Master Mix II (Applied Biosystems). The primers used were TaqMan Gene Expression Assays ID Mm04337153_m1 for KLHL3 and Mm03928990_g1 for 18S (Rn18s) containing FAM dye. mRNA expression levels were calculated with the comparative threshold cycle method (22 DDCt) and 18S mRNA was used for normalization.
Blood Analysis and Urinalysis
Blood was collected via cardiac puncture under isoflurane anesthesia and transferred into heparinized tubes; 95 μl was loaded into a Chem8+ cartridge for electrolyte measurement by i-STAT analyzer (Abbot Point of Care Inc., Princeton, NJ).
BP Measurement
BP was measured in male Cul3+/−/Klhl3+/− mice by radiotelemetry using PA-C10 catheters (Data Sciences International, St. Paul, MN) implanted via the left carotid artery into the aortic arch, with the transmitter inserted into a pocket in the body cavity, as previously described.22 Isoflurane (4% in O2 to induce, 1.5%–2% to maintain; Piramel Enterprises) was used to anesthetize the animals. Mice received buprenorphine (0.5 mg/kg sc; Zoopharm, Windsor, CO) for postsurgical analgesia. Animals were monitored for alertness, activity, and loss of weight, and they were given ≥7 days to recover from surgery before measurements were recorded for 10 seconds every 1 minute. Hourly averages were used to calculate mean systolic BP (SBP). After 4-day acclimation, 24-hour mean SBP was defined as baseline, and delta SBP was calculated by the difference from 24-hour mean SBP under HS/NK or HS/LK diet.
Renal Clearance
Renal clearance was performed as described previously.29 Mice were infused with saline intravenously for 4 hours (0.2–0.3 ml/1 hour and total 0.8–1.2 ml) to maintain hemodynamics. Urine collection started 1 hour after infusion of 0.3 ml saline, and two collections (every 30 minutes) were performed. Hydrochlorothiazide (25 mg/kg body weight) was then infused intravenously as a bolus and four collections every 30 minutes were performed. Urinary [Na+] was determined by flame photometry, and delta [Na+] excretion was calculated as the difference between control and hydrochlorothiazide collection periods.
Statistics
The null hypothesis was tested using two-tailed unpaired t tests, one-way ANOVA, and two-way ANOVA for repeated measures using GraphPad Prism 9 as indicated in the figures. Post hoc analysis was performed using unpaired t tests with Bonferroni correction. All data are plotted as mean±SEM. Significant P values are described as *P<0.05, **P<0.01, and ***P<0.001.
Results
Mutant CUL3 Promotes KLHL3 Degradation In Vivo
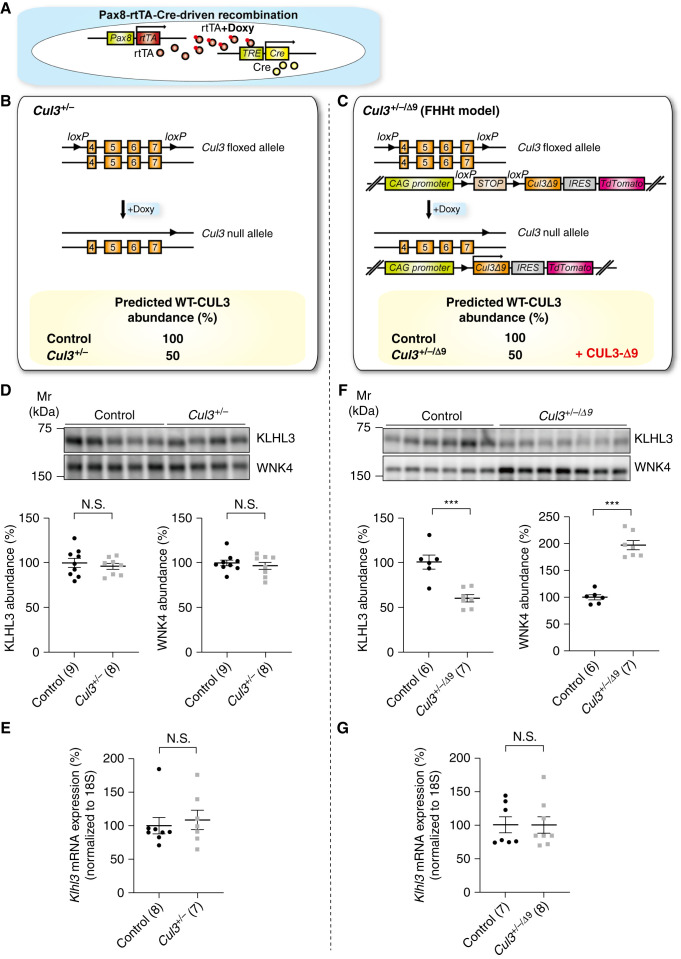
We previously reported that CUL3-Δ9 is highly neddylated and promotes KLHL3 degradation in vitro, and proposed a central role for anomalous KLHL3 degradation in CUL3-Δ9–mediated FHHt.17 This idea is supported by data from a mouse model of FHHt model, showing approximately 75% reduction in KLHL3 protein abundance.21 However, no decrease in KLHL3 was shown in three other CUL3-Δ9 FHHt models including Cul3+/−/Δ9 mice that we generated,19,22,23 so whether KLHL3 is degraded by CUL3-Δ9 in vivo remains controversial. One explanation for these discrepant findings is technical issues with KLHL3 Western blotting, so in this study we determined KLHL3 abundance using an antibody and protocol previously validated in global Klhl3−/− mice.24 Furthermore, although all CUL3 mutations identified to date are heterozygous,10,30 the effect of CUL3 heterozygosity on KLHL3 expression has not been determined. To clarify these issues, we determined KLHL3 abundance in inducible tubule-specific Cul3+/− mice, and in mice that express a CUL3-Δ9 transgene on a Cul3 heterozygous background (Cul3+/−/Δ9), by using the Pax8-rtTA-Cre driver system22 (Figure 2, A–C). In these models, administration of doxycycline causes Cre-mediated excision at the floxed Cul3 allele and additionally induction of CUL3-Δ9 expression in Cul3+/−/Δ9 mice.
Figure 2.
KLHL3 is reduced in a model of FHHt. (A) The Pax8-rtTA-LC1 system (present in all mice) was used for doxycycline-induced Cre-mediated recombination at loxP sites specifically in renal epithelia. (B) In addition to Pax8-rtTA-LC1, Cul3+/− mice carried “floxed” exons 4–7 and doxycycline treatment induced recombination, disrupting one allele and predicted to reduce CUL3 expression to about 50%. (C) Cul3+/−/Δ9 mice, a model of FHHt,22 were the same as Cul3+/− mice but also carried a transgene fragment consisting of a strong synthetic promoter (CAG) promoter, a floxed transcription blocker (STOP), cDNA encoding Cul3 Δ403–459 (CUL3-Δ9), an internal ribosome entry site (IRES), and cDNA encoding the fluorophore tdTomato. After induction by doxycycline, in addition to inactivation of one Cul3 allele, the IRES permitted translation of CUL3-Δ9 and tdTomato separately from the same transcript. Control mice were genetically identical to either Cul3+/− or Cul3+/−/Δ9 mice and were given vehicle only. (D) Abundances of WNK4 and KLHL3 did not differ between controls and Cul3+/− mice. (E) Quantitative real-time PCR showed that Klhl3 mRNA did not differ between controls and Cul3+/− mice. (F) Cul3+/−/Δ9 mice displayed significantly lower protein KLHL3 protein abundance and WNK4 abundance compared with control mice. (G) Klhl3 mRNA expression was not significantly different from control mice in Cul3+/−/Δ9. Individual values and mean±SEM are shown, with n in parentheses. Statistical differences were determined by two-tailed unpaired t tests. ***P<0.001; N.S., P>0.05.
