Abstract
AKI affects approximately 13.3 million people around the world each year, causing CKD and/or mortality. The mammalian kidney cannot generate new nephrons after postnatal renal damage and regenerative therapies for AKI are not available. Human kidney tissue culture systems can complement animal models of AKI and/or address some of their limitations. Donor-derived somatic cells, such as renal tubule epithelial cells or cell lines (RPTEC/hTERT, ciPTEC, HK-2, Nki-2, and CIHP-1), have been used for decades to permit drug toxicity screening and studies into potential AKI mechanisms. However, tubule cell lines do not fully recapitulate tubular epithelial cell properties in situ when grown under classic tissue culture conditions. Improving tissue culture models of AKI would increase our understanding of the mechanisms, leading to new therapeutics. Human pluripotent stem cells (hPSCs) can be differentiated into kidney organoids and various renal cell types. Injury to human kidney organoids results in renal cell-type crosstalk and upregulation of kidney injury biomarkers that are difficult to induce in primary tubule cell cultures. However, current protocols produce kidney organoids that are not mature and contain off-target cell types. Promising bioengineering techniques, such as bioprinting and “kidney-on-a-chip” methods, as applied to kidney nephrotoxicity modeling advantages and limitations are discussed. This review explores the mechanisms and detection of AKI in tissue culture, with an emphasis on bioengineered approaches such as human kidney organoid models.
Keywords: acute renal failure, cisplatin, cisplatin nephrotoxicity, tubule cells, renal proximal tubule cell, podocyte, kidney organoids, kidney-on-a-chip, nephrotoxicity, renal cell biology
AKI refers to the rapid decline of renal function, which is diagnosed by increased serum creatinine (sCr) levels. AKI can be the result of various insults, such as drugs, toxins, sepsis, or ischemia-reperfusion injury (IRI), causing renal cell failure.1 Damage to the major structures of the kidney, including tubule cells, glomeruli, interstitial cells, and vascular cells, leads to the initiation of hemodynamic and inflammatory pathways, resulting in decreased GFR.2 Since the concept of toxic nephropathy was introduced in 1965, clinicians and scientists have sought to prevent, detect, and treat nephrotoxicity.3 Nephrotoxicants and nephrotoxins are toxic substances that injure the kidney. Resulting kidney damage includes interstitial nephritis,4 glomerulonephritis,5 rhabdomyolysis,6 crystal nephropathy,7 tubular cell toxicity,8 and glomerulosclerosis.9
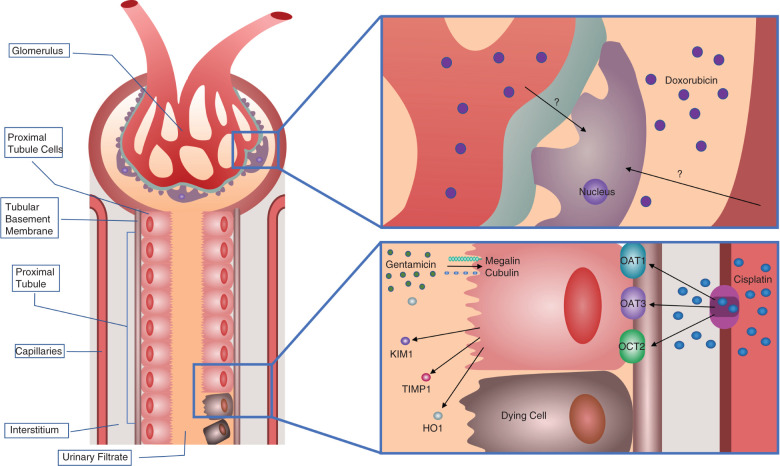
In 1987, a renal biopsy study of approximately 100 patients with acute renal failure identified the drugs associated with AKI.10 This research suggested that nonsteroidal anti-inflammatory drugs, antibiotics, glafenin, contrast media, diuretics, chemotherapy, and acetaminophen may cause kidney injury.10 On the basis of the affected nephron segment, the mechanisms of drug-induced nephrotoxicity can be classified into various types.11 The categories include hemodynamically mediated kidney injury, tubular epithelial cell damage, tubulointerstitial disease, glomerular disease, renal vasculitis, thrombosis, and obstructive nephropathy. Most drug-induced nephrotoxicity involves injury to the proximal tubular epithelial cells and podocytes. The epithelial cells in the proximal tubules express specific transporters, so chemicals with an affinity for those transporters can enter the cell and induce damage.12 The entry of drugs into the proximal tubules is mediated by members of the solute carrier family (SLC), such as organic cation transporter 2 (OCT2/SLC22A2), the organic anion transporters 1 and 3 (OAT1/SLC22A6, OAT3/SLC22A8), and receptors such as megalin and cubulin (Figure 1).13–15 In podocytes, the plasma membrane monoamine transporter and bisphosphonates mediate disruption of the podocyte cytoskeleton, causing cell death.16,17
Figure 1.
Mechanism of tubular injury during AKI. Nephrotoxicants enter cells through receptor-mediated transport. For cisplatin, transport into the proximal tubule epithelial cell is facilitated by OAT1, OAT3, and OCT2 receptors on the basolateral membrane. Inflammatory and vasoactive mediators and ROS damage the tubular cells and lead to the shedding of the proximal tubule brush border, loss of polarity, and cell death by apoptosis and necrosis. Proximal tubule cell damage releases kidney injury markers, including KIM-1, NGAL, TIMP1, and HO1, into the urinary filtrate. For Dox, the transporters are unknown, but the drug induces oxidative stress resulting in podocyte apoptosis.
Modeling of kidney injury relies on both in vivo and in vitro methods. The in vivo animal models have been utilized for nephrotoxicity studies for a number of years, with mice and rats being the most popular animals. Meanwhile, in vitro models using human kidney cells such as tubule cells, podocytes, or other epithelial cells have also been used to support and further explore findings in vivo. In this review, we will focus on the in vitro human AKI models. We will discuss biomarkers for nephrotoxicity in humans, advantages and disadvantages of available tissue culture models of AKI, and the latest advances, including human kidney organoids derived from human pluripotent stem cells (hPSCs) and kidney-on-a-chip microfluidic systems.
Biomarkers of AKI
Diagnosis of AKI is on the basis of levels of preclinical or clinical biomarkers. The clinical biomarkers are sCr and BUN, both late-stage markers of AKI. Preclinical studies have identified many early-stage AKI biomarkers, such as enzymes18 including alanine aminopeptidase, alkaline phosphatase, α-glutathione–S-transferase, γ-glutamyl transpeptidase, and N-acetyl–β-glucosaminidase; proteins including kidney injury molecule-1 (KIM-1),19 neutrophil gelatinase-associated lipocalin (NGAL), and liver-type fatty acid-binding protein; and cytokines including IL-18. Recently discovered AKI biomarkers include heme oxygenase-1 (HO1) and urinary miRNA.20 Translation of more sensitive markers into the clinic may facilitate earlier diagnosis to implement testing of therapeutics for earlier and more successful treatment of AKI.
KIM-1, also known as hepatitis A virus cellular receptor 1 (HAVCR1) or T cell immunoglobulin and mucin domain-containing protein 1, is a candidate urinary biomarker for detecting drug-induced AKI.19 In the setting of AKI, the glycoprotein KIM-1 acts as a phagocytic receptor on the apical surface of proximal tubule cells, which enables the removal of apoptotic cellular debris. After a metalloproteinase cleavage, the ectodomain of KIM-1 is shed in the urine. KIM-1 has been tested as a biomarker of drug-induced nephrotoxicity in preclinical trials.21 In 2002, Han et al. reported the presence of KIM-1 protein in the urine of 23 patients with AKI and nine patients with CKD.19 Compared with conventional biomarkers, such as creatinine, BUN, urinary N-acetyl–β-glucosaminidase, γ-glutamyl transpeptidase, or alkaline phosphatase, urinary KIM-1 is an earlier diagnostic indicator of AKI.19,22 A study by Timmeren et al. showed that KIM-1 expression in kidney tissues correlated with urinary KIM-1, suggesting potential as an early biomarker.23
HO1 is a 32 kDa enzyme induced in response to oxidative stress.24 It is a cryoprotective antioxidant present in the proximal tubules that is expressed at higher levels, both in plasma and urine, in patients with AKI.25 Deficiency of HO1 after a toxic insult can promote epithelial–mesenchymal transition (EMT) and lead to AKI to CKD transition.26,27 Therefore, sustained expression of HO1 after injury might suggest a reduced risk of CKD. After injury, HO1 is expressed in proximal tubules, glomeruli, and interstitial cells.28,29
NGAL, or lipocalin 2, is a protein belonging to the lipocalin superfamily. In a healthy kidney, tubules endocytose NGAL. During injury, NGAL levels rise in both distal tubules and collecting ducts, causing shedding of NGAL into the urine. NGAL found in the urine is comprised of different molecular forms of NGAL originating from various cell types. The increased translation within epithelial cells, secretion by infiltrating neutrophils, and impaired reabsorption all contribute to elevated, detectable levels during injury.30 Elevated levels of NGAL in the kidneys of patients with AKI were first identified in 2005.31 The first clinical study evaluating NGAL as an AKI predictor was performed in children at risk of AKI.32 NGAL is under development as a biomarker for the detection of kidney injury, both in vivo and in vitro.33 In addition to AKI, NGAL is a promising marker for CKD.34
ILs are the cytokines expressed by lymphocytes, monocytes, macrophages, endothelial cells, and fibroblasts. After kidney injury, tubular epithelial cells and podocytes produce certain ILs as part of the inflammatory response. For example, IL-8 expressed in proximal tubule cells and intercalated renal cells accumulates in urine in response to AKI.35,36
Urinary miRNAs have also been investigated for detecting AKI. Studies using miRNome profiling revealed that various urinary miRNAs, including miR-21, miR-200c, miR-423, and miR-4640, were differentially expressed by patients with AKI.37 However, the technique does not provide information on the status of renal cells and is not cell specific.38
Preclinical Kidney Models
Animal Models and Potential Drawbacks
Animal models have contributed a great deal to our understanding of kidney physiology and pathophysiology. Studies in rats and mice have allowed us to explore the pathogenesis, genetics, and underlying mechanisms of kidney diseases. In vivo models of nephrotoxic AKI are usually obtained by the administration of toxic drugs to animals.