As previously reported22 and predicted, WT CUL3 expression levels were approximately 50% lower in both Cul3+/− and Cul3+/−/Δ9 mice, but CUL3-Δ9 was detected only in Cul3+/−/Δ9 mice (Supplemental Figure 2). Specificity of the anti-KLHL3 antibody was confirmed in a newly generated doxycycline-inducible tubule-specific Klhl3−/− mouse model (Supplemental Figure 3). Cul3+/− mice, which showed no increase in WNK4 or FHHt phenotype (Figure 2D and 22) showed no difference in KLHL3 protein or mRNA levels compared with controls (Figures 2, D and E). In Cul3+/−/Δ9 mice, which displayed increased WNK4 (Figure 2F, and 22) and a FHHt phenotype,22 KLHL3 protein abundance was almost 50% lower than controls, with no difference in Klhl3 mRNA abundance (Figure 2, F and G).
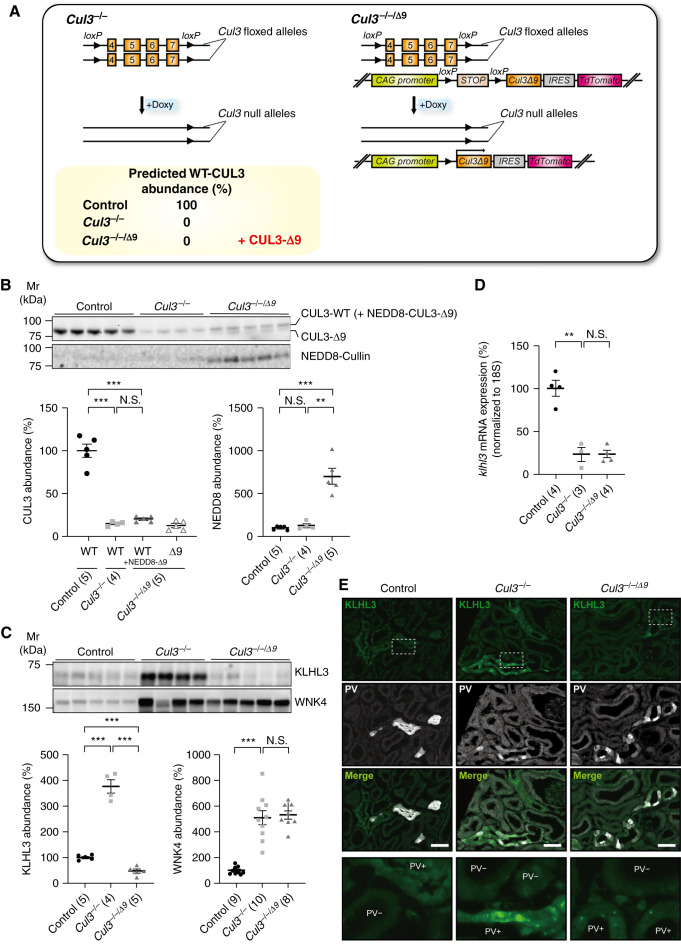
We recently reported that WT CUL3 competes with CUL3-Δ9 and inhibits CUL3-Δ9–mediated KLHL3 degradation in vitro.18 To further clarify the effects of WT CUL3 and CUL3-Δ9 on KLHL3 degradation in vivo, we compared Cul3−/− mice (Figure 3A) with littermates expressing CUL3-Δ9 on the Cul3−/− background (Cul3−/−/Δ9 mice) (Figure 3A), using the inducible Pax8-rtTA-Cre driver system (Figure 2A).17,22,25 Doxycycline treatment dramatically reduced CUL3 abundance in both strains (Figure 3B), and induced CUL3-Δ9 (Figure 3B) and tdTomato (Supplemental Figure 4) expression in Cul3−/−/Δ9 mice. Despite CUL3-Δ9 expression being driven by the strong CAG promoter present in the transgene, CUL3-Δ9 protein abundance was low, consistent with observations in knock-in models.19,21,23 This is most likely due to CUL3-Δ9 autoubiquitination, which has been observed in vitro for both CUL3-Δ9 and a newly identified FHHt-causing mutation in the Jab1 binding site of CUL3.16,19 Abundance of NEDD8 was also significantly higher in Cul3−/−/Δ9 mice, suggesting a high degree of cullin neddylation (Figure 3B). Western blotting showed that compared with controls, KLHL3 abundance was dramatically elevated in Cul3−/− mice, but lower in Cul3−/−/Δ9 mice (Figure 3C). In contrast, WNK4 protein abundance was greatly increased in both Cul3−/− and Cul3−/−/Δ9 mice (Figure 3C), similar to our previous results in HEK293 cells and in a different cohort of Cul3−/− mice.17 Klhl3 mRNA expression was lower in Cul3−/− mice despite increased KLHL3 protein abundance; Cul3−/−/Δ9 mice displayed similar Klhl3 mRNA levels (Figure 3D), suggesting effects of CUL3-Δ9 on KLHL3 abundance on the Cul3−/− background were not transcriptional; higher NEDD8 also supports a post-translational effect. Shorter-term (4 days) doxycycline treatment confirmed that CUL3 deletion and transgene induction were concurrent, and showed similar but less striking effects on abundances of NEDD8, KLHL3, and WNK4 in Cul3−/−/Δ9 mice (Supplemental Figure 5). Together, these results demonstrate that KLHL3 is a substrate for WT CUL3, and although CUL3-Δ9 strongly stimulates KLHL3 degradation in the context of Cul3 deficiency, it lacks ubiquitin ligase activity toward WNK4 in vivo.
Figure 3.
CUL3-Δ9 prevents cytoplasmic accumulation and aggregation of KLHL3 along the distal convoluted tubule (DCT) in Cul3−/− mice. (A) Tubule-specific Cul3−/− mice were made by generating mice with two “floxed” Cul3 alleles and the Pax8-rtTA-LC1 system (left). To express CUL3-Δ9 on the Cul3−/− background (Cul3−/−/Δ9 mice), a transgene fragment consisting of a strong synthetic promoter (CAG) promoter, a floxed transcription blocker (STOP), cDNA encoding Cul3 Δ403–459 (CUL3-Δ9), an internal ribosome entry site (IRES), and cDNA encoding the fluorophore tdTomato was introduced (right). Doxycycline treatment is predicted to completely ablate CUL3 expression in both Cul3−/− and Cul3−/−/Δ9 mice, and induce CUL3-Δ9 expression in Cul3−/−/Δ9 mice. (B) Doxycycline-induced Cul3 deletion and CUL3-Δ9 expression. Controls were uninduced Cul3fl/fl mice, administered 5% sucrose in drinking water. Western blotting showed that both doxycycline-induced Cul3−/− and Cul3−/−/Δ9 mice displayed significantly lower CUL3 abundance than controls. Total CUL3 expression did not differ between Cul3−/− and Cul3−/−/Δ9 mice. CUL3-Δ9 expression was detected only in Cul3−/−/Δ9 mice. Western blotting for NEDD8 showed higher abundance in Cul3−/−/Δ9 mice. Note that neddylated CUL3-Δ9 runs at the same Mw as WT CUL3. (C) Western blotting revealed that Cul3−/− mice displayed significantly higher KLHL3 protein abundance compared with controls, but Cul3−/−/Δ9 mice did not. WNK4 protein abundance was greatly increased in both Cul3−/− and Cul3−/−/Δ9 mice indicating loss of CUL3-mediated WNK4 degradation in both models. (D) Quantitative real-time PCR revealed significantly lower Klhl3 mRNA expression in Cul3−/− mice, but expression was similar between Cul3−/− and Cul3−/−/Δ9 mice. Individual values and mean±SEM are shown, with n in parentheses. Statistical differences were examined by one-way ANOVA, followed by post hoc unpaired t tests with Bonferroni correction. **P<0.01; ***P<0.001; N.S., P>0.05. (E) Immunofluorescence revealed cytoplasmic accumulation of KLHL3 (green) and aggregation along DCT1 (PV [D, white] of Cul3−/− mice (middle), whereas KLHL3 was only detected in small aggregates in Cul3−/−/Δ9 mice (right). Representative image, three mice total were analyzed with similar results. Areas marked by rectangles are enlarged at the bottom. Scale bars: 50 µm.