Differences in gene composition and expression in the mouse and human genomes must be carefully considered when using mouse models.39 Practical drawbacks of animal models include the management, approvals, and cost of veterinary surgical equipment. Training personnel, genotyping, and intensive specialization of labor that is often required to carry out such animal work is time consuming. Species differences in genetics or physiology may result in unexpected outcomes during first-in-human early-stage clinical trials.40,41 The failure of many clinical trials of therapeutic approaches to treat patients with AKI could be due to multiple areas of misalignment between the AKI animal models and patients with AKI. This includes the younger age of animals used compared with older patients, the high toxicant dosage usually used to induce AKI in animals as opposed to lower doses given over a longer time to patients who have cancer,42 and physiologic differences between species. Although the animal models do not fully mimic clinical AKI pathology, human in vitro studies can complement them to address some of their limitations.
Primary Human Kidney Cells
Primary cells are isolated or harvested from living tissues or organs. Renal primary cells permit studies of renal function and the effects of nephrotoxicants. Human primary renal proximal tubular cells (HPTC) express proximal tubule cell-specific markers during early passages. These cells express various transporters43 and produce drug metabolism enzymes.44,45 However, disadvantages of their use include donor variability,46 expression of some transporters and enzymes below physiologic levels, and loss of tubule cell identity during passaging.47 All approaches relying on primary human kidney cells have production limitations, including sourcing from discarded kidneys that are unsuitable for transplantation and limited proliferative capacity in tissue culture.48
Other Adult-derived Kidney Cells: Advanced Culture and Reprogramming Strategies
Similar to primary cells, kidney organoids derived from kidney biopsy samples can also be used to model nephrotoxicity.49 Such human kidney–derived organoids may come from an epithelial progenitor cell type without a stromal component and express a mixed set of markers including aquaporin-1, aquaporin-3, podocin, synaptopodin, and nephrin.
Interestingly, Kaminski et al. reprogrammed mouse fibroblast cells with transcription factors Emx2, Hnf1b, Hnf4a, and Pax8 into early phenotype renal tubular epithelial cells.50 Translation of this study to human cells could provide an alternative option to generate tubule cells from other kinds of primary cell types in the future. In a separate direct reprogramming study, human nephron progenitors were generated from adult primary renal tubular epithelial cells by the expression of SNAI2, SIX1, and EYA1, which under certain conditions could be differentiated back into tubular epithelium.51 Further development of direct reprogramming strategies is needed for large-scale production of desired kidney cells for nephrotoxicity testing.52
Immortalized Human Kidney Cells
To overcome the lack of reliability over many passages and heterogeneity associated with primary cells, researchers have immortalized primary cell types to derive kidney cell lines. Commonly used fully immortalized kidney cell lines include HK-253 and RPTEC/TERT1.54 Primary cells have been immortalized with human telomerase reverse transcription,55 human papillomavirus 16 E6/E7 genes,56 and/or SV40 large T antigen (SV40T).57 Conditionally immortalized proximal tubule epithelial cells (ciPTEC),57 and podocytes (CIHP-1)58 have the advantage of temperature-controlled states of proliferation versus differentiation (Table 1). Conditionally immortalized cell lines expressing temperature-sensitive SV40T proliferate when incubated at the permissive temperature of 33°C when SV40T is active. On turning off the SV40T by incubating the cells at 37°C, these lines are induced to mature.57 The cell type characteristics of the cell lines were confirmed by expression of markers OAT1, OAT3, and OCT2 on the basolateral membrane and ABCB1 (P-gp), SLC47A1 (MATE1), SLC47A2 (MATE2), ABCC2 (MRP2), ABCC4 (MRP4), ABCG2 (BCRP), and the endocytosis receptors megalin and cubilin on the apical membrane.59
Table 1.
In vitro models of AKI in immortalized cells
| Cell Line | Markers | Function | Reference(s) |
|---|---|---|---|
| RPTEC/TERT1 | Aminopeptidase-N, E-cadherin, MRP2, MRP4, OAT4, MDR1, MATE1, OCTN2, OCT3 | Response to PTH treatment by increased cAMP levels GGT activity, Megalin/cubilin transport system | 168 , 169 |
| ciPTEC | Aminopeptidase-N, Pgp, AQP1, dpp-IV, MRP2/4,OCT2, OAT1, OAT3, BCRP, MATE1/2, CaSR, megalin/cubilin receptors | ALP activity, albumin endocytosis, sodium-dependent phosphate uptake, OCT2, and P-gp activity, UGT activity | 57 , 170 |
| HK-2 | Vimentin, cytokeratin, α3β1 integrin, leucine aminopeptidase, fibronectin, MCT1, MDR1, OATP4C1 | Gluconeogenesis, and Na+ -dependent glucose uptake, GGT and alkaline phosphatase activity, increased cAMP levels in response to PTH, synthesis and secretion of plasma proteins | 171 |
| Nki-2 | E-cadherin, CK8/18/19, GGT1, OAT1, OAT4 | Stable cell line, potentially valuable in toxicity and drug ???? | 172 |
| CIHP-1 | Synaptopodin, CD2AP, nephrin, podocin, ZO-1, P-cadherin, α, β, γ-catenin | A valuable tool in the study of human glomerular disease | 173 |
PTH, Parathyroid hormone; cAMP, Cyclic adenosine monophosphate; AQP1, aquaporin-1; ALP, Alkaline Phosphatase; UGT, Uridine 5'-diphospho-glucuronosyltransferase ; GGT, Gamma glutamyl-transferase.
Kidney-on-a-chip Microfluidic Systems
Microelectromechanical systems allow researchers to apply microfluidics in tissue culture to engineer an “organ-on-a-chip.” “Kidney-on-a-chip” refers to the use of microfluidic devices for growing renal cells, which can be utilized for preclinical drug development and toxicity screening. The first kidney-on-a-chip study by Jang and coworkers used a microfluidic device lined by living human kidney epithelial cells to produce a system that better mimicked the in vivo responses to cisplatin toxicity and phosphoglycolate phosphatase efflux transporter activity than conventional culture conditions.60 Fabrication of three-dimensional (3D) renal proximal convoluted tubules embedded within an extracellular matrix on perfusion chips also showed an improvement in phenotypic and functional properties as compared with two-dimensional (2D) controls.61 In 2018, Vriend et al. grew immortalized proximal tubule epithelial cells expressing the OAT1 transporter (ciPTEC-OAT1) on chips to allow for high-throughput screening and compatibility with high-content imaging platforms.61 In 2017, Musah et al. cocultured podocytes derived from human-induced pluripotent stem cells (hiPSCs) with a layer of human kidney glomerular endothelium in an organ-on-a-chip microfluidic device.62 They reported improved tissue–tissue interface and molecular filtration properties in the “glomerulus-on-a-chip.”62 A recent study using human RPTEC cells cultured on a chip reported improved polarized tight junctions, cilia formation in the apical brush borders, and OCT2 transporter expression on the basolateral membrane, as compared with 2D cultures.63
The kidney-on-a-chip method has many advantages including extended life, biocompatibility, higher gas permeability and sensitivity, and low cost. The major advantage of the kidney-on-a-chip is the ability to apply a nephrotoxicant to either the apical or basolateral surface of the epithelium, which is not possible in a hiPSC-derived organoid. Commercially available kidney-on-a-chip devices such as the TissUse device, OrganoPlate, and the Nortis device are utilized for nephrotoxicity testing and pharmacokinetic studies. Further studies in the field may lead to a more advanced AKI-on-a-chip model and/or a treatment option through the development of the implantable bioartificial kidney.64
Stem Cell–derived Human Kidney Models
Although immortalized kidney cells are highly utilized because they are generally easy to grow, they also have reduced function and altered morphologic characteristics as compared with matching cell types in vivo.65 Because of these limitations, although in vitro AKI models on the basis of these cell lines may report decreased cell viability or proliferation, the data do not always translate to follow-up studies conducted in vivo.66 Therefore, another approach under development is to differentiate stem cells toward a renal lineage for AKI modeling. Of interest for AKI research, there are now protocols for the creation of kidney cells induced from hPSCs.67–70 For example, hPSCs have been differentiated into other renal cell types including ureteric bud cells, podocytes, tubule cells, and renal progenitor cells.70–78
Self-organizing kidney organoids generated from hPSCs contain various renal cell lineages.79–84 The kidney houses two progenitor cell types originating from the posterior primitive streak in the blastocyst, the metanephric mesenchyme and ureteric bud. the metanephric mesenchyme is derived from the intermediate mesoderm whereas ureteric bud is derived from the anterior intermediate mesoderm.67 A protocol for the induction of mesoderm and intermediate mesoderm was first published in 2013.68 A “kidney-in-a-dish” can be obtained from human stem cells differentiated by providing the appropriate signals and factors at the right times to mimic the developing embryonic kidney.79–81,83–85 Kidney organoids developed using various protocols are morphologically and transcriptionally more similar to a human fetal kidney than to an adult kidney.79
Most kidney organoid protocols rely on activation of canonical Wnt, Activin A signaling to reach the intermediate mesoderm stage, followed by treatment with fibroblast growth factor 9 for nephrogenesis. Some protocols use a pulse of Wnt activation at the nephrogenesis stage to trigger mesenchymal-to-epithelial transition to increase the number of nephrons formed within the aggregate.79 Kidney organoids express markers for nephron segments including distal tubules, proximal tubules, early loops of Henle, presumptive collecting ducts, glomeruli, and podocytes, and endothelial and interstitial cells.79,80 Transport into proximal tubules within kidney organoids as measured by dextran uptake suggests the absorptive function is at least somewhat present in these immature organoids.80
Although kidney organoids contain many renal cell types, they are not without practical drawbacks including high cost, complexity, skill requirement, variable reproducibility, and limitations to large-scale culture (Table 2). To facilitate scale-up, 3D kidney organoids may be grown in bioreactor spinner flasks.86 To lower costs, “KnockOut Serum Replacement” has been substituted for fibroblast growth factor 987 or special swirling media.86 Kidney organoid protocols with improved maturity and reproducibility are needed. Development of more mature organoids expressing drug transporters at levels that are closer to those found in the adult human kidney would further increase the utility of organoid models of nephrotoxicity. Three-dimensional bioprinting permits complex structure fabrication with different materials, cell types, and growth factors.88 Bioprinting of hPSC-derived kidney organoids created reproducible, higher-quality organoids, suggesting the translational promise of these methods for large-scale production.89,90 At the same time, bioprinting approaches are generally not regarded as simple enough to be widely adopted across research laboratories.
Table 2.
Advantages and disadvantages of the in vitro human AKI models
| Method | Advantages | Disadvantages | Reference |
|---|---|---|---|
| Primary cell lines | Intact genetic and phenotypic properties Used with real-time imaging techniques | Interdonor variability Short lifespan Unsuitable for transplantation Source issue | 43 , 44 , 48 |
| Immortalized cell lines | Long lifespan Less variability Easiest to culture | Absence of important transporters Least differentiated option available | 53 , 54 , 160 |
| Conditionally immortalized cell lines | Long lifespan Less variability Easy to culture Temperature-controlled state of proliferation | Absence of important transporters | 57 , 58 , 63 |
| Kidney organoids | System enabling more physiologic modeling Multiple cell types are represented so interactions occur Used with real-time imaging techniques Resources available online at gudmap.org | Highly variable results between cell lines, protocols, and batches Long culture time with a short usage span High cost Maturity level is fetal kidney Presence of off-target cell types Nonadult kidney cell types such as nephron progenitors are present | 79 , 80 , 89 , 174 |
| Kidney-on-a-chip | Fluid flow and sheer stress encourages differentiation Multicompartment systems for cell type crosstalk Low cell number eases setup | Uses specialized devices Bioengineering knowledge required Low cell number complicates scale-up and extraction | 60 , 175 , 176 |
Kidney organoids have many advantages over the cell lines, but they have a short lifespan of approximately 30 days and contain immature endothelial cells that are not well organized into a functional vasculature. New approaches suggest the vascularization challenge is addressable. Organoids grown on chick chorioallantoic membrane had vascularization and circulation of chick blood within kidney organoids.91 Alternatively, kidney organoids grown under flow on 3D-printed millifluidic chips with a controlled wall shear enhanced the vascular network formation in developing kidney organoids.92 Although these studies reported improved vascularization within kidney organoids cultured under flow, the resulting vasculature was still not perfusable.92,93 Another approach using renal subcapsular transplantation of hPSC-kidney organoids in mice resulted in vasculogenesis and improved glomerular and tubular maturation.94 Three-dimensional bioprinting may provide another avenue for improvement of vascularization and therefore oxygenation within human kidney organoids.95,96
Another challenge is the presence of off-target cell types that are not desired such as neuronal, muscle, and melanoma cells, which increase in relative population as the organoids age.97,98 Uncommitted mesodermal cells that exist transiently during kidney organoid differentiation are likely to give rise to these undesirable cell types. Inhibition of brain-derived neurotrophic factor and its receptor NTRK2 greatly reduced the number of neurons without inhibiting differentiation of kidney cell types.97 Kidney organoids also do not have stromal patterning, a renal pelvis, an immune system, and a lymphatic system. Although nephrons are patterned appropriately from glomerulus to collecting duct, they do not loop, preventing tubular-glomerular crosstalk.
Nephrotoxicants for Modeling AKI
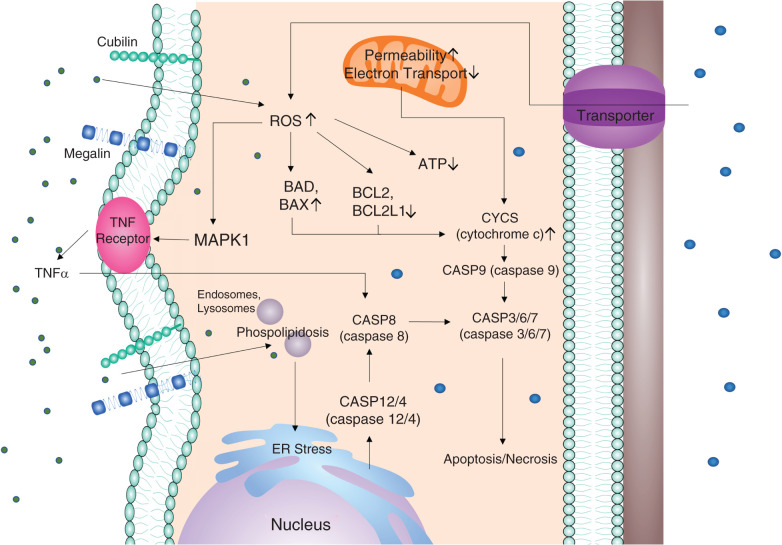
Cisplatin is a chemotherapeutic agent used to treat several cancers including testicular, bladder, ovarian, esophageal, breast, and others. Cisplatin is a cationic drug that enters proximal tubule cells via cationic OCT2 and OAT transporters. Cellular injury results from nuclear and mitochondrial DNA damage and oxidative stress, with some efflux occurring via multidrug and toxin extrusion (MATE) transporters.99,100 Cisplatin mainly injures the S3 segment of the proximal tubule in the medullary region, causing cell death via inflammation and generation of reactive oxygen species (ROS).101
The pathways involved in cisplatin-induced cell death within renal tubular epithelial cells are well established (Figure 1). The damaged mitochondrial DNA reduces oxidative phosphorylation, disturbing mitochondrial ROS (mtROS) production, and leading to apoptosis and necrosis.102–104 Cisplatin also causes endoplasmic reticulum stress by activating caspase-12, upregulating Grp78/BiP, and activating the caspase cascade of apoptosis.105 TNF-α may play a central role in the pathogenesis of cisplatin-induced renal injury by activating the cytokine response.106
Cisplatin-induced AKI processes have been explored both in animal models107–109 and conventional in vitro models.110–112 For cisplatin, rodent models have AKI biomarkers and histology within 72 hours after one injection of 6–30 mg/kg body weight.107–109,113 In HK-2 cells, cisplatin-induced apoptosis causes downregulation of BCL2 and activation of CASP3, suggesting that HK-2–based models mimic the apoptosis pathways in vivo.114 Treatment of 3D transwells or suspension kidney organoids with a single dose of 5–50 μM cisplatin for 24 hours induced toxicity, damaging all cellular compartments in the organoids including podocytes, proximal tubule cells, distal tubule cells, and interstitial cells.79,83,84,115
Digby et al. reported that 3D kidney organoids treated with a higher dose of 50 μM cisplatin for 48 hours had upregulation of proximal tubule injury marker HAVCR1 and the inflammatory cytokine C-X-C motif chemokine ligand 8 (CXCL8), and DNA damage and cell death.116(preprint),117 This is consistent with the acute toxicity, high mortality, and absence of fibrosis found in animal models at higher doses.116(preprint),117 Digby et al. also showed that a repeated low-dose regimen of cisplatin avoided acute cell death and resulted in a milder but cumulative injury phenotype compared with a single high dose in kidney organoids.116(preprint) Such low-dose regimens better mimic the nephrotoxicity found in patients receiving chemotherapy.
Gentamicin
Gentamicin is a highly charged polycationic aminoglycoside antibiotic that is also a potent nephrotoxicant. Gentamicin enters the cells through megalin, a multiligand endocytic receptor located in the apical brush border, and damages the proximal convoluted renal tubule.15,118–120 Gentamicin forms ternary complexes with iron ions, and after oxidation, triggers the generation of ROS.121 Gentamicin accumulates in lysosomes and produces lysosomal phospholipidosis,122 leading to the impairment of the phosphatidylinositol cascade.123 Gentamicin exposure causes an early increase in the proapoptotic protein Bax (Figure 2).120
Figure 2.
Pathways of nephrotoxicant-induced tubular cell death. Nephrotoxicants enter cells through specific transporters such as OCT2 and OAT1, or endocytic renal receptors such as megalin and cubilin. The drugs damage both nuclear and mitochondrial DNA, leading to the production of ROS and subsequently activation of both mitochondrial and nonmitochondrial pathways of apoptosis and necrosis.62,129,167 Mitochondrial dysfunction activates the CASP3 cascade, leading to cell death, whereas the increase of ROS decreases levels of survival proteins such as BCL2 and increases levels of prodeath proteins such as BAX. BCL2, B-cell lymphoma 2; BAX, BCL2 associated ×.
Rodents are injected with 80–100 mg/kg of gentamicin per day for ≤8 days to induce AKI.124–126 Treatment of HK-2 cells with a 500 μg/ml dose of gentamicin for 24–48 hours resulted in cellular toxicity.127 The gentamicin treatment inhibited the lysosomal activity of phospholipases in the proximal tubular epithelial cells and generated ROS in the cells.128 Treatment of kidney organoids with 5 mg/ml gentamicin resulted in KIM-1 expression at the luminal surface of lotus tetragonolobus lectin (LTL)–positive tubules, suggesting that gentamicin treatment injured proximal tubules within the organoids.84
Doxorubicin/Adriamycin
Doxorubicin (Dox), sold under the brand name Adriamycin, is a chemotherapeutic drug that increases intracellular ROS production in renal tissue, resulting in cell death via apoptosis.129 Dox suppresses the activity of topoisomerase 2b, which results in the reduction of both p53-based antiapoptotic activity and antioxidant protein expression.130 Dox-induced oxidative stress disrupts the mitochondrial membrane. Mitochondria then release cytochrome c and proapoptotic factors into the cytosol. Dox upregulates the proapoptotic proteins BAD and BAX and downregulates the antiapoptotic BCL-2 family proteins BCL2 and BCL2L1 (Bcl-XL) in the renal tissue (Figure 2).131 Studies show that Dox also upregulates BID and caspase-8. Dox mediates cell death through the TNF-α pathway by activating MAPKs, a family of serine/threonine kinases.129
Dox is distinct from other nephrotoxicants in that it injures podocytes in the glomerulus132 and tubular epithelial cells.133 Injection of 10 mg/kg body weight of Dox has been used to generate a mouse model of podocytopathy.134 Kidney organoids have also been used to create Dox-based podocytopathy models.86,89 Kidney organoids treated with 2 µM or 10 µM of Dox for 24–72 hours have reduced glomerular structures and tubule networks.86,89
Sodium Cyanide
Cell-culture modeling of hypoxic-anoxic injury is a system parallel to the popular in vivo AKI rodent models of IRI.135 Anoxia from chemical treatment can model the metabolic stress that results from AKI in vitro. ATP depletion via sodium cyanide treatment was first reported in 1996.136 Cells were treated with sodium cyanide and 2-deoxy-d-glucose (5–10 mM each) for up to 2 hours to induce stress via tyrosine phosphorylation of β-catenin, JUP (plakoglobin), and the Bax cofactor NPM1 (nucleophosmin).137,138 The cells had ATP content reduced to <10% of normal within 10 minutes. This method has been used for human kidney monolayer cultures including RPTEC139 and HK-2140 cells. Implementing the same method to induce anoxia in kidney organoids might further improve this model of AKI to mimic IRI.
Quantifying Nephrotoxic Injury In Vitro
Kidney-specific In Vitro Biomarkers
KIM-1 expression levels and/or staining are often used to assess the extent of cellular damage for AKI studies in vitro. To permit in vitro nephrotoxicity screening, luciferase and mCherry transgenes were knocked into the KIM-1 locus via CRISPR/Cas to generate a KIM-1 reporter HK-2 cell line.141 The KIM-1 reporter cell line expressed luciferase in response to hypoxia, cisplatin, and glucose treatments under carefully monitored, specific cell-culture conditions.141 However, some in vitro studies have found that primary proximal tubule cells and some cell lines did not show significant differences in KIM-1 expression after nephrotoxic drug treatment compared with negative controls.33,142 Kidney organoids treated with doses of cisplatin ranging from 5 to 100 μM have increased expression of KIM-1 (Figure 3).83,87 Kidney organoids treated with gentamicin also have upregulation of KIM-1 expression at LTL+ tubules in a dose-dependent manner.84 Although kidney organoids upregulate KIM-1, they resemble fetal kidneys, so they may lack the full cadre of transporters involved in drug-induced toxicity.117
Figure 3.