In control mice, immunofluorescence showed cytoplasmic KLHL3 localization along distal convoluted tubule 1 (DCT1) (parvalbumin colocalization) (Figure 3E) and DCT2/CNT (calbindin colocalization) (Supplemental Figure 6) with relatively lower signal intensity along DCT1. Although we previously reported significant KLHL3 along the thick ascending limb (TAL),17 the antibody used was not validated in Klhl3−/− mice. Consistent with analysis of recent mouse single-cell transcriptomic datasets31,32 (Supplemental Figure 7), immunofluorescence did not reveal significant KLHL3 signal along TAL using the newly validated antibody (Supplemental Figure 3E). In Cul3−/− mice, KLHL3 accumulated in the cytoplasm and in aggregates in DCT1 and DCT2/CNT segments, but in Cul3−/−/Δ9 mice KLHL3 was only observed in aggregates (Figure 3E and Supplemental Figure 6A). KLHL3 signals did not appear different in TAL of Cul3−/− mice (Supplemental Figure 6, C and D), suggesting KLHL3 accumulation in Cul3−/− mice is DCT specific. As previously reported,22 KLHL3 was exclusively cytoplasmic in Cul3+/−/Δ9 mice (Supplemental Figure 6B), suggesting WT CUL3 expression is needed for normal KLHL3 distribution. These data suggest that in Cul3−/− mice, KLHL3 accumulates in the cytoplasm of DCT and forms aggregates, but the high activity of CUL3-Δ9 toward KLHL3 limits aggregation in Cul3−/−/Δ9 mice. Together, these data are consistent with our previous in vitro findings that showed WT CUL3 can compete with CUL3-Δ9 and inhibit CUL3-Δ9–mediated KLHL3 degradation.18
Combined CUL3 and KLHL3 Deficiency Causes an FHHt-like Phenotype
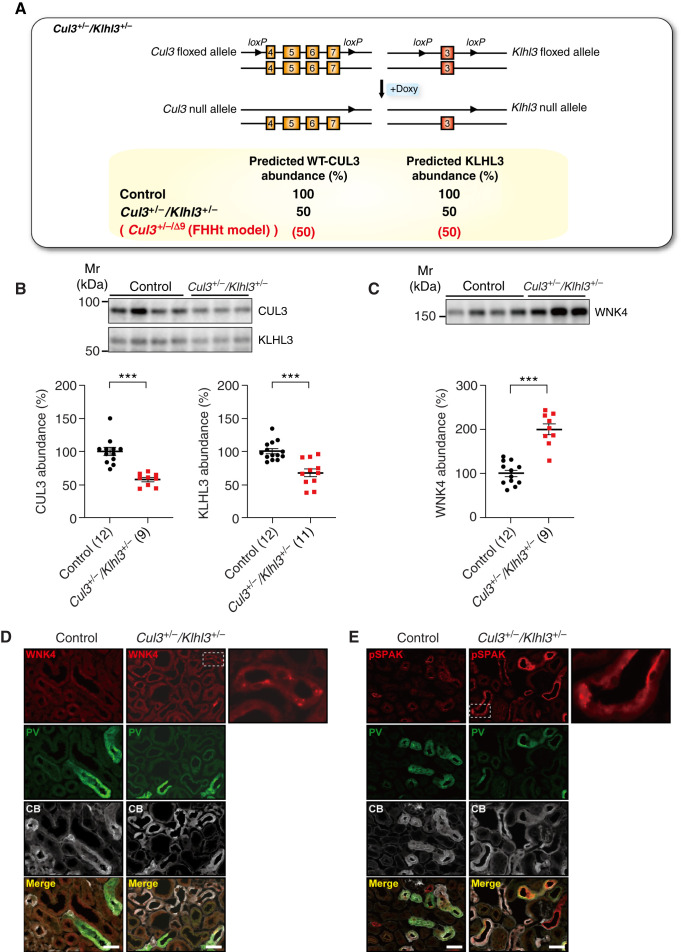
FHHt-causing CUL3 mutants induce their own degradation, because they display enhanced autoubiquitination,16,19 and CUL3-Δ9 expression is extremely low in several mouse models.19,21–23 In these models, WT CUL3 is expressed at 50% of normal levels, which led to the hypothesis that CUL3 haploinsufficiency causes FHHt, but Cul3+/− mice do not display an FHHt phenotype.22 Consistent with data from CUL3WT/Δex9 mice,21 our data show that CUL3-Δ9 promotes KLHL3 degradation in vivo. Whereas KLHL3 is about 50% lower in Cul3+/−/Δ9 mice, mice heterozygous for KLHL3 do not phenocopy FHHt.24 This raises the possibility that the combination of lower WT CUL3 and KLHL3 abundances ultimately causes FHHt. To test this, we generated mice heterozygous for both CUL3 and KLHL3 using the Pax8-rtTA-Cre driver system (Figure 4A). Lower abundances of CUL3 and KLHL3 were confirmed by Western blot (Figure 4B). WNK4 abundance was two-fold higher compared with controls (Figure 4C), consistent with data from Cul3+/−/Δ9 mice (Figure 3C). Immunofluorescence showed higher WNK4 and phosphorylated-SPAK signal in DCT of Cul3+/−/Klhl3+/− mice with some aggregation (Figures 4, D and E). The WNK4 and phosphorylated-SPAK aggregates appeared smaller than those in Klhl3−/− or Cul3−/− mice (Supplemental Figures 8 and 9), but were similar to those seen in Cul3+/−/Δ9 mice (Supplemental Figure 9). WNK4 regulates NKCC2 activity along the TAL,33 but WNK4 aggregates did not colocalize with NKCC2 (Supplemental Figures 9 and 10). These data suggest that combined CUL3 and KLHL3 deficiency is sufficient to activate the WNK4-SPAK pathway specifically along the DCT.
Figure 4.
Combined CUL3 and KLHL3 deficiency activates the WNK4-SPAK pathway. (A) Cul3 and Klhl3 heterozygous mice (Cul3+/−/Klhl3+/− mice) carried one WT Cul3 allele and one with exons 4–7 floxed, and one WT Klhl3 allele and one with exon 3 floxed. The Pax8-rtTA-LC1 system was used for tubule-specific inducible recombination and doxycycline treatment was predicted to reduce abundances of both CUL3 and KLHL3 by 50%, similar to what we observed in the Cul3+/−/Δ9 model of FHHt (Figure 1, shown in red). (B) Western blotting revealed significantly lower abundances of CUL3 and KLHL3 in Cul3+/−/Klhl3+/− mice compared with control mice. (C) Compared with control mice, Cul3+/−/Klhl3+/− mice showed significantly higher WNK4 abundance. Individual values and mean±SEM are shown. For (A)–(C) statistical differences were examined by two-tailed unpaired t tests (B and C). ***P<0.001. (D) Immunofluorescence revealed higher WNK4 (red) aggregation in parvalbumin-positive (PV, green) and calbindin-positive (CB, white) cells. (E) Cul3+/−/Klhl3+/− mice showed higher phosphorylated SPAK (pSPAK) abundance with some aggregation (red) in PV- and CB-positive cells. Representative image, three mice total were analyzed with similar results. Scale bars: 50 µm.