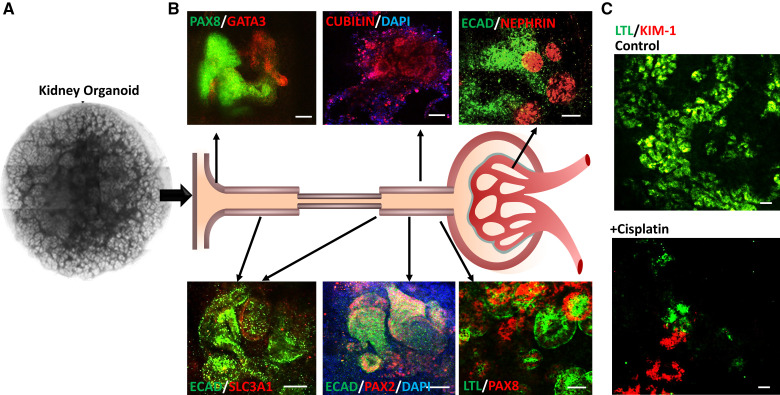
Modeling kidney injury in human kidney organoids. (A) Fully differentiated day 25 kidney organoids created from hiPSCs on transwells. (B) Immunofluorescence of kidney organoids at day 25 shows the presence of various kidney cell types including collecting ducts (GATA3), distal tubules (ECAD), proximal tubules (LTL, PAX8, PAX2, SLC3A1, CUBILIN), and glomeruli (NEPHRIN). Cell nuclei were labeled with DAPI (blue). (C) Representative immunofluorescence of kidney organoid treated with 5 μM of cisplatin from day 21–23. Cisplatin treatment upregulated expression of kidney injury molecule KIM-1 (red) in the proximal tubule (LTL) compared with the control. GATA3, GATA binding protein 3; ECAD, epithelial cadherin; LTL, lotus tetragonolobus lectin; PAX2, PAX8, paired box homeotic gene 2 and 8. Scale bar 50 μm.
An in vitro primary proximal tubule cell model of exposure to nephrotoxicants revealed a positive correlation between HO1 transcript expression and exposure to nephrotoxic compounds. On the basis of analysis of gene-drug pairs, the HO1 gene was overexpressed with increasing doses of multiple compounds, including cadmium chloride (CdCl2), cisplatin, gentamicin, and FK-506.143 A kidney-on-a-chip model of exposure to CdCl2 also upregulated HO1, whereas KIM-1 expression was only marginally increased.143 This suggests that HO1 may be a more sensitive readout than KIM-1 when measuring responses to nephrotoxic metals in vitro.
Another reported in vitro biomarker is tissue inhibitor of metalloproteinase (TIMP-1). Treatment with cisplatin or gentamicin increased the levels of TIMP-1 in animal models.144 In HK-2 cells, TIMP-1 was significantly increased after exposure to 10 μM cisplatin.114 Another study in RPTEC/TERT1 cells found that treatment with cisplatin and gentamicin increased TIMP-1 expression in the treatment groups compared with the vehicle control group.145 TIMP-1 expression has not been studied in the organoid-based in vitro AKI models yet, but may be a useful biomarker for future exploration.
Inflammatory Cytokines
A toxicity model based on HPTC demonstrated an increase in the expression of IL-6 and/or IL-8 at the study endpoint, suggesting that production of these ILs may predict renal proximal tubular toxicity and the potential for preclinical drug development.46 Upregulation of IL-6 and IL-8 expression was also found in proximal tubule cell lines in response to gentamicin, CdCl2, and uremic toxins. The upregulation of IL-6 and IL-8 was greater than that of KIM-1 in response to these compounds.46 Toxic drugs also induced IL- 6 and IL-8 in both the HPTC cell line and in hPSC-derived HPTCs.146,147
Cytotoxicity Assays
To evaluate nephrotoxicity in vitro, studies commonly rely on assays of acute toxicity, cell viability, cell damage, and/or metabolic activity. Cellular integrity may be measured with dyes such as trypan blue, whereas the metabolic activity may be measured by lactate production or reduction of the tetrazolium dye MTT 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.148–150 Some cytotoxicity assays are not transferrable to 3D kidney organoids, because the compounds do not reach the center of the organoids, but a few studies have explored the possibilities. Apoptosis within the proximal tubule cells can be detected by the presence of cleaved caspase-3. γH2AX antibody staining and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay may also be used to determine the extent of DNA damage and cell death.117 Colocalization of CC3, γH2AX, and TUNEL staining together with other markers can provide information regarding the specificity of the toxic drugs for certain cell types or states. CellTiter-Glo luminescent assay can also detect cell viability by measuring bioluminescence activity in renal organoids.49
Functional Assays
Several assays are available that test renal cell functionality. Functional assays for permeability include the transport of substrates such as para-aminohippurate and inulin leakage. These have been used for evaluating the nephrotoxicity in animal-based models151,152 and for some human-based in vitro models.153 FITC-labeled BSA transport can also test the functionality of human kidney-derived cells, podocytes,154 and kidney organoids.82,86 Because FITC can enter the cells through solute channels, it may not be a valid functional assay in all patients. Selective endocytosis of dextran, Alexa FluorTM 488 by LTL+ tubule cells has also been used to demonstrate the functionality of organoids.79
In Vitro Models of Nephrotoxicity
Conventional In Vitro Cell Culture Models of AKI
A common in vitro model of AKI utilizes the HK-2 cell line. HK-2 cells treated with 10 μM cisplatin for 24–48 hours can model some aspects of AKI in vitro, including cellular apoptosis and increased kidney injury biomarker expression. HK-2 cells exposed to 20 μM cisplatin for 48 hours had increased secretion of the inflammatory cytokines IL-6 and IL-8 into the culture media.155 HK-2 cells treated with 5 μM Dox also had a significant reduction in cell survival. However, HK-2 cells do not express OAT1, OAT3, OCT2, MRP2, and BCRP,141 and immortalized cell lines express approximately 20-fold more KIM-1 at baseline compared with primary cells, making further induction of KIM-1 expression during in vitro nephrotoxicity studies problematic.156
RPTEC/TERT1 and ciPTEC cell lines express more of the expected tubule cell markers than do HK-2 cells (Table 1). Exposing RPTEC/TERT1 cells to different nephrotoxicants, such as cisplatin, gentamicin, aristolochic acid, or cyclosporine A, induced primary proximal tubule toxicity.145 RPTEC that were treated with higher doses of cisplatin exhibited a 50%–100% decrease in viability as measured by increased LDH release.157 Cisplatin treatment resulted in the phosphorylation of p65, increasing the downstream gene expression of IL6, IL8, and C-X-C motif chemokine ligand 10 in RPTECs.155 The ciPTEC cell line was also used for screening various drugs.158 Among the different tubule cell lines, ciPTEC cells express the most proximal tubule markers. As discussed above, certain transporters (OAT1, OAT3, and OCT2) and receptors (megalin/cubilin) are important for internalization of drugs. Therefore, ciPTEC cells may be the best model to study human kidney toxicity. Improved toxic drug sensitivity was observed when the ciPTEC cells were grown as 3D structures.159,160
Cultures of immortalized cells on 2D surfaces have the apical surface exposed, so substances cannot enter easily via basal transporters. Studies of 3D culture of immortalized cell lines have apical and basal expression and therefore are likely improved.161 Most of these cell lines were derived from the S1 and S2 segments of the proximal tubules in the kidney cortex, so their best application is to S1 and S2 proximal tubule injury.
Immortalized human podocyte cell lines were developed because mature podocytes lose their proliferating capacity and dedifferentiate after isolation. Conditionally immortalized human podocyte cell lines, such as Nki-2 and CIHP-1, are used for modeling podocytopathies in vitro.58,162,163 A Dox-based in vitro study of podocyte injury in conditionally immortalized human podocyte cell lines showed generation of free radicals (ROS).164 Primary cell line cultures exhibit less sensitivity to drug exposure than do 3D primary organoids.66
Advanced In Vitro Modeling of AKI in Human Kidney Organoids
hiPSC-derived kidney organoid models have utility for disease modeling and drug screening. The kidney organoids generated are comprised of fetal-stage nephrons expressing both tubule segments, primitive glomeruli, and stromal populations. Patient-derived and CRISPR-mutated kidney organoids derived from hPSCs can recapitulate genetic kidney diseases.80,83 Correction of patient hiPSC–derived kidney organoids can restore the function of specific cell types in vitro.85 Modeling of AKI with nephrotoxicants is facilitated in organoids that have a basal-out pattern that exposes the transporters.
The dosages of drugs required to induce toxicity in kidney cells varies, depending on the method of culture. Between 5–20 μM cisplatin is required to cause proximal tubular cell apoptosis in organoids cultured using either the transwell or monolayer methods.79,84 In our experience, kidney organoids cultured on transwells with 5 μM cisplatin treatment for 48 hours have upregulated KIM-1 in the proximal tubules accompanied by loss of expression of the proximal tubule marker LTL (Figure 3). However, cell death in cisplatin-induced AKI organoid studies may not be completely specific to the proximal tubule, because distal tubule cells and interstitial cells are damaged as well.117
Comparative analysis of fetal and adult human kidney transcripts to better understand the lack of proximal tubule specificity was conducted.117 Although matured kidney organoids express SLC31A1/copper transporter 1, SLC31A2/copper transporter 2, ATPase copper transporting beta, and SLC47A1/MATE1, the expression of proximal tubule-specific SLC22A2/OCT2 and SLC47A2/MATE2K transporters was very low.116(preprint) In the case of 3D organoids, a higher 50 µM dose of cisplatin induced both HAVCR1/KIM-1 and CXCL8 expression and DNA damage and cell death in the organoids.116(preprint) Transcriptional profiling of the corresponding injured organoids showed increased expression of injury biomarkers, such as CXCL2, CCL2, HAVCR1, and NGAL, with high enrichment in TNF-α signaling.116(preprint) The study suggested that cisplatin-induced kidney organoid damage might be mediated through TNF-α receptors (Figure 2).