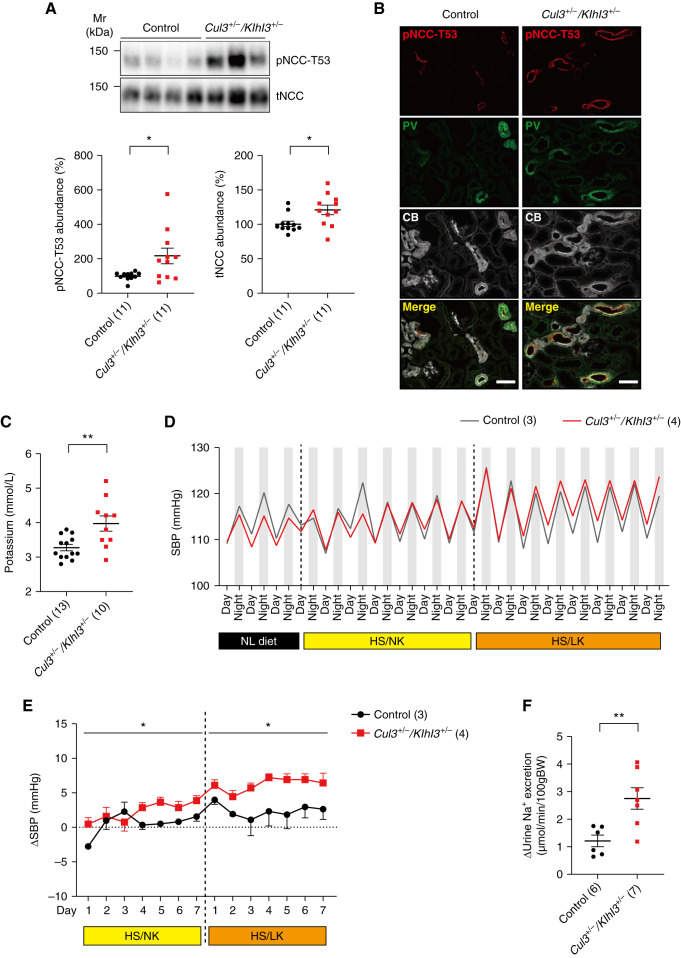
FHHt is characterized by hyperkalemia and hypertension, caused by increased NCC phosphorylation.34 We next determined whether Cul3+/−/Klhl3+/− mice phenocopy FHHt. Abundances of phospho-T53 NCC (pNCC) (a surrogate for NCC activity) and total NCC (tNCC) were significantly higher in Cul3+/−/Klhl3+/− mice compared with controls (Figure 5A) and immunofluorescence showed higher pNCC signal (Figure 5B). Cul3+/−/Klhl3+/− mice displayed significantly higher plasma [K+] levels than control mice (Figure 5C), but other blood chemistry parameters including TCO2 did not differ (Supplemental Figure 11). We next measured SBP using radiotelemetry in mice on normal, HS/NK, and HS/LK diets (low K+ was added to maximally activate NCC35). There were no significant differences in SBP on normal diet (Supplemental Figure 12), but the 24-hour ΔSBP was significantly higher in Cul3+/−/Klhl3+/− mice consuming HS/NK or HS/LK diets compared with control mice (Figure 5, D and E, and Supplemental Figure 12). The pNCC/tNCC ratio was also higher in Cul3+/−/Klhl3+/− mice fed HS/LK diet compared with controls (Supplemental Figure 13). Higher NCC activity was confirmed by renal clearance, with Cul3+/−/Klhl3+/− mice showing a greater increase in urinary Na+ excretion in response to hydrochlorothiazide infusion (Figure 5F). Together, these results indicate that combined CUL3 and KLHL3 deficiency is sufficient to cause an FHHt-like phenotype.
Figure 5.
Combined CUL3 and KLHL3 deficiency causes an FHHt-like phenotype. (A) Western blotting revealed pNCC-T53 and tNCC abundances were significantly higher in Cul3+/−/Klhl3+/− mice compared with control mice. (B) Immunofluorescence revealed higher pNCC signal in Cul3+/−/Klhl3+/− mice parvalbumin-positive (PV, green) and calbindin-positive (CB, white) cells, consistent with Western blot data. Scale bars: 50 µm. (C) Plasma [K+] was significantly higher in Cul3+/−/Klhl3+/− mice. (D and E) Radiotelemetric blood pressure measurement revealed no significant difference in SBP was detected on a normal diet, but there was a significantly higher increase in SBP (ΔSBP) in Cul3+/−/Klhl3+/− mice fed HS/NK and HS/LK diets compared with control mice (zero in E represents baseline SBP on the day before diet change). (F) Renal clearance showed that Cul3+/−/Klhl3+/− mice displayed a greater increase in Na+ excretion in response to hydrochlorothiazide administration (HCTZ). Individual values and mean±SEM are shown, with n in parentheses. Statistical differences were examined by two-tailed unpaired t tests (A and C) and two-way repeated measures ANOVA (D). *P<0.05; **P<0.01.
Disruption of CUL3 Deneddylation Lowers CUL3 and KLHL3 Abundance and Increases NCC Phosphorylation
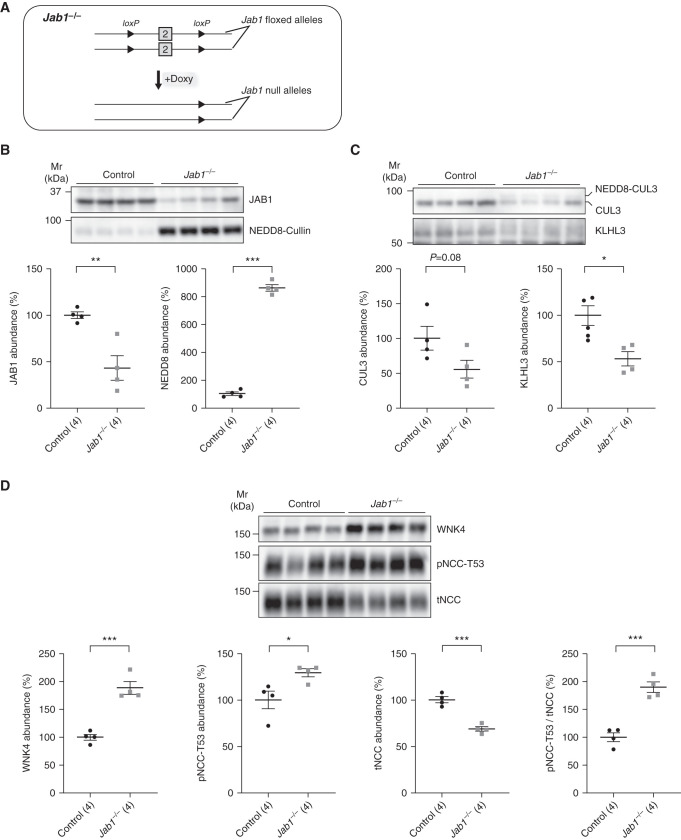
Activity and integrity of the CRL is dependent on cycles of addition (neddylation) and removal (deneddylation) of NEDD8 to CUL3.17,18 FHHt-causing CUL3 mutants are unable to interact with JAB1, the catalytic subunit of the COP9 signalosome, an enzyme that catalyzes NEDD8 removal from CUL3,16,18,19 and the resulting dysregulation of deneddylation is likely the ultimate cause of FHHt. Because global Jab1 disruption is embryonically lethal,36 we generated inducible tubule-specific Jab1−/− mice, but similar to inducible tubule-specific Cul3−/−, renal injury occurs with long-term disruption resulting in lower pNCC and tNCC, despite a dramatic increase in WNK4 abundance (20 and Supplemental Figure 14). Therefore, to determine the contribution of loss of JAB1-CUL3-Δ9 interactions to FHHt, we performed Western blots on kidneys from mice after shorter-term Jab1 disruption (Figures 6A, Supplemental Figures 14 and 15). Compared with controls, JAB1 abundance was about 60% lower and abundance of neddylated cullins was significantly higher in Jab1−/− mice (Figure 6B). Abundance of total CUL3 trended lower in Jab1−/− mice with a shift to the neddylated form, and KLHL3 abundance was significantly lower (Figure 6C). Abundances of WNK4 and pNCC, and the pNCC/tNCC ratio were higher in Jab1−/− mice (Figure 6D). Although not as dramatic as our previous findings,20 tNCC was still lower suggesting onset of injury even at the earlier time point of 3 weeks. At an earlier time point with no effect on tNCC abundance, results were similar with pNCC trending higher (Supplemental Figure 15). Plasma [K+] was not higher in Jab1−/− mice despite increased pNCC (data not shown), consistent with effects of Jab1 disruption on other segments. Thus, loss of deneddylation activates the WNK4-NCC pathway consistent with FHHt and mimics the effects of CUL3-Δ9 expression on CUL3 and KLHL3 abundance, further supporting our hypothesis that decreases in CUL3 and KLHL3 are integral to CUL3-Δ9–mediated FHHt.
Figure 6.
Acute disruption of the deneddylase Jab1 mimics the effects of CUL3-Δ9 expression. (A) Tubule-specific Jab1−/− mice were made by generating mice with two “floxed” Jab1 alleles and the Pax8-rtTA-LC1 system.20 Mice were treated with doxycycline for 3 weeks to induce recombination to acutely disrupt Jab1, then samples collected 2 weeks later. (B) Western blotting showed that JAB1 expression was significantly reduced, whereas abundance of neddylated Cullins (NEDD8-Cullin) was increased. (C) Jab1−/− mice displayed higher abundance of neddylated CUL3, and abundances of total (neddylated and unneddylated) CUL3 and KLHL3 were about 50% lower than in controls. (E) Abundances of WNK4 and NCC phosphorylated at T-53 (pNCC-T53) were significantly higher, but total NCC was lower; pNCC-T53/tNCC was significantly higher. Individual values and mean±SEM are shown. Statistical differences were examined by two-tailed unpaired t tests (B and C). *P<0.05; **P<0.01, ***P<0.001.
Discussion
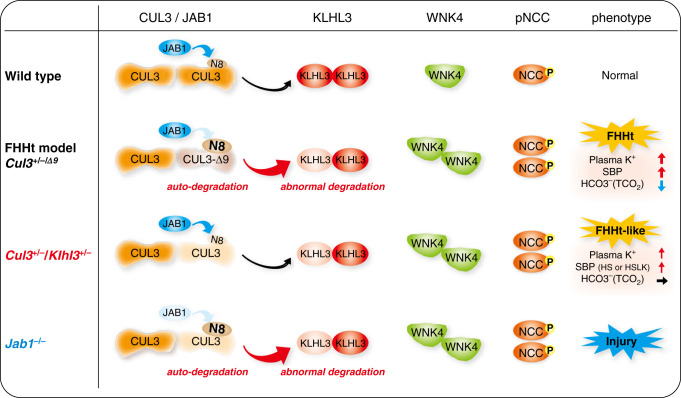
In vitro studies have identified multiple functional defects in FHHt-causing CUL3 mutants, including autoubiquitination and degradation,16,19 altered affinity for substrate adaptors,17,19,37 anomalous degradation of KLHL3,17 and reduced interaction with WT CUL3.37 The relative contribution of these to FHHt has been unclear, but this study implicates the complex functional haploinsufficiency of CUL3 and KLHL3 resulting from impaired CUL3 deneddylation as a central mechanism (Figure 7).
Figure 7.
Renal mechanisms of CUL3-Δ9–mediated FHHt. Wild type, under normal conditions, CUL3 constitutively degrades KLHL3 at a low level, and forms a normal Cullin-Ring ubiqutin ligase with KLHL3 that mediates WNK4 degradation. CUL3 is activated by covalent linkage of the ubiquitin-like protein NEDD8 (N8), which is removed by the enzyme JAB1. Cycling of N8 conjugation (neddylation) and removal (deneddylation) is required for stability of the Cullin-Ring ubiqutin ligase. FHHt models, in CUL3 heterozygous mice expressing CUL3-Δ9 from a transgene (Cul3+/−/Δ9) and knock-in mice with exon 9 deletion (CUL3WT/Δex9), CUL3-Δ9 cannot interact with JAB1, resulting in hyperneddylation. This leads to CUL3-Δ9 autoubiquitination and degradation, and induces KLHL3 degradation. Abundances of WNK4 and pNCC increase, leading to an FHHt phenotype. Cul3+/−/Klhl3+/−, combination of lower CUL3 and KLHL3 abundance results in increased abundance WNK4 and pNCC, and an FHHt-like phenotype. The full FHHt phenotype may require additional effects such as sequestration of adaptors in non-functional complexes and formation of unstable WT-CUL3/CUL3-Δ9 heterodimers. Jab1−/−, acute Jab1 disruption reduces abundances of CUL3 and KLHL3, leading to increased abundances of WNK4 and pNCC. However, due to effects along the whole nephron, there is renal injury rather than an FHHt phenotype.
On the basis of in vitro experiments, we proposed a central role for anomalous KLHL3 degradation in CUL3-Δ9–mediated FHHt.17,18 However, a study using the newly identified CUL3Δ474–477 mutant reported no difference in KLHL3 abundance between normal and mutant primary human fibroblasts, despite increased WNK4 expression in the mutant cells.16 Both CUL3-Δ9 and CUL3Δ474–477 display enhanced interaction with KLHL3.16,19 One possibility is that the formation of aberrant complexes with KLHL3 is more important in CUL3Δ474–477–mediated FHHt, because greater structural flexibility in CUL3-Δ919 resulting from the larger deletion could lead to additional functional defects. However, this new study has several limitations. The ubiquitination status of KLHL3 in the presence of CUL3Δ474–477 was not determined, the conclusion that KLHL3 was unchanged was on the basis of a single sample, fibroblasts were used rather than kidney cells or tissue, and specificity of the anti-KLHL3 antibody was not confirmed with the Western blot protocol used. Further studies, both in vitro and in vivo, are therefore needed to more conclusively exclude a role for enhanced KLHL3 degradation in CUL3Δ474–477–mediated FHHt.