Human embryonic stem cell–derived kidney organoids treated with 5 mg/ml of gentamicin for 48 hours had KIM-1 expression in LTL+ but not E-cadherin+ tubules.84 Dox-induced renal cell death has also been evaluated in kidney organoids derived from hPSCs. Bioprinted 3D kidney organoids treated with 10 µM Dox for 72 hours exhibited near-complete collapse of glomerular structures and tubule networks.89 Organoids treated with Dox did not show podocyte staining for MAFB, so they may have suffered from podocyte loss via apoptosis.89 Gene-expression analysis found an increase in both BAX and caspase-3 after Dox treatment. Bioprinted 3D kidney organoids have also been used for glomerular toxicity screening.89 Dox treatment of these bioprinted organoids collapsed glomerular structures and reduced their MAFB staining. Kidney micro-organoids treated with Dox had TUNEL positivity in the podocytes, suggesting the feasibility of using these kidney micro-organoids for drug toxicity screening.86
Transplantation of human kidney organoids improved both polarization and development of apical brush border,165 but become an in vivo model system without the advantages of an in vitro model system. The long-term follow-up study reported chondrogenesis and cyst formation within the grafts, which might be caused by the nonkidney or partially differentiated cells within the transplant.165 Additionally, there are no studies documenting the effects of nephrotoxic agents on transplanted kidney organoids. Improvements in kidney organoid culture to address these issues would increase the utility of organoid models of AKI.
Kidney-on-a-chip Microfluidic Models of AKI
In 2016, Adler and coworkers reported the use of kidney-on-a-chip to model nephrotoxicity. In the study, HPTCs were successfully self-assembled to recapitulate the in vivo structure within the chips. Exposure of HPTCs with 25 µM CdCl2 resulted in upregulation of HO1 and KIM-1, suggesting toxicity.143 Another nephrotoxicity model used RPTECs-on-a-chip treated with cisplatin, gentamycin, or cyclosporin A. Cell viability was significantly decreased after exposure to the toxicants.166
In 2017, Musah et al. used a microfluidic device to generate “glomerulus-on-a-chip” by seeding primary glomerular endothelial cells in the lower channel and hiPSC-derived podocytes in the upper channel to study the tissue–tissue interaction. They reported that Dox treatment resulted in dose-dependent delamination and decreasing cell viability. Nonselective albumin leakage in the glomerular filtration barrier enhanced the uptake of albumin by the hiPSC-derived podocytes lining the device’s urine compartment.62 A study using human RPTEC cells on chips modeled cisplatin toxicity in cells exposed via the basolateral membrane, which was abolished in the presence of the OCT2 inhibitor cimetidine.63 These findings show that advanced models may permit greater understanding of the role of kidney epithelial polarization and membrane localization for drug influx mechanisms, and achieving drug sensitivity at clinically relevant levels.
No AKI model completely mimics clinical AKI, but combining animal models with human tissue culture has proven to be the most robust option available so far. Primary renal tubule epithelial cells and immortalized tubule cell lines have many transporters and are susceptible to injury by nephrotoxic drugs. Compared with immortalized tubule cell lines, tubule cells within kidney organoids derived from hPSC are more sensitive to nephrotoxic injury. However, the younger technologies have their own drawbacks because they are still under development. These limitations include lack of proper maturation and vascularization, the presence of off-target nonrenal cell types in organoid models, and variation between batches. Advancing biomedical engineering approaches including microfluidics, 3D bioprinting, and in vivo transplantation may help to mature and vascularize kidney organoids, providing many new avenues for kidney nephrotoxicity modeling.
Disclosures
L. Woodard reports receiving research funding from Bayer and SalioGen Therapeutics; reports having consulting agreements with AlfaSights and Biogeneration Ventures; reports receiving honoraria from the National Institutes of Health (NIH); reports serving on an advisory committee to the American Society of Gene and Cell Therapy; and reports other interests/relationships with American Society of Gene & Cell Therapy. All remaining authors have nothing to disclose.
Funding
This work was supported by the US Department of Veterans Affairs (BX004845) and the Vanderbilt O’Brien Kidney Center (5P30DK114809-02) (to L. Woodard). This material is the result of work supported with resources and use of facilities at the Veterans Affairs Tennessee Valley Healthcare System. The Vanderbilt Cell Imaging Shared Resource is supported by NIH grants (CA68485, DK20593, DK58404, DK59637, and EY08126). The project described was supported by the National Center for Advancing Translational Sciences Clinical and Translational Science Award UL1 TR002243 to the Vanderbilt Institute for Clinical and Translational Research.
Acknowledgments
J. Bejoy conceptualized the study and wrote the original draft; L. Woodard provided supervision; J. Bejoy and L. Woodard were responsible for the methodology, funding acquisition, and investigation; J. Bejoy, E. Qian, and L. Woodard were responsible for the revision, editing, and visualization. This study is solely the responsibility of the authors and does not necessarily represent official views of the National Center for Advancing Translational Sciences or the NIH.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Makris K, Spanou L: Acute kidney injury: Definition, pathophysiology and clinical phenotypes. Clin Biochem Rev 37: 85–98, 2016 [PMC free article] [PubMed] [Google Scholar]
- 2.Basile DP, Anderson MD, Sutton TA: Pathophysiology of acute kidney injury. Compr Physiol 2: 1303–1353, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schreiner GE: Toxic nephropathy: Adverse renal effects caused by drugs and chemicals. JAMA 191: 849–850, 1965 [DOI] [PubMed] [Google Scholar]
- 4.Markowitz GS, Perazella MA: Drug-induced renal failure: A focus on tubulointerstitial disease. Clinica chimica acta 351: 31–47, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Fervenza FC, Fitzpatrick PM, Mertz J, Erickson SB, Liggett S, Popham S, et al. : Acute rapamycin nephrotoxicity in native kidneys of patients with chronic glomerulopathies. Nephrology Dialysis Transplantation 19: 1288–1292, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Coco TJ, Klasner AE: Drug-induced rhabdomyolysis. Curr Opin Pediatr 16: 206–210, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Perazella MA: Crystal-induced acute renal failure. Am J Med 106: 459–465, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Zager RA: Pathogenetic mechanisms in nephrotoxic acute renal failure. Semin Nephrol 17: 3–14, 1997 [PubMed] [Google Scholar]
- 9.Markowitz GS, Appel GB, Fine PL, Fenves AZ, Loon NR, Jagannath S, et al. : Collapsing focal segmental glomerulosclerosis following treatment with high-dose pamidronate. J Am Soc Nephrol 12: 1164–1172, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Kleinknecht D, Landais P, Goldfarb B: Drug-associated acute renal failure: A prospective collaborative study of 81 biopsied patients. Adv Exp Med Biol 212: 125–128, 1987 [DOI] [PubMed] [Google Scholar]
- 11.Nolin T, Himmelfarb J: Adverse drug reactions, edited by Uetrecht J, Springer, Berlin, Heidelberg, 2010, pp 111–130 [Google Scholar]
- 12.Burckhardt G: Drug transport by organic anion transporters (OATs). Pharmacol Ther 136: 106–130, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Nigam SK, et al. : Handling of drugs, metabolites, and uremic toxins by kidney proximal tubule drug transporters. CJSAN 10: 2039–2049, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ciarimboli G, Ludwig T, Lang D, Pavenstädt H, Koepsell H, Piechota HJ, et al. : Cisplatin nephrotoxicity is critically mediated via the human organic cation transporter 2. Am J Pathol 167: 1477–1484, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jado JC, Humanes B, González-Nicolás MÁ, Camaño S, Lara JM, López B, et al. : Nephroprotective effect of cilastatin against gentamicin-induced renal injury in vitro and in vivo without altering its bactericidal efficiency. Antioxidants 9: 821, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xia L, Zhou M, Kalhorn TF, Ho HTB, Wang J: Podocyte-specific expression of organic cation transporter PMAT: Implication in puromycin aminonucleoside nephrotoxicity. 296: F1307 –F1313, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perazella MA, Markowitz GS: Bisphosphonate nephrotoxicity. Kidney Int 74: 1385–1393, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Westhuyzen J, et al. : Measurement of tubular enzymuria facilitates early detection of acute renal impairment in the intensive care unit. Nephrol Dial Transplant 18: 543–551, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV: Kidney injury molecule-1 (KIM-1): A novel biomarker for human renal proximal tubule injury. Kidney Int 62: 237–244, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Weber EJ, Himmelfarb J, Kelly EJ: Concise review: Current and emerging biomarkers of nephrotoxicity. Curr Opin Toxicol 4: 16–21, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Griffin BR, Faubel S, Edelstein CL: Biomarkers of drug-induced kidney toxicity. Ther Drug Monit 41: 213–226, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vaidya VS, Ramirez V, Ichimura T, Bobadilla NA, Bonventre JV: Urinary kidney injury molecule-1: A sensitive quantitative biomarker for early detection of kidney tubular injury. Am J Physiol Renal Physiol 290: F517–F529, 2006 [DOI] [PubMed] [Google Scholar]
- 23.van Timmeren MM, et al. : Tubular kidney injury molecule‐1 (KIM‐1) in human renal disease. 212: 209–217, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Gozzelino R, Jeney V, Soares MP: Mechanisms of cell protection by heme oxygenase-1. Annu Rev Pharmacol Toxicol 50: 323–354, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Nath KA: Human AKI and heme oxygenase-1. J Am Soc Nephrol 23: 971–974, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kie J-H, Kapturczak MH, Traylor A, Agarwal A, Hill-Kapturczak N: Heme oxygenase-1 deficiency promotes epithelial-mesenchymal transition and renal fibrosis. J Am Soc Nephrol 19: 1681–1691, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nath KA: Heme oxygenase-1 and acute kidney injury. Curr Opin Nephrol Hypertens 23: 17–24, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lever JM, Boddu R, George JF, Agarwal A: Heme oxygenase-1 in kidney health and disease. Antioxid Redox Signal 25: 165–183, 2016. 26906116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zager RA, Johnson AC, Becker K: Plasma and urinary heme oxygenase-1 in AKI. J Am Soc Nephrol 23: 1048–1057, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mårtensson J, Bellomo R: The rise and fall of NGAL in acute kidney injury. Blood Purif 37: 304–310, 2014 [DOI] [PubMed] [Google Scholar]