The issue of antibody specificity is particularly important. No decrease in KLHL3 was reported in several mouse models of FHHt, including the Cul3+/−/Δ9 model used in this study.19,22,23 It has been shown more recently that the anti-KLHL3 antibody used in most studies does not discriminate KLHL3 from similar CUL3 adaptors, including KLHL2, expressed along the TAL,38 and that putative KLHL3 bands on Western blots are still apparent in mice lacking the protein, calling these studies into question. Sasaki and colleagues recently validated a KLHL3 antibody in constitutive, global Klhl3−/− mice.24 We confirmed specificity of this antibody using the same Western blot protocol in a newly generated inducible tubule-specific Klhl3−/− model. Similar to Yoshida and colleagues, who reported a 75% reduction in KLHL3 in their Cul3WT/Δex9 model of FHHt,21 we found that KLHL3 abundance is about 50% lower in Cul3+/−/Δ9 mice. We also found WT CUL3 constitutively promotes KLHL3 degradation, because KLHL3 was dramatically elevated in Cul3−/− mice, whereas the mutant CUL3-Δ9 on a Cul3−/− background reduced KLHL3 abundance, showing it displays a potent gain-of-function toward KLHL3. Despite the enhanced activity toward KLHL3, however, CUL3-Δ9 does not form a CRL that can promote WNK4 degradation, because WNK4 abundance remains high in mice expressing CUL3-Δ9 on a Cul3−/− background.
We also demonstrate that the combination of reduced KLHL3 and reduced CUL3 is mechanistically important in CUL3-Δ9–mediated FHHt (Figure 7). Tubule-specific heterozygous Cul3+/−/Klhl3+/− mice with reduced abundances of both WT CUL3 and KLHL3 displayed increased WNK4-SPAK activation, pNCC abundance, and an FHHt-like phenotype (increased plasma [K+] and a salt-sensitive increase in SBP). This was despite the fact that KLHL3 abundance was reduced less than in CUL3-Δ9–expressing mice (30% compared with 50%–75%). In Cul3+/−/Klhl3+/− mice, pNCC abundance was two-fold higher than controls, but SBP was not higher on normal diet. This is consistent with the finding that constitutive global Klhl3−/− mice, with a seven-fold increase in pNCC, display normal baseline blood pressure that is salt sensitive.24 Thus, although Cul3+/−/Klhl3+/− mice display an FHHt-like phenotype, extrarenal effects of CUL3-Δ9 are likely to be important for the development of severe hypertension. Patients with CUL3-Δ9 mutations exhibit hypertension much earlier in life compared with those with WNK1, WNK4, or KLHL3 mutations,10 consistent with the higher blood pressure observed in systemic CUL3-Δ9 FHHt models.19,21,23 CUL3-Δ9–mediated increases in RhoA abundance in the vasculature leading to increased vascular tone are a key mechanism.23,26,37 Cul3+/−/Klhl3+/− mice did not display higher plasma [Cl-] or metabolic acidosis (Supplemental Figure 11, P=0.16 for TCO2), two other features of FHHt. In contrast, Klhl3−/− mice displayed both, despite being normotensive at baseline.24 One possibility is that a greater reduction in KLHL3 abundance, resulting in a greater increase in NCC activity, may be needed. In support of this, mice expressing constitutively active SPAK specifically along the DCT1 display NCC hyperactivity and an FHHt-like phenotype featuring metabolic acidosis.3 This is driven by secondary, thiazide-reversible, effects of hyperkalemia that lower proximal tubule ammonia generation and collecting duct ammonia reabsorption.39 Another possibility is that adaptor “sequestration” or abnormal CRL ubiquitin ligase activity is also required to elicit the full phenotype.16,37 Regardless, Cul3+/−/Klhl3+/− mice displayed higher plasma [K+], the sine qua non of FHHt.10
Constitutive Cul3 disruption in mice is embryonically lethal, suggesting it has essential systemic effects40; yet when one mutant CUL3-Δ9 allele is present, a phenotype limited to the distal nephron and the vasculature results.19,21–23 This suggests the mutation affects only a small set of CUL3 actions. In the kidney, the DCT may be more sensitive to the effects of CUL3-Δ9 than other segments. KLHL3 appears to be uniquely sensitive to CUL3-Δ9–mediated degradation, because abundances of several other CUL3 adaptors were unchanged in knock-in mice with exon 9 deletion,21 and the DCT expresses KLHL3 at much higher levels than other segments at both the mRNA9,17,31,32 (Supplemental Figure 7) and protein level (Supplemental Figure 3). The structural basis for the high sensitivity of KLHL3 to CUL3-Δ9–mediated degradation remains to be determined. Our immunofluorescence data indeed suggest the effects on KLHL3 and WNK4 abundances are specific to the DCT. The observation that mice expressing constitutively active SPAK along the DCT1 phenocopy FHHt3 suggests a minimal role for effects of CUL3-Δ9 on other segments. However, in this study we used the Pax8-Cre system, results in CUL3-Δ9 expression in all segments. Generation of mice expressing CUL3-Δ9 specifically along the DCT will be necessary to address this limitation. In vitro studies have revealed that CUL3-Δ9 weakly interacts with JAB1, the enzyme that catalyzes removal of NEDD8 from CUL3. Because cycling of neddylation and deneddylation is required for stability of the CRL and its normal function, the resulting hyperneddylation has been proposed to be central in CUL3-Δ9–mediated FHHt. In this study, we show that short-term Jab1 disruption mimics the effects of CUL3-Δ9 expression, with lower WT CUL3 and KLHL3 abundances, and increased WNK4 and pNCC abundances. However, these mice did not display higher plasma [K+], most likely because Jab1 affects all cullins along the tubule, leading to pleiotropic effects and injury.20 DCT-specific Jab1 disruption will be needed to more conclusively confirm the contribution of loss of JAB1-CUL3-Δ9 interactions to FHHt, but recently identification of a patient with FHHt with a mutation in the region of CUL3 that directly interacts with JAB1 binding strongly suggests this is primary.16
Although FHHt is a rare disease, identification of causative mutations in WNK kinases led to the identification of a novel pathway that stimulates NCC activity. Conversely, mutations in KCNJ10, encoding the basolateral K+ channel Kir4.1, cause EAST/SeSAME syndrome, with loss of NCC activity leading to hypokalemia.41 These discoveries have led to the development of a model in which the DCT acts as a “sensor” of plasma [K+], with alterations in NCC activity maintaining K+ homeostasis by metering Na+ delivery to downstream K+-secreting segments.5 Modulation of CUL3 neddylation leading to altered CRL function and/or KLHL3 abundance may add another level of complexity to this scheme. Dietary K+ restriction reduced KLHL3 abundance and increased KLHL3 phosphorylation (which prevents WNK binding7), increasing WNK4 abundance.6 Recent work from the Fenton group suggests neddylation of several cullins, including CUL3 changes in dietary K+ intake in mice,42(preprint) pointing to a possible role for modulation of JAB1 activity under normal physiologic conditions. Dysregulation of CUL3 neddylation may also contribute to diabetic kidney disease, because KLHL3 abundance is lower in db/db diabetic mice,14 in streptozotocin-induced type 1 diabetic mice,15 and in a mouse model of pre-eclampsia,43 with increased NCC phosphorylation observed in all three models. We also reported that renal tubular Cul3−/− mice develop renal fibrosis, and that lower CUL3 abundance is observed in several mouse renal injury models.44 CUL3 dysregulation may therefore play a broader role in kidney disease.
In summary, we show KLHL3 is strongly targeted for degradation by CUL3-Δ9. The unique sensitivity of KLHL3 to CUL3-Δ9 may explain why FHHt is a “DCT-specific” disease. In support of this, our data suggest that loss of JAB1-mediated deneddylation and the resulting lower abundances of CUL3 and KLHL3 are mechanistically important in FHHt, although this needs to be confirmed in patients with the disease. Finally, our data suggest FHHt may reflect dysregulation of a physiologic mechanism governing CUL3 activity in the kidney.