- 31.Mori K, et al. : Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. 115: 610–621, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mishra J, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. 365: 1231–1238, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Luo QH, Chen ML, Sun FJ, Chen ZL, Li MY, Zeng W, et al. : KIM-1 and NGAL as biomarkers of nephrotoxicity induced by gentamicin in rats. Mol Cell Biochem 397: 53–60, 2014 [DOI] [PubMed] [Google Scholar]
- 34.Gacka E, Życzkowski M, Bogacki R, Paradysz A, Hyla-Klekot L: The usefulness of determining neutrophil gelatinase-associated lipocalin concentration excreted in the urine in the evaluation of cyclosporine a nephrotoxicity in children with nephrotic syndrome. Dis Markers 2016: 6872149, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zubowska M, Wyka K, Fendler W, Młynarski W, Zalewska-Szewczyk B: Interleukin 18 as a marker of chronic nephropathy in children after anticancer treatment. Dis Markers 35: 811–818, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin X, Yuan J, Zhao Y, Zha Y: Urine interleukin-18 in prediction of acute kidney injury: A systemic review and meta-analysis. J Nephrol 28: 7–16, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramachandran K, Saikumar J, Bijol V, Koyner JL, Qian J, Betensky RA, et al. : Human miRNome profiling identifies microRNAs differentially present in the urine after kidney injury. Clin Chem 59: 1742–1752, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lyu L-L, Feng Y, Liu B-C: Urinary biomarkers for chronic kidney disease with a focus on gene transcript. Chin Med J (Engl) 130: 2251–2256, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morizane R, Bonventre JV: Kidney organoids: A translational journey. Trends Mol Med 23: 246–263, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jo SK, Rosner MH, Okusa MD: Pharmacologic treatment of acute kidney injury: Why drugs haven’t worked and what is on the horizon. CJASN 2: 356–365, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Palevsky PM, et al. : Design of clinical trials in acute kidney injury: Report from an NIDDK workshop on trial methodology. CJASN 7: 844–850, 2012 [DOI] [PubMed] [Google Scholar]
- 42.Sharp CN, Siskind LJ: Developing better mouse models to study cisplatin-induced kidney injury. 313: F835–F841, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oo ZY, Deng R, Hu M, Ni M, Kandasamy K, bin Ibrahim MS, et al. : The performance of primary human renal cells in hollow fiber bioreactors for bioartificial kidneys. Biomaterials 32: 8806–8815, 2011 [DOI] [PubMed] [Google Scholar]
- 44.McLaren J, Whiting P, Simpson J, Hawksworth G: Isolation and characterisation of human proximal tubular cells derived from kidney cortical segments. 14: 916–922, 1995 [DOI] [PubMed] [Google Scholar]
- 45.Atilano-Roque A, Wen X, Aleksunes LM, Joy MS: Nrf2 activators as potential modulators of injury in human kidney cells. Toxicol Rep 3: 153–159, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Y, et al. : An in vitro method for the prediction of renal proximal tubular toxicity in humans. Toxicol Res (Camb) 2: 352–365, 2013 [Google Scholar]
- 47.Vesey DA, Qi W, Chen X, Pollock CA, Johnson DW: Isolation and primary culture of human proximal tubule cells. Methods Mol Biol 466: 19–24, 2009 [DOI] [PubMed] [Google Scholar]
- 48.Sharpe CC, Dockrell ME: Primary culture of human renal proximal tubule epithelial cells and interstitial fibroblasts. Methods Mol Biol 806: 175–185, 2012 [DOI] [PubMed] [Google Scholar]
- 49.Ding B, Sun G, Liu S, Peng E, Wan M, Chen L, et al. : Three-dimensional renal organoids from whole kidney cells: Generation, optimization, and potential application in nephrotoxicology in vitro. Cell Transplant 29: 963689719897066, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaminski MM, Tosic J, Kresbach C, Engel H, Klockenbusch J, Müller AL, et al. : Direct reprogramming of fibroblasts into renal tubular epithelial cells by defined transcription factors. Nat Cell Biol 18: 1269–1280, 2016 [DOI] [PubMed] [Google Scholar]
- 51.Vanslambrouck JM, et al. : Direct reprogramming to human nephron progenitor-like cells using inducible piggyBac transposon expression of SNAI2-EYA1-SIX1. 95: 1153–1166, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Little MH: Returning to kidney development to deliver synthetic kidneys. Dev Biol 474: 22–36, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ryan MJ, Johnson G, Kirk J, Fuerstenberg SM, Zager RA, Torok-Storb B: HK-2: An immortalized proximal tubule epithelial cell line from normal adult human kidney. Kidney Int 45: 48–57, 1994 [DOI] [PubMed] [Google Scholar]
- 54.Simon-Friedt BR, Wilson MJ, Blake DA, Yu H, Eriksson Y, Wickliffe JK: The RPTEC/TERT1 cell line as an improved tool for in vitro nephrotoxicity assessments. biol Trace Elem Res 166: 66–71, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee KM, Choi KH, Ouellette MMJC: Use of exogenous hTERT to immortalize primary human cells. 45, 33–38, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Halbert CL, Demers GW, Galloway DA: The E7 gene of human papillomavirus type 16 is sufficient for immortalization of human epithelial cells. 65: 473–478, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wilmer MJ, Saleem MA, Masereeuw R, Ni L, van der Velden TJ, Russel FG, et al. : Novel conditionally immortalized human proximal tubule cell line expressing functional influx and efflux transporters. Cell Tissue Res 339: 449–457, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saleem MA, O’Hare MJ, Reiser J, Coward RJ, Inward CD, Farren T, et al. : A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol 13: 630–638, 2002 [DOI] [PubMed] [Google Scholar]
- 59.Bajaj P, Chowdhury SK, Yucha R, Kelly EJ, Xiao G: Emerging kidney models to investigate metabolism, transport, and toxicity of drugs and xenobiotics. Drug Metabolism Disposition 46: 1692–1702, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jang K-J, et al. : Human kidney proximal tubule-on-a-chip for drug transport and nephrotoxicity assessment. Integrative Biology 5: 1119–1129, 2013 [DOI] [PubMed] [Google Scholar]
- 61.Vriend J, Nieskens TTG, Vormann MK, van den Berge BT, van den Heuvel A, Russel FGM, et al. : Screening of drug-transporter interactions in a 3D microfluidic renal proximal tubule on a chip. AAPS J 20: 87, 2018 [DOI] [PubMed] [Google Scholar]
- 62.Musah S, et al. : Mature induced-pluripotent-stem-cell-derived human podocytes reconstitute kidney glomerular-capillary-wall function on a chip. Nature Biomedical Engineering 1: 0069, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nieskens TTG, Persson M, Kelly EJ, Sjögren A-K: A multicompartment human kidney proximal tubule-on-a-chip replicates cell polarization–dependent cisplatin toxicity. Drug Metabol Disposit 48: 1303–1311, 2020 [DOI] [PubMed] [Google Scholar]
- 64.Shiva N, Sharma N, Kulkarni YA, Mulay SR, Gaikwad AB: Renal ischemia/reperfusion injury: An insight on in vitro and in vivo models. Life Sci 256: 117860, 2020 [DOI] [PubMed] [Google Scholar]
- 65.Baer PC, Tunn UW, Nunez G, Scherberich JE, Geiger H: Transdifferentiation of distal but not proximal tubular epithelial cells from human kidney in culture. Exp Nephrol 7: 306–313, 1999 [DOI] [PubMed] [Google Scholar]
- 66.Astashkina AI, Mann BK, Prestwich GD, Grainger DW: Comparing predictive drug nephrotoxicity biomarkers in kidney 3-D primary organoid culture and immortalized cell lines. Biomaterials 33: 4712–4721, 2012 [DOI] [PubMed] [Google Scholar]
- 67.Taguchi A, Kaku Y, Ohmori T, Sharmin S, Ogawa M, Sasaki H, et al. : Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell 14: 53–67, 2014 [DOI] [PubMed] [Google Scholar]
- 68.Mae S-I, Shono A, Shiota F, Yasuno T, Kajiwara M, Gotoda-Nishimura N, et al. : Monitoring and robust induction of nephrogenic intermediate mesoderm from human pluripotent stem cells. Nat Commun 4: 1367, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Howden SE, Wilson SB, Groenewegen E, Starks L, Forbes TA, Tan KS, et al. : Plasticity of distal nephron epithelia from human kidney organoids enables the induction of ureteric tip and stalk. Cell Stem Cell 28: 671–684.e6, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Narayanan K, et al. : Human embryonic stem cells differentiate into functional renal proximal tubular–like cells. 83: 593–603, 2013 [DOI] [PubMed] [Google Scholar]
- 71.Araoka, T, et al. : Efficient and rapid induction of human iPSCs/ESCs into nephrogenic intermediate mesoderm using small molecule-based differentiation methods. 9: e84881, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kang M, Han, Y-M: Differentiation of human pluripotent stem cells into nephron progenitor cells in a serum and feeder free system. 9: e94888, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lam AQ, et al. : Rapid and efficient differentiation of human pluripotent stem cells into intermediate mesoderm that forms tubules expressing kidney proximal tubular markers. 25: 1211–1225, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mae SI, Ryosaka M, Sakamoto S, Matsuse K, Nozaki A, Igami M, et al. : Expansion of human iPSC-derived ureteric bud organoids with repeated branching potential. Cell Rep 32: 107963, 2020 [DOI] [PubMed] [Google Scholar]
- 75.Ciampi O, Iacone R, Longaretti L, Benedetti V, Graf M, Magnone MC, et al. : Generation of functional podocytes from human induced pluripotent stem cells. Stem Cell Res (Amst) 17: 130–139, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rauch C, Feifel E, Kern G, Murphy C, Meier F, Parson W, et al. : Differentiation of human iPSCs into functional podocytes. PLoS One 13: e0203869–e0203869, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Song B, Smink AM, Jones CV, Callaghan JM, Firth SD, Bernard CA, et al. : The directed differentiation of human iPS cells into kidney podocytes. PLoS One 7: e46453, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chandrasekaran V, Carta G, da Costa Pereira D, Gupta R, Murphy C, Feifel E, et al. : Generation and characterization of iPSC-derived renal proximal tubule-like cells with extended stability. Sci Rep 11: 11575, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Takasato M, Er PX, Chiu HS, Maier B, Baillie GJ, Ferguson C, et al. : Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 526: 564–568, 2015 [DOI] [PubMed] [Google Scholar]
- 80.Low JH, Li P, Chew EGY, Zhou B, Suzuki K, Zhang T, et al. : Generation of human PSC-derived kidney organoids with patterned nephron segments and a de novo vascular network. Cell Stem Cell 25: 373–387.e9, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Taguchi A, Nishinakamura R: Higher-order kidney organogenesis from pluripotent stem cells. Cell Stem Cell 21: 730–746.e6, 2017 [DOI] [PubMed] [Google Scholar]
- 82.Xinaris C, Benedetti V, Rizzo P, Abbate M, Corna D, Azzollini N, et al. : In vivo maturation of functional renal organoids formed from embryonic cell suspensions. J Am Soc Nephrol 23: 1857–1868, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Freedman BS, Brooks CR, Lam AQ, Fu H, Morizane R, Agrawal V, et al. : Modelling kidney disease with CRISPR-mutant kidney organoids derived from human pluripotent epiblast spheroids. Nat Commun 6: 8715, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Morizane R, Lam AQ, Freedman BS, Kishi S, Valerius MT, Bonventre JV: Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat Biotechnol 33: 1193–1200, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Forbes TA, Howden SE, Lawlor K, Phipson B, Maksimovic J, Hale L, et al. : Patient-iPSC-derived kidney organoids show functional validation of a ciliopathic renal phenotype and reveal underlying pathogenetic mechanisms. Am J Hum Genet 102: 816–831, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kumar SV, et al. : Kidney micro-organoids in suspension culture as a scalable source of human pluripotent stem cell-derived kidney cells. Development 146: dev172361, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Przepiorski A, Sander V, Tran T, Hollywood JA, Sorrenson B, Shih JH, et al. : A simple bioreactor-based method to generate kidney organoids from pluripotent stem cells. Stem Cell Reports 11: 470–484, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Murphy SV, Atala A: 3D bioprinting of tissues and organs. JNb 32: 773–785, 2014 [DOI] [PubMed] [Google Scholar]
- 89.Higgins JW, Chambon A, Bishard K, Hartung A, Arndt D, Brugnano J, et al. : Bioprinted pluripotent stem cell-derived kidney organoids provide opportunities for high content screening. bioRxiv. 10.1101/505396 (Preprint posted December 23, 2018)
- 90.Lawlor KT, Vanslambrouck JM, Higgins JW, Chambon A, Bishard K, Arndt D, et al. : Cellular extrusion bioprinting improves kidney organoid reproducibility and conformation. Nat Mater 20: 260–271, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Garreta E, Prado P, Tarantino C, Oria R, Fanlo L, Martí E, et al. : Fine tuning the extracellular environment accelerates the derivation of kidney organoids from human pluripotent stem cells. Nat Mater 18: 397–405, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Homan KA, Gupta N, Kroll KT, Kolesky DB, Skylar-Scott M, Miyoshi T, et al. : Flow-enhanced vascularization and maturation of kidney organoids in vitro. Nat Methods 16: 255–262, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Little MH, Combes AN: Kidney organoids: Accurate models or fortunate accidents. Genes Dev 33: 1319–1345, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.van den Berg CW, Ritsma L, Avramut MC, Wiersma LE, van den Berg BM, Leuning DG, et al. : Renal subcapsular transplantation of PSC-derived kidney organoids induces neo-vasculogenesis and significant glomerular and tubular maturation in vivo. Stem Cell Reports 10: 751–765, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhu W, et al. : Direct 3D bioprinting of prevascularized tissue constructs with complex microarchitecture. 124: 106–115, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jia W, et al. : Direct 3D bioprinting of perfusable vascular constructs using a blend bioink. 106: 58–68, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wu H, et al. : Comparative analysis and refinement of human PSC-derived kidney organoid differentiation with single-cell transcriptomics. 23: 869–881, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Combes AN, Zappia L, Er PX, Oshlack A, Little MHJGm: Single-cell analysis reveals congruence between kidney organoids and human fetal kidney. 11: 3, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Miller RP, Tadagavadi RK, Ramesh G, Reeves WB: Mechanisms of cisplatin nephrotoxicity. Toxins (Basel) 2: 2490–2518, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nakamura T, Yonezawa A, Hashimoto S, Katsura T, Inui K: Disruption of multidrug and toxin extrusion MATE1 potentiates cisplatin-induced nephrotoxicity. Biochem Pharmacol 80: 1762–1767, 2010 [DOI] [PubMed] [Google Scholar]
- 101.Ozkok A, Edelstein CL: Pathophysiology of cisplatin-induced acute kidney injury. BioMed Res Int 2014: 967826, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kleih M, Böpple K, Dong M, Gaißler A, Heine S, Olayioye MA, et al. : Direct impact of cisplatin on mitochondria induces ROS production that dictates cell fate of ovarian cancer cells. Cell Death Dis 10: 851, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Matsushima H, Yonemura K, Ohishi K, Hishida A: The role of oxygen free radicals in cisplatin-induced acute renal failure in rats. J Lab Clin Med 131: 518–526, 1998 [DOI] [PubMed] [Google Scholar]
- 104.Choi Y-M, Kim HK, Shim W, Anwar MA, Kwon JW, Kwon HK, et al. : Mechanism of cisplatin-induced cytotoxicity is correlated to impaired metabolism due to mitochondrial ROS generation. PLoS One 10: e0135083, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mandic A, Hansson J, Linder S, Shoshan MCJJoBC: Cisplatin induces endoplasmic reticulum stress and nucleus-independent apoptotic signaling. 278: 9100–9106, 2003 [DOI] [PubMed] [Google Scholar]
- 106.Ramesh G, Reeves WB: TNF-α mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J Clin Invest 110: 835–842, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Morsy MA, Heeba GH: Nebivolol ameliorates cisplatin-induced nephrotoxicity in rats. Basic Clin Pharmacol Toxicol 118: 449–455, 2016 [DOI] [PubMed] [Google Scholar]
- 108.Lu LH, et al. : Increased macrophage infiltration and fractalkine expression in cisplatin-induced acute renal failure in mice. J Pharmacol Experi Therap 324: 111–117, 2008 [DOI] [PubMed] [Google Scholar]
- 109.Ko J-W, Lee IC, Park SH, Moon C, Kang SS, Kim SH, et al. : Protective effects of pine bark extract against cisplatin-induced hepatotoxicity and oxidative stress in rats. Lab Anim Res 30: 174–180, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li Y, et al. : Activation of sirtuin 3 by silybin attenuates mitochondrial dysfunction in cisplatin-induced acute kidney injury. 8: 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Meng X-M, Ren GL, Gao L, Yang Q, Li HD, Wu WF, et al. : NADPH oxidase 4 promotes cisplatin-induced acute kidney injury via ROS-mediated programmed cell death and inflammation. Lab Invest 98: 63–78, 2018 [DOI] [PubMed] [Google Scholar]
- 112.Tang J, et al. : Blockade of histone deacetylase 6 protects against cisplatin-induced acute kidney injury. Clinical Science 132: 339–359, 2018 [DOI] [PubMed] [Google Scholar]
- 113.Zhang J, Rudemiller NP, Patel MB, Wei Q, Karlovich NS, Jeffs AD, et al. : Competing actions of type 1 angiotensin ii receptors expressed on T lymphocytes and kidney epithelium during cisplatin-induced AKI. J Am Soc Nephrol 27: 2257–2264, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sohn SJ, Kim SY, Kim HS, Chun YJ, Han SY, Kim SH, et al. : In vitro evaluation of biomarkers for cisplatin-induced nephrotoxicity using HK-2 human kidney epithelial cells. Toxicol Lett 217: 235–242, 2013 [DOI] [PubMed] [Google Scholar]
- 115.Soo JY, Jansen J, Masereeuw R, Little MH: Advances in predictive in vitro models of drug-induced nephrotoxicity. Nat Rev Nephrol 14: 378–393, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Digby JLM, Przepiorski A, Davidson AJ, Sander V: Modeling acute kidney injury in kidney organoids with cisplatin. bioRxiv. 10.1101/2019.12.22.886572 (Preprint posted December 23, 2019) [Google Scholar]
- 117.Digby JLM, Vanichapol T, Przepiorski A, Davidson AJ, Sander V: Evaluation of cisplatin-induced injury in human kidney organoids. 318: F971–F978, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kaloyanides GJ, Pastoriza-Munoz E: Aminoglycoside nephrotoxicity. Kidney Int 18: 571–582, 1980 [DOI] [PubMed] [Google Scholar]
- 119.Tulkens PM: Experimental studies on nephrotoxicity of aminoglycosides at low doses. Mechanisms and perspectives. Am J Med 80(6B): 105–114, 1986 [DOI] [PubMed] [Google Scholar]
- 120.Martínez-Salgado C, Eleno N, Morales AI, Pérez-Barriocanal F, Arévalo M, López-Novoa JM: Gentamicin treatment induces simultaneous mesangial proliferation and apoptosis in rats. Kidney Int 65: 2161–2171, 2004 [DOI] [PubMed] [Google Scholar]
- 121.Lesniak W, Pecoraro VL, Schacht JJC: Ternary complexes of gentamicin with iron and lipid catalyze formation of reactive oxygen species. 18: 357–364, 2005 [DOI] [PubMed] [Google Scholar]
- 122.Shanley PF, Burke TJ: Differential susceptibility to gentamicin toxicity within the proximal convoluted tubule. Ren Fail 12: 83–87, 1990 [DOI] [PubMed] [Google Scholar]
- 123.De Broe ME, Paulus GJ, Verpooten GA, Roels F, Buyssens N, Wedeen R, et al. : Early effects of gentamicin, tobramycin, and amikacin on the human kidney. Kidney Int 25: 643–652, 1984 [DOI] [PubMed] [Google Scholar]
- 124.Hur E, Garip A, Camyar A, Ilgun S, Ozisik M, Tuna S, et al. : The effects of vitamin d on gentamicin-induced acute kidney injury in experimental rat model. Int J Endocrinol 2013: 313528, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Huang H, et al. : Gentamicin-induced acute kidney injury in an animal model involves programmed necrosis of the collecting duct. JASN 31: 2097–2115, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.He L, et al. Protective effects of curcumin on acute gentamicin-induced nephrotoxicity in rats. 93: 275–282, 2015 [DOI] [PubMed] [Google Scholar]
- 127.Zager RA, Johnson ACM, Geballe A: Gentamicin suppresses endotoxin-driven TNF-α production in human and mouse proximal tubule cells. 293: F1373–F1380, 2007 [DOI] [PubMed] [Google Scholar]