Disclosures
D. Ellison reports receiving honoraria from Augusta University and Johns Hopkins; reports having an advisory or leadership role for the American Heart Association, as an Author for Up To Date, Consulting Editor for Hypertension, and the Editorial Board of American Journal of Physiology: Renal Physiology; and reports other interests or relationships with the Leadership Council for American Society of Nephrology. J.A. McCormick reports having an advisory or leadership role for Editorial Boards of the American Journal of Physiology: Renal Physiology, Frontiers in Physiology: Renal and Epithelial Physiology, and Kidney360. S. Gurley reports having employment with United Therapeutics. All remaining authors have nothing to disclose.
Funding
This work is supported by a Uehara Foundation postdoctoral award (to Y. Maeoka), an American Heart Association Postdoctoral Fellowship 17POST33670206 (to M. Ferdaus), Ben J. Lipps postdoctoral fellowship (to X-T. Su), National Institute of Diabetes and Digestive and Kidney Diseases grants DK051496 and DK054983 (to D. Ellison), DK098141 (to J. McCormick), Mentored Research Scientist Career Development Award, DK120790 (to R. Cornelius), Veterans Affairs grant 1I01BX002228, and a Fondation LeDucq Transatlantic Network of Excellence grant (to D. Ellison).
Supplementary Material
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Author Contributions
R. Cornelius, D. Ellison, M. Ferdaus, S. Gurley, Y. Maeoka, J. McCormick, and C.-L. Yang were responsible for the conceptualization; R. Cornelius, M. Ferdaus, Y. Maeoka, J. McCormick, L. Miller, J. Robertson, A. Sharma, and X.-T. Su were responsible for the data curation; R. Cornelius, M. Ferdaus, Y. Maeoka, J. McCormick, L. Miller, and A. Sharma were responsible for the formal analysis; D. Ellison and J. McCormick were responsible for the funding acquisition; R. Cornelius, L. Miller, J. McCormick, A. Sharma, and X.-T. Su were responsible for the investigation; S. Gurley and J. McCormick were responsible for the methodology; J. McCormick was responsible for the project administration; M. Ferdaus, Y. Maeoka, and J. McCormick were responsible for the visualization; Y. Maeoka and J. McCormick wrote the original draft; and R. Cornelius, D. Ellison, S. Gurley, Y. Maeoka, J. McCormick, and C.-L. Yang reviewed and edited the manuscript.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2021081099/-/DCSupplemental.
Supplemental Table 1. List of antibodies used.
Supplemental Figure 1. Normalization of Western blot data by Coomassie gel compared with actin normalization.
Supplemental Figure 2. CUL3 expression levels in Cul3+/− and Cul3+/−/Δ9 mice.
Supplemental Figure 3. Generation of inducible renal epithelial-specific Klhl3−/− mice and anti-KLHL3 antibody validation.
Supplemental Figure 4. Doxycycline-induced CUL3-Δ9 transgene activation in Cul3−/−/Δ9 mice.
Supplemental Figure 5. The effect of CUL3-Δ9 on KLHL3 after shorter-term doxycycline treatment.
Supplemental Figure 6. KLHL3 aggregates in DCT of Cul3−/−/Δ9 mice.
Supplemental Figure 7. Cul3 and Klhl3 expression pattern along the nephron.
Supplemental Figure 8. KLHL3 deficiency activates the WNK4-SPAK/NCC cascade.
Supplemental Figure 9. WNK aggregation in Cul3−/−, Cul3−/−/Δ9, and Cul3+/−/Δ9 mice.
Supplemental Figure 10. WNK aggregation was not observed along the TAL in Cul3+/−/Klhl3+/− mice.
Supplemental Figure 11. iSTAT analysis of Cul3+/−/Klhl3+/− mice.
Supplemental Figure 12. SBP is salt sensitive in Cul3+/−/Klhl3+/− mice.
Supplemental Figure 13. pNCC-T53 and tNCC abundances after HS/LK diet for 7 days.
Supplemental Figure 14. Hyperneddylation of CUL3 causes lowered abundances of CUL3 and KLHL3 and WNK4 accumulation.
Supplemental Figure 15. Acute disruption of the deneddylase Jab1 mimics the effects of CUL3-Δ9 expression.
References
- 1.McCormick JA, Mutig K, Nelson JH, Saritas T, Hoorn EJ, Yang CL, et al. : A SPAK isoform switch modulates renal salt transport and blood pressure. Cell Metab 14: 352–364, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grimm PR, Coleman R, Delpire E, Welling PA: Constitutively active SPAK causes hyperkalemia by activating NCC and remodeling distal tubules. J Am Soc Nephrol 28: 2597–2606, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grimm PR, Taneja TK, Liu J, Coleman R, Chen YY, Delpire E, et al. : SPAK isoforms and OSR1 regulate sodium-chloride co-transporters in a nephron-specific manner. J Biol Chem 287: 37673–37690, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin SH, Yu IS, Jiang ST, Lin SW, Chu P, Chen A, et al. : Impaired phosphorylation of Na(+)-K(+)-2Cl(-) cotransporter by oxidative stress-responsive kinase-1 deficiency manifests hypotension and Bartter-like syndrome. Proc Natl Acad Sci U S A 108: 17538–17543, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoorn EJ, Gritter M, Cuevas CA, Fenton RA: Regulation of the renal NaCl cotransporter and its role in potassium homeostasis. Physiol Rev 100: 321–356, 2020 [DOI] [PubMed] [Google Scholar]
- 6.Ishizawa K, Xu N, Loffing J, Lifton RP, Fujita T, Uchida S, et al. : Potassium depletion stimulates Na-Cl cotransporter via phosphorylation and inactivation of the ubiquitin ligase Kelch-like 3. Biochem Biophys Res Commun 480: 745–751, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shibata S, Arroyo JP, Castañeda-Bueno M, Puthumana J, Zhang J, Uchida S, et al. : Angiotensin II signaling via protein kinase C phosphorylates Kelch-like 3, preventing WNK4 degradation. Proc Natl Acad Sci U S A 111: 15556–15561, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson FH, Disse-Nicodème S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, et al. : Human hypertension caused by mutations in WNK kinases. Science 293: 1107–1112, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Louis-Dit-Picard H, Barc J, Trujillano D, Miserey-Lenkei S, Bouatia-Naji N, Pylypenko O, et al. : KLHL3 mutations cause familial hyperkalemic hypertension by impairing ion transport in the distal nephron. Nat Genet 44: 456–460, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Boyden LM, Choi M, Choate KA, Nelson-Williams CJ, Farhi A, Toka HR, et al. : Mutations in Kelch-like 3 and cullin 3 cause hypertension and electrolyte abnormalities. Nature 482: 98–102, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J, Zou J, Littlejohn R, Liu J, Su H: Neddylation, an emerging mechanism regulating cardiac development and function. Front Physiol 11: 612927, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He X, Zhu A, Feng J, Wang X: Role of neddylation in neurological development and diseases [published online ahead of print]. Biotechnol Appl Biochem 2021 [DOI] [PubMed] [Google Scholar]
- 13.Zou T, Zhang J: Diverse and pivotal roles of neddylation in metabolism and immunity. FEBS J 288: 3884–3912, 2021 [DOI] [PubMed] [Google Scholar]
- 14.Ishizawa K, Wang Q, Li J, Xu N, Nemoto Y, Morimoto C, et al. : Inhibition of sodium glucose cotransporter 2 attenuates the dysregulation of Kelch-Like 3 and NaCl cotransporter in obese diabetic mice. J Am Soc Nephrol 30: 782–794, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo Q, Zhang Y, Jiang GR, Zhang C: Decreased KLHL3 expression is involved in the activation of WNK-OSR1/SPAK-NCC cascade in type 1 diabetic mice. Pflugers Arch 473: 185–196, 2021 [DOI] [PubMed] [Google Scholar]