- 128.Wang Z, Liu L, Mei Q, Liu L, Ran Y, Zhang R: Increased expression of heat shock protein 72 protects renal proximal tubular cells from gentamicin-induced injury. J Korean Med Sci 21: 904–910, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pal S, Sil PCA: A 43 kD protein from the leaves of the herb Cajanus indicus L. modulates doxorubicin induced nephrotoxicity via MAPKs and both mitochondria dependent and independent pathways. Biochimie 94: 1356–1367, 2012 [DOI] [PubMed] [Google Scholar]
- 130.Mobaraki M, et al. : Molecular mechanisms of cardiotoxicity: a review on major side-effect of doxorubicin. 79: 335–344, 2017 [Google Scholar]
- 131.Su Z, Ye J, Qin Z, Ding X: Protective effects of madecassoside against Doxorubicin induced nephrotoxicity in vivo and in vitro. Sci Rep 5: 18314, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lee VW, Harris DC: Adriamycin nephropathy: A model of focal segmental glomerulosclerosis. 16: 30–38, 2011 [DOI] [PubMed] [Google Scholar]
- 133.Javaid B, Olson JL, Meyer TW: Glomerular injury and tubular loss in adriamycin nephrosis. JASN 12: 1391–1400, 2001 [DOI] [PubMed] [Google Scholar]
- 134.Sang Y, et al. : Semaphorin3A-inhibitor ameliorates doxorubicin-induced podocyte injury. 21: 4099, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Skrypnyk NI, Harris RC, de Caestecker MP: Ischemia-reperfusion model of acute kidney injury and post injury fibrosis in mice. JoVE 78: e50495, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang YH, Borkan SC: Prior heat stress enhances survival of renal epithelial cells after ATP depletion. 270: F1057–F1065, 1996 [DOI] [PubMed] [Google Scholar]
- 137.Schwartz JH, Shih T, Menza SA, Lieberthal W: ATP depletion increases tyrosine phosphorylation of β-catenin and plakoglobin in renal tubular cells. 10: 2297–2305, 1999 [DOI] [PubMed] [Google Scholar]
- 138.Wang Z, Gall JM, Bonegio R, Havasi A, Illanes K, Schwartz JH, et al. : Nucleophosmin, a critical Bax cofactor in ischemia-induced cell death. Mol Cell Biol 33: 1916–1924, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang Z, et al. : T95 nucleophosmin phosphorylation as a novel mediator and marker of regulated cell death in acute kidney injury. 319: F552–F561, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kishi S, et al. : Meclizine preconditioning protects the kidney against ischemia–reperfusion injury. 2: 1090–1101, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Veach RA, Wilson MH: CRISPR/Cas9 engineering of a KIM-1 reporter human proximal tubule cell line. PLoS One 13: e0204487, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rached E, et al. : Evaluation of putative biomarkers of nephrotoxicity after exposure to ochratoxin a in vivo and in vitro. Toxicological Sciences 103: 371–381, 2008 [DOI] [PubMed] [Google Scholar]
- 143.Adler M, Ramm S, Hafner M, Muhlich JL, Gottwald EM, Weber E, et al. : A quantitative approach to screen for nephrotoxic compounds in vitro. J Am Soc Nephrol 27: 1015–1028, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sieber M, et al. : Comparative analysis of novel noninvasive renal biomarkers and metabonomic changes in a rat model of gentamicin nephrotoxicity. Toxicological Sciences 109: 336–349, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Qiu X, Miao Y, Geng X, Zhou X, Li B: Evaluation of biomarkers for in vitro prediction of drug-induced nephrotoxicity in RPTEC/TERT1 cells. Toxicol Research 9: 91–100, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kandasamy K, Chuah JK, Su R, Huang P, Eng KG, Xiong S, et al. : Prediction of drug-induced nephrotoxicity and injury mechanisms with human induced pluripotent stem cell-derived cells and machine learning methods. Sci Rep 5: 12337, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Li Y, Kandasamy K, Chuah JK, Lam YN, Toh WS, Oo ZY, et al. : Identification of nephrotoxic compounds with embryonic stem-cell-derived human renal proximal tubular-like cells. Mol Pharm 11: 1982–1990, 2014 [DOI] [PubMed] [Google Scholar]
- 148.Murphy RA, Stafford RM, Petrasovits BA, Boone MA, Valentovic MA: Establishment of HK-2 cells as a relevant model to study tenofovir-induced cytotoxicity. Int J Mol Sci 18: 531, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Meng X-M, Li HD, Wu WF, Ming-Kuen Tang P, Ren GL, Gao L, et al. : Wogonin protects against cisplatin-induced acute kidney injury by targeting RIPK1-mediated necroptosis. Lab Invest 98: 79–94, 2018 [DOI] [PubMed] [Google Scholar]
- 150.Li W, Yang Y, Li Y, Zhao Y, Jiang H: Sirt5 attenuates cisplatin-induced acute kidney injury through regulation of Nrf2/HO-1 and Bcl-2. BioMed Res Int 2019: 4745132, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tiong HY, Huang P, Xiong S, Li Y, Vathsala A, Zink D: Drug-induced nephrotoxicity: Clinical impact and preclinical in vitro models. Mol Pharm 11: 1933–1948, 2014 [DOI] [PubMed] [Google Scholar]
- 152.Cuppage FE, Setter K, Sullivan P, Reitzes EJ, Melnykovych AO: Gentamicin nephrotoxicity. II. Physiological, biochemical and morphological effects of prolonged administration to rats. Virchows Arch B Cell Pathol Incl Mol Pathol 24: 121–138, 1977 [PubMed] [Google Scholar]
- 153.Huang X, et al. : The activation of antioxidant and apoptosis pathways involved in damage of human proximal tubule epithelial cells by PM2.5 exposure. Environ Sci Eur 32: 2, 2020 [Google Scholar]
- 154.Gianesello L, Priante G, Ceol M, Radu CM, Saleem MA, Simioni P, et al. : Albumin uptake in human podocytes: a possible role for the cubilin-amnionless (CUBAM) complex. Sci Rep 7: 13705, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Maekawa H, Inoue T, Ouchi H, Jao TM, Inoue R, Nishi H, et al. : Mitochondrial damage causes inflammation via cgas-sting signaling in acute kidney injury. Cell Rep 29: 1261–1273.e6, 2019 [DOI] [PubMed] [Google Scholar]
- 156.Sakolish C, Weber EJ, Kelly EJ, Himmelfarb J, Mouneimne R, Grimm FA, et al. : Technology transfer of the microphysiological systems: A case study of the human proximal tubule tissue chip. Sci Rep 8: 14882, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.King SM, Higgins JW, Nino CR, Smith TR, Paffenroth EH, Fairbairn CE, et al. : 3D proximal tubule tissues recapitulate key aspects of renal physiology to enable nephrotoxicity testing. Front Physiol 8: 123, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sjögren AK, Breitholtz K, Ahlberg E, Milton L, Forsgard M, Persson M, et al. : A novel multi-parametric high content screening assay in ciPTEC-OAT1 to predict drug-induced nephrotoxicity during drug discovery. Arch Toxicol 92: 3175–3190, 2018 [DOI] [PubMed] [Google Scholar]
- 159.Secker PF, Luks L, Schlichenmaier N, Dietrich DR: RPTEC/TERT1 cells form highly differentiated tubules when cultured in a 3D matrix. ALTEX 35: 223–234, 2018 [DOI] [PubMed] [Google Scholar]
- 160.Fedecostante M, et al. : Recellularized native kidney scaffolds as a novel tool in nephrotoxicity screening. Drug Metabol Disposition 46: 1338–1350, 2018 [DOI] [PubMed] [Google Scholar]
- 161.Mizuguchi K, Aoki H, Aoyama M, Kawaguchi Y, Waguri-Nagaya Y, Ohte N, et al. : Three-dimensional spheroid culture induces apical-basal polarity and the original characteristics of immortalized human renal proximal tubule epithelial cells. Exp Cell Res 404: 112630, 2021 [DOI] [PubMed] [Google Scholar]
- 162.Liu X, Cao W, Qi J, Li Q, Zhao M, Chen Z, et al. : Leonurine ameliorates adriamycin-induced podocyte injury via suppression of oxidative stress. Free Radic Res 52: 952–960, 2018 [DOI] [PubMed] [Google Scholar]
- 163.Chen Z, An X, Liu X, Qi J, Ding D, Zhao M, et al. : Hyperoside alleviates adriamycin-induced podocyte injury via inhibiting mitochondrial fission. Oncotarget 8: 88792–88803, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Magnasco A, Corselli M, Bertelli R, Ibatici A, Peresi M, Gaggero G, et al. : Mesenchymal stem cells protective effect in adriamycin model of nephropathy. Cell Transplant 17: 1157–1167, 2008 [DOI] [PubMed] [Google Scholar]
- 165.Nam SA, Seo E, Kim JW, Kim HW, Kim HL, Kim K, et al. : Graft immaturity and safety concerns in transplanted human kidney organoids. Exp Mol Med 51: 1–13, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yin L, Du G, Zhang B, Zhang H, Yin R, Zhang W, et al. : Efficient drug screening and nephrotoxicity assessment on co-culture microfluidic kidney chip. Sci Rep 10: 6568, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lopez-Novoa JM, Quiros Y, Vicente L, Morales AI, Lopez-Hernandez FJ: New insights into the mechanism of aminoglycoside nephrotoxicity: An integrative point of view. Kidney Int 79: 33–45, 2011 [DOI] [PubMed] [Google Scholar]
- 168.Aschauer L, Carta G, Vogelsang N, Schlatter E, Jennings P: Expression of xenobiotic transporters in the human renal proximal tubule cell line RPTEC/TERT1. Toxicol In Vitro 30(1 Pt A): 95–105, 2015 [DOI] [PubMed] [Google Scholar]
- 169.Aschauer L, Limonciel A, Wilmes A, Stanzel S, Kopp-Schneider A, Hewitt P, et al. : Application of RPTEC/TERT1 cells for investigation of repeat dose nephrotoxicity: A transcriptomic study. Toxicol In Vitro 30(1 Pt A): 106–116, 2015 [DOI] [PubMed] [Google Scholar]
- 170.Jansen J, Schophuizen CMS, Wilmer MJ, Lahham SHM, Mutsaers HAM, Wetzels JFM, et al. : A morphological and functional comparison of proximal tubule cell lines established from human urine and kidney tissue. Exp Cell Res 323: 87–99, 2014 [DOI] [PubMed] [Google Scholar]
- 171.Jenkinson SE, Chung GW, van Loon E, Bakar NS, Dalzell AM, Brown CD: The limitations of renal epithelial cell line HK-2 as a model of drug transporter expression and function in the proximal tubule. Pflugers Arch 464: 601–611, 2012 [DOI] [PubMed] [Google Scholar]
- 172.DesRochers TM, Suter L, Roth A, Kaplan DL: Bioengineered 3D human kidney tissue, a platform for the determination of nephrotoxicity. PLoS One 8: e59219, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sakairi T, Abe Y, Kajiyama H, Bartlett LD, Howard LV, Jat PS, et al. : Conditionally immortalized human podocyte cell lines established from urine. Am J Physiol Renal Physiol 298: F557–F567, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Romero-Guevara R, Ioannides A, Xinaris C: Kidney organoids as disease models: Strengths, weaknesses and perspectives. Front Physiol 11: 563981, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Petrosyan A, Cravedi P, Villani V, Angeletti A, Manrique J, Renieri A, et al. : A glomerulus-on-a-chip to recapitulate the human glomerular filtration barrier. Nat Commun 10: 3656, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Vormann MK, Vriend J, Lanz HL, Gijzen L, van den Heuvel A, Hutter S, et al. : Implementation of a human renal proximal tubule on a chip for nephrotoxicity and drug interaction studies. J Pharm Sci 110: 1601–1614, 2021 [DOI] [PubMed] [Google Scholar]