- 16.Chatrathi HE, Collins JC, Wolfe LA, Markello TC, Adams DR, Gahl WA, et al. : Novel CUL3 variant causing familial hyperkalemic hypertension impairs regulation and function of ubiquitin ligase activity. Hypertension 79: 60–75, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCormick JA, Yang CL, Zhang C, Davidge B, Blankenstein KI, Terker AS, et al. : Hyperkalemic hypertension-associated cullin 3 promotes WNK signaling by degrading KLHL3. J Clin Invest 124: 4723–4736, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cornelius RJ, Zhang C, Erspamer KJ, Agbor LN, Sigmund CD, Singer JD, et al. : Dual gain and loss of cullin 3 function mediates familial hyperkalemic hypertension. Am J Physiol Renal Physiol 315: F1006–F1018, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schumacher FR, Siew K, Zhang J, Johnson C, Wood N, Cleary SE, et al. : Characterisation of the Cullin-3 mutation that causes a severe form of familial hypertension and hyperkalaemia. EMBO Mol Med 7: 1285–1306, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cornelius RJ, Si J, Cuevas CA, Nelson JW, Gratreak BDK, Pardi R, et al. : Renal COP9 signalosome deficiency alters CUL3-KLHL3-WNK signaling pathway. J Am Soc Nephrol 29: 2627–2640, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoshida S, Araki Y, Mori T, Sasaki E, Kasagi Y, Isobe K, et al. : Decreased KLHL3 expression is involved in the pathogenesis of pseudohypoaldosteronism type II caused by cullin 3 mutation in vivo. Clin Exp Nephrol 22: 1251–1257, 2018 [DOI] [PubMed] [Google Scholar]
- 22.Ferdaus MZ, Miller LN, Agbor LN, Saritas T, Singer JD, Sigmund CD, et al. : Mutant Cullin 3 causes familial hyperkalemic hypertension via dominant effects. JCI Insight 2: 96700, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abdel Khalek W, Rafael C, Loisel-Ferreira I, Kouranti I, Clauser E, Hadchouel J, et al. : severe arterial hypertension from cullin 3 mutations is caused by both renal and vascular effects. J Am Soc Nephrol 30: 811–823, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sasaki E, Susa K, Mori T, Isobe K, Araki Y, Inoue Y, et al. : KLHL3 knockout mice reveal the physiological role of KLHL3 and the pathophysiology of pseudohypoaldosteronism type II caused by mutant KLHL3. Mol Cell Biol 37: e00508-16, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Traykova-Brauch M, Schönig K, Greiner O, Miloud T, Jauch A, Bode M, et al. : An efficient and versatile system for acute and chronic modulation of renal tubular function in transgenic mice. Nat Med 14: 979–984, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agbor LN, Ibeawuchi SC, Hu C, Wu J, Davis DR, Keen HL, et al. : Cullin-3 mutation causes arterial stiffness and hypertension through a vascular smooth muscle mechanism. JCI Insight 1: e91015, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McDonough AA, Veiras LC, Minas JN, Ralph DL: Considerations when quantitating protein abundance by immunoblot. Am J Physiol Cell Physiol 308: C426–C433, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferdaus MZ, Mukherjee A, Nelson JW, Blatt PJ, Miller LN, Terker AS, et al. : Mg2+ restriction downregulates NCC through NEDD4-2 and prevents its activation by hypokalemia. Am J Physiol Renal Physiol 317: F825–F838, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang MX, Cuevas CA, Su XT, Wu P, Gao ZX, Lin DH, et al. : Potassium intake modulates the thiazide-sensitive sodium-chloride cotransporter (NCC) activity via the Kir4.1 potassium channel. Kidney Int 93: 893–902, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cornelius RJ, Yang CL, Ellison DH: Hypertension-causing cullin 3 mutations disrupt COP9 signalosome binding. Am J Physiol Renal Physiol 318: F204–F208, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen L, Chou CL, Knepper MA: A comprehensive map of mRNAs and their isoforms across all 14 renal tubule segments of mouse. J Am Soc Nephrol 32: 987–912, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ellison DH: Me or your own eyes: RNA-Seq and the kidney. J Am Soc Nephrol 32: 768–771, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Terker AS, Castañeda-Bueno M, Ferdaus MZ, Cornelius RJ, Erspamer KJ, Su XT, et al. : With no lysine kinase 4 modulates sodium potassium 2 chloride cotransporter activity in vivo. Am J Physiol Renal Physiol 315: F781–F790, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoorn EJ, Nelson JH, McCormick JA, Ellison DH: The WNK kinase network regulating sodium, potassium, and blood pressure. J Am Soc Nephrol 22: 605–614, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Terker AS, Zhang C, McCormick JA, Lazelle RA, Zhang C, Meermeier NP, et al. : Potassium modulates electrolyte balance and blood pressure through effects on distal cell voltage and chloride. Cell Metab 21: 39–50, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tian L, Peng G, Parant JM, Leventaki V, Drakos E, Zhang Q, et al. : Essential roles of Jab1 in cell survival, spontaneous DNA damage and DNA repair. Oncogene 29: 6125–6137, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ibeawuchi SR, Agbor LN, Quelle FW, Sigmund CD: Hypertension-causing mutations in cullin3 protein impair RhoA protein ubiquitination and augment the association with substrate adaptors. J Biol Chem 290: 19208–19217, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kasagi Y, Takahashi D, Aida T, Nishida H, Nomura N, Zeniya M, et al. : Impaired degradation of medullary WNK4 in the kidneys of KLHL2 knockout mice. Biochem Biophys Res Commun 487: 368–374, 2017 [DOI] [PubMed] [Google Scholar]
- 39.Harris AN, Grimm PR, Lee HW, Delpire E, Fang L, Verlander JW, et al. : Mechanism of hyperkalemia-induced metabolic acidosis. J Am Soc Nephrol 29: 1411–1425, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singer JD, Gurian-West M, Clurman B, Roberts JM: Cullin-3 targets cyclin E for ubiquitination and controls S phase in mammalian cells. Genes Dev 13: 2375–2387, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bockenhauer D, Feather S, Stanescu HC, Bandulik S, Zdebik AA, Reichold M, et al. : Epilepsy, ataxia, sensorineural deafness, tubulopathy, and KCNJ10 mutations. N Engl J Med 360: 1960–1970, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murali SK, Little R, Poulsen SB, Ferdaus MZ, Ellison DH, McCormick JA, et al. : Potassium effects on NCC are attenuated during inhibition of Cullin E3-ubiquitin ligases. bioRxiv. 10.1101/2021.11.30.470531 (Preprint posted December 1, 2021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Y, Jiang G, Zhang C: Downregulation of Cullin 3 ligase signaling pathways contributes to hypertension in preeclampsia. Front Cardiovasc Med 8: 654254, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saritas T, Cuevas CA, Ferdaus MZ, Kuppe C, Kramann R, Moeller MJ, et al. : Disruption of CUL3-mediated ubiquitination causes proximal tubule injury and kidney fibrosis. Sci Rep 9: 4596, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